Abstract
Converging lines of evidence suggest that the cerebellum plays an integral role in cognitive function through its interactions with association cortices like the medial frontal cortex (MFC). It is unknown precisely how the cerebellum influences the frontal cortex and what type of information is reciprocally relayed between these two regions. A subset of neurons in the cerebellar dentate nuclei, or the homologous lateral cerebellar nuclei (LCN) in rodents, express D1 dopamine receptors (D1DRs) and may play a role in cognitive processes. We investigated how pharmacologically blocking LCN D1DRs influences performance in an interval timing task and impacts neuronal activity in the frontal cortex. Interval timing requires executive processes such as working memory, attention, and planning and is known to rely on both the frontal cortex and cerebellum. In our interval timing task, male rats indicated their estimates of the passage of a period of several seconds by making lever presses for a water reward. We have shown that a cue-evoked burst of low-frequency activity in the MFC initiates ramping activity (i.e., monotonic increases or decreases of firing rate over time) in single MFC neurons. These patterns of activity are associated with successful interval timing performance. Here we explored how blocking right LCN D1DRs with the D1DR antagonist SCH23390 influences timing performance and neural activity in the contralateral (left) MFC. Our results indicate that blocking LCN D1DRs impaired some measures of interval timing performance. Additionally, ramping activity of MFC single units was significantly attenuated. These data provide insight into how catecholamines in the LCN may drive MFC neuronal dynamics to influence cognitive function.
Keywords: Cerebellum, medial frontal cortex, interval timing, lateral cerebellar nuclei, dopamine
1. Introduction
Dopamine is a vital neurotransmitter for cognitive function (Goldman-Rakic, 1998; Ott and Nieder, 2019). In the rat, the deep cerebellar nuclei are more enriched with dopamine than the cerebellar cortex, with concentrations similar to those observed in frontal cortex (Versteeg et al., 1976). Tyrosine hydroxylase positive fibers richly innervate the cerebellar nuclei of the rodent (Nelson et al., 1997). D1 dopamine receptors (D1DRs) are expressed in the striatum, amygdala, olfactory bulb, cerebellum, and frontal cortex (Huang et al., 1992; Ariano and Sibley, 1994; Bergson et al., 1995). However, little is known about the functional role dopamine plays in the cerebellum. Here we focus on a highly understudied population of D1DR-expressing neurons in the lateral cerebellar nuclei (LCN) of the cerebellum (Nelson et al., 1997; Barili et al., 2000; Melchitzky and Lewis, 2000; Delis et al., 2004) that have only recently been implicated in cognitive functions (Locke et al., 2018).
The rodent LCN, homologous to the human dentate nuclei, appear to carry the majority of cerebellar cognitive output, sending projections to areas including the contralateral frontal cortex via the thalamus (Kim et al., 1994; Dum et al., 2002; Tellmann et al., 2015). D1DR-expressing neurons of the LCN have been demonstrated to be critical for cognitive tasks such as response inhibition, spatial navigation memory, social recognition, and prepulse inhibition (Locke et al., 2018). D1DR neurons of the LCN have been specifically implicated in cognitive processes and mark a medial-caudal-ventral aspect of the nucleus (Giompres and Delis, 2005; Locke et al., 2018). The majority of these D1DR neurons appear to be GABAergic or glycinergic, with projections to the cerebellar cortex and locally within the nucleus (Locke et al., 2018). The source of dopaminergic innervation to the LCN may include the VTA (Simon et al., 1979; Panagopoulos et al., 1991; Ikai et al., 1992) and the locus coeruleus (Oliver et al., 1971), but has yet to be established.
Although typically associated with motor learning in the sub-second range, (Garcia and Mauk, 1998; Bracha, 2004) there is evidence that the cerebellum may be recruited for interval timing in the range of seconds (Ohmae et al., 2017; Parker et al., 2017). Furthermore, human lesion studies and primate neural recordings indicate the caudal portion of the dentate nucleus is involved in the internal monitoring of time (Gooch et al., 2010; Ashmore and Sommer, 2013; Ohmae et al., 2017). Dopaminergic circuitry and binding of both D1 and D2-type dopamine receptors is necessary for successful interval timing (Buhusi, 2003; Drew et al., 2003; Meck, 2006; Parker et al., 2014a; De Corte et al., 2019). Interval timing also requires frontal executive processes such as working memory, attention, and planning (Parker et al., 2014a; Xu et al., 2014; Kononowicz, 2015). Interval timing can be measured in rodents by presenting rats with a cue (e.g., houselight) that requires them to make a response (e.g., lever press) after a certain interval of time has elapsed to earn reward (e.g., water delivery). Time frequency analyses reveal that when the cue is presented at trial-start, there is a burst of low-frequency (delta/theta) activity in the frontal cortex (Parker et al., 2014a) and cerebellum (Parker et al., 2017) that may cause single neurons to begin ramping (i.e., monotonically increasing or decreasing activity) to encode the passage of time. These activity patterns are essential for timing and are impacted in patients with schizophrenia (Parker et al., 2017) and first-degree relatives (Penney et al., 2005). Recently, we reported similar absence of low-frequency brain rhythms in patients with schizophrenia and in animals with D1DRs pharmacologically blocked in the frontal cortex (Parker et al., 2017). Optogenetically stimulating the LCN at 2Hz throughout the interval reinstated normal rhythms and ramping activity in single units in the frontal cortex, as well as recovered timing performance (Parker et al., 2017). These data indicate that the cerebellum may influence MFC network dynamics during timing tasks.
To further understand the nature of this circuit and explore cerebellar modulation of frontal networks, we investigated how pharmacologically manipulating D1DR-expressing neurons in the cerebellum influences timing performance and neuronal activity in the frontal cortex. In this study, animals trained in a fixed-interval timing task received an infusion of D1DR antagonist SCH23390 into the right LCN. Neuronal activity was recorded in the left medial frontal cortex (MFC). Our results indicate that blocking LCN D1DRs impairs interval timing performance. Additionally, ramping activity of MFC single units was significantly attenuated when LCN D1DRs were blocked. These data provide insight into how the cerebellum influences medial frontal networks in behaving animals and indicates that cerebellar neurons expressing D1DRs may modify cognitive capacities that rely on the frontal cortex.
2. Materials and Methods
2.1. Rodents
A total of 13 male Long-Evans rats (aged 3 months) were included in this study, 6 of which had neuronal ensemble recordings. One additional animal was excluded on the basis of a change in timing efficiency between the saline and DIDR-block conditions that was greater than 2 standard deviations (SD) beyond the mean change in efficiency of the other animals. Animals were well trained in a fixed interval timing task prior to surgery and testing. Surgical procedures, neurophysiological recordings, and focal drug infusions were performed according to procedures described previously (Parker et al., 2014a, 2015a, 2015c). Single housing and a 12 hour light/dark cycle were used; all experiments took place during the light cycle. Rats were maintained at ~90% of free-access body weight during the course of these experiments and received one day of free access to water per week. All procedures were approved by the Animal Care and Use Committee at the University of Iowa.
2.2. Rodent Interval timing task
All behavioral training and testing took place in operant chambers (MedAssociates, St Albans, VT). All rats were trained to perform an interval timing task according to previously published methods (Narayanan et al., 2012; Parker et al., 2014a, 2015a, 2015c). Water restricted rats were trained to make operant lever presses to receive water rewards. After a period of fixed-ratio lever training, 12 s fixed-interval trials were introduced (cued by a house light) in which rewards were delivered for responses made after a 12 s interval elapsed. Rewarded presses were signaled by an auditory click stimulus, extinguishing of the house light, and delivery of water. Presses made before the target duration elapsed were not rewarded. Once animals were well-trained (average response curves indicated lever pressing clustered around the 12 s interval), a second trial type was introduced and also rewarded with water. For this, a second light cue located on the right side of the lever was presented in addition to the house light cue to indicate a shorter 3s target interval. Cues turned on at trial onset and lasted until the onset of the intertrial interval, which coincided with a rewarded press or, if no press was made after 12 s, after 18 s from trial onset. Each rewarded trial was followed by a 6, 8, 10 or 12 s pseudorandom intertrial interval. A time-out occurred after 18s on trials with no responses after 12 s. Training and testing sessions were 60 minutes in duration. Trial type order (i.e., 3 s or 12 s target duration) was randomized throughout the session. Operant chambers were housed in sound-attenuating cabinets (MedAssociates). Water rewards were delivered via a pump (MedAssociates) connected to a metal drinking tube (AnCare) via Tygon tubing. Animals were motivated by regulated access to water, while food was available ad libitum. Rats consumed 10–15 mL of water during each behavioral session and additional water (5–10 mL) was provided 1–3 hours after each behavioral session in the home cage.
To assess interval timing performance, we first quantified average response times between the time of trial start and the time of reward availability. Second, we analyzed how efficient an animal was at estimating the interval as the number of responses between 11–12 s divided by the overall number of responses during the 12 s trial. We performed the same analysis for 3 s trials, but only assessed responses within the first 3 s of this trial type. Efficiency scores closer to 1 reflect a greater number of responses occurring near the to-be-timed interval, indicating more temporally guided performance (Parker et al., 2015c, 2015b). Finally, we assessed single-trial start times for the 12 s trials only. This analysis focuses on the fact that, during individual trials, rats begin by responding at a constant, low rate. Then, as the time of reward approaches, they abruptly start responding at a constant, high rate in anticipation of reward (i.e., start-time). To identify single-trial start times, we fit 2 flat lines (first low, second high) to individual-trial response rates across time, using conventional 1 s time bins. We iteratively moved the transition point between the two lines across bins until absolute residuals were minimized (Guilhardi and Church, 2004; De Corte et al., 2018).
2.3. Rodent surgery
Rats trained in the interval timing task were implanted with a custom 16-channel microwire array (Fig 2A; MicroProbes) in the left MFC (n=6; AP: +3.2, ML: ±1.2, DV: −3.5 @ 12° in the lateral plane). Based on electrode geometry and the midpoint of placement, the vast majority of recordings were made in prelimbic cortex. However, it is possible that a few single units from anterior cingulate or orbital frontal cortex were recorded. Silent channels were recovered from the white matter of the corpus callosum. Rats were also implanted with a single 22-gauge infusion cannula (Plastics One) in the right LCN (n=13; AP:−10.8, ML: ±3.6, DV:−6.2). The right cerebellum was specifically targeted in this experiment based on reports of laterality to the cerebellar contribution to cognition. The right cerebellar lobules that project to right deep nuclei and the left MFC are preferentially activated during cognitive functional neuroimaging tasks (Stoodley et al., 2012). Therefore, we also unilaterally target the left MFC which is one of the projection targets of the contralaterally projecting cerebellar thalamic pathway. A surgical level of anesthesia was maintained with hourly (or as needed) ketamine supplements (10 mg/kg). The electrode array was inserted while concurrently recording neuronal activity to verify implantation in layer II/III of the MFC. The craniotomy was sealed with cyanoacrylate (‘SloZap’, Pacer Technologies, Rancho Cucamonga, CA) accelerated by ‘ZipKicker’ (Pacer Technologies), and methyl methacrylate (i.e., dental cement; AM Systems, Port Angeles, WA). Following implantation, animals recovered for one week before being reacclimatized to behavioral and recording procedures.
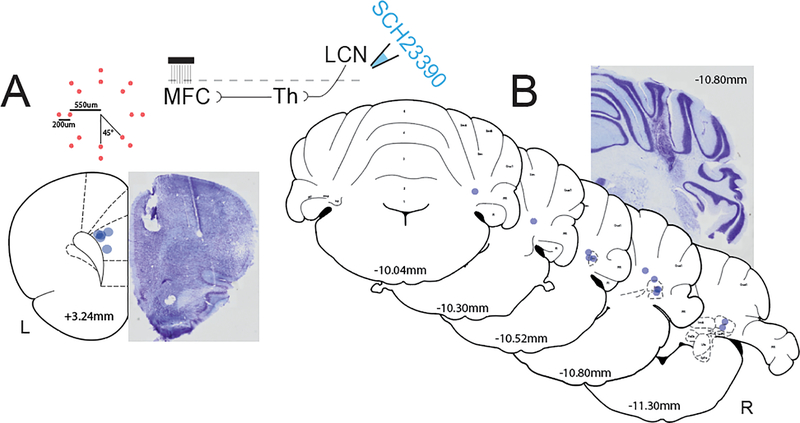
Fig 2: Histological reconstruction of LCN infusion cannulae and MFC electrode arrays.
A) Central placement of MFC electrode arrays (blue dots, N=5) with distance from bregma and example thionin-stained section. Inset top left: top-down view diagram of custom electrode geometry with measurements from center, red dots indicate individual channels. Inset top middle: circuit diagram illustrating thalamic relay from LCN D1DRs to the MFC. B) LCN cannula placements (blue dots, N=12) with distance from bregma ranging from −10.04mm to −11.30mm. Example thionin-stained section with LCN cannula tract at −10.80mm from bregma. Coronal MFC and cerebellar templates adapted from Paxinos & Watson (2007).
2.4. Rodent perfusions and histology
When experiments were complete, rats were anesthetized, euthanized by injections of 100 mg/kg sodium pentobarbital, and transcardially perfused with 10% formalin. Brains were post fixed in a solution of 10% formalin and 20% sucrose before being sectioned on a freezing microtome. Brain slices were mounted on gelatin-subbed slides and stained for cell bodies using DAPI (ThermoFisher). Histological reconstruction was completed using post mortem analysis of electrode and cannula placements and confocal microscopy in each animal. These data were used to determine locations for the electrode array and infusion cannula (Fig 2). To verify the presence of D1DR-expressing neurons in the LCN, a D1DR antibody (rat anti-D1 dopamine receptor; Sigma-D2944) and tyrosine hydroxylase (rabbit anti-TH; Millipore-AB152; 1:500) were also used on cerebellar tissue.
2.5. Focal drug infusions
Focal drug infusions into the LCN were performed according to procedures described previously (Parker et al., 2014a, 2015a, 2015c). Prior to behavioral testing, the LCN was infused with either 0.9% saline (Phoenix Scientific, St. Joseph, MO) during control sessions or D1-dopamine receptor antagonist SCH23390 (0.5 μg of 1.0 μg/μL) (Parker et al., 2013, 2014a). Infusions were carried out while the animal was lightly anesthetized with isoflurane. An injector was inserted into the guide cannula and 0.5 μL of fluid was delivered at a rate of 30 μL/hr (0.5 μL/min) via a syringe infusion pump (KDS Scientific, Holliston, MA). After completing an infusion, the injector was left in place for 2 minutes to allow for diffusion and the experiment began after a 30 minute period for recovery from isoflurane. Subjects were first tested under saline infusion conditions, followed by SCH23390 infusion at least 24 hours later.
2.6. Neurophysiological analyses
Neuronal ensemble recordings in the MFC of awake behaving animals were acquired using a multi-electrode recording system (Plexon, Dallas, TX). Putative single neurons were identified on-line using an oscilloscope and audio monitor. Plexon off-line sorter was used to analyze the signals after the experiments and to remove artifacts. Spike activity was analyzed for all cells that fired at rates above 0.1 Hz. Statistical summaries were based on all recorded neurons. Principal component analysis (PCA) and waveform shape were used for spike sorting. Single units were identified as having 1) consistent waveform shape, 2) separable clusters in PCA space, and 3) a consistent refractory period of at least 2 ms in interspike interval histograms. Preliminary analysis of neuronal activity and quantitative analysis of basic firing properties were carried out using NeuroExplorer (Nex Technologies, Littleton, MA), and quantitative analyses were performed with custom routines for MATLAB. Peri-event rasters and average histograms were constructed around light on, lever release, lever press, and lick events. Peri-event time histograms were calculated by recording the time of each putative action potential around cue onset with 0.01 s bins. Each occurrence of the cue was considered a trial, and putative action potentials were plotted relative to these events (0–12 s) using a raster plot. Histograms were calculated by taking the average firing rate and smoothing over 1 s using a Gaussian window. Data were tested for normality prior to subsequent analyses.
We defined time-related ramping activity as firing rate that increased or decreased over the interval. We measured this in two ways: PCA and linear regression. PCA can be used to identify key patterns in a population of neurons using orthogonal basis functions from peri-event histograms during the 12 s interval (Parker et al., 2014a, 2015c). This unbiased, data driven approach is an objective way to define neuronal firing patterns as it does not make prior assumptions or average activity over trials and between units. To analyze PCA, all neurons are pooled from both drug conditions. The same principal components were projected onto experiments with saline and LCN SCH23390, and PC weights were compared via a t-test (Parker et al., 2014a). Secondly, neuronal activity fitting a linear model of firing rate over the interval was characterized using fitlm in MATLAB (p<0.05).
3. Results
3.1. Histology
3.1.1. D1DR expression in cerebellum
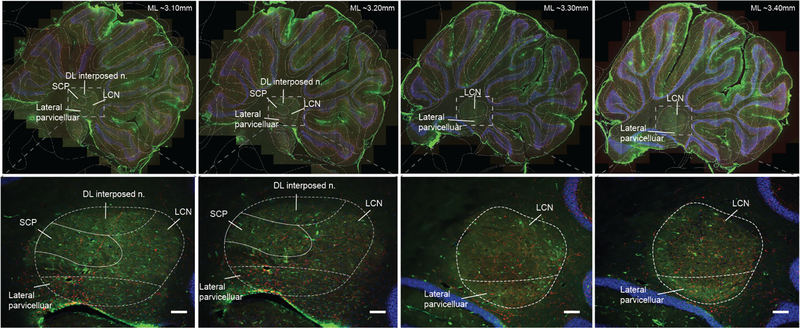
A subset of neurons in the LCN express D1DRs. Prior work indicates that these neurons are restricted to the ventral-caudal aspect of the LCN which is consistent with an identified cognitive region in the homologous dentate nucleus in humans (Dum et al., 2002; Küper et al., 2011; Tellmann et al., 2015; Locke et al., 2018). The presence of these neurons in the rat LCN was confirmed here using a D1DR antibody (green; Fig 1). The regions selected represent −3.10 to −3.40 mm in the medial-lateral plane as identified using the Allen Rat Brain Atlas. Further histological analyses will need to be done to determine if the D1DR expressing neurons are organized in a similar pattern in the rat. Additionally, tyrosine hydroxylase (red; Fig1) identifies axons of dopaminergic neurons likely originating in the VTA and/or the locus coeruleus (Locke et al., 2018) that may innervate this region in rats. Future studies will further define catecholamines in the LCN.
Fig 1: D1DRs are expressed on neurons in the LCN.
D1DRs (green, D1 dopamine antibody) are present in the lateral cerebellar nuclei −3.10 to −3.40 mm in the medial-lateral plane as identified using the Allen Rat Brain Atlas. Additionally, staining for tyrosine hydroxylase (a dopamine precursor, red) identifies axons of dopaminergic neurons likely originating in the VTA and/or the locus coeruleus. Outlined areas indicate putative subregions of sagittal sections through the cerebellar nuclei based on the Allen Rat Brain Atlas from −3.10 to −3.40 mm in the medial-lateral plane. These areas include: superior cerebellar peduncle (SCP), dorsal lateral hump of the interposed nuclei (DL interposed n.), lateral parvicellular, and lateral cerebellar nuclei (LCN).
3.1.2. Cannula and Electrode Placements
Histological reconstruction of LCN cannula (n=12) and MFC electrode placements (n=5) was completed using a combination of DAPI and thionin stained sections (one animal was unable to be included for histological reconstruction; Fig 2). The MFC location was successfully targeted in all 5 animals (Fig 2A). There was increased variability with the LCN cannula placement with two animals having the estimated target more rostral than preferred (Fig 2B). Given the large cannula diameter (700um external/400um internal), large volume of the drug infusions (0.5 μL which is estimated to have a diffusion radius of about 1 mm (Martin, 1991)), it is likely that even at the most rostral location, the drug had the desired of effect of binding D1DRs in the LCN. This was confirmed by a robust decrease in timing efficiency on 12s trials for the two animals with the most rostral cannula placements following SCH23390 in the LCN in comparison to saline, consistent with the rest of the sample. All other cannula placements were within or above the LCN.
3.2. Behavioral results
3.2.1. Interval timing
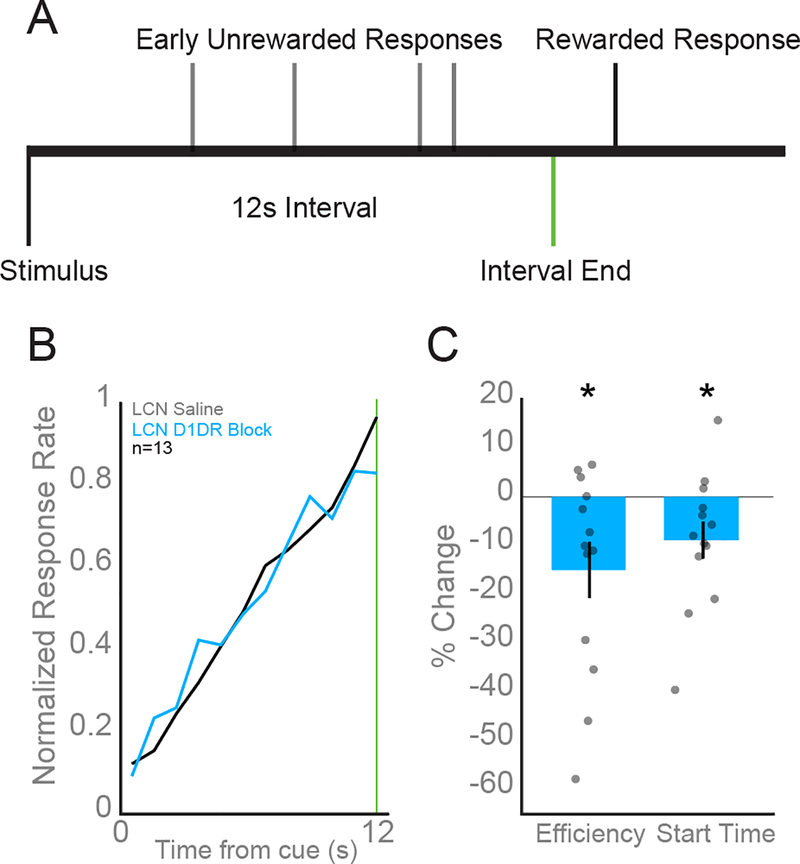
Sub-second timing is a well-characterized capability of the cerebellum (Ivry and Spencer, 2004) while its role in supra-second timing is less understood. The cerebellum may participate in supra-second processing and timing at these intervals also recruits regions of the contralateral frontal cortex (Ohmae et al., 2017; Parker et al., 2017). To determine the effect of cerebellar D1DRs on interval timing performance, we infused either 0.5 μL saline or 1 mg/mL D1DR antagonist SCH23390 into the right LCN prior to performance in animals well-trained in the fixed interval timing task. The behavioral responses of animals well-trained in the timing task show clustering near the end of the 3 s or 12 s interval. Rodents with saline infused in the LCN had an average response time of 7.80 ± 0.14s while animals with SCH23390 had average response time of 7.56 ± 0.14 s (Fig 3B) in 12 s trials and 2.00 ± 0.05 s vs 2.05 ± 0.07 s on 3s trials. Although the average response times were not significantly different, we found LCN D1DR blockade significantly impaired performance on 12s trials as measured by efficiency (saline 0.16 ± 0.006 vs SCH23390: 0.13 ± 0.01; t(12) = −2.65; p = 0.02; Fig 3C) and start times (saline: 5.19 ± 0.19 vs SCH23390: 4.76 ± 0.30; t(12) = −2.24; p = 0.045; Fig 3C). There was no significant effect of LCN D1DR blockade on timing efficiency during 3s trials (saline 0.55 ± 0.037 vs SCH23390: 0.60 ± 0.05; t(12) = 0.64; p = 0.54).
Fig 3: D1DR antagonist SCH23390 in the LCN impairs interval timing performance.
A) Rodents estimated the passage of a 12 s period by making a lever press. The house light turning on served as the stimulus that signaled the start of each trial. Water reward was dispensed for the first lever press that occurred following the elapse of 12 s. B) Average response histograms indicate animals with D1DRs in the LCN blocked using 0.5μl of 1 mg/mL SCH 23390 (blue) responded as if the interval had elapsed earlier compared to responses in saline sessions (black). C) Additionally, animals with LCN D1DRs blocked had significantly impaired interval timing efficiency and response start times compared to control sessions with LCN saline in 12 s trials. All data are presented as mean +/− SEM. Asterisks indicate significance at p<0.05.
3.2.2. Motor Performance
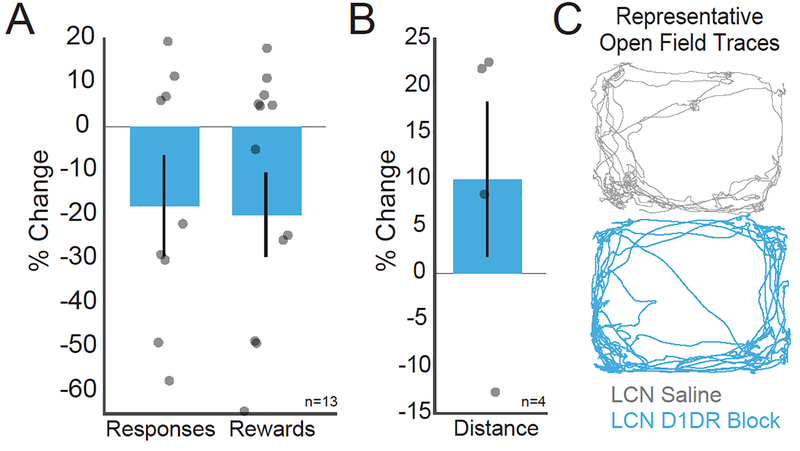
To effectively study cognitive processes, it is essential to confirm that manipulations do not alter motor function. There were no significant differences in motor function between saline and LCN D1DR blockade conditions as measured by number of responses (saline vs SCH23390: 508.31 ± 64.87 vs 405.77 ± 75.50; t(12) = −1.44, p = 0.18) and rewards earned (103.69 ± 10.54 vs 82.85 ± 13.58; t(12) = −1.74, p = 0.11; Fig 4A, B). Additionally, in four subjects, there were no significant differences in the total distance traveled or speed (mm/sec) in 5 minutes of open field between LCN saline or D1DR block (saline vs SCH23390, distance: 16599 ± 1618 vs 17906 ± 935; t(3) = 0.98, p = 0.4; Fig 4C). These data suggest that LCN D1DR blockade specifically influenced timing of responses without influencing gross motor demands of the task.
Fig 4: Blocking D1DRs in the LCN does not impair motor output.
There were no clear motor effects following D1DR blockade in the LCN in comparison to control infusions of saline. The percent change between LCN D1DR blockade and LCN saline infusions for A) Reponses per behavioral session, B) rewards per behavioral session, and C) open field speed (mm per sec) were non-significantly different (see example trace of open field distance traveled at right).
3.3. Cerebellar modulation of frontal cortical single unit activity
Previous studies indicate that neurons in the frontal cortex ramp during successful performance of the interval timing task (Matell et al., 2003; Kim et al., 2013; Parker et al., 2014a, 2015a, 2017; Donnelly et al., 2015; Gouvea et al., 2015; Narayanan, 2016). This monotonically increasing or decreasing ramping activity in the frontal cortex is hypothesized to encode the passage of time (Niki and Watanabe, 1979; Durstewitz, 2003; Kim et al., 2013; Parker et al., 2014a; Xu et al., 2014). Using granger causality analyses, we have also shown that low-frequency activity in the cerebellum leads activity in the frontal cortex during interval timing, and that LCN stimulation can reinstate neuronal ramping patterns in a suboptimally functioning MFC (Parker et al., 2017). If LCN D1DR-expressing neurons are essential for cognitive function, inactivation should disrupt frontal ramping activity, indicating a potential mechanism for the decreased performance on the interval timing task. To test this hypothesis, six rodents were implanted with recording electrodes in the left MFC and an infusion cannula in the right LCN. D1DRs in the LCN were blocked using a microinfusion of 0.5 μL of 1 mg/mL SCH23390. As animals specifically showed impairments following LCN D1DR blockade during 12 s trials, neuronal recording data from only 12 s trials are discussed. 137 neurons from six rats were isolated in saline sessions (1.43 ± 0.08 neurons per electrode; 22.83 ± 1.33 neurons per rodent) and 110 neurons were identified from the same rats in MFC D1DR receptor blockade sessions (1.15 ± 0.23 neurons per electrode or 18.33 ± 3.64 neurons per rodent). These neurons are assumed to be independent populations because the recordings took place on different days.
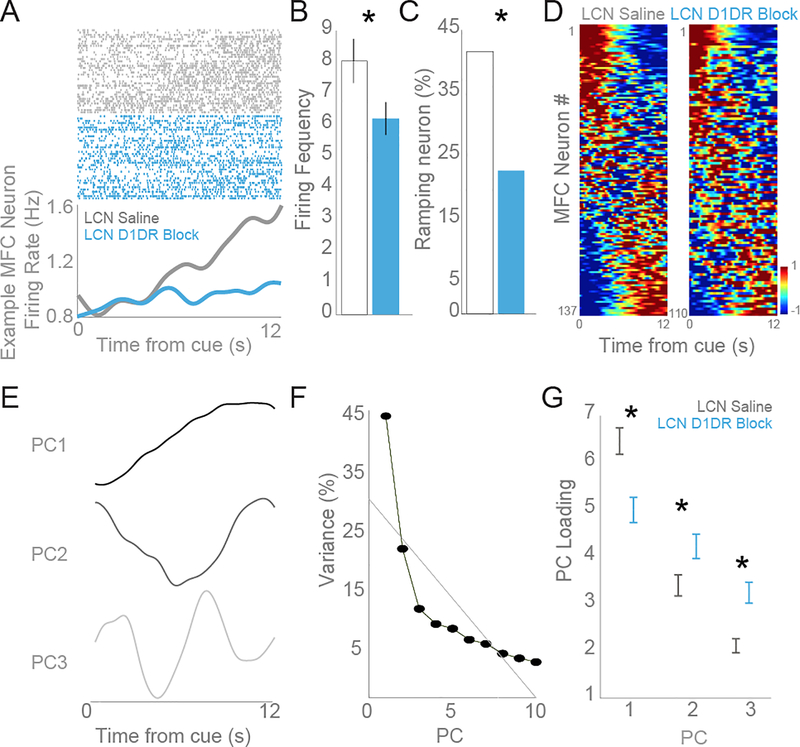
Consistent with our previous studies, we found that more than a third of the neurons in the frontal cortex showed a ramping pattern of activity, namely a consistent increase or decrease in firing rate over time within individual trials (Fig 5A), as revealed by linear regression. The ratio of neurons with a ramping pattern we observed is consistent with previous reports of frontal neurons during timing tasks (Matell et al., 2003; Narayanan and Laubach, 2009; Kim et al., 2013; Parker et al., 2014a; Xu et al., 2014). In saline control sessions, 57 of 137 (42%) neurons had significant ramping activity while LCN D1DR blockade significantly reduced the number of MFC neurons with ramping activity (23%, 25 of 110 neurons; χ2 = 9.80; p = 0.0017; Fig 5C). These data provide evidence that LCN D1DR blockade attenuates MFC ramping activity without significantly influencing the slope of ramping neurons (saline vs SCH23390: 0.098 ± 0.001 vs .0107 ± 0.021; t(80)=−0.40, p = 0.69) or motor performance. However, there was a significant decrease in the firing frequency of neurons following LCN D1DR blockade (saline vs SCH23390: 8.04 ± 0.70 vs 6.22 ± 0.52; t(245) = 2.00, p = 0.046; Fig 5B). Despite this difference in average MFC firing frequency between conditions, ramping activity was measured using the normalized firing rate so the difference in absolute firing rate does not mitigate the main finding of attenuated MFC ramping activity following LCN D1DR blockade.
Fig 5: Blocking D1DRs in the LCN attenuated ramping activity in the frontal cortex.
Neurons in the frontal cortex ramp to encode the passage of time. A) An individual example of a neuron with robust time-related ramping – i.e, neural activity that consistently increased / decreased activity over the interval is shown. Peri-event rasters are sorted with respect to mean response time, with trials with a short mean response time on the bottom and trials with a longer mean response time on top. Single neurons were isolated based on waveform and interspike intervals. A raster plot (top) indicates the activity of a single unit where each dot represents an action potential. Rasters are sorted by mean response time. The average activity histogram (bottom) shows that the ramping activity from a putative single unit in the saline condition (gray) has an attenuated ramping pattern following D1DR blockade (blue). From six animals, a total of 137 frontal neurons were recorded following saline infusion in the LCN and 110 following LCN D1DR blockade. B) The firing frequency was reduced from 8.04 to 6.22 following LCN D1DR blockade. C) Regression analyses revealed that 57 of 137 MFC neurons (42%) had a consistent ramp over time following LCN saline while LCN D1DR blockade diminished the number of MFC ramping neurons to 25 of 110 neurons (23%). D) Heat-maps of peri-event time histograms revealed that time-related ramping was a ubiquitous feature of MFC neurons in the saline condition (left) and this pattern was changed following LCN D1DR blockade (right). Neurons are sorted based on PC1. Color scale is at bottom right. E) Principal component analysis, a data-driven technique, identified interval-related ramping as the first principal component in the MFC. F) The amount of variance explained by PC1 was 44%, 21% for PC2, 11% for PC3. G) The weight of PC1 was significantly reduced following LCN D1DR blockade in comparison to saline condition whereas the weight of PC2 significantly increased following LCN D1DR blockade. Asterisk indicates significance at p<0.05 via t-test, displayed as mean +/− SEM.
Principal component analysis was used as a data driven approach to further investigate patterns in MFC neural activity. Similar to the regression results, the most prominent principal component (PC1) was a ramp (Fig 5D). The pattern of activity identified by PC1 explained 44% of the variance in our dataset while the second principal component (PC2) explained only 21% variance (Fig 5D). Progressively less variance was explained by the smaller components. If LCN D1DR activity is involved in time estimation, we expect that blocking LCN D1DR activity would decrease frontal cortical ramping activity and explain less variance by PC1 with LCN D1DR blockade than with saline. To analyze this, we calculated the strength of each principle component in the two drug conditions (see heatmaps of all neurons sorted by PC1 in Fig 5D). We found that LCN D1DR blockade significantly reduced the loading of PC1 (saline vs SCH23390: 6.47 ± 0.28 vs 5.00 ± 0.27; t(245)= 3.69, p = 0.0003; Fig 5E), consistent with the regression analysis showing a reduction in ramping neurons during these sessions. Furthermore, PC2 had a significantly higher weight when LCN D1DRs were blocked compared to the saline condition (saline vs SCH23390: 3.39 ± 0.22 vs 4.23 ± 0.26; t(245)=−2.44, p < 0.015). PC3, like PC2, also had a significantly higher weight when LCN D1DRs were blocked relative to the saline condition (saline vs SCH23390: 2.11 ± 0.15 vs 3.24 ± 0.22; t(245)=−4.34, p < 0.0001).
4. Discussion
In this study, we tested the hypothesis that neurons expressing D1DRs in the LCN are required for interval timing and drive essential timing-related patterns of activity in the frontal cortex. Our results indicate that blocking D1DRs in the LCN impairs some measures of interval timing performance and attenuates downstream single unit ramping activity without strongly affecting gross motor performance. These data provide novel evidence that cerebellar D1DR-expressing neurons are involved in supra-second timing.
The cerebellum is typically associated with precise timing in the sub-second range such as that required for eyeblink conditioning (Spencer and Ivry, 2013). However, we have recently shown that inactivating the LCN with the GABAA agonist muscimol impairs timing performance on a supra-second interval timing task (Parker et al., 2017). Additionally, stimulating cerebellar projections to the frontal cortex in the thalamus reinstates ramping activity in single units in the frontal cortex and rescues timing performance during medial frontal D1DR blockade (Parker et al., 2017). Taken together, these past findings generally implicate the LCN and its thalamo-frontal projections as instrumental in interval timing tasks, but do not provide a more detailed understanding of what cell populations and transmitters within the LCN are necessary for successful performance. Here, we demonstrated specifically that D1-expressing neurons in the LCN are necessary for interval timing and appear to modulate medial frontal ramping activity during task performance. Collectively, these data implicate the cerebellum as an essential node in a distributed supra-second interval timing system (Meck, 2005).
Beyond supporting the involvement of the cerebellum in interval timing, our results further refine this position by providing evidence that dopaminergic signaling in the cerebellum is vital for its participation in interval timing. Stimulus-evoked bursts of dopamine release from the midbrain to multiple distributed brain regions (e.g., the frontal cortex and striatum) are hypothesized to serve as “start signals” during interval timing tasks that cause these regions to begin functioning as a unified network (Matell and Meck, 2000, 2004; Meck, 2005; Teki et al., 2012). By blocking D1 receptors in the LCN, we may have prevented the cerebellum from receiving this start signal, thereby preventing its recruitment and disrupting the network as a whole. Additional studies are needed to integrate research about precise dopaminergic inputs to the LCN and the cognitive role of the cerebellum. Furthermore, the cerebellum may participate in both the initiation and adjustment components of interval timing under certain task conditions, making it instrumental in reducing variability in timing (Petter et al., 2016). Consistent with this, our data indicate that blocking D1DRs in the LCN impaired timing behavior during 12 s trials and reduced frontal ramping activity, which has been shown to be critical for successful timing performance (Emmons et al., 2017). However, D1DR blockade in the right LCN did not significantly affect timing performance during 3 s trials. This suggests that D1 activation in the LCN may only be necessary for timing more difficult (i.e., longer) durations. Broadly, these findings suggest that the cerebellum is providing some type of relevant information to other regions in the timing network. Further studies focusing on this corticocerebellar circuit and dopaminergic inputs to the cerebellum in the context of interval timing will be necessary to elucidate the type of information being conveyed by the cerebellum.
There are a few caveats to the present study. First is the lack of counterbalanced infusion order with saline infusions always preceding SCH23390. Second, the compounded light cues for the 3 s (house light + second light) and 12 s (house light only) fixed interval trial types represent a potential confound of cue discrimination in the behavioral data. Namely, the D1DR blockade related deficits described on the 12 s interval may be due to either difficulty in timing the interval or difficulty determining which trial type is being presented. Third, while it is likely that the LCN was sufficiently targeted by all infusions it is possible that nearby structures, such as the interpositus nucleus, also received some volume of infusion due to the volume and spread parameters described above. Fourth, with only four animals included in the open field condition, we may not have significant power to conclude that no motor impairments were induced by LCN D1DR blockade. Additional work should investigate the LCN D1DR blockade on additional motor tasks. Lastly, although SCH23390 has been reported to robustly bind D1DRs (Schulz et al., 1985), the use of this drug in the LCN has not been previously reported. However, Klitenick et. al (2005) reported a decrease in Fos-like immunoreactivity in cerebellar lobules I-X in animals exposed to an IP infusion of SCH23390 (versus those receiving distilled water) and then given a systemic infusion of d-amphetamine and cocaine indicating some binding of D1 receptors in the cerebellar cortex (Klitenick et al., 1995). Despite this finding, we cannot exclude the role of SCH23390 off target binding to D5 receptors. Additional work is necessary to investigate the properties of SCH23390 binding in the cerebellar cortex versus the cerebellar deep nuclei.
To our knowledge, our present study and our previous report (Parker et al., 2017) are the first to show that optogenetically or pharmacologically manipulating the cerebellum results in electrophysiological changes in the prefrontal cortex. Prior work has only implicated neocortical activity driving changes in the cerebellar cortex (Roš et al., 2009). Based on the work of Locke et al., we hypothesized that the LCN may be modulating the MFC through dopaminergic signaling (Locke et al., 2018) and we found that specifically manipulating cerebellar D1DRs modifies downstream neuronal dynamics and influences performance on a timing task that probes elementary cognitive function. Electrical stimulation of the LCN causes dopamine release in the frontal cortex (Mittleman et al., 2008). We have previously shown that ramping activity in the frontal cortex is dopamine dependent, and that loss of ramping impairs interval timing performance (Parker et al., 2013). Our previous data support the hypothesis that ramping activity originates in the cerebellum and is relayed to the frontal cortex via connections in the thalamus (Parker et al., 2017). However, it should be noted that output from the LCN may also influence timing performance through projections relayed in the thalamus to the striatum, another region essential for interval timing (Petter et al., 2016; Emmons et al., 2017).
The notion that cerebellar output is required for cognition and a powerful modulator of frontal activity makes cerebellar circuitry an appealing target for future therapeutics. In particular, patients with schizophrenia may benefit from therapies that modulate frontal activity by means of cerebellar manipulation without influencing gross motor function. In patients with schizophrenia, there is a decrease in D1 dopamine binding potential in the frontal cortex, which correlates to impairments in working memory (Abi-Dargham et al., 2002). Ramping patterns in the frontal cortex rely on dopamine which is also known to be essential for a range of cognitive functions (Matell et al., 2003; Parker et al., 2014a; Donnelly et al., 2015; Narayanan, 2016; Kim et al., 2017). There have been many studies targeting frontal dopamine in schizophrenia, however, the main target of antipsychotic medications is D2 dopamine receptors with smaller off-target effects on D1 receptors. Cerebellar transcranial magnetic theta burst stimulation effectively relieves some cognitive and negative symptoms in treatment-resistant schizophrenia patients (Demirtas-Tatlidede et al., 2010; Garg et al., 2016). Additionally, cerebellar stimulation influences frontal cortical activity (Schutter et al., 2003; Halko et al., 2014). We have previously shown that stimulating LCN projections in the thalamus boosts cognitive control signals and rescues frontal D1DR-blockade induced dysfunction (Parker et al., 2017). Taken together, these data incite the potential for cerebellar-targeted therapeutics to influence frontal cortical function in human diseases of impaired cognition such as schizophrenia.
4.1. Conclusions
Our current results suggest that specifically stimulating LCN D1DRs may be uniquely effective in modulating MFC circuitry and cognitive processing. Future studies will investigate how manipulating dopamine and other neurotransmitter systems in the LCN influences local field activity in frontal networks. Future directions will further define the role of these neurons in fine motor function and explore a possible role for these GABAergic D1DRs in synchronization of LCN output, similar to GABAergic interneurons of the frontal cortex (Gonzalez-Burgos and Lewis, 2008). These data provide a novel and highly clinically relevant insight into cerebellar-frontal interactions which could inform future translational efforts targeting the cerebellum for patients with schizophrenia (Parker et al., 2014b).
Table 1:
Significant Classification Statistics – 12s Interval
| Within Subjects T-Test | stat | df | p-value | Figure |
|---|---|---|---|---|
| Sal vs SCH23390 Start Times | t = −2.24 | 12 | 0.045 | Fig 3D |
| Sal vs SCH23390 Efficiency | t = −2.65 | 12 | 0.021 | Fig 3D |
| Sal vs SCH23390 Firing Frequency | t = 2.00 | 245 | 0.046 | Fig 5B |
| Sal vs SCH23390 Ramping Neurons | χ2 = 9.80 | 0.0017 | Fig 5C | |
| Sal vs SCH23390 PC1 Loading | t = 3.69 | 245 | 0.0003 | Fig 5G |
| Sal vs SCH23390 PC2 Loading | t = −2.44 | 245 | 0.0155 | Fig 5G |
| Sal vs SCH23390 PC3 Loading | t = −4.35 | 245 | 0.00002 | Fig 5G |
Highlights.
D1 dopamine receptors expressing neurons are located in the lateral cerebellar nuclei and may play a key role in cognitive function.
Blocking D1 dopamine receptors in the lateral cerebellar nuclei impairs performance on an interval timing task
Manipulating D1 dopamine receptor expressing neurons does not influence motor performance.
Blocking D1 dopamine receptors in the lateral cerebellar nuclei attenuates monotonic increases or decreases in individual neurons in the medial frontal cortex that are necessary for the estimation of the elapse of time.
Acknowledgements
K.L.P has received generous funding to complete this research from the Brain & Behavior Foundation Young Investigator NARSAD Award, The Nellie Ball Research Trust, NIMH K01 MH106824, NIMH R01MH118240, the University of Iowa Department of Psychiatry, and the Iowa Neuroscience Institute. ESC NIMH R01MH1116883 was also used to fund this work.
Abbreviations
- LCN
Lateral cerebellar nucle
- MFC
medial frontal cortex
- TMS
transcranial magnetic stimulation
- PCA
Principal component analysis
Footnotes
Competing Interests
We do not have any competing interests to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abi-Dargham A, Mawlawi O, Lombardo I, Gil R, Martinez D, Huang Y, Hwang D-R, Keilp J, Kochan L, Van Heertum R, Gorman JM, Laruelle M (2002) Prefrontal dopamine D1 receptors and working memory in schizophrenia. J Neurosci 22:3708–3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariano MA, Sibley DR (1994) Dopamine receptor distribution in the rat CNS: elucidation using anti-peptide antisera directed against D1A and D3 subtypes. Brain Res 649:95–110. [DOI] [PubMed] [Google Scholar]
- Ashmore RC, Sommer MA (2013) Delay activity of saccade-related neurons in the caudal dentate nucleus of the macaque cerebellum. Journal of Neurophysiology:jn.00906.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barili P, Bronzetti E, Ricci A, Zaccheo D, Amenta F (2000) Microanatomical localization of dopamine receptor protein immunoreactivity in the rat cerebellar cortex. Brain Res 854:130–138. [DOI] [PubMed] [Google Scholar]
- Bergson C, Mrzljak L, Smiley JF, Pappy M, Levenson R, Goldman-Rakic PS (1995) Regional, cellular, and subcellular variations in the distribution of D1 and D5 dopamine receptors in primate brain. J Neurosci 15:7821–7836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracha V (2004) Role of the cerebellum in eyeblink conditioning. Prog Brain Res 143:331–339. [DOI] [PubMed] [Google Scholar]
- Buhusi C (2003) Dopaminergic Mechanisms of Interval Timing and Attention In: Functional and Neural Mechanisms of Interval Timing (Meck W, ed). CRC Press; Available at: http://www.crcnetbase.com/doi/abs/10.1201/9780203009574.ch12 [Accessed March 26, 2019]. [Google Scholar]
- De Corte BJ, Della Valle RR, Matell MS (2018) Recalibrating timing behavior via expected covariance between temporal cues. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Corte BJ, Wagner LM, Matell MS, Narayanan NS (2019) Striatal dopamine and the temporal control of behavior. Behavioural Brain Research 356:375–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis F, Mitsacos A, Giompres P (2004) Dopamine receptor and transporter levels are altered in the brain of Purkinje Cell Degeneration mutant mice. Neuroscience 125:255–268. [DOI] [PubMed] [Google Scholar]
- Demirtas-Tatlidede A, Freitas C, Cromer JR, Safar L, Ongur D, Stone WS, Seidman LJ, Schmahmann JD, Pascual-Leone A (2010) Safety and proof of principle study of cerebellar vermal theta burst stimulation in refractory schizophrenia. Schizophr Res 124:91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly NA, Paulsen O, Robbins TW, Dalley JW (2015) Ramping single unit activity in the medial prefrontal cortex and ventral striatum reflects the onset of waiting but not imminent impulsive actions. Eur J Neurosci:n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew MR, Fairhurst S, Malapani C, Horvitz JC, Balsam PD (2003) Effects of dopamine antagonists on the timing of two intervals. Pharmacol Biochem Behav 75:9–15. [DOI] [PubMed] [Google Scholar]
- Dum RP, Li C, Strick PL (2002) Motor and Nonmotor Domains in the Monkey Dentate. Annals of the New York Academy of Sciences 978:289–301. [DOI] [PubMed] [Google Scholar]
- Durstewitz D (2003) Self-Organizing Neural Integrator Predicts Interval Times through Climbing Activity. J Neurosci 23:5342–5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmons EB, Corte BJD, Kim Y, Parker KL, Matell MS, Narayanan NS (2017) Rodent medial frontal control of temporal processing in the dorsomedial striatum. J Neurosci:1376–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia KS, Mauk MD (1998) Pharmacological analysis of cerebellar contributions to the timing and expression of conditioned eyelid responses. Neuropharmacology 37:471–480. [DOI] [PubMed] [Google Scholar]
- Garg S, Sinha VK, Tikka SK, Mishra P, Goyal N (2016) The efficacy of cerebellar vermal deep high frequency (theta range) repetitive transcranial magnetic stimulation (rTMS) in schizophrenia: A randomized rater blind-sham controlled study. Psychiatry Res 243:413–420. [DOI] [PubMed] [Google Scholar]
- Giompres P, Delis F (2005) Dopamine transporters in the cerebellum of mutant mice. Cerebellum 4:105–111. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS (1998) The cortical dopamine system: role in memory and cognition. Adv Pharmacol 42:707–711. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Lewis DA (2008) GABA neurons and the mechanisms of network oscillations: implications for understanding cortical dysfunction in schizophrenia. Schizophr Bull 34:944–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooch CM, Wiener M, Wencil EB, Coslett HB (2010) Interval Timing Disruptions in Subjects with Cerebellar Lesions. Neuropsychologia 48:1022–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouvea TS, Monteiro T, Motiwala A, Soares S, Machens C, Paton JJ (2015) Striatal dynamics explain duration judgments. Elife 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilhardi P, Church RM (2004) Measures of temporal discrimination in fixed-interval performance: a case study in archiving data. Behav Res Methods Instrum Comput 36:661–669. [DOI] [PubMed] [Google Scholar]
- Halko MA, Farzan F, Eldaief MC, Schmahmann JD, Pascual-Leone A (2014) Intermittent Theta-Burst Stimulation of the Lateral Cerebellum Increases Functional Connectivity of the Default Network. J Neurosci 34:12049–12056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q, Zhou D, Chase K, Gusella JF, Aronin N, DiFiglia M (1992) Immunohistochemical localization of the D1 dopamine receptor in rat brain reveals its axonal transport, pre- and postsynaptic localization, and prevalence in the basal ganglia, limbic system, and thalamic reticular nucleus. Proc Natl Acad Sci USA 89:11988–11992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikai Y, Takada M, Shinonaga Y, Mizuno N (1992) Dopaminergic and non-dopaminergic neurons in the ventral tegmental area of the rat project, respectively, to the cerebellar cortex and deep cerebellar nuclei. Neuroscience 51:719–728. [DOI] [PubMed] [Google Scholar]
- Ivry RB, Spencer RM (2004) The neural representation of time. Current Opinion in Neurobiology 14:225–232. [DOI] [PubMed] [Google Scholar]
- Kim J, Ghim J-W, Lee JH, Jung MW (2013) Neural correlates of interval timing in rodent prefrontal cortex. J Neurosci 33:13834–13847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SG, Uğurbil K, Strick PL (1994) Activation of a cerebellar output nucleus during cognitive processing. Science 265:949–951. [DOI] [PubMed] [Google Scholar]
- Kim Y-C, Han S-W, Alberico SL, Ruggiero RN, De Corte B, Chen K-H, Narayanan NS (2017) Optogenetic Stimulation of Frontal D1 Neurons Compensates for Impaired Temporal Control of Action in Dopamine-Depleted Mice. Curr Biol 27:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klitenick MA, Tham CS, Fibiger HC (1995) Cocaine and d-amphetamine increase c-fos expression in the rat cerebellum. Synapse 19:29–36. [DOI] [PubMed] [Google Scholar]
- Kononowicz TW (2015) Dopamine-dependent oscillations in frontal cortex index “start-gun” signal in interval timing. Front Hum Neurosci 9:331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küper M, Dimitrova A, Thürling M, Maderwald S, Roths J, Elles HG, Gizewski ER, Ladd ME, Diedrichsen J, Timmann D (2011) Evidence for a motor and a non-motor domain in the human dentate nucleus--an fMRI study. Neuroimage 54:2612–2622. [DOI] [PubMed] [Google Scholar]
- Locke TM, Soden ME, Miller SM, Hunker A, Knakal C, Licholai JA, Dhillon KS, Keene CD, Zweifel LS, Carlson ES (2018) Dopamine D1 receptor positive neurons in the lateral nucleus of the cerebellum contribute to cognitive behavior. Biological Psychiatry 0 Available at: http://www.biologicalpsychiatryjournal.com/article/S0006-3223(18)30067-2/abstract [Accessed February 9, 2018]. [DOI] [PMC free article] [PubMed]
- Martin JH (1991) Autoradiographic estimation of the extent of reversible inactivation produced by microinjection of lidocaine and muscimol in the rat. Neuroscience Letters 127:160–164. [DOI] [PubMed] [Google Scholar]
- Matell MS, Meck WH (2000) Neuropsychological mechanisms of interval timing behavior. Bioessays 22:94–103. [DOI] [PubMed] [Google Scholar]
- Matell MS, Meck WH (2004) Cortico-striatal circuits and interval timing: coincidence detection of oscillatory processes. Brain Res Cogn Brain Res 21:139–170. [DOI] [PubMed] [Google Scholar]
- Matell MS, Meck WH, Nicolelis MAL (2003) Interval timing and the encoding of signal duration by ensembles of cortical and striatal neurons. Behavioral Neuroscience 117:760–773. [DOI] [PubMed] [Google Scholar]
- Meck WH (2005) Neuropsychology of timing and time perception. Brain Cogn 58:1–8. [DOI] [PubMed] [Google Scholar]
- Meck WH (2006) Neuroanatomical localization of an internal clock: A functional link between mesolimbic, nigrostriatal, and mesocortical dopaminergic systems. Brain Research 1109:93–107. [DOI] [PubMed] [Google Scholar]
- Melchitzky DS, Lewis DA (2000) Tyrosine hydroxylase- and dopamine transporter-immunoreactive axons in the primate cerebellum. Evidence for a lobular- and laminar-specific dopamine innervation. Neuropsychopharmacology 22:466–472. [DOI] [PubMed] [Google Scholar]
- Mittleman G, Goldowitz D, Heck DH, Blaha CD (2008) Cerebellar modulation of frontal cortex dopamine efflux in mice: Relevance to autism and schizophrenia. Synapse 62:544–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan NS (2016) Ramping activity is a cortical mechanism of temporal control of action. Curr Opin Behav Sci 8:226–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan NS, Land BB, Solder JE, Deisseroth K, DiLeone RJ (2012) Prefrontal D1 dopamine signaling is required for temporal control. Proc Natl Acad Sci USA 109:20726–20731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan NS, Laubach M (2009) Methods for studying functional interactions among neuronal populations. Methods Mol Biol 489:135–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson TE, King JS, Bishop GA (1997) Distribution of tyrosine hydroxylase-immunoreactive afferents to the cerebellum differs between species. J Comp Neurol 379:443–454. [DOI] [PubMed] [Google Scholar]
- Niki H, Watanabe M (1979) Prefrontal and cingulate unit activity during timing behavior in the monkey. Brain Res 171:213–224. [DOI] [PubMed] [Google Scholar]
- Ohmae S, Kunimatsu J, Tanaka M (2017) Cerebellar Roles in Self-Timing for Sub- and Supra-Second Intervals. The Journal of Neuroscience 37:3511–3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver AP, Hoffer BJ, Bloom FE, Siggins GR (1971) Activation of a Central Noradrenergic Projection to Cerebellum. Nature 233:481. [DOI] [PubMed] [Google Scholar]
- Ott T, Nieder A (2019) Dopamine and Cognitive Control in Prefrontal Cortex. Trends in Cognitive Sciences Available at: https://linkinghub.elsevier.com/retrieve/pii/S1364661319300130 [Accessed February 7, 2019]. [DOI] [PubMed] [Google Scholar]
- Panagopoulos NT, Papadopoulos GC, Matsokis NA (1991) Dopaminergic innervation and binding in the rat cerebellum. Neurosci Lett 130:208–212. [DOI] [PubMed] [Google Scholar]
- Parker KL, Kim Y, Kelley RM, Nessler AJ, Chen K-H, Muller-Ewald VA, Andreasen NC, Narayanan NS (2017) Delta-frequency stimulation of cerebellar projections can compensate for schizophrenia-related medial frontal dysfunction. Molecular Psychiatry:647–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KL, Alberico SL, Miller AD, Narayanan NS (2013) Prefrontal D1 dopamine signaling is necessary for temporal expectation during reaction time performance. Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KL, Chen K-H, Kingyon JR, Cavanagh JF, Narayanan NS (2014a) D1-Dependent 4 Hz Oscillations and Ramping Activity in Rodent Medial Frontal Cortex during Interval Timing. J Neurosci 34:16774–16783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KL, Chen K-H, Kingyon JR, Cavanagh JF, Naryanan NS (2015a) Medial frontal ~4 Hz activity in humans and rodents is attenuated in PD patients and in rodents with cortical dopamine depletion. Journal of Neurophysiology:jn.00412.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KL, Chen K-H, Kingyon JR, Cavanagh JF, Naryanan NS (2015b) Medial frontal ~4 Hz activity in humans and rodents is attenuated in PD patients and in rodents with cortical dopamine depletion. J Neurophysiol:jn.00412.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KL, Narayanan NS, Andreasen NC (2014b) The therapeutic potential of the cerebellum in schizophrenia. Front Syst Neurosci 8:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KL, Ruggiero RN, Narayanan NS (2015c) Infusion of D1 Dopamine Receptor Agonist into Medial Frontal Cortex Disrupts Neural Correlates of Interval Timing. Front Behav Neurosci 9:294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penney TB, Meck WH, Roberts SA, Gibbon J, Erlenmeyer-Kimling L (2005) Interval-timing deficits in individuals at high risk for schizophrenia. Brain and cognition 58:109–118. [DOI] [PubMed] [Google Scholar]
- Petter EA, Lusk NA, Hesslow G, Meck WH (2016) Interactive roles of the cerebellum and striatum in sub-second and supra-second timing: Support for an initiation, continuation, adjustment, and termination (ICAT) model of temporal processing. Neuroscience & Biobehavioral Reviews 71:739–755. [DOI] [PubMed] [Google Scholar]
- Roš H, Sachdev RNS, Yu Y, Šestan N, McCormick DA (2009) Neocortical Networks Entrain Neuronal Circuits in Cerebellar Cortex. J Neurosci 29:10309–10320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz DW, Stanford EJ, Wyrick SW, Mailman RB (1985) Binding of [3H]SCH23390 in rat brain: regional distribution and effects of assay conditions and GTP suggest interactions at a D1-like dopamine receptor. J Neurochem 45:1601–1611. [DOI] [PubMed] [Google Scholar]
- Schutter DJLG, van Honk J, d’Alfonso AAL, Peper JS, Panksepp J (2003) High frequency repetitive transcranial magnetic over the medial cerebellum induces a shift in the prefrontal electroencephalography gamma spectrum: a pilot study in humans. Neurosci Lett 336:73–76. [DOI] [PubMed] [Google Scholar]
- Simon H, Le Moal M, Calas A (1979) Efferents and afferents of the ventral tegmental-A10 region studied after local injection of [3H]leucine and horseradish peroxidase. Brain Research 178:17–40. [DOI] [PubMed] [Google Scholar]
- Spencer DRMC Ivry PRB (2013) Cerebellum and Timing In: Handbook of the Cerebellum and Cerebellar Disorders (Manto M, Schmahmann JD, Rossi F, Gruol DL, Koibuchi N, eds), pp 1201–1219. Springer Netherlands; Available at: http://link.springer.com/referenceworkentry/10.1007/978-94-007-1333-8_52 [Accessed March 3, 2017]. [Google Scholar]
- Stoodley CJ, Valera EM, Schmahmann JD (2012) Functional topography of the cerebellum for motor and cognitive tasks: an fMRI study. Neuroimage 59:1560–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teki S, Grube M, Griffiths TD (2012) A Unified Model of Time Perception Accounts for Duration-Based and Beat-Based Timing Mechanisms. Front Integr Neurosci 5 Available at: https://www.frontiersin.org/articles/10.3389/fnint.2011.00090/full [Accessed November 26, 2017]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellmann S, Bludau S, Eickhoff S, Mohlberg H, Minnerop M, Amunts K (2015) Cytoarchitectonic mapping of the human brain cerebellar nuclei in stereotaxic space and delineation of their co-activation patterns. Front Neuroanat 9 Available at: http://journal.frontiersin.org/article/10.3389/fnana.2015.00054/full [Accessed September 12, 2017]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versteeg DHG, Van der Gugten J, De Jong W, Palkovits M (1976) Regional concentrations of noradrenaline and dopamine in rat brain. Brain Research 113:563–574. [DOI] [PubMed] [Google Scholar]
- Xu M, Zhang S, Dan Y, Poo M (2014) Representation of interval timing by temporally scalable firing patterns in rat prefrontal cortex. Proc Natl Acad Sci USA 111:480–485. [DOI] [PMC free article] [PubMed] [Google Scholar]