Abstract
Background.
Adolescents will increasingly be involved in decisions about return of genomic results. We examined adolescents’ and parents’ decisions about learning actual genomic research results for the adolescent and whether choices were associated with participants’ demographic factors.
Methods.
Adolescents between 13–17 years old and a parent (dyads) were recruited through flyers, social media, employee emails, and clinic visits at a pediatric hospital. Dyads used a decision tool to independently choose the categories of conditions they wanted to learn about the adolescent. They then came together to discuss their independent decisions and make final joint decisions. Conditions were categorized by preventability, treatability, adult-onset and carrier status. Participants could make granular choices by including or excluding conditions in each category. Categorical choices were collapsed into the “aggregate choice” to learn all or not all results.
Results.
Study visits were completed by 163 dyads. Adolescents were less likely than their parents to independently choose to learn all results (64.4% vs. 76.1%; p=.0056). Parents were less likely to independently choose to learn all results for their daughters than their sons (OR=0.41, 95% CI (0.18–0.96); p=.032). Black adolescents were less likely to independently choose to learn all results than white adolescents (OR=0.22; 95% CI (0.08–0.55); p=.0015). After making joint decisions, 70.6% of dyads chose to learn all results.
Conclusions.
Adolescents independently wanted to learn less genomic information than their parents. While adolescents’ cannot legally make genomic testing decisions without parental permission, adolescents’ should be engaged in decisions about return of genomic results.
Keywords: Adolescents, decision making, genetic testing, parents, children, genetic research results
There has been a longstanding ethical principle to only test children for genetic conditions that can be monitored, prevented or treated during childhood. Ethical deliberations have focused on preserving children’s future autonomy until the age of majority by delaying offers of genetic testing for carrier status which could have reproductive implications, and risk for adult-onset conditions for which nothing can be done during childhood (1–4). However this perspective was challenged when the American College of Medical Genetics and Genomics (ACMG) recommended mandatory opportunistic analysis and return of a subset of genes, including some for adult-onset conditions, regardless of patient age when genome sequencing is performed for clinical purposes (5). The ACMG justified this decision stating identification of genetic risk for an adult-onset condition in the child may be the only indicator that a parent is also at risk for the condition (5, 6). In response to concerns raised by ACMG members and stakeholders, ACMG subsequently changed their recommendations to allow adult patients and parents of children the choice to opt out of analysis and return of the entire subset of genes when clinical sequencing is performed (7). While the change was responsive to protection of adult autonomy and parents’ roles as surrogate decision makers, considerations of a child’s future autonomy remained unchanged and the role of the adolescent in assent and dissent for elective procedures continued to be ignored. More recently, ACMG’s Social, Ethical, Legal Issues committee published a separate statement stressing the importance of ensuring adolescents are engaged in the decisions about return of findings related to clinical genomic testing (8).
Increasing access to genomic information about a child’s carrier status and risk for some adult-onset conditions is already available through direct-to-consumer genetic testing options (3, 9, 10). Access to genomic information not actionable in childhood is also becoming available in a pediatric research context (11). A recent consensus study report on the return of individual research results, in particular results generated from genomic analysis of biospecimens, includes recommendations and principles that may result in the more frequent return of research results to participants (12). Others have acknowledged participants ought to be given choices about learning clinically actionable genomic results identified in a research setting (13). We found no studies that prospectively engaged adolescents and parents to make actual choices about the adolescent’s genomic results they wanted to learn during a study.
Here we report the first aim of our site-specific Electronic Medical Record and Genomics III (e3) implementation project; to examine adolescents’ and parents’ choices about the categories of conditions they wanted to learn that were informed by the e3 gene panel. Participants were aware they would learn both negative and positive results based on their choices. We hypothesized that adolescents’ and parents’ choices to learn all results would differ.
Methods
Adolescents between 13–17 years of age and one parent or legal guardian (dyads) were prospectively recruited through flyers, social media postings, employee emails, and direct clinic recruitment. Eligibility criteria included being registered or willing to register the adolescent for an electronic health record (EHR) patient portal, having the capacity to make decisions, and being able to read and speak English. The presence or absence of a specific diagnosis was not an eligibility criterion. Parents and adolescents provided written informed consent and assent respectively during an in-person study visit. Participants were informed that any returned results would be placed in the adolescents’ EHR and that a copy of the results would be sent to the adolescents’ primary care provider. A blood sample was obtained from the adolescent at the end of the study visit for genetic testing. The Cincinnati Children’s Hospital Medical Center (CCHMC) Institutional Review Board approved this study.
Dyads attended a 90–120 minute study visit at CCHMC, a full-service nonprofit pediatric academic medical center, from July 2016 - March 2018. Study visit procedures have been previously described (14). Briefly, dyads were asked to watch a 17-minute video prior to the study visit. The video explained the meaning, limitations, and potential risks and benefits of learning genomic results meaning (15). During the study visit, participants completed a demographic questionnaire and a knowledge assessment developed by the study team to assess understanding of the video. Answers to the knowledge assessment were reviewed and participants’ questions were answered before continuing with study procedures.
Adolescents and parents were separated and independently completed a decision tool to indicate the categories of results they wanted to learn. The categories were modeled on an existing decision tool designed to capture parents’ preferences for learning severe and preventable genomic research results for their child (16). We modified the tool for our study with input from local adolescents and parents (17). Final categories included choices to learn about conditions that were preventable, not preventable, or both; treatable, not treatable, or both; adult-onset; and carrier results for autosomal recessive conditions. Definitions of each category were provided. High cholesterol was provided as an example of a health condition that could be inherited, begin in childhood or adulthood, and be preventable with diet and exercise and treatable with medications (Supplemental Material).
After independently completing the decision tool, participants were given a list of 32 conditions informed by the e3 panel’s returnable genes. The list provided brief descriptions of each condition and indicated how each condition was categorized so participants could review the conditions they would learn based on their choices. The list was organized by condition rather than gene since more than one gene could increase the risk for a particular condition (17). All conditions on the list were treatable. Participants could include or exclude specific conditions on this list (i.e. make granular choices). Granular choices were considered a fifth category of choices for data analysis. The 32 conditions were informed by 73 genes (including the original set of genes recommended by ACMG (5), as well as 13 single nucleotide variants (SNVs) of 11 genes (84 genes total)). Details of the CLIA compliant genetic testing pipeline can be found in supplemental materials.
After completing the decision tool independently, dyads reconvened to discuss their choices and arrive at a joint decision. During this facilitated discussion, participants were asked to share the reasons for their choices, what they thought about one another’s choices, and perceived risks and benefits of learning about the various conditions (Supplemental Material). To minimize parental influence, adolescents were asked to answer all questions before their parent. Dyads were reminded that only results for which they agreed upon would be returned. At the end of the discussion, dyads jointly recompleted the decision tool (joint decisions) to inform the final results that would be returned (18).
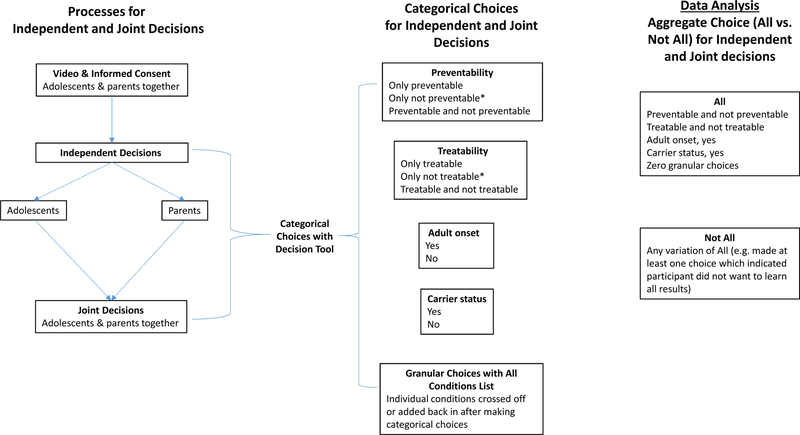
Our primary outcome variable was participants’ aggregate choice (whether or not to learn all results). We also explored associations with categorical choices (preventable, treatable, adult-onset, carrier, and granular choices). Participants’ independent and dyads’ joint decisions were captured (Figure 1).
Figure 1:
Overview of Independent and Joint Decision-Making Processes and Choices
Participant demographics included age, sex, and race. We also collected parent education, income, type of insurance, and marital status. Health-related variables included perceived health and prior genetics exposure (participant reported previous genetic testing or that they or a family member had a genetic condition).
Statistical Methods:
Participant responses were entered into a REDCap (19) database by a study team member. A different study team member reviewed data entry for errors. Analyses were performed using JMP, version 14.0 (SAS Institute, Cary NC). Descriptive statistics included frequencies and means ± standard deviation.
To compare whether choices differed between parents and adolescents, we performed McNemar’s test to account for the paired nature within the dyad. Choices were further stratified by adolescent age (per year), self-reported sex, and race. We performed simple logistic regression to assess whether adolescents’ age, race, sex, health status and prior genetics exposure; as well as parents’ education, income, and prior genetics exposure were singularly associated with participants’ independent decisions. Sex and prior genetics exposure were treated as two-level predicator variables. Race was treated as a three-level predictor variable. Age was treated as a continuous variable with the odds ratio (OR) reflecting the change per year. Income, education, and health status were treated as ordinal variables with responses ranked from lowest to highest (lowest to highest income/education or poor to excellent health) and assigned a numeric value. ORs reported for ordinal variables were based on the range of responses rather than unit increases.
Predictor variables which were significant when evaluated singularly were included in multivariable regression models. Adolescent health status and participants’ prior genetics exposure were not significant in simple regression analysis and were therefore not included in multivariable regression analysis. Income and education were also excluded as they were associated with race. Separate analyses were run for adolescents and parents. Because some predictors were associated with outcomes in only the adolescent or parent models, we performed combined regression analyses which included who the respondent was (adolescent or parent) as a predictor variable. In these combined models, we tested whether the effect of adolescent age, sex, and race differed according to who the respondent was by adding an interaction term. To minimize concerns about overfitting models, only one interaction term was included in each model.
Missing data were excluded from descriptive results, with the exception of income which was not reported by 11% of participants. Overall, the degree of missingness was low, and thus for comparative testing these were excluded. P<.05 was the minimal level accepted for statistical significance.
Results
Participant Characteristics
Of 184 dyads who scheduled study visits, 21 dyads cancelled (n=16) or did not show-up (n=5). Recruitment sources of those who attended a study visit included email (n=53), social media (n=38), flyer (n=37), clinic (n=27), and other (e.g. word of mouth) (n=8). There was no difference in recruitment source between those who did and did not attend a study visit. Independent and joint decisions were made by 163 dyads. Two adolescents, whose responses are included in the analyses, made categorical choices but after reviewing the list of conditions, decided to learn no results.
The mean age of adolescents was 15.4 years and that of parents was 45 years. Most participants were female (62% of adolescents; 90.2% of parents), white (79.1% of adolescents and parents) and reported their health as good or better (88% of adolescents; 87.6% of parents). Less than 10% of adolescents and 13.5% of parents reported previous genetic testing. Among parents, 59.8% had at least a college degree and 55.8% made $60,000 or more. Additional participant characteristics are described in Table 1.
Table 1.
Participant Characteristics
| Adolescent (n = 163) No. (%) |
Parent (n = 163) No. (%) |
|
|---|---|---|
| Age, mean (± SD), y | 15.4 ± 1.2 | 45.0 ±7.3 |
| Sex | ||
| Female | 101 (62.0) | 147 (90.2) |
| Male | 61 (37.4) | 16 (9.8) |
| Othera | 1 (0.6) | |
| Race | ||
| White | 123 (79.1) | 129 (79.1) |
| Black or African American | 25 (15.3) | 27 (16.6) |
| Multiple Races/Other | 15 (9.2) | 7 (4.3) |
| Hispanic or Latino ethnicity | ||
| Hispanic | 8 (4.9) | 3 (1.8) |
| Not Hispanic | 147 (90.2) | 158 (96.9) |
| Unknown | 8 (4.9) | 2 (1.2) |
| Education level | ||
| Some HS | 4 (2.5) | |
| HS/GED | 14 (8.8) | |
| Post HS | 20 (12.6) | |
| Associates | 26 (16.4) | |
| Bachelors | 49 (30.8) | |
| Masters | 40 (25.2) | |
| Doctoral/professional | 6 (3.8) | |
| Adolescent Age (years) | ||
| 13 | 23 (14.1) | |
| 14 | 39 (23.9) | |
| 15 | 44 (27.0) | |
| 16 | 41 (25.2) | |
| 17 | 16 (9.8) | |
| Household Income | ||
| Less than $15,000 | 7 (4.3) | |
| $15,000 – $29,999 | 16 (9.9) | |
| $30,000 – $44,999 | 17(10.5) | |
| $45,000 – $59,999 | 13 (8.0) | |
| $60,000 – $89,999 | 29 (17.9) | |
| $90,000 – $149,000 | 30 (18.5) | |
| $150,000 or more | 32 (19.8) | |
| No answer | 18 (11.1) | |
| Health Insurance (Parent) | ||
| Private | 123 (75.9) | |
| Not Private | 39 (24.1) | |
| Marital Status (Parent) | ||
| Married | 110 (67.9) | |
| Never Married | 14 (8.6) | |
| Divorced | 27 (16.7) | |
| Separated | 6 (3.7) | |
| Widowed | 2 (1.2) | |
| Living with Partner | 3 (1.9) | |
| Health | ||
| Poor | 3 (1.9) | 3 (1.9) |
| Fair | 16(10.1) | 17 (10.5) |
| Good | 47 (29.6) | 66 (40.7) |
| Very Good | 49 (30.8) | 52 (32.1) |
| Excellent | 44 (27.7) | 24 (14.8) |
| Participate in research at same institution in past two years? | ||
| Yes | 53 (32.5) | |
| No | 110 (67.5) | |
| Ever had a genetic test? | ||
| Yes | 16 (9.8) | 22 (13.5) |
| No | 132 (80.1) | 136 (83.4) |
| Not Sure | 15 (9.2) | 5 (3.1) |
| Ever been told by a doctor you have a genetic condition? | ||
| Yes | 22 (13.6) | 16 (9.9) |
| No | 126 (77.8) | 140 (87.0) |
| Not sure | 14 (8.6) | 5 (3.1) |
| Prior genetics exposure | ||
| Yes | 45 (27.6) | 52 (31.9) |
| No | 118 (72.4) | 111 (68.1) |
| Anyone in immediate family been told they have a genetic condition? | ||
| Yes | 30 (18.6) | 37 (22.8) |
| No | 102 (63.4) | 110 (67.9) |
| Not sure | 29 (18.0) | 15 (9.3) |
Dyad excluded from analyses that examined associations with sex.
Choices
Aggregate Choices:
Overall, adolescents were significantly less likely than parents to independently choose to learn all results (64.4% vs. 76.1% respectively; p=.0056). All dyads came to agreement on choices during the joint discussion, after which, 70.6% of dyads jointly chose to learn all results (Table 2). Of the 68 dyads who had at least one discordant independent choice, 35 (51.5%) made final joint decision(s) that all matched the adolescent’s independent choice(s), 14 (20.6%) made joint decision(s) that all matched the parent’s independent choices(s), and 19 (27.9%) made mixed joint decisions (at least one joint decision matched one of the adolescent’s and at least one joint decision matched one of the parent’s independent choices).
Table 2.
Adolescents’ and Parents’ Categorical and Aggregate Choices made Independently and Jointly
| Categorical Choices | Adolescent Independent Decisions (N=163) No. (%) | Parent Independent Decisions (N=163) No. (%) | P-Valuea | Dyads’ Final Joint Decisions (N=163) No. (%) |
|---|---|---|---|---|
| Preventableb | ||||
| Preventable + Not Preventable | 133 (81.6) | 147 (90.2) | .016 | 142 (87.1) |
| Only Preventable | 30 (18.4) | 16 (9.8) | 21 (12.9) | |
| Treatableb | ||||
| Treatable + Not Treatable | 125 (76.7) | 136 (83.4) | .078 | 129 (79.1) |
| Only Treatable | 38 (23.3) | 27 (16.6) | 34 (20.9) | |
| Adult-onset Conditions | ||||
| Yes | 138 (84.7) | 152 (93.3) | .0006 | 149 (91.4) |
| Carrier Results for Autosomal Recessive Conditions | 151 (92.6) | 156 (95.7) | .23 | 154 (94.5) |
| Yes | ||||
| Granular Choices | ||||
| Yes | 22 (13.5) | 15 (9.2) | .21 | 23 (14.1) |
| Aggregate Choice (Learn All vs. Not All Results) | ||||
| All | 105 (64.4) | 124 (76.1) | .0056 | 115 (70.6) |
| Not All | 58 (35.6) | 39 (23.9) | 48 (29.4) |
Differences between parents’ and adolescents’ independent choices based on McNemar’s test
No participants chose to only learn “Not Preventable” or “Not Treatable.” These choices are therefore not represented in Table 2.
Categorical Choices:
Carrier status was the category of information that adolescents and parents most frequently chose to learn (both independently and jointly), followed by adult-onset conditions, preventable conditions, and treatable conditions. Less than 15% of participants made granular choices. Adolescents were significantly less likely than parents to independently choose to learn results for conditions that were “preventable and not preventable” (81.6% and 90.2% respectively; p=.016) as compared to only preventable; and adult-onset (84.7% and 93.3% respectively; p=.0006) (Table 2). These findings persisted in combined regression analysis when we included who the respondent was (adolescent or parent) as a predictor variable and controlled for adolescent age, race, and sex (Supplemental Material).
Parents’ and Adolescents’ Independent Aggregate Choices by Age, Sex, and Race
When stratified by age, fewer 13 year olds (60.9%) independently chose to learn all results than their parents’ (82.6%) (n=23; p=.025) (Supplemental Table 1). Of 61 male adolescents, 59.0% choose to learn all results compared to 85.3% of their parents (p=.0017) (Supplemental Table 2). Additionally, among 25 adolescents who self-reported as black, 36% chose to learn all results compared to 76% of their parents (p=.0023) (Supplemental Table 3).
Associations of Adolescent Age, Sex, and Race with Independent Choices in Regression Analysis
Aggregate Choices:
In simple (Supplemental Table 4) and multivariable (Table 3) regression analyses, adolescent age was not associated with participants’ aggregate choices. However, adolescent sex was associated with parents’ aggregate choices and adolescent race was associated with adolescents’ aggregate choices. When controlling for adolescent age and race, the odds of parents choosing to learn all results was less for their daughters than their sons (OR=0.41; 95% CI (0.18–0.96); p=.032). When controlling for age and sex, the odds of adolescents choosing to learn all results was less for self-reported black adolescents than white adolescents (OR=0.22; 95% CI (0.08–0.55; p=0015) (Table 3).
Table 3:
Multivariable Regression Models: Associations of Sex, Age, and Race with Adolescents’ and Parents’ Choices made Independentlya
| Choicesb | Preventable | Treatable | Adult Onset | Carrier | Granular | All Results | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variablesc | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | p | OR (95% CI) | P | OR (95% CI) | P | |
| Age | Adolescent | 1.1 (0.77 – 1.6) | .62 | 1.2(0.88 – 1.7) | .24 | 1.4(0.98 – 2.1) | .076 | 1.3 (0.77 – 2.3) | .30 | 1.1 (0.72 – 1.6) | .68 | 1.0(0.75 – 1.3) | .98 |
| Parent | 0.66 (0.41 – 1.0) | .069 | 0.63 (0.43 – 0.91) | .011 | 0.81 (0.48 – 1.4) | .42 | 1.4 (0.71 – 2.7) | .32 | 0.74 (0.46 – 1.2) | .19 | 0.81 (0.60 – 1.1) | .19 | |
| Sex (ref male) | Adolescent | 1.9 (0.80 – 4.4) | .15 | 0.67 (0.30 – 1.5) | .32 | 0.74 (0.28 – 1.9) | .73 | 0.79 (0.21 – 3.0) | .73 | 2.9 (1.1 – 7.8) | .031 | 1.3 (0.64 – 2.6) | .48 |
| Parent | 0.09 (0.01 −0.69) | .0018 | 0.41 (0.15 – 1.1) | .070 | 0.14 (0.02 – 1.2) | .025 | 0.23 (0.03 – 2.0) | .13 | 0.32 (0.08 – 1.3) | .078 | 0.41 (0.18 – 0.96) | .032 | |
| Race (ref white, black and other OR reported) | Adolescent | 0.20 (0.08 – 0.53) 2.6 (0.31 – 21.0) | .0017 | 0.30 (0.11 – 0.76) 0.95 (0.24 – 3.7) | .042 | 0.27 (0.10 – 0.74) NEd | .003 | 0.33 (0.08 – 1.3) NE | .10 | 0.18(0.06 – 0.51) 1.7 (0.19 – 14.3) | .0038 | 0.22 (0.08 – 0.55) 1.8 (0.48–6.8) | .0015 |
| Parent | 0.46 (0.11 – 2.0) NE | .13 | 0.75 (0.22 – 2.6) 1.2 (0.23 – 5.9) | .87 | 0.59 (0.11 – 3.2) NE | .30 | 0.41 (0.07 – 2.4) NE | .34 | 0.32 (0.08 – 1.2) 1.1 (0.12 – 9.4) | .26 | 0.82 (0.29 – 2.3) 2.0 (0.41 – 9.6) | .58 | |
| Goodness of fit | Adolescent | 0.84 | 0.24 | 0.97 | 1.0 | 0.99 | 0.017 (0.42 exclude age) | ||||||
| Parent | 1.0 | 0.87 | 1.0 | 1.0 | 1.0 | 0.19 | |||||||
Each model includes age, sex and race as predictor variables.
Choices
Preventable: Chose to learn “preventable and not preventable” vs. only preventable
Treatable: Chose to learn “treatable and not treatable” vs. only treatable
Adult Onset: Chose to learn or did not chose to learn
Carrier Status: Chose to learn or did not chose to learn
Granular Choices: No, did not make granular choices or yes, did make granular choices
All Results: Chose to learn all or did not choose to learn all
Age, sex, and race are all from adolescent. Age OR is reported for increase for one year.
NE – Not estimated
Categorical Choices:
The odds of parents choosing to learn “treatable and non-treatable” conditions (as compared to only treatable) declined with increasing adolescent age (OR=0.63 for each 1 year increase in age; 95% CI (0.43–0.91); p=.011; Table 3). With respect to preventable conditions, the odds of parents choosing to learn “preventable and non-preventable” conditions was less for their daughters than for their sons (OR = 0.09; 95% CI 0.01–0.69; p=.0018). The odds of parents choosing to learn adult-onset conditions was also less for their daughters than their sons (OR = 0.14; 95% CI 0.02 − 1.2; p=.025). There were no significant associations with carrier status in multivariate models.
For adolescents, race was associated with adolescents’ decisions for all conditions except carrier status. Across these categories, black adolescents chose to learn less information than white adolescents (Table 3).
We then tested whether the regression coefficients for adolescent age, sex and race differed by who made the decision. For preventable choices, the regression coefficient was significant for age (p=.048), sex (p=.0002), and race (p=.042), adolescents’ and parents’ preferences for learning preventable conditions differed based on these three variables. The only other significant interaction terms were between age and treatable conditions (p=.004) and sex and granular choices (p=.003) (Supplemental Table 5).
Discussion
This study is the first to demonstrate that adolescents without clinical indication who participate in research offering the return of predisposition genetic testing results make different decisions about learning genomic information than their parents make for them. Although most participants in our study independently chose to learn all results, adolescents chose to learn less information about themselves than their parents chose to learn about the adolescent. Current ACMG guidelines recommend enabling parents to opt in or out of analysis and return of their child’s results for a subset of genes unrelated to the diagnostic purpose for which sequencing was performed. However, our findings demonstrate that while parents might consider the best interest of their child when acting as their child’s surrogate decision maker, they can make choices that differ from their child’s. Our findings provide empirical evidence that support expert opinion regarding the importance of engaging adolescents in decisions about genomic testing and the type of results to learn, and honoring the adolescents’ decision when possible (3, 8, 20).
The overwhelming majority of adolescents and parents independently and jointly chose to learn results for carrier status and risk for adult-onset conditions. Expert contributors to professional statements recommending deferment of such choices (1, 4) might consider modifications that promote adolescent engagement and assent for genomic sequencing when possible. Our study is not the first to suggest that parents and adolescents want more genomic information than is currently supported by guidelines (21–24). Research has found high hypothetical interest in learning all potentially returnable genomic findings among adolescents with genetic conditions and parents whose children have developmental disorders or genetic conditions (23, 25, 26), and among “healthy” adolescents (24). However our finding that fewer adolescents than parents chose to learn certain categories of results highlights the importance of engaging adolescents in these choices rather than relying on parents decisions alone.
Adolescence is an opportunity for parents to progressively empower their adolescents in decision-making and for adolescents to learn skills to transition to independent decision-making (27). While adolescent age was not associated with participants’ aggregate choices in our study, others have reported that parents’ interest in receiving their adolescent’s health information varies by adolescent age depending on the salience of the topic (28).
Parents, who were predominantly mothers, were less likely to choose to learn all results for their daughters than their sons. Women are often the primary healthcare decision makers and caregivers (29). However it is not clear why mothers would make different choices for their sons than daughters and this finding warrants further investigation. Although we did not find differences in aggregate choices between adolescent males and females, others have found that male adolescents express less interest in learning hypothetical genomic results than females (30, 31). Further research is needed to understand the societal reasons for these differences as well as to examine whether fathers make different choices for their sons and daughters.
In our study, adolescents who self-reported as black chose to learn fewer results than those who self-reported as white. Our finding is in contrast to hypothetical decisions made by 282 adolescents within a classroom setting in the same catchment area (24) as our study. In the latter study, 83.6% of 177 black/African-American adolescents and 83.1% of 65 white/Caucasian adolescents wanted to learn incidental findings that were not medically actionable. These disparate findings could reflect different considerations when making hypothetical versus actual genomic testing. For example, actual decisions may be influenced by growing concerns about societal discrimination among black adolescents (32) resulting in decisions to learn less genomic information. Although we did not find a difference in choices by race among parents, others have found preferences about learning genomic sequencing results for themselves or their child to be lower among black or African-American adults, suggesting a need for culturally tailored strategies to facilitate decision-making about return of genomic results (33, 34). The small numbers of black participants in our study is a limitation and further research is needed to understand the factors that might influence different genomic choices among black adolescents.
Similar to other health care decisions (35), adolescents and parents did not always agree after making independent decisions. In our study all dyads were able to reach agreement about which results to learn. Parent-child collaboration is an important aspect in decision-making, particularly as children mature developmentally and cognitively (36, 37). Facilitation of joint decision-making by a provider or researcher may increase the likelihood of keeping both parents and adolescents engaged in decisions about return of genomic results rather than relying on parental preferences alone (38) and may foster congruence when decisional preferences are discordant (17). To encourage participation and build trust, we chose to solicit the adolescents’ perspectives before that of the parents, an approach that others may want to consider when facilitating shared decision-making. Such an approach recognizes adolescents’ agency and growing medical decision-making autonomy and empowers them to be stakeholders in decisions about their health (17, 39, 40).
Limitations
Our findings are informed by a subset of dyads, whose parents gave permission for the adolescent to undergo genomic testing without a clinical indication and may not be generalizable to all healthy adolescents and parents. Overall, 11% of scheduled dyads did not attend their study visit. Given the challenges of finding times when both adolescents and parents were available to attend a 90 minute study visit, we consider this rate to be low. However, participation bias may be present, particularly if those who participated were information seekers and more interested in learning genomic results than the general population. In addition, participants in our study were predominantly white, over half reported family incomes greater than $60,000, and nearly 60% of parents had at least a bachelor’s degree. In spite of these limitations, we found that adolescents independently made different choices than their parents. Our numbers were too small to assess whether specific conditions were more likely to be included or excluded when granular choices were made. However, one might expect greater discrepancy between adolescent and parent choices, or perhaps less interest in learning any genomic results, among populations who do not volunteer to participate in a research study such as ours. Additional empirical research on adolescents’ and parents’ choices in research and clinical settings, including the roles of race, age, sex and ethnicity on choices is needed, as is the impact of returning genomic information to adolescents based on their choices.
Implications
Adolescent health will increasingly include consideration of genomic information. Our findings support that adolescents are capable of making decisions about learning non-diagnostic genomic results in the context of a facilitated decision-making conversation involving the adolescent, parent and researcher. Because our understanding of genomic information is evolving and interpretation of results is anticipated to change as our knowledge improves, there is no compelling reason to report all possible results at the time genomic sequencing is done for clinical or research purposes.
Supplementary Material
Implications and Contributions:
Adolescents unselected for clinical indication chose to learn less genomic information than their parents. Rather than relying solely on parents’ decisions, shared decision making should be facilitated among adolescents and parents as opportunities to learn genomic information in clinical and research settings become increasingly available.
Acknowledgements of Support and Assistance:
The authors would like to thank the study clinical research coordinators who recruited participants and managed the study visit procedures (Larragem Parsley, Paul Gecaine, Matthew Veerkamp) and the genetic counseling trainees who helped with facilitated joint discussions (Alanna Kongkriangkai, Jessica Shank, Josie Pervola, Kayleigh Swaggart, Preethi Pillai and Bryana Rivers). The authors would also like to thank the families who participated in this study.
Findings have been presented orally and by poster at the American College of Medical Genetics and Genomics in Seattle, WA, April, 2019.
Funding Sources: This research was part of a single site eMERGE III network project initiated and funded by the NHGRI through grant U01HG8666 (Cincinnati Children’s Hospital Medical Center, John B. Harley, PI). Research reported in this publication was also supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1 TR001425. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
Abbreviations:
- ASHG
American Society of Human Genetics
- ACMG
American College of Medical Genetics and Genomics
- CCHMC
Cincinnati Children’s Hospital Medical Center
- CI
Confidence Interval
- e3 or eMERGE III
Electronic Medical Record and Genomics III
- OR
Odds Ratio
- SNV
Single Nucleotide Variant
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: The authors have no financial relationships relevant to this article to disclose.
Potential Conflicts of Interest: The authors have no conflicts of interest relevant to this article to disclose.
References
- [1].Ross LF, Saal HM, David KL, et al. Technical report: Ethical and policy issues in genetic testing and screening of children. Genetics in medicine : official journal of the American College of Medical Genetics 2013;15:234–245. [DOI] [PubMed] [Google Scholar]
- [2].Abdul-Karim R, Berkman BE, Wendler D, et al. Disclosure of incidental findings from next-generation sequencing in pediatric genomic research. Pediatrics 2013;131:564–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Botkin JR, Belmont JW, Berg JS, et al. Points to Consider: Ethical, Legal, and Psychosocial Implications of Genetic Testing in Children and Adolescents. American journal of human genetics 2015;97:6–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Committee On Bioethics, Committee On Genetics AND, The American College Of Medical Genetics AND, et al. Ethical and policy issues in genetic testing and screening of children. Pediatrics 2013;131:620–622. [DOI] [PubMed] [Google Scholar]
- [5].Green RC, Berg JS, Grody WW, et al. ACMG recommendations for reporting of incidental findings in clinical exome and genome sequencing. Genetics in medicine : official journal of the American College of Medical Genetics 2013;15:565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Watson M, Genomics ACMG. Incidental findings in clinical genomics: a clarification. Genetics in Medicine 2013;15:664–666. [DOI] [PubMed] [Google Scholar]
- [7].ACMG Board of Directors. ACMG policy statement: updated recommendations regarding analysis and reporting of secondary findings in clinical genome-scale sequencing. Genetics in medicine : official journal of the American College of Medical Genetics 2015;17:68–69. [DOI] [PubMed] [Google Scholar]
- [8].Bush LW, Bartoshesky LE, David KL, et al. Pediatric clinical exome/genome sequencing and the engagement process: encouraging active conversation with the older child and adolescent: points to consider-a statement of the American College of Medical Genetics and Genomics (ACMG). Genetics in medicine : official journal of the American College of Medical Genetics 2018. [DOI] [PubMed] [Google Scholar]
- [9].Borry P, Howard HC, Senecal K, et al. Health-related direct-to-consumer genetic testing: a review of companies’ policies with regard to genetic testing in minors. Fam Cancer 2010;9:51–59. [DOI] [PubMed] [Google Scholar]
- [10].23andMe. 23andMe And The FDA. Available at: https://customercare.23andme.com/hc/en-us/articles/211831908-23andMe-and-the-FDA Accessed March 11 2019. [Google Scholar]
- [11].Berg JS, Agrawal PB, Bailey DB Jr., et al. Newborn Sequencing in Genomic Medicine and Public Health. Pediatrics 2017;139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].National Academies of Sciences Engineering and Medicine. Returning individual research results to participants: Guidance for a new research paradigm. Washington, DC: The National Academies Press, 2018. [PubMed] [Google Scholar]
- [13].Jarvik GP, Amendola LM, Berg JS, et al. Return of genomic results to research participants: the floor, the ceiling, and the choices in between. American journal of human genetics 2014;94:818–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Pervola J, Myers MF, McGowan ML, et al. Giving adolescents a voice: the types of genetic information adolescents choose to learn and why. Genetics in medicine : official journal of the American College of Medical Genetics 2019;21:965–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lynch J, Prows CA, Myers MF, et al. Genome Testing: Expectations and Results. Cincinnati, OH: YouTube, 2016. [Google Scholar]
- [16].Bacon PL, Harris ED, Ziniel SI, et al. The development of a preference-setting model for the return of individual genomic research results. Journal of empirical research on human research ethics : JERHRE 2015;10:107–120. [DOI] [PubMed] [Google Scholar]
- [17].McGowan ML, Prows CA, DeJonckheere M, et al. Adolescent and Parental Attitudes About Return of Genomic Research Results: Focus Group Findings Regarding Decisional Preferences. Journal of Empirical Research on Human Research Ethics 2018:1556264618776613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Pervola J, Myers MF, McGowan ML, et al. Giving Adolescents a Voice: The Types of Genetic Information Adolescents Choose to Learn and Why. Genetics in medicine : official journal of the American College of Medical Genetics under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Holm IA, Savage SK, Green RC, et al. Guidelines for return of research results from pediatric genomic studies: deliberations of the Boston Children’s Hospital Gene Partnership Informed Cohort Oversight Board. Genetics in medicine : official journal of the American College of Medical Genetics 2014;16:547–552. [DOI] [PubMed] [Google Scholar]
- [21].Tercyak KP, Hensley Alford S, Emmons KM, et al. Parents’ attitudes toward pediatric genetic testing for common disease risk. Pediatrics 2011;127:e1288–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Shkedi-Rafid S, Fenwick A, Dheensa S, et al. Genetic testing of children for adult-onset conditions: opinions of the British adult population and implications for clinical practice. European journal of human genetics : EJHG 2015;23:1281–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Harris ED, Ziniel SI, Amatruda JG, et al. The beliefs, motivations, and expectations of parents who have enrolled their children in a genetic biorepository. Genetics in medicine : official journal of the American College of Medical Genetics 2012;14:330–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hufnagel SB, Martin LJ, Cassedy A, et al. Adolescents’ preferences regarding disclosure of incidental findings in genomic sequencing that are not medically actionable in childhood. American journal of medical genetics Part A 2016;170:2083–2088. [DOI] [PubMed] [Google Scholar]
- [25].Levenseller BL, Soucier DJ, Miller VA, et al. Stakeholders’ opinions on the implementation of pediatric whole exome sequencing: implications for informed consent. Journal of genetic counseling 2014;23:552–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sapp JC, Dong D, Stark C, et al. Parental attitudes, values, and beliefs toward the return of results from exome sequencing in children. Clinical genetics 2014;85:120–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Patton GC, Sawyer SM, Santelli JS, et al. Our future: a Lancet commission on adolescent health and wellbeing. Lancet 2016;387:2423–2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ford CA, Cheek C, Culhane J, et al. Parent and Adolescent Interest in Receiving Adolescent Health Communication Information From Primary Care Clinicians. J Adolesc Health 2016;59:154–161. [DOI] [PubMed] [Google Scholar]
- [29].Matoff-Stepp S, Applebaum B, Pooler J, et al. Women as health care decision-makers: implications for health care coverage in the United States. J Health Care Poor Underserved 2014;25:1507–1513. [DOI] [PubMed] [Google Scholar]
- [30].Sabatello M, Chen Y, Sanderson SC, et al. Increasing genomic literacy among adolescents. Genetics in medicine : official journal of the American College of Medical Genetics 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Harel A, Abuelo D, Kazura A. Adolescents and genetic testing: what do they think about it? J Adolesc Health 2003;33:489–494. [DOI] [PubMed] [Google Scholar]
- [32].Leventhal AM, Cho J, Andrabi N, et al. Association of Reported Concern About Increasing Societal Discrimination With Adverse Behavioral Health Outcomes in Late Adolescence. Jama Pediatrics 2018;172:924–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Yu JH, Crouch J, Jamal SM, et al. Attitudes of African Americans toward return of results from exome and whole genome sequencing. American journal of medical genetics Part A 2013;161A:1064–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lewis MA, Stine A, Paquin RS, et al. Parental preferences toward genomic sequencing for non-medically actionable conditions in children: a discrete-choice experiment. Genetics in medicine : official journal of the American College of Medical Genetics 2018;20:181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Wilfond BS, Carpenter KJ. Incidental findings in pediatric research. The Journal of law, medicine & ethics : a journal of the American Society of Law, Medicine & Ethics 2008;36:332–340, 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Miller VA, Reynolds WW, Nelson RM. Parent-child roles in decision making about medical research. Ethics Behav 2008;18:161–181. [Google Scholar]
- [37].Lipstein EA, Brinkman WB, Fiks AG, et al. An emerging field of research: challenges in pediatric decision making. Medical decision making : an international journal of the Society for Medical Decision Making 2015;35:403–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Miller VA, Werner-Lin A, Walser SA, et al. An Observational Study of Children’s Involvement in Informed Consent for Exome Sequencing Research. Journal of empirical research on human research ethics : JERHRE 2017;12:6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Werner-Lin A, Tomlinson A, Miller V, et al. Adolescent engagement during assent for exome sequencing. AJOB Empirical Bioethics 2016;7:275–284. [Google Scholar]
- [40].Sabatello M, Appelbaum PS. Raising Genomic Citizens: Adolescents and the Return of Secondary Genomic Findings. The Journal of law, medicine & ethics : a journal of the American Society of Law, Medicine & Ethics 2016;44:292–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.