Abstract
Introduction
Cardiovascular disease is a major cause of death in both men and women. While women are protected until the onset of menopause, after menopause women have increased risk of adverse cardiovascular disease events. Animal models of myocardial infarction recapitulate many of the sex differences observed in humans, and proteomics evaluations offer mechanistic insights to explain sex differences.
Areas covered
In this review, we will discuss how proteomics has helped us understand the hormonal, chromosomal, and immune mechanisms behind sex differences in response to ischemic injury and the development of heart failure.
Expert opinion
There are a number of ways in which proteomics has and will continue to facilitate our understanding of sex differences in cardiac remodeling after myocardial infarction.
Keywords: cardiovascular disease, myocardial infarction, cardiac remodeling, proteomics, sex differences, inflammation
1. Introduction
Cardiovascular disease is the major cause of death in developed countries for both men and women.[1] Men develop heart disease earlier and usually present with more severe coronary artery plaque formation than women. As a consequence, myocardial infarction (MI) incidences occurs 7 to 10 years earlier and is associated with more widespread plaque formation in men than in women. Even though women have decreased frequency of MI, acute mortality in the first days after MI is greater in younger women than in age-matched men.[2] Cardiac rupture of the left ventricle (LV) after acute MI has also been reported more frequently in young women than young men.[3] The risk of heart failure (HF) with preserved ejection fraction is also higher in women; however, men are more likely to develop HF with reduced ejection fraction.[2, 4, 5] In some but not all studies, a higher total in-hospital mortality rate after MI in women was accounted for on the basis of differences in age and comorbidities.[2, 6, 7] In addition, delayed or misdiagnosis also means fewer women receive current standard of care.[8] Overall, women have a higher mortality rate than men, dying less from arrhythmia and more from LV rupture independent of whether thrombolytic therapy was used or not.[2, 9] Despite being protected early in life, after menopause women have increased risk of adverse cardiovascular disease events; yet the mechanisms behind this phenomenon are not clearly understood.
Animal studies have added a better understanding of sex differences during the development of cardiovascular disease. In mice, young females have higher day 7 survival rates and decreased LV dilation.[10] Young females have increased expression of genes and proteins related to angiogenesis and extracellular matrix remodeling, with a blunted immune response compared to young male mice. Overall, this facilitates better prognosis after MI.[10–12] With age, the immune response including production of reactive oxygen species (ROS) becomes amplified in old female mice compared to young female counterparts.[10] An increase in immune response does not occur in male mice with age. While much of the literature focuses on the role of hormones, estrogen in particular, recent studies evaluating the effect of the sex chromosomes on disease progression have escalated. This includes evaluating the role of genetic regulation of the immune response.
Proteomics has been used extensively to determine mechanisms and identify biomarkers of cardiovascular disease development and prognosis.[13–19] Proteomics is commonly used to: 1) catalog protein constituents and quantify protein abundance; 2) refine genome annotation; 3) identify differences in post-translational modification on proteins and peptides; 4) investigate protein interactions and formation of complexes; 5) examine complex biological mixtures to enrich for distinct protein groups; and 6) obtain structural details.[14] While most associate proteomics to use of mass spectrometry, proteomics includes other approaches such as flow cytometry, multiplex immunoassays, surface plasmon resonance, and X-ray crystallography. In this review, we will discuss the benefit of using proteomics to uncover mechanisms behind sex differences to the response to ischemic injury and the development of heart failure.
2. Clinical assessment of sex differences
Before menopause, women have lower risk of MI than age-matched men. After menopause, estrogen levels decline and the risk of MI increases, implying that hormones play a role in cardiovascular disease protection in women. The role of estrogen and its receptors are corroborated by the fact that men who have non-functional estrogen receptors have early onset of coronary artery disease.[20] While the Women’s Health Initiative found no link between hormone replacement therapy and reduced risk of MI in postmenopausal women, there were a number of issues with the experimental design and technical aspects of that study.[21] The timing hypothesis revealed that when hormone replacement therapy was initiated made a difference. In 2013, the WHI Estrogen-Alone Trial published outcomes for a 10-year follow-up and found women in the 50- to 59-year-old group had significant reduction in MI risk (0.54 [0.34–0.86]; p-interaction=0.007), coronary heart disease (0.59 [0.38–0.90]; p-interaction=0.049), and total mortality (0.73 [0.59–1.00]; p-interaction=0.040).[21–24] Similar findings did not hold true for older women, especially those who had established atherosclerosis. Standard doses of oral hormonal therapy in the older group resulted in increased risk for coronary plaque thrombosis, instability, and mural rupture.[24–26]
3. Hormonal regulation of sex differences
Estrogen has direct benefits to many aspects of MI remodeling. Both estrogen receptors ERα and ERβ activate the Gα and Gβγ proteins, triggering transduction pathways including the phosphatidylinositol-3 kinase (PI3K)/protein kinase B (Akt) and p38/mitogen-activated protein kinases (MAPK) pathways.[27–30] Ex vivo, ERα binds to the regulatory subunit, p85a, of PI3K to enhance activation of Akt in the presence of estrogen.[27] Akt promotes cell survival by intervening before cytochrome c release and caspase activation.[31] Estrogen has also been linked to increased endothelial nitric oxide synthase (eNOS) activity and decreased leukocyte accumulation after ischemia and reperfusion injury.[27, 32–35] One possible mechanism is estrogen generates a feed-forward loop by G protein-coupled estrogen receptor 1 enhancement of calmodulin mediated eNOS activation.[36, 37]
Similarly to ERα, ERβ is protective after ischemia and reperfusion through increased PI3K/AKT and anti-apoptotic signaling.[38] Estrogen activation of ERβ in females blocks hypoxia-induced activation of JNK.[38, 39] In males, ablation of ERβ did not affect myocardial activation of JNK but instead increased ERK activity, indicating specific sex differences exist in ERβ-mediated signaling. ERβ is also protective through transforming growth factor (Tgf)β stimulation of cardiac ECM production after MI to help form a stable scar.[40]
While the majority of studies have highlighted the benefits of estrogen, others have noted abnormal concentrations of estrogen are detrimental.[41, 42] For example, Smith et al. concluded that estrogen during the early phase of MI (days 1–7) can be detrimental resulting in increased infarct expansion.[41] One caveat is that in this study, ovarectomized rats were given estrogen at elevated levels compared to what was found in intact animals. The detrimental effects of estrogen, therefore, were likely due to supraphysiological concentrations. This is in line with studies that have shown physiological levels of estrogen had no significant effect on mortality but improved cardiac physiology, reduced fibrosis, and increased capillary density.[42] Doses that raised plasma estrogen far beyond the physiological level exacerbated cardiac fibrosis, hypertrophy, and LV dysfunction and dilatation. These studies highlight the complexity of estrogen in cardiovascular disease and how estrogen concentrations or extent and type of receptor activation (ERα versus ERβ versus receptor independent actions), estrogen may play beneficial or detrimental roles.
While estrogen has been shown to be protective, testosterone has been shown to be the opposite.[43, 44] Testosterone promotes inflammation and significant myocardial expansion in both male and female mice during the acute MI remodeling phase.[44] During the latter MI phase, testosterone induces myocardial hypertrophy with a significant increase in LV mass.[45] Interestingly, at 8 weeks after MI, LV end-diastolic pressure and wall stress decreased with testosterone exposure. Future experiments using proteomic evaluations will help to dissect out additional mechanisms that distinguish direct and indirect effects of sex hormones on MI remodeling.
4. Chromosomal regulation of sex differences
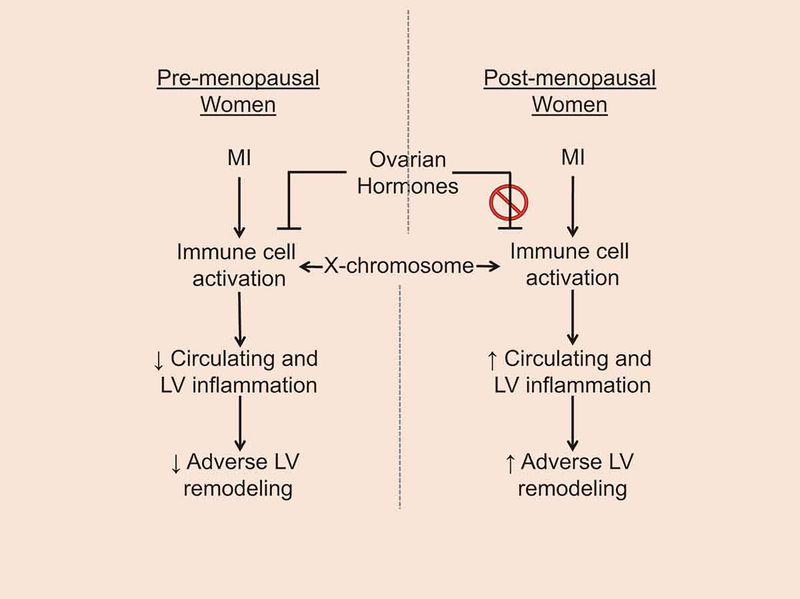
The contribution of the X chromosome in the development of cardiovascular disease is not fully understood. This is highlighted by the fact that the majority of genome-wide association studies do not include sex chromosomes.[46] Klinefelter patients (males born with an extra copy of the X chromosome) exhibit slightly lower testosterone concentrations compared to other men but much higher than women. Nonetheless, Klinefelter patients have a cytokine profile similar to that of women, suggesting that sex chromosomes are more influential than sex steroids in regards to the inflammatory response.[47] In gonadectomized female mice that have estrogen effects removed, the X chromosome promoted cardiac injury due to elevated expression of inflammatory genes (e.g., CD40 ligand, interleukin-1 receptor-associated kinase 1, FoxP3) and genes that promote apoptosis, lipid oxidation, and generation of oxygen-derived free radicals.[11, 48, 49] This provides an additional mechanism where women are protected by estrogens early in life, but after menopause, they are more susceptible to MI due to the detrimental effects of the genes encoded on the X chromosome (Figure 1).
Figure 1.
Schematic that illustrates immune cells as regulators of adverse cardiac remodeling after myocardial infarction through both X-chromosomal and hormonal mechanisms.
There are three proposed mechanisms for the role of genetic regulation in sex differences, and all three involve variations of X chromosome inactivation. X chromosome inactivation exists in two different forms: random and imprinted. Random X inactivation occurs early in the female embryo, where both maternal and paternal X chromosomes have an equal chance of becoming inactive.[50] Imprinting occurs during gamete production when DNA from the paternal parent becomes methylated to render the gene inactive.[51] Tissues of the female offspring therefore consist of mosaics of maternal and paternal X-chromosome genes.
In general, mosaicism is viewed as beneficial for women. For example, X chromosome mosaicism for gp91phox (NOX2) expression in females translates into a mixture of pro- and anti-inflammatory leukocytes during endotoxemia.[52, 53] This is due to the fact that females are a mixture of two kinds of cells: each with different functional chromosomes. In some cells, the copy from the mother is expressed, while the copy from the father is expressed in the other cells. This contributes to female mice having a dampened yet more efficient inflammatory response to ischemic injury.[10, 12]
X chromosome inactivation provides dosage compensation for X-linked genes between XX females and XY males.[51] For example, if a mother has a deleterious X mutation, the mutation is expressed in all cells of XY individuals but only half of the cells of XX individuals, making males more susceptible to X-linked mutations. Under normal conditions, a balanced gene expression between males and females is achieved due to X inactivation. However, X inactivation is not always 100% complete. In women, 15% of X chromosomal genes are overexpressed due to activation in both X chromosomes, while 3% are overexpressed in female mice.[54] Due to chromosomal differences, the use of mice may not fully recapitulate the role of the X chromosome. Spolarics et al. showed that blood neutrophils could be assessed for the X-linked protein marker glucose 6-phosphate dehydrogenase as a means to investigate female immune cell mosaicism using the unique inheritance pattern of X-linked polymorphisms.[55] Using a X-linked protein marker, cells can be separated and assessed for physiological differences linking mosaicism to changes in cellular function. Coupling this approach with a proteomics output would be a way to connect molecular to cell physiology.
In a mouse model of ischemia and reperfusion, four genes were more highly expressed in the myocardium from XX relative to XY mice: Eif2s3x, Kdm5c, Kdm6a, and Usp9x.[11] Eif2s3x serves as a translation initiation factor and regulates the rate of protein translation through interactions with eukaryotic translation initiation factor 2.[56] In the brain, sex differences in Eif2s3x transcription are not preserved at the protein level [56]; whether this holds true in the heart is unknown. Kdm5c and Kdm6a are two histone demethylases that affect transcription of numerous autosomal genes. Kdm6a is required for proper embryonic development of the heart as deletion leads to severe congenital heart defects.[57, 58] Usp9x, a ubiquitin-specific protease, is a novel mTORC1 and −2 binding partner that negatively regulates mTOR activity indicating it may be protective after MI.[59, 60] These four genes provide potential mechanistic sex differences in MI remodeling.
While most genes are consistently silent in one of the two X chromosomes, some genes exhibit tissue-specific differences. Assessment of adipose, liver, muscle, and brain tissue in a mouse showed striking differences in genes differentially expressed across individual tissues of males and females.[61] Across the four tissues profiled, a total of 71 genes displayed differential expression between sexes.[61] Out of these, only 27 showed either female-bias or male-bias in all tissues. The only common patterns were between liver and adipose tissue: steroid and lipid metabolism, oxidoreductase activity, and defense response were common to both.[61] In a separate study, X inactivation differed not only between tissues but also between developmental stages.[62] For example, histone methylation profiles for Mid1 was enriched only in female embryos but not in adult liver tissue, indicating Mid1 may be initially silenced during the embryonic stage only to escape X-inactivation later in development. Using proteomics to evaluate how genetic differences affect protein expression is needed to fully understand the role of sex chromosomes in cardiovascular disease.
5. Sex differences in immune regulation
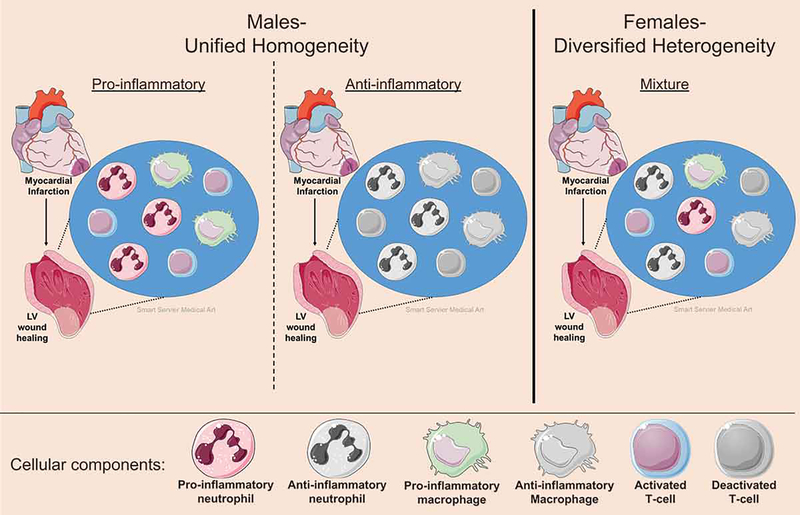
The effects of sex hormones and X chromosome mosaics are pertinent in the immune system response to MI. Men have a more homogeneous unified polarized cellular machinery compared to women whose immune cells are a mixture of diversified activation status (Figure 2). The resulting mosaic cellular variants provides fluctuations in the degree of cell trafficking, recruitment to injury sites, rates in necrosis or apoptosis, and cell proliferation in women.[52, 53, 63] Consequently, female mosaicism buffers the inflammatory response by downregulating hyper-active cell populations during excessive inflammation or compensates for a prolonged compensatory anti-inflammatory immune response, thereby improving the clinical course.[64]
Figure 2.
X-chromosomal cellular mosaicism results in phenotype diversity in females but increased functional polarity in males. Females are a mixture of cells that express genes from the mother or father, giving rise to a mosaic of cellular responses to ischemic injury. While cells from males are either pro- or anti-inflammatory, females can be a mixed bag containing both pro- and anti-inflammatory cells. This would improve the clinical course in females by downregulating hyper-active cell populations during excessive inflammation or compensating for a prolonged compensatory anti-inflammatory response. The figure was generated using Servier Medical Art (http://www.servier.com).
While estrogen has a significant impact on T-lymphocyte populations, recent evidence reveals a strong chromosomal role in T-cell mediated immunity.[65] CD8+ T-cells from pre- and postmenopausal women have upregulated pro-inflammatory genes to a greater extent than CD8+ T-cells from men in multiple inflammatory diseases including in HIV infection, lupus, and allergic asthma.[66–68] In vitro, upon activation mediated by CD3 and CD28 signaling, both mouse and human female lymphocytes partially reactivate the inactive X-chromosome, resulting in activation of both X chromosomes and an overexpression of immunity-related genes.[69]
Higher mortality rates in male mice after MI correlate with increased neutrophil infiltration into the ischemic and border zones.[10, 70] In a mouse MI model, neutrophils isolated from the infarct of females showed reduced pro-inflammatory N1 and greater anti-inflammatory N2 polarization.[10] In addition, neutrophils from females had a greater tissue clearance capacity compared to neutrophils isolated from males. Matrix metalloprotease-9, a neutrophil granule component, was elevated in males at MI day 4 when there is a maximal LV rupture risk.[70] This was opposite to what was observed in vitro, where apolipoprotein F stimulation of neutrophils increased tissue clearance capacity in both sexes.[10] These results signify the stimulus needed to activate female neutrophils is heterogeneous and supports the theory of mosaicism differentially altering female responses.
The effect of female hormones on neutrophils varies depending on the experimental context and tissue type.[71] After carotid artery injury, estrogen down-regulated neutrophil chemotaxis by decreasing expression of three adhesion molecules (P-selectin, intercellular adhesion molecule-1, and vascular cell adhesion molecule).[72] In contrast, estrogen given to healthy ovarectomized mice led to an upregulation in neutrophil numbers in the spleen, peripheral blood, and bone marrow, and increase degranulation compared to the placebo group.[71, 73] The female sex hormones estrogen and progesterone also mediate delayed neutrophil apoptosis in both sexes and enhance female intracellular production of ROS.[74]
Macrophage mediated inflammation is attenuated in female compared to male animals.[10, 75, 76] Female peritoneal macrophages exhibit greater toll-like receptor expression, as well as enhanced phagocytosis, providing female resident macrophages with a more efficient acute inflammatory response.[65] Infiltrating macrophages within the myocardium from male mice following coxsackievirus B3 (CVB3) infection express high levels of classically activated macrophages (M1) markers, whereas females show enhanced expression of alternatively activated macrophage (M2) phenotype.[77] Interestingly, glycoproteomic evaluation revealed that older women (average age of 73±9 years) who developed heart failure after MI had an increase in plasma proteins associated with macrophage mediated ROS production compared to men, indicating after menopause the protection from macrophage mediated inflammation was lost.[10]
Cellular metabolism plays a role in immune cell physiology. For example, pro-inflammatory (M1) macrophage metabolism is characterized by high glycolysis and relatively low oxidative phosphorylation, whereas M2 macrophage metabolism is characterized by oxidative phosphorylation, fatty acid oxidation, and upregulated arginase 1 activity.[78] Estrogen-signaling mainly through ERα is known to regulate metabolic pathways by altering glucose homeostasis.[79, 80] In addition, estrogen promotes an anti-inflammatory and pro-resolving macrophage phenotype through activation of ERα.[28, 81, 82] Glycoproteomic analysis of plasma from patients with MI who developed heart failure mapped to an increase in LXR/RXR pathway activation in men but not women, signifying heart failure in men is dependent on cholesterol and fatty acid homeostasis.[10] While strong evidence indicate hormones play a role in cellular metabolism, the impact of immunometabolism on cellular physiology requires further examination. In addition, dissecting out the hormonal versus chromosomal mechanisms at play during disease development using proteomic approaches is needed to fully understand sex differences in cardiovascular wound healing.
6. Conclusion
Proteomics has helped and will continue to help us understand the hormonal, chromosomal, and immune mechanisms behind sex differences in response to ischemic injury and the development of heart failure.
7. Expert Opinion
In this review, we summarized three possible mechanisms behind sex differences in MI response and the development of heart failure. While the majority of the research has focused on sex hormones, emerging evidence, including proteomic examinations, suggest that additional female-specific factors, such as X chromosomes in cardiovascular and immune cells, might act to increase, rather than decrease, risk for cardiovascular disease. Understanding the roles each play and dissecting the overall effect will facilitate in our understanding of sex differences in cardiovascular disease.
There are a number of future directions for this line of research (Table 1). Additional studies evaluating alterations to protein abundance or post-translational modification that may be involved in cardiovascular disease pathogenesis would facilitate our ability to distinguish direct and indirect effects of sex hormones on MI remodeling. Using protein markers associated with X-linked polymorphisms, cardiac derived cells can be sorted using an antibody based technique and assessed for cell activation status to facilitate our understanding of the role of genetic influences. Assessment of immunometabolism on cellular physiology using a multi-omics approach including metabolomics, lipidomics, and proteomics could also provide a system-wide view of the metabolic changes in immune cells that occur during MI and dissect out the hormonal versus chromosomal mechanisms.
Table 1:
Proteomic experiments needed to reveal further sex differences in MI remodeling.
| 1. Proteomic assessment of immune cells as well as circulating and myocardial proteins for cell activation status to facilitate in our understanding of the role of ovarian hormones versus genetic influences. |
| 2. Proteomic evaluations to dissect out additional mechanisms that distinguish direct and indirect effects of sex hormones on MI remodeling. |
| 3. Proteomics to evaluate how genetic differences affect protein expression to understand the role of sex chromosomes in cardiovascular disease. |
| 5. Proteomic data can facilitate our understanding of mosaicism to link protein expression differences to remodeling responses in males and females. |
| 6. Proteomics can help to assess the impact of immunometabolism on cellular physiology by dissecting out the hormonal versus chromosomal mechanisms at play during disease development. |
As highlighted in a recent report of the NHLBI Working Group on Sex Differences Research in Cardiovascular Disease, to address these questions one must choose an appropriate experimental model.[83] In addition to the usual consideration of whether the model mimics the human condition, it is also important to consider whether the model mimics the disease in the appropriate sex. In regards to preclinical studies, one must consider that physiologically estrogen does not remain elevated throughout the lifespan of a woman. Instead, estrogen and other ovarian hormones cycle making evaluation of sex hormones and their roles in wound healing difficult to study if the right model is not used.
In addition, transparency in the sex of cells used for in vitro studies is necessary. An estimated <30% of articles published related to CVD report the sex of the cells used. This lack of transparency may in part, contribute to the growing concern over reproducibility of research findings in preclinical studies.[83] Of note, in vitro studies are not the only ones that have not reported sex of animals used. In a recent report published in AJP-Heart and Circulatory Physiology, 16% of cardiovascular studies that measured echocardiography did not report sex of the animal used; of note, 21% of the studies used both sexes while over half only used males only.[84]
Targeted inclusion of both sexes in current research programs will improve public health. In order to understand the basic biology of sex differences, more preclinical studies evaluating differences at the genetic, proteomic, cellular, and physiological level are needed. While the endpoint is the same, the path to disease development is often different by gender.[10] In addition, closer evaluation of underlying causes such as age at onset, severity of progression, and symptom presentation are necessary.[51, 83, 85]
Proteomics will continue to provide insight into the mechanisms that define sex differences in cardiac remodeling after myocardial infarction, which will impact diagnosis, treatment, and outcomes for both men and women.
Article highlights.
Myocardial infarction (MI) incidences occurs 7 to 10 years earlier and is associated with more widespread plaque formation in men than in women.
Estrogen has direct benefits to many aspects of MI remodeling, while testosterone has detrimental effects.
While research has focused on sex hormones, additional female-specific factors, such as X chromosomes in cardiovascular and immune cells, might act to alter risk for cardiovascular disease.
Proteomics is a useful technology to uncover sex differences in cardiac remodeling after myocardial infarction.
Acknowledgments
Funding
This paper was funded by the National Institutes of Health under award numbers HL075360, HL129823, and HL137319, U54DA016511, and from the Biomedical Laboratory Research and Development Service of the Veterans Affairs Office of Research and Development under award numbers 5I01BX000505 and IK2BX003922. This work was also financially supported, in part, by the 2019 S&R Foundation Ryuji Ueno Award that was bestowed to Dr. DeLeon-Pennell by the American Physiological Society. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the Veterans Administration, or the American Physiological Society.
Footnotes
Declaration of Interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as:
* of interest
** of considerable interest
- 1.Benjamin EJ, Virani SS, Callaway CW, et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation 2018;137:e67–e492. [DOI] [PubMed] [Google Scholar]
- 2.Mehta LS, Beckie TM, DeVon HA, et al. Acute Myocardial Infarction in Women: A Scientific Statement From the American Heart Association. Circulation 2016;133:916–47. [DOI] [PubMed] [Google Scholar]
- 3.Hutchins KD, Skurnick J, Lavenhar M, et al. Cardiac rupture in acute myocardial infarction: a reassessment. Am J Forensic Med Pathol 2002;23:78–82. [DOI] [PubMed] [Google Scholar]
- 4.Azad N, Kathiravelu A, Minoosepeher S, et al. Gender differences in the etiology of heart failure: A systematic review. J Geriatr Cardiol 2011;8:15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duca F, Zotter-Tufaro C, Kammerlander AA, et al. Gender-related differences in heart failure with preserved ejection fraction. Sci Rep 2018;8:1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vaccarino V, Parsons L, Peterson ED, et al. Sex differences in mortality after acute myocardial infarction: changes from 1994 to 2006. Arch Intern Med 2009;169:1767–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matura LA. In-hospital Mortality Characteristics of Women With Acute Myocardial Infarction. J Clin Med Res 2009;1:262–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia M, Mulvagh SL, Merz CN, et al. Cardiovascular Disease in Women: Clinical Perspectives. Circ Res 2016;118:1273–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marrugat J, Sala J, Masia R, et al. Mortality differences between men and women following first myocardial infarction. RESCATE Investigators. Recursos Empleados en el Sindrome Coronario Agudo y Tiempo de Espera. JAMA 1998;280:1405–9. [DOI] [PubMed] [Google Scholar]
- 10.DeLeon-Pennell KY, Mouton AJ, Ero OK, et al. LXR/RXR signaling and neutrophil phenotype following myocardial infarction classify sex differences in remodeling. Basic Res Cardiol 2018;113:40.** This article defined the proteomic basis for differences in both humans and mice after myocardial infarction.
- 11.Li J, Chen X, McClusky R, et al. The number of X chromosomes influences protection from cardiac ischaemia/reperfusion injury in mice: one X is better than two. Cardiovasc Res 2014;102:375–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Q, Williams R, Healy CL, et al. An association between gene expression and better survival in female mice following myocardial infarction. J Mol Cell Cardiol 2010;49:801–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindsey ML, Weintraub ST and Lange RA. Using extracellular matrix proteomics to understand left ventricular remodeling. Circ Cardiovasc Genet 2012;5:o1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindsey ML, Mayr M, Gomes AV, et al. Transformative Impact of Proteomics on Cardiovascular Health and Disease: A Scientific Statement From the American Heart Association. Circulation 2015;132:852–72. [DOI] [PubMed] [Google Scholar]
- 15.Lindsey ML, Hall ME, Harmancey R, et al. Adapting extracellular matrix proteomics for clinical studies on cardiac remodeling post-myocardial infarction. Clin Proteomics 2016;13:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lam MP, Ping P and Murphy E. Proteomics Research in Cardiovascular Medicine and Biomarker Discovery. J Am Coll Cardiol 2016;68:2819–2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edwards AV, White MY and Cordwell SJ. The role of proteomics in clinical cardiovascular biomarker discovery. Mol Cell Proteomics 2008;7:1824–37. [DOI] [PubMed] [Google Scholar]
- 18.Anderson L Candidate-based proteomics in the search for biomarkers of cardiovascular disease. J Physiol 2005;563:23–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vasan RS. Biomarkers of cardiovascular disease: molecular basis and practical considerations. Circulation 2006;113:2335–62. [DOI] [PubMed] [Google Scholar]
- 20.Sudhir K, Chou TM, Chatterjee K, et al. Premature coronary artery disease associated with a disruptive mutation in the estrogen receptor gene in a man. Circulation 1997;96:3774–7. [DOI] [PubMed] [Google Scholar]
- 21.Hsia J, Langer RD, Manson JE, et al. Conjugated equine estrogens and coronary heart disease: the Women’s Health Initiative. Arch Intern Med 2006;166:357–65. [DOI] [PubMed] [Google Scholar]
- 22.Espeland MA, Rapp SR, Shumaker SA, et al. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women’s Health Initiative Memory Study. JAMA 2004;291:2959–68. [DOI] [PubMed] [Google Scholar]
- 23.LaCroix AZ, Chlebowski RT, Manson JE, et al. Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy: a randomized controlled trial. JAMA 2011;305:1305–14.*This study defined the time hypothesis of estrogen treatment and explained the previous null effects of estrogen in the Women’s Helath Initiative Trial.
- 24.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA 2002;288:321–33. [DOI] [PubMed] [Google Scholar]
- 25.Manson JE, Hsia J, Johnson KC, et al. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med 2003;349:523–34. [DOI] [PubMed] [Google Scholar]
- 26.Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA 1998;280:605–13. [DOI] [PubMed] [Google Scholar]
- 27.Simoncini T, Hafezi-Moghadam A, Brazil DP, et al. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature 2000;407:538–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang QG, Raz L, Wang R, et al. Estrogen attenuates ischemic oxidative damage via an estrogen receptor alpha-mediated inhibition of NADPH oxidase activation. J Neurosci 2009;29:13823–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Acconcia F and Kumar R. Signaling regulation of genomic and nongenomic functions of estrogen receptors. Cancer Lett 2006;238:1–14. [DOI] [PubMed] [Google Scholar]
- 30.Kumar P, Wu Q, Chambliss KL, et al. Direct interactions with G alpha i and G betagamma mediate nongenomic signaling by estrogen receptor alpha. Mol Endocrinol 2007;21:1370–80. [DOI] [PubMed] [Google Scholar]
- 31.Kennedy SG, Kandel ES, Cross TK, et al. Akt/Protein kinase B inhibits cell death by preventing the release of cytochrome c from mitochondria. Mol Cell Biol 1999;19:5800–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zakeri R, Vallejo-Giraldo C, Taylor KE, et al. Using heart failure with preserved ejection fraction to understand an ‘omics approach to evaluating vascular dysfunction and cardiovascular disease. J Neurol Neurophysiol 2011;S1:006. [Google Scholar]
- 33.Chang Y, Han Z, Zhang Y, et al. G protein-coupled estrogen receptor activation improves contractile and diastolic functions in rat renal interlobular artery to protect against renal ischemia reperfusion injury. Biomed Pharmacother 2019;112:108666. [DOI] [PubMed] [Google Scholar]
- 34.Veenema R, Casin KM, Sinha P, et al. Inorganic arsenic exposure induces sex-disparate effects and exacerbates ischemia-reperfusion injury in the female heart. Am J Physiol Heart Circ Physiol 2019;316:H1053–H1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang S, Bae L and Zhang L. Estrogen increases eNOS and NOx release in human coronary artery endothelium. J Cardiovasc Pharmacol 2000;36:242–7. [DOI] [PubMed] [Google Scholar]
- 36.Jayachandran M, Hayashi T, Sumi D, et al. Temporal effects of 17beta-estradiol on caveolin-1 mRNA and protein in bovine aortic endothelial cells. Am J Physiol Heart Circ Physiol 2001;281:H1327–33. [DOI] [PubMed] [Google Scholar]
- 37.Tran QK, Firkins R, Giles J, et al. Estrogen Enhances Linkage in the Vascular Endothelial Calmodulin Network via a Feedforward Mechanism at the G Protein-coupled Estrogen Receptor 1. J Biol Chem 2016;291:10805–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang M, Wang Y, Weil B, et al. Estrogen receptor beta mediates increased activation of PI3K/Akt signaling and improved myocardial function in female hearts following acute ischemia. Am J Physiol Regul Integr Comp Physiol 2009;296:R972–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang M, Tsai BM, Reiger KM, et al. 17-beta-Estradiol decreases p38 MAPK-mediated myocardial inflammation and dysfunction following acute ischemia. J Mol Cell Cardiol 2006;40:205–12. [DOI] [PubMed] [Google Scholar]
- 40.Zhang JB and Guo CL. Protective effect and mechanism of estrogen receptor beta on myocardial infarction in mice. Exp Ther Med 2017;14:1315–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith PJ, Ornatsky O, Stewart DJ, et al. Effects of estrogen replacement on infarct size, cardiac remodeling, and the endothelin system after myocardial infarction in ovariectomized rats. Circulation 2000;102:2983–9. [DOI] [PubMed] [Google Scholar]
- 42.Zhan E, Keimig T, Xu J, et al. Dose-dependent cardiac effect of oestrogen replacement in mice post-myocardial infarction. Exp Physiol 2008;93:982–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cavasin MA, Sankey SS, Yu AL, et al. Estrogen and testosterone have opposing effects on chronic cardiac remodeling and function in mice with myocardial infarction. Am J Physiol Heart Circ Physiol 2003;284:H1560–9. [DOI] [PubMed] [Google Scholar]
- 44.Cavasin MA, Tao ZY, Yu AL, et al. Testosterone enhances early cardiac remodeling after myocardial infarction, causing rupture and degrading cardiac function. Am J Physiol Heart Circ Physiol 2006;290:H2043–50. [DOI] [PubMed] [Google Scholar]
- 45.Nahrendorf M, Frantz S, Hu K, et al. Effect of testosterone on post-myocardial infarction remodeling and function. Cardiovascular research 2003;57:370–8. [DOI] [PubMed] [Google Scholar]
- 46.Regitz-Zagrosek V and Kararigas G. Mechanistic Pathways of Sex Differences in Cardiovascular Disease. Physiol Rev 2017;97:1–37.** Provides a comprehensive review of sex differences in cardiovascular disease.
- 47.Lefevre N, Corazza F, Valsamis J, et al. The Number of X Chromosomes Influences Inflammatory Cytokine Production Following Toll-Like Receptor Stimulation. Front Immunol 2019;10:1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stamova B, Tian Y, Jickling G, et al. The X-chromosome has a different pattern of gene expression in women compared with men with ischemic stroke. Stroke 2012;43:326–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brooks WH and Renaudineau Y. Epigenetics and autoimmune diseases: the X chromosome-nucleolus nexus. Front Genet 2015;6:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Borsani G, Tonlorenzi R, Simmler MC, et al. Characterization of a murine gene expressed from the inactive X chromosome. Nature 1991;351:325–9. [DOI] [PubMed] [Google Scholar]
- 51.Arnold AP. A general theory of sexual differentiation. J Neurosci Res 2017;95:291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chandra R, Federici S, Hasko G, et al. Female X-chromosome mosaicism for gp91phox expression diversifies leukocyte responses during endotoxemia. Crit Care Med 2010;38:2003–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chandra R, Federici S, Nemeth ZH, et al. Female X-chromosome mosaicism for NOX2 deficiency presents unique inflammatory phenotype and improves outcome in polymicrobial sepsis. J Immunol 2011;186:6465–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Disteche CM. Escape from X inactivation in human and mouse. Trends Genet 1995;11:17–22. [DOI] [PubMed] [Google Scholar]
- 55.Spolarics Z The X-files of inflammation: cellular mosaicism of X-linked polymorphic genes and the female advantage in the host response to injury and infection. Shock 2007;27:597–604.* An example of how to use proteomics techniques to evaluate mosiacsim.
- 56.Xu J, Watkins R and Arnold AP. Sexually dimorphic expression of the X-linked gene Eif2s3x mRNA but not protein in mouse brain. Gene Expr Patterns 2006;6:146–55. [DOI] [PubMed] [Google Scholar]
- 57.Shpargel KB, Sengoku T, Yokoyama S, et al. UTX and UTY demonstrate histone demethylase-independent function in mouse embryonic development. PLoS Genet 2012;8:e1002964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee S, Lee JW and Lee SK. UTX, a histone H3-lysine 27 demethylase, acts as a critical switch to activate the cardiac developmental program. Dev Cell 2012;22:25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Agrawal P, Chen YT, Schilling B, et al. Ubiquitin-specific peptidase 9, X-linked (USP9X) modulates activity of mammalian target of rapamycin (mTOR). J Biol Chem 2012;287:21164–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Buss SJ, Muenz S, Riffel JH, et al. Beneficial effects of Mammalian target of rapamycin inhibition on left ventricular remodeling after myocardial infarction. J Am Coll Cardiol 2009;54:2435–46. [DOI] [PubMed] [Google Scholar]
- 61.Yang X, Schadt EE, Wang S, et al. Tissue-specific expression and regulation of sexually dimorphic genes in mice. Genome Res 2006;16:995–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang F, Babak T, Shendure J, et al. Global survey of escape from X inactivation by RNA-sequencing in mouse. Genome Res 2010;20:614–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chandra R, Federici S, Nemeth ZH, et al. Cellular mosaicism for X-linked polymorphisms and IRAK1 expression presents a distinct phenotype and improves survival following sepsis. J Leukoc Biol 2014;95:497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Spolarics Z, Pena G, Qin Y, et al. Inherent X-Linked Genetic Variability and Cellular Mosaicism Unique to Females Contribute to Sex-Related Differences in the Innate Immune Response. Front Immunol 2017;8:1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Scotland RS, Stables MJ, Madalli S, et al. Sex differences in resident immune cell phenotype underlie more efficient acute inflammatory responses in female mice. Blood 2011;118:5918–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ito C, Okuyama-Dobashi K, Miyasaka T, et al. CD8+ T Cells Mediate Female-Dominant IL-4 Production and Airway Inflammation in Allergic Asthma. PLoS One 2015;10:e0140808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hewagama A, Patel D, Yarlagadda S, et al. Stronger inflammatory/cytotoxic T-cell response in women identified by microarray analysis. Genes Immun 2009;10:509–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Meier A, Chang JJ, Chan ES, et al. Sex differences in the Toll-like receptor-mediated response of plasmacytoid dendritic cells to HIV-1. Nat Med 2009;15:955–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang J, Syrett CM, Kramer MC, et al. Unusual maintenance of X chromosome inactivation predisposes female lymphocytes for increased expression from the inactive X. Proc Natl Acad Sci U S A 2016;113:E2029–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fang L, Gao XM, Moore XL, et al. Differences in inflammation, MMP activation and collagen damage account for gender difference in murine cardiac rupture following myocardial infarction. J Mol Cell Cardiol 2007;43:535–44.* Provides a comprehensive assessement of sex differences in mice after myocardial infarction.
- 71.Dai R, Cowan C, Heid B, et al. Neutrophils and neutrophil serine proteases are increased in the spleens of estrogen-treated C57BL/6 mice and several strains of spontaneous lupus-prone mice. PLoS One 2017;12:e0172105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Miller AP, Feng W, Xing D, et al. Estrogen modulates inflammatory mediator expression and neutrophil chemotaxis in injured arteries. Circulation 2004;110:1664–9. [DOI] [PubMed] [Google Scholar]
- 73.Plackett TP, Deburghraeve CR, Palmer JL, et al. Effects of Estrogen on Bacterial Clearance and Neutrophil Response After Combined Burn Injury and Wound Infection. J Burn Care Res 2016;37:328–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Molloy EJ, O’Neill AJ, Grantham JJ, et al. Sex-specific alterations in neutrophil apoptosis: the role of estradiol and progesterone. Blood 2003;102:2653–9. [DOI] [PubMed] [Google Scholar]
- 75.Li J, Ao L, Zhai Y, et al. Gender disparity in the role of TLR2 in post-ischemic myocardial inflammation and injury. Int J Clin Exp Med 2015;8:10537–47. [PMC free article] [PubMed] [Google Scholar]
- 76.Kararigas G, Dworatzek E, Petrov G, et al. Sex-dependent regulation of fibrosis and inflammation in human left ventricular remodelling under pressure overload. Eur J Heart Fail 2014;16:1160–7. [DOI] [PubMed] [Google Scholar]
- 77.Li K, Xu W, Guo Q, et al. Differential macrophage polarization in male and female BALB/c mice infected with coxsackievirus B3 defines susceptibility to viral myocarditis. Circ Res 2009;105:353–64. [DOI] [PubMed] [Google Scholar]
- 78.Diskin C and Palsson-McDermott EM. Metabolic Modulation in Macrophage Effector Function. Front Immunol 2018;9:270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Michalek RD, Gerriets VA, Nichols AG, et al. Estrogen-related receptor-alpha is a metabolic regulator of effector T-cell activation and differentiation. Proc Natl Acad Sci U S A 2011;108:18348–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mauvais-Jarvis F, Clegg DJ and Hevener AL. The role of estrogens in control of energy balance and glucose homeostasis. Endocr Rev 2013;34:309–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Campbell L, Emmerson E, Williams H, et al. Estrogen receptor-alpha promotes alternative macrophage activation during cutaneous repair. J Invest Dermatol 2014;134:2447–2457. [DOI] [PubMed] [Google Scholar]
- 82.Villa A, Rizzi N, Vegeto E, et al. Estrogen accelerates the resolution of inflammation in macrophagic cells. Sci Rep 2015;5:15224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Maric-Bilkan C, Arnold AP, Taylor DA, et al. Report of the National Heart, Lung, and Blood Institute Working Group on Sex Differences Research in Cardiovascular Disease: Scientific Questions and Challenges. Hypertension 2016;67:802–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lindsey ML, Kassiri Z, Virag JAI, et al. Guidelines for Measuring Cardiac Physiology in Mice. Am J Physiol Heart Circ Physiol 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Arnold AP, Cassis LA, Eghbali M, et al. Sex Hormones and Sex Chromosomes Cause Sex Differences in the Development of Cardiovascular Diseases. Arterioscler Thromb Vasc Biol 2017;37:746–756. [DOI] [PMC free article] [PubMed] [Google Scholar]