Abstract
Background:
Maladaptive approach-avoidance behavior has been implicated in the pathophysiology of major depressive disorder (MDD), but the neural basis of these abnormalities in decision-making remains unclear. Capitalizing on recent preclinical findings, we adapted an approach-avoidance conflict task from non-human primate research for use in human functional MRI.
Methods:
Forty-two female participants, including 18 unmedicated individuals with current MDD (mean age 25.2 ± 5.1) and 24 psychiatrically healthy controls (mean age 26.3 ± 7.6) completed the adapted approach-avoidance task during functional MRI. To probe potential mechanistic factors underlying the observed behavioral and fMRI findings and inform interpretation of putative group differences, we examined electrophysiological data from two female Macaca mulatta monkeys performing the approach-avoidance conflict task mimicked in the fMRI study.
Results:
Findings demonstrated congruent neural correlates of approach-avoidance conflict and aversive responsiveness in the anterior cingulate cortex, including pregenual cortex, of human subjects and macaques (humans p<0.05 whole-brain corrected; macaques p<0.05). The MDD group exhibited aberrant task-related activations in the anterior cingulate cortex, prefrontal cortex and striatum (all ps<0.05). Neural effects in the MDD group were cross-sectionally associated with stress and depressive symptoms. Importantly, they also prospectively predicted stress at six-month follow-up (all ps<0.05).
Conclusions:
Findings indicate there is conservation of anterior cingulate regions of activation across species and that frontal and striatal regions, in unmedicated humans with MDD, are abnormally responsive during cost-benefit decision-making. We suggest that these disruptions could be valuable candidates for translational biomarkers.
Keywords: major depressive disorder, approach-avoidance conflict, primate, anterior cingulate cortex, fMRI, accumbens
Introduction
Major depressive disorder (MDD) is a complex condition characterized by multiple abnormalities, including blunted approach and increased avoidance behavior. Decreased approach behavior predicts future depression (1,2), poor treatment outcomes (3–5), and chronicity (6). Similarly, heightened avoidance contributes to the initiation, maintenance and relapse of MDD (7–13). Despite these findings, little is known about neural mechanisms underlying maladaptive approach-avoidance (Ap-Av) decision-making in MDD. Most prior studies focused on approach and avoidance separately; yet in daily life, decisions are made in conflict situations by balancing rewarding and aversive outcomes. Moreover, prior human studies used paradigms with few correlates in animals. Developing cross-species comparisons could therefore be important for understanding mechanisms linked to MDD (14–16) and potentially reduce current setbacks in drug discovery in clinical neuroscience (17). As a first step, we adapted a non-human primate (NHP) Ap-Av conflict paradigm (14) for humans. By ensuring functional equivalency between human and NHP tasks, our goal was to evaluate with more precision mechanisms implicated in dysregulated Ap-Av behaviors in MDD.
In preclinical studies, Ap-Av decision-making is instantiated by a cortico-striato-limbic network (16). In rodents, Ap-Av paradigms recruit the striatum, medial prefrontal cortex (MPFC), hippocampus, and amygdala (16,18–21). Notably, aberrant approach behavior emerged in rodents when the MPFC was disconnected from the striatum (20). Chronic stress increased this non-optimal behavior, as did optogenetic manipulation of the MPFC-striatal pathway, providing causal evidence that an intrastriatal circuit engaged by the MPFC underlies neural processing of Ap-Av decisions (21).
Studies in NHPs complement these findings by highlighting dissociable roles for the dorsolateral prefrontal cortex (DLPFC) and pregenual anterior cingulate cortex (pACC) in Ap-Av behavior, with the pACC preferentially encoding reward and aversiveness and the DLPFC preferentially encoding low motivation (14,22). In particular, the pACC and striatum have emerged as key regions for avoidance-related neural activity in NHPs. Specifically, microstimulation in the pACC and the caudate nucleus can increase avoidance, and these effects are blocked by the anxiolytic diazepam (14,23).
In humans, Ap-Av fMRI paradigms uncovered conflict-related activation in the pACC, dorsal ACC (dACC), caudate, DLPFC and insula (15,24–27), and stimulation of the subthalamic nucleus (STN) enhanced avoidance (28). Here, we tested individuals with MDD and healthy controls as they were presented with stimuli identical to those used in macaques, which simultaneously, but independently, indicated varied levels of rewarding and aversive outcomes. This protocol created multiple combinations of rewarding and aversive offers and thus multiple levels of conflict. We compared fMRI findings to electrophysiological data acquired in NHPs from regions shown to be implicated in MDD (pACC, DLPFC, striatum). To our knowledge, our study is the first to adapt task design, modeling and data analysis from a microstimulation-electrophysiological study in NHPs to probe neural circuitry underlying Ap-Av behaviors in MDD. We hypothesize that, compared to healthy controls, MDD participants would show reduced activation associated with reward (striatum) and conflict resolution (ACC), but increased activation associated with aversiveness (STN, amygdala). We further hypothesized these neural abnormalities would correlate with current and future symptoms.
Methods and Materials
Human Participants
Twenty-one unmedicated female adults with current MDD (MDD group, mean age: 25.2±5.1) and 35 psychiatrically healthy control (HC group, mean age: 26.3±7.6) female adults participated after providing written informed consent to a protocol approved by the Partners Human Research Committee. For details see Supplemental Information.
Procedures
After screening, participants underwent an imaging session, during which they performed a computerized Ap-Av task. After the scan, they rated stimuli for their valence and arousal. Six months later, participants completed a follow-up clinical session, and repeated the self-report questionnaires.
Human Task
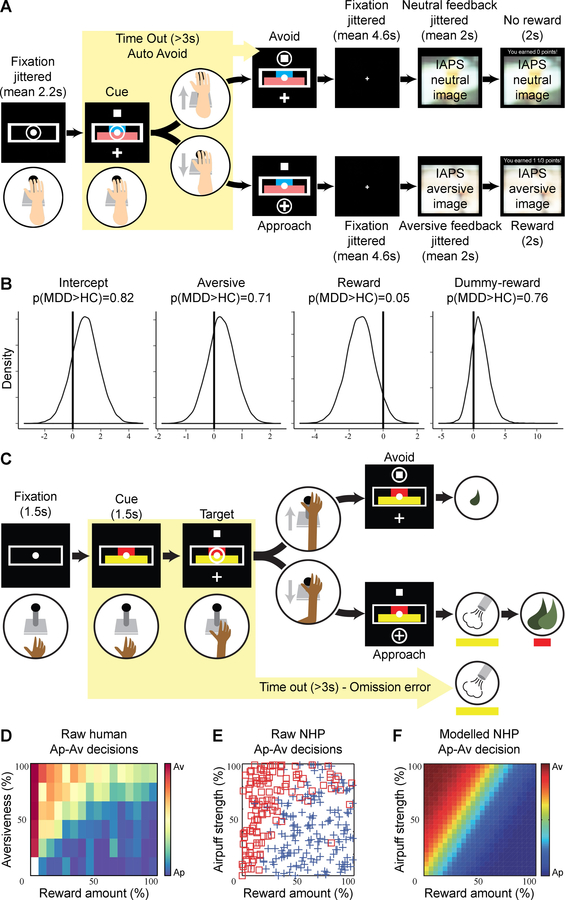
The human Ap-Av task (Fig. 1A) was adapted from a prior NHP study (14) (Supplemental Information). In each trial, participants had to decide (using a joystick) whether to approach or avoid an offer. Approach decisions led to the receipt of a reward (points) but also presentation of an aversive picture with a matching aversive sound; avoidance decisions led to no reward and presentation of a neutral picture. The lengths of two parametrically varied, horizontal bars denoted the size of, respectively, the offered reward points and aversiveness of the outcome picture. The task included 105 trials: (1) approach-reward trials (only reward outcomes; n=15), (2) avoid-threat trials (only aversive outcomes; n=15); and (3) conflict trials (a combination of reward and aversive outcome; n=75).
Figure 1: Approach vs. avoidance – Task and behavior across species.
(A) Human approach-avoidance (Ap-Av) task: The length of the blue and pink bars indicated, respectively, the offered points and normative aversiveness of the image/sound presented after approach choice. The participant could move the joystick to the cross target to indicate an approach choice or to the square target for an avoidance choice. (B) Results of Bayesian hierarchical regression: Patients with MDD were less sensitive to reward than healthy controls. (C) NHP Ap-Av task: During the cue period, the red and yellow horizontal bars, respectively, signaling the offered amounts of reward and punishment, appeared on the monitor. The monkeys made a decision between acceptance and rejection of the combined offer and reported this by choosing either of two targets (cross for acceptance; square for rejection) that appeared during the response period. (D) Raw behavior in the human Ap-Av task. (E) Avoidance (red square) and approach (blue cross) decisions made by the monkey in a single session of the Ap–Av task. (F) The behavioral model derived by logistic regression with the dataset shown in E. The color scale indicating the probability of choosing avoidance (red) or approach (blue) is shown on the right.
Behavioral Data Analysis in Humans
To quantify the influence of reward and aversiveness on choosing to approach or avoid offers, we estimated Bayesian hierarchical logistic regression models using the brms package (29) in R (30). We compared models with different transformations of offered reward and aversiveness using the leave-one out cross-validation method (31). All models were run as hierarchical models simultaneously estimating individual and group parameters. We report effects of MDD on parameters as credibly different when more than 95% of the posterior distribution is above/below zero.
Human fMRI Data Acquisition, Pre-processing and Analysis
Data acquisition and preprocessing details are in the Supplemental Information. The first-level general linear model included five regressors (offered choice presentation onsets for approach-reward decisions, avoid-threat decisions, conflict-approach decisions, conflict-avoidance decisions, and feedback). Presentation onsets were also parametrically modulated by trial-by-trial offered reward and aversiveness, and convolved with a hemodynamic response function. For whole-brain analyses, conditions were contrasted to examine (1) approach vs. avoidance, averaged across conflict conditions to examine approach or avoidance regardless of conflict; (2) conflict-approach vs. approach-reward to examine the effect of conflict without the potential confound of avoidance activation. For ROI analyses, mean activation was extracted from 8 a priori ROIs: bilateral ROIs in the DLPFC (Fig. 2A), STN (Fig. 2C), NAc (Fig. 3A), insula, amygdala and caudate, and single ROIs for pACC (Fig. 2B) and dACC. These values were entered in a mixed-effects linear regression with a between-subjects factor of Group (MDD, HC), and within-subjects factors of Choice (approach, avoid), Conflict (conflict, non-conflict), and, for bilateral ROIs Laterality (left, right). All significant regression interactions were followed up with t-tests (two-tailed) to examine group differences in approach/avoid and conflict/non-conflict. Effect sizes were estimated using Cohen’s d. Degrees of freedom differ across contrasts due to bilateral vs unilateral ROIs, outlier exclusions, and because the Satterthwaite approximation was used, which considers random effects in mixed-effects models.
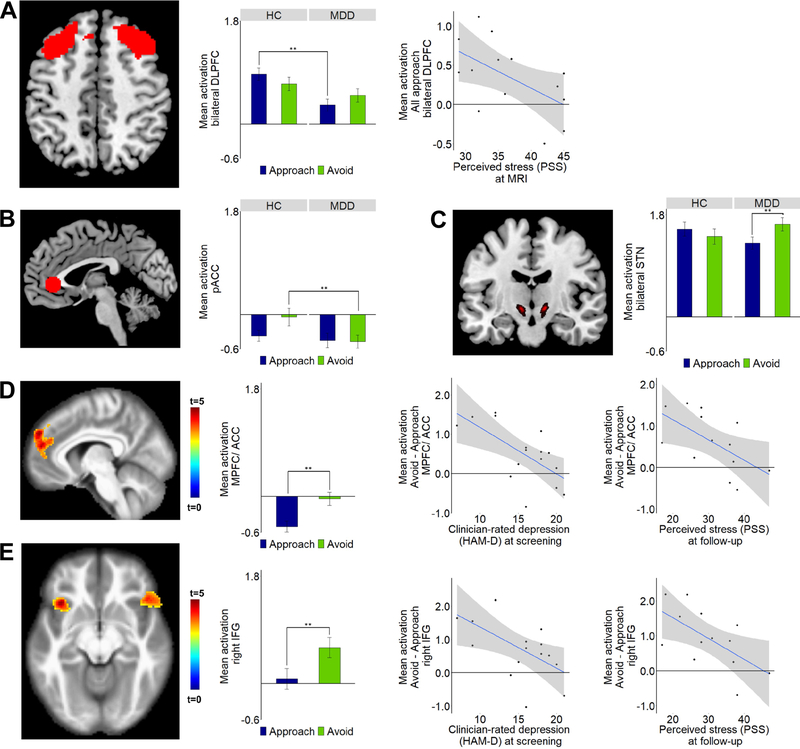
Figure 2: Approach vs. avoidance – fMRI findings.
Differences between the MDD and HC groups as well as Pearson correlations between approach- or avoidance-related activation and clinical symptoms are shown for three regions-of-interest: (A) bilateral dorsolateral prefrontal cortex (DLPFC); (B) Pregenual ACC (pACC) (based on a 10mm sphere placed on coordinates from a meta-analysis (55)); (C) Bilateral subthalamic nucleus (STN). (D, E) Thresholded statistical map showing increased activation for avoidance vs. approach trials in the (D) MPFC (superior medial gyrus (SMG), superior frontal gyrus (SFG)) and pregenual/dorsal anterior cingulate cortex (pACC/dACC) and (E) bilateral IFG*. In all these regions, among the MDD group, reduced avoidance-related activation was associated with greater severity of clinician-rated depression (Hamilton Depression Rating Scale – HAM-D) at screening and higher levels of perceived stress at follow-up. Note: Whole-brain correction performed using a voxel height threshold of p<0.001 and a cluster correction threshold of p<0.05 FWE. ROI analyses performed using mixed-effects linear regression with a P threshold of 0.05 (uncorrected). *See Supplemental Information (Supplemental Fig. 3) for plots and correlations for left IFG. All follow-up correlations remain significant when controlling for baseline measures with hierarchical regression. See Supplemental Information for full statistics.
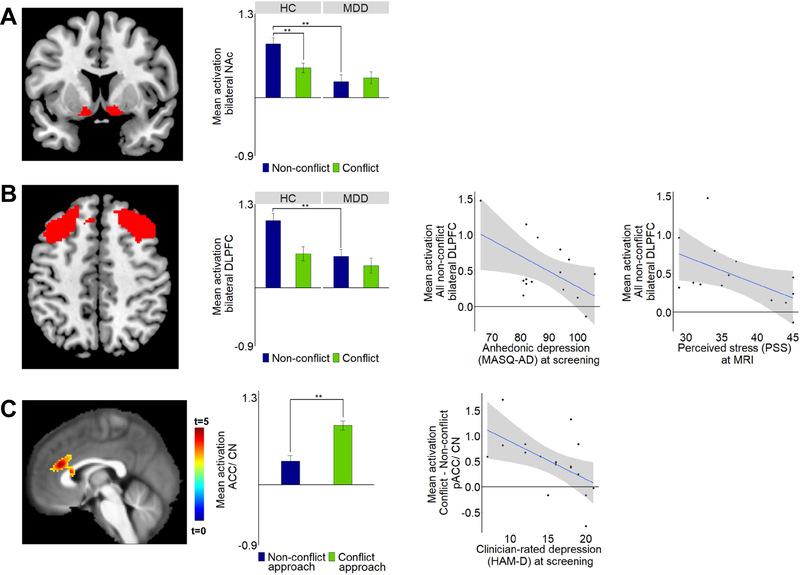
Figure 3: Conflict vs. non-conflict – fMRI findings.
Differences between the MDD and HC groups as well as Pearson correlations between conflict- or non-conflict-related activation and clinical symptoms are shown for two regions-of-interest: (A) Bilateral nucleus accumbens (NAc). (B) Bilateral dorsolateral 30 prefrontal cortex (DLPFC). (C) Thresholded statistical map showing increased activation across all participants for conflict-approach vs. approach-reward trials in the pACC/dorsal ACC and caudate. Mean activation extracted and plotted for the cluster shown in C. In the MDD group, reduced conflict-related activation was associated with increased severity of clinician-rated depression (Hamilton Depression Rating Scale – HAM-D) at screening. Note: Whole-brain correction performed using a voxel height threshold of p<0.001 and a cluster correction threshold of p=0.05 FWE. ROI analyses performed using mixed-effects linear regression with a P threshold of 0.05 (uncorrected). All follow-up correlations remain significant when controlling for baseline measures with hierarchical regression. See Supplemental Information for full statistics.
Animal Subjects and Procedure
We studied two female Macaca mulatta monkeys (monkey A, age:7 years, 6.8 kg; monkey S, age:6 years, 7.5 kg) in experiments conducted following the Guide for Care and Use of Laboratory Animals of United States National Research Council. All procedures were approved by the Committee on Animal Care of the Massachusetts Institute of Technology. Monkeys were trained to perform an Ap–Av task previously described (14,22,23). For details see the Supplemental Information.
NHP Ap-Av Task
As in the human version, in each trial, the monkey had to decide whether to approach or avoid an offer, and to indicate her decision by moving a joystick that guided a cursor on a screen (Fig. 1C). Two red and yellow horizontal bars appeared on the screen after a 2-s pre-cue period. The lengths of two horizontal bars, which were parametrically varied, denoted the offered amount of food reward (red) and the offered pressure of an aversive airpuff directed at the monkey’s face (yellow). After the 1.5-s cue period, single targets appeared above and below the bars. If the monkey chose the cross target (approach choice), an airpuff and food were given at the indicated amounts. When the monkey chose the square target (avoid choice), no airpuff was given, but a minimal reward was delivered to maintain motivation to perform the task. Target locations were randomly varied.
Neuronal Recording and Analysis in Macaques
Monkey A had an initial 36 electrodes implanted in neocortical targets (DLPFC: n=24; ACC: n=12) followed by 42 electrodes implanted in a separate session (DLPFC: n=18; ACC: n=24). Monkey S had 12 electrodes first implanted into the ACC and then, in a separate session, had 30 electrodes implanted (DLPFC: n=12; ACC: n=18). The DLPFC region targeted corresponded to Walker’s area 46 (32). The ACC consisted of the dorsal (areas 8 and 9) and pregenual ACC (areas 24 and 32) (33). Data were classified into single-unit activities using Offline Sorter (Plexon) and analyzed using MATLAB (Mathworks). To model parametrically the monkey’s choice pattern, we adopted the econometric conditional logit model (34,35) to infer subjective internal variables. To examine decision-related activity, we analyzed spike activity during the cue period, during which the monkeys had to make a decision, but they did not yet know the direction of joy stick movement required to approach or avoid the offer. To decode neuronal activity during the Ap-Av task, we performed stepwise regression using MATLAB (Mathworks) with explanatory variables and added parameters derived from theoretical modeling. Details of neuronal recording, modelling and statistical analyses are in the Supplemental Information.
Results
Human Study
Reduced reward sensitivity in MDD
Hierarchical Bayesian regression showed that reaction times were significantly increased by conflict (β=0.21, 95% CI=0.14,0.28) and avoidance (β=0.14, 95% CI=0.07,0.21), indicating the task elicited the expected effects. To investigate the impact of reward and aversiveness on Ap-Av behavior, we compared multiple Bayesian hierarchical logistic regression models. The models differed in the transformations of reward and aversiveness used to capture observed approach and avoidance (see Supplemental Table 3). The model that best accounted for choice patterns modeled choice (approach=1, avoid=0) on trial t dependent on a logarithmic transformation of the value of offered reward, a direct linear mapping of the value of the offered aversiveness, and a dummy-coded variable for whether offered reward was zero (Dnoreward=1) or not (Dnoreward=0):
The analysis confirmed that higher offered reward increased probability to approach (,), whereas stronger aversiveness increased probability to avoid (,). The model identified a credible effect of sensitivity to reward across groups: individuals with MDD were less sensitive to reward than HC. None of the other coefficients differed between groups (Fig. 1B).
Imaging Results
Complete tables of imaging results are presented in the Supplemental Information (Supplemental Tables 4,5).
Aberrant Ap-Av activation in MDD is related to clinical symptoms and stress
When considering approach vs. avoidance (i.e., averaged across conflict conditions), relative to HC, the MDD group showed reduced approach-related activation in the DLPFC (t(156)=3.54, p<0.001, d=−0.55, Fig. 2A) and reduced avoidance-related pACC activation (t(79)=2.24, p=0.03, d=−0.46, Fig. 2B). Moreover, within-group analyses revealed the MDD group showed increased avoidance-related STN activation compared to approach (t(138)=−2.06, p=0.04, d=0.35, Fig. 2C). Among the MDD group, reduced approach-related DLPFC activation correlated with higher perceived stress (Perceived Stress Scale (PSS)) (r=−0.57, p=0.04).
Whole-brain corrected analyses across all participants demonstrated avoidance-related activation in three clusters: one cluster in the MPFC and pACC/dACC (p<0.001, whole-brain corrected, Fig. 2D) and bilateral clusters in the inferior frontal gyrus (IFG) (ps<0.01, whole-brain corrected, Fig. 2E). Among the MDD group, decreasing levels of avoidance-related activation in these regions were associated with increased baseline depressive symptoms (Hamilton Depression Rating Scale (HAM-D)) and higher perceived stress at follow-up (ps<0.03). Thus, ROI and whole-brain analyses implicated different parts of the ACC (ROI and whole brain cluster not overlapping) in avoidance behavior, and reduced ACC activation was associated with depressive symptoms and perceived stress.
Aberrant conflict-related activation in MDD is associated with clinical symptoms and stress
We next probed the effect of conflict by comparing conflict trials (i.e., trials with a combination of reward and aversive offers) and non-conflict trials (i.e., approach-reward or avoid-threat trials). Compared to HC, the MDD group showed reduced NAc (t(143)=4.16, p<0.001, d=−0.66, Fig. 3A) and DLPFC (t(155)=3.62, p<0.001, d=−0.56, Fig. 3B) activation during non-conflict trials. In the MDD group, decreasing non-conflict DLPFC activation was associated with higher baseline anhedonia (Mood and Anxiety Symptoms Questionnaire: Anhedonic Depression (MASQ-AD)) (r=−0.54, p=0.04) and perceived stress at MRI (r=−0.54, p=0.05). Thus, in ROI analyses, the MDD group showed no differentiation between conflict and non-conflict activations and decreased DLPFC activation was associated with anhedonia and perceived stress.
Approached conflict was examined by contrasting conflict-approach and approach-reward trials. Using whole-brain corrected statistics across all participants, we found conflict-related activation in the pACC/dACC and caudate (p<0.001, whole-brain corrected, Fig. 3C). In MDD, decreasing conflict-related activation was associated with greater baseline depression severity (r=−0.57, p=0.02). Thus, whole-brain corrected analysis implicated the ACC in conflict monitoring and aberrant conflict-related activation was associated with depressive symptoms.
Aversiveness is tracked by the human ACC
Using parametric modulation of all approached offers, we found no group differences in how aversiveness modulated activation in the Ap-Av task (ps>0.05). However, across all participants (n=42), we identified whole-brain corrected clusters tracking trial-by-trial aversiveness in approach trials, in a large cluster in the orbital gyrus, pACC and dACC and another cluster in the right IFG and insula (IFG/insula) (ps<0.001, whole-brain corrected, Supplemental Fig. 4). These clusters did not survive correction for multiple comparisons in a model comparing reward and aversiveness and no correlations with clinical measures emerged.
Results of Non-Human Primate Study
The goal of the NHP analyses was to address two hypotheses pertinent to the human ACC findings in order to inform MDD-HC findings. First, whether the ACC of NHPs contained neurons specifically responding to conflict. Second, whether the ACC contained neurons exhibiting activation related to aversiveness. The data analyzed here were not previously published, except as noted. Most of the methods have been fully described (14,22); accordingly, we describe newly introduced analysis, but only summarize other methodology. In total, we isolated 3109 neocortical units from the bilateral DLPFC (mainly cortical area 46) and ACC (areas 8, 9, 24 and 32) of the two monkeys, and classified using stepwise regression of 11 explanatory variables (Supplemental Information).
Conflict activation in the NHP ACC
Our human fMRI findings showed activation of the pACC/dACC and caudate associated with conflict in approach decisions (Fig. 3C). We thus tested whether the ACC of NHPs contained neurons parametrically responding to conflict. The conflict units consist of two groups of units encoding decision-making conflict. We defined the entropy (Ep+) units as those with cue-period activity showing a positive correlation with entropy (Fig. 4A) and standard deviation (Sd+) units as those with cue-period activity showing a positive correlation with the standard deviation of the Ap-Av choices (Fig. 4B). Previous NHP studies have not reported units responding to behavioral conflict in the dACC (36,37), but have in the DLPFC (38). We thus compared the proportions of the Ap-Av conflict neurons in the ACC and DLPFC. These conflict units were observed significantly more frequently in the ACC than DLPFC (p<0.05, Fig. 4C), suggesting the ACC contained units with decision-period activity responding specifically for Ap-Av conflict. The distribution of these conflict units was not limited to pACC (area 32) but was also observed in a broader region including dACC (area 9) (Fig. 4D), resembling conflict activation of the human pACC/dACC (Fig. 3C).
Figure 4: Properties of conflict units.
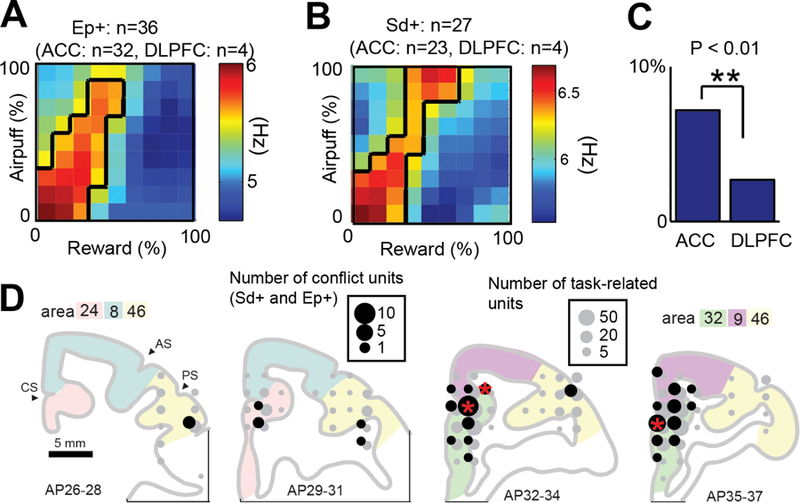
(A, B) Population activity of Ep+ (A) and Sd+ (B) neurons. (C) Proportion of conflict neurons among all classified neurons in the ACC (cortical areas 8, 24, 32) and DLPFC (mainly area 46). We observed conflict units more frequently in the ACC than DLPFC (**p<0.01, Fisher’s exact test). (D) Distribution of conflict units (Ep+ and Sd+). The size of black and gray circles indicate, respectively, the number of conflict and task-related neurons at each location. Red stars indicate locations in which proportions of conflict units to task-related units were significantly larger than the average (*p<0.05, Fisher’s exact test). Note: Units classified with activity correlated positively (+) with standard deviation of decision (Sd) and entropy of decision (Ep).
The observation of NHP single unit activity specifically responding to Ap-Av conflict is important, given prior negative results for conflict-specific neuronal responses in the ACC (36–38). Our results clearly show similar neural pACC/dACC responding in humans and NHPs, suggesting a common neuronal mechanism of ACC response to Ap-Av conflict.
Aversiveness is tracked by the NHP ACC
The human fMRI data indicate greater activation in the pACC and surrounding regions for the degree of aversiveness of the offer (Supplemental Fig. 4). We thus tested whether neuronal activity in the macaques exhibited a similar regional bias. Units encoding aversiveness consisted of two groups encoding potential and chosen aversiveness. We defined aversiveness (Ave+) units as those with cue-period activity exhibiting positive correlation with the offered airpuff (Fig. 5A) and chosen aversiveness (ChA+) units as those with cue-period activity showing positive correlation with the size of the airpuff to be delivered as a result of the monkey’s decision (Fig. 5B). These aversiveness units were observed significantly more frequently in the ACC than DLPFC (p<0.05, Fig. 5C). Although these units were found in both the dorsal and ventral banks of the cingulate sulcus, the proportion of the aversiveness units to the task-related units was significantly larger than the average specifically in the pACC (area 32 or 24) (Fig. 5D). These spatial biases in aversiveness unit distribution thus corresponded to the human fMRI data demonstrating pACC activation for aversiveness.
Figure 5: Properties of aversiveness neurons.
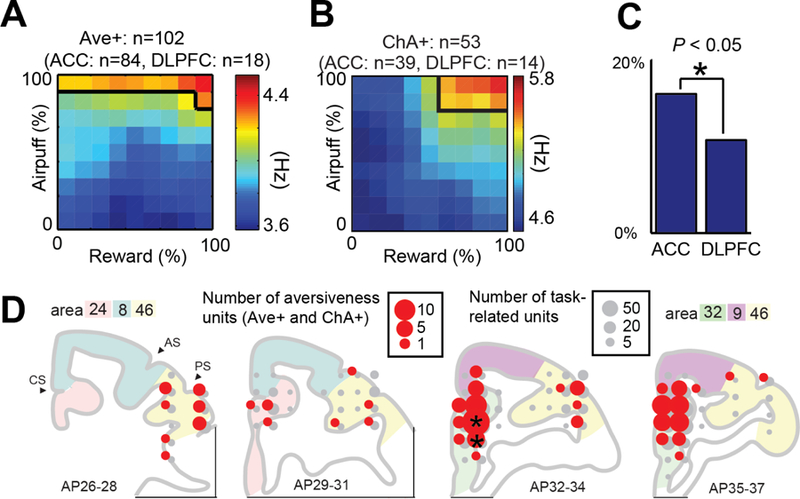
(A, B) Population activity of Ave+ (A) and ChA+ (B) neurons. (C) Proportion of aversiveness neurons among all classified neurons in the ACC and DLPFC. We observed aversiveness neurons in the ACC significantly more frequently in the DLPFC (*p<0.05, Fisher’s exact test). (D) Distribution of aversiveness neurons (Ave+ and ChA+). The size of red and gray circles indicate, respectively, the number of aversiveness and task-related neurons at each location. Black stars indicate the locations in which proportions of conflict units to task-related units were significantly larger than the average (*p<0.05, Fisher’s exact test). Note: Units classified with activity correlated positively (+) with offered aversiveness (Ave) and chosen aversiveness (ChA).
Discussion
Cross-species models of Ap-Av conflict should be valuable in providing mechanistic information for translational research. For technical reasons, such studies have been lacking. Here, we designed a coordinated study in humans and non-human primates with similar experimetal protocols in an effort to use the NHP findings to inform interpretation of putative differences in neural activity observed through fMRI of individuals with MDD. Relative to HCs, unmedicated participants with MDD exhibited reduced (1) reward sensitivity, (2) ventral striatal and DLPFC activation in non-conflict trials, (3) approach-related DLPFC and (4) avoidance-related pACC activation. Moreover, unlike HCs, the MDD group showed larger STN activation during avoidance than approach. These patterns were bolstered by two additional sets of findings. First, neural abnormalities during the Ap-Av task were correlated with current stress appraisal and depressive symptoms and predicted stress appraisal six months later. Second, across species, conflict and aversiveness were associated with activation in regions of the ACC, validating targets emerging from fMRI analyses. Collectively, findings point to network-level alterations highlighting dysregulation in complex interactions between reward valuation, cost-benefit integration and conflict resolution.
Aberrant reward sensitivity and avoidance signaling in MDD
Individuals with MDD were less sensitive to reward than HCs. In addition, relative to HCs, the MDD group exhibited reduced pACC activation during avoidance (Fig. 2B), suggesting a reduction in normative avoidance activation. Given literature highlighting maladaptive avoidance in MDD (10,13), we speculate the pACC abnormality might reflect a more automatic (lacking cost-benefit integration) avoidance decision-making style in MDD, potentially reflecting the lack of behavioral sensitivity to reward driving less need for conflict resolution. This hypothesis suggests maladaptive avoidance in MDD might be linked to abnormalities within network circuitry including regions examined here, the neocortex, striatum and STN. In accord with this speculation, the MDD group showed relatively increased STN activation during avoid decisions (Fig. 2C). The STN is proposed to raise decision thresholds in cortico-basal ganglia circuits to prevent approach responses (39), which might be accentuated in MDD. Bilateral STN stimulation induces immobility in the forced swim test in rats (40) and increases avoidance (28) and depressive symptoms (41) in humans.
As hypothesized, and confirming cross-species participation of the ACC and ventrolateral PFC in avoidance behavior and conditioned fear (14,20,22,42–44), whole-brain analyses demonstrated avoidance-related activation in the MPFC/pACC/dACC and bilateral IFG. In MDD, reduced avoidance-related activation correlated with depression severity (Fig. 2D) and predicted higher levels of perceived stress 6 months later. These findings draw parallels to studies in rodent Ap-Av behavior implicating the MPFC-striatal circuit in aberrant valuation of rewards and punishments, demonstrating similar effects of chronic stress and optogenetic inhibition of the medial prefronto-striosomal circuit (20,21). In prior analyses of our NHP sample, stimulation of the pACC increased avoidance decisions, and administration of diazepam blocked this effect (14). Future work would benefit from administration of diazepam in humans.
Blunted DLPFC and NAc activation in MDD
The DLPFC has been implicated in approach and anticipation of aversiveness in low-conflict decisions (left DLPFC) (24, 45) and avoidance and high-conflict decisions (right DLPFC) (15, 46). EEG research has linked these asymmetries to MDD (47), but this relationship has not consistently emerged with fMRI. No evidence of laterality emerged, but findings extend earlier reports by showing MDD is characterized by reduced bilateral DLPFC approach-related activation (Fig. 2A). Additionally, MDD was associated with reduced DLPFC activation during non-conflict trials (Fig. 3B), and blunted DLPFC activation correlated with increasing anhedonic symptoms and perceived stress. Prior NHP findings demonstrated that activation of DLPFC neurons signaled low motivation (22). Therefore, reduced abililty to engage the DLPFC to complete low-conflict/low motivation trials in MDD represents a potential neural underpinning of impaired anticipation of aversion, stress appraisal and anhedonia.
Neuroimaging implicates the ventral striatum, particularly the NAc, in the anticipation and valuation of rewards (48, 49). Alterations in NAc activations are implicated in a range of psychiatric conditions (50) and are thought to underlie deficits in reinforcement learning and motivation. The reduced NAc activation reported here may thus suggest blunted neural response related to the anticipation and evaluation of reward in a given offer in MDD, an effect that has hitherto not been directly linked to Ap-Av behavior. We found the MDD group was behaviorally less sensitive to reward. In addition, in the absence of conflict (in approach-reward/avoid-threat trials), the NAc was activated in the HC group but not MDD group (Fig. 3A). Maladaptive NAc responses to non-conflict choice situations (e.g., easy choices) may be a key underlying feature contributing to impaired approach behavior in MDD.
Cross-species function of the ACC
The role of the human ACC in conflict monitoring is well established by prior work in healthy controls (15). However, conflict activation in the ACC of NHPs has been debated (Supplemental Discussion). The prior gap between humans and NHPs could stem from differences in task requirements (51) or cognitive demands (52). Here, we focused on Ap-Av conflict in which participants need reconciliation between positive and negative emotional responses. Surprisingly, the two prior neuroimaging studies of Ap-Av-conflict in MDD reported group differences in multiple regions (including the striatum) but not in the ACC (53,54). Here, we found neural correlates of conflict in pACC/dACC and caudate (Fig. 3C). Reduced conflict-related activation, in a region similarly activated by conflict in the NHPs (Fig. 4), was associated with depression severity and perceived stress in MDD.
Further cross-species integration stems from the comparison of findings on chosen aversiveness being encoded in the pACC of NHPs. The region identified in the monkeys was in the ventral bank of the cingulate sulcus (posterior part of cortical area 32 and/or anterior part of area 24) (Fig. 5). Using parametric modulation, we found a large region of the ACC (including/adjoining the pACC) also encoded chosen aversiveness in humans (Supplemental Fig. 4) (see Supplemental Information for a model comparing reward and aversiveness). Thus, the current NHP and human findings concur in highlighting a role of the ACC in conflict and aversion processing. Given prior NHP findings (14,42), this cross-species integration should aid future investigations of interventions in humans with MDD and anxiety disorders that could remediate Ap-Av-abnormalities.
Limitations
Despite our integration of behavioral assessments, brain activity measures, and computational modeling, limitations exist. First, behavioral modeling indicated reduced reward sensitivity in MDD, but groups did not differ in avoidance. This pattern points to possible specificity, but participants made significantly more approach than avoid decisions, indicating the aversiveness of the affective images may have not been potent enough to produce behavioral group differences. Second, whole-brain fMRI analyses were corrected, but the ROI-based regression analyses report corrected and uncorrected statistics. When applying a Bonferroni correction, only NAc and DLPFC group differences remain significant. Also, when comparing aversiveness and reward post-hoc in the parametrically modulated findings, aversiveness related clusters do not survive correction, limiting specificity. Third, although there was high correlation between the normative and subjective ratings for most participants, the assumption that aversive stimuli meant the same to all participants is another limitation. Fourth, the putative interaction between reward valuation and conflict resolution prevented us from separating these two aspects of Ap-Av conflict, and it is possible MDD-related abnormalities in one or both domains might have driven findings. Fifth, because the NHPs did not show depressive-like phenotypes, their data do not immediately inform models of MDD. However, confluence in computational parameters (e.g., avoidance-related pACC activation) modulated by MDD (in humans) and stimulations (in NHPs) allowed us to draw stronger conclusions about fMRI findings in MDD. Future integration of preclinical studies using manipulations relevant to depression (e.g., chronic stress) (19,20) and studies in MDD will be needed for stronger mechanistic models. Finally, only female human participants and macaques were included, limiting generalizability. Despite these limitations, the current cross-species study takes the first steps in developing an Ap-Av conflict model that can be used in humans and NHPs, defining a neural model of avoidance and conflict that correlates with and predicts the symptoms of MDD.
Supplementary Material
KEY RESOURCES TABLE
| Resource Type | Specific Reagent or Resource | Source or Reference | Identifiers | Additional Information |
|---|---|---|---|---|
| Add additional rows as needed for each resource type | Include species and sex when applicable. | Include name of manufacturer, company, repository, individual, or research lab. Include PMID or DOI for references; use “this paper” if new. | Include catalog numbers, stock numbers, database IDs or accession numbers, and/or RRIDs. RRIDs are highly encouraged; search for RRIDs at https://scicrunch.org/resources. | Include any additional information or notes if necessary. |
| Organism/Strain | Rhesus Macaque (Macaca mulatta) | Primate Center | N/A | |
| Software; Algorithm | SPM12 | The FIL Methods group | RRID: SCR_007_037 | |
| Software; Algorithm | R | The R Project for Statistical Computing | SCR:001905 | |
| Software; Algorithm | Matlab 2017a | Mathworks | RRID: SCR_001622 | |
| Software; Algorithm | bspmview | Spunt, B (2014) | https://github.com/spunt/bspmview | |
| Software; Algorithm | CORTEX (VCortex) | National Institute of Mental Health | https://www.nimh.nih.gov/research/research-conducted-at-nimh/research-areas/clinics-and-labs/ln/shn/software-projects.shtml | |
| Software; Algorithm | Offline Sorter | Plexon | https://plexon.com/products/offline-sorter/ | |
| Software; Algorithm | Chronux | Bokil et al., 2010 | http://chronux.org/ | |
| Other | A 3T Tim Trio Siemens scanner | Siemens Medical Systems | ||
| Other | Connectome EPI sequence | Feinberg et al., 2010; Xu et al., 2013 | N/A | |
| Other | Eyelink 1000 | SR Research | Cat#UEPLEDVMCN1X | |
| Other | Custom built microdrives | Feingold et al., 2012 | N/A | |
| Other | Digital Lynx | Neuralynx | N/A | |
| Other | Master 8 | AMPI | N/A | |
| Other | A365 | WPI | N/A | |
| Other | L/S Masterflex, | Cole-Parmer | N/A | |
| Other | 20-A | Silent Air | N/A | |
| Other | 900-EIA | Control Air | N/A | |
Acknowledgments
The authors would like to thank Poornima Kumar and Stefanie Nickels for their guidance on the human fMRI analyses, Jeffrey Curry for his assistance with non-human primate analyses, Dan Gibson for his advice on these analyses, Amit Etkin for advice on human anatomical masks and Malavika Mehta for her assistance with human data collection.
Funding
The work in humans was supported by NIMH grant R37 MH068376 (awarded to D.A.P), a Kaplen Fellowship on Depression (C.L.M), and Livingston Fellowship (C.L.M). M.I and C.L.M were partially supported by the John and Charlene Madison Cassidy Fellowship in Translational Neuroscience. The work in NHPs was supported by the NIH (R01 NS025529), the CHDI Foundation (A-5552), the Office of Naval Research (N00014-07-1-0903), the Army Research Office (W911NF-16-1-0474), MEXT KAKENHI (18H04943 and 18H05131), the Simons Center for the Social Brain, the Naito Foundation, the Uehara Memorial Foundation and the Saks Kavanaugh Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Over the past three years, D.A.P has received consulting fees from Akili Interactive Labs, BlackThorn Therapeutics, Boehringer Ingelheim, and Takeda, and an honorarium from Alkermes for activities unrelated to the current paper. No funding from these entities was used to support the current work, and all views expressed are solely those of the authors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
All other authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Wardenaar KJ, Giltay EJ, van Veen T, Zitman FG, Penninx BWJH (2012): Symptom dimensions as predictors of the two-year course of depressive and anxiety disorders. J Affect Disord. 136: 1198–1203. [DOI] [PubMed] [Google Scholar]
- 2.McFarland BR, Shankman SA, Tenke CE, Bruder GE, Klein DN (2006): Behavioral activation system deficits predict the six-month course of depression. J Affect Disord. 91: 229–234. [DOI] [PubMed] [Google Scholar]
- 3.McMakin DL, Olino TM, Porta G, Dietz LJ, Emslie G, Clarke G, et al. (2012): Anhedonia predicts poorer recovery among youth with selective serotonin reuptake inhibitor treatment resistant depression. J Am Acad Child Adolesc Psychiatry. 51: 404–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spijker J, Bijl R V, de Graaf R, Nolen WA (2001): Determinants of poor 1-year outcome of DSM-III-R major depression in the general population: results of the Netherlands Mental Health Survey and Incidence Study (NEMESIS). Acta Psychiatr Scand. 103: 122–130. [DOI] [PubMed] [Google Scholar]
- 5.Vrieze E, Pizzagalli DA, Demyttenaere K, Hompes T, Sienaert P, de Boer P, et al. (2013): Reduced reward learning predicts outcome in major depressive disorder. Biol Psychiatry. 73: 639–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moos RH, Cronkite RC (1999): Symptom-based predictors of a 10-year chronic course of treated depression. J Nerv Ment Dis. 187: 360–368. [DOI] [PubMed] [Google Scholar]
- 7.Ferster CB (1973): A functional anlysis of depression. Am Psychol. 28: 857–70. [DOI] [PubMed] [Google Scholar]
- 8.Krantz SE, Moos RH (1988): Risk factors at intake predict nonremission among depressed patients. J Consult Clin Psychol. 56: 863–869. [DOI] [PubMed] [Google Scholar]
- 9.Richter J, Eisemann M, Richter G (2000): Temperament and character during the course of unipolar depression among inpatients. Eur Arch Psychiatry Clin Neurosci. 250: 40–7. [DOI] [PubMed] [Google Scholar]
- 10.Ottenbreit ND, Dobson KS (2004): Avoidance and depression: the construction of the cognitive-behavioral avoidance scale. Behav Res Ther. 42: 293–313. [DOI] [PubMed] [Google Scholar]
- 11.Holahan CJ, Moos RH, Holahan CK, Brennan PL, Schutte KK (2005): Stress generation, avoidance coping, and depressive symptoms: A 10-year model. J Consult Clin Psychol. 73: 658–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aldao A, Nolen-Hoeksema S, Schweizer S (2010): Emotion-regulation strategies across psychopathology: A meta-analytic review. Clin Psychol Rev. 30: 217–37. [DOI] [PubMed] [Google Scholar]
- 13.Ottenbreit ND, Dobson KS, Quigley L (2014): An examination of avoidance in major depression in comparison to social anxiety disorder. Behav Res Ther. 56: 82–90. [DOI] [PubMed] [Google Scholar]
- 14.Amemori K, Graybiel AM (2012): Localized microstimulation of primate pregenual cingulate cortex induces negative decision-making. Nat Neurosci. 15: 776–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aupperle RL, Melrose AJ, Francisco A, Paulus MP, Stein MB (2015): Neural substrates of approach-avoidance conflict decision-making. Hum Brain Mapp. 36: 449–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirlic N, Young J, Aupperle RL (2017): Animal to human translational paradigms relevant for approach avoidance conflict decision making. Behav Res Ther. 96: 14–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robbins TW (2017): Cross-species studies of cognition relevant to drug discovery: a translational approach. Br J Pharmacol. 174: 3191–3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Millan MJ (2003): The neurobiology and control of anxious states. Prog Neurobiol. 70: 83–244. [DOI] [PubMed] [Google Scholar]
- 19.File SE, Seth P (2003): A review of 25 years of the social interaction test. Eur J Pharmacol. 463: 35–53. [DOI] [PubMed] [Google Scholar]
- 20.Friedman A, Homma D, Gibb LG, Amemori K, Rubin SJ, Hood AS, et al. (2015): A corticostriatal path targeting striosomes controls decision-making under conflict. Cell. 161: 1320–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friedman A, Homma D, Bloem B, Gibb LG, Amemori K, Hu D, et al. (2017): Chronic stress alters striosome-circuit dynamics, leading to aberrant decision-making. Cell. 171: 1191–1205.e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amemori K, Amemori S, Graybiel AM (2015): Motivation and affective judgments differentially recruit neurons in the primate dorsolateral prefrontal and anterior cingulate cortex. J Neurosci. 35: 1939–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amemori K, Amemori S, Gibson DJ, Graybiel AM (2018): Striatal microstimulation induces persistent and repetitive negative decision-making predicted by striatal beta-band oscillation. Neuron. 99: 829–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schlund MW, Brewer AT, Magee SK, Richman DM, Solomon S, Ludlum M, Dymond S (2016): The tipping point: Value differences and parallel dorsal-ventral frontal circuits gating human approach-avoidance behavior. Neuroimage. 136: 94–105. [DOI] [PubMed] [Google Scholar]
- 25.Shenhav A, Straccia MA, Cohen JD, Botvinick MM (2014): Anterior cingulate engagement in a foraging context reflects choice difficulty, not foraging value. Nat Neurosci. 17: 1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tom SM, Fox CR, Trepel C, Poldrack RA (2007): The Neural Basis of Loss Aversion in Decision-Making Under Risk. Science (80- ). 315: 515 LP–518. [DOI] [PubMed] [Google Scholar]
- 27.Talmi D, Dayan P, Kiebel SJ, Frith CD, Dolan RJ (2009): How humans integrate the prospects of pain and reward during choice. J Neurosci. 29: 14617–14626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel SR, Herrington TM, Sheth SA, Mian M, Bick SK, Yang JC, et al. (2018): Intermittent subthalamic nucleus deep brain stimulation induces risk-aversive behavior in human subjects. Elife. 7: e36460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bürkner P-C (2017): brms: An R Package for Bayesian Multilevel Models Using Stan. J Stat Softw. 1. [Google Scholar]
- 30.R Development Core Team (2013): R: A language and environment for statistical computing.
- 31.Vehtari A, Gelman A, Gabry J (2017): Practical Bayesian model evaluation using leave-one-out cross-validation and WAIC. Stat Comput. 27: 1413–1432. [Google Scholar]
- 32.Walker AE (1940): A cytoarchitectural study of the prefrontal area of the macaque monkey. J Comp Neurol. 73: 59–86. [Google Scholar]
- 33.Saleem KS, Logothetis NK (2012): A combined MRI and histology atlas of the rhesus monkey brain in stereotaxic coordinates. Academic Press. [Google Scholar]
- 34.Train K (2003): Discrete Choice Models using Simulation. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 35.McFadden D (1973): Conditional logit analysis of qualitative choice behavior. In: Zarembka P, editor. Front Econom. New York, NY: Academic Press. [Google Scholar]
- 36.Nakamura K, Roesch MR, Olson CR (2005): Neuronal activity in macaque SEF and ACC during performance of tasks involving conflict. J Neurophysiol. 93: 884–908. [DOI] [PubMed] [Google Scholar]
- 37.Ito S, Stuphorn V, Brown JW, Schall JD (2003): Performance monitoring by the anterior cingulate cortex during saccade countermanding. Science (80- ). 302: 120–122. [DOI] [PubMed] [Google Scholar]
- 38.Mansouri FA, Buckley MJ, Tanaka K (2007): Mnemonic function of the dorsolateral prefrontal cortex in conflict-induced behavioral adjustment. Science (80- ). 318: 987–990. [DOI] [PubMed] [Google Scholar]
- 39.Frank MJ (2006): Hold your horses: A dynamic computational role for the subthalamic nucleus in decision making. Neural Networks. 19: 1120–1136. [DOI] [PubMed] [Google Scholar]
- 40.Temel Y, Boothman LJ, Blokland A, Magill PJ, Steinbusch HWM, Visser-Vandewalle V, Sharp T (2007): Inhibition of 5-HT neuron activity and induction of depressive-like behavior by high-frequency stimulation of the subthalamic nucleus. Proc Natl Acad Sci. 104: 17087–17092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strutt AM, Simpson R, Jankovic J, York MK (2012): Changes in cognitive◻emotional and physiological symptoms of depression following STN◻DBS for the treatment of Parkinson’s disease. Eur J Neurol. 19: 121–127. [DOI] [PubMed] [Google Scholar]
- 42.Clarke HF, Horst NK, Roberts AC (2015): Regional inactivations of primate ventral prefrontal cortex reveal two distinct mechanisms underlying negative bias in decision making. Proc Natl Acad Sci. 112: 4176 LP–4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wallis CU, Cockcroft GJ, Cardinal RN, Roberts AC, Clarke HF (2019): Hippocampal interaction with Area 25, but not Area 32, regulates marmoset approach–avoidance behavior. Cereb Cortex. doi: 10.1093/cercor/bhz015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Agustin-Pavon C, Braesicke K, Shiba Y, Santangelo AM, Mikheenko Y, Cockroft G, et al. (2012): Lesions of ventrolateral prefrontal or anterior orbitofrontal cortex in primates heighten negative emotion. Biol Psychiatry. 72: 266–272. [DOI] [PubMed] [Google Scholar]
- 45.Spielberg JM, Miller GA, Warren SL, Engels AS, Crocker LD, Banich MT, et al. (2012): A brain network instantiating approach and avoidance motivation. Psychophysiology. 49: 1200–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chrysikou EG, Gorey C, Aupperle RL (2017): Anodal transcranial direct current stimulation over right dorsolateral prefrontal cortex alters decision making during approach-avoidance conflict. Soc Cogn Affect Neurosci. 12: 468–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davidson RJ (1998): Anterior electrophysiological asymmetries, emotion, and depression: Conceptual and methodological conundrums. Psychophysiology. 35: 607–614. [DOI] [PubMed] [Google Scholar]
- 48.Schultz W (2000): Multiple reward signals in the brain. Nat Rev Neurosci. 1: 199. [DOI] [PubMed] [Google Scholar]
- 49.Haber SN, Knutson B (2009): The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology. 35: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Whitton AE, Treadway MT, Pizzagalli DA (2015): Reward processing dysfunction in major depression, bipolar disorder and schizophrenia. Curr Opin Psychiatry. 28: 7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schall JD, Emeric EE (2010): Conflict in cingulate cortex function between humans and macaque monkeys: more apparent than real. Brain Behav Evol. 75: 237–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boschin EA, Brkic MM, Simons JS, Buckley MJ (2016): Distinct roles for the anterior cingulate and dorsolateral prefrontal cortices during conflict between abstract rules. Cereb Cortex. 27: 34–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Derntl B, Seidel E-M, Eickhoff SB, Kellermann T, Gur RC, Schneider F, Habel U (2011): Neural correlates of social approach and withdrawal in patients with major depression. Soc Neurosci. 6: 482–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chandrasekhar Pammi VS, Pillai Geethabhavan Rajesh P, Kesavadas C, Rappai Mary P, Seema S, Radhakrishnan A, Sitaram R (2015): Neural loss aversion differences between depression patients and healthy individuals: A functional MRI investigation. Neuroradiol J. 28: 97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marusak HA, Thomason ME, Peters C, Zundel C, Elrahal F, Rabinak CA (2016): You say ‘prefrontal cortex’ and I say ‘anterior cingulate’: meta-analysis of spatial overlap in amygdala-to-prefrontal connectivity and internalizing symptomology. Transl Psychiatry. 6: e944. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.