Abstract
Context
Polycystic ovary syndrome (PCOS), a condition of androgen excess in women, is associated with cardiometabolic risk factors; however, this association is not fully characterized in a population-based sample of premenopausal women and high-risk groups such as Hispanics/Latinas.
Objective
We examined the association of PCOS signs and metabolic syndrome (MetS) in premenopausal Hispanic/Latina women.
Methods
This cross-sectional analysis includes 1427 women age 24 to 44 years from the Hispanic Community Health Study/Study of Latinos. PCOS signs included menstrual cycle greater than 35 days or irregular, self-reported PCOS, and oral contraceptive use to regulate periods or acne, and a composite of 1 or more PCOS signs. We calculated odds ratios (OR) and 95% CI for MetS, accounting for sociodemographic factors and the complex survey design; an additional model included body mass index (BMI).
Results
The mean age was 34 years and 30% reported any PCOS sign. The odds of MetS were higher in women reporting cycles greater than 35 days or irregular (OR 1.63; CI: 1.07-2.49) vs cycles 24 to 35 days, self-reported PCOS (OR 2.49; CI: 1.38-4.50) vs no PCOS, and any PCOS sign (OR 1.58; CI: 1.10-2.26) vs none. We found no association between OC use to regulate periods or acne and MetS (OR 1.1; CI: 0.6-1.8). When adjusting for BMI, only the association of self-reported PCOS and MetS was attenuated (OR 1.78; CI: 0.92-3.44).
Conclusions
In Hispanic/Latina women, irregular menstrual cycles, self-reported PCOS, and any PCOS sign were associated with MetS and could indicate women at metabolic disease risk.
Keywords: polycystic ovary syndrome, androgen excess, testosterone, cardiometabolic risk factors, women’s health
Women with polycystic ovary syndrome (PCOS), an endocrine disorder characterized by androgen excess (1), are more likely to have metabolic abnormalities (2, 3) and metabolic syndrome (MetS) (4, 5) compared with women without PCOS. Irregular menstrual cycles, which are commonly observed in women with PCOS, are associated with insulin resistance in women with PCOS (6, 7) and with metabolic diseases (8, 9). However, these studies have generally been limited to symptomatic women presenting in clinics. Because PCOS signs may also be related to metabolic disease, population-based studies of women are necessary to understand the association of PCOS and PCOS signs with risk for metabolic disease.
Prior reports of PCOS and androgen excess and MetS did not include Hispanic/Latina women, a high-risk group for cardiometabolic disease. In the United States, compared with non-Hispanic white women, Hispanic/Latina women have a disproportionally higher burden of metabolic diseases, such as MetS (10), nonalcoholic fatty liver disease (11), and type 2 diabetes (12). The few studies of PCOS in Hispanic/Latina women suggest Hispanic/Latina women with PCOS have a greater burden of obesity and insulin resistance vs women with PCOS of other races/ethnicities (13–15). Similarly, Hispanic/Latina women have a high burden of abdominal adiposity (16) and insulin resistance (12), offering an opportunity to evaluate the relationship between androgen excess and metabolic dysregulation in this population.
Our objective was to examine the association of PCOS signs, an indication of androgen excess, with the prevalence of MetS in premenopausal Hispanic/Latina women using data from the largest cohort of Hispanic/Latino adults in the United States, the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). In addition, we sought to evaluate whether obesity modified the association between PCOS signs and MetS. We hypothesized that women with PCOS signs had a higher prevalence of MetS. Prior research has been limited in premenopausal women, an age when metabolic abnormalities and adiposity are developing. An improved understanding of the relationships between PCOS signs and health outcomes in women would inform risk management and prevention at a time when primary prevention efforts are likely to be more effective.
Materials and Methods
Study design and population
The HCHS/SOL was designed to examine the prevalence among Hispanic/Latinos of risk factors and protective factors for chronic diseases, and their associations with the incidence of various chronic diseases (https://sites.cscc.unc.edu/hchs/). The HCHS/SOL baseline study design and sampling methods have been published previously (17, 18). Between March 2008 and June 2011, a total of 16 415 self-identified Hispanic/Latino individuals age 18 to 74 years were recruited from randomly selected households in the Bronx, New York; San Diego, California; Chicago, Illinois; and Miami, Florida. The study was designed to include participants from Cuban, Dominican, Mexican, Puerto Rican, Central American, and South American backgrounds. Households were chosen using a stratified 2-stage area probability sample design. Census block groups were randomly selected in specified geographic areas of each study site, and households were randomly selected in each sample block group. Households were screened for eligibility and self-identified Hispanic/Latino individuals age 18 to 74 years were selected in each household. Oversampling occurred at each stage (block groups in areas of high concentration of Hispanic/Latinos, households associated with a Hispanic/Latino surname, and individuals age 45 to 74 years at rates higher than younger household members).
The visit 2 examination was a call-back of the HCHS/SOL cohort starting on average 5 to 6 years after baseline. Overall, 11 623 participants were reexamined between October 2014 and December 2017. Approval by institutional review boards was obtained at each participating institution, and written informed consent was obtained from all study participants. This cross-sectional study includes women of reproductive age (24-44 years) who participated in visit 2 (N = 1663), which was when PCOS symptoms were assessed. We excluded women who self-reported as postmenopausal (n = 19), had had a hysterectomy (n = 44), had had ovaries removed (n = 33), had had breast or cervical cancer (n = 6), or who were missing outcomes or covariates of interest (n = 134). The final sample size was 1427.
Study measurements
All examinations and interviewer-administered questionnaires were obtained at the field centers by certified bilingual study personnel, following a standardized protocol with ongoing quality assurance procedures. Study participants were asked to fast and to abstain from smoking for 12 hours before the examination and to avoid vigorous physical activity the morning of the examination. Body weight was measured to the nearest 0.1 kg and height was recorded to the nearest centimeter. Waist circumference (WC) was measured at the uppermost lateral border of the right ilium to the nearest 0.1 cm using a measuring tape. Body mass index (BMI) was calculated as weight (kilograms) divided by height (meters) squared. Three seated blood pressure measurements were obtained after a 5-minute rest using an oscillometric automated sphygmomanometer (Omron HEM-907XL) and averaged for these analyses.
Blood samples were obtained and processed. Fresh as well as frozen specimens were shipped to the HCHS/SOL Central Laboratory for assays and long-term storage. High-density lipoprotein (HDL) cholesterol was measured by a magnesium/dextran sulfate method. Plasma glucose was measured using a hexokinase enzymatic method (Roche Diagnostics). Triglycerides were measured in serum on a Roche Modular P chemistry analyzer using a glycerol blanking enzymatic method (Roche Diagnostics). Diabetes and prediabetes were based on the American Diabetes Association definition as a hemoglobin A1c greater than or equal to 6.5%, post–oral glucose tolerance test glucose greater than or equal to 200 mg/dL, fasting glucose greater than or equal to 126 mg/dL, nonfasting glucose greater than or equal to 200 mg/dL, or self-reported use of antidiabetes medication (19) plus an additional self-reported diabetes criterion.
Interviewer-administered questionnaires were used to obtain information on demographic factors, education and income, country of origin and generational status, length of residence in the United States, and language preference. Study staff asked women about their reproduction history and pregnancy-related complications. Participants were asked to bring all prescription and nonprescription medications taken during the 4 weeks preceding the examination. Study staff recorded all medications for coding.
MetS was defined according to the American Heart Association/National Heart, Lung, and Blood Institute 2009 Joint Scientific Statement (20), which for women was having 3 or more of the following: 1) WC greater than or equal to 88 cm, 2) triglycerides greater than or equal to 150 mg/dL, 3) HDL less than 50 mg/dL, 4) blood pressure greater than or equal to 130 mm Hg systolic and/or greater than or equal to 85 mm Hg diastolic and/or on drug treatment, 5) fasting glucose greater than or equal to 100 mg/dL and/or on drug treatment.
Polycystic ovary syndrome signs in women
Our exposures of interest were the following PCOS signs: 1) prior self-reported diagnosis of PCOS, 2) menstrual cycles greater than 35 days or irregular, 3) oral contraceptive (OC) use to regulate menstrual cycles or acne, and 4) a composite measure of any PCOS sign that included any of the above. Participants were asked, “Has a health care provider ever told you that you have polycystic ovary syndrome or PCOS?” to indicate self-reported PCOS. Menstrual cycle length was indicated by the response to “How many days did your typical menstrual cycle last, that is, how many days were between the beginning of one menstrual period to the beginning of bleeding of the next period?” with options of less than 24 days, 24 to 35 days, greater than 35 days (ie, oligomenorrhea), or too variable or irregular to say. Women were asked to think about their menstrual periods at age 20 to 40 years when they were not using birth control pills or other hormone medications and were not pregnant or breastfeeding. For the analysis, we created a 3-level variable for menstrual cycle length by combining greater than 35 days (unweighted n = 40) and too variable or irregular to say (unweighted n = 209) because of small numbers for cycles greater than 35 days. OC use was defined by asking participants who reported OC use, “Why have you used this/these hormonal preparations?” Possible choices were taking it for birth control, acne, menstrual cramps or painful periods, to regulate periods, to treat vaginal bleeding, or other. We categorized OC use as not taking OCs, taking OCs to regulate periods or acne, and taking OCs for another reason. Women not taking OCs were the referent group.
Statistical analysis
Summary statistics and their variances accounted for HCHS/SOL complex survey design and were weighted to adjust for sampling probability and visit 2 nonresponse(18) using SAS version 9.4 (SAS Institute) and SUDAAN release 10.0.0 (RTI). Means and prevalence estimates for demographic and health characteristics were computed by PCOS signs. The prevalence of MetS and of each of its 5 components was estimated by PCOS signs. We used multivariable logistic regression to estimate the association between each PCOS sign and the prevalence of MetS adjusting for covariates. Model 1 adjusted only for site (Bronx, Chicago, Miami, and San Diego); model 2 further adjusted for age; model 3 further adjusted for Hispanic/Latina background, education (< high school, high school, or more), and current smoking (yes, no); model 4 further adjusted for continuous BMI. Because WC is a MetS criterion, we considered model 3 as the final model because of concerns about including BMI in the model. First-order interactions between PCOS signs and BMI greater than or equal to 30 kg/m2 were tested by adding an interaction term in the respective models, and results were stratified if significant interactions were detected (P < .1). All statistical tests were 2-sided at a significance level of .05.
Results
Overall, 18.2% women had menstrual cycles greater than 35 days or irregular, 14% reported OC use to regulate periods or acne, 6% self-reported PCOS, and 30% had any PCOS sign. On average, these women were age 33.6 years; 46% were of Mexican background; 11% each of Dominican, Cuban, or Puerto Rican background; and 27% had less than a high school education (Table 1). Women with any PCOS sign tended to have a higher burden of cardiometabolic risk factors compared to those without PCOS signs (Table 1). Similar patterns were seen with self-reported PCOS and menstrual cycle length (Table 2). Women self-reporting PCOS tended to have higher BMI, WC, systolic blood pressure, low-density lipoprotein cholesterol, and fasting insulin and glucose measures compared to women not self-reporting PCOS (Table 2). Women with menstrual cycles greater than 35 days or irregular tended to have higher WC, low-density lipoprotein cholesterol, triglycerides, and fasting insulin compared to women with 24- to 35-day cycles (Table 2). All PCOS signs tended to have a higher prevalence of diabetes compared to their respective comparison groups (Tables 1 and 2). The distribution of Hispanic/Latino background varied across each group of PCOS signs, particularly for self-reported PCOS.
Table 1.
Demographic and Health Characteristics Overall and by PCOS Signs Among Premenopausal Women, HCHS/SOL (2014-2017; Unweighted n = 1427)
| Overall | Any PCOS Sign | |||
|---|---|---|---|---|
| (Unweighted n = 1427) | No (Unweighted n = 1018) | Yes (Unweighted n = 409) | ||
| Characteristic | Unweighted No. | Mean or % (SE) | Mean or % (SE) | Mean or % (SE) |
| Age, y | 1427 | 33.6 (0.2) | 34.0 (0.2) | 32.6 (0.3) |
| Hispanic background, % | ||||
| Dominican | 132 | 11.2 (1.4) | 12.2 (1.6) | 8.8 (2.2) |
| Central American | 183 | 9.2 (1.1) | 9.6 (1.2) | 8.4 (1.5) |
| Cuban | 107 | 11.6 (1.6) | 13.8 (1.9) | 6.5 (1.5) |
| Mexican | 716 | 46.1 (2.3) | 45.7 (2.4) | 47.2 (3.5) |
| Puerto Rican | 151 | 11.9 (1.3) | 9.9 (1.3) | 16.5 (2.4) |
| South American | 73 | 4.0 (0.6) | 4.0 (0.8) | 4.0 (1.1) |
| Other/Mixed | 65 | 6.1 (0.9) | 5.0 (0.9) | 8.6 (1.8) |
| < High school education, % | 406 | 27.4 (1.6) | 28.5 (1.8) | 25.0 (2.7) |
| Current smoking, % | 162 | 13.5 (1.2) | 12.8 (1.4) | 15.0 (2.3) |
| BMI ≥ 30, kg/m2 | 628 | 42.7 (1.8) | 41.5 (2.1) | 45.4 (3.2) |
| BMI, kg/m2 | 1427 | 30.0 (0.3) | 29.7 (0.3) | 30.8 (0.5) |
| Waist circumference, cm | 1424 | 96.4 (0.6) | 95.3 (0.7) | 98.9 (1.1) |
| SBP, mm Hg | 1427 | 107.7 (0.4) | 107.7 (0.5) | 107.6 (0.8) |
| DBP, mm Hg | 1427 | 68.9 (0.3) | 68.8 (0.4) | 69.1 (0.7) |
| LDL-C, mg/dL | 1416 | 104.6 (1.0) | 104.0 (1.2) | 105.9 (1.7) |
| HDL-C, mg/dL | 1427 | 53.5 (0.5) | 53.6 (0.6) | 53.3 (1.1) |
| Triglycerides, mg/dL | 1426 | 94.9 (2.0) | 91.6 (2.1) | 102.7 (4.5) |
| Fasting insulin, pmol/L | 1425 | 87.2 (2.4) | 81.9 (2.8) | 99.4 (4.5) |
| Fasting glucose, mg/dL | 1426 | 97.4 (1.1) | 97.2 (1.4) | 97.9 (1.7) |
| Diabetes, % | ||||
| No diabetes | 874 | 62.7 (1.7) | 65.2 (1.9) | 56.7 (3.0) |
| Prediabetes | 415 | 28.4 (1.5) | 27.2 (1.8) | 31.4 (3.0) |
| Diabetes | 138 | 8.9 (0.9) | 7.6 (1.0) | 12.0 (2.0) |
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; HCHS/SOL, Hispanic Community Health Study/Study of Latinos; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; PCOS, polycystic ovary syndrome; SBP, systolic blood pressure.
Table 2.
Demographic and Health Characteristics Overall and by Self-Reported PCOS and Menstrual Cycle Length Among Premenopausal Women in the HCHS/SOL (unweighted n = 1427)
| Overall | Self-Reported PCOS | Menstrual Cycle Length | |||||
|---|---|---|---|---|---|---|---|
| (Unweighted n = 1427) | No (Unweighted n = 1340) | Yes (Unweighted n = 87) | < 24 d (Unweighted n = 104) | 24 to 35 d (Unweighted n = 1074) | > 35 d or Irregular (Unweighted n = 249) | ||
| Characteristic | Unweighted No. | Mean or % (SE) | Mean or % (SE) | Mean or % (SE) | Mean or % (SE) | Mean or % (SE) | Mean or % (SE) |
| Age, y | 1427 | 33.6 (0.2) | 33.6 (0.2) | 33.7 (0.8) | 33.8 (0.8) | 33.8 (0.2) | 33.0 (0.4) |
| Hispanic background, % | |||||||
| Dominican | 132 | 11.2 (1.4) | 10.6 (1.3) | 20.0 (5.5) | 16.0 (4.6) | 11.4 (1.5) | 8.1 (2.4) |
| Central American | 183 | 9.2 (1.1) | 9.4 (1.1) | 5.6 (2.8) | 9.1 (3.2) | 9.4 (1.2) | 8.3 (2.0) |
| Cuban | 107 | 11.6 (1.6) | 11.9 (1.6) | 6.3 (2.7) | 9.7 (4.0) | 12.9 (1.8) | 7.3 (2.1) |
| Mexican | 716 | 46.1 (2.3) | 46.5 (2.3) | 40.8 (6.4) | 45.1 (6.6) | 46.1 (2.4) | 46.7 (4.2) |
| Puerto Rican | 151 | 11.9 (1.3) | 11.6 (1.3) | 16.1 (4.7) | 15.9 (5.3) | 9.6 (1.2) | 19.4 (3.2) |
| South American | 73 | 4.0 (0.6) | 4.0 (0.7) | 2.9 (2.0) | 1.7 (1.6) | 4.4 (0.8) | 3.3 (1.3) |
| Other/Mixed | 65 | 6.1 (0.9) | 5.9 (0.9) | 8.4 (3.1) | 2.6 (2.5) | 6.2 (1.0) | 7.0 (2.3) |
| < High school education, % | 406 | 27.4 (1.6) | 27.8 (1.6) | 21.8 (5.8) | 35.0 (6.0) | 25.9 (1.8) | 30.3 (3.6) |
| Current smoking, % | 162 | 13.5 (1.2) | 13.6 (1.2) | 12.4 (4.3) | 12.8 (4.4) | 12.5 (1.3) | 17.7 (3.2) |
| BMI ≥ 30, kg/m2 | 628 | 42.7 (1.8) | 41.5 (1.8) | 60.8 (6.2) | 44.0 (6.6) | 41.9 (2.0) | 45.4 (4.0) |
| BMI, kg/m2 | 1427 | 30.0 (0.3) | 29.7 (0.3) | 34.1 (1.5) | 29.3 (1.0) | 29.8 (0.3) | 31.1 (0.7) |
| Waist circumference, cm | 1424 | 96.4 (0.6) | 95.8 (0.6) | 105.8 (3.2) | 95.8 (2.4) | 95.7 (0.6) | 99.4 (1.5) |
| SBP, mm Hg | 1427 | 107.7 (0.4) | 107.6 (0.4) | 109.5 (2.3) | 109.7 (2.0) | 107.4 (0.5) | 107.9 (1.0) |
| DBP, mm Hg | 1427 | 68.9 (0.3) | 68.8 (0.3) | 70.2 (1.3) | 69.8 (1.3) | 68.7 (0.4) | 69.0 (0.9) |
| LDL-C, mg/dL | 1416 | 104.6 (1.0) | 104.4 (1.1) | 107.4 (3.2) | 104.1 (3.8) | 103.9 (1.1) | 107.4 (2.4) |
| HDL-C, mg/dL | 1427 | 53.5 (0.5) | 53.7 (0.6) | 51.5 (1.4) | 55.4 (2.1) | 53.7 (0.5) | 52.0 (1.7) |
| Triglycerides, mg/dL | 1426 | 94.9 (2.0) | 94.9 (2.1) | 95.7 (5.8) | 90.8 (5.3) | 92.5 (2.2) | 106.3 (5.2) |
| Fasting insulin, pmol/L | 1425 | 87.2 (2.4) | 85.1 (2.4) | 119.6 (13.1) | 77.9 (7.4) | 84.3 (2.7) | 102.3 (5.7) |
| Fasting glucose, mg/dL | 1426 | 97.4 (1.1) | 96.7 (1.1) | 108.1 (6.6) | 94.8 (3.0) | 97.2 (1.3) | 99.6 (2.5) |
| Diabetes, % | |||||||
| No diabetes | 874 | 62.7 (1.7) | 63.2 (1.7) | 53.5 (6.3) | 68.2 (6.4) | 64.2 (1.9) | 54.0 (4.0) |
| Prediabetes | 415 | 28.4 (1.5) | 28.5 (1.6) | 27.1 (5.5) | 27.0 (5.9) | 27.5 (1.7) | 32.9 (3.7) |
| Diabetes | 138 | 8.9 (0.9) | 8.3 (0.9) | 19.4 (5.7) | 4.8 (2.2) | 8.3 (1.0) | 13.2 (2.7) |
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; HCHS/SOL, Hispanic Community Health Study/Study of Latinos; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; PCOS, polycystic ovary syndrome; SBP, systolic blood pressure.
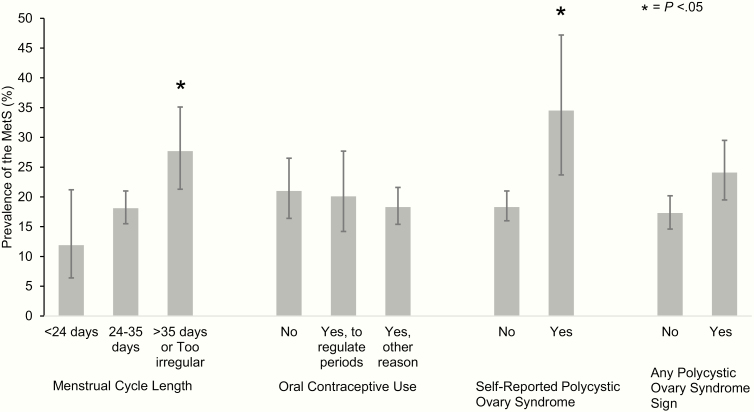
Overall, the prevalence of MetS was 19.3% (95% CI: 16.9%-21.8%). Women self-reporting PCOS had a significantly higher MetS prevalence (34.5%; 95% CI: 23.7%-47.2%) compared to women not reporting PCOS (18.3%; 95% CI: 16.0%-21.0%; Fig. 1). Women reporting menstrual cycle length greater than 35 days or irregular cycles also had a significantly higher MetS prevalence (27.7%; 95% CI: 21.3%-35.1%) compared to women with menstrual cycles of 24 to 35 days (18.1%; 95% CI: 15.5%-21.0%). OC use to regulate periods or acne had similar MetS prevalence compared to those with no OC use (20.1%; 95% CI: 14.2%-27.4% vs 21.0%; 95% CI: 16.4%-26.5%, respectively). MetS prevalence tended to be higher among women reporting any PCOS sign (24.1%; 95% CI: 19.5%-29.5%) compared to those who did not (17.3%; 95% CI: 14.6%-20.2%), but this difference was not statistically significant.
Figure 1.
Prevalence of metabolic syndrome by polycystic ovary syndrome signs in premenopausal Hispanic/Latina women in the Hispanic Community Health Study/Study of Latinos (unweighted n = 1427).
Menstrual cycle length greater than 35 days or irregular, self-reported PCOS, and any PCOS sign were significantly associated with higher odds of MetS prevalence adjusted for study site, age, Hispanic/Latino background, education, and smoking status (Table 3; model 3). Women reporting cycles greater than 35 days or irregular cycles had 1.63 (95% CI: 1.07-2.49) times the odds of MetS compared with women reporting cycles of 24 to 35 days. When considering the 4-levels for menstrual cycles, the effect is stronger for irregular cycles (OR 1.8; 95% CI: 1.1, 2.9), but weaker for cycles greater than 35 days (OR 0.6; 95% CI: 0.2-2.3) compared with women reporting cycles of 24 to 35 days. Self-reported PCOS had 2.49 (95% CI: 1.38-4.50) times the odds of MetS compared to women not reporting PCOS. Women with any PCOS sign had 1.58 (95% CI: 1.10-2.26) times the odds of MetS compared to women without PCOS signs. We found no significant associations between MetS and OC use to regulate periods or acne. When additionally adjusting for BMI in model 4, the results were similar except for self-reported PCOS, for which the association was attenuated (OR: 1.78; 95% CI: 0.92-3.44). The interaction with BMI greater than or equal to 30 kg/m2 was not statistically significant (P > .26).
Table 3.
Association of PCOS Signs and Prevalence of Metabolic Syndrome Among Premenopausal Women in the HCHS/SOL (Unweighted n = 1427)
| Model 1 | Model 2 | Model 3 | Model 4 | |
|---|---|---|---|---|
| PCOS Sign | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) |
| Menstrual cycle length | ||||
| < 24 days | 0.61 (0.30-1.26) | 0.60 (0.29-1.25) | 0.54 (0.25-1.16) | 0.51 (0.25-1.06) |
| 24-35 days | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| > 35 days or too irregular | 1.72 (1.16-2.56) | 1.86 (1.23-2.80) | 1.63 (1.07-2.49) | 1.59 (1.02-2.49) |
| Overall P value | .007 | .004 | .016 | .014 |
| Oral contraceptive use | ||||
| No | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| Yes, to regulate periods or acne | 0.90 (0.53-1.53) | 1.11 (0.63-1.95) | 1.05 (0.60-1.83) | 0.99 (0.55-1.79) |
| Yes, other reason | 0.80 (0.55-1.16) | 0.80 (0.55-1.17) | 0.77 (0.53-1.13) | 0.84 (0.55-1.31) |
| Overall P value | .491 | .269 | .252 | .661 |
| Self-reported PCOS | ||||
| No | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| Yes | 2.33 (1.33-4.08) | 2.38 (1.30-4.37) | 2.49 (1.38-4.50) | 1.78 (0.92-3.44) |
| Any PCOS sign | ||||
| No | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| Yes | 1.52 (1.08-2.13) | 1.72 (1.21-2.44) | 1.58 (1.10-2.26) | 1.48 (1.02-2.15) |
Separate models were fit for each sign. Model 1: adjusted for study site. Model 2: adjusted for study site and age. Model 3: adjusted for study site, age, Hispanic/Latino background, education (less than high school, high school, and more than high school), and smoking status (current vs other); Model 4: Model 3 plus BMI. Overall P values are provided for variables with more than 2 groups.
Abbreviations: BMI: body mass index; HCHS/SOL, Hispanic Community Health Study/Study of Latinos; OR: odds ratio; PCOS: polycystic ovary syndrome.
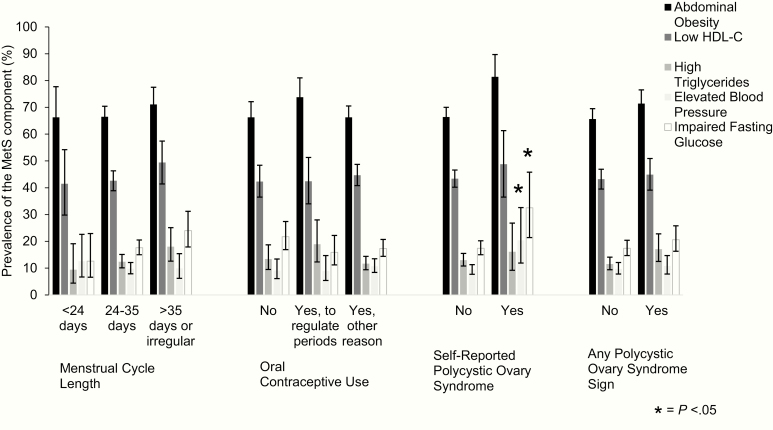
The 2 most common prevalent MetS components were abdominal obesity (> 66%) and low HDL-C (> 42%), irrespective of PCOS signs (Fig. 2). The profile of MetS abnormalities differed within PCOS signs. In general, all women with PCOS signs tended to have a higher prevalence of MetS components compared with their respective referent group, but not all statistically significant. Women with self-reported PCOS had a significantly higher prevalence of elevated blood pressure (20.4%; 95% CI: 11.9%-23.6%) compared with women not reporting PCOS (9.4%; 95% CI: 7.7%-11.3%). Women reporting PCOS also had a higher prevalence of impaired fasting glucose (32.5%; 95% CI: 21.4%-45.8%) compared with women not reporting PCOS (17.4%; 95% CI: 15.0%-20.2%).
Figure 2.
Prevalence of metabolic syndrome components by polycystic ovary syndrome signs in premenopausal Hispanic/Latina women in the Hispanic Community Health Study/Study of Latinos (unweighted n = 1427).
Discussion
In this large cohort study of Hispanic/Latina premenopausal women living in the United States, having menstrual cycles greater than 35 days or irregular cycles, self-reported PCOS, and having any PCOS sign were associated with a higher prevalence of MetS. OC use to regulate periods or acne was not statistically associated with MetS. Our results suggest that PCOS signs are associated with prevalent metabolic disease.
Although prior studies have shown an association between irregular menstrual cycles and higher androgen levels (21–23), metabolic dysregulation (8), and type 2 diabetes (9), few have assessed the prevalence of MetS. One study showed an association between irregular menstrual cycles and MetS in adolescents (24). In this study, irregular menstrual cycles were associated with almost a 2-fold greater risk of having MetS. In a study of women with PCOS, the prevalence of oligomenorrhea (menstrual cycles > 35 days) was higher in those with MetS compared to those without MetS (25), suggesting menstrual irregularities could indicate a greater risk for metabolic disease in women with PCOS. Irregular menstrual cycles are a phenotype of PCOS, a common cause of menstrual cycle disorders (26–28). Menstrual cycle disturbances are reported in more than two-thirds of women with PCOS (21, 29, 30). In this population-based study of women, irregular menstrual cycles were associated with MetS and could be an indicator of PCOS in this population.
Studies have consistently shown an association between PCOS and MetS. The prevalence of MetS is higher in women with PCOS compared with the general population (31, 32). In a meta-analysis of 18 studies, compared to women without PCOS, women with PCOS had 2.9 higher odds of the prevalence of MetS (95% CI: 2.4-3.5) (33). According to a recent prospective study, women suspected to have PCOS developed MetS about 3 years earlier compared with women not suspected to have PCOS (34). Similarly in this study, women who self-reported PCOS had more than double the prevalence of MetS than those who did not, and women reporting any PCOS sign had a higher prevalence of MetS than those who did not. Although women self-reported PCOS and PCOS signs, they could be important markers of metabolic disease.
In this study, we found a higher prevalence of elevated fasting glucose and blood pressure in women with self-reported PCOS compared to women not reporting PCOS. In contrast, in a study of 102 Brazilian women with PCOS, the most prevalent MetS components were low HDL-C (70%) and abdominal adiposity (58%) (35). Similarly, a study of racial and ethnic differences in MetS among women with PCOS showed that the most prevalent MetS components were low HDL-C (59%) and BMI greater than or equal to 30 (42%) among women with PCOS from Brazil (36). Interestingly, US black women with PCOS had a similar prevalence of elevated BMI (74%) and elevated glucose (22%) as seen in this study, suggesting a commonality among US minority groups with PCOS. Differences observed between these studies and the current study may be due to the study populations. These studies included only women from Brazil, limiting inferences to Hispanic/Latina women. In this study, we included a diverse group of Hispanic/Latina women, of whom 4% had a South American background. Further, these studies included only women with PCOS, limiting the ability to compare to a reference group as we did in this study. Our results and prior studies point to racial and ethnic differences in MetS components in women with PCOS.
Limited data exist on the prevalence of PCOS in Hispanic/Latina women. About 6% to 10% of reproductive-age women in the United States have PCOS (37, 38). In this study, 6% self-reported PCOS, although the prevalence is likely higher because about 70% of women with PCOS are not diagnosed (39). In 156 Mexican American women, the prevalence of PCOS was 13%; however, PCOS was based on signs of PCOS including menstrual irregularity and clinical hyperandrogenism (40). Another study of 150 Mexican American women found the prevalence of PCOS to be either 6% (95% CI: 1.9%-10.1%) using the National Institutes of Health criteria or 6.6% (95% CI: 2.3%-10.9%) using the Rotterdam criteria (41). Given the limitations in the field, large population-based studies are needed to characterize PCOS in Hispanic/Latina women, a group at high risk of metabolic disease.
Cardiometabolic abnormalities were prevalent among women at the first HCHS/SOL examination: 17% had diabetes (42), 36% had MetS (16), and 78% of women were overweight or obese (43). These observations indicate a high burden of metabolic and cardiovascular risk factors across the age spectrum. The lack of knowledge about risk factors for metabolic disease specific to women, such as PCOS and androgen excess, is a major barrier to understanding health disparities in Hispanic/Latina women. Most studies of androgens in women included primarily older women or women with PCOS. Thus, future population-based studies of premenopausal women would provide a better understanding of the role of PCOS and androgens on long-term health in women.
Our study has several limitations to consider. The cross-sectional design precludes the assessment of temporality and causality in the observed associations. Obesity, a MetS component, could be related to menstrual irregularities (44), and a higher BMI is a common feature in women with PCOS; thus, prospective studies are needed to further examine the observed associations. We used self-reported PCOS that could be subject to misclassification; however, women who self-reported PCOS had a higher prevalence of PCOS signs compared to women not self-reporting PCOS. Lastly, we lack information on sex hormone levels and assessment of ovarian structure or function. Because PCOS signs are proxies of high androgen levels, our observed associations are likely attenuated. However, in a general population similar to this study, women with self-reported oligomenorrhea had higher androgen levels compared with women not reporting oligomenorrhea (45). Future studies to measure androgen levels will be critical to further characterize these relationships.
This is the first study of PCOS signs and MetS in Hispanic/Latina women, a high-risk group for metabolic disease and for whom studies are lacking. Menstrual cycles greater than 35 days or irregular, self-reported PCOS, and any PCOS sign were cross-sectionally associated with MetS. The prevalence of the cardiometabolic abnormities considered components of MetS is high in Hispanic/Latinos, particularly abdominal adiposity. Reducing the burden of MetS and its sequelae is a high priority that will benefit from future, prospective studies with androgen levels to understand the role of PCOS and androgen excess as markers of health and provide insights into targeted screening and prevention of metabolic disease in women.
Acknowledgments
Financial Support: The Hispanic Community Health Study/Study of Latinos (HCHS/SOL) is a collaborative study supported by contracts from the National Heart, Lung, and Blood Institute (NHLBI) to the University of North Carolina (HHSN268201300001I/N01-HC-65233), University of Miami (HHSN268201300004I/N01-HC-65234), Albert Einstein College of Medicine (HHSN268201300002I/N01-HC-65235), University of Illinois at Chicago (HHSN268201300003I/N01-HC-65236 Northwestern University), and San Diego State University (HHSN268201300005I/N01-HC-65237). The following Institutes/Centers/Offices have contributed to the HCHS/SOL through a transfer of funds to the NHLBI: National Institute on Minority Health and Health Disparities, National Institute on Deafness and Other Communication Disorders, National Institute of Dental and Craniofacial Research, National Institute of Diabetes and Digestive and Kidney Diseases, National Institute of Neurological Disorders and Stroke, NIH Institution–Office of Dietary Supplements. M.L.M. was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Building Interdisciplinary Research Careers in Women’s Health (5K12HD001441).
Glossary
Abbreviations
- BMI
body mass index;
- HCHS/SOL
Hispanic Community Health Study/Study of Latinos
- MetS
metabolic syndrome
- PCOS
polycystic ovary syndrome
Additional Information
Disclosure Summary: The authors have no conflicts of interest.
Data Availability: Availability of data and detailed policies for accessing HCHS/SOL study data can be found online (https://sites.cscc.unc.edu/hchs/). The HCHS/SOL study data are made available through the NHLBI BioLINCC repository (https://biolincc.nhlbi.nih.gov/studies/hchssol/).
References
- 1. Zawadzki J, Dunaif A.. Diagnostic Criteria for Polycystic Ovary Syndrome: Towards a Rationale Approach. Consensus Conference on Polycystic Ovary Syndrome Current Issues in Endocrinology and Metabolism. Bethesda, MD: Blackwell Scientific Publications; 1992. [Google Scholar]
- 2. Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000;21(6):697–738. [DOI] [PubMed] [Google Scholar]
- 3. Hammond GL, Wu TS, Simard M. Evolving utility of sex hormone-binding globulin measurements in clinical medicine. Curr Opin Endocrinol Diabetes Obes. 2012;19(3):183–189. [DOI] [PubMed] [Google Scholar]
- 4. Brand JS, van der Tweel I, Grobbee DE, Emmelot-Vonk MH, van der Schouw YT. Testosterone, sex hormone-binding globulin and the metabolic syndrome: a systematic review and meta-analysis of observational studies. Int J Epidemiol. 2011;40(1):189–207. [DOI] [PubMed] [Google Scholar]
- 5. Golden SH, Ding J, Szklo M, Schmidt MI, Duncan BB, Dobs A. Glucose and insulin components of the metabolic syndrome are associated with hyperandrogenism in postmenopausal women: the Atherosclerosis Risk in Communities study. Am J Epidemiol. 2004;160(6):540–548. [DOI] [PubMed] [Google Scholar]
- 6. Brower M, Brennan K, Pall M, Azziz R. The severity of menstrual dysfunction as a predictor of insulin resistance in PCOS. J Clin Endocrinol Metab. 2013;98(12):E1967–E1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Panidis D, Tziomalos K, Chatzis P, et al. Association between menstrual cycle irregularities and endocrine and metabolic characteristics of the polycystic ovary syndrome. Eur J Endocrinol. 2013;168(2):145–152. [DOI] [PubMed] [Google Scholar]
- 8. Pinola P, Lashen H, Bloigu A, et al. Menstrual disorders in adolescence: a marker for hyperandrogenaemia and increased metabolic risks in later life? Finnish general population-based birth cohort study. Hum Reprod. 2012;27(11):3279–3286. [DOI] [PubMed] [Google Scholar]
- 9. Solomon CG, Hu FB, Dunaif A, et al. Long or highly irregular menstrual cycles as a marker for risk of type 2 diabetes mellitus. JAMA. 2001;286(19):2421–2426. [DOI] [PubMed] [Google Scholar]
- 10. Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287(3):356–359. [DOI] [PubMed] [Google Scholar]
- 11. Lazo M, Hernaez R, Eberhardt MS, et al. Prevalence of nonalcoholic fatty liver disease in the United States: the Third National Health and Nutrition Examination Survey, 1988-1994. Am J Epidemiol. 2013;178(1):38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Narayan KM, Boyle JP, Thompson TJ, Sorensen SW, Williamson DF. Lifetime risk for diabetes mellitus in the United States. JAMA. 2003;290(14):1884–1890. [DOI] [PubMed] [Google Scholar]
- 13. Kauffman RP, Baker VM, Dimarino P, Gimpel T, Castracane VD. Polycystic ovarian syndrome and insulin resistance in white and Mexican American women: a comparison of two distinct populations. Am J Obstet Gynecol. 2002;187(5):1362–1369. [DOI] [PubMed] [Google Scholar]
- 14. Kauffman RP, Baker TE, Graves-Evenson K, Baker VM, Castracane VD. Lipoprotein profiles in Mexican American and non-Hispanic white women with polycystic ovary syndrome. Fertil Steril. 2011;96(6):1503–1507. [DOI] [PubMed] [Google Scholar]
- 15. Lo JC, Feigenbaum SL, Yang J, Pressman AR, Selby JV, Go AS. Epidemiology and adverse cardiovascular risk profile of diagnosed polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91(4):1357–1363. [DOI] [PubMed] [Google Scholar]
- 16. Heiss G, Snyder ML, Teng Y, et al. Prevalence of metabolic syndrome among Hispanics/Latinos of diverse background: the Hispanic Community Health Study/Study of Latinos. Diabetes Care. 2014;37(8):2391–2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sorlie PD, Avilés-Santa LM, Wassertheil-Smoller S, et al. Design and implementation of the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol. 2010;20(8):629–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lavange LM, Kalsbeek WD, Sorlie PD, et al. Sample design and cohort selection in the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol. 2010;20(8):642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl 1):S62–S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Alberti KG, Eckel RH, Grundy SM, et al. ; International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity Circulation. 2009;120(16):1640–1645. [DOI] [PubMed] [Google Scholar]
- 21. Balen AH, Conway GS, Kaltsas G, et al. Polycystic ovary syndrome: the spectrum of the disorder in 1741 patients. Hum Reprod. 1995;10(8):2107–2111. [DOI] [PubMed] [Google Scholar]
- 22. Van Anders SM, Watson NV. Menstrual cycle irregularities are associated with testosterone levels in healthy premenopausal women. Am J Hum Biol. 2006;18(6):841–844. [DOI] [PubMed] [Google Scholar]
- 23. Wei S, Schmidt MD, Dwyer T, Norman RJ, Venn AJ. Obesity and menstrual irregularity: associations with SHBG, testosterone, and insulin. Obesity (Silver Spring). 2009;17(5):1070–1076. [DOI] [PubMed] [Google Scholar]
- 24. Bouzas IC, Cader SA, Leão L, Kuschnir MC, Braga C. Menstrual cycle alterations during adolescence: early expression of metabolic syndrome and polycystic ovary syndrome. J Pediatr Adolesc Gynecol. 2014;27(6):335–341. [DOI] [PubMed] [Google Scholar]
- 25. Korhonen S, Hippeläinen M, Niskanen L, Vanhala M, Saarikoski S. Relationship of the metabolic syndrome and obesity to polycystic ovary syndrome: a controlled, population-based study. Am J Obstet Gynecol. 2001;184(3):289–296. [DOI] [PubMed] [Google Scholar]
- 26. Conway G, Dewailly D, Diamanti-Kandarakis E, et al. ; ESE PCOS Special Interest Group The polycystic ovary syndrome: a position statement from the European Society of Endocrinology. Eur J Endocrinol. 2014;171(4):P1–P29. [DOI] [PubMed] [Google Scholar]
- 27. Legro RS, Arslanian SA, Ehrmann DA, et al. ; Endocrine Society Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98(12):4565–4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hull MG. Epidemiology of infertility and polycystic ovarian disease: endocrinological and demographic studies. Gynecol Endocrinol. 1987;1(3):235–245. [DOI] [PubMed] [Google Scholar]
- 29. Franks S. Polycystic ovary syndrome: a changing perspective. Clin Endocrinol (Oxf). 1989;31(1):87–120. [DOI] [PubMed] [Google Scholar]
- 30. Panidis D, Tziomalos K, Papadakis E, et al. Associations of menstrual cycle irregularities with age, obesity and phenotype in patients with polycystic ovary syndrome. Hormones (Athens). 2015;14(3):431–437. [DOI] [PubMed] [Google Scholar]
- 31. Essah PA, Wickham EP, Nestler JE. The metabolic syndrome in polycystic ovary syndrome. Clin Obstet Gynecol. 2007;50(1):205–225. [DOI] [PubMed] [Google Scholar]
- 32. Glueck CJ, Papanna R, Wang P, Goldenberg N, Sieve-Smith L. Incidence and treatment of metabolic syndrome in newly referred women with confirmed polycystic ovarian syndrome. Metabolism. 2003;52(7):908–915. [DOI] [PubMed] [Google Scholar]
- 33. Moran LJ, Misso ML, Wild RA, Norman RJ. Impaired glucose tolerance, type 2 diabetes and metabolic syndrome in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2010;16(4):347–363. [DOI] [PubMed] [Google Scholar]
- 34. Peng Q, Karvonen-Gutierrez CA, Randolph JF, Nan B, McConnell D, Harlow SD. Age at onset of metabolic syndrome among women with and without polycystic ovary syndrome-like status. J Clin Endocrinol Metab. 2019;104(5):1429–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Soares EM, Azevedo GD, Gadelha RG, Lemos TM, Maranhão TM. Prevalence of the metabolic syndrome and its components in Brazilian women with polycystic ovary syndrome. Fertil Steril. 2008;89(3):649–655. [DOI] [PubMed] [Google Scholar]
- 36. Chan JL, Kar S, Vanky E, et al. Racial and ethnic differences in the prevalence of metabolic syndrome and its components of metabolic syndrome in women with polycystic ovary syndrome: a regional cross-sectional study. Am J Obstet Gynecol. 2017;217(2):189.e1–189.e8. [DOI] [PubMed] [Google Scholar]
- 37. Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89(6):2745–2749. [DOI] [PubMed] [Google Scholar]
- 38. Knochenhauer ES, Key TJ, Kahsar-Miller M, Waggoner W, Boots LR, Azziz R. Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: a prospective study. J Clin Endocrinol Metab. 1998;83(9):3078–3082. [DOI] [PubMed] [Google Scholar]
- 39. March WA, Moore VM, Willson KJ, Phillips DI, Norman RJ, Davies MJ. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. 2010;25(2):544–551. [DOI] [PubMed] [Google Scholar]
- 40. Goodarzi MO, Quiñones MJ, Azziz R, Rotter JI, Hsueh WA, Yang H. Polycystic ovary syndrome in Mexican-Americans: prevalence and association with the severity of insulin resistance. Fertil Steril. 2005;84(3):766–769. [DOI] [PubMed] [Google Scholar]
- 41. Moran C, Tena G, Moran S, Ruiz P, Reyna R, Duque X. Prevalence of polycystic ovary syndrome and related disorders in Mexican women. Gynecol Obstet Invest. 2010;69(4):274–280. [DOI] [PubMed] [Google Scholar]
- 42. Schneiderman N, Llabre M, Cowie CC, et al. Prevalence of diabetes among Hispanics/Latinos from diverse backgrounds: the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Diabetes Care. 2014;37(8):2233–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Daviglus ML, Talavera GA, Avilés-Santa ML, et al. Prevalence of major cardiovascular risk factors and cardiovascular diseases among Hispanic/Latino individuals of diverse backgrounds in the United States. JAMA. 2012;308(17):1775–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Metwally M, Li TC, Ledger WL. The impact of obesity on female reproductive function. Obes Rev. 2007;8(6):515–523. [DOI] [PubMed] [Google Scholar]
- 45. Taponen S, Martikainen H, Järvelin MR, et al. Hormonal profile of women with self-reported symptoms of oligomenorrhea and/or hirsutism: Northern Finland birth cohort 1966 study. J Clin Endocrinol Metab. 2003;88(1):141–147. [DOI] [PubMed] [Google Scholar]