Abstract
Penicillium genus constituted by over 200 species is one of the largest and fascinating groups of fungi, particularly well established as a source of antibiotics. Endophytic Penicillium has been reported to colonize their ecological niches and protect their host plant against multiples stresses by exhibiting diverse biological functions that can be exploited for countless applications including agricultural, biotechnological, and pharmaceutical. Over the past 2 decades, endophytic Penicillium species have been investigated beyond their antibiotic potential and numerous applications have been reported. We comprehensively summarized in this review available data (2000–2019) regarding bioactive compounds isolated from endophytic Penicillium species as well as the application of these fungi in multiple agricultural and biotechnological processes. This review has shown that a very large number (131) of endophytes from this genus have been investigated so far and more than 280 compounds exhibiting antimicrobial, anticancer, antiviral, antioxidants, anti-inflammatory, antiparasitics, immunosuppressants, antidiabetic, anti-obesity, antifibrotic, neuroprotective effects, and insecticidal and biocontrol activities have been reported. Moreover, several endophytic Penicillium spp. have been characterized as biocatalysts, plant growth promoters, phytoremediators, and enzyme producers. We hope that this review summarizes the status of research on this genus and will stimulate further investigations.
Electronic supplementary material
The online version of this article (10.1007/s13205-020-2081-1) contains supplementary material, which is available to authorized users.
Keywords: Endophytes, Penicillium genus, Agricultural, Biotechnological, Pharmaceutical
Introduction
Microbial life has fascinated mankind for centuries. Microbes are considered today as vital to every ecosystem and the pillars of life on Earth (Gilbert and Neufeld 2014; Gibbons and Gilbert 2015). It has been estimated that the number of microbial cells on Earth is about a nonillion (1030), a number that exceeds the estimated number of stars in the Universe (Lennon and Locey 2018). Microbes colonize all ecological niches on the planet and, by their omnipresence, impact the entire biosphere. Their ability to contribute substantially to the function of every ecosystem reflects their tremendous biological diversity. For centuries, this ability has been harnessed to improve our quality of life. Microbes are used to produce alternative fuels to meet growing energy demands, new crops to feed our rapidly growing population, and medicines to fight emerging infectious diseases (Lennon and Locey 2018).
Indeed, among microbial species, the Penicillium genus is one of the largest groups of fungi, composed of over 200 recognized species (Pitt et al. 2000). Species of Penicillium are ubiquitous fungi because of their undemanding nutritional requirements and their ability to grow over a wide range of conditions and environments (Kirk et al. 2008). These species have been isolated as endophytes of multiple and various plant species (Nicoletti et al. 2014). This endophytic lifestyle has been reported to confer to Penicillium species the ability to protect plants species against biotic stresses, to promote plant growth (Waqas et al. 2015; Hassan 2017), and protect the host against pathogens attack via the production of antagonist compounds. Indeed, Penicillium has become one of the most well-known genera of fungi for the discovery of bioactive compounds (Nicoletti and Trincone 2016). Over the years, the investigation of Penicillium species, particularly endophytes have expanded far beyond their ability to produce antibiotic compounds and a wide range of biological activities and applications has been reported. This review offers an overview of the agricultural, pharmaceutical and biotechnological applications of endophytic Penicillium species isolated from medicinal plants growing in diverse habitats. We aim to comprehensively present the current status of the exploration of endophytic Penicillium species while shedding light upon future directions.
Methodology
For this review, existing pieces of literature (research articles, reviews, books, reports) were collected from multiple scientific journals, and worldwide databases such as Scopus, ScienceDirect, PubMed, Web of Science, Medline, Springer, and Google Scholar. The following keywords were searched, “endophytic Penicillium spp.”, “bioactive potential of endophytic penicillium”, “Penicillium spp. from medicinal plants”, “bioactive molecules from Penicillium sp.”, “biocontrol potential of Penicillium species”. Overall, more than 300 different publications (2000–2019) were collected and used for this review. From the analysis of data collected, more than 100 different Penicillium species isolated from more than 110 plants belonging to over 63 families have already been investigated for their bioactive potential (Suppl 1). Briefly, Penicillium species were investigated for their ability to produce compounds with the potential to be used for drug discovery against multiples diseases. We also found that endophytic Penicillium spp. have been investigated for agricultural purposes particularly against phytopathogens and insects as well as to reduce the pollution of agricultural farms by pollutants such as chemical pesticides and heavy metals. Besides, Penicillium has also been investigated for its multiple biotechnological applications including biotransformation, enzyme production, or their ability to facilitate the production of nanoparticles (Fig. 1).
Fig. 1.
Schematic representation of the multiple applications of endophytic Penicillium species isolated from medicinal plants. Briefly, our investigation showed that endophytes isolated from plant tissues were identified using morphological and molecular tools. For their applications, these species were cultured in a liquid or solid medium to produce metabolites purified and identified using various chromatographic and spectroscopic tools. Their biological activities were evaluated using multiples set of assays for drug discovery and agricultural purposes. Also, the ability of the whole organism to sequester heavy metals and toxic compounds for the detoxification of nature, to produce important enzymes or to biotransform compounds were intensively investigated
Pharmacologically active compounds from Penicillium species
Antimicrobial from Penicillium
The major scientific and medical breakthrough in treating microbial infections was the discovery of penicillin by Sir Alexander Fleming, who observed the ongoing lysis of Staphylococcus colonies on a plate contaminated by Penicillium notatum (Fleming 1929). Since then, countless investigations have been conducted to investigate diverse Penicillium species for new antibiotics. These investigations are currently motivated by the ongoing threats of multi-resistant pathogens (Tacconelli et al. 2018). In pursuit of the investigation of endophytic fungi for new antimicrobial agents, the main approach used for the identification of potent compounds was the screening and bio-guided fractionation of extracts. Using this methodology, several investigations led to the identification of dozens of Penicillium species as potent producers of antibiotics. In fact, extracts from 263 endophytes isolated from seven medicinal plants including Alhagi graecorum, Cressa cretica, Citrullus colocynthis, Tamarix nilotica, Achillea fragrantissima, Artemisia sieberi, and Nitraria retusa, were screened for antibacterial activity and P. chrysogenum was the most potent isolate with strong activity against a wide range of pathogens including Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, and Klebsiella pneumoniae (Gashgari et al. 2016). Similarly, 91 endophytic fungi isolates obtained from ethnomedicinal plant Melastoma malabathricum L. were tested against several bacterial and fungal pathogens and the results showed that extract from P. chermesinum KM405640 displayed the most significant activity (Mishra et al. 2016). Toghueo et al. (2016) also reported the antibacterial activity (MIC 0.625–0.019 mg/mL) of an extract of P. chermesinum, an endophyte from Terminalia catappa against nine bacteria pathogens. In addition, extracts from several other Penicillium species isolated from Vellozia gigantea (Ferreira et al. 2017), P. turbatum BLH34 isolated from Macleaya cordata (Wang et al. 2016a, b, c), P. amestolkiae elv609 from Orthosiphon stamineus Benth (Rozman et al. 2017), P. citrinum TDPEF34 (Ben Mefteh et al. 2018), P. funiculosum Fes1711 from Ficus elastic (Ding et al. 2019), P. glabrum (Zhang et al. 2009), P. commune and P. glabrum from Bauhinia forficate (Bezerra et al. 2015), and P. commune and P. canescens from Olea europaea L. (Malhadas et al. 2017) were also reported for their broad antimicrobial activity. These investigations are supporting the trends of knowledge on the potential of Penicillium spp. as sources of potential antibiotics and suggest that further investigation of these species could lead to the identification of potent compounds.
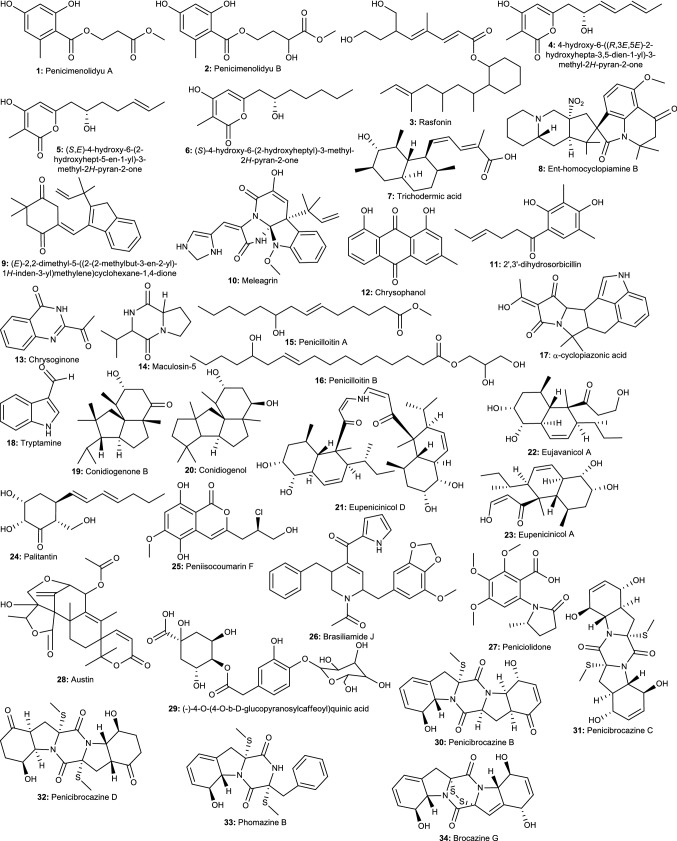
Indeed, to date, dozens of active compounds from endophytic Penicillium spp. have been reported (Fig. 2). In this regard, the screening of 58 fungal endophytes from Ginkgo biloba L. led to the identification of P. cataractum SYPF 7131, displaying the strongest activity. The fractionation led to the isolation of four new compounds, alteryulactone, penicimenolidying, penicimenolidyu A, penicimenolidyu B along with isobenzofuranbutanoic acid, hydroxy-6-methoxy-5-methylisobenzofuran, rasfonin, and 7-hydroxy-2,5-dimethyl-chromen. All the compounds were tested and penicimenolidyu A (1), penicimenolidyu B (2) and rasfonin (3) exhibited potency against five bacteria pathogens with MIC ranging from 10 to 90 μg/mL (Wu et al. 2018). Recently, the bio-guided fractionation of extracts of P. ochrochloron associated with Taxus media led to the purification of four compounds including three new 3,4,6-trisubstituted α-pyrone derivatives, namely 6-(2′R-hydroxy-3′E,5′E-diene-1′-heptyl)-4-hydroxy-3-methyl-2H-pyran-2-one (4), 6-(2′S-hydroxy-5′E-ene-1′-heptyl)-4-hydroxy-3-methyl-2H-pyran-2-one (5), and 6-(2′S-hydroxy-1′-heptyl)-4-hydroxy-3-methyl-2H-pyran-2-one (6), together with trichodermic acid (7), all displaying broad antimicrobial activity with MIC values ranging from 12.5 to 100 μg/mL (Zhao et al. 2019). Another similar study led to the isolation of a broad antibacterial compound, Ent-homocyclopiamine B (8) from extract of P. concentricum, an endophyte of Trichocolea tomentella (Ali et al. 2019). The bio-guided fractionation of active extract from P. chrysogenum MTCC 5108, another endophytic fungus of the mangrove plant Porteresia coarctata (Roxb.) led to the isolation of 3,1′-didehydro-3[2″(3′″,3′″-dimethyl-prop-2-enyl)-3″-indolylmethylene]-6-methyl pipera-zine-2,5-dione (9) exhibiting specific activity against Vibrio cholerae with inhibition diameter of 14–16 mm (Devi et al. 2012). From the ethyl acetate extract from another P. chrysogenum, several compounds were isolated and tested. Meleagrin (10) was active against Candida albicans, 2′,3′-dihydrosorbicillin (11) and meleagrin (10) inhibited K. pneumoniae while, chrysophanol (12), 2′,3′-dihydrosorbicillin (11), chrysoginone (13), and maculosin-5 (14) moderately inhibited the growth of A. niger, E. coli, B. subtilis, and B. megaterium (Hawas et al. 2013). Compounds, penicilloitins A–B (15–16), along with α-cyclopiazonic acid (17), and tryptamine (18), isolated from extracts of a marine endophytic Penicillium species were reported to display antimicrobial activity (19–22 mm) against S. aureus and E. coli (Mourshid et al. 2016). Previously, the bio-guided fractionation of endophytic P. chrysogenum led to the purification of seven compounds including two new tetracyclic diterpenes. When tested against a range of pathogens, conidiogenone B (19) showed potency against methicillin-resistant S. aureus (MRSA), P. fluorescens, P. aeruginosa, and S. epidermidis (MIC 8 μg/mL), while conidiogenol (20) was only active against P. fluorescens and S. epidermidis with MIC values of 16 μg/mL (Gao et al. 2011a). Eujavanicol A, eupenicinicol A, eupenicinicols C and D were isolated from Eupenicillium sp. LG41, an endophyte from the Chinese medicinal plant Xanthium sibiricum. Eupenicinicol D (21) exhibited very good activity against S. aureus (MIC 0.1 μg/mL), eujavanicol A (22) was active against E. coli (MIC 5 μg/mL), while eupenicinicol A (23) was active against B. subtilis with MIC of 10 μg/mL (Li et al. 2017). An antimycobacterial-guided fractionation of extract of a Penicillium sp. HL4-159-41B isolated from the Canadian medicinal plant Aralia nudicaulis led to the purification of palitantin (24) moderately active against M. tuberculosis H37Ra (Li et al. 2015a, b, c). Similarly, 13 compounds were isolated from a P. commune QQF-3 endophyte of the mangrove plant Kandelia candel. Among these compounds, peniisocoumarin F (25) exhibited potency against M. tuberculosis protein tyrosine phosphatase B (MptpB) with an IC50 value of 20.7 μM (Cai et al. 2018).
Fig. 2.
Selected antimicrobial compounds produced by endophytic Penicillium species
Antibacterial screening of extract from 180 endophytes isolated from roots, stems, and leaves of Panax notoginseng was conducted and P. janthinellum SYPF 7899 displaying the strongest antibacterial activity was identified as the most potent. The bio-guided fractionation led to the isolation of ten compounds including three newly described metabolites. Among these compounds, brasiliamide J (26) was active against the five bacterial strains with MIC values 15–85 μg/mL, while peniciolidone (27) and austin (28) were active against S. aureus, B. subtilis and E. coli with MIC value ranging from 35 to 85 μg/mL. Besides, brasiliamide J showed a strong binding affinity to the filamentous temperature-sensitive protein Z (FtsZ) from B. subtilis and S. aureus, suggesting that this compound may constitute a promising antibacterial agent, with the ability to inhibit FtsZ in various bacteria pathogens (Xie et al. 2018). In a previous study, (−)-4-O-(4-O-β-d-glucopyranosylcaffeoyl) quinic acid (29) isolated from another P. citrinum, an endophyte of Avicennia marina, was found to exhibit potent chemoreversal activity, through the inhibition of the function of P-glycoprotein efflux pump in bacteria (Liu et al. 2015a). Extract from P. setosum sp. nov., an endophyte of Withania somnifera, was found to exhibit antibacterial activity against E. coli and S. aureus. In a further experiment, the scanning electron micrographs of treated cells show morphological changes such as shortening of size, bubbles, and blisters on the surface of E. coli, while open holes and deep craters were found on the surface of S. aureus. Intracellular changes were also noticed through different membrane permeabilization assays in addition to the release of intracellular material including Na+, K+, and cytoplasmic β-galactosidase. This potent extract was fractionated and tested. The most active fraction (MIC 8 μg/mL) was found to contain leucodelphinidin, dihydroquercetin, kaempferol, quercetin and patulin. In in silico study, these compounds were able to bind to multiple drug targets in bacteria pathogens with great affinity (George et al. 2019). Further investigation of these compounds could lead to new antibiotics with multi-target activity; the feature needs to prevent the future development of pathogen’s resistance to new drugs.
Several other studies also reported the isolation of compounds with broad antimicrobial activity from endophytic Penicillium species. For instance, six compounds including five new sulfide diketopiperazine derivatives were isolated from an extract of P. brocae MA-231, an endophytic fungus from Avicennia marina. Penicibrocazines B–D (30–32) and phomazine B (33) exhibited antimicrobial activity against S. aureus with MIC values ranging from 0.25 to 32 μg/mL, while only penicibrocazine C (33) was active against M. luteus with MIC of 0.25 μg/mL (Meng et al. 2015). The further investigation of this isolate led to the purification of four new diketopiperazines among which brocazine G (34) was strongly potent and selective against S. aureus (MIC 0.25 μg/mL), spirobrocazine A (35) exhibited moderate activity against E. coli, S. aureus, and Vibrio harveyi, with MIC values of 32, 16, and 64 μg/mL, respectively, while spirobrocazine C (36) was active against E. coli, Aeromonas hydrophilia, and V. harveyi, with a MIC value of 32 μg/mL (Meng et al. 2016). In another study, the chemical investigation of an extract of P. chermesinum EN-480, an endophytic fungus obtained from Pterocladiella tenuis led to the purification of chermesins A–D, four new spiromeroterpenoids. In antimicrobial assay, chermesins A (37) and B (38) were potent against C. albicans, E. coli, M. luteus, and Vibrio alginolyticus (MIC 8–64 μg/mL), while chermesin D (39) only inhibited E. coli with MIC of 64 μg/mL (Liu et al. 2016a). Similarly, arisugacin K (40) isolated from an extract of P. echinulatum pt-4, an endophytic fungus isolated from Chondrus ocellatus, was also reported as an inhibitor of E. coli (Li et al. 2014a, b, c, d, e). Among the 11 compounds isolated from Penicillium sp. AS-79 endophyte of Haliplanella luciae, paspaline (41) only inhibited E. coli (MIC 0.5 μg/mL), 6-hydroxylpaspalinine (42) and 3-deoxo-4b-deoxypaxilline (43) inhibited V. parahaemolyticus (MIC 64 and 16 μg/mL, respectively), while paspalitrem C (44), emindole SB (45) and 10,23-dihydro-24,25-dehydroaflavinine (46) showed activity against E. coli, P. aeruginosa, Vibrio parahaemolyticus and Vibrio alginolyticus with MIC values of 0.5–8 μg/mL (Hu et al. 2017). In another investigation, eight compounds were isolated from P. citrinum, an endophyte from Salicornia herbacea Torr, among which (3β,5α,8α,22E)-5,8-epidioxyergosta-6,9,22-trien-3-ol (47) was potent against Clostridium perfringens and Micrococcus tetragenus with a MIC value of 23.5 μM, stigmasta-7,22-diene-3β,5α,6α-triol (48) and 3β,5α-dihydroxy-(22E,24R)-ergosta-7,22-dien-6β-yl oleate (49) inhibited B. subtilis and M. phlei with MIC values of 22.5 and 14.4 μM, respectively, while, ergone (50), exhibited broad-spectrum antimicrobial activity against C. albicans, C. perfringens, Mycobacterium smegmatis, and Mycobacterium phlei with MIC values of 25.5, 25.5, 18.5, and 51 μM, respectively (Wang et al. 2014).
Six other compounds were isolated from P. citrinum HL-5126, an endophyte of Bruguiera sexangula var. rhynchopetala. When tested against Bacillus subtilis, B. cereus, and Micrococcus tetragenus, 1-(2,6-dihydroxyphenyl)butan-1-one (51) was potent against all pathogens with MIC values of 6.94 μM (Zheng et al. 2016a, b). Continue investigation of this fungus led to the purification of five other compounds among which 2′-acetoxy-7-chlorocitreorosein (52) was active against S. aureus (MIC 22.8 μM) and Vibrio parahaemolyticus (MIC 10 μM), while chloroisosulochrin dehydrate (53), citreorosein (54) and MT-1 (55) were active against all bacteria with the MIC ranging from 22.8 to 50 μM (He et al. 2017). In a later study, six other compounds were isolated from the same endophyte and only penibenzophenone A (56) showed activity against S. aureus with a MIC value of 20 μg/mL (Zheng et al. 2019). Similarly, (−)-3-carboxypropyl-7-hydroxyphthalide (57) and (−)-3-carboxypropyl-7-hydroxyphthalide methyl ester (58), two new phthalide derivatives were also isolated from Penicillium vulpinum, an endophyte of Sophora tonkinensis. Compound 57 exhibited broad activity against B. subtilis, Shigella dysenteriae and Enterobacter areogenes with MIC values of 12.5–25 μg/mL while, compound 58 only inhibited E. aerogenes with MIC value of 12.5 μg/mL (Qin et al. 2019a, b). In another study, penialidin A–C (59–61), citromycetin (62), p-hydroxyphenylglyoxalaldoxime (63) and brefelfin A (64) isolated from Penicillium sp., an endophytic fungus of Garcinia nobilis were reported to inhibit Vibrio cholerae and Shigella flexneri with MIC value ranging from 0.5 to 128 µg/mL (Jouda et al. 2016a, b), while penialidin C was the most potent against Mycobacterium smegmatis with MIC of 15.6 μg/mL (Jouda et al. 2016a, b). Ten compounds including five rare dichloro-aromatic polyketides were isolated from the extract of Penicillium sp., an endophytic fungus of Pinellia ternata growing in China. These compounds were tested against S. aureus ATCC 25923, B. subtilis ATCC 6633, E. coli ATCC 25922, P. aeruginosa ATCC 9027 and C. albicans ATCC 24433. The results showed that helvolic acid (65) was active against the four bacteria with MIC ranging from 4.6 to 75 μg/mL. (+)-(2R)-3′-methoxyl citreovirone (66) and citreovirone (67) were moderately active against E. coli and S. aureus (MIC 62.6 and 76.6 μg/mL). Trypacidin A (68) inhibited S. aureus (MIC 76 μg/mL) and B. subtilis (MIC 54.1 μg/mL), while cis-bis-(methylthio)-silvatin (69) inhibited only S. aureus with a MIC value of 43.4 μg/mL (Yang et al. 2017). A new isoquinolone alkaloid, 5-hydroxy-8-methoxy-4-phenylisoquinolin-1(2H)-one (70), along with 3-O-methylviridicatin (71) and viridicatol (72), was isolated from the extract of Penicillium sp. R22, an endophytic fungus of Nerium indicum. The three compounds displayed antibacterial activity against E. coli, P. aeruginosa, S. aureus, and Streptococcus lactis with MIC values ranging from 15.6 to 125 μg/mL (Ma et al. 2017a). Among the compounds isolated from P. brasilianum endophyte of Melia azedarach, only brasiliamide A moderately inhibited B. subtilis with MIC of 250 µg/mL (Fill et al. 2009). It is clear from these investigations that active crude extracts from Penicillium species are the source of not only broad-spectrum antimicrobial compounds but also compounds with specific activity against a target pathogen.
Several studies focus on the identification of potent inhibitors of S. aureus; the most dangerous of all the Staphylococcal bacteria have been reported with interesting findings. Indeed, 96 endophytic fungi isolated from Aralia elata were screened for their anti-Staphylococcus aureus activity. Several isolates exhibited activity with Penicillium sp. G22 as the most potent. This isolate was found to produce two well-known potent compounds: ginsenosides Re and Rb2 (Wu et al. 2012). In another investigation, four compounds, cladosporin, epiepoformin, phyllostine, and patulin isolated from Penicillium sp., an endophyte of Fucus spiralis, were found active against S. aureus (Flewelling et al. 2013). Similarly, methyl (Z)-3-(3,4-dihydroxyphenyl)-2-formamidoacrylate (73) isolated from EtOAc extracts of the marine algae-derived endophytic fungus P. oxalicum EN-290 was found to strongly inhibit the growth of S. aureus with a MIC value of 2 μg/mL (Li et al. 2015a, b, c). Lai et al. (2013) previously reported the activity of perinadine A, alternariol, and citrinin against S. aureus ATCC 29213 with MIC of 64 µg/mL. Many compounds with activity against both sensitive and resistant strains of S. aureus have also been identified from Penicillium. For instance, compounds, 7-hydroxy-deoxytalaroflavone and deoxytalaroflavone isolated from the extract of Penicillium sp. FJ-1, an endophytic fungus of Ceriops tagal were found to inhibit S. aureus and methicillin-resistant S. aureus (Jin et al. 2013). Recently, compounds, GKK1032C (74), pyrrospirone E (75) and F (76), and GKK1032B (77) and GKK1032A2 (78) isolated from the endophytic fungus Penicillium sp. CPCC 400817 exhibited potency against both methicillin-susceptible and methicillin-resistant S. aureus with MIC ranging from 1.6 to 25.8 μg/mL (Qi et al. 2019). Previously, the investigation of extract of P. restrictum from Silybum marianum led to the purification of 2-hydroxyemodic acid, 1′-hydroxyisorhodoptilometrin, 1′-hydroxy-2′-ketoisorhodoptilometrin, desmethyl dermoquinone, 2-chloroemodic acid, ω-hydroxyemodin, emodic acid, (+)-2′S-isorhodoptilometrin, and emodin exhibiting quorum sensing inhibition in a clinical isolate of methicillin-resistant S. aureus (MRSA), with IC50 values ranging from 8 to 120 μM (Figueroa et al. 2014). Similarly, among the seven compounds isolated from the Chinese mangrove endophytic fungus Penicillium sp. GD6, 2-deoxy-sohirnone C (79) was potent against methicillin-resistant S. aureus with MIC value of 80 μg/mL (Jiang et al. 2018). It is clear from these investigations that Penicillium species can produce compounds that can be developed as drugs to treat infections caused by both sensitive and multi-resistant S. aureus, infections currently difficult to manage with available medicines.
Although Penicillium species are well known for their antibacterial activity, several compounds with activity against human fungal pathogens have also been reported. Indeed, a new aurone glycoside, (Z)-7,4′-dimethoxy-6-hydroxy-aurone-4-O-β-glucopyranoside, was isolated from Penicillium sp. FJ-1 of mangrove plant Avicennia marina and showed potency against Candida species. This compound also showed the ability to inhibit in a dose-dependent manner the extracellular secretion of phospholipase (Song et al. 2015). Another compound (−)-(1R,4R)-1,4-(2,3)-indolmethane-1-methyl-2,4-dihydro-1H-pyrazino-[2,1-b]-quinazoline-3,6-dione (80), a unique quinazoline alkaloid was isolated from P. vinaceum no. X17 endophyte of Crocus sativus. When tested against C. albicans ATCC 76615, C. neoformans ATCC 32609, T. rubrum and A. fumigatus, this compound was potent with MIC values ranging from 16 to 64 μg/mL (Zheng et al. 2012). Pyrenocines A and B along with a new pyrone derivative penicillone (81) were isolated from the endophytic fungus P. paxilli PSU-A71. The three compounds were potent against M. gypseum SH-MU-4 with MICs of 128, 32 and 64 μg/mL, respectively (Rukachaisirikul et al. 2007). Six compounds including orcinol, cyclo-(L-Pro-L-Val), uracil, dihydroisocoumarins, 4-hydroxymellein, 8-methoxymellein, and 5-hydroxymellein were isolated from two Penicillium spp. from leaves of Alibertia macrophylla. The six compounds exhibited antifungal activity against Cladosporium cladosporioides and C. sphaerospermum with MIC ranging from 5 to 50 μg/mL (Oliveira et al. 2009). Six derivatives of the well-known antifungal drug griseofulvin including dechlorogriseofulvin, dechlorodehydrogriseofulvin, griseofulvin, dehydrogriseofulvin, mevastatin acid, and mevastatin along with griseophenone C and peniprequinolone were isolated from an extract of the endophytic fungus P. namyslowskii (Wubshet et al. 2013). These investigations showed that Penicillium spp. can also produce compounds that can serve as a good starting point for the discovery of new antifungal drugs. Therefore, further investigations are needed for the discovery of new antifungal drugs.
Antiparasitic and antiviral agents
Aiming to evaluate the antiparasitic potential of endophytes from diverse medicinal plants, several investigations led to the identification of some potent fungi from the Penicillium genus. In fact, among the 65 crude extracts from 51 selected endophytic fungi isolated from Garcinia plants growing in Thailand, Penicillium spp. were among the 14% of extract that exhibited potency (Phongpaichit et al. 2007). Similarly, 12 endophytes from Symphonia globulifera were evaluated against a chloroquine-resistant strain of Plasmodium falciparum and the results showed that extract from P. janthinellum with IC50 of 0.2 µg/mL was among the most potent (Ateba et al. 2018). From another screening of extracts from 152 endophytic fungi isolated from Annona muricata, extract from P. citrinum AMrb11 was among the seven most potent with IC50 0.84–0.93 μg/mL (Toghueo et al. 2019). These results could suggest that endophytic Penicillium spp. are promising sources of potent compounds that might be further investigated as novel drugs against malaria. Indeed, recently Maehara et al. (2019) showed that Penicillium sp. isolated from young stems of Cinchona ledgeriana cultivated in Japan was able to produce Cinchona alkaloids although the quantity was much smaller. This could justify a further chemical investigation of these species for novel antimalarial drug discovery.
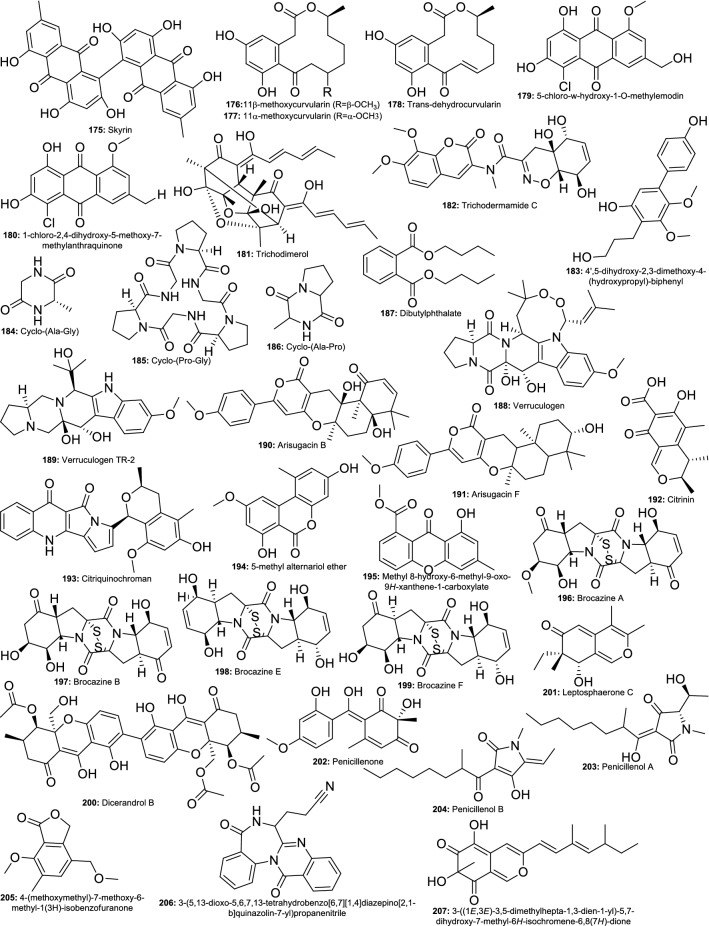
In the quest for potent endophytic fungi with the ability to produce broad antiparasitic agents, 121 endophytic fungi isolated from four Brazilian plant species Ageratum myriadenia, Palicourea tetraphylla, Piptadenia adiantoides, and Trixis vauthieri were investigated against Leishmania amazonensis, Trypanosoma cruzi, and the enzyme trypanothione reductase (TryR) from Trypanosoma cruzi. From the results, 24 extracts (19.8%) inhibited the activity of TryR, and three showed the ability to inhibit the growth of T. cruzi with IC50 values ranging from 1 to 10 µg/mL. Eleven extracts (9%) were able to inhibit the growth of L. amazonensis with IC50 values ranging from 4.6 to 24.4 µg/mL. Potent fungi belonged to seven genera including Penicillium (Rosa et al. 2010). The chemical investigation of Penicillium sp., an endophytic fungus from Limonium tubiflorum from Egypt led to four new compounds along with 12 known metabolites. Among these compounds, 11β-methoxycurvularin (82), 11α-methoxycurvularin (83), 5-chloro-6,8,10-trihydroxy-1-methoxy-3-methyl-9(10H)-anthracenone (84) and trichodimerol (85) showed pronounced antitrypanosomal activity with MIC values ranging from 4.96 to 9.75 μM (Aly et al. 2011). These results indicate that Penicillium can produce bioactive molecules (Fig. 3) needed for drug development against neglected tropical diseases.
Fig. 3.
Antiparasitic and antiviral agents from endophytic Penicillium species
Hepatitis C virus (HCV) is the causal agent of hepatitis C, the liver disease affecting an estimated 71 million people globally. WHO estimated that in 2015, there were 1.75 million new HCV infections in the world and approximately 399,000 people died from hepatitis C in 2016 (WHO 2019). Because of its important role in replication, hepatitis C virus (HCV) NS3–NS4A protease represents an attractive target for new anti-HCV drug discovery. Several investigations have been conducted to evaluate the anti-HCV potential of metabolites from Penicillium spp. (Fig. 3). In a recent study, extracts from endophytes P. polonicum MERVA43, and P. chrysogenum MERVA42 were reported to exhibit significant hepatitis C virus (HCV) inhibition (El-Gendy et al. 2018). Previously, the ethyl acetate extract of P. chrysogenum, endophyte from Liagora viscida exhibiting potent activity against HCV NS3-NS4A protease (IC50 20 µg/mL), was fractionated to obtain 12 known metabolites among which emodin (86) and ω-hydroxyemodin (87) were strongly potent with IC50 values of 22.5 and 10.6 μg/mL. Moreover, a predictive mechanism of action using docking studies revealed that the higher activity of ω-hydroxyemodin could be due to its ability to bind with the meta-coupled phenolic hydroxy groups to Gln41 and His57, one of the active triad amino acids in the active site, while emodin was predicted to form an H-bond between the meta-coupled phenolic hydroxy groups and Gln41 and Gly137 (Hawas et al. 2013). These compounds can serve as a basis for the synthesis of new potent anti-HCV agents for the management of hepatitis C. In addition to the anti-HCV activity, the potential of Penicillium to produce anti-HIV compounds has also been reported. In this regard, (+)-sclerotiorin (88) isolated from endophytic fungus P. sclerotiorum PSU-A13 was reported to exhibit anti-HIV-1 protease and anti-HIV-1 integrase activities with the IC50 values of 14.5 and 62.7 μg/mL, respectively (Arunpanichlert et al. 2010). Overall, these studies showed that Penicillium species can produce compounds with the potential to serve as leads for the development of new antiviral agents. Therefore, further antiviral-guided investigations of these fungi species are urgently needed.
The vector control is also one of the means used in the management of viral and parasitic diseases. The identification of active compounds with the ability to exhibit larvicidal activity is needed. Hamisonine (89) a limonoid compound from P. oxalicum LA-1 endophyte from Limonia acidissima was tested against III and IV instar Culex quinquefasciatus larvae. The result shows that hamisonine possesses strong larvicidal activity with the LC50 of 1.779 ppm against III instar larvae and 3.031 ppm against IV instar larvae of C. quinquefasciatus, respectively. Further studies also showed that hamisonine can damage peritrophic membrane and epithelial cells of mosquito larvae (Seetharaman et al. 2017). This finding is particularly important since Culex quinquefasciatus is a vector of parasites and viruses responsible for diseases such as lymphatic filariasis and several arboviruses including St. Louis encephalitis virus and West Nile virus (Bartholomay et al. 2010).
Antidiabetic and anti-obesity agents
Diabetes mellitus is a chronic disorder that affects millions of population worldwide. Global estimates published in 2010 reported the world’s diabetic prevalence as 6.4%, affecting 285 million adults. In 2016, diabetes was the direct cause of 1.6 million deaths (WHO 2018). Many natural products are used for the management of this disease. For instance, gymnemagenin, the triterpenoid found in the medicinal plant Gymnema sylvestre is used in the pharmaceutical industry as an antidiabetic agent (Manika et al. 2013). To investigate other sources for the mass production of this compound, metabolomes of endophytic fungi isolated from leaves of G. sylvestre were investigated using analytical and spectroscopic tools. Penicillium oxalicum was identified as able to produce gymnemagenin (90), and the structure was confirmed by FTIR, UV, and NMR analyses (Parthasarathy and Sathiyabama 2014). Other investigations were conducted to evaluate the potential of Penicillium spp. to produce compounds that can be used as antidiabetic agents. Eight compounds were isolated from an extract of Penicillium sp. HN29-3B1 endophyte from Cerbera manghas, among which 6-demethylpenisimplicissin (91) and 2ʺ-epihydroxydihydrovermistatin (92) exhibited α-glucosidase inhibitory activity with IC50 values of 9.5 and 8 µM, respectively (Liu et al. 2014b). Continuing the investigation, on the same endophyte, Liu et al. (2015b) reported the isolation of ten metabolites including five new. Among these compounds, pinazaphilone B (93), Sch 1385568 (94), 6′-methyl-[1,1′-biphenyl]-3,3′,4′,5-tetraol (95), and (±)-penifupyrone (96) inhibited α-glucosidase with IC50 values of 28, 16.6, 2.2, and 14.4 μM, respectively.
Ten new isocoumarins, along with three known analogs were isolated from the extract of P. commune QQF-3 endophyte of the mangrove plant Kandelia candel and tested. Peniisocoumarin C (97), peniisocoumarin G (98), peniisocoumarin I (99) and J (100) exhibited potent inhibitory effects against α-glucosidase with IC50 values ranging from 38.1 to 78.1 μM (Cai et al. 2018). Similarly, the investigation of P. chermesinum ZH4-E2 isolated from another Kandelia candel plant growing in South China sea led to the purification of eight metabolites among which 6′-O-desmethylterphenyllin (101), 3,3ʺ-dihydroxy-6′-O-desmethylterphenyllin (102), 3-hydroxy-6′-O-desmethylterphenyllin (103) and chermesinone A (104) strongly inhibited α-glucosidase activity with IC50 values of 0.9, 2.5, 4.9 and 24.5 µM, respectively (Huang et al. 2011). Eight compounds including six new peaurantiogriseols A–F (105–110) and two known aspermytin A (111), 1-propanone,3-hydroxy-1-(1,2,4a,5,6,7,8,8a-octahydro-2,5-dihydroxy-1,2,6-trimethyl-1-naphthalenyl) (112) were isolated from the extract of mangrove endophytic fungus P. aurantiogriseum 328#. All the compounds were tested and showed low inhibitory activity against human aldose reductase (Ma et al. 2015). However, these small molecules offer the opportunity for further optimization that can lead to more potent compounds. Several endophytes from Boswellia sacra were screened for their ability to inhibit α-glucosidase and P. citrinum was identified as the most potent. The bio-guided fractionation led to the isolation of five active compounds including, 11-oxoursonic acid benzyl ester, n-nonane, 3-decene-1-ol, 2-hydroxyphenyl acetic acid, and glochidacuminoside A (Ali et al. 2017a, b). It is now clear from this investigation that endophytic Penicillium species can produce structurally diverse and potent compounds (Fig. 4) that can be further investigated for the development of new antidiabetic agents. Further investigations in this direction are needed to boost the antidiabetic drug discovery.
Fig. 4.
Antidiabetic and anti-obesity agents from Penicillium
Pancreatic lipase (PL) is considered today as one of the safest targets for diet-induced anti-obesity drug development. The only one PL inhibitor approved for anti-obesity treatment to date is Orlistat. Therefore, new inhibitors are needed. Searching for potential inhibitors, the in vitro screening of extracts from 70 endophytic fungi led to the identification of Penicillium sp. #57TBBALM as the most potent with IC50 of 3.69 µg/mL (Gupta et al. 2015). In a more recent study, three new compounds, purpurolide A (113), along with two new 6/4/5/5 tetracyclic sesquiterpene lactones, purpurolides B (114) and C (115), were isolated from endophytic fungus P. purpurogenum IMM003. They displayed potent inhibition of the activity of pancreatic lipase with IC50 values of 2.83, 5.45, and 6.63 μM, respectively (Wang et al. 2018). Several other compounds with the potential to inhibit the activity of other anti-obesity drug targets have also been reported (Fig. 4). Eight compounds were isolated from an extract of P. commune endophyte from Vitis vinifera and tested for binding affinity and inhibitory activity against 11β-hydroxysteroid dehydrogenase type 1. The results showed that penicopeptide A (116), cyclopenol (117), and cyclopenin (118) showed potency with Kd (9.07–68.05 μM) and IC50 (82.1–513 μM) for binding affinity and inhibitory activity, respectively. Compound 116, the most potent (Kd 9.07 μM; IC50 82.1 μM) also decreased the lipid droplet accumulation associated with the inhibition of 11β-HSD1 expression in differentiation-induced 3T3-L1 preadipocytes (Sun et al. 2016). Two other compounds, penicillactones B (119) and C (120) isolated from an extract of the endophytic fungus P. dangeardii Pitt were reported to inhibit the release of β-glucuronidase with IC50 values of 2.58 and 1.57 μM (Liu et al. 2013). Endophytic Penicillium spp. isolated from stems of Scurrula atropurpurea produces cholic acid (121), deoxycholic acid (122) and glycine conjugates (Ohashi et al. 2008). These compounds are approved by the FDA for the treatment of children and adults with bile acid synthesis disorders due to single enzyme defects and peroxisomal disorders.
Anti-inflammatory and antioxidant metabolites
Several metabolites produced by endophytic fungi from Penicillium genus have already been reported for their anti-inflammatory and antioxidant activities (Fig. 5). The chemical investigation of extract from Penicillium chrysogenum MT-12 isolated from Huperzia serrata led to the purification of 12 new polyketides, penicichrysogenins A–L along with five known compounds. When tested for their anti-inflammatory activity, penicichrysogenin D (123), penicichrysogenin E (124), penicichrysogenin H (125), penicichrysogenin I (126), penicichrysogenin K (127) and penicichrysogenin L (128), (+) asperlone A (129) and (–) asperlone A (130) exhibited inhibition of nitric oxide production in lipopolysaccharide (LPS)-stimulated RAW264.7 macrophage cells with IC50 values ranging from 17.5 to 98.4 μM (Qi et al. 2017a). The further investigation of this endophyte led to the isolation of the other eight new chrysogenolides (A–H) along with seven known 3,5-dimethylorsellinic acid-derived meroterpenoids among which, chrysogenolide C (131), chrysogenolide D (132), chrysogenolide F (133), berkeleyacetal C (134), and purpurogenolide C (135) also inhibited nitric oxide production with IC50 values ranging from 4.3 to 78.2 μM (Qi et al. 2017b). These compounds with low micromolar activity represents good candidate for further investigation. In another investigation, the bio-guided fractionation of the ethyl acetate extract of an endophytic fungus, P. polonicum from Taxus fuana led to the isolation of a dimeric anthraquinone, (R)-1,1′,3,3′,5,5′-hexahydroxy-7,7′-dimethyl[2,2′-bianthracene]-9,9′,10,10′-tetraone (136), a steroidal furanoid (−)-wortmannolone (137), along with three other compounds. Compound 136 showed moderate inhibition while 137 showed inhibitory activities in the TNF-α-induced NF-κB assay with an IC50 value of 0.47 µM (Fatima et al. 2017). Agarospirol (138) exhibiting anti-inflammatory activity was also identified in the extract of P. polonicum isolated from Aquilaria malaccensis Lam by Chhipa and Kaushik (2017).
Fig. 5.
Anti-inflammatory and antioxidant metabolites from Penicillium spp.
Gallic acid is a natural phenolic compound with diverse industrial applications and exhibiting a wide range of biological activities such as antioxidant, anti‐inflammatory, anti‐microbial and anti‐cancer (Peyrat-Maillard et al. 2000; Mukherjee and Banerjee 2003). In the search for a potential source of gallic acid, several endophytic fungi from Acer ginnala including one Penicillium sp. were found to exhibit gallic acid-production ability in culture (Qi et al. 2009). Many other antioxidant compounds were isolated from the culture of Penicillium species. For example, penicitriketo (139), a new polyketide isolated from Penicillium sp. was reported to exhibit moderate DPPH radical scavenging activity with an IC50 value of 85.33 μM (Wang et al. 2014). The investigation of extract from Penicillium sp. YY-20, an endophyte from the root of Ginkgo biloba led to six known metabolites among which adenosine, adenine and 2′-deoxyadenosine, exhibited potential DPPH-scavenging activities (Yuan et al. 2014). Among the five compounds isolated from an extract from P. chrysogenum no. 005, an endophytic fungus associated with Cistanche deserticola Y. C. Ma, chrysogenamide A (140) exhibited a neurocyte protection effect against oxidative stress-induced cell death in SH-SY5Y cells (Lin et al. 2008a).
Anticancer compounds from Penicillium
Nature has been and will remain an inspiration for modern chemistry for new and more efficient anticancer compounds. Endophytic fungi from Penicillium genus, well known for their outstanding ability to produce structurally diverse and active metabolites have been continuously investigated over the years as a source of anticancer compounds. Potent extracts from endophytes belonging to this genus have been identified from anticancer screening in many studies over the years. For instance, among the 65 crude extracts from the 51 selected endophytic fungi isolated from Garcinia species, Penicillium sclerotiorum was identified among the potent strains (Phongpaichit et al. 2007). In another study, extracts from ten endophytic strains isolated from Lycium barbarum were screened for anti-tumor activity and the five most potent strains belonged to three genera including Penicillium (Dai and Du 2017). Another 17 endophytic fungi isolated from Cassia fistula were tested for their cytotoxicity against cancer cells line and identified P. sclerotiorum as being the most potent. Further analysis of the mechanism of action of this extract revealed interesting findings. Extract from P. sclerotiorum was able to arrest cell cycle at S and G2/M phase in a dose-dependent manner. The downregulation of proapoptotic protein BCL2 and upregulation of BAX (BCL2-associated X), tumor suppressor protein, p53, and Apaf-1 (apoptotic peptidase activating factor 1) suggested that the extract potentiates apoptosis rather than necrosis in cells. This activation of the mitochondrial pathway of apoptosis was further confirmed by observing the loss of mitochondrial membrane potential and a distinct DNA fragmentation pattern in treated cells (Kuriakose et al. 2018). In another investigation, the ethyl acetate extract of P. crustosum, an endophyte from Phoenix dactylifera was reported to exhibit higher antiproliferative activity against HepG2 (IC50 82 μg/mL) and MCF7 (IC50 126 μg/mL). Moreover, this extract significantly reduced the level of LDH and the expression of IL-6 transcript in the MCF7 cell line but not in the HepG2 cell line (Abutaha et al. 2018). These results greatly indicate that Penicillium spp. isolated from medicinal plants may serve as potential sources of the anticancer compounds with multiple mode of action. Therefore, further investigation to isolate the active compounds (Fig. 6) followed by and mode of action studies could reveal new potential drugs against multiple cancer diseases.
Fig. 6.
Few anticancer compounds isolated from endophytic Penicillium species
In the continuous efforts to identify new anticancer molecules, 23 compounds isolated from an extract of P. concentricum endophyte from Trichocolea tomentella were tested against HeLa cervical, HT-29 colon, MDA-MB-321 breast, PC-3, and DU-145 prostate cancer cell lines using the sulforhodamine B assay. The results showed that all the compounds exhibited a varying degree of potency. 2-Bromogentisyl alcohol (141) and 3-hydroxy-benzenemethanol (142) exhibited the highest cytotoxic activity against different cancer cell lines while Epoxydon (143) showed selectivity against DU-145 prostate cancer cells (IC50 1.2 μM) and was found to induce cell cycle arrest in G2/M phase. In the mitochondrial transmembrane potential (MTP) assay, compounds 141, 142, and 143 were potent with IC50 values of 0.1, 0.2, and 7 μM, respectively, while only compound 142 exhibited potent inhibition (IC50 0.3 μM) in nuclear factor kappa B (NF-κB) target-based assay. These compounds exhibiting very strong inhibition against multiple cancer cells could be useful for the development of a new lead molecule for cancer therapy (Anaya-Eugenio et al. 2019). In the earlier report, 21 compounds were isolated from the extract of this P. concentricum and tested for antiproliferative activity against HT-29 (colon cancer) and MCF-7 (breast cancer) cell lines. The results showed that 141, 143, 6-dehydroxy-6-bromogabosine C (144), 2-chlorogentisyl alcohol (145), gentisyl alcohol (146), and griseofulvin (147) were active against MCF-7 cell line with IC50 of 8.4, 5.7, 9.7, 14.9, 17.1, and 13.9 μM, respectively, while only 143, and 146 were active against HT-29 cell line with IC50 values of 14.1 and 6.4 μM, respectively (Ali et al. 2017a, b).
Nine compounds including two new chaetoglobosins, penochalasin I (148) and penochalasin J (149), along with chaetoglobosins G (150), F (151), C (152), A (153), E, armochaetoglobosin I and cytoglobosin C (154) were isolated from extract the of culture of P. chrysogenum V11 endophyte from Myoporum bontioides A. Gray. These compounds were tested against the human breast cancer cell line (MDA-MB-435), a human gastric cancer cell line (SGC-7901), and a human lung adenocarcinoma epithelial cell line (A549). The results showed that compounds 148–153 and 154 were potent against the three cell lines with IC50 values ranging from 3.35 to 38.77 µM while, chaetoglobosin E (155) was active only against A549 with IC50 of 36.63 µM (Huang et al. 2016). Similarly, the antiproliferative extract from P. chrysogenum endophytes of Olea europea was fractionated to purify six compounds including three known indole alkaloids. Among these compounds, roquefortine C (156) exhibited moderate inhibition against the six cancer cell lines (MDA-MB-231, MDA-468, BT-474, SK BR-3, MCF7, and MCF7-dox) with IC50 ranging from 21.7 to 28.9 μM, while DHTD (157) was only moderately active against MCF7 (IC50 23.8 μM) and MDA-MB-231 (IC50 31.8 μM) cell line. Meleagrin (158) was the most potent (IC50 1.9–8.9 μM) against all cell lines and showed excellent ATP competitive c-Met inhibitory activity in Z-Lyte assay, confirmed by Western blot analysis and molecular docking studies. HGF-induced cell migration and invasion of breast cancer cell lines were also inhibited by meleagrin treatment in a dose-dependent manner. This study demonstrated that meleagrin could be a novel lead c-Met inhibitor that can be useful for the control of c-Met-dependent metastatic and invasive breast malignancies. Therefore, more investigation of this compound for new drug development is required (Mady et al. 2016). The chromatographic separation of extract from another P. chrysogenum AD-1540, from Grateloupia turuturu, yielded two new benzophenone derivatives, chryxanthones A (159) and B (160). These two compounds also exhibited potency against six human cancer cell lines with IC50 values ranging from 20.4 to 46.4 μM (Zhao et al. 2018a, b). These molecules with unique properties could be developed as drug candidates for cancer therapy.
Another potential target in breast cancer therapy is aromatase, a cytochrome P450 enzyme complex that converts androgens to estrogens (Jongen et al. 2005), involved in important physiological processes and played a key role in diseases like endometrial and breast cancers (Maiti et al. 2007). Aromatase inhibitors can block the production of estrogens and may be effective against estrogen receptor-positive breast cancer. Therefore, Fatima et al. (2016) showed that extract from endophytic P. polonicum exhibiting aromatase inhibition with IC50 of 10.5 μg/mL could be a good source of new potential aromatase inhibitors, useful for cancer treatment. Indeed, recently, two new compounds β-lactone polonicin A and enoic acid polonicin B together with seven known compounds were isolated from P. polonicum obtained from Camptotheca acuminata and tested for anticancer activity. The results showed that fusarubin (161), 3-methyl ether-fusarubin (162), Javanicin (163), anhydrofusarubin (164), 2-isopropanol-3-methyl-7-methoxy-naphthazarin (165) were active against HepG2 hepatocellular carcinoma (HCC) cell line with IC50 ranging from 4.01 to 77.09 μg/mL (Wen et al. 2019). Wortmannin (166) another cytotoxic compound isolated from P. wortmannii and P. funiculosum endophyte of medicinal herbs were reported to target PIK3CA protein which is a part of the PI3K/AKT/mTOR signaling pathway in multi-resistant cancer cells (Kuete et al. 2015).
Eight compounds were isolated from extracts of endophytic fungi, Penicillium melinii Yuan-25 and P. janthinellum, both from the roots of Panax ginseng, using the anti-Pyricularia oryzae-guided fractionation. When evaluated for their cytotoxicity against six human cancer cell lines (MKN45, LOVO, A549, MDA-MB-435, HepG2, and HL-60), all exhibited a varying degree of potency. Brefeldin A (167) was the most potent against the six cell lines with IC50 < 0.12 μg/mL, followed by ginsenocin (168) and penicillic acid (169) (IC50 0.49–7.46 μg/mL) (Zheng et al. 2013). The chemical investigation of extract from another mangrove endophytic fungus P. janthinellum HDN13-309 led to the isolation of six new epipolythiodioxopiperazine alkaloids, penicisulfuranols A–F. Penicisulfuranol A (170), B (171) and C (172) showed cytotoxicity with IC50 values ranging from 0.1 to 3.9 μM (Zhu et al. 2017a, b). In a recent study, penicisulfuranol A was shown to cause the depletion of multiple Hsp90 client proteins without the induction of heat-shock protein 70 (Hsp70). Moreover, the treatment with penicisulfuranol A induced apoptosis and inhibited the growth of xerograph tumor of HCT116 cells in vitro as well as in vivo. A more detail study showed that this compound was able to bind to the C terminus of Hsp90 at the site different from the ATP-binding domain (Dai et al. 2019). Using the 2D-SEPBOX bioactivity-guided fractionation, oxyskyrin, skyrin, dicatenarin, and 1,6,8-trihydroxy-3-hydroxy methylanthraquinone were isolated from the extract of P. pinophilum, an endophyte from Allium schoenoprasum. When tested against the NCI-60 panel of human cancer cell lines, oxyskyrin (173) was active only against HCT-116 (IC50 48 μg/mL), while dicatenarin (174) and skyrin (175) were potent against all cancer cells with IC50 ranging from 12 to 38 μg/mL. Furthermore, these two compounds were able to generate intracellular reactive oxygen species which led to the induction of a significant change in the mitochondrial transmembrane potential and the mitochondrial-mediated apoptotic pathway. An increased induction of caspase-3 apoptotic proteins in human pancreatic cancer (MIA PaCa-2) cells was also noted (Koul et al. 2016). The activity of these compounds could be further optimized through medicinal chemistry for the development of potential anticancer drug candidates.
The chemical investigation of extract from Penicillium sp., an endophytic fungus from Limonium tubiflorum growing in Egypt led to the purification of four new compounds including penilactone, 10,11-epoxycurvularin, neobulgarone G, and sulfimarin, along with 12 known metabolites. 11β-methoxycurvularin (176), 11α-methoxycurvularin (177), trans-dehydrocurvularin (178), 5-chloro-ω-hydroxy-1-O-methylemodin (179), 1-chloro-2,4-dihydroxy-5-methoxy-7-methylanthraquinone (180) and trichodimerol (181) inhibited K562, Jurkat and U937 cell lines with IC50 values ranging from 2.5 to 74.4 μM and inhibited TNFα-induced NF-κB activity in K562 cells with IC50 values ranging from 1.6 to 47.8 μM (Aly et al. 2011). Trichodermamide C (182), a modified dipeptide isolated from the endophytic fungus Eupenicillium sp. was reported to display cytotoxicity towards the human colorectal carcinoma (HCT116) and A549 with IC50 values of 0.68 and 4.28 µg/mL, respectively (Davis et al. 2008). Another chemical investigation of extracts of P. thomi, an endophyte from the root of Bruguiera gymnorrhiza led to the isolation of a new compound, along with 11 known compounds. Five including 4′,5-dihydroxy-2,3-dimethoxy-4-(hydroxypropyl)-biphenyl (183), cyclo-(Ala-Gly) (184), cyclo-(Pro-Gly) (185), cyclo-(Ala-Pro) (186), and dibutylphthalate (187) exhibited potency against A549, HepG2 and HT29 cancer cell line with IC50 values ranging from 8.9 to 20.1 µM (Chen et al. 2007). P. brasilianum isolated from Melia azedarach was reported to produce verruculogen (188) and verruculogen TR-2 (189), two compounds known for their activity against resistant cancer cell lines (Fill et al. 2013). Seven compounds including two new meroterpenoids were isolated from an extract of endophytic fungus Penicillium sp. SXH-65 and tested against Hela, HL-60, and K562 cell lines. Only arisugacin B (190) and arisugacin F (191) exhibited activity with IC50 values ranging from 24 to 60 μM (Sun et al. 2014a, b). Similarly, among the three compounds isolated from the mangrove endophytic fungus Penicillium sp. (SBE-8), only citrinin (192) showed potency against KB and KBv cell lines (Guo et al. 2009). Recently, Guo et al. (2019) also reported the weak activity of (S)-6-(sec-butyl)-5-(hydroxymethyl)-4-methoxy-2H-pyran-2-one, (5S,7R)-7-ethyl-4,5-dimethoxy-7-methyl-5,7-dihydro-2H-furo[3,4-b]pyran-2-one and (5R,7R)-7-ethyl-4,5-dimethoxy-7-methyl-5,7-dihydro-2H-furo[3,4-b]pyran-2-one, three new compounds isolated from culture of Penicillium herquei, endophyte of Cordyceps sinensis. Nineteen metabolites including six new compounds were isolated from the extracts of P. citrinum isolated from the stem of the Moroccan plant Ceratonia siliqua and tested for their activity against the murine lymphoma L5178Y cell line. Eighteen compounds were inactive (IC50 > 10 μM) and only citriquinochroman (193) was potent with an IC50 value of 6.1 μM (El-Neketi et al. 2013). Following the investigation of this endophyte, twenty compounds were isolated and tested against the same cell line L5178Y. The results showed that only 5-methyl alternariol ether (194) and methyl 8-hydroxy-6-methyl-9-oxo-9H-xanthene-1-carboxylate (195) showed significant activity with IC50 values of 3.7 μM and 2.7 μM, respectively (Lai et al. 2013).
The fractionation of the cytotoxic extract of P. brocae MA-231, an endophyte of the marine mangrove plant Avicennia marina, led to the purification of seven compounds including six new disulfide-bridged diketopiperazine derivatives brocazines A–F, and one known analog. When tested against nine different cancer cell lines, brocazine A (196), B (197), E (198), F (199) exhibited very good potency with IC50 values ranging from 1.2 to 12.4 μM (Meng et al. 2014). Penicillium sp. endophyte isolated from Costa Rican rainforest was identified through an anticancer high-throughput screening and the bioassay-guided fractionation led to the identification of five xanthones including penexanthone A, monomer penexanthone B, dicerandrols B, A, and C. All these compounds exhibited a varying degree of potency against 15 cancer cell lines. Dicerandrol B (200) was the most potent against all the cell lines with IC50 values ranging from 1 to 17 μM (Cao et al. 2012). In another investigation, leptosphaerone C, penicillenone, arugosin I, 9-demethyl FR-901235, bacillosporin A, bacillosporin C, sequoiamonascin D, sequoiatone A, and sequoiatone B were isolated from the Penicillium sp. JP-1, an endophyte from Aegiceras corniculatum and tested against cancer cells. Leptosphaerone C (201) showed activity against A-549 cells (IC50 1.45 μM), while penicillenone (202) was active against P388 cells with an IC50 value of 1.38 μM (Lin et al. 2008b). The investigation of another Penicillium sp. GQ-7 from the same plant led to the isolation of penicillenols A–C along with citrinin, phenol A acid, phenol A, and dihydrocitrinin. Only penicillenol A (203) and B (204) showed potency against HL-60 cell line with IC50 values of 0.76 μM and 3.20 μM, respectively (Lin et al. 2008c). A new isobenzofuranone, 4-(methoxymethyl)-7-methoxy-6-methyl-1(3H)-isobenzofuranone (205), isolated from an extract of the mangrove endophytic fungus, Penicillium sp. ZH58 obtained from the South China sea coast was reported to exhibit cytotoxicity against KB and KBV200 cells with IC50 values of 6 and 10 μg/mL, respectively (Yang et al. 2013). Similarly, a new benzodiazepine alkaloid, 3-(5,13-dioxo-5,6,7,13-tetrahydrobenzo[6,7][1,4]diazepino[2,1-b]quinazolin-7-yl)propanenitrile (206) was isolated from Penicillium sp. 299# endophyte of the mangrove plant Acanthus ilicifolius. This compound inhibited the growth of human cancer lines MDA-MB-435, HepG2, HCT-116, and Calu-3 (Li et al. 2015a, b, c). In an earlier report, the investigation of Penicillium sp. 303# endophyte of the same mangrove plant led to 14 compounds among which compounds 207, 208, 209, and 210 were active against human cancer lines MDA-MB-435, HepG2, HCT-116, and A549 with IC50 ranging from 7.13 to 39.64 μg/mL (Li et al. 2014a). Li et al. (2014b) also showed that meroterpenoids isolated from a culture of Penicillium sp., an endophytic fungus from Dysosma versipellis, showed weak cytotoxic activity against HCT-116, HepG2, BGC-823, NCI-H1650, and A2780 cell lines with IC50 > 10 μM. Extract of Penicillium decumbens CP-4, an endophytic fungus isolated from Cephalotaxus mannii Hook. f., was investigated to isolated 12 compounds including one new peniproline A. All the compounds were evaluated for their cytotoxic activities against Bel-7402 and Hela cell lines by MTT method and only peniproline A (211) exhibited potency with IC50 values of 8.1 and 15.5 μM, respectively (Wang et al. 2017).
Four new eudesmane-type sesquiterpenoids, penicieudesmol A–D were isolated from an extract of the mangrove-derived endophytic fungus Penicillium sp. J-54 and only penicieudesmol B (212) exhibited weak cytotoxicity against K-562 cells with IC50 of 90.1 μM (Qiu et al. 2018). Compound, 15 α-hydroxy-(22E, 24R)-ergosta-3,5,8(14),22-tetraen-7-one (213) from Penicillium sp. FJ-1 was reported to show potency against glioma cell lines (Liu et al. 2014a). Among the four compounds isolated from Penicillium sp. N-175-1, sporogen-AO1 (214) and petasol (215) exhibited cytotoxicity against HL60 and HeLa cells and showed the robust growth-restoring activity of a Saccharomyces cerevisiae (cdc2-1 rad9Δ) mutant strain. Therefore, these compounds may inhibit the growth of HL60 and HeLa cells by blocking the cell cycle and can be further developed as lead inhibitors of survival signal transduction pathways (Shiono et al. 2017). Four compounds, a new funicone derivative, penifupyrone (216), along with funicone (217), deoxyfunicone (218), and 3-O-methylfunicone (219) were isolated from Penicillium sp. HSZ-43, an endophytic fungus of Tripterygium wilfordii. All the isolated compounds exhibited potency against human oral epidermoid carcinoma KB cells with IC50 values of 4.7, 13.2, 22.6 and 35.3 μM, respectively (Chen et al. 2014). Three alkaloids named penicidones A–C (220-222) along with lumichrome, physcion, and emodin-1,6-dimethyl ether were isolated from the culture of Penicillium sp. IFB-E022, an endophyte of Quercus variabilis. The three new compounds exhibited moderate potency against SW1116, K562, KB and HeLa cells line with IC50 values ranging from 21.1 to 80.8 μM (Ge et al. 2008). A new pyrrolyl 4-quinolinone alkaloid, penicinoline (223) was isolated from Penicillium sp., a mangrove endophytic fungus. Penicinoline showed potency against 95-D and HepG2 cell lines with IC50 values of 0.57 and 6.5 μg/mL, respectively, and was inactive against Hela, KB, KBv200, and Hep2 cell lines (Shao et al. 2010). A new furanocoumarin, 5-methyl-8-(3-methylbut-2-enyl) furanocoumarin (224), isolated from another mangrove endophytic fungus, Penicillium sp. ZH16 was also found to inhibit KB and KBv200 cell lines with IC50 values 5 and 10 µg/mL, respectively (Huang et al. 2012).
The chemical investigation of an extract of Penicillium sp. ZO-R1-1, isolated from roots of the medicinal plant Zingiber officinale, resulted in the purification of 22 compounds including nine new indole diterpenoids. When tested for their cytotoxicity against L5178Y, A2780, J82, and HEK-293 cell lines, all the compounds exhibited varying degree of potency with shearilicine (225), paspalinine-13-ene (226), shearinine O (227), and shearinine P (228) being the more potent with IC50 ranging from 3.6 to 40.6 μM. Compound 225 was the most potent against both L5178Y and A2780 cells with IC50 of 3.6 and 8.7 μM, respectively (Ariantari et al. 2019). In another study, three novel meroterpenoids, isopenicins A–C were isolated from an extract of Penicillium sp. sh18, an endophyte from Isodon eriocalyx var. laxiflora and tested against HCT116, SW480, Caco2, SW620, MCF-7, A549, and SMMC-7721 cells line. From the results, isopenicin A (229) and B (230) exhibited potency with IC50 values ranging from 7.91 to 37.06 μM. Furthermore, isopenicin A was able to inhibit the luciferase activity in a concentration-dependent manner in human colorectal cancer (CRC), to suppress the expression of Wnt target genes, Axin 2, c-myc, and survivin, the expression of total and active β-catenin as well as induced dramatic apoptosis in SW620 and HCT116 cells (Tang et al. 2019).
In addition to their ability to produce novel anticancer compounds, Penicillium spp. have also been reported as capable of producing well-known anticancer drugs. For instance, 107 endophytic fungi associated with Taxus globosa were screened for paclitaxel using a competitive inhibition enzyme immunoassay. The results showed that among the 16 potent strains, Penicillium sp. SGLMf44 was among the top 5 with around 150 ng/L of paclitaxel produced in the M1D medium (Soca-Chafre et al. 2011). In another investigation, the combination of the liquid chromatography–mass spectrometry and proton nuclear magnetic resonance was used to demonstrate the ability of Penicillium aurantiogriseum NRRL 62431 isolated from Corylus avellana L. to produce paclitaxel (231). Further investigation into the genome of P. aurantiogriseum NRRL 62431 revealed the presence of gene candidates that may be involved in the paclitaxel biosynthesis (Yang et al. 2014).
Resveratrol (232) considered as a potential candidate for the prevention and treatment of several types of cancer has received great attention over the past decades (Salehi et al. 2018). Therefore, endophytic fungi producing resveratrol have been investigated. Sixty-five endophytes isolated from three medicinal plants Vitis vinifera L. cv. Merlot, Vitis quinquangularis Rehd. and Polygonum cuspidatum Siebold & Zucc. were screened. Among the 21 producing-fungi identified, Penicillium sp. YP30 and Penicillium sp. YP2 were able to produce 17 and 24 µg/L in liquid culture (Shi et al. 2012). In another screening of 53 endophytic fungi from Vitis vinifera, two Penicillium spp. isolated from leaf and stem were found to produce 15.3 and 21.9 μg/mL, respectively (Dwibedi and Saxena 2018). These findings are of great interest since using the metabolic engineering approach, these endophytic Penicillium species can be optimized for the large industrial production of these potent drugs.
Antifibrotic and immunosuppressive compounds from Penicillium
Idiopathic pulmonary fibrosis (IPF) is the most common and deadly form of idiopathic interstitial pneumonia, particularly in elderly adults. The mortality is estimated between 3 and 5 years after the diagnosis (Fernandez and Eickelberg 2012). Over the past decade, growing shreds of evidence are supporting the clinical benefits of antifibrotic treatment in IPF. Moreover, two antifibrotic drugs are now recommended in patients in the mild-moderate stage of the disease (Raghu et al. 2015). New antifibrotic agents could boost the development of new drugs needed for the management of this disease. To search for new and potent antifibrotic agents from endophytes, extract from Penicillium sp. sh18 endophyte of Isodon eriocalyx var. laxiflora was fractionated to purify two compounds, among which penicilfuranone A (233) displayed significant antifibrotic effect in activated hepatic stellate cells via negative regulation of transforming growth factor-β (TGF-β)/Smad signaling (Wang et al. 2016a, b, c). This compound (Fig. 7) can constitute a starting point for further medicinal chemistry study for the synthesis of semi-natural compounds with improved potency.
Fig. 7.
Antifibrotic and immunosuppressive agents
Searching for natural immunosuppressive agents, the two new benzophenone derivatives, peniphenone, and methyl peniphenone, along with seven known xanthones isolated from Penicillium sp. ZJ-SY2 endophyte isolated from the leaves of Sonneratia apetala were tested. The results showed that peniphenone (234), conioxanthone A (235), pinselin (236), and sydowinin A (237) showed potent immunosuppressive activity with IC50 values ranging from 5.9 to 9.3 μg/mL (Liu et al. 2016b). More investigation of Penicillium spp. could lead to more active compounds to accelerate the discovery of drugs.
Neuroprotective effects of compounds from Penicillium species
Acetylcholinesterase by rapid hydrolysis of the neurotransmitter acetylcholine in numerous cholinergic pathways in the central and peripheral nervous systems is involved in the termination of impulse transmission (Colović et al. 2013). This enzyme has been identified as a drug target in Alzheimer’s disease (AD) considered today as the most common single cause of dementia in our aging society. The development of drug inhibitors of acetylcholinesterase is based on the finding that cholinergic pathways in the cerebral cortex and basal forebrain are compromised and the resultant cholinergic deficit contributes to the cognitive impairment of these patients (Becker 1991; Katzman and Saitoh 1991). Although many acetylcholinesterase inhibitors are currently used, they are toxic and have several side effects. Therefore, new and less toxic drugs are needed for the management of this disease.
Huperzine A (238) is a well-known acetylcholinesterase inhibitor currently isolated from the medicinal plant Huperzia serrata. To search for a new source to produce this compound, over 120 endophytic fungi isolated from this plant were screened and nine fungi including Penicillium sp. were identified as potent (Wang et al. 2011b). In a similar study, extracts from 127 strains of endophytic fungi isolated from roots, branches, and leaves of Huperzia serrata were screened for their anti-acetylcholinesterase activity. As a result, 39 potent fungi belonging to 15 genera including Penicillium were identified (Wang et al. 2011a). This research opened new avenues for searching future AChE inhibitors from endophytes and justified continue efforts in investigating these microorganisms as a potential source of new medicines (Fig. 8). Indeed, eight compounds isolated from endophyte P. chermesinum ZH4-E2 were tested and two compounds, 3ʺ-deoxy-6′-O-desmethylcandidusin B (239) and 6′-O-desmethylcandidusin B (240) exhibited strong inhibitory activity toward acetylcholinesterase with IC50 values of 7.8 and 5.2 μM, respectively (Huang et al. 2011). In a later study, three compounds including arigsugacin I (241), a new α-pyrone meroterpene, arigsugacins F (242) and territrem B (243), isolated from the extract of Penicillium sp. sk5GW1L endophyte of Kandelia candel showed very strong inhibitory activities against acetylcholinesterase with IC50 values of 0.64 µM, 0.37 µM, and 7.03 nM, respectively (Huang et al. 2013). The further investigation of Penicillium sp. SK5GW1L led to the purification of five compounds, including 3-epiarigsugacin E (244), a new compound along with arisugacin D (245), arisugacin B (246), territrem C (247), and terreulactone C (248). The five compounds showed strong inhibitory activities against acetylcholinesterase with IC50 values ranging from 0.028 to 53.39 μM (Ding et al. 2016). These compounds with strong activity and the low molecular weight (≈ 500) represent to be good candidates for further optimization to develop new and more potent acetylcholinesterase inhibitors.
Fig. 8.
Neuroprotective agents from Penicillium spp.
Among the two new compounds isolated from an extract of Penicillium sp. FJ-1, the endophytic fungus of Acanthus ilicifolius Linn, (2R,3S)-pinobanksin-3-cinnamate (249), exhibited potent neuroprotective effects on corticosterone-damaged PC12 cells by upregulating endogenous antioxidant enzymes (Liu et al. 2014a). Another natural neuroprotective compound is paeoniflorin (250), mainly isolated from Paeonia lactiflora Pallas. Over the past decades, the neuroprotective potential of this compound has been the subject of several investigations (Manayi et al. 2017). Searching for a potential source to produce this compound, extracts from 22 endophytic fungi isolated from Paeonia lactiflora were investigated. Three paeoniflorin-producing fungi including an isolate of Penicillium commune were identified (Cheng et al. 2018). Echinacoside (251), a natural phenylethanoid glycoside, known for its numerous pharmacologically beneficial activities for human health, especially the neuroprotective effects as the potential for the treatment of Parkinson’s and Alzheimer’s diseases has become increasingly important. Recently, Xu et al. (2018) reported an alternative method to produce echinacoside (37.16 mg/L) by fermentation using Penicillium sp. H1, an endophyte isolated from Ligustrum lucidum Ait. These studies confirm the hypothesis that endophytes can be used for the industrial production of important neuroprotective drugs.
Treatment with withanolide A (252), a major active constituent isolated from Withania somnifera predominantly induces axonal outgrowth in normal cortical neurons (Kuboyama et al. 2002). Supplementation of withanolide-enriched extract of Withania somnifera restored hypoxia-induced depleted antioxidant glutathione level and free radical scavenging enzyme system in the brain (Baitharu et al. 2013). Since glutathione is the major antioxidant in the brain, modulation of its biosynthesis by withanolide A under hypoxic condition could ameliorate oxidative stress-induced neurodegeneration and consequent memory dysfunction (Baitharu et al. 2014). In a recent study, several endophytes from Withania somnifera were found to increase the production yield of this compound in leaves and roots. For instance, in root treated with Penicillium oxalicum strain 5aWF, the production increased by up to 52%. This fungal also upregulated several genes involved in the withanolide and sterol biosynthetic pathways and host resistant-related genes (Kushwaha et al. 2019). This study demonstrates that endophytic Penicillium spp. can also be used to improve the production of desire neuroprotective metabolites in plants.
Agricultural importance of Penicillium species
Phytoremediation and plant growth-promoting properties
The contamination of soil by diverse pollutants such as metals has been one of the most challenging problems affecting the agricultural and fishing industries. In recent years, phytoremediation has emerged as a technology to help in cleaning the soil and water bodies from these noxious pollutants. Several studies demonstrating the potential of endophytic Penicillium spp. to improve the phytoremediation efficacy of host plants have been reported. In fact, among endophyte isolated from stems and roots of Brassica napus, Penicillium sp. CBRF65 showed the ability to tolerate 2 mM Cd and 20 mM Pb. Moreover, this fungal showed the highest phosphate-solubilizing activity and significantly increased the rape biomass and promoted the extraction efficacy of Pb and Cd (Shi et al. 2017). Four different endophytic fungi isolated from soybean plants were screened for their potential to bio-accumulate metal, and P. funiculosum LHL06 with a growth diameter of 5.3 cm2 was identified as the most Cu- and Cd-resistant strain. The inoculation of plants with P. funiculosum LHL06 was found to not only improve the biomass but also to increase resistance to the toxic effects of metal contamination (Khan and Lee 2013). The results suggested that this fungus can be applied to bioremediate polluted agricultural fields. Recently, Penicillium funiculosum LHL06 was found to tolerate and remediate combined heavy metal contamination while upregulating gibberellins (GA1, GA3, GA4, GA7, and GA9) and indole-3-acetic acid production. The authors showed that P. funiculosum LHL06-inoculated roots significantly upregulated the expression of stress-related proteins to combat metal toxicity. Furthermore, LHL06-inoculated plants exhibited higher antioxidant activity and transcript accumulation of glutathione S-transferase (GmGST8 and GmGST3), G6PDH, and GmSOD1 [Cu–Zn], which decreased metal-induced reactive oxygen species (Bilal et al. 2019).
In another investigation aiming to identify endophytes with the ability to detoxify soils polluted with chromium (Cr), 114 endophytic fungi were screened. Four strains among which P. radicum was able to induce resistance in L. sativa against Cr and detoxified up to 95% of extracellular Cr. This isolate was able to biotransform Cr from highly toxic hexavalent to the least toxic trivalent form, thus helped the Cr-stressed Lactuca sativa to restore its normal growth (Bibi et al. 2018). Another endophyte Penicillium ruqueforti was found to induce resistance in wheat-inoculated plants grown in heavy metal-contaminated soil. These wheat plants also showed a higher plant growth, nutrient uptake, and low concentrations of heavy metals in shoot and roots (Ikram et al. 2018). P. oxalicum isolated from Artemisia annua L. was investigated for its ability to decontaminate Triclosan (TCS), an emerging contaminant in aqueous and soil environment. From the results, it appears that P. oxalicum exhibits a high TCS uptake capacity and can reduce this compound to 2-chlorohydroquinone, 2,4-dichlorophenol, and hydroquinone, three non-toxic metabolites (Tian et al. 2018). Overall, data accumulated revealed that endophytic Penicillium spp. can establish a symbiotic relationship with host plants and act as biofertilizer for healthy and safe crop production. The application of these endophytes for bioremediation in crop farms can help promote the growth and tolerance of plants and reduce metal-accumulation, making plants safer for consumption (Fig. 9). Heavy metal pollution in crop fields is one of the major issues in sustainable agriculture production. Improving crop growth while reducing the accumulation of the toxic metal is an ideal strategy. Therefore, investigating gibberellin-producing endophytic fungi associated with crop plants in metal-contaminated agriculture fields could be an important step towards reducing agrochemical pollutions. This phytoremediation strategy using microbial endophytes can provide a low-cost and sustainable way to improve the agricultural economies, particularly for developing countries.
Fig. 9.
Endophytic fungi phytoremediation and plant growth promotion abilities. Plant-associated fungi have been reported to detoxify contaminated soil and plants via multiple mechanisms including efflux of heavy metals from the cell, metal sorption, bioaccumulation, and enzymatic oxidation or reduction to a non-toxic form by biotransformation. These studies also demonstrated the upregulation of the production of various plant hormones such as gibberellins and indole-3-acetic acid, leading to growth promotion
Plant hormones (gibberellins and indole-3-acetic acid) are well known to regulate many different aspects of plant growth and development including cell division and elongation, seed germination, stem and hypocotyl elongation, root growth, and flowering induction (Fu et al. 2015; Gomez et al. 2016). Several endophytic fungi belonging to Penicillium genus have been reported for their ability to produce plant hormones and enhance the growth of crop plants (Ahmad et al. 2010; Khan et al. 2008, 2011a, b 2013a, b; Khan and Lee 2013; Waqas et al. 2012; Kurchenko et al. 2013; Khan et al. 2015a, b; You et al. 2012; Hamayun et al. 2010; Min et al. 2014; Yuan et al. 2014). These investigations were summarised in a review by Leitão and Enguita (2016). In the continuation, endophytic fungi isolated from Stanhopea tigrina were tested for their gibberellin-producing ability. The results showed that among the 21 potent isolates, half belonged to Penicillium genus (Salazar-Cerezo et al. 2018). Penicillium chrysogenum and P. crustosum isolated from Teucrium polium were evaluated for their plant growth-promoting properties. The results revealed that these endophytes differentially produced indole acetic acid, ammonia and exhibited variable phosphate solubilization capacity (Hassan 2017).
Biological control of plant pathogens
Plant pathogens are known in today’s agricultural industry for their negative effect not only on seedling survival and growth but also on crop production and quality. This often results in a massive economic loss. It is well established that endophytes protect plants by producing antagonist antibiotic compounds. Therefore, over the past decades, the natural antagonistic potential of endophytic fungi has been investigated for the development of natural pesticides needed to reduce the harmful effects of diverse phytopathogens. To search for potential biocontrol agents, 123 endophytic fungi isolated from healthy maize and rice plants were screened for in vitro plant growth promotion, stress tolerance, and disease suppressive activity. From the results, P. simplicissimum ENF22, isolated from rice exhibited potent antagonist activity against Pyricularia oryzae ITCC 4511, Rhizoctonia solani ITCC 6491, Sclerotium oryzae ITCC 4107, and Pythium ultimum ITCC 1650 with an inhibition diameter range from 35.48 to 62.29 mm. This endophyte also showed growth ability in a wide range of pH (3–12) and extreme salinity (10%). Besides, the study of root colonization showed that ENF22 can colonize from 0.98 to 1.24 log10CFU/g root in rice and 1.01 to 1.24 log10CFU/g root in maize. This fungus presented good ability and can, therefore, be exploited as a biocontrol agent to improve control plant phytopathogens and crop productivity (Potshangbam et al. 2017). The non-volatile substances produced by P. simplicissimum CEF-818, an endophyte from Gossypium hirsutum, was previously reported to strongly inhibit the growth of the highly virulent plant pathogen Verticillium dahliae isolate Vd080 (Li et al. 2014a, b, c, d, e). In further investigation, Yuan et al. (2017) reported the ability of P. simplicissimum CEF-818 to reduce in greenhouse, the disease incidence and disease index by 67% in cotton attacked by verticillium wilt. This fungus provided a protective effect of 69.5% in cotton plants. In the harvest, the available cotton bolls of a plant treated with CEF-818 increased to 13.1 and the seed cotton yield significantly increased to 3442.04 kg/ha as well. Therefore, P. simplicissimum possesses biocontrol ability that can be harnessed to limit the damage caused by verticillium wilt on cotton. Katoch and Pull (2017) also demonstrated the capability of P. commune MC-9 L isolated from Monarda citriodora Cerv. ex Lag to exhibit biocontrol ability against Sclerotinia sp. in fumigation assay. In addition, extract from Penicillium sp. isolated from Camptotheca acuminata was reported to inhibit the growth of plant pathogens, Rhizoctonia solani, Gibberella fujikuroi, Pyricularia grisea, Gibberella zeae, Fusarium oxysporum f. sp. vasinfectum (Ding et al. 2010). These extracts exhibit inhibition via the production of active compounds (Fig. 10) that can be purified and further investigated for the formulation of new fungicides.
Fig. 10.
Compounds with activity against wide range of plants pathogens
Indeed, the chemical investigation of extract P. chrysogenum QEN-24S, an endophytic fungus of a marine Laurencia sp., led to the purification of five compounds among which penicimonoterpene (253) displayed potency against Alternaria brassicae (Gao et al. 2010). Continuing the investigation of this endophyte, nine compounds including two new polyoxygenated steroids were later isolated. Only penicisteroids A and B, and anicequol were tested for antimicrobial activity and the results showed that penicitide A (254) and penicimonoterpene (253) inhibited Alternaria brassicae and Aspergillus niger with an inhibition zone of 7 and 17 mm (Gao et al. 2011b). Peniginsengin A (255), a new polyoxygenated farnesylcyclohexenone, was isolated from the fermentation of Penicillium sp. YIM PH30003, an endophytic fungus associated with Panax notoginseng (Burk.) F. H. Chen was found to possess inhibitory ability against F. solani with a MIC of 64 μg/mL (Yang et al. 2016). A new chaetoglobosin, penochalasin K along with chaetoglobosin C, penochalasin I, and chaetoglobosin A were isolated from endophytic fungus P. chrysogenum V11. Among these compounds, penochalasin K (256) was very potent against Colletotrichum gloeosporioides and R. solani with MIC values of 6.13 μM and MIC 12.26 μM, respectively (Zhu et al. 2017a, b). Similarly, Huang et al. (2016) reported the activity of chaetoglobosin C (152), A (153), E (155), and armochaetoglobosin I (257) against the plant pathogenic fungus R. solani (MIC 11.79–23.66 μM), and penochalasin J (149), chaetoglobosin A (153), and chaetoglobosin E (155) against C. gloeosporioides (MIC 23.58–47.35 μM). Compounds penicibrocazine B (30), penicibrocazine D (32), phomazine B (33), and penicibrocazine E (258) were also reported as potent inhibitors of plant pathogen Gaeumannomyces graminis with MIC values of 0.25, 8, 0.25, and 64 μg/mL (Meng et al. 2015).
Penicillium canescens, isolated from Polygonatum cyrtonema was found to exhibit antifungal activity. The fractionation led to o-acetylbenzeneamidinocarboxylic acid (259), griseofulvin (260) and naphtho[1,2-b] furan-3-carboxylic acid, 4-hydroxy-5-methoxy-2-methyl (261). These compounds exhibited activity against a broad-spectrum antifungal activity with griseofulvin being the most active Botrytis cinerea, Colletotrichum orbiculare, Didymella bryoniae and Sclerotinia sclerotiorum with IC50 was 0.68, 0.38, 0.91, and 0.61 mg/L, respectively (Wang et al. 2010). Brasiliamide A, viridicatumtoxin along with two new compounds peniciadametizine A and B were isolated from a marine endophyte Penicillium adametzioides AS-53. When tested against a set of pathogenic microbes, peniciadametizine A (262) and B (263) exhibited potency against Alternaria brassicae with MIC values of 4 and 32 μg/mL, respectively (Liu et al. 2015c). A new isoquinolone alkaloid, 5-hydroxy-8-methoxy-4-phenylisoquinolin-1(2H)-one (264), along with 3-O-methylviridicatin (265) and viridicatol (266) were isolated from the extract of Penicillium sp. R22, an endophytic fungus of Nerium indicum. The three compounds displayed strong antifungal activity against Alternaria brassicae, Alternaria alternata and Valsa mali with MIC value of 31.2 μg/mL (Ma et al. 2016). In continuation of their investigation, Ma et al. (2017b) in a later study reported the activity (MIC 31.2 μg/mL) of the new compound, 4-(2′R, 4′-dihydroxybutoxy) benzoic acid (267) isolated from the same endophyte Penicillium sp. R22 against C. gloeosporioides, Alternaria alternata, and Alternaria brassicae. In another study, among the three compounds, isolated from P. raciborskii, an endophytic fungus isolated from Rhododendron tomentosum, outovirin C (268) at 0.38 mM inhibited the growth of F. oxysporum, B. cinerea, and V. dahlia (Kajula et al. 2016). Pseurotin A (269) isolated from an extract of Penicillium janczewskii K. M. Zalessky, an endophyte from tissue Prumnopitys andina, was reported to inhibit phytopathogenic bacteria Erwinia carotovora and Pseudomonas syringae, with IC50 values of 220 and 112 μg/mL, respectively (Schmeda-Hirschmann et al. 2008). A Penicillium sp. isolated from the leaves of indoor ornamentals was able to inhibit the plant pathogen B. cinerea by the production of antifungal volatile organic compounds (Mahnert et al. 2018). Two plant pathogens, Erwinia carotovora subsp. Carotovora (Jones) and Sclerotium rolfsii Sacc were also inhibited by penicitroamide (270), a new metabolite isolated from the ethyl acetate extract of Penicillium sp. no. 24, an endophyte from Tapiscia sinensis Oliv. This compound also exhibits 60% lethality against brine shrimp at 10 μg/mL (Feng et al. 2016). Overall, Penicillium species are a source of compounds exhibiting activity against a wide range of plant pathogens. Therefore, the continuous investigation of these species may lead to the discovery of new fungicides needed to replace toxic chemical pesticides currently used in agricultural industries.
Insecticidal activity
Among the worst pests of grasslands are species of Wiseana, an indigenous genus which attacks pasture over a wide area of New Zealand (Barlow et al. 1986). The use of insecticides is the main strategy for the management of these insects and reduces their devastating effects. Metabolites with insecticidal activity are currently investigated. Recently, four new compounds including janthitrem A, janthitrem D, janthitrem B and janthitrem C along with 10 knowns were isolated from endophyte Penicillium janthinellum and tested for insecticidal activity. Compounds janthitrem A (271) and janthitrem B (272) were found to reduce weight gain and food consumption of Wiseana cervinata larvae (Babu et al. 2018). Two other compounds, penicinoline (273) and its semi-synthetic analog, penicinotam (274), were tested for their insecticidal activity against Aphis gossypii, Plutella xylostella, Heliothis virescens, Septoria tritici, and Uromyces fabae. The results showed that penicinoline was strongly potent against A. gossypii with 100% mortality on a mixed population at 1000 ppm while, penicinotam in addition to his potency against A. gossypii also inhibited larvae of P. xylostella at 500 ppm and H. virescens at 1000 ppm (Shao et al. 2010). These results argue in favor of investigating natural products as a source of new insecticidal compounds (Fig. 11) and suggest that these bioactive compounds could serve as a template for the synthesis of more potent molecules needed to control insects attacking a wide variety of crops.
Fig. 11.
Insecticidal agents from Penicillium
The use of endophytic Penicillium spp. for biotechnological purposes
Biotransformation
Over the past decade, microbial biotransformations have gained importance. This mechanism currently used by microbes to acclimatize to environmental changes is useful in a wide range of biotechnological processes. This approach has been extensively utilized to generate metabolites in bulk amounts with more specificity and represents a valuable strategy to build molecules, like parent drug in the drug discovery program. The most significant aspect of biotransformation is that it maintains the original carbon skeleton after obtaining the products. This approach has been used to produce dozens of active compounds (Fig. 12).
Fig. 12.
Metabolites produced through microbial transformation using endophytic Penicillium species
Gentiopicroside is a popular ingredient in Chinese herbal medicine and health products because of its protective effect on the liver, against liver dysfunction, and its ability to promote gastric acid secretion, antimicrobial and anti-inflammatory activities (Kim et al. 2009). The biotransformation of gentiopicroside by the endophytic fungus P. crustosum 2T01Y01 isolated from Dendrobium candidum Wall. ex Lindl led to seven deglycosylated derivatives including four novel ones, 5α-(hydroxymethyl)-6β-methyl-3,4,5,6-tetrahydropyrano[3,4-c]pyran-1(8H)-one (275), (Z)-4-(1-hydroxybut-3-en-2-yl)-5,6-dihydropyran-2-one (276), (E)-4-(1-hydroxybut-3-en-2-yl)-5,6-dihydropyran-2-one (277), and 5-hydroxymethyl-3,4,5,6-tetrahydroisochromen-1-one (278) along with known ones, 5α-(hydroxymethyl)-6β-methyl-1H,3H-5,6-dihydropyrano[3,4-c]pyran-1(3H)-one (279), 5α-(hydroxymethyl)-6α-methyl-5,6-dihydropyrano[3,4-c]pyran-1(3H)-one (280), and 5-hydroxymethyl-5,6-dihydroisochromen-1-one (281). Compounds 275, 278, and 279 exhibited hepatoprotective activities against human hepatocyte line HL-7702 injury induced by hydrogen peroxide (Zeng et al. 2014). In another investigation, prednisolone, a current anti-inflammatory medicine was biotransformed by a marine endophytic fungus Penicillium lapidosum isolated from an alga to produce compound, 20β-hydroxyprednisolone (282) (Sultan et al. 2014). Fill et al. (2012) also reported the biotransformation of 1-indanone into dihydrocoumarin (283) and (−)-(R)-3-hydroxy-1-indanone (284) by the endophytic fungus P. brasilianum isolated from Melia azedarach. Among the 426 fungi isolates from the medicinal plant Kadsura angustifolia, 39 exhibited remarkable biocatalytic activity among which several Penicillium spp. (Huang et al. 2015).
Piplartine, an amide alkaloid originally isolated from Piper longum is a lead anticancer agent in addition to other reported properties such as antiplatelet, antinociceptive, anxiolytic, antidepressant, neuroprotective, antiatherosclerotic, and antimicrobial (Piska et al. 2018). The endophytic Penicillium crustosum VR4 isolated from Viguiera robusta was reported to metabolize piplartine to produce two new compounds named 3,4-dihydropiplartine (285) and 8,9-dihydropiplartine (286) (da Silva-Junior et al. 2017). This finding is of interest and can be useful characterizing piplartine metabolites during pre‐clinical trials, as the compound is considered promising for anticancer therapy.
Production of enzymes
Endophytes have been reported for their ability to produce enzymes with the wide industrial application (Toghueo et al. 2017; Mishra et al. 2019). Several Penicillium spp. have been reported to produce an array of industrially important enzymes. To cite just a few as per illustration, 89 endophytes isolated from Cymbopogon citratus, Murraya koenigii, Oldenlandia diffusa and Pereskia bleo were screened in vitro for new L-asparaginase enzyme. The results showed that the 25 potent endophytes belonged to four genera including Penicillium (Chow and Ting 2015). In a more recent study, 20 endophytic isolates from Tillandsia catimbauensis were screened for their ability to produce l-asparaginase enzyme. The results showed that Penicillium sp. 4 URM 7827 was among the most potent (1.28 U/g) strains identified (Silva et al. 2018). Penicillium spp. can constitute a good means for the industrial production of this important enzyme also used in the treatment of leukemia.
Endophytic Penicillium funiculosum isolated from the bog plants of Ukrainian Polesie was reported to display polygalacturonase activity. The author proposed that this endophyte plays a significant role in the hydrolysis of pectic substances of plant tissues and the migration of mineral elements in wetland ecosystems (Kurchenko 2013). 16 other endophytic P. funiculosum were reported by Yurieva et al. (2016) to produce a varied amount of cellulase and xylanase enzymes. In the previous study, Cabezas et al. (2012) reported the ability of P. glabrum to exhibit CMCase, exoglucanase and β-glucosidase enzyme activities of 44.5, 48.3, and 0.45 U/mL respectively. A strain P. glandicola isolated from Opuntia ficus indica Mill. was also reported to produce pectinase, cellulase, xylanase, and protease (Bezerra et al. 2012). In another study, endophytes isolated from B. forficate were screened for their enzymatic activity. Among active isolates, Penicillium glabrum was able to produce cellulase, lipase, xylanase, and protease (Bezerra et al. 2015). The proteolytic activity of P. bilaiae endophyte of palm roots was recently investigated. From the results, factors such as temperature, pH and glucose concentration dramatically affected protease yield and the optimization of culture condition led to a 1086-fold enhancement of protease production (Ben Mefteh et al. 2019). These studies are demonstrating that endophytic Penicillium spp. are producers of varied hydrolytic enzymes with multiple biotechnological applications. Therefore, these fungi can be fully exploited for the cost-effective production of enzymes.
Synthesis of nanoparticles
The development of eco-friendly and reliable processes for the synthesis of nanoparticles has attracted considerable interest in nanotechnology because of its tremendous impetus in modulating metals into nanosize to their potential use for human benefits. Extracts from microbial species have been identified as the eco-friendly means for the synthesis of bioactive nanoparticles. A gold nanoparticle synthesized (AuNPs) from an extract of Penicillium citrinum was reported to exhibit significant antioxidant potential compared to the standard ascorbic acid (Manjunath et al. 2017). A silver nanoparticles (AgNPs) synthesized from the extract of P. polonicum ARA 10, isolated from the marine green alga Chaetomorpha antennina, was tested for antimicrobial activity against Salmonella enterica serovar Typhimurium MTCC 1251 and exhibited activity with MIC value of 7.81 μg/mL (Neethu et al. 2018a). In another study, Neethu et al. (2018b) reported the antibacterial efficacy of AgNPs against multi-drug-resistant, biofilm-forming Acinetobacter baumannii with MIC of 15.62 μg/mL. Extract and nanoparticles from another P. chrysogenum isolated from the medicinal plant Calotropis procera Ait were also reported to exhibit very good inhibition of seven bacteria pathogens (Mohamed et al. 2019). In an earlier report, silver nanoparticles synthesized from Penicillium sp. from leaves of Curcuma longa were found to inhibit MDR E. coli and S. aureus with an inhibition diameter of 17 and 16 mm (Singh et al. 2014). These investigations are demonstrating that Penicillium species can effectively harness the synthesis of more potent nanoparticles for multiple medical applications.
Conclusion
This investigation into the current trend of knowledge concerning the multiple application endophytic Penicillium species showed that this genus has been intensively exploited over the last 2 decades not only as sources of bioactive compounds against a wide array of disease but also as means to reduce environmental pollution and improve agriculture. These species have also been used in multiple biotechnological processes as biocatalyst and sources of important enzymes. The current review has shown that a very large number (131) of endophytes from this genus have been investigated so far and more than 280 compounds exhibiting antimicrobial, anticancer, antiviral, antioxidants, anti-inflammatory, antiparasitics, immunosuppressants, antidiabetic, anti-obesity, antifibrotic, neuroprotective effects, insecticidal, and biocontrol activities have been reported. However, this study also revealed that many Penicillium species were investigated only at the crude extract level. Therefore, their chemical investigation is still needed to identify potential active ingredients. On the other hand, many new compounds isolated were not investigated for any biological activities (Suppl 2). The future investigation of these compounds could lead to the identification of a potential active molecule to initiate new drug discovery program. Overall, the diversity of bioactive secondary metabolites produced by Penicillium endophytes opens avenues of developing new chemical skeletons as a prototype for new drugs. This investigation of literature data also showed that only a few studies have been conducted to evaluate the phytoremediation, plant growth-promoting properties and enzymatic ability of endophytic Penicillium spp. Data collected up to this time are valuable for understanding the extent of the potential of endophytic Penicillium species, and demonstrate that this genus represents a true microbial factory that can be fully exploited for a wide range of applications to improve human life.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Rufin Marie Kouipou Toghueo is a postdoctoral researcher at the Drug Discovery Unit, University of Dundee. This integrated program is developed by the Drug Discovery Unit with the support of the Wellcome Centre for Anti-Infective Research as a special program for leadership research, capacity building, and training in drug discovery against tropical diseases.
Author contributions
RMKT wrote the manuscript and FFB provided critical inputs.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest in the publication.
References
- Abutaha N, Semlali A, Baabbad A, Al-Shami M, Alanazi M, Wadaan MA. Anti-proliferative and anti-inflammatory activities of entophytic Penicillium crustosum from Phoenix dactylifer. Pak J Pharm Sci. 2018;31(2):421–427. [PubMed] [Google Scholar]
- Ahmad N, Hamayun M, Khan SA, Khan AL, Lee IJ, Shin DH. Gibberellin-producing endophytic fungi isolated from Monochoria vaginalis. J Microbiol Biotechnol. 2010;20(12):1744–1749. [PubMed] [Google Scholar]
- Ali S, Khan AL, Ali L, Rizvi TS, Khan SA, Hussain J, Hamayun M, Al-Harrasi A. Enzyme inhibitory metabolites from endophytic Penicillium citrinum isolated from Boswellia sacra. Arch Microbiol. 2017;199(5):691–700. doi: 10.1007/s00203-017-1348-3. [DOI] [PubMed] [Google Scholar]
- Ali T, Inagaki M, Chai H, Wieboldt T, Rapplye C, Rakotondraibe LH. Halogenated compounds from directed fermentation of Penicillium concentricum, an endophytic fungus of the Liverwort Trichocolea tomentella. J Nat Prod. 2017;80(5):1397–1403. doi: 10.1021/acs.jnatprod.6b01069. [DOI] [PubMed] [Google Scholar]
- Ali T, Pham TM, Ju KS, Rakotondraibe HL. Ent-homocyclopiamine B, a prenylated indole alkaloid of biogenetic interest from the endophytic fungus Penicillium concentricum. Molecules. 2019 doi: 10.3390/molecules24020218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aly AH, Debbab A, Clements C, Edrada-Ebel R, Orlikova B, Diederich M, Wray V, Lin W, Proksch P. NF kappa B inhibitors and antitrypanosomal metabolites from endophytic fungus Penicillium sp. isolated from Limonium tubiflorum. Bioorg Med Chem. 2011;19(1):414–421. doi: 10.1016/j.bmc.2010.11.012. [DOI] [PubMed] [Google Scholar]
- Anaya-Eugenio GD, Ali T, Rakotondraibe LH, Carcache de Blanco E. Cytotoxic constituents from Penicillium concentricum, an endophytic fungus from Trichocolea tomentella. Anticancer Drugs. 2019;30(4):323–329. doi: 10.1097/cad.0000000000000759. [DOI] [PubMed] [Google Scholar]
- Ariantari NP, Ancheeva E, Wang C, Mándi A, Knedel TO, Kurtán T, Chaidir C, Müller WEG, Kassack MU, Janiak C, Daletos G, Proksch P. Indole diterpenoids from an endophytic Penicillium sp. J Nat Prod. 2019;82(6):1412–1423. doi: 10.1021/acs.jnatprod.8b00723. [DOI] [PubMed] [Google Scholar]
- Arunpanichlert J, Rukachaisirikul V, Sukpondma Y, Phongpaichit S, Tewtrakul S, Rungjindamai N, Sakayaroj J. Azaphilone and isocoumarin derivatives from the endophytic fungus Penicillium sclerotiorum PSU-A13. Chem Pharm Bull (Tokyo) 2010;58(8):1033–1036. doi: 10.1248/cpb.58.1033. [DOI] [PubMed] [Google Scholar]
- Ateba JET, Toghueo RMK, Awantu AF, Mba’ning BM, Gohlke S, Sahal D, Rodrigues-Filho E, Tsamo E, Boyom FF, Sewald N, Lenta BN. Antiplasmodial properties and cytotoxicity of endophytic fungi from Symphonia globulifera (Clusiaceae) J Fungi (Basel) 2018;4(2):E70. doi: 10.3390/jof4020070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu JV, Popay AJ, Miles CO, Wilkins AL, di Menna ME, Finch SC. Identification and structure elucidation of Janthitrems A and D from Penicillium janthinellum and determination of the Tremorgenic and anti-insect activity of Janthitrems A and B. J Agric Food Chem. 2018;66(50):13116–13125. doi: 10.1021/acs.jafc.8b04964. [DOI] [PubMed] [Google Scholar]
- Baitharu I, Jain V, Deep SN, Hota KB, Hota SK, et al. Withania somnifera root extract ameliorates hypobaric hypoxia induced memory impairment in rats. J Ethnopharmacol. 2013;145:431–441. doi: 10.1016/j.jep.2012.10.063. [DOI] [PubMed] [Google Scholar]
- Baitharu I, Jain V, Deep SN, Shroff S, Sahu JK, Naik PK, et al. Withanolide A prevents neurodegeneration by modulating hippocampal glutathione biosynthesis during hypoxia. PLoS One. 2014;9(10):e105311. doi: 10.1371/journal.pone.0105311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow ND, French RA, Pearson JF. Population ecology of Wiseana cervinata, a pasture pest in New Zealand. J Appl Ecol. 1986;23(2):415. doi: 10.2307/2404026. [DOI] [Google Scholar]
- Bartholomay LC, Waterhouse RM, Mayhew GF, Campbell CL, Michel K, Zou Z, Ramirez JL, Das S, Alvarez K, Arensburger P, Bryant B, Chapman SB, Dong Y, Erickson SM, Karunaratne SH, Kokoza V, Kodira CD, Pignatelli P, Shin SW, Vanlandingham DL, Atkinson PW, Birren B, Christophides GK, Clem RJ, Hemingway J, Higgs S, Megy K, Ranson H, Zdobnov EM, Raikhel AS, Christensen BM, Dimopoulos G, Muskavitch MA. Pathogenomics of Culex quinquefasciatus and meta-analysis of infection responses to diverse pathogens. Science. 2010;330(6000):88–90. doi: 10.1126/science.1193162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker RE. Therapy of the cognitive deficit in Alzheimer’s disease; the cholinergic system. In: Becker RE, Giacobini E, editors. Cholinergic basis of Alzheimer therapy. Boston: Berkhauser; 1991. pp. 1–22. [Google Scholar]
- Ben Mefteh F, Daoud A, Chenari Bouket A, Thissera B, Kadri Y, Cherif-Silini H, Eshelli M, Alenezi FN, Vallat A, Oszako T, Kadri A, Ros-García JM, Rateb ME, Gharsallah N, Belbahri L. Date palm trees root-derived endophytes as fungal cell factories for diverse bioactive metabolites. Int J Mol Sci. 2018;19(7):E1986. doi: 10.3390/ijms19071986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Mefteh F, Frikha F, Daoud A, Chenari Bouket A, Luptakova L, Alenezi FN, Al-Anzi BS, Oszako T, Gharsallah N, Belbahri L. Response surface methodology optimization of an acidic protease produced by Penicillium bilaiae isolate TDPEF30, a newly recovered endophytic fungus from healthy roots of date palm trees (Phoenix dactylifera L) Microorganisms. 2019;7(3):E74. doi: 10.3390/microorganisms7030074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezerra JD, Santos MG, Svedese VM, Lima DM, Fernandes MJ, Paiva LM, Souza-Motta CM. Richness of endophytic fungi isolated from Opuntia ficus-indica Mill. (Cactaceae) and preliminary screening for enzyme production. World J Microbiol Biotechnol. 2012;28(5):1989–1995. doi: 10.1007/s11274-011-1001-2. [DOI] [PubMed] [Google Scholar]
- Bezerra JD, Nascimento CC, Barbosa Rdo N, da Silva DC, Svedese VM, Silva-Nogueira EB, Gomes BS, Paiva LM, Souza-Motta CM. Endophytic fungi from medicinal plant Bauhinia forficata: diversity and biotechnological potential. Braz J Microbiol. 2015;46(1):49–57. doi: 10.1590/S1517-838246120130657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibi S, Hussain A, Hamayun M, Rahman H, Iqbal A, Shah M, Irshad M, Qasim M, Islam B. Bioremediation of hexavalent chromium by endophytic fungi; safe and improved production of Lactuca sativa L. Chemosphere. 2018;211:653–663. doi: 10.1016/j.chemosphere.2018.07.197. [DOI] [PubMed] [Google Scholar]
- Bilal S, Shahzad R, Khan AL, Al-Harrassi A, Kim CK, Lee I-J. Phytohormones enabled endophytic Penicillium funiculosum LHL06 protects Glycine max L. from synergistic toxicity of heavy metals by hormonal and stress-responsive proteins modulation. J Hazard Mater. 2019;379:120824. doi: 10.1016/j.jhazmat.2019.120824. [DOI] [PubMed] [Google Scholar]
- Cabezas L, Calderon C, Medina LM, Bahamon I, Cardenas M, Bernal AJ, Gonzalez A, Restrepo S. Characterization of cellulases of fungal endophytes isolated from Espeletia spp. J Microbiol. 2012;50(6):1009–1013. doi: 10.1007/s12275-012-2130-5. [DOI] [PubMed] [Google Scholar]
- Cai R, Wu Y, Chen S, Cui H, Liu Z, Li C, She Z. Peniisocoumarins A–J: isocoumarins from Penicillium commune QQF-3, an endophytic fungus of the mangrove plant Kandelia candel. J Nat Prod. 2018;81(6):1376–1383. doi: 10.1021/acs.jnatprod.7b01018. [DOI] [PubMed] [Google Scholar]
- Cao S, McMillin DW, Tamayo G, Delmore J, Mitsiades CS, Clardy J. Inhibition of tumor cells interacting with stromal cells by xanthones isolated from a Costa Rican Penicillium sp. J Nat Prod. 2012;75(4):793–797. doi: 10.1021/np2009863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Zhu Y, Wang HZ, Wang SJ, Zhang RQ. The metabolites of a mangrove endophytic fungus, Penicillium thomi. J Asian Nat Prod Res. 2007;9(2):159–164. doi: 10.1080/10286020500480423. [DOI] [PubMed] [Google Scholar]
- Chen MJ, Fu YW, Zhou QY. Penifupyrone, a new cytotoxic funicone derivative from the endophytic fungus Penicillium sp HSZ-43. Nat Prod Res. 2014;28(19):1544–1548. doi: 10.1080/14786419.2014.924932. [DOI] [PubMed] [Google Scholar]
- Cheng X, Wei Z, Pu S, Xiang M, Yan A, Zhang Y, Wang X. Diversity of endophytic fungi of Paeonia lactiflora Pallas and screening for fungal paeoniflorin producers. FEMS Microbiol Lett. 2018 doi: 10.1093/femsle/fny263. [DOI] [PubMed] [Google Scholar]
- Chhipa H, Kaushik N. Fungal and bacterial diversity isolated from Aquilaria malaccensis tree and soil, induces agarospirol formation within 3 months after artificial infection. Front Microbiol. 2017;8:1286. doi: 10.3389/fmicb.2017.01286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow Y, Ting AS. Endophytic l-asparaginase-producing fungi from plants associated with anticancer properties. J Adv Res. 2015;6(6):869–876. doi: 10.1016/j.jare.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colović MB, Krstić DZ, Lazarević-Pašti TD, Bondžić AM, Vasić VM. Acetylcholinesterase inhibitors: pharmacology and toxicology. Curr Neuropharmacol. 2013;11(3):315–335. doi: 10.2174/1570159X11311030006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva-Junior EA, Paludo CR, Gouvea DR, Kato MJ, Furtado NAJC, Lopes NP, Vessecchi R, Pupo MT. Gas-phase fragmentation of protonated piplartine and its fungal metabolites using tandem mass spectrometry and computational chemistry. J Mass Spectrom. 2017;52(8):517–525. doi: 10.1002/jms.3955. [DOI] [PubMed] [Google Scholar]
- Dai JX, Du XN. Antimicrobial and anti-tumor activities of endophytes from Lycium barbarum of Ningxia. Zhongguo Zhong Yao Za Zhi. 2017;42(11):2072–2077. doi: 10.19540/j.cnki.cjcmm.20170415.001. [DOI] [PubMed] [Google Scholar]
- Dai J, Chen A, Zhu M, Qi X, Tang W, Liu M, Li D, Gu Q, Li J. Penicisulfuranol A, a novel C-terminal inhibitor disrupting molecular chaperone function of Hsp90 independent of ATP binding domain. Biochem Pharmacol. 2019;163:404–415. doi: 10.1016/j.bcp.2019.03.012. [DOI] [PubMed] [Google Scholar]
- Davis RA, Longden J, Avery VM, Healy PC. The isolation, structure determination and cytotoxicity of the new fungal metabolite, trichodermamide C. Bioorg Med Chem Lett. 2008;18(9):2836–2839. doi: 10.1016/j.bmcl.2008.03.090. [DOI] [PubMed] [Google Scholar]
- Devi P, Rodrigues C, Naik CG, D’Souza L. Isolation and characterization of antibacterial compound from a mangrove-endophytic fungus, Penicillium chrysogenum MTCC 5108. Indian J Microbiol. 2012;52(4):617–623. doi: 10.1007/s12088-012-0277-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding T, Jiang T, Zhou J, Xu L, Gao ZM. Evaluation of antimicrobial activity of endophytic fungi from Camptotheca acuminata (Nyssaceae) Genet Mol Res. 2010;9(4):2104–2112. doi: 10.4238/vol9-4gmr809. [DOI] [PubMed] [Google Scholar]
- Ding B, Wang Z, Huang X, Liu Y, Chen W, She Z. Bioactive α-pyrone meroterpenoids from mangrove endophytic fungus Penicillium sp. Nat Prod Res. 2016;30(24):2805–2812. doi: 10.1080/14786419.2016.1164702. [DOI] [PubMed] [Google Scholar]
- Ding Z, Tao T, Wang L, Zhao Y, Huang H, Zhang D, Liu M, Wang Z, Han J. Bioprospecting of novel and bioactive metabolites from endophytic fungi isolated from rubber tree Ficus elastica leaves. J Microbiol Biotechnol. 2019;29(5):731–738. doi: 10.4014/jmb.1901.01015. [DOI] [PubMed] [Google Scholar]
- Dwibedi V, Saxena S. Arcopilus aureus, a resveratrol-producing endophyte from Vitis vinifera. Appl Biochem Biotechnol. 2018;186(2):476–495. doi: 10.1007/s12010-018-2755-x. [DOI] [PubMed] [Google Scholar]
- El-Gendy MMAA, Yahya SMM, Hamed AR, Soltan MM, El-Bondkly AMA. Phylogenetic analysis and biological evaluation of marine endophytic fungi derived from red sea sponge hyrtios erectus. Appl Biochem Biotechnol. 2018;185:755–777. doi: 10.1007/s12010-017-2679-x. [DOI] [PubMed] [Google Scholar]
- El-Neketi M, Ebrahim W, Lin W, Gedara S, Badria F, Saad HE, Lai D, Proksch P. Alkaloids and polyketides from Penicillium citrinum, an endophyte isolated from the Moroccan plant Ceratonia siliqua. J Nat Prod. 2013;76(6):1099–1104. doi: 10.1021/np4001366. [DOI] [PubMed] [Google Scholar]
- Fatima N, Kondratyuk TP, Park EJ, Marler LE, Jadoon M, Qazi MA, Mehboob Mirza H, Khan I, Atiq N, Chang LC, Ahmed S, Pezzuto JM. Endophytic fungi associated with Taxus fuana (West Himalayan Yew) of Pakistan: potential bio-resources for cancer chemopreventive agents. Pharm Biol. 2016;54(11):2547–2554. doi: 10.3109/13880209.2016.1170154. [DOI] [PubMed] [Google Scholar]
- Fatima N, Sripisut T, Youn UJ, Ahmed S, Ul-Haq I, Munoz-Acuna U, Simmons CJ, Qazi MA, Jadoon M, Tan GT, de Blanco EJC, Chang LC. Bioactive constituents from an endophytic fungus, Penicillium polonicum NFW9, associated with Taxus fauna. Med Chem. 2017;13(7):689–697. doi: 10.2174/1573406413666170216145121. [DOI] [PubMed] [Google Scholar]
- Feng ZW, Lv MM, Li XS, Zhang L, Liu CX, Guo ZY, Deng ZS, Zou K, Proksch P. Penicitroamide, an antimicrobial metabolite with high carbonylization from the endophytic fungus Penicillium sp. (No. 24) Molecules. 2016;21(11):1438. doi: 10.3390/molecules21111438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez IE, Eickelberg O. New cellular and molecular mechanisms of lung injury and fibrosis in idiopathic pulmonary fibrosis. Lancet. 2012;380(9842):680–688. doi: 10.1016/S0140-6736(12)61144-1. [DOI] [PubMed] [Google Scholar]
- Ferreira MC, Cantrell CL, Wedge DE, Gonçalves VN, Jacob MR, Khan S, Rosa CA, Rosa LH. Antimycobacterial and antimalarial activities of endophytic fungi associated with the ancient and narrowly endemic neotropical plant Vellozia gigantea from Brazil. Mem Inst Oswaldo Cruz. 2017;112(10):692–697. doi: 10.1590/0074-02760170144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa M, Jarmusch AK, Raja HA, El-Elimat T, Kavanaugh JS, Horswill AR, Cooks RG, Cech NB, Oberlies NH. Polyhydroxyanthraquinones as quorum sensing inhibitors from the guttates of Penicillium restrictum and their analysis by desorption electrospray ionization mass spectrometry. J Nat Prod. 2014;77(6):1351–1358. doi: 10.1021/np5000704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fill TP, Geris dos Santos RM, Barisson A, Rodrigues-Filho E, Souza AQ. Co-production of bisphenylpropanoid amides and meroterpenes by an endophytic Penicillium brasilianum found in the root bark of Melia azedarach. Z Naturforsch C. 2009;64(5–6):355–360. doi: 10.1515/znc-2009-5-609. [DOI] [PubMed] [Google Scholar]
- Fill TP, da Silva JV, de Oliveira KT, da Silva BF, Rodrigues-Fo E. Oxidative potential of some endophytic fungi using 1-indanone as a substrate. J Microbiol Biotechnol. 2012;22(6):832–837. doi: 10.4014/jmb.1112.12014. [DOI] [PubMed] [Google Scholar]
- Fill TP, Asenha HB, Marques AS, Ferreira AG, Rodrigues-Fo E. Time course production of indole alkaloids by an endophytic strain of Penicillium brasilianum cultivated in rice. Nat Prod Res. 2013;27(11):967–974. doi: 10.1080/14786419.2012.701210. [DOI] [PubMed] [Google Scholar]
- Fleming A. On the antibacterial action of cultures of a Penicillium, with special reference to their use in the isolation of B. influenzæ. Br J Exp Pathol. 1929;10(3):226–236. [Google Scholar]
- Flewelling AJ, Johnson JA, Gray CA. Antimicrobials from the marine algal endophyte Penicillium sp. Nat Prod Commun. 2013;8(3):373–374. [PubMed] [Google Scholar]
- Fu SF, Wei JY, Chen HW, Liu YY, Lu HY, Chou JY. Indole-3-acetic acid: a widespread physiological code in interactions of fungi with other organisms. Plant Signal Behav. 2015;10(8):e1048052. doi: 10.1080/15592324.2015.1048052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao SS, Li XM, Du FY, Li CS, Proksch P, Wang BG. Secondary metabolites from a marine-derived endophytic fungus Penicillium chrysogenum QEN-24S. Mar Drugs. 2010;9(1):59–70. doi: 10.3390/md9010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao SS, Li XM, Li CS, Proksch P, Wang BG. Penicisteroids A and B, antifungal and cytotoxic polyoxygenated steroids from the marine alga-derived endophytic fungus Penicillium chrysogenum QEN-24S. Bioorg Med Chem Lett. 2011;21(10):2894–2897. doi: 10.1016/j.bmcl.2011.03.076. [DOI] [PubMed] [Google Scholar]
- Gao SS, Li XM, Zhang Y, Li CS, Wang BG. Conidiogenones H and I, two new diterpenes of Cyclopiane class from a marine-derived endophytic fungus Penicillium chrysogenum QEN-24S. Chem Biodivers. 2011;8(9):1748–1753. doi: 10.1002/cbdv.201000378. [DOI] [PubMed] [Google Scholar]
- Gashgari R, Gherbawy Y, Ameen F, Alsharari S. Molecular characterization and analysis of antimicrobial activity of endophytic fungi from medicinal plants in Saudi Arabia. Jundishapur J Microbiol. 2016;9(1):e26157. doi: 10.5812/jjm.26157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge HM, Shen Y, Zhu CH, Tan SH, Ding H, Song YC, Tan RX. Penicidones A–C, three cytotoxic alkaloidal metabolites of an endophytic Penicillium sp. Phytochemistry. 2008;69(2):571–576. doi: 10.1016/j.phytochem.2007.07.014. [DOI] [PubMed] [Google Scholar]
- George TK, Joy A, Divya K, Jisha MS. In vitro and in silico docking studies of antibacterial compounds derived from endophytic Penicillium setosum. Microb Pathog. 2019;131:87–97. doi: 10.1016/j.micpath.2019.03.033. [DOI] [PubMed] [Google Scholar]
- Gibbons SM, Gilbert JA. Microbial diversity—exploration of natural ecosystems and microbiomes. Curr Opin Genet Dev. 2015;35:66–72. doi: 10.1016/j.gde.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert JA, Neufeld JD. Life in a world without microbes. PLoS Biol. 2014;12(12):e1002020. doi: 10.1371/journal.pbio.1002020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez MD, Ventimilla D, Sacristan R, Perez-Amador MA. Gibberellins regulate ovule integument development by interfering with the transcription factor ATS. Plant Physiol. 2016;172(4):2403–2415. doi: 10.1104/pp.16.01231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Cheng F, Zou K, Wang J, She Z, Lin Y. Secondary metabolites from the mangrove endophytic fungus Penicillium sp. (SBE-8) Nat Prod Commun. 2009;4(11):1481–1483. [PubMed] [Google Scholar]
- Guo DL, Qiu L, Feng D, He X, Li XH, Cao ZX, Gu YC, Mei L, Deng F, Deng Y. Three new ɑ-pyrone derivatives induced by chemical epigenetic manipulation of Penicillium herquei, an endophytic fungus isolated from Cordyceps sinensis. Nat Prod Res. 2019;2:1–7. doi: 10.1080/14786419.2018.1544974. [DOI] [PubMed] [Google Scholar]
- Gupta M, Saxena S, Goyal D. Potential pancreatic lipase inhibitory activity of an endophytic Penicillium species. J Enzyme Inhib Med Chem. 2015;30(1):15–21. doi: 10.3109/14756366.2013.871007. [DOI] [PubMed] [Google Scholar]
- Hamayun M, Khan SA, Iqbal I, Ahmad B, Lee IJ. Isolation of a gibberellin-producing fungus (Penicillium sp. MH7) and growth promotion of Crown daisy (Chrysanthemum coronarium) J Microbiol Biotechnol. 2010;20(1):202–207. [PubMed] [Google Scholar]
- Hassan SE. Plant growth-promoting activities for bacterial and fungal endophytes isolated from medicinal plant of Teucrium polium L. J Adv Res. 2017;8(6):687–695. doi: 10.1016/j.jare.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawas UW, El-Halawany AM, Ahmed EF. Hepatitis C virus NS3-NS4A protease inhibitors from the endophytic Penicillium chrysogenum isolated from the red alga Liagora viscida. Z Naturforsch C. 2013;68(9–10):355–366. [PubMed] [Google Scholar]
- He KY, Zhang C, Duan YR, Huang GL, Yang CY, Lu XR, Zheng CJ, Chen GY. New chlorinated xanthone and anthraquinone produced by a mangrove-derived fungus Penicillium citrinum HL-5126. J Antibiot (Tokyo) 2017;70(7):823–827. doi: 10.1038/ja.2017.52. [DOI] [PubMed] [Google Scholar]
- Hu XY, Meng LH, Li X, Yang SQ, Li XM, Wang BG. Three new indole diterpenoids from the sea-anemone-derived fungus Penicillium sp. AS-79. Mar Drugs. 2017;15(5):137. doi: 10.3390/md15050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Feng X, Xiao Z, Liu L, Li H, Ma L, Lu Y, Ju J, She Z, Lin Y. Azaphilones and p-terphenyls from the mangrove endophytic fungus Penicillium chermesinum (ZH4-E2) isolated from the South China Sea. J Nat Prod. 2011;74(5):997–1002. doi: 10.1021/np100889v. [DOI] [PubMed] [Google Scholar]
- Huang Z, Yang J, Cai X, She Z, Lin Y. A new furanocoumarin from the mangrove endophytic fungus Penicillium sp (ZH16) Nat Prod Res. 2012;26(14):1291–1295. doi: 10.1080/14786419.2011.569502. [DOI] [PubMed] [Google Scholar]
- Huang X, Sun X, Ding B, Lin M, Liu L, Huang H, She Z. A new anti-acetylcholinesterase α-pyrone meroterpene, arigsugacin I, from mangrove endophytic fungus Penicillium sp. sk5GW1L of Kandelia candel. Planta Med. 2013;79(16):1572–1575. doi: 10.1055/s-0033-1350896. [DOI] [PubMed] [Google Scholar]
- Huang Q, An H, Song H, Mao H, Shen W, Dong J. Diversity and biotransformative potential of endophytic fungi associated with the medicinal plant Kadsura angustifolia. Res Microbiol. 2015;166(1):45–55. doi: 10.1016/j.resmic.2014.12.004. [DOI] [PubMed] [Google Scholar]
- Huang S, Chen H, Li W, Zhu X, Ding W, Li C. Bioactive Chaetoglobosins from the mangrove endophytic fungus Penicillium chrysogenum. Mar Drugs. 2016;14(10):172. doi: 10.3390/md14100172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikram M, Ali N, Jan G, Jan FG, Rahman IU, Iqbal A, Hamayun M. IAA producing fungal endophyte Penicillium roqueforti Thom., enhances stress tolerance and nutrients uptake in wheat plants grown on heavy metal contaminated soils. PLoS One. 2018;13(11):e0208150. doi: 10.1371/journal.pone.0208150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang CS, Zhou ZF, Yang XH, Lan LF, Gu YC, Ye BP, Guo YW. Antibacterial sorbicillin and diketopiperazines from the endogenous fungus Penicillium sp. GD6 associated Chinese mangrove Bruguiera gymnorrhiza. Chin J Nat Med. 2018;16(5):358–365. doi: 10.1016/S1875-5364(18)30068-2. [DOI] [PubMed] [Google Scholar]
- Jin PF, Zuo WJ, Guo ZK, Mei WL, Dai HF. Metabolites from the endophytic fungus Penicillium sp FJ-1 of Ceriops tagal. Yao Xue Xue Bao. 2013;48(11):1688–1691. [PubMed] [Google Scholar]
- Jongen V, Thijssen J, Hollema H, Donker GH, Santema JG, Van der Zee AG, Heineman MJ. Is aromatase cytochrome P450 involved in the pathogenesis of endometrioid endometrial cancer? Int J Gynecol Cancer. 2005;15:529–536. doi: 10.1111/j.1525-1438.2005.15320.x. [DOI] [PubMed] [Google Scholar]
- Jouda JB, Mawabo IK, Notedji A, Mbazoa CD, Nkenfou J, Wandji J, Nkenfou CN. Anti-mycobacterial activity of polyketides from Penicillium sp. endophyte isolated from Garcinia nobilis against Mycobacterium smegmatis. Int J Mycobacteriol. 2016;5(2):192–196. doi: 10.1016/j.ijmyco.2016.02.007. [DOI] [PubMed] [Google Scholar]
- Jouda JB, Tamokou JD, Mbazoa CD, Sarkar P, Bag PK, Wandji J. Anticancer and antibacterial secondary metabolites from the endophytic fungus Penicillium sp. CAM64 against multi-drug resistant Gram-negative bacteria. Afr Health Sci. 2016;16(3):734–743. doi: 10.4314/ahs.v16i3.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajula M, Ward JM, Turpeinen A, Tejesvi MV, Hokkanen J, Tolonen A, Häkkänen H, Picart P, Ihalainen J, Sahl HG, Pirttilä AM, Mattila S. Bridged epipolythiodiketopiperazines from Penicillium raciborskii, an endophytic fungus of Rhododendron tomentosum Harmaja. J Nat Prod. 2016;79(4):685–690. doi: 10.1021/np500822k. [DOI] [PubMed] [Google Scholar]
- Katoch M, Pull S. Endophytic fungi associated with Monarda citriodora, an aromatic and medicinal plant and their biocontrol potential. Pharm Biol. 2017;55(1):1528–1535. doi: 10.1080/13880209.2017.1309054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzman R, Saitoh T. Advances in Alzheimer’s disease. FASEB J. 1991;5:278–286. [PubMed] [Google Scholar]
- Khan AL, Lee IJ. Endophytic Penicillium funiculosum LHL06 secretes gibberellin that reprograms Glycine max L. growth during copper stress. BMC Plant Biol. 2013;13:86. doi: 10.1186/1471-2229-13-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan SA, Hamayun M, Yoon H, Kim HY, Suh SJ, Hwang SK, Kim JM, Lee IJ, Choo YS, Yoon UH, Kong WS, Lee BM, Kim JG. Plant growth promotion and Penicillium citrinum. BMC Microbiol. 2008;8:231. doi: 10.1186/1471-2180-8-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AL, Hamayun M, Ahmad N, Hussain J, Kang SM, Kim YH, Adnan M, Tang DS, Waqas M, Radhakrishnan R, Hwang YH, Lee IJ. Salinity stress resistance offered by endophytic fungal interaction between Penicillium minioluteum LHL09 and Glycine max L. J Microbiol Biotechnol. 2011;21(9):893–902. doi: 10.4014/jmb.1103.03012. [DOI] [PubMed] [Google Scholar]
- Khan AL, Hamayun M, Kim YH, Kang SM, Lee IJ. Ameliorative symbiosis of endophyte (Penicillium funiculosum LHL06) under salt stress elevated plant growth of Glycine max L. Plant Physiol Biochem. 2011;49(8):852–861. doi: 10.1016/j.plaphy.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Khan AL, Waqas M, Hamayun M, Al-Harrasi A, Al-Rawahi A, Lee IJ. Co-synergism of endophyte Penicillium resedanum LK6 with salicylic acid helped Capsicum annuum in biomass recovery and osmotic stress mitigation. BMC Microbiol. 2013;13:51. doi: 10.1186/1471-2180-13-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AL, Waqas M, Khan AR, Hussain J, Kang SM, Gilani SA, Hamayun M, Shin JH, Kamran M, Al-Harrasi A, Yun BW, Adnan M, Lee IJ. Fungal endophyte Penicillium janthinellum LK5 improves growth of ABA-deficient tomato under salinity. World J Microbiol Biotechnol. 2013;29(11):2133–2144. doi: 10.1007/s11274-013-1378-1. [DOI] [PubMed] [Google Scholar]
- Khan AL, Waqas M, Hussain J, Al-Harrasi A, Hamayun M, Lee IJ. Phytohormones enabled endophytic fungal symbiosis improve aluminum phytoextraction in tolerant Solanum lycopersicum: an examples of Penicillium janthinellum LK5 and comparison with exogenous GA3. J Hazard Mater. 2015;295:70–78. doi: 10.1016/j.jhazmat.2015.04.008. [DOI] [PubMed] [Google Scholar]
- Khan AL, Waqas M, Lee IJ. Resilience of Penicillium resedanum LK6 and exogenous gibberellin in improving Capsicum annuum growth under abiotic stresses. J Plant Res. 2015;128(2):259–268. doi: 10.1007/s10265-014-0688-1. [DOI] [PubMed] [Google Scholar]
- Kim JA, Son NS, Son JK, et al. Two new secoiridoid glycosides from the rhizomes of Gentiana scabra Bunge. Arch Pharm Res. 2009;32:863–867. doi: 10.1007/s12272-009-1608-0. [DOI] [PubMed] [Google Scholar]
- Kirk PM, Cannon PF, David JC, Stalpers JA. Ainsworth & Bisby’s dictionary of the fungi. 10. Wallingford: CAB International; 2008. [Google Scholar]
- Koul M, Meena S, Kumar A, Sharma PR, Singamaneni V, Riyaz-Ul-Hassan S, Hamid A, Chaubey A, Prabhakar A, Gupta P, Singh S. Secondary metabolites from endophytic fungus Penicillium pinophilum induce ROS-mediated apoptosis through mitochondrial pathway in pancreatic cancer cells. Planta Med. 2016;82(4):344–355. doi: 10.1055/s-0035-1558308. [DOI] [PubMed] [Google Scholar]
- Kuboyama T, Tohda C, Zhao J, Nakamura N, Hattori M, et al. Axon- or dendrite-predominant outgrowth induced by constituents from Ashwagandha. NeuroReport. 2002;13:1715–1720. doi: 10.1097/00001756-200210070-00005. [DOI] [PubMed] [Google Scholar]
- Kuete V, Saeed ME, Kadioglu O, Börtzler J, Khalid H, Greten HJ, Efferth T. Pharmacogenomic and molecular docking studies on the cytotoxicity of the natural steroid wortmannin against multidrug-resistant tumor cells. Phytomedicine. 2015;22(1):120–127. doi: 10.1016/j.phymed.2014.11.011. [DOI] [PubMed] [Google Scholar]
- Kurchenko IN. Polygalacturonase activity of microscopic fungi of different trophic groups. Mikrobiol Z. 2013;75(2):57–66. [PubMed] [Google Scholar]
- Kurchenko IN, Vasilevskaia AI, Artyshkova LV, Nakonechnaia LT, Iur’eva EM. Utilization of different carbon sources by soil and endophytic strains of Penicillium funiculosum Thom. Mikrobiol Z. 2013;75(3):12–23. [PubMed] [Google Scholar]
- Kuriakose GC, Lakshmanan MD, Bp A, Rs HK, Th AK, Ananthaswamy KCJ. Extract of Penicillium sclerotiorum an endophytic fungus isolated from Cassia fistula L. induces cell cycle arrest leading to apoptosis through mitochondrial membrane depolarization in human cervical cancer cells. Biomed Pharmacother. 2018;105:1062–1071. doi: 10.1016/j.biopha.2018.06.094. [DOI] [PubMed] [Google Scholar]
- Kushwaha RK, Singh S, Pandey SS, Kalra A, Babu CSV. Fungal endophytes attune withanolide biosynthesis in Withania somnifera, prime to enhanced withanolide A content in leaves and roots. World J Microbiol Biotechnol. 2019;35(2):20. doi: 10.1007/s11274-019-2593-1. [DOI] [PubMed] [Google Scholar]
- Lai D, Brötz-Oesterhelt H, Müller WEG, Wray V, Proksch P. Bioactive polyketides and alkaloids from Penicillium citrinum, a fungal endophyte isolated from Ocimum tenuiflorum. Fitoterapia. 2013;91:100–106. doi: 10.1016/j.fitote.2013.08.017. [DOI] [PubMed] [Google Scholar]
- Leitão AL, Enguita FJ. Gibberellins in Penicillium strains: challenges for endophyte-plant host interactions under salinity stress. Microbiol Res. 2016;183:8–18. doi: 10.1016/j.micres.2015.11.004. [DOI] [PubMed] [Google Scholar]
- Lennon JT, Locey KJ (2018) There are more microbial species on Earth than stars in the galaxy. Aeon, 10 Sept 2018
- Li J, Wang J, Jiang CS, Li G, Guo YW. (+)-Cyclopenol, a new naturally occurring 7-membered 2,5-dioxopiperazine alkaloid from the fungus Penicillium sclerotiorum endogenous with the Chinese mangrove Bruguiera gymnorrhiza. J Asian Nat Prod Res. 2014;16(5):542–548. doi: 10.1080/10286020.2014.911290. [DOI] [PubMed] [Google Scholar]
- Li J, Yang X, Lin Y, Yuan J, Lu Y, Zhu X, Li J, Li M, Lin Y, He J, Liu L. Meroterpenes and azaphilones from marine mangrove endophytic fungus Penicillium 303#. Fitoterapia. 2014;97:241–246. doi: 10.1016/j.fitote.2014.06.011. [DOI] [PubMed] [Google Scholar]
- Li JW, Duan RG, Zou JH, Chen RD, Chen XG, Dai JG. Meroterpenoids and isoberkedienolactone from endophytic fungus Penicillium sp associated with Dysosma versipellis. Yao Xue Xue Bao. 2014;49(6):913–920. [PubMed] [Google Scholar]
- Li XD, Miao FP, Liang XR, Ji NY. Meroterpenes from an algicolous strain of Penicillium echinulatum. Magn Reson Chem. 2014;52(5):247–250. doi: 10.1002/mrc.4049. [DOI] [PubMed] [Google Scholar]
- Li ZF, Wang LF, Feng ZL, Zhao LH, Shi YQ, Zhu HQ. Diversity of endophytic fungi from different Verticillium-wilt-resistant Gossypium hirsutum and evaluation of antifungal activity against Verticillium dahliae in vitro. J Microbiol Biotechnol. 2014;24(9):1149–1161. doi: 10.4014/jmb.1402.02035. [DOI] [PubMed] [Google Scholar]
- Li H, Doucet B, Flewelling AJ, Jean S, Webster D, Robichaud GA, Johnson JA, Gray CA. Antimycobacterial natural products from endophytes of the medicinal plant Aralia nudicaulis. Nat Prod Commun. 2015;10(10):1641–1642. [PubMed] [Google Scholar]
- Li J, Zhong YS, Yuan J, Zhu X, Lu YJ, Lin YC, Liu L. A new terminal cyano group-containing benzodiazepine alkaloid from the mangrove endophytic fungus Penicillium sp. Nat Prod Commun. 2015;10(9):1549–1551. [PubMed] [Google Scholar]
- Li X, Li XM, Zhang P, Wang BG. A new phenolic enamide and a new meroterpenoid from marine alga-derived endophytic fungus Penicillium oxalicum EN-290. J Asian Nat Prod Res. 2015;17(12):1204–1212. doi: 10.1080/10286020.2015.1117454. [DOI] [PubMed] [Google Scholar]
- Li G, Kusari S, Golz C, Laatsch H, Strohmann C, Spiteller M. Epigenetic modulation of endophytic Eupenicillium sp LG41 by a histone deacetylase inhibitor for production of decalin-containing compounds. J Nat Prod. 2017;80(4):983–988. doi: 10.1021/acs.jnatprod.6b00997. [DOI] [PubMed] [Google Scholar]
- Lin Z, Wen J, Zhu T, Fang Y, Gu Q, Zhu W. Chrysogenamide A from an endophytic fungus associated with Cistanche deserticola and its neuroprotective effect on SH-SY5Y cells. J Antibiot (Tokyo). 2008;61(2):81–85. doi: 10.1038/ja.2008.114. [DOI] [PubMed] [Google Scholar]
- Lin Z, Zhu T, Fang Y, Gu Q, Zhu W. Polyketides from Penicillium sp. JP-1, an endophytic fungus associated with the mangrove plant Aegiceras corniculatum. Phytochemistry. 2008;69(5):1273–1278. doi: 10.1016/j.phytochem.2007.10.030. [DOI] [PubMed] [Google Scholar]
- Lin ZJ, Lu ZY, Zhu TJ, Fang YC, Gu QQ, Zhu WM. Penicillenols from Penicillium sp. GQ-7, an endophytic fungus associated with Aegiceras corniculatum. Chem Pharm Bull (Tokyo). 2008;56(2):217–221. doi: 10.1248/cpb.56.217. [DOI] [PubMed] [Google Scholar]
- Liu Y, Ding G, Li Y, Qu J, Ma S, Lv H, Liu Y, Wang W, Dai J, Tang Y, Yu S. Structures and absolute configurations of penicillactones A–C from an endophytic microorganism Penicillium dangeardii Pitt. Org Lett. 2013;15(20):5206–5209. doi: 10.1021/ol4023485. [DOI] [PubMed] [Google Scholar]
- Liu JF, Chen WJ, Xin BR, Lu J. Metabolites of the endophytic fungus Penicillium sp. FJ-1 of Acanthus ilicifolius. Nat Prod Commun. 2014;9(6):799–801. [PubMed] [Google Scholar]
- Liu Y, Xia G, Li H, Ma L, Ding B, Lu Y, He L, Xia X, She Z. Vermistatin derivatives with α-glucosidase inhibitory activity from the mangrove endophytic fungus Penicillium sp. HN29-3B1. Planta Med. 2014;80(11):912–917. doi: 10.1055/s-0034-1382859. [DOI] [PubMed] [Google Scholar]
- Liu J, Xu M, Zhu MY, Feng Y. Chemoreversal metabolites from the endophytic fungus Penicillium citrinum isolated from a mangrove Avicennia marina. Nat Prod Commun. 2015;10(7):1203–1205. [PubMed] [Google Scholar]
- Liu Y, Mándi A, Li XM, Meng LH, Kurtán T, Wang BG. Peniciadametizine A, a Dithiodiketopiperazine with a unique Spiro[furan-2,7′-pyrazino[1,2-b][1,2]oxazine] skeleton, and a related analogue, Peniciadametizine B, from the marine sponge-derived fungus Penicillium adametzioides. Mar Drugs. 2015;13(6):3640–3652. doi: 10.3390/md13063640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Yang Q, Xia G, Huang H, Li H, Ma L, Lu Y, He L, Xia X, She Z. Polyketides with α-glucosidase inhibitory activity from a mangrove endophytic fungus, Penicillium sp. HN29-3B1. J Nat Prod. 2015;78(8):1816–1822. doi: 10.1021/np500885f. [DOI] [PubMed] [Google Scholar]
- Liu T, Greenslade A, Yang S. Levels of rhizome endophytic fungi fluctuate in Paris polyphylla var yunnanensis as plants age. Plant Divers. 2016;39(1):60–64. doi: 10.1016/j.pld.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Chen S, Liu W, Liu Y, Huang X, She Z. Polyketides with immunosuppressive activities from mangrove endophytic fungus Penicillium sp. ZJ-SY2. Mar Drugs. 2016;14(12):217. doi: 10.3390/md14120217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Li J, Huang M, Liu L, Wang J, Lin Y. Six new polyketide decalin compounds from mangrove endophytic fungus Penicillium aurantiogriseum 328. Mar Drugs. 2015;13(10):6306–6318. doi: 10.3390/md13106306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Qiao K, Kong Y, Li M, Guo L, Miao Z, Fan C. A new isoquinolone alkaloid from an endophytic fungus R22 of Nerium indicum. Nat Prod Res. 2016;31(8):951–958. doi: 10.1080/14786419.2016.1258556. [DOI] [PubMed] [Google Scholar]
- Ma YM, Qiao K, Kong Y, Guo LX, Li MY, Fan C. A new p-hydroxybenzoic acid derivative from an endophytic fungus Penicillium sp. of Nerium indicum. J Asian Nat Prod Res. 2017;19(12):1245–1251. doi: 10.1080/10286020.2017.1313240. [DOI] [PubMed] [Google Scholar]
- Ma YM, Qiao K, Kong Y, Li MY, Guo LX, Miao Z, Fan C. A new isoquinolone alkaloid from an endophytic fungus R22 of Nerium indicum. Nat Prod Res. 2017;31(8):951–958. doi: 10.1080/14786419.2016.1258556. [DOI] [PubMed] [Google Scholar]
- Mady MS, Mohyeldin MM, Ebrahim HY, Elsayed HE, Houssen WE, Haggag EG, Soliman RF, El Sayed KA. The indole alkaloid meleagrin, from the olive tree endophytic fungus Penicillium chrysogenum, as a novel lead for the control of c-Met-dependent breast cancer proliferation, migration and invasion. Bioorg Med Chem. 2016;24(2):113–122. doi: 10.1016/j.bmc.2015.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maehara S, Agusta A, Tokunaga Y, Shibuya H, Hata T. Endophyte composition and Cinchona alkaloid production abilities of Cinchona ledgeriana cultivated in Japan. J Nat Med. 2019;73(2):431–438. doi: 10.1007/s11418-018-1273-z. [DOI] [PubMed] [Google Scholar]
- Mahnert A, Ortega RA, Berg C, Grube M, Berg G. Leaves of indoor ornamentals are biodiversity and functional hotspots for fungi. Front Microbiol. 2018;9:2343. doi: 10.3389/fmicb.2018.02343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiti A, Cuendet M, Croy VL, Endringer DC, Pezzuto JM, Cushman M. Synthesis and biological evaluation of (±)-abyssinone II and its analogues as aromatase inhibitors for chemoprevention of breast cancer. J Med Chem. 2007;50:2799–2806. doi: 10.1021/jm070109i. [DOI] [PubMed] [Google Scholar]
- Malhadas C, Malheiro R, Pereira JA, de Pinho PG, Baptista P. Antimicrobial activity of endophytic fungi from olive tree leaves. World J Microbiol Biotechnol. 2017;33(3):46. doi: 10.1007/s11274-017-2216-7. [DOI] [PubMed] [Google Scholar]
- Manayi A, Omidpanah S, Barreca D, Ficarra S, Daglia M, Nabavi SF. Neuroprotective effects of paeoniflorin in neurodegenerative diseases of the central nervous system. Phytochem Rev. 2017;16:1173. doi: 10.1007/s11101-017-9527-z. [DOI] [Google Scholar]
- Manika N, Singh S, Verma RK, Bagchi GD. Extraction efficacy, stability assessment and seasonal variation of bioactive “gymnemagenin” in Gymnema sylvestre. Ind Crops Prod. 2013;44:572–576. doi: 10.1016/j.indcrop.2012.09.020. [DOI] [Google Scholar]
- Manjunath HM, Joshi CG, Raju NG. Biofabrication of gold nanoparticles using marine endophytic fungus—Penicillium citrinum. IET Nanobiotechnol. 2017;11(1):40–44. doi: 10.1049/iet-nbt.2016.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng LH, Li XM, Lv CT, Huang CG, Wang BG. Brocazines A–F, cytotoxic bisthiodiketopiperazine derivatives from Penicillium brocae MA-231, an endophytic fungus derived from the marine mangrove plant Avicennia marina. J Nat Prod. 2014;77(8):1921–1927. doi: 10.1021/np500382k. [DOI] [PubMed] [Google Scholar]
- Meng LH, Zhang P, Li XM, Wang BG. Penicibrocazines A–E, five new sulphide diketopiperazines from the marine-derived endophytic fungus Penicillium brocae. Mar Drugs. 2015;13(1):276–287. doi: 10.3390/md13010276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng LH, Wang CY, Mándi A, Li XM, Hu XY, Kassack MU, Kurtán T, Wang BG. Three diketopiperazine alkaloids with spirocyclic skeletons and one bisthiodiketopiperazine derivative from the mangrove-derived endophytic fungus Penicillium brocae MA-231. Org Lett. 2016;18(20):5304–5307. doi: 10.1021/acs.orglett.6b02620. [DOI] [PubMed] [Google Scholar]
- Min YJ, Park MS, Fong JJ, Quan Y, Jung S, Lim YW. Diversity and saline resistance of endophytic fungi associated with Pinus thunbergii in coastal shelterbelts of Korea. J Microbiol Biotechnol. 2014;24(3):324–333. doi: 10.4014/jmb.1310.10041. [DOI] [PubMed] [Google Scholar]
- Mishra VK, Singh G, Passari AK, Yadav MK, Gupta VK, Singh BP. Distribution and antimicrobial potential of endophytic fungi associated with ethnomedicinal plant Melastoma malabathricum L. J Environ Biol. 2016;37(2):229–237. [PubMed] [Google Scholar]
- Mishra R, Kushveer JS, Revanthbabu P, Sarma VV. Endophytic fungi and their enzymatic potential. In: Singh B, editor. Advances in endophytic fungal research fungal biology. Cham: Springer; 2019. pp. 283–337. [Google Scholar]
- Mohamed NH, Ismail MA, Abdel-Mageed WM, Mohamed Shoreit AA. Antimicrobial activity of green silver nanoparticles from endophytic fungi isolated from Calotropis procera (Ait) latex. Microbiology. 2019 doi: 10.1099/mic.0.000832. [DOI] [PubMed] [Google Scholar]
- Mourshid SS, Badr JM, Risinger AL, Mooberry SL, Youssef DT. Penicilloitins A and B, new antimicrobial fatty acid esters from a marine endophytic Penicillium species. Z Naturforsch C. 2016;71(11–12):387–392. doi: 10.1515/znc-2015-0242. [DOI] [PubMed] [Google Scholar]
- Mukherjee G, Banerjee R. Production of gallic acid: biotechnological routes (Part 1) Chim Oggi Chem Today. 2003;21:59–62. [Google Scholar]
- Neethu S, Midhun SJ, Radhakrishnan EK, Jyothis M. Green synthesized silver nanoparticles by marine endophytic fungus Penicillium polonicum and its antibacterial efficacy against biofilm forming, multidrug-resistant Acinetobacter baumanii. Microb Pathog. 2018;116:263–272. doi: 10.1016/j.micpath.2018.01.033. [DOI] [PubMed] [Google Scholar]
- Neethu S, Midhun SJ, Sunil MA, Soumya S, Radhakrishnan EK, Jyothis M. Efficient visible light induced synthesis of silver nanoparticles by Penicillium polonicum ARA 10 isolated from Chetomorpha antennina and its antibacterial efficacy against Salmonella enterica serovar Typhimurium. J Photochem Photobiol, B. 2018;180:175–185. doi: 10.1016/j.jphotobiol.2018.02.005. [DOI] [PubMed] [Google Scholar]
- Nicoletti R, Trincone A. Bioactive compounds produced by strains of Penicillium and Talaromyces of marine origin. Mar Drugs. 2016;14(2):37. doi: 10.3390/md14020037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoletti R, Fiorentino A, Scognamiglio M. Endophytism of Penicillium species in woody plants. Open Mycol J. 2014;8:1–26. [Google Scholar]
- Ohashi K, Miyagawa Y, Nakamura Y, Shibuya H. Bioproduction of bile acids and the glycine conjugates by Penicillium fungus. J Nat Med. 2008;62(1):83–86. doi: 10.1007/s11418-007-0190-3. [DOI] [PubMed] [Google Scholar]
- Oliveira CM, Silva GH, Regasini LO, Zanardi LM, Evangelista AH, Young MC, Bolzani VS, Araujo AR. Bioactive metabolites produced by Penicillium sp. 1 and sp. 2, two endophytes associated with Alibertia macrophylla (Rubiaceae) Z Naturforsch C. 2009;64(11–12):824–830. doi: 10.1515/znc-2009-11-1212. [DOI] [PubMed] [Google Scholar]
- Parthasarathy R, Sathiyabama M. Gymnemagenin-producing endophytic fungus isolated from a medicinal plant Gymnema sylvestre R.Br. Appl Biochem Biotechnol. 2014;172(6):3141–3152. doi: 10.1007/s12010-014-0754-0. [DOI] [PubMed] [Google Scholar]
- Peyrat-Maillard MN, Bonnely S, Berset C. Determination of the antioxidant activity of phenolic compounds by coulometric detection. Talanta. 2000;51:709–716. doi: 10.1016/s0039-9140(99)00331-8. [DOI] [PubMed] [Google Scholar]
- Phongpaichit S, Nikom J, Rungjindamai N, Sakayaroj J, Hutadilok-Towatana N, Rukachaisirikul V, Kirtikara K. Biological activities of extracts from endophytic fungi isolated from Garcinia plants. FEMS Immunol Med Microbiol. 2007;51(3):517–525. doi: 10.1111/j.1574-695X.2007.00331.x. [DOI] [PubMed] [Google Scholar]
- Piska K, Gunia-Krzyżak A, Koczurkiewicz P, Wójcik-Pszczoła K, Pękala E. Piperlongumine (piplartine) as a lead compound for anticancer agents—synthesis and properties of analogues: a mini-review. Eur J Med Chem. 2018;156:13–20. doi: 10.1016/j.ejmech.2018.06.057. [DOI] [PubMed] [Google Scholar]
- Pitt JI, Samson RA, Frisvad JC. List of accepted species and their synonyms in the family Trichocomaceae. In: Samson RA, Pitt JI, editors. Integration of modern taxonomic methods for Penicillium and Aspergillus classification. Amsterdam: Harwood Academic Publishers; 2000. pp. 9–79. [Google Scholar]
- Potshangbam M, Devi SI, Sahoo D, Strobel GA. Functional characterization of endophytic fungal community associated with Oryza sativa L. and Zea mays L. Front Microbiol. 2017;8:325. doi: 10.3389/fmicb.2017.00325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi FH, Jing TZ, Wang ZX, Zhan YG. Fungal endophytes from Acer ginnala Maxim: isolation, identification and their yield of gallic acid. Lett Appl Microbiol. 2009;49(1):98–104. doi: 10.1111/j.1472-765X.2009.02626.x. [DOI] [PubMed] [Google Scholar]
- Qi B, Liu X, Mo T, Li SS, Wang J, Shi XP, Wang XH, Zhu ZX, Zhao YF, Jin HW, Tu PF, Shi SP. Nitric oxide inhibitory polyketides from Penicillium chrysogenum MT-12, an endophytic fungus isolated from Huperzia serrata. Fitoterapia. 2017;123:35–43. doi: 10.1016/j.fitote.2017.09.014. [DOI] [PubMed] [Google Scholar]
- Qi B, Liu X, Mo T, Zhu Z, Li J, Wang J, Shi X, Zeng K, Wang X, Tu P, Abe I, Shi S. 3,5-Dimethylorsellinic acid derived meroterpenoids from Penicillium chrysogenum MT-12, an endophytic fungus isolated from Huperzia serrata. J Nat Prod. 2017;80(10):2699–2707. doi: 10.1021/acs.jnatprod.7b00438. [DOI] [PubMed] [Google Scholar]
- Qi X, Li X, Zhao J, He N, Li Y, Zhang T, Wang S, Yu L, Xie Y. GKK1032C, a new alkaloid compound from the endophytic fungus Penicillium sp. CPCC 400817 with activity against methicillin-resistant S. aureus. J Antibiot (Tokyo) 2019;72(4):237–240. doi: 10.1038/s41429-019-0144-5. [DOI] [PubMed] [Google Scholar]
- Qin D, Shen W, Wang J, Han M, Chai F, Duan X, Yan X, Guo J, Gao T, Zuo S, Dong J. Enhanced production of unusual triterpenoids from Kadsura angustifolia fermented by a symbiont endophytic fungus, Penicillium sp. SWUKD4.1850. Phytochemistry. 2019;158:56–66. doi: 10.1016/j.phytochem.2018.11.005. [DOI] [PubMed] [Google Scholar]
- Qin Y, Liu X, Lin J, Huang J, Jiang X, Mo T, Xu Z, Li J, Yang R. Two new phthalide derivatives from the endophytic fungus Penicillium vulpinum isolated from Sophora tonkinensis. Nat Prod Res. 2019;5:1–7. doi: 10.1080/14786419.2019.1636237. [DOI] [PubMed] [Google Scholar]
- Qiu L, Wang P, Liao G, Zeng Y, Cai C, Kong F, Guo Z, Proksch P, Dai H, Mei W. New eudesmane-type sesquiterpenoids from the mangrove-derived endophytic fungus Penicillium sp. J-54. Mar Drugs. 2018 doi: 10.3390/md16040108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghu G, Rochwerg B, Zhang Y, Garcia CA, Azuma A, Behr J, et al. An official ATS/ERS/JRS/ALAT clinical practice guideline: treatment of idiopathic pulmonary fibrosis. An update of the 2011 clinical practice guideline. Am J Respir Crit Care Med. 2015;192(2):e3–e19. doi: 10.1164/rccm.201506-1063ST. [DOI] [PubMed] [Google Scholar]
- Rosa LH, Gonçalves VN, Caligiorne RB, Alves TM, Rabello A, Sales PA, Romanha AJ, Sobral ME, Rosa CA, Zani CL. Leishmanicidal, trypanocidal, and cytotoxic activities of endophytic fungi associated with bioactive plants in Brazil. Braz J Microbiol. 2010;41(2):420–430. doi: 10.1590/S1517-838220100002000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozman NASB, Hamin NSBMN, Ring LC, Nee TW, Mustapha MB, Yenn TW. Antimicrobial efficacy of Penicillium amestolkiae elv609 extract treated cotton fabric for diabetic wound care. Mycobiology. 2017;45(3):178–183. doi: 10.5941/myco.2017.45.3.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rukachaisirikul V, Kaeobamrung J, Panwiriyarat W, Saitai P, Sukpondma Y, Phongpaichit S, Sakayaroj J. A new pyrone derivative from the endophytic fungus Penicillium paxilli PSU-A71. Chem Pharm Bull (Tokyo). 2007;55(9):1383–1384. doi: 10.1248/cpb.55.1383. [DOI] [PubMed] [Google Scholar]
- Salazar-Cerezo S, Martinez-Montiel N, Cruz-Lopez MDC, Martinez-Contreras RD. Fungal diversity and community composition of culturable fungi in Stanhopea trigrina cast Gibberellin producers. Front Microbiol. 2018;9:612. doi: 10.3389/fmicb.2018.00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi B, Mishra AP, Nigam M, Sener B, Kilic M, Sharifi-Rad M, Fokou PVT, Martins N, Sharifi-Rad J. Resveratrol: a double-edged sword in health benefits. Biomedicines. 2018;6(3):91. doi: 10.3390/biomedicines6030091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmeda-Hirschmann G, Hormazabal E, Rodriguez JA, Theoduloz C. Cycloaspeptide A and pseurotin A from the endophytic fungus Penicillium janczewskii. Z Naturforsch C. 2008;63(5–6):383–388. doi: 10.1515/znc-2008-5-612. [DOI] [PubMed] [Google Scholar]
- Seetharaman P, Gnanasekar S, Chandrasekaran R, Chandrakasan G, Syed A, Hodhod MS, Ameen F, Sivaperumal S. Isolation of limonoid compound (Hamisonine) from endophytic fungi Penicillium oxalicum LA-1 (KX622790) of Limonia acidissima L. for its larvicidal efficacy against LF vector, Culex quinquefasciatus (Diptera: Culicidae) Environ Sci Pollut Res Int. 2017;24(26):21272–21282. doi: 10.1007/s11356-017-9770-2. [DOI] [PubMed] [Google Scholar]
- Shao CL, Wang CY, Gu YC, Wei MY, Pan JH, Deng DS, She ZG, Lin YC. Penicinoline, a new pyrrolyl 4-quinolinone alkaloid with an unprecedented ring system from an endophytic fungus Penicillium sp. Bioorg Med Chem Lett. 2010;20(11):3284–3286. doi: 10.1016/j.bmcl.2010.04.043. [DOI] [PubMed] [Google Scholar]
- Shi J, Zeng Q, Liu Y, Pan Z. Alternaria sp MG1, a resveratrol-producing fungus: isolation, identification, and optimal cultivation conditions for resveratrol production. Appl Microbiol Biotechnol. 2012;95(2):369–379. doi: 10.1007/s00253-012-4045-9. [DOI] [PubMed] [Google Scholar]
- Shi Y, Xie H, Cao L, Zhang R, Xu Z, Wang Z, Deng Z. Effects of Cd- and Pb-resistant endophytic fungi on growth and phytoextraction of Brassica napus in metal-contaminated soils. Environ Sci Pollut Res Int. 2017;24(1):417–426. doi: 10.1007/s11356-016-7693-y. [DOI] [PubMed] [Google Scholar]
- Shiono Y, Muslihah NI, Suzuki T, Ariefta NR, Anwar C, Nurjanto HH, Aboshi T, Murayama T, Tawaraya K, Koseki T, Yoshida J, Usukhbayar N, Uesugi S, Kimura KI. New eremophilane and dichlororesorcinol derivatives produced by endophytes isolated from Ficus ampelas. J Antibiot (Tokyo). 2017;70(12):1133–1137. doi: 10.1038/ja.2017.125. [DOI] [PubMed] [Google Scholar]
- Silva LF, Freire KTLS, Araújo-Magalhães GR, Agamez-Montalvo GS, Sousa MA, Costa-Silva TA, Paiva LM, Pessoa-Junior A, Bezerra JDP, Souza-Motta CM. Penicillium and Talaromyces endophytes from Tillandsia catimbauensis, a bromeliad endemic in the Brazilian tropical dry forest, and their potential for L-asparaginase production. World J Microbiol Biotechnol. 2018;34(11):162. doi: 10.1007/s11274-018-2547-z. [DOI] [PubMed] [Google Scholar]
- Singh D, Rathod V, Ninganagouda S, Hiremath J, Singh AK, Mathew J. Optimization and characterization of silver nanoparticle by endophytic fungi Penicillium sp. isolated from Curcuma longa (Turmeric) and application studies against MDR E. coli and S. aureus. Bioinorg Chem Appl. 2014;2014:408021. doi: 10.1155/2014/408021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soca-Chafre G, Rivera-Orduña FN, Hidalgo-Lara ME, Hernandez-Rodriguez C, Marsch R, Flores-Cotera LB. Molecular phylogeny and paclitaxel screening of fungal endophytes from Taxus globosa. Fungal Biol. 2011;115(2):143–156. doi: 10.1016/j.funbio.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Song YX, Ma Q, Li J. A new aurone glycoside with antifungal activity from marine-derived fungus Penicillium sp. FJ-1. Zhongguo Zhong Yao Za Zhi. 2015;40(6):1097–1101. [PubMed] [Google Scholar]
- Sultan S, Noor MZ, el Anouar H, Shah SA, Salim F, Rahim R, Al Trabolsy ZB, Weber JF. Structure and absolute configuration of 20β-Hydroxyprednisolone, a biotransformed product of predinisolone by the marine endophytic fungus Penicillium lapidosum. Molecules. 2014;19(9):13775–13787. doi: 10.3390/molecules190913775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun SS, Chen XM, Guo SX. Analysis of endophytic fungi in roots of Santalum album Linn. and its host plant Kuhnia rosmarinifolia Vent. J Zhejiang Univ Sci B. 2014;15(2):109–115. doi: 10.1631/jzus.B1300011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Kong X, Gao H, Zhu T, Wu G, Gu Q, Li D. Two new meroterpenoids produced by the endophytic fungus Penicillium sp. SXH-65. Arch Pharm Res. 2014;37(8):978–982. doi: 10.1007/s12272-013-0268-2. [DOI] [PubMed] [Google Scholar]
- Sun W, Chen X, Tong Q, Zhu H, He Y, Lei L, Xue Y, Yao G, Luo Z, Wang J, Li H, Zhang Y. Novel small molecule 11β-HSD1 inhibitor from the endophytic fungus Penicillium commune. Sci Rep. 2016;6:26418. doi: 10.1038/srep26418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, Monnet D, Pulcini C, Kahlmeter G, Kluytmans J, Carmeli Y, WHO Pathogens Priority List Working Group et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018;18(3):318–327. doi: 10.1016/S1473-3099(17)30753-3. [DOI] [PubMed] [Google Scholar]
- Tang JW, Kong LM, Zu WY, Hu K, Li XN, Yan BC, Wang WG, Sun HD, Li Y, Puno PT. Isopenicins A–C: two types of antitumor meroterpenoids from the plant endophytic fungus Penicillium sp. sh18. Org Lett. 2019;21(3):771–775. doi: 10.1021/acs.orglett.8b04020. [DOI] [PubMed] [Google Scholar]
- Tian H, Ma YJ, Li WY, Wang JW. Efficient degradation of triclosan by an endophytic fungus Penicillium oxalicum B4. Environ Sci Pollut Res Int. 2018;25(9):8963–8975. doi: 10.1007/s11356-017-1186-5. [DOI] [PubMed] [Google Scholar]
- Toghueo RMK, Zeuko’o Mnkem E, Mbekou Kanko MI, Jesus Marie Arc-en-Ce, Ngo Mback N, Eke P, Vázquez de Aldana BR, Íñigo Z, Fekam Boyom F (2016) Antimicrobial and antiradical activities of ethyl acetate extracts from endophytic fungi isolated from Cameroonian medicinal plants. J Med Stud 4(4): 290–295
- Toghueo RMK, Zabalgogeazcoa I, Vázquez de Aldana BR, Boyom FF. Enzymatic activity of endophytic fungi from the medicinal plants Terminalia catappa, Terminalia mantaly and Cananga odorata. S Afr J Bot. 2017;109:146–153. [Google Scholar]
- Toghueo RMK, Kemgne EAM, Eke P, Kanko MIM, Dize D, Sahal D, Boyom FF. Antiplasmodial potential and GC–MS fingerprint of endophytic fungal extracts derived from Cameroonian Annona muricata. J Ethnopharmacol. 2019;235:111–121. doi: 10.1016/j.jep.2019.02.010. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wang G, Wang L, Xu X, Xia J, Huang X, Wu Y, Zhang C. Isolation and identification of an endophytic fungus of Polygonatum cyrtonema and its antifungal metabolites. Wei Sheng Wu Xue Bao. 2010;50(8):1036–1043. [PubMed] [Google Scholar]
- Wang Y, Zeng Q, Zhang Z, Yan R, Wang L, Du Z. Isolation of endophytic fungi from Huperzia serrata and their acetylcholinesterase inhibitory activity. Zhongguo Zhong Yao Za Zhi. 2011;36(6):734–740. [PubMed] [Google Scholar]
- Wang Y, Zeng QG, Zhang ZB, Yan RM, Wang LY, Zhu D. Isolation and characterization of endophytic huperzine A-producing fungi from Huperzia serrata. J Ind Microbiol Biotechnol. 2011;38(9):1267–1278. doi: 10.1007/s10295-010-0905-4. [DOI] [PubMed] [Google Scholar]
- Wang X, Wang H, Liu T, Xin Z. A PKS I gene-based screening approach for the discovery of a new polyketide from Penicillium citrinum Salicorn 46. Appl Microbiol Biotechnol. 2014;98(11):4875–4885. doi: 10.1007/s00253-014-5572-3. [DOI] [PubMed] [Google Scholar]
- Wang W, Zhai Y, Cao L, Tan H, Zhang R. Endophytic bacterial and fungal microbiota in sprouts, roots and stems of rice (Oryza sativa L.) Microbiol Res. 2016;188–189:1–8. doi: 10.1016/j.micres.2016.04.009. [DOI] [PubMed] [Google Scholar]
- Wang WG, Li A, Yan BC, Niu SB, Tang JW, Li XN, Du X, Challis GL, Che Y, Sun HD, Pu JX. LC–MS-guided isolation of Penicilfuranone A: a new antifibrotic furancarboxylic acid from the plant endophytic fungus Penicillium sp. sh18. J Nat Prod. 2016;79(1):149–155. doi: 10.1021/acs.jnatprod.5b00814. [DOI] [PubMed] [Google Scholar]
- Wang XJ, Min CL, Han PL, Qian J. Identification and fermentation optimization of an endophytic fungus BLH34 with antibacterial activity from Macleaya cordata. Zhong Yao Cai. 2016;39(8):1728–1733. [PubMed] [Google Scholar]
- Wang X, Li J, Yu S, Ye L, Feng M, Li J. Peniproline A, a new 1-phenylamino-2-pyrrolidone metabolite from the endophytic fungus Penicillium decumbens CP-4. Nat Prod Res. 2017;31(15):1772–1777. doi: 10.1080/14786419.2017.1290623. [DOI] [PubMed] [Google Scholar]
- Wang YN, Xia GY, Wang LY, Ge GB, Zhang HW, Zhang JF, Wu YZ, Lin S. Purpurolide A, 5/5/5 spirocyclic sesquiterpene lactone in nature from the endophytic fungus Penicillium purpurogenum. Org Lett. 2018;20(22):7341–7344. doi: 10.1021/acs.orglett.8b03323. [DOI] [PubMed] [Google Scholar]
- Waqas M, Khan AL, Kamran M, Hamayun M, Kang SM, Kim YH, Lee IJ. Endophytic fungi produce gibberellins and indoleacetic acid and promotes host-plant growth during stress. Molecules. 2012;17(9):10754–10773. doi: 10.3390/molecules170910754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waqas M, Khan AL, Hamayun M, Shahzad R, Kang SM, Kim JG, et al. Endophytic fungi promote plant growth and mitigate the adverse effects of stem rot: an example of Penicillium citrinum and Aspergillus terreus. J Plant Interact. 2015;10:280–287. [Google Scholar]
- Wen Y, Lv Y, Hao J, Chen H, Huang Y, Liu C, Huang H, Ma Y, Yang X. Two new compounds of Penicillium polonicum, an endophytic fungus from Camptotheca acuminata Decne. Nat Prod Res. 2019;13:1–5. doi: 10.1080/14786419.2019.1569003. [DOI] [PubMed] [Google Scholar]
- WHO (2018) Diabetes. https://www.who.int/news-room/fact-sheets/detail/diabetes. Accessed 30 Oct 2018
- WHO (2019) Hepatitis C. https://www.who.int/news-room/fact-sheets/detail/hepatitis-c. Accessed 9 Jul 2019
- Wu H, Yang H, You X, Li Y. Isolation and characterization of saponin-producing fungal endophytes from Aralia elata in Northeast China. Int J Mol Sci. 2012;13(12):16255–16266. doi: 10.3390/ijms131216255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YY, Zhang TY, Zhang MY, Cheng J, Zhang YX. An endophytic fungi of Ginkgo biloba L. produces antimicrobial metabolites as potential inhibitors of FtsZ of Staphylococcus aureus. Fitoterapia. 2018;128:265–271. doi: 10.1016/j.fitote.2018.05.033. [DOI] [PubMed] [Google Scholar]
- Wubshet SG, Nyberg NT, Tejesvi MV, Pirttilä AM, Kajula M, Mattila S, Staerk D. Targeting high-performance liquid chromatography–high-resolution mass spectrometry-solid-phase extraction-nuclear magnetic resonance analysis with high-resolution radical scavenging profiles—bioactive secondary metabolites from the endophytic fungus Penicillium namyslowskii. J Chromatogr A. 2013;1302:34–39. doi: 10.1016/j.chroma.2013.05.032. [DOI] [PubMed] [Google Scholar]
- Xie J, Wu YY, Zhang TY, Zhang MY, Peng F, Lin B, Zhang YX. New antimicrobial compounds produced by endophytic Penicillium janthinellum isolated from Panax notoginseng as potential inhibitors of FtsZ. Fitoterapia. 2018;131:35–43. doi: 10.1016/j.fitote.2018.10.006. [DOI] [PubMed] [Google Scholar]
- Xu F, Cao H, Cui X, Guo H, Han C. Optimization of fermentation condition for Echinacoside yield improvement with Penicillium sp. H1, an endophytic fungus isolated from Ligustrum lucidum ait using response surface methodology. Molecules. 2018;23(10):E2586. doi: 10.3390/molecules23102586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Huang R, Qiu SX, She Z, Lin Y. A new isobenzofuranone from the mangrove endophytic fungus Penicillium sp. (ZH58) Nat Prod Res. 2013;27(20):1902–1905. doi: 10.1080/14786419.2013.784870. [DOI] [PubMed] [Google Scholar]
- Yang Y, Zhao H, Barrero RA, Zhang B, Sun G, Wilson IW, Xie F, Walker KD, Parks JW, Bruce R, Guo G, Chen L, Zhang Y, Huang X, Tang Q, Liu H, Bellgard MI, Qiu D, Lai J, Hoffman A. Genome sequencing and analysis of the paclitaxel-producing endophytic fungus Penicillium aurantiogriseum NRRL 62431. BMC Genomics. 2014;15:69. doi: 10.1186/1471-2164-15-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Yang F, Zhao L, Duang R, Chen G, Li X, Li Q, Qin S, Ding Z. A new polyoxygenated farnesylcyclohexenone from fungus Penicillium sp. Nat Prod Res. 2016;30(1):65–68. doi: 10.1080/14786419.2015.1034712. [DOI] [PubMed] [Google Scholar]
- Yang MH, Li TX, Wang Y, Liu RH, Luo J, Kong LY. Antimicrobial metabolites from the plant endophytic fungus Penicillium sp. Fitoterapia. 2017;116:72–76. doi: 10.1016/j.fitote.2016.11.008. [DOI] [PubMed] [Google Scholar]
- You YH, Yoon H, Kang SM, Shin JH, Choo YS, Lee IJ, Lee JM, Kim JG. Fungal diversity and plant growth promotion of endophytic fungi from six halophytes in Suncheon Bay. J Microbiol Biotechnol. 2012;22(11):1549–1556. doi: 10.4014/jmb.1205.05010. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Tian JM, Xiao J, Shao Q, Gao JM. Bioactive metabolites isolated from Penicillium sp. YY-20, the endophytic fungus from Ginkgo biloba. Nat Prod Res. 2014;28(4):278–281. doi: 10.1080/14786419.2013.850686. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Feng H, Wang L, Li Z, Shi Y, Zhao L, Feng Z, Zhu H. Potential of endophytic fungi isolated from cotton roots for biological control against Verticillium Wilt disease. PLoS One. 2017;12(1):e0170557. doi: 10.1371/journal.pone.0170557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurieva OM, Kurchenko IM, Syrchin SO, Kharkevych OS, Pavlychenko AK, Nakonechna LT. Cellulase and xylanase activities of endophytic and soil Penicillium funiculosum strains. Mikrobiol Z. 2016;78(5):75–82. [PubMed] [Google Scholar]
- Zeng WL, Li WK, Han H, Tao YY, Yang L, Wang ZT, Chen KX. Microbial biotransformation of gentiopicroside by the endophytic fungus Penicillium crustosum 2T01Y01. Appl Environ Microbiol. 2014;80(1):184–192. doi: 10.1128/aem.02309-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Mu J, Feng Y, Kang Y, Zhang J, Gu PJ, Wang Y, Ma LF, Zhu YH. Broad-spectrum antimicrobial epiphytic and endophytic fungi from marine organisms: isolation, bioassay and taxonomy. Mar Drugs. 2009;7(2):97–112. doi: 10.3390/md7020097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao DL, Yuan XL, Du YM, Zhang ZF, Zhang P. Benzophenone derivatives from an algal-endophytic isolate of Penicillium chrysogenum and their cytotoxicity. Molecules. 2018;23(12):E3378. doi: 10.3390/molecules23123378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Yuan LY, Guo DL, Ye Y, Da-Wa ZM, Wang XL, Ma FW, Chen L, Gu YC, Ding LS, Zhou Y. Bioactive halogenated dihydroisocoumarins produced by the endophytic fungus Lachnum palmae isolated from Przewalskia tangutica. Phytochemistry. 2018;148:97–103. doi: 10.1016/j.phytochem.2018.01.018. [DOI] [PubMed] [Google Scholar]
- Zhao T, Xu LL, Zhang Y, Lin ZH, Xia T, Yang DF, Chen YM, Yang XL. Three new α-pyrone derivatives from the plant endophytic fungus Penicillium ochrochloronthe and their antibacterial, antifungal, and cytotoxic activities. J Asian Nat Prod Res. 2019;21(9):851–858. doi: 10.1080/10286020.2018.1495197. [DOI] [PubMed] [Google Scholar]
- Zheng CJ, Li L, Zou JP, Han T, Qin LP. Identification of a quinazoline alkaloid produced by Penicillium vinaceum, an endophytic fungus from Crocus sativus. Pharm Biol. 2012;50(2):129–133. doi: 10.3109/13880209.2011.569726. [DOI] [PubMed] [Google Scholar]
- Zheng CJ, Xu LL, Li YY, Han T, Zhang QY, Ming QL, Rahman K, Qin LP. Cytotoxic metabolites from the cultures of endophytic fungi from Panax ginseng. Appl Microbiol Biotechnol. 2013;97(17):7617–7625. doi: 10.1007/s00253-013-5015-6. [DOI] [PubMed] [Google Scholar]
- Zheng CJ, Huang GL, Xu Y, Song XM, Yao J, Liu H, Wang RP, Sun XP. A new benzopyrans derivatives from a mangrove-derived fungus Penicillium citrinum from the South China Sea. Nat Prod Res. 2016;30(7):821–825. doi: 10.1080/14786419.2015.1072712. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Dai JJ, Guan YX, Jiang YY, Xing QQ. Isolation, identification and inhibition activity of endophytes in Fengdan. Zhongguo Zhong Yao Za Zhi. 2016;41(1):45–50. doi: 10.4268/cjcmm20160109. [DOI] [PubMed] [Google Scholar]
- Zheng CJ, Liao HX, Mei RQ, Huang GL, Yang LJ, Zhou XM, Shao TM, Chen GY, Wang CY. Two new benzophenones and one new natural amide alkaloid isolated from a mangrove-derived Fungus Penicillium citrinum. Nat Prod Res. 2019;33(8):1127–1134. doi: 10.1080/14786419.2018.1460832. [DOI] [PubMed] [Google Scholar]
- Zhu M, Zhang X, Feng H, Dai J, Li J, Che Q, Gu Q, Zhu T, Li D. Penicisulfuranols A–F, alkaloids from the mangrove endophytic fungus Penicillium janthinellum HDN13-309. J Nat Prod. 2017;80(1):71–75. doi: 10.1021/acs.jnatprod.6b00483. [DOI] [PubMed] [Google Scholar]
- Zhu X, Zhou D, Liang F, Wu Z, She Z, Li C. Penochalasin K, a new unusual chaetoglobosin from the mangrove endophytic fungus Penicillium chrysogenum V11 and its effective semi-synthesis. Fitoterapia. 2017;123:23–28. doi: 10.1016/j.fitote.2017.09.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.