Abstract
GABAergic interneurons in the hippocampus are critically involved in almost all hippocampal circuit functions including coordinated network activity. Somatostatin-expressing orienslacunosum moleculare (O-LM) interneurons are a major subtype of dendritically projecting interneurons in hippocampal subregions (e.g., CA1), and express group I metabotropic glutamate receptors (mGluRs), specifically mGluR1 and mGluR5. Group I mGluRs are thought to regulate hippocampal circuit functions partially through GABAergic interneurons. Previous studies suggest that a group I/II mGluR agonist produces slow supra-threshold membrane oscillations (<0.1 Hz), which are associated with high-frequency action potential (AP) discharges in O-LM interneurons. However, the properties and underlying mechanisms of these slow oscillations remain largely unknown. We performed whole-cell patch-clamp recordings from mouse interneurons in the stratum oriens/alveus (O/A interneurons) including CA1 O-LM interneurons. Our study revealed that the selective mGluR1/5 agonist (S)-3,5-dihydroxyphenylglycine (DHPG) induced slow membrane oscillations (< 0.1 Hz), which were associated with gamma frequency APs followed by AP-free perithreshold gamma oscillations. The selective mGluR1 antagonist (S)-(+)-α-Amino-4-carboxy-2-methylbenzeneacetic acid (LY367385) reduced the slow oscillations, and the selective mGluR5 antagonist 2-methyl-6-(phenylethynyl)pyridine hydrochloride (MPEP) partially blocked them. Blockade of nonselective cation-conducting transient receptor potential channels, L-type Ca2+ channels, or ryanodine receptors all abolished the slow oscillations, suggesting the involvement of multiple mechanisms. Our findings suggest that group I mGluR activation in O/A interneurons may play an important role in coordinated network activity, and O/A interneuron vulnerability to excitotoxicity, in disease states like seizures, is at least in part due to an excessive rise in intracellular Ca2+.
Keywords: Somatostatin-expressing interneurons, metabotropic glutamate receptors, perithreshold membrane oscillations, excitotoxicity, seizures
1. Introduction
GABAergic interneurons are known to contribute to almost all aspects of cortical circuit functions through their molecular, anatomical, and physiological diversity (Pelkey et al., 2017). In the hippocampus, distinct subtypes of GABAergic interneurons perform specific circuit operations, including coordinated network oscillations (e.g., theta and gamma network oscillations) and feedback/feedforward inhibition (Freund and Katona, 2007; Klausberger and Somogyi, 2008; Pelkey et al., 2017). GABAergic interneurons in the hippocampus express a variety of cell type-specific G protein-coupled receptors that regulate neurotransmission and excitability through extrinsic (e.g., acetylcholine and serotonin) and endogenous (e.g., endocannabinoids) neuromodulators (Armstrong and Soltesz, 2012; Freund and Katona, 2007; Pelkey et al., 2017). In addition to neuromodulators, glutamate binds to a group of G protein-coupled metabotropic glutamate receptors (mGluRs) that are expressed in certain types of hippocampal interneurons (Niswender and Conn, 2010; Shigemoto et al., 1997; van Hooft et al., 2000). mGluRs can be divided into three subgroups, groups I-III, based on their amino-acid sequence homology and G protein coupling (Yin and Niswender, 2014). Group I mGluRs, specifically mGluR1 and mGluR5, are highly expressed in GABAergic interneurons in hippocampal subregions (e.g., CA1) (Ferraguti et al., 2004; Lujan et al., 1996; Nagy et al., 2013; Shigemoto et al., 1997; van Hooft et al., 2000), where they regulate interneuron excitability, synaptic plasticity, and coordinated network oscillations (Kullmann and Lamsa, 2007; Le Duigou et al., 2015, 2011; Mcbain et al., 1994; Peterfi et al., 2012; Topolnik et al., 2006; Woodhall et al., 1999).
Oriens lacunosum-moleculare (O-LM) interneurons are one of the major subtypes of dendritically projecting interneurons that express somatostatin in the CA1 subregion of the hippocampus (Katona et al., 1999; Mcbain et al., 1994; Sik et al., 1995). As the name “O-LM interneuron” indicates, this interneuron subtype typically has its soma and dendrites in the stratum oriens, and its axons are predominantly localized in the stratum lacunosum moleculare, forming inhibitory synapses primarily on the distal apical dendrite tuft of pyramidal cells (Katona et al., 1999). O-LM interneurons receive excitatory inputs originating primarily from CA1 pyramidal cells (Blasco-Ibanez and Freund, 1995). This unique characteristic allows O-LM interneurons to perform a prototypical feedback inhibitory circuit operation, which plays a key role in gating external glutamatergic inputs from the entorhinal cortex (Katona et al., 1999; Pelkey et al., 2017). O-LM interneurons are also known to participate in hippocampal network oscillations (e.g., theta and gamma oscillations) (Katona et al., 2014; Varga et al., 2012). They express one of the alternative splicing isoforms of mGluR1, mGluR1α, and mGluR5 (Ferraguti et al., 2004; Lujan et al., 1996; Nagy et al., 2013; Shigemoto et al., 1997; van Hooft et al., 2000). Published studies suggest that group I mGluRs in hippocampal interneurons contribute not only to synchronized network oscillations induced by mGluR activation (Martin, 2001; Pálhalmi et al., 2004; Whittington et al., 1995), but also to their vulnerability to excitotoxicity in pathological conditions such as seizures (Sanon et al., 2010).
The group I/II mGluR agonist, 1S,3R-1-aminocyclopentane-1,3-dicarboxylic acid (ACPD), is known to induce membrane potential oscillations selectively in oriens/alveus (O/A) interneurons including CA1 O-LM interneurons, but not in other interneuron subtypes (Mcbain et al., 1994; van Hooft et al., 2000; Woodhall et al., 1999). The same studies also revealed that slow oscillations are associated with high-frequency action potential (AP) discharges during suprathreshold depolarizing phases. However, the properties and underlying mechanisms of mGluR-mediated membrane oscillations remain largely unknown. In this study, we performed whole-cell patch-clamp recordings from O/A interneurons (including O-LM interneurons) in the CA1 subregion of the mouse hippocampus to examine the actions and underlying mechanisms of group I mGluRs on membrane potential oscillations. The data show that mGluR1 and mGluR5 activation in CA1 O/A interneurons generate intrinsic slow membrane oscillations that are associated with intrinsic gamma oscillations during depolarizing phases. We also found that non-selective transient receptor potential (TRP) channels, voltage-gated L-type Ca2+ channels, and ryanodine-sensitive internal Ca2+ stores contribute to group I mGluR-mediated slow oscillations in CA1 O/A interneurons.
2. Materials and Methods
All experimental procedures were carried out in accordance with the National Institute of Health (NIH) and approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Arkansas for Medical Sciences (UAMS).
2.1. Animals
Four to eight week old C57BL/6 mice (The Jackson Laboratory stock # 000664) of either sex were used for electrophysiological recordings from CA1 O-LM interneurons. All efforts were made to minimize pain and suffering and to reduce the number of animals used.
2.2. Brain slice preparation
Brain slice preparation procedures were similar to those described previously (Kang et al., 2018). Animals were deeply anaesthetized with isoflurane then decapitated, and their brains were submerged in cold, oxygenated (95% O2 and 5% CO2) slicing medium containing (in mM): 85 NaCl, 1.25 NaH2PO4, 4 MgCl2, 0.5 CaCl2, 24 NaHCO3. 2.5 KC1, 75 sucrose, and 25 glucose. Horizontal hippocampal slices (300 μm thick) were cut using a vibrating microtome (Leica VT 1200, Leica Microsystems, Buffalo Grove, IL, USA) tissue slicer. The solution was bubbled with 95% O2 and 5% CO2. Slices were maintained at 33°C for 30 min, then at room temperature until they were used for whole-cell patch-clamp recordings.
2.3. Electrophysiology
Whole-cell patch-clamp recording procedures used in the present study were similar to those described previously (Kang et al., 2018). Electrophysiological recordings were performed from hippocampal O/A interneurons at 33°C in oxygenated (95% O2 and 5% CO2) artificial cerebrospinal fluid (ACSF) containing (in mM): 126 NaCl, 2.5 KC1, 26 NaHCO3, 2 CaCl2, 2 MgCl2, 1.25 NaH2PO4, and 10 glucose. Slices were superfused with ACSF at a rate of 2.5 ml/min. Individual neurons were visualized using an upright microscope (Eclipse FN1, Nikon Instruments Inc., Melville, NY, USA) with infrared differential interference contrast (DIC) optics. The interneuron subtypes were initially distinguished by their location, size and electrophysiological properties. A subpopulation of recorded interneurons were filled with 0.2 % biocytin, and their subtypes were later confirmed by their morphological and immunochemical properties (see the section 2.6 Histology and result section). Whole-cell patch-clamp recordings were obtained from O/A interneurons in the CA1 region of the hippocampus with borosilicate patch pipettes (3–5 MΩ) filled with an internal solution containing (in mM): 126 K-gluconate, 4 KCl, 10 HEPES, 4 ATP-Mg, 0.3 GTP-Na, and 10 phosphocreatine. The pH and osmolarity of the internal solution were adjusted to 7.2 and 290 mOsm respectively. Recordings were obtained using MultiClamp700B amplifiers (Molecular Devices, Sunnyvale, CA, USA). After forming the whole-cell configuration, the recording was allowed to rest for at least 3 min prior to data acquisition. Signals were sampled at 10 kHz, low-pass filtered at 3 KHz using Digidata 1440A analog-to-digital digitizer (Molecular Devices, Sunnyvale, CA, USA), and stored on computer for subsequent analysis using pClamp 10 software (Molecular Devices, Sunnyvale, CA,USA). Series resistances were continuously monitored and recordings were discarded if the series resistance increased >20%.
2.4. Data analyses
The data were analyzed using Clampfit 10 software (Molecular Devices). Peak depolarization amplitude was measured from baseline (resting membrane potential) following exposure to drugs. Since (S)-3,5-dihydroxyphenylglycine (DHPG) produced long-lasting membrane depolarization along with membrane potential oscillations, the peak amplitude of either oscillations or slow depolarization was considered for all drug treatments. Exposure to DHPG for 60 sec led to slow membrane oscillations for 2-5 min following application. Thus, the number of plateau-like slow oscillations were counted for 5 min after exposure to pharmacological agents. AP discharges and AP-free perithreshold oscillations of 0.5-2 sec during peak depolarization (approximately −25 mV) were used to construct the power spectra of voltage recordings using pClamp 10 software in a way similar to that previously described (Kang et al., 2018). The power spectra showed distinct peaks at gamma frequencies. Peak frequency is referred to as the maximal frequency of AP discharges or intrinsic perithreshold oscillations in O/A interneurons in response to DHPG.
2.5. Administration of pharmacological agents
Concentrated stock solutions of pharmacological agents were dissolved in appropriate diluents and stored as recommended by the manufacturer. These drugs were diluted in physiological solution to the required concentration just before use. Pharmacological agents were obtained from Tocris Bioscience (Ellisville, MO, USA), Alomone Labs (Jerusalem, Israel) or Sigma-Aldrich (St. Louis, MO, USA). 2-Amino-5-phosphonopentanoic acid (APV), (2S)-3-[[(1S)-1-(3,4- Dichlorophenyl)ethyl]amino-2-hydroxypropyl](phenylmethyl)phosphinic acid hydrochloride (CGP55845), 2,3-Dihydroxy-6-nitro-7-sulfamoyl-benzo(F) quinoxaline (NBQX), and 6-Imino-3-(4- methoxyphenyl)-1(6H)-pyridazinebutanoic acid hydrobromide (SR95531) were purchased from Alomone Laboratories. DHPG, (S)-(+)-α-Amino-4-carboxy-2-methylbenzeneacetic acid (LY 367385), 2-methyl-6-(phenylethynyl)pyridine hydrochloride (MPEP), 2-aminoethoxydiphenylborate (2-APB), flufenamic acid (FFA), Tetrodotoxin (TTX), ryanodine, nimodipine, and 1,2-Bis(2-aminophenoxy)ethane-N,N,N',N',-tetraacetic acid (BAPTA) were purchased from Tocris Bioscience. DHPG was bath applied for 45-60 s. Group I mGluRs were blocked using either LY 367385 (100 μM) or MPEP (50 μM), selective blockers of mGluR1 and mGluR5, respectively. Brain slices were either pre-incubated with mGluR antagonists or blockers (e.g., nimodipine) for 15-30 min before recording when the chemicals were used. BAPTA was included in the recording pipette.
2.6. Histology
Some putative O/A interneurons were filled with 0.2 % biocytin during recordings in order to reconstruct their morphology and test their immunopositivity for somatostatin and mGluR1α. After recording for >15 min, slices were transferred to a fixative containing 4 % paraformaldehyde and 0.2 % picric acid in 0.1 M phosphate buffer (pH 7.4) at 4°C for 2 days. Then, the slices were washed 3 times with a 0.1 M phosphate buffer (5 min incubation for each wash), and transferred to a 30 % sucrose in 0.1 M phosphate buffer for incubation at 4°C. Brain slices were embedded in O.C.T. compound (Fisher Scientific, Hampton, NH, USA) and frozen on dry ice. The frozen brain tissues in O.C.T. compound were used for cryosectioning to obtain 50 μm slices. These brain slices were used for post hoc analyses of recorded cells based on immuno-reactivities to somatostatin (EMD Millipore, Burlington, MA, USA; Clone YC7, MAB354MI; 1:1,000, rat) and mGluR1α (Frontier Institute Co. Ltd., Ishikari, Hokkaido, Japan; mGluRlα-GP-Af660; 1:1,000, guinea pig). Secondary antibodies conjugated to Alexa 488/647 (Invitrogen, Carlsbad, CA, USA) or CF633 (Biotium, Fremont, CA, USA) that were raised in goat against rat or guinea pig were used to detect the location of the primary antibodies. Streptavidin conjugated to Rhodamin Red-X (Jackson ImmunoResearch, West Grove, CA, USA; 1:500) was used to detect biocytin. All primary and secondary antibodies were diluted in Tris-buffered saline containing 0.3% Triton-X 100 and 1% normal goat serum. The sections were then mounted in Vectashield (Vector Laboratories, Burlingame, CA, USA) and subjected to Z-stack image acquisitions using a Nikon C1 confocal microscope equipped with S Fluor 40x NA 1.3 objective lens (Nikon Instruments Inc., Melville, NY, USA). To visualize the axonal and dendritic arbors, the biocytin-filled cells were subsequently treated with a Vectastain ABC kit (Vector Laboratories, Burlingame, CA, USA; PK-4000), and a DAB peroxidase substrate kit (Vector Laboratories, Burlingame, CA, USA; , SK-4100), then reconstructed using a Nikon Eclipse NiU Microscope equipped with a Y-IDT drawing tube and CFI Fluor 40× NA 0.8 objective lens (Nikon Instruments Inc., Melville, NY, USA) for detailed morphological analysis as previously described (Kang et al., 2018).
2.7. Statistical analyses
To analyze the data from whole-cell patch-clamp recordings, unpaired or paired two-tailed Student’s t tests were used. In cases where the data did not show a normal distribution on the basis of the Shapiro–Wilk test, then Mann–Whitney U or Wilcoxon’s signed-rank tests were used for unpaired and paired data, respectively. ANOVAs were followed by Tukey–Kramer test for mean comparisons. Data are presented as mean ± SEM. A p value < 0.05 was considered as significant. The number of cells tested is indicated by “n”. All statistical analyses were performed using Sigma Plot (Systat Software Inc.) or Origin Pro 2015 (OriginLab Corporation) software.
3. Results
3.1. Group I mGluR activation produces intrinsic slow oscillations in CA1 O/A interneurons
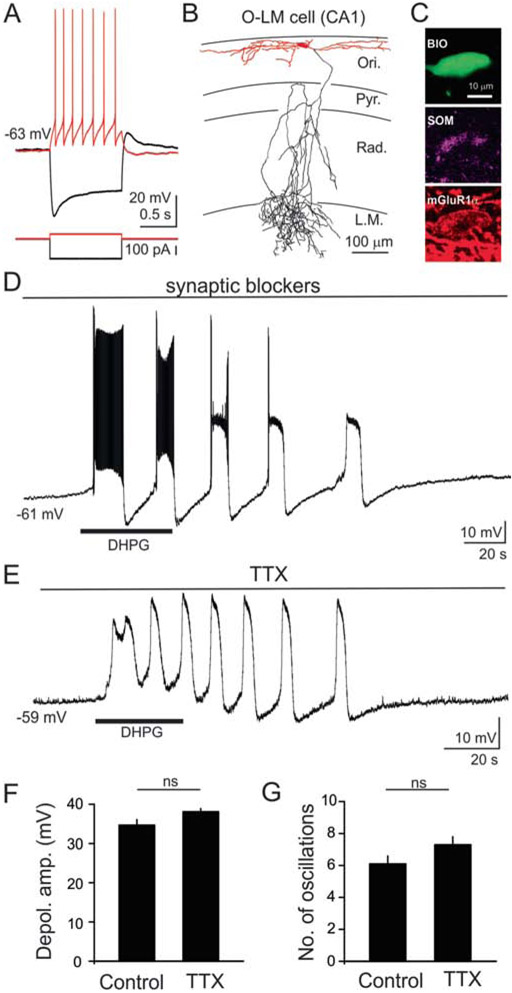
Whole-cell patch-clamp recordings were obtained from CA1 O/A interneurons. Putative O-LM interneurons were initially distinguished based on their somatic location and shape under DIC microscopy (large oval somata and horizontally projecting dendrites in the stratum oriens/alveus), and their subsequent electrophysiological properties. Under current clamp mode, we obtained voltage responses of these neurons with 1 sec hyperpolarizing and depolarizing current steps (−140 to +140 pA with 40 pA increments or −200 to +200 pA with 50 pA increments from their resting membrane potential). As previously described regarding O-LM interneurons in the stratum oriens/alveus (Maccaferri and McBain, 1996; Nicholson and Kullmann, 2013; Zhang and McBain, 1995), the recorded O/A interneurons exhibited notable membrane depolarizing “sag” upon hyperpolarization and moderate frequency accommodating AP discharges with deep hyperpolarization during sustained depolarization (Fig. 1A). If the recorded neurons did not show these characteristic electrophysiological properties, those interneurons were discarded. Fifteen O/A interneurons were filled with biocytin during whole-cell patch-clamp recordings. The cell types were post hoc confirmed by their morphological and neurochemical characteristics (Fig. 1B,C). Anatomically, the somata and horizontally projecting dendrites of O/A interneurons were found in the stratum oriens/alveus, while the axons primarily projected to the stratum lacunosum-moleculare, where they formed extensive branches in 7 of 15 O/A interneurons as previously described regarding O-LM interneurons (Mcbain et al., 1994) (Fig. 1B). In the remaining 8 of 15 O/A interneurons, no axons or truncated axons were found, probably because the axons were cut when the brains were initially sectioned. Immunohistochemical analysis revealed that almost all O/A interneurons were positive for both somatostatin (13/15 cells) and mGluR1α (15/15 cells) (Fig.1C). Thus, a subpopulation of the recorded O/A interneurons were positively identified as O-LM interneurons based on their characteristic electrophysiological, anatomical, and neurochemical properties (Baude et al., 1993; Katona et al., 1999; van Hooft et al., 2000; Varga et al., 2012). Since some of the recorded O/A cells lost their characteristic axon during the sectioning process we were not able to anatomically identified these cells as O-LM interneurons, therefore, we refer collectivly to the recorded interneurons in this study as O/A interneurons. Meanwhile, specific cells that were electrophysiologically, anatomically, and neurochemically identified as O-LM cells will be referred to as such.
Figure 1. Activation of group I mGluRs produced slow oscillations (<0.1 Hz) in O/A interneurons.
(A) Example voltage response of a CA1 O/A interneuron to hyperpolarizing and depolarizing current pulses (−200 to +50 pA, 1 sec). This interneuron exhibited a prominent membrane depolarizing “sag” upon hyperpolarization, and moderate spike frequency adaptation with deep hyperpolarization upon sustained depolarization. (B) Representative morphological reconstruction of a biocytin-filled CA1 O-LM interneuron showing extensive axonal branching in the stratum lacunosum moleculare. Axons are shown in black and dendrites in red. The soma is shown in red and located in the stratum oriens with dendrites projecting horizontally. The major axon traverses the stratum pyramidale and radiatum before forming extensive arborization in the stratum lacunosum moleculare. (C) A representative biocytin filled O-LM interneuron (top) showing expression of somatostatin (middle) and mGluR1α (bottom). (D) Sample voltage trace from an O/A interneuron revealing that exposure to DHPG (10 μM, 60 sec, indicated by bar) produced slow membrane depolarization along with oscillatory responses in the presence of synaptic blockers. These large, slow oscillatory responses (depolarization/hyperpolarization cycles) were accompanied by high frequency firing during the initial phase and increased baseline activity at later stages. (E) In the same cell, DHPG-induced oscillations persisted in the presence of the sodium channel blocker tetrodotoxin (TTX, 1 μM). (F) Histogram of population data showing peak membrane depolarization in control and in the presence of TTX. No significant differences in peak amplitude were found between control and TTX. (G) Histogram illustrating the number of oscillations induced by DHPG under control condition and in the presence of TTX. No significant differences in peak depolarization amplitude, or in the number of oscillations were observed between the control (n=14) and TTX (n=32). Abbreviations: Pyr: stratum pyramidale; Ori: stratum oriens; Rad: stratum radiatum; LM: stratum lacunosum moleculare; ns: not significant.
Previous studies showed that the group I/II mGluR agonist, 1S,3R-1-aminocyclopentane-1,3-dicarboxylic acid (ACPD), selectively produced large inward oscillatory currents or membrane potential oscillations in CA1 O/A interneurons, but not in other interneuron subtypes (Mcbain et al., 1994; van Hooft et al., 2000; Woodhall et al., 1999). We initially tested the actions of the selective group I mGluR agonist, DHPG, on membrane oscillations in O/A interneurons. To rule out the potential involvement of network activity in DHPG-mediated actions, synaptic blockers (10 μM NBQX, 10 μM APV, 10 μM SR95531, and 2 μM CGP55845) were added to the ACSF to block AMPA, NMDA, GABAA and GABAB receptors, respectively. After obtaining a stable baseline for 2–3 min, DHPG (10 μM, 60 s) was bath applied. As illustrated in Fig. 1D, DHPG produced a high-amplitude, long-lasting membrane depolarization (34.7 ± 1.4 mV, n = 11), which was associated with slow (0.03–0.08 Hz) membrane oscillations. The membrane depolarization showed oscillatory responses (depolarization/hyperpolarization cycles) for up to 5 min after the termination of DHPG application. These slow oscillations were associated with high frequency AP discharges during the early phase and increased baseline activity during later stages of depolarization (Fig. 1D). The DHPG-induced membrane depolarization and associated oscillatory responses persisted in the presence of the sodium channel blocker, TTX (1 μM; Fig. 1E). There were no significant differences in DHPG-induced peak depolarization amplitude (Control: 34.7 ± 1.4 mV, n = 11; TTX: 38.1 ± 0.8 mV, n = 32, p > 0.05, Fig. 1F), or the number of oscillations between control and TTX (Control: 6.1 ± 0.5, n = 14; TTX: 7.3 ± 0.5, n = 32, p > 0.1, Fig. 1G). These findings suggest that AP discharges are not required for intrinsically generated slow oscillations in CA1 O/A interneurons induced by group I mGluR activation.
3.2. Group I mGluR activation induces intrinsic gamma frequency oscillations in O/A interneurons
The group I/II mGluR agonist, ACPD, is known to produce suprathreshold membrane depolarization with high frequency AP discharges in CA1 O-LM interneurons (Mcbain et al., 1994; van Hooft et al., 2000). Hippocampal interneurons are known to generate intrinsic theta to gamma frequency membrane potential oscillations when membrane potentials remain close to AP threshold (Cea-del Rio et al., 2011; Chapman and Lacaille, 1999; Fuentealba et al., 2010; Kang et al., 2018). Since group I mGluR activation produced suprathreshold depolarization in O/A interneurons as described in Fig. 1, we sought to determine the frequencies of AP discharges and perithreshold membrane oscillations produced by group I mGluR activation.
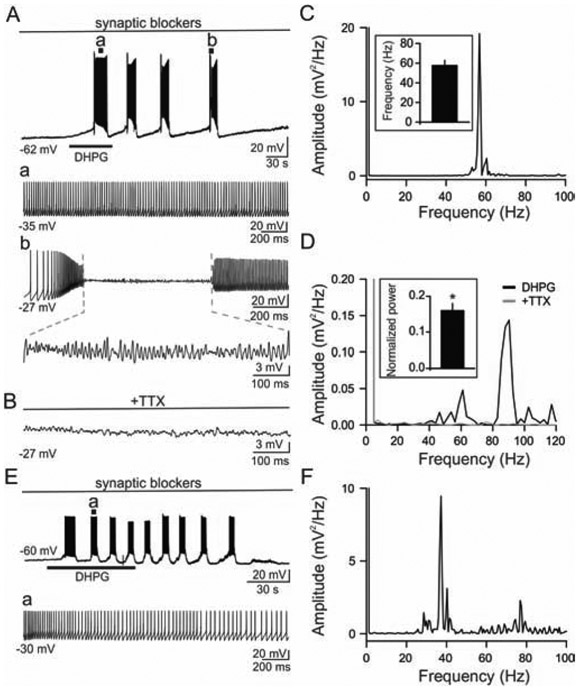
Voltage responses were obtained following bath exposure to DHPG (10 μM, 60 sec). As shown in Fig. 1D and 2A, DHPG produced multiple slow depolarization/repolarization cycles in CA1 O/A interneurons. These slow oscillations exhibited gamma frequency AP discharges during peak membrane depolarization (57.6 ± 5.3 Hz, n = 14; Fig. 2Aa, C). In addition, the slow oscillations exhibited perithreshold gamma oscillations during depolarization in 10 out of 14 O/A interneurons tested (59.5 ± 5.8 Hz, n = 10, Fig. 2Ab). In the remaining 4 O/A interneurons, DHPG produced gamma frequency AP dishcarges without perithreshold gamma oscillations during slow depolarization (Fig. 2E, F). Since persistent sodium current is known to be critically involved in intrinsic perithreshold oscillations of hippocampal interneurons (Chapman and Lacaille, 1999; Kang et al., 2018), we determined if DHPG-induced perithreshold gamma oscillations in O/A interneurons were attenuated by blockers of persistent sodium current. We found that DHPG-induced perithreshold gamma oscillations were inhibited by 1 μM TTX (Fig. 2B, D). In the presence of TTX, theta to gamma power of intrinsic subthreshold oscillations (i.e., the area under the curve of power spectrum from 4 Hz to 100 Hz) were reduced to 0.15 ± 0.02 mV2 from control conditions (1.04 ± 0.20 mV2, n = 6; p < 0.05; Fig. 2D). These results indicate that, in addition to slow oscillations, DHPG also generated AP discharges at gamma frequencies, as well as intrinsic perithreshold gamma oscillations in CA1 O/A interneurons.
Figure 2. Group I mGluR activation induced intrinsic gamma oscillations in O/A interneurons.
(A) Representative voltage trace revealing slow oscillations following exposure to DHPG (10 μM, 60 sec, indicated by bar). AP discharges (a), and perithreshold membrane oscillations (b) during the time marked by vertical lines in the top trace are shown on the fast time base. (B) Application of 1 μM TTX selectively attenuated DHPG induced gamma oscillations. (C) The power spectrum from an O/A interneuron in “Aa” showing the AP frequency at the range of gamma frequency (56.7 Hz). Inset, histogram of population data showing firing frequency obtained during peak depolarization induced by DHPG (n=14). (D) The power spectrum from “Ab” revealing perithreshold oscillations at the frequency of 90.3 Hz in DHPG (black) compared to that in TTX (gray). Inset, histogram of population data showing that TTX significantly abolishes DHPG-induced perithreshold gamma oscillations. (E) Representative voltage trace revealing slow oscillations and AP discharges following exposure to DHPG (10 μM, 60 sec, indicated by bar) without AP-free periods in an O/A interneuron. (F) The power spectrum from the O/A interneuron in E showing the AP frequency at the range of gamma frequency (37.2 Hz). *p < 0.05 in this and following figures.
3.3. Slow membrane oscillations are mediated by both mGhiR1 and mGluR5
Since O-LM interneurons express both mGluR1 and mGluR5 (van Hooft et al., 2000), both the group I/II mGluR agonists (e.g., ACPD) (Mcbain et al., 1994; Woodhall et al., 1999), and the selective group I agonist DHPG produced slow membrane oscillations or increased excitability, we suspected that mGluR1 and/or mGluR5 may contribute to DHPG-mediated slow oscillations. To dissect the contributions of specific group I mGluR subtypes to intrinsic slow oscillations in O-LM interneurons, we used selective mGluR subtype antagonists.
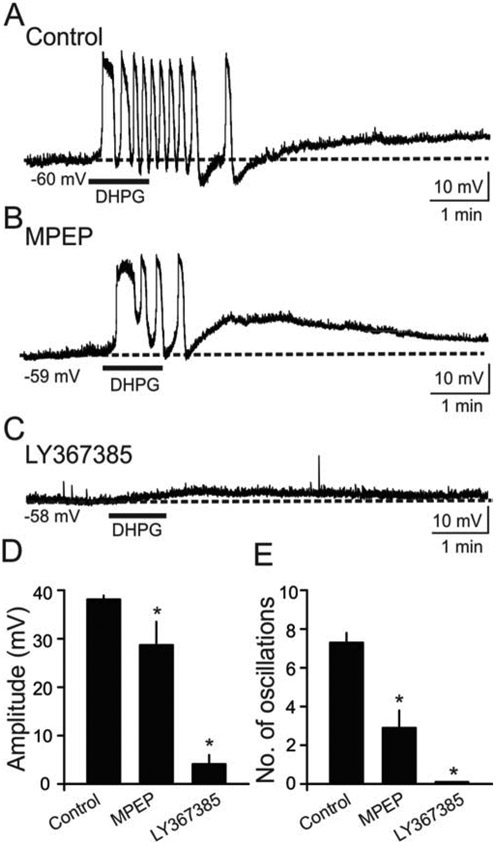
Under control conditions, bath application of DHPG (10 μM, 60 sec) induced robust membrane depolarizations that were associated with oscillations in the presence of TTX (Fig. 3A). In O/A interneurons (n = 7), slices were pre-incubated in MPEP for 15–30 min before the bath application of DHPG. In the presence of MPEP, DHPG elicited slow oscillations with reduced peak depolarization amplitude compared to control conditions (Control: 38.1 ± 0.8 mV, n = 32; MPEP: 28.7 ± 4.8 mV, n = 7; Fig. 3B, D). The number of DHPG-induced oscillations was also significantly reduced by MPEP (Control: 7.3 ± 0.5, n = 32; MPEP: 2.9 ± 0.9; n = 7, p < 0.05; Fig. 3B, E). We then tested the contribution of mGluR1 to DHPG-mediated slow oscillations in O/A interneurons using the selective mGluR1 antagonist LY 367385. For 10 cells, slices were pre-incubated in LY 367385 (100 μM) for 15–30 min. LY 367385 also attenuated the peak amplitude of DHPG-induced depolarization (10 % of control; n = 10, p < 0.05, Fig. 3C, D) and the number of oscillations (0 % of control; n = 10, p < 0.05, Fig. 3C, E). These results indicate that both mGluR1 and mGluR5 contribute to slow oscillations, with greater contribution coming from mGluR1.
Figure 3. Contribution of mGluR1 and mGluR5 subtypes to slow oscillations in O/A interneurons.
(A) Sample voltage trace showing that exposure to DHPG (indicated by bar) induced slow oscillations in the presence of TTX. (B) In a different O/A cell, oscillatory responses to DHPG were partially antagonized by a selective mGluR5 antagonist, MPEP (50 μM).The number of DHPG-induced oscillations and peak amplitude were partially antagonized by MPEP. (C) Slow oscillations were abolished by a selective mGluR1 antagonist LY367385 (100 μM) in a diffent O/A interneuron. The slice was preincubated with LY367385 for 15-30 min, and subsequently bath applied during the whole-cell patch-clamp recordings. (D) Bar graph of population data illustrating the antagonistic actions of MPEP and LY367385 on DHPG-induced peak membrane depolarization. The peak depolarization was significantly altered by MPEP (n=7, p<0.1) and LY367385 (n=10, p<0.001) compared to control conditions (n=32). (E) Population data illustrating the antagonistic actions of MPEP and LY367385 on DHPG-induced slow oscillations. The number of oscillations was significantly reduced by both MPEP (n=7, p<0.05) and LY367385 (n= 10, p<0.001) compared to control conditions (n=32).
3.4. Activation of nonselective transient receptor potential (TRP) channels contributes to oscillations
Group I mGluR activation regulates multiple types of ion channels in hippocampal interneurons including O-LM interneurons (Camiré et al., 2012). Given that nonselective cation currents through transient receptor potential (TRP) channels are known to be functionally linked to group I mGluR activation in O-LM interneurons (Huang et al., 2004; Topolnik et al., 2006) TRP channels may be critically involved in group I mGluR-mediated slow oscillations in O/A interneurons. Accordingly, we first tested if TRP channels contribute to the mGluR-mediated oscillations by using two selective TRP channel blockers, flufenamic acid (FFA) and 2-aminoethoxydiphenylborate (2-APB), which are known to block TRP channels in hippocampal interneurons (Clapham, 2007; Lee et al., 2011).
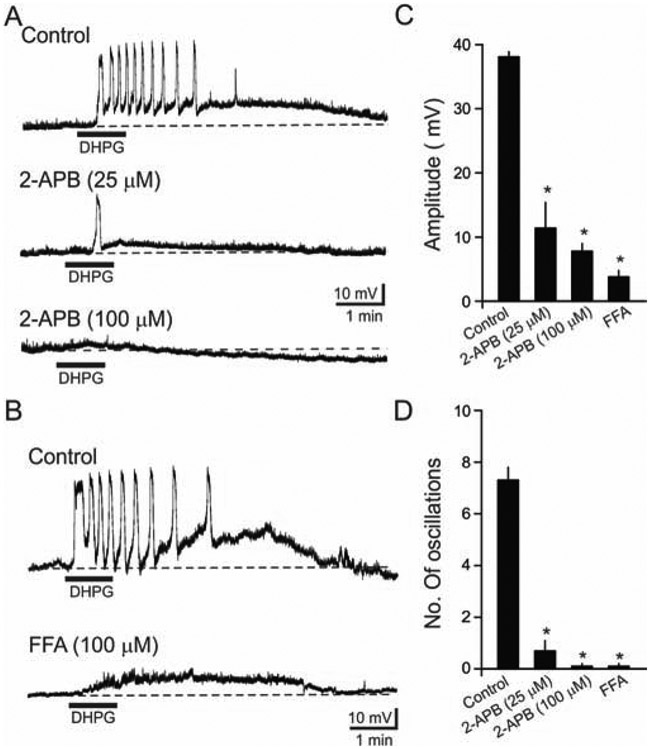
Brain slices were pre-incubated with 2-APB or FFA for at least 30 min before DHPG application. As shown in Fig. 4A, 2-APB attenuated slow oscillations in a dose-dependent manner. The peak depolarization amplitude was significantly reduced by 2-APB (Control: 38.1 ± 0.8 mV, n = 32; 25 μM 2-APB: 11.4 ± 4.1 mV, p < 0.05, n = 7; 100 μM 2-APB: 7.8 ± 1.2 mV, n = 8; p < 0.05; Fig. 4A, C). Similarly, the number of DHPG-induced slow oscillations were attenuated by 2-APB, often with a single depolarization or no response to DHPG (Control: 7.3 ± 0.5, n = 32; 25 μM 2-APB: 0.7 ± 0.4, p < 0.05, n = 7; 100 μM 2-APB: 0.1 ± 0.1, n = 8; p < 0.05; Fig. 4A, D).
Figure 4. DHPG-induced slow oscillations were blocked by the nonselective cation channel blockers 2-APB and FFA.
(A) Representative voltage traces showing that DHPG-induced slow oscillations were reduced by 25 μM and 100 μM 2-APB. In the examples, the slices were preincubated with 2-APB for 30 min before application of DHPG. (B) In a different cell, the DHPG-induced slow oscillations were blocked by 100 μM FFA (bottom). (C) Histogram of quantitative data depicting the actions of 2-APB and FFA on DHPG-induced peak depolarization amplitude. The peak amplitude was significantly reduced by 2-APB (25 μM: n=7, p<0.001; 100 μM: n=8, p<0.001) and FFA (n=8, p<0.001), (D) Histogram of quantitative data illustrating the actions of 2-APB and FFA on the number of oscillations induced by DHPG. The number of oscillations was attenuated both by 2-APB (25 μM: n=7, p<0.001; 100 μM: n=8, p<0.0001) and FFA (n=8, p<0.0001).
The TRP channel blocker FFA had similar effects on DHPG-induced depolarization and oscillations. FFA (100 μM) reduced the DHPG-induced depolarization amplitude (Control: 38.1 ± 0.8 mV, n = 32; FFA: 3.8 ± 1.1 mV, n = 8, p < 0.05; Fig. 4B, C), and blocked oscillations (Control: 7.3 ± 0.5, n = 32; FFA: 0.1 ± 0.1, n = 8; p < 0.05; Fig. 4B, D). These findings suggest that TRP channels contribute to mGluR1/5-mediated depolarization and associated oscillations in O/A interneurons.
3.5. Contribution of L-Type Ca2+ channels to slow oscillations
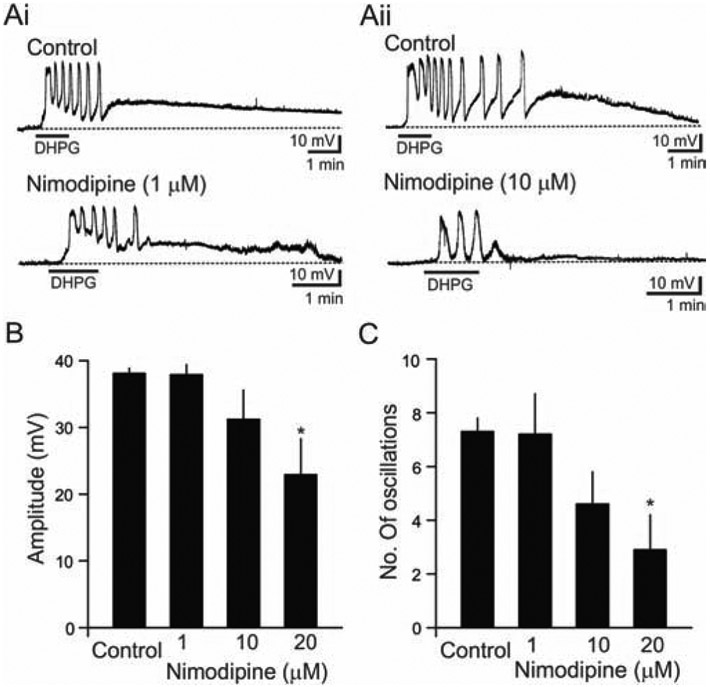
O-LM interneurons are known to express a variety of voltage-gated calcium channels (VGCCs) in their dendrites such as T-, L-, N- and P/Q-types (Topolnik et al., 2009). Among VGCCs localized in O-LM interneurons, L-type Ca2+ channels are reportedly coupled to mGluR5 and facilitate long-term potentiation (LTP) of dendritic Ca2+ signaling (Camiré et al., 2012; Topolnik et al., 2009). Moreover, putative O-LM interneurons expressing mGluR1α or somatostatin are known to express Cav 1.3 (> 70 %) as well as Cav 1.2 (< 20 %) (Vinet and Sik, 2006). Thus, we sought to examine the involvement of L-type Ca2+ channels in DHPG-mediated slow oscillations in O/A interneurons using the L-type Ca2+ channel blocker, nimodipine.
In O/A interneurons (n = 23), slices were pre-incubated in nimodipine (1,10, or 20 μM) for 15–30 min before the bath application of DHPG. A low concentration (1 μM) of nimodipine did not alter DHPG-mediated depolarization amplitude (Control: 38.1 ± 0.8 mV, n = 32; Nimodipine: 37.9 ± 1.5 mV, n = 6; Fig. 5Ai, B), or the number of oscillations (Control: 7.3 ± 0.5, n = 32; Nimodipine: 7.2 ± 1.5, n = 6; Fig., 5Ai, C). In contrast, nimodipine (10 and 20 μM) blocked the DHPG-induced oscillations in the presence of TTX (1 μM) as shown in Fig. 5Aii and C. Nimodipine (10 and 20 μM) reduced both DHPG-mediated depolarization amplitude (Control: 33.1 ± 0.8 mV, n = 32; 10 μM Nimodipine: 31.2 ± 4.4 mV, n = 9; 20 μM Nimodipine: 22.9 ± 5.4 mV, n = 8; p < 0.05; Fig. 5B) and the number of oscillations (Control: 7.3 ± 0.5, n = 32; 10 μM Nimodipine: 4.6 ± 1.2, n = 9; 20 μM Nimodipine: 2.9 ± 1.3, n = 8, p < 0.05; Fig. 5C). Together, these findings suggest that L-type Ca2+ channels are involved in DHPG-mediated depolarization and slow oscillations in O/A interneurons.
Figure 5. Effects of the L-type Ca2+ channel blocker nimodipine on DHPG-induced membrane oscillations.
(A) Sample voltage traces illustrate the effects of nimodipine on DHPG-induced membrane depolarization and slow oscillations. Low level of nimodipine (1 μM) did not reduce depolarization amplitude or the number of oscillations (Ai). Higher levels of nimodipine (10 μM) reduced slow oscillations (Aii). (B) Bar graph illustrating the dose-dependent actions of nimodipine on DHPG-induced depolarization. The amplitude was reduced by 10 μM (n=9) and 20 μM nimodipine (n=8, p<0.05), but not by 1 μM (n=6). (C) The dose-dependent actions of nimodipine on DHPG-induced oscillations. The number of slow oscillations were reduced by 20 μM nimodipine (n=8, p<0.05), but not by 1 μM (n=6, p>0.5).
3.6. mGluR-mediated actions involve Ca2+ release from ryanodine-sensitive stores
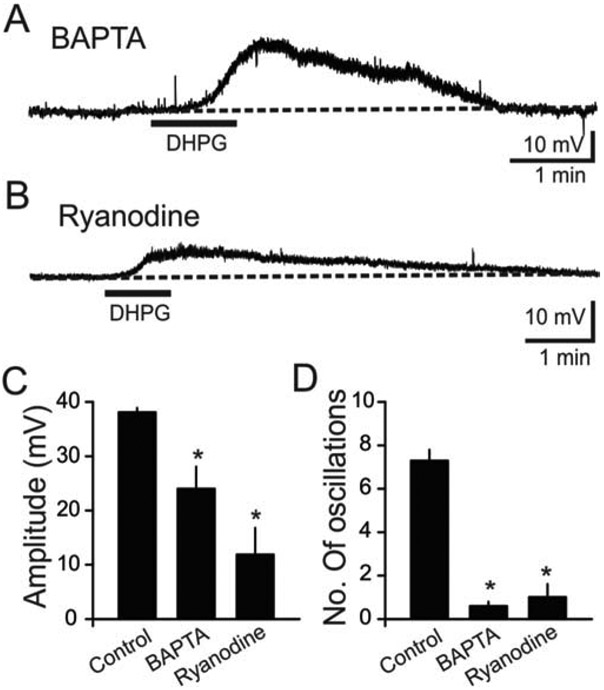
Our findings suggest that L-type Ca2+ channels and TRP channels are critically involved in group I mGluR-mediated slow oscillations in O/A interneurons. Ryanodine-sensitive Ca2+ stores can functionally interact with either L-type Ca2+ channels or TRP channels following activation of G protein-coupled receptors in hippocampal interneurons (Lee et al., 2011; Topolnik et al., 2009; Woodhall et al., 1999). Since ryanodine-sensitive stores, L-type Ca2+ channels, and TRP channels can all contribute to increased intracellular [Ca2+], modulation of [Ca2+]i triggered by group I mGluR activation could be responsible for the slow membrane potential oscillations. Hence, we used high intracellular Ca2+-buffering conditions in O/A interneurons to determine if this would abolish DHPG-mediated slow oscillations. We first compared data with a standard pipette solution (without Ca2+ chelator) to data obtained with a pipette solution containing the Ca2+ chelator BAPTA (30 mM). In the presence of BAPTA, application of DHPG produced smaller membrane depolarizations (63.1 % of control, n = 8, p < 0.05; Fig. 6A, C). In addition, exposure to DHPG produced reduced slow oscillatory responses in the presence of BAPTA (8.2 % of control, n = 8, p < 0.05; Fig. 6D). In the presence of a lower BAPTA concentration (10 mM), DHPG produced fewer slow oscillations compared to data with a standard pipette solution (1.0 ± 0.0, n = 4). These findings suggest not only that intracellular Ca2+ signaling is required for DHPG-mediated slow oscillations in O/A interneurons, but also that DHPG-mediated slow oscillations in O/A interneurons are generated by intrinsic Ca2+-dependent mechanisms, and not by network activity. The latter is in agreement with the findings demonstrated in Fig. 1 showing that DHPG-mediated slow oscillations persist in the presence of synaptic blockers.
Figure 6. Intracellular Ca2+ stores were involved in mGluR-mediated slow oscillations.
(A) Sample current trace showing that exposure to DHPG produced reduced membrane depolarization and slow oscillations in the presence of BAPTA. The Ca2+ chelator BAPTA (30 mM) was included in the pipette to clamp intracellular Ca2+ to lower levels. (B) Blockade of ryanodine receptors by ryanodine (10 μM) attenuated DHPG-mediated depolarization and slow oscillations. Slices were pre-incubated with ryanodine for 30 min before exposure to DHPG. (C) Population data revealed that the DHPG-induced depolarization was significantly attenuated by BAPTA (n=8, p<0.01), and ryanodine (n=10, p<0.001). (D) Population data showing that the number of oscillations were blocked by BAPTA (n=8, p<0.001) and ryanodine (n=10, p<0.001).
Previous studies have shown that the oscillatory responses generated by a group I/II mGluR agonist involve Ca2+ entry through voltage-gated Ca2+ channels, and Ca2+ release from internal stores in O/A interneurons (Woodhall et al., 1999). To determine whether mGluR-mediated depolarization and oscillatory activity involve the release of Ca2+ from intracellular stores, ryanodine was used to deplete Ca2+ from endoplasmic reticulum and other intracellular storage sites. We pre-incubated (> 30 min) slices in 10 μM ryanodine before exposure to DHPG. Ryanodine significantly reduced the depolarization amplitude (31.2 % of control, n = 10, p < 0.05) and the number of oscillations (13.6 % of control, n = 10, p < 0.05, Fig. 6B, D). The data suggest that ryanodine-sensitive intracellular Ca2+ stores are important for group I mGluR-mediated depolarization and oscillations in O/A interneurons.
4. Discussion
In the current study, we examined the properties and underlying mechanisms of group I mGluR-mediated intrinsic oscillations in O/A interneurons. The key findings are: 1) Group I mGluR activation induced membrane depolarization associated with intrinsic slow membrane potential oscillations (< 0.1 Hz), gamma frequency AP discharges, and perithreshold oscillations in CA1 O/A interneurons; 2) The slow oscillations were mediated by both mGluR1 and mGluR5, with a greater mGluR1 contribution; 3) TRP channels, L-type Ca2+ channels, and ryanodine receptors were involved in the slow oscillations. These results indicate that multiple mechanisms are involved in group I mGluR-mediated intrinsic slow oscillations, gamma frequency AP discharges, and perithreshold oscillations in O/A interneurons, which may contribute to group I mGluR-mediated network oscillations and O/A interneuron vulnerability to excitotoxicity in seizures.
4.1. The contribution of mGluR1 and mGluR5 to DHPG-mediated slow oscillations
Group I mGluR activation produces diverse effects on neuronal excitability and synaptic plasticity in CA1 interneurons, including O-LM interneurons (Kullmann and Lamsa, 2007; Le Duigou et al., 2015, 2011; Mcbain et al., 1994; Peterfi et al., 2012; Topolnik et al., 2006; Woodhall et al., 1999). Our findings suggest that both mGluR1 and mGluR5 have distinct contributions to depolarization and generation of membrane potential oscillations in O/A interneurons, with significantly greater contribution from mGluR1. This is in agreement with observed expression of mGluR1α and mGluR5 in O-LM interneurons (Baude et al., 1993; Ferraguti et al., 2004; Lujan et al., 1996; Nagy et al., 2013; Shigemoto et al., 1997; van Hooft et al., 2000). The differential contributions of mGluR1α and mGluR5 might be attributable to differences in expression level and/or differential intracellular signaling. Indeed, published studies suggest that mGluR1α activation produces increased intracellular [Ca2+] through TRP channel activation and Ca2+-induced Ca2+ release from ryanodine-sensitive stores in the CA1 stratum oriens interneurons, including O-LM interneurons, whereas mGluR5 activation appears to be preferentially coupled to Ca2+ release, but not to TRP channel activation (Camiré et al., 2012). Prior studies (Mcbain et al., 1994; van Hooft et al., 2000; Woodhall et al., 1999) have reported mGluR-mediated excitation in striatum oriens interneurons, including O-LM cells. Our current study expands these findings with evidence that mGluR1 and mGluR5 cooperatively contribute to group I mGluR-induced excitation in O-LM cells. Similarly, both mGluR1 and mGluR5 are required for induction of LTP and long-term depression (LTD) of afferent excitatory synapses in oriens interneurons, including O-LM interneurons (Le Duigou and Kullmann, 2011; Peterfi et al., 2012). Thus, cooperative activation of mGluR1 and mGluR5 appears to also play a role in synaptic plasticity at afferent excitatory synapses in O-LM interneurons.
4.2. Molecular mechanisms underlying DHPG-mediated oscillations
Multiple mechanisms contribute to group I mGluR-mediated depolarization and slow oscillations. TRP channels are known to be activated by a variety of Gq/11-coupled receptors including mGluR1/5 receptors in the hippocampus (Huang et al., 2004; Nagy et al., 2013). Transient receptor potential canonical channel-6 (TRPC6) forms Ca2+-permeable non-selective cation channels in neurons including hippocampal interneurons (Nagy et. al. 2013). Interestingly, mGluR1 and TRPC6 were found to be co-localized in stratum oriens interneurons (Nagy et. al. 2013). This anatomical evidence is in agreement with our current findings as well as prior studies indicating that Ca2+ entry through TRP channels in CA1 stratum oriens interneurons contributes to LTP (Topolnik et. al. 2006). Thus, co-localization of mGluR1 and TRPC6 in these interneurons likely plays a role in regulation of neuronal excitability and synaptic plasticity.
Based on our findings that blockade of L-type Ca2+ channels abolished mGluR1/5 mediated slow oscillations in a majority of O/A interneurons tested, our working model of mGluR1/5-mediated slow oscillations in O/A interneurons is that mGluR1/5 activation leads to opening of TRP channels, resulting in membrane depolarization, which then activates L-type Ca2+ channels. Thus, both TRP channels and L-type Ca2+ channels contribute to slow oscillations. These findings are in accordance with published studies indicating that most CA1 stratum oriens interneurons expressing markers for O-LM interneurons (e.g., mGluR1α or somatostatin) express Cav 1.3 and/or Cav 1.2 (Vinet and Sik, 2006). Cav 1.2 and Cav 1.3 are known to show differential sensitivities to dihydropyridine L-type Ca2+ channel blockers (e.g., nimodipine), such as the one used in this study. Specifically, Cav 1.2-mediated components are reportedly blocked at low concentrations of nimodipine (< 2 μM), whereas Cav 1.3-mediated components are blocked at high concentrations of nimodipine (> 2 μM) (Olson et al., 2005). Our study showed that higher concentration of nimodipine (20 μM) reduced DHPG-mediated oscillations (Fig. 5B, C), whereas a lower concentration of nimodipine (1 μM) has no effect on DHPG-mediated oscillations, suggesting that Cav 1.3 channels are selectively involved in DHPG-mediated depolarization. Cav 1.3 channels are of particular interest, not only because they are known to be critically involved in major types of neurological and psychiatric disorders (Ortner and Striessnig, 2016), but also because they are much less abundant in the brain compared to Cav 1.2 channels, which are the predominant subtype of L-type Ca2+ channels outside the central nervous system (Sinnegger-Brauns et al., 2009). Thus, previous attempts have been made to develop selective Cav 1.3 antagonists, which could be used for treatments of brain disorders including Parkinson’s disease, without the side effects caused by non-selective L-type Ca2+ channel blockers (Kang et al., 2012).
Ca2+ release from intracellular stores is critically involved in group I mGluR-mediated responses in O/A interneurons (Woodhall et al., 1999). In agreement with that report, we also found that either ryanodine or the Ca2+ chelator, BAPTA, blocked DHPG-mediated slow oscillations. In interneurons within as well as outside of the hippocampus, Gq/11-coupled or Gi/o-coupled receptors activate TRP channels and L-type Ca2+ channels, which are known to be functionally coupled to ryanodine receptors (Lee et al., 2011; Topolnik et al., 2006). Our current findings also suggest that the functional connection of TRP channels and L-type Ca2+ channels to ryanodine receptors is critically involved in DHPG-mediated slow oscillations.
It is interesting that all of the key molecules related to DHPG-mediated slow oscillations (i.e., group I mGluRs, TRP channels, L-type Ca2+ channels, and ryanodine receptors) are known to be involved in the synthesis of endocannabinoids (Bardell and Barker, 2010; Isokawa and Alger, 2006; Kreitzer and Malenka, 2005; Maejima et al., 2001). Diacylglycerol lipase-α, a synthesizing enzyme of the endocannabinoid, 2-arachidonoylglycerol, is expressed in somatostatin-expressing interneurons in the hippocampus (Peterfi et al., 2012). Furthermore, studies by the Katona group suggest that group I mGluR activation produces endocannabinoid signaling-dependent long-term suppression of afferent excitatory synapses on O-LM interneurons (Peterfi et al., 2012). Thus, it is possible that when excitation of O-LM interneurons is reduced through endocannabinoid-mediated retrograde signaling (following group I mGluR activation), hippocampal networks may be more prone to pathological increased excitability and seizures due to decreased O-LM interneuron-mediated feedback inhibition.
4.3. Limitations of this study
A subpopulation of horizontally oriented O/A interneurons in this study were identified anatomically as O-LM interneurons. Although almost all tested O/A interneurons expressed somatostatin and mGluR1α, we could not exclude the possibility that other GABAergic neuronal subtypes (e.g., bistratified cells, long-range projecting cells) expressing somatostatin and mGluR1α (Ferraguti et al., 2004; Katona et al., 2017; Maccaferri et al., 2000) were included in the current study in addition to O-LM interneurons. While the current study provids the evidence for the generation of DHPG-mediated oscillations in O/A interneurons, it will be critical to determine whether there are differences in DHPG-mediated oscillations among functionally distinct O/A interneuron subtypes expressing somatostatin in future studies.
Lower MPEP concentrations (10-30 μM) have been more often used when authors studied the contribution of mGluR5 to the effects of synaptically released glutamate on synaptic activity. However, in other published studies of slice whole-cell patch-clamp recordings, 50 μM MPEP, which was used in this study, has been used to selectively block mGluR5 in conjunction with DHPG (e.g., Govindaiah and Cox, 2006; Young et al., 2013, 2008). Thus, we felt 50 μM MPEP was most relevant for our current study. Gasparini et al. showed that MPEP (up to 30 μM) had no antagonist activity on cells expressing mGluR1 (Gasparini et al., 1999). Although the authors did not explicitly claim that higher concentration of MPEP (100 μM) had antagonist activities at mGluR1, their Figure 2A appears to show that 100 μM MPEP blocked mGluR1. They did not test the antagonistic effects of 50 μM MPEP on mGluR1 in that paper. Thus, it remains unknown whether 50 μM MPEP affects mGluR1 in addition to mGluR5. We could not exclude the possibility that that concentration of MPEP might affect mGluR1. In the future, it will be important to determine whether lower MPEP concentration (10-30 μM) also reduces DHPG-mediated oscillations in O/A interneurons.
Although we used 2-APB and flufenamic acid to block TRP channels in this study, they are known to have a broad spectrum of action in addition to TRP channels. For example, 2-APB can also block IP3 receptors (Maruyama et al., 1997), whereas flufenamic acid can also block calcium-activated chloride channels (White and Aylwin, 1990). There are recently developed specific TRP channel blockers, which are commercially available (e.g., SAR7334 and clemizole hydrochloride, selective TRPC blockers; Maier et al., 2015; Richter et al., 2014). Therefore, future studies should test whether the aforementioned selective TRP channel blockers inhibit DHPG-mediated oscillations in O/A interneurons and identity TRP channel subtypes involved in slow oscillations, which should be interpreted with caution due to their potential undiscovered off-target effects.
4.4. Functional relevance
Hippocampal GABAergic interneurons are critically involved in network oscillations including theta, gamma, and ripple oscillations (Colgin 2016). O-LM interneurons are known to fire in vivo at specific phases of hippocampal network oscillations including theta, gamma, and ripple oscillations suggesting their involvement in hippocampal network oscillations (Katona et al., 2014; Varga et al., 2012). While it is still largely unknown which in vivo conditions may activate group I mGluRs in GABAergic interneurons, selective mGluR agonists have been used to produce hippocampal gamma oscillations (Martin, 2001; Pálhalmi et al., 2004; Whittington et al., 1995). Such published studies suggest that GABAergic interneurons are critically involved in group I/II mGluR-mediated gamma oscillations in the hippocampus (Whittington et al., 1995). These findings are in general agreement with later publications showing that activation of group I/II mGluR produces suprathreshold depolarization in specific subtypes of GABAergic interneurons including O-LM interneurons (Mcbain et al., 1994; van Hooft et al., 2000; Woodhall et al., 1999). However, group I mGluR-mediated firing properties of O-LM interneurons and the mechanisms underlying high-frequency APs remained largely unknown until our current studies. Our study reveals that O/A interneurons (including O-LM interneurons) generate gamma frequency AP discharges and sodium-dependent intrinsic perithreshold gamma oscillations in response to the selective group I mGluR agonist DHPG. It is important to note that DHPG-mediated slow suprathreshold membrane depolarization, which triggered gamma frequency AP discharges, was produced in presence of synaptic blockers, indicating that O/A interneurons have intrinsic properties to generate slow and gamma oscillations. Thus, the intrinsic properties of O/A interneurons may play a role, at least in part, in group I/II mGluR-mediated network gamma oscillations.
O-LM interneurons are also involved in in vitro seizure-like events produced by 4-aminopyrridine (Ziburkus et al., 2006). Published studies showed that O-LM interneurons generated repeated gamma frequency AP discharges, followed by an AP-free period during ictal discharge (Ziburkus et al., 2006). Interstingly, our current results showed that activation of group I mGluRs produced similar repeated gamma frequency AP discharges, followed by an AP-free period in O/A interneurons (including O-LM cells), raising the possibility that group I mGluRs in O-LM interneurons could be activated strongly enough to generate slow and gamma frequency activity in seizures. Based on the correlation analyses of pyramidal cell and O-LM interneuron activity, Ziburkus et al. (2006) suggested that synaptic interaction between those two cell types were critically involved in the generation of ictal discharges. It is also possible that the mechanisms underlying DHPG-mediated slow and gamma oscillations in O-LM interneurons, which are described in the current study, also play a supporting role in these ictal discharges.
Group I mGluRs have been implicated in seizures (Bianchi et al., 2012; Niswender and Conn, 2010) as group I mGluR activation produces long-lasting epileptiform discharges at the network level (Bianchi et al., 2012; Qian and Tang, 2016). The DHPG-mediated slow oscillations in O/A interneurons described in this study are reminiscent of prominent epileptiform burst discharges and paroxysmal depolarizations observed in pyramidal cells, mediated by synaptic activation of mGluR1 and mGluR5 in temporal lobe epilepsy (Sanon et al., 2010). Furthermore, group I mGluR antagonists are neuroprotective in animal models of temporal lobe epilepsy, suggesting that activation of these receptors on interneurons during epileptic seizures may cause excitotoxicity (Renaud et al., 2002), as previously predicted (Woodhall et al., 1999). In fact, many prior studies have demonstrated the loss of GABAergic interneurons (particularly somatostatin-expressing interneurons) in epilepsy (Best et al., 1993; Hofmann et al., 2016; Houser and Esclapez, 1996; Morin et al., 1998). Seizures and neuronal injuries induced by selective mGluR agonists were reduced by dantrolene, which is known to inhibit intracellular calcium mobilization (McDonald et al., 1993). This is in general agreement with the critical role of intracellular calcium overload in neuronal excitotoxicity (Choi, 1994). Thus, based on our current findings regarding the involvement of TRP channels, L-type calcium channels, and ryanodine receptors in slow oscillations, it is reasonable to assume that excessive activation of O/A interneurons by group I mGluR elevates intracellular Ca2+ which could trigger excitotoxicity and explain their vulnerability during seizure events.
Given that O-LM interneurons, a subgroup of O/A interneurons, are thought to be critically involved in hippocampus-dependent cognitive tasks through their contributions to coordinated network oscillations (Dugladze et al., 2007; Muller and Remy, 2014; Varga et al., 2012), their cell death could lead not only to hyperexcitability and spontaneous seizures, but also to cognitive deficits associated with temporal lobe epilepsy (Bui et al., 2018; Groticke et al., 2008). Therefore, mGluR1α, mGluR5, and related molecular pathways in O-LM interneurons may represent potential therapeutic targets for prevention of seizures and/or cognitive deficits associated with temporal lobe epilepsy.
Highlights.
Group I mGluR activation in O/A interneurons produced slow membrane oscillations
Slow oscillations were associated with gamma oscillations
Slow oscillations were mediated by both mGluR1 and mGluR5
TRP channels and L-type Ca2+ channels were involved in the slow oscillations
Ryanodine receptors were also involved in the slow oscillations
ACKNOWLEDGMENTS
This work was supported by the College of Medicine, University of Arkansas for Medical Sciences to S-HL, Bogard Neurology Research and Stroke Prevention Fund to S-HL, and Core Facilities of the Center for Translational Neuroscience, Award P30 GM110702 from the IDeA program at NIGMS to EGR.
Abbreviations
- ACPD
1S,3R-1-aminocyclopentane-1,3-dicarboxylic acid
- ACSF
artificial cerebrospinal fluid
- AMPA
α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- AP
action potential
- APV
2-Amino-5-phosphonopentanoic acid
- BAPTA
1,2-Bis(2-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid
- CGP55845
(2S)-3-[[(1S)-1-(3,4-Dichlorophenyl)ethyl]amino-2-hydroxypropyl](phenylmethyl)phosphinic acid hydrochloride
- DHPG
(S)-3,5-dihydroxyphenylglycine
- DIC
differential interference contrast
- FFA
flufenamic acid
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- LY 367385
(S)-(+)-α-Amino-4-carboxy-2-methylbenzeneacetic acid
- mGluR
metabotropic glutamate receptor
- MPEP
2-methyl-6-(phenylethynyl)pyridine hydrochloride
- NBQX
2,3-Dihydroxy-6-nitro-7-sulfamoyl-benzo(F) quinoxaline
- NMDA
N-methyl-D-aspartate
- O/A interneurons
interneurons in the stratum oriens/alveus
- O-LM interneurons
oriens lacunosum-moleculare interneurons
- SR95531
6-Imino-3-(4-methoxyphenyl)-1(6H)-pyridazinebutanoic acid hydrobromide
- TRP channels
non-selective transient receptor potential channels
- TTX
tetrodotoxin
- 2-APB
2-aminoethoxydiphenylborate
Footnotes
DECLARATIONS OF INTEREST
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Armstrong C, Soltesz I, 2012. Basket cell dichotomy in microcircuit function. J. Physiol 590, 683–94. doi: 10.1113/jphysiol.2011.223669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardell TK, Barker EL, 2010. Activation of TRPC6 channels promotes endocannabinoid biosynthesis in neuronal CAD cells. Neurochem. Int 57, 76–83. doi: 10.1016/j.neuint.2010.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baude A, Nusser Z, Roberts JDB, Mulvihill E, Jeffrey Mcllhinney RA, Somogyi P, 1993. The metabotropic glutamate receptor (mGluRlα) is concentrated at perisynaptic membrane of neuronal subpopulations as detected by immunogold reaction. Neuron 11, 771–787. doi: 10.1016/0896-6273(93)90086-7 [DOI] [PubMed] [Google Scholar]
- Best N, Mitchell J, Baimbridge KG, Wheal HV, 1993. Changes in parvalbumin-immunoreactive neurons in the rat hippocampus following a kainic acid lesion. Neurosci. Lett 155, 1–6. doi: 10.1016/0304-3940(93)90660-D [DOI] [PubMed] [Google Scholar]
- Bianchi R, Wong RKS, Merlin LR, 2012. Glutamate receptors in epilepsy: Group I mGluR-mediated epileptogenesis, 4th editio. ed, Jasper’s Basic Mechanisms of the Epilepsies. National Center for Biotechnology Information, Bethesda, Maryland. [PubMed] [Google Scholar]
- Blasco-Ibanez JM, Freund TF, 1995. Synaptic Input of Horizontal Interneurons in Stratum Oriens of the Hippocampal CA1 Subfield: Structural Basis of Feed-back Activation. Eur. J. Neurosci 7, 2170–2180. doi: 10.1111/j.1460-9568.1995.tb00638.x [DOI] [PubMed] [Google Scholar]
- Bui AD, Nguyen TM, Limouse C, Kim HK, Szabo GG, Felong S, Maroso M, Soltesz I, 2018. Dentate gyrus mossy cells control spontaneous convulsive seizures and spatial memory. Science (80-, ). 359, 787–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camiré O, Lacaille JC, Topolnik L, 2012. Dendritic signaling in inhibitory interneurons: Local tuning via group I metabotropic glutamate receptors. Front. Physiol doi: 10.3389/fphys.2012.00259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cea-del Rio CA, Lawrence JJ, Erdelyi F, Szabo G, McBain CJ, 2011. Cholinergic modulation amplifies the intrinsic oscillatory properties of CA1 hippocampal cholecystokinin-positive interneurons. J. Physiol 589, 609–27. doi: 10.1113/jphysiol.2010.199422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman CA, Lacaille JC, 1999. Intrinsic theta-frequency membrane potential oscillations in hippocampal CA1 interneurons of stratum lacunosum-moleculare. J. Neurophysiol 81, 1296–1307. [DOI] [PubMed] [Google Scholar]
- Choi D, 1994. Calcium and excitotoxic neuronal injury. Ann. N. Y. Acad. Sci 747, 162–171. [DOI] [PubMed] [Google Scholar]
- Clapham DE, 2007. SnapShot: Mammalian TRP Channels. Cell 129, 220. doi: 10.1016/j.cell.2007.03.034 [DOI] [PubMed] [Google Scholar]
- Colgin LL, 2016. Rhythms of the hippocampal network. Nat. Rev. Neurosci 17, 239–249. doi: 10.1038/nrn.2016.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugladze T, Vida I. Tort AB, Gross A, Otahal J, Heinemann U, Kopell NJ, Gloveli T, 2007. Impaired hippocampal rhythmogenesis in a mouse model of mesial temporal lobe epilepsy. Proc. Natl. Acad. Sci. U. S. A 104, 17530–17535. doi: 10.1073/pnas.0708301104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraguti F, Cobden P, Pollard M, Cope D, Shigemoto R, Watanabe M, Somogyi P, 2004. Immunolocalization of metabotropic glutamate receptor 1 alpha (mGluR1alpha) in distinct classes of interneuron in the CA1 region of the rat hippocampus. Hippocampus 14, 193–215. doi: 10.1002/hipo.10163 [DOI] [PubMed] [Google Scholar]
- Freund TF, Katona I, 2007. Perisomatic Inhibition. Neuron 56, 33–42. doi: 10.1016/j.neuron.2007.09.012 [DOI] [PubMed] [Google Scholar]
- Fuentealba P, Klausberger T, Karayannis T, Suen WY, Huck J, Tomioka R, Rockland K, Capogna M, Studer M, Morales M, Somogyi P, 2010. Expression of COUP-TFII nuclear receptor in restricted GABAergic neuronal populations in the adult rat hippocampus. J. Neurosci 30, 1595–609. doi: 10.1523/JNEUROSCI.4199-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparini F, Lingenhohl K, Stoehr N, Flor PJ, Heinrich M, Vranesic I, Biollaz M, Allgeier H, Heckendorn R, Urwyler S, Varney MA, Johnson EC, Hess SD, Rao SP, Sacaan AI, Santori EM, Veliçelebi G, Kuhn R, 1999. 2-Methyl-6-(phenylethynyl)-pyridine (MPEP), a potent, selective and systemically active mGlu5 receptor antagonist. Neuropharmacology 38, 1493–1503. doi: 10.1016/S0028-3908(99)00082-9 [DOI] [PubMed] [Google Scholar]
- Govindaiah G, Cox CL, 2006. Metabotropic Glutamate Receptors Differentially Regulate GABAergic Inhibition in Thalamus. J. Neurosci 26, 13443–13453. doi: 10.1523/JNEUROSCI.3578-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groticke I, Hoffmann K, Loscher W, 2008. Behavioral alterations in a mouse model of temporal lobe epilepsy induced by intrahippocampal injection of kainate. Exp. Neurol 213, 71–83. doi: 10.1016/j.expneurol.2008.04.036 [DOI] [PubMed] [Google Scholar]
- Hofmann G, Balgooyen L, Mattis J, Deisseroth K, Buckmaster PS, 2016. Hilar somatostatin interneuron loss reduces dentate gyrus inhibition in a mouse model of temporal lobe epilepsy. Epilepsia 57, 977–983. doi: 10.1111/epi.13376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houser CR, Esclapez M, 1996. Vulnerability and plasticity of the GABA system in the pilocarpine model of spontaneous recurrent seizures, in: Epilepsy Research. pp. 207–218. doi: 10.1016/S0920-1211(96)00054-X [DOI] [PubMed] [Google Scholar]
- Huang Y, Sinha S, Tanaka K, Rothstein J, Bergles D, 2004. Astrocyte Glutamate Transporters Regulate Metabotropic Glutamate Receptor-Mediated Excitation of Hippocampal Interneurons. J. Neurosci 24, 4551–4559. doi: 10.1523/JNEUROSCI.5217-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isokawa M, Alger BE, 2006. Ryanodine Receptor Regulates Endogenous Cannabinoid Mobilization in the Hippocampus. J. Neurophysiol 95, 3001–3011. doi : 10.1152/jn.00975.2005 [DOI] [PubMed] [Google Scholar]
- Kang S, Cooper G, Dunne SF, Dusel B, Luan C-H, Surmeier DJ, Silverman RB, 2012. CaV1.3-selective L-type calcium channel antagonists as potential new therapeutics for Parkinson’s disease. Nat. Commun 3, 1146. doi: 10.1038/ncomms2149 [DOI] [PubMed] [Google Scholar]
- Kang Y-J, Lewis HES, Young MW, Govindaiah G, Greenfield LJ, Garcia-Rill E, Lee S-H, 2018. Cell type-specific intrinsic perithreshold oscillations in hippocampal GABAergic interneurons. Neuroscience in press, doi: 10.1016/j.neuroscience.2018.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona T, Acsády L, Freund TF, 1999. Postsynaptic targets of somatostatinimmunoreactive interneurons in the rat hippocampus. Neuroscience 88, 37–55. doi: 10.1016/S0306-4522(98)00302-9 [DOI] [PubMed] [Google Scholar]
- Katona L, Lapray D, Viney T, Oulhaj A, Borhegyi Z, Micklem B, Klausberger T, Somogyi P, 2014. Sleep and movement differentiates actions of two types of somatostatin-expressing GABAergic interneuron in rat hippocampus. Neuron 82, 872–886. doi: 10.1016/j.neuron.2014.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona L, Micklem B, Borhegyi Z, Swiejkowski DA, Valenti O, Viney TJ, Kotzadimitriou D, Klausberger T, Somogyi P, 2017. Behavior-dependent activity patterns of GABAergic long-range projecting neurons in the rat hippocampus. Hippocampus 27, 359–377. doi: 10.1002/hipo.22696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T, Somogyi P, 2008. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science 321, 53–7. doi: 10.1126/science.1149381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC, 2005. Dopamine Modulation of State-Dependent Endocannabinoid Release and Long-Term Depression in the Striatum. J. Neurosci 25, 10537–10545. doi: 10.1523/JNEUROSCI.2959-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann DM, Lamsa KP, 2007. Long-term synaptic plasticity in hippocampal interneurons. Nat Rev Neurosci 8, 687–699. doi: 10.1038/nrn2207 [DOI] [PubMed] [Google Scholar]
- Le Duigou C, Holden T, Kullmann DM, 2011. Short- and long-term depression at glutamatergic synapses on hippocampal interneurons by group i mGluR activation. Neuropharmacology 60, 748–756. doi: 10.1016/j.neuropharm.2010.12.015 [DOI] [PubMed] [Google Scholar]
- Le Duigou C, Kullmann DM, 2011. Group I mGluR agonist-evoked long-term potentiation in hippocampal oriens interneurons. J. Neurosci 31, 5777–81. doi : 10.1523/JNEUROSCI.6265-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Duigou C, Savary E, Kullmann DM, Miles R, 2015. Induction of Anti-Hebbian LTP in CA1 Stratum Oriens Interneurons: Interactions between Group I Metabotropic Glutamate Receptors and M1 Muscarinic Receptors. J. Neurosci 35, 13542–13554. doi: 10.1523/JNEUROSCI.0956-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SY, Földy C, Szabadics J, Soltesz I, 2011. Cell-type-specific CCK2 receptor signaling underlies the cholecystokinin-mediated selective excitation of hippocampal parvalbumin-positive fast-spiking basket cells. J. Neurosci 31, 10993–11002. doi: 10.1523/JNEUROSCI.1970-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujan R, Nusser Z, Roberts JD, Shigemoto R, Somogyi P, 1996. Perisynaptic location of metabotropic glutamate receptors mGluR1 and mGluR5 on dendrites and dendritic spines in the rat hippocampus. Eur. J. Neurosci 8, 1488–1500. doi:DOI 10.1111/j.1460-9568.1996.tb01611.x [DOI] [PubMed] [Google Scholar]
- Maccaferri G, David J, Roberts B, Szucs P, Cottingham CA, Somogyi P, 2000. Cell surface domain specific postsynaptic currents evoked by identified GABAergic neurones in rat hippocampus in vitro. J. Physiol 524, 91–116. doi: 10.1111/j.1469-7793.2000.t01-3-00091.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccaferri G, McBain CJ, 1996. The hyperpolarization-activated current (Ih) and its contribution to pacemaker activity in rat CA1 hippocampal stratum oriens-alveus interneurones. J. Physiol 497, 119–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maejima T, Hashimoto K, Yoshida T, Aiba a, Kano M, 2001. Presynaptic inhibition caused by retrograde signal from metabotropic glutamate to cannabinoid receptors. Neuron 31, 463–75. [DOI] [PubMed] [Google Scholar]
- Maier T, Follmann M, Hessler G, Kleemann H-W, Hachtel S, Fuchs B, Weissmann N, Linz W, Schmidt T, Löhn M, Schroeter K, Wang L, Rütten H, Strübing C, 2015. Discovery and pharmacological characterization of a novel potent inhibitor of diacylglycerol-sensitive TRPC cation channels. Br. J. Pharmacol 172, 3650–60. doi: 10.1111/bph.13151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SJ, 2001. Activation of metabotropic glutamate receptors induces gamma frequency oscillations in the rat dentate gyrus in vivo. Neuropharmacology 40, 634–637. [DOI] [PubMed] [Google Scholar]
- Maruyama T, Kanaji T, Nakade S, Kanno T, Mikoshiba K, 1997. 2APB, 2-aminoethoxydiphenyl borate, a membrane-penetrable modulator of Ins(1,4,5)P3-induced Ca2+release. J. Biochem 122, 498–505. doi: 10.1093/oxfordjournals.jbchem.a021780 [DOI] [PubMed] [Google Scholar]
- Mcbain CJ, Dichiara TJ, Kauer JA, 1994. Activation of Metabotropic Glutamate Receptors Differentially Affects Two Classes of Hippocampal Interneurons and Potentiates Excitatory Synaptic Transmission. J. Neurosci 14, 4433–4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald JW, Fix AS, Tizzano JP, Schoepp DD, 1993. Seizures and brain injury in neonatal rats induced by 1 S,3R-ACPD, a metabotropic glutamate receptor agonist. J Neurosci 13, 4445–4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin F, Beaulieu C, Lacaille JC, 1998. Selective loss of GABA neurons in area CA1 of the rat hippocampus after intraventricular kainate. Epilepsy Res. 32, 363–369. doi: 10.1016/S0920-1211(98)00033-3 [DOI] [PubMed] [Google Scholar]
- Muller C, Remy S, 2014. Dendritic inhibition mediated by O-LM and bistratified interneurons in the hippocampus. Front. Synaptic Neurosci 6. doi: 10.3389/fnsyn.2014.00023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy GA, Botond G, Borhegyi Z, Plummer NW, Freund TF, Hájos N, 2013. DAG-sensitive and Ca2+ permeable TRPC6 channels are expressed in dentate granule cells and interneurons in the hippocampal formation. Hippocampus 23, 221–232. doi : 10.1002/hipo.22081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson E, Kullmann DM, 2013. Long-term potentiation in hippocampal oriens interneurons: postsynaptic induction, presynaptic expression and evaluation of candidate retrograde factors. Philos. Trans. R. Soc. B Biol. Sci 369, 20130133. doi: 10.1098/rstb.2013.0133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswender CM, Conn PJ, 2010. Metabotropic Glutamate Receptors: Physiology, Pharmacology, and Disease. Annu. Rev. Pharmacol. Toxicol 50, 295–322. doi: 10.1146/annurev.pharmtox.011008.145533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson PA, Tkatch T, Hernandez-Lopez S, Ulrich S, Ilijic E, Mugnaini E, Zhang H, Bezprozvanny I, Surmeier DJ, 2005. G-protein-coupled receptor modulation of striatal Cav1.3 L-type Ca2+ channels is dependent on a Shank-binding domain. J. Neurosci 25, 1050–1062. doi: 10.1523/JNEUROSCI.3327-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortner NJ, Striessnig J, 2016. L-type calcium channels as drug targets in CNS disorders. Channels. doi: 10.1080/19336950.2015.1048936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pálhalmi J, Paulsen O, Freund T., Hájos N, 2004. Distinct properties of carbachol-and DHPG-induced network oscillations in hippocampal slices. Neuropharmacology 47, 381–389. doi: 10.1016/j.neuropharm.2004.04.010 [DOI] [PubMed] [Google Scholar]
- Pelkey KA, Chittajallu R, Craig MT, Tricoire L, Wester JC, McBain CJ, 2017. Hippocampal GABAergic Inhibitory Interneurons. Physiol. Rev 97, 1619–1747. doi: 10.1152/physrev.00007.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterfi Z, Urban GM, Papp OI, Nemeth B, Monyer H, Szabo G, Erdelyi F, Mackie K, Freund TF, Hajos N, Katona I, 2012. Endocannabinoid-Mediated Long-Term Depression of Afferent Excitatory Synapses in Hippocampal Pyramidal Cells and GABAergic Interneurons. J. Neurosci 32, 14448–14463. doi: 10.1523/JNEUROSCI.1676-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian F, Tang F-R, 2016. Metabotropic Glutamate Receptors and Interacting Proteins in Epileptogenesis. Curr. Neuropharmacol 14, 551–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaud J, Emond M, Meilleur S, Psarropoulou C, Carmant L, 2002. AIDA, a class I metabotropic glutamate-receptor antagonist limits kainate-induced hippocampal dysfunction. Epilepsia 43, 1306–1317. doi: 10.1046/j.1528-1157.2002.10402.x [DOI] [PubMed] [Google Scholar]
- Richter JM, Schaefer M, Hill K, 2014. Clemizole Hydrochloride Is a Novel and Potent Inhibitor of Transient Receptor Potential Channel TRPC5. Mol. Pharmacol 86, 514–521. doi: 10.1124/mol.114.093229 [DOI] [PubMed] [Google Scholar]
- Sanon NT, Pelletier JG, Carmant L, Lacaille JC, 2010. Interneuron subtype specific activation of mGluR15 during epileptiform activity in hippocampus. Epilepsia 51, 1607–1618. doi: 10.1111/j.1528-1167.2010.02689.x [DOI] [PubMed] [Google Scholar]
- Shigemoto R, Kinoshita A, Wada E, Nomura S, Ohishi H, Takada M, Flor PJ, Neki A, Abe T, Nakanishi S, Mizuno N, 1997. Differential presynaptic localization of metabotropic glutamate receptor subtypes in the rat hippocampus. J. Neurosci 17, 7503–22. doi:9295396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sik A, Penttonen M, Ylinen A, Buzsáki G, 1995. Hippocampal CA1 interneurons: an in vivo intracellular labeling study. J. Neurosci 15, 6651–6665. doi: 10.1002/cne.903390204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnegger-Brauns MJ, Huber IG, Koschak A, Wild C, Obermair GJ, Einzinger U, Hoda J-C, Sartori SB, Striessnig J, 2009. Expression and 1, 4-dihydropyridine-binding properties of brain L-type calcium channel isoforms. Mol. Pharmacol 75, 407–414. doi: 10.1124/mol.108.049981.pore-forming [DOI] [PubMed] [Google Scholar]
- Topolnik L, Azzi M, Morin F, Kougioumoutzakis A, Lacaille J-C, 2006. mGluR1/5 subtype-specific calcium signalling and induction of long-term potentiation in rat hippocampal oriens/alveus interneurones. J. Physiol 575, 115–131. doi: 10.1113/jphysiol.2006.112896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topolnik L, Chamberland S, Pelletier J, Ran L, Lacaille J, 2009. Activity-Dependent Compartmentalized Regulation of Dendritic Ca 2+ Signaling in Hippocampal Interneurons. J. Neurosci 29, 4658–4663. doi: 10.1523/JNEUROSCI.0493-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hooft JA, Giuffrida R, Blatow M, Monyer H, 2000. Differential expression of group I metabotropic glutamate receptors in functionally distinct hippocampal interneurons. J Neurosci 20, 3544–3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga C, Golshani P, Soltesz L, 2012. Frequency-invariant temporal ordering of interneuronal discharges during hippocampal oscillations in awake mice. Proc. Natl. Acad. Sci. U. S. A 109, E2726–34. doi: 10.1073/pnas.1210929109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinet J, Sík A, 2006. Expression pattern of voltage-dependent calcium channel subunits in hippocampal inhibitory neurons in mice. Neuroscience 143, 189–212. doi: 10.1016/j.neuroscience.2006.07.019 [DOI] [PubMed] [Google Scholar]
- White MM, Aylwin M, 1990. Niflumic and flufenamic acids are potent reversible blockers of Ca2(+)-activated Cl-channels in Xenopus oocytes. Mol. Pharmacol 37, 720–4. [PubMed] [Google Scholar]
- Whittington MA, Traub RD, Jefferys JGR, 1995. Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature 373, 612–615. doi: 10.1038/373612a0 [DOI] [PubMed] [Google Scholar]
- Woodhall G, Gee CE, Robitaille R, Lacaille JC, 1999. Membrane Potential and Intracellular Ca2+ Oscillations Activated by mGluRs in Hippocampal Stratum Oriens/Alveus Interneurons. J. Neurophysiol 81, 371–382. [DOI] [PubMed] [Google Scholar]
- Yin S, Niswender CM, 2014. Progress toward advanced understanding of metabotropic glutamate receptors: structure, signaling and therapeutic indications. Cell. Signal 26, 2284–2297. doi: 10.1016/j.cellsig.2014.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SR, Bianchi R, Wong RKS, 2008. Signaling mechanisms underlying group I mGluR-induced persistent AHP suppression in CA3 hippocampal neurons. J. Neurophysiol 99, 1105–18. doi: 10.1152/jn.00435.2007 [DOI] [PubMed] [Google Scholar]
- Young SR, Chuang S-C, Zhao W, Wong RKS, Bianchi R, 2013. Persistent Receptor Activity Underlies Group I mGluR-Mediated Cellular Plasticity in CA3 Neuron. J. Neurosci 33, 2526–2540. doi: 10.1523/JNEUROSCI.3338-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, McBain CJ, 1995. Potassium conductances underlying repolarization and after-hyperpolarization in rat CA1 hippocampal interneurones. J. Physiol 488, 661–672. doi: 10.1113/jphysiol.1995.sp020998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziburkus J, Cressman J, Barreto E, Schiff S, 2006. Interneuron and Pyramidal Cell Interplay During In Vitro Seizure-Like Events. J. Neurophysiol 95, 3948–3954. doi : 10.1152/jn.01378.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]