Abstract
The genus Dicliptera (Justicieae, Acanthaceae) consists of approximately 150 species distributed throughout the tropical and subtropical regions of the world. Newly obtained chloroplast genomes (cp genomes) are reported for five species of Dilciptera (D. acuminata, D. peruviana, D. montana, D. ruiziana and D. mucronata) in this study. These cp genomes have circular structures of 150,689–150,811 bp and exhibit quadripartite organizations made up of a large single copy region (LSC, 82,796–82,919 bp), a small single copy region (SSC, 17,084–17,092 bp), and a pair of inverted repeat regions (IRs, 25,401–25,408 bp). Guanine-Cytosine (GC) content makes up 37.9%–38.0% of the total content. The complete cp genomes contain 114 unique genes, including 80 protein-coding genes, 30 transfer RNA (tRNA) genes, and four ribosomal RNA (rRNA) genes. Comparative analyses of nucleotide variability (Pi) reveal the five most variable regions (trnY-GUA-trnE-UUC, trnG-GCC, psbZ-trnG-GCC, petN-psbM, and rps4-trnL-UUA), which may be used as molecular markers in future taxonomic identification and phylogenetic analyses of Dicliptera. A total of 55-58 simple sequence repeats (SSRs) and 229 long repeats were identified in the cp genomes of the five Dicliptera species. Phylogenetic analysis identified a close relationship between D. ruiziana and D. montana, followed by D. acuminata, D. peruviana, and D. mucronata. Evolutionary analysis of orthologous protein-coding genes within the family Acanthaceae revealed only one gene, ycf15, to be under positive selection, which may contribute to future studies of its adaptive evolution. The completed genomes are useful for future research on species identification, phylogenetic relationships, and the adaptive evolution of the Dicliptera species.
Keywords: Dicliptera, Chloroplast genome, Species identification, Phylogeny, Adaptive evolution, Molecular markers
Introduction
The genus Dicliptera Juss. belongs to tribe Justicieae of the family Acanthaceae; it consists of approximately 150 species, which are typically found in the tropical and subtropical regions of the world (Scotland & Vollesen, 2000; Mabberley, 2017; Hu et al., 2011). It is readily recognized by umbellately arranged, rarely solitary, cymose inflorescence units (cymules) subtended by conspicuously paired bracts, anthers with two partially or completely superposed thecae and, in the Palaeotropics, resupinate corollas that lack a rugula (Darbyshire, 2008). Eleven species of Dicliptera are found in Peru, most of which are located in the Andes (Brako & Zarucchi, 1993; León, 2006). Species such as Dicliptera chinensis, D. peruviana, and D. verticillata are used in traditional herbal medicines in China and Peru (Bussmann & Glenn, 2010; Horacio, Graciela & Percy, 2007; Telefo, Moundipa & Tchouanguep, 2002; Zhang, Zhu & Gao, 2010). Species delimitation within Dicliptera is difficult (Balkwill, Norris & Balkwill, 1996) due, in part, to the remarkable uniformity of the floral morphology in the majority of the taxa. Its taxonomy is also confounded by the presence of several widespread species complexes (Darbyshire, 2008). The taxonomic difficulties make it important to analyze the species using molecular analysis, infrageneric classification, and the relationships within Dicliptera. Kiel et al. (2017) conducted the only phylogenetic analysis of the tribe Justicieae, using five chloroplast regions (ndhF-trnL, trnT-trnL-UAG, trnS-trnG, ndhA, rpl16) and one nuclear region (nrITS). However, the interspecific relationships within Dicliptera have not been determined because of the limited number of samples available.
Five common Peruvian species were collected, representing the genus Dicliptera (D. acuminata, D. peruviana, D. montana, D. mucronata, and D. ruiziana). D. acuminata, D. montana, and D. peruviana are used in the agroindustrial industry (Victor, Jhan & Raúl, 2017); D. peruviana is a traditional herbal medicine used by the Andeans of Canta, Lima, Peru to alleviate stomach aches (Horacio, Graciela & Percy, 2007); D. mucronata is distributed mainly in Central America and is easily confused with D. scabra (Victor, Jhan & Raúl, 2017); D. ruiziana is found throughout Peru to elevations of about 3,000 m (Antonio Galán et al., 2009). All five species were collected in Southeast Peru and were distinguished from each other by the character of their leaves, bracts, bracteoles, and calyxes (Table 1). However, species delimitation is difficult without the aid of the flowers and the distribution of the five Dicliptera species is often overlapping. Species determinations in Central America are predominantly made by morphological comparisons as opposed to molecular comparisons. The study of the complete cp genomes of five Dicliptera species may encourage more effective species identification within the genus Dicliptera, especially in Central America.
Table 1. Morphological differences among Dicliptera acuminata, D. peruviana, D. montana, D. mucronata and D. ruiziana.
| Species | D. acuminata | D. peruviana | D. montana | D. mucronata | D. ruiziana |
|---|---|---|---|---|---|
| Plant height | Ca. 60 cm | Ca. 60 cm | Ca. 50 cm | 60–130 cm | Ca. 30 cm |
| Stem | Erect, branched, sulcate, hirsute | Erect, branched, sulcate, hirsute | Erect, branched, sulcate, pubescent | Erect, branched, sulcate, pubescent | Erect, branched, sulcate, pubescent |
| Leaf blade | Oblong-lanceolate, 3.5–7.0 × 1.5–2.5 cm, villous | Ovate, 3.5–6.0 × 2.5–4.0 cm, pubescent | Ovate, 1.0–1.5 × 0.8–1.0 cm, pilose when young, then glabrescent | Ovate, 3.0–3.5 × 1.5–2.0 cm, scarcely pilose | Ovate, 1.0–1.5 × 0.8–1.0 cm, pubescent |
| Leaf apex | Acuminate-acute | Acute | Acuminate | Acuminate-acute | Acute |
| Inflorescence | Verticillaster | Verticillaster | Spikelike thyrse | Verticillaster | Pedunculate cyme |
| Bracts | Lanceolate-linear, ciliate | Ovate, ciliate | Spatulate, gland-tipped pilose | Obovate-rhombic, pilose | Obovate, gland-tipped pilose |
| Bracteoles | Subulate, ciliate | Subulate, | Hyaline, asymmetrical, minute pilose | Linear-subulate, pilose | Subulate |
| Calyx lobes | Subulate, ciliate | Linear, hirsute | Lanceolate, minute pilose | Linear-lanceolate, margin minutely pubescent | Lanceolate, gland-tipped pilose |
| Corolla | Purple, outside pubescent | Purple, outside pubescent | Pale purple, outside pilose | Purplish red, outside pilose | Pink, outside pubescent |
| Style | scarcely pilose | glabrous | scarcely pilose | glabrous | scarcely pilose |
The chloroplast genome (cp genome) is an independent genome that has been used in many evolutionary studies (Fan et al., 2018; Gao, Wang & Deng, 2018; George et al., 2015; Chen et al., 2018; Inkyu et al., 2018; Kim & Lee, 2004; Wang et al., 2016; Mader et al., 2018; Meng et al., 2018; Ma et al., 2017; Raubeson et al., 2007; Wang et al., 2008; Wu et al., 2018). It has a simple structure with a low molecular weight and multiple copies. Most of the cp genomes have circular structures with quadripartite organizations composed of one large single copy region (LSC), one small single copy region (SSC), and a pair of inverted regions (IRs). However, there are numerous exceptions to the common structure, like the IR-lacking clade (IRLC) in Fabaceae (Fan et al., 2018; Gao, Wang & Deng, 2018; George et al., 2015; Chen et al., 2018; Inkyu et al., 2018; Kim & Lee, 2004; Wang et al., 2016; Mader et al., 2018; Meng et al., 2018; Schwarz et al., 2015). The complete cp genomes of more than 2,400 plants have been published, to date, in the NCBI database (http://www.ncbi.nlm.nih.gov/genome). The majority of plant cp genomes are 110 to 170 kb in length (Olmstead & Palmer, 1994; Weng et al., 2013; Wicke et al., 2011). The family Acanthaceae is a large family with approximately 230 genera and 4,300 species, yet only ten species from this family have fully sequenced cp genomes (Table S1).
The complete chloroplast genome is widely used for species identification, phylogenic studies, and studies in adaptive evolution (Wang et al., 2016; Ma et al., 2017; Fan et al., 2018; Gao, Wang & Deng, 2018). Adaptive evolution is defined as the suitability for the improvement of a species during its evolutionary processes. It is always driven by evolutionary processes such as natural selection and leads to biological pressures and biodiversity at all levels of biological organization (Yang & Swanson, 2002; Scottphillips et al., 2013; Hall & Strickberger, 2008). The non-synonymous (KA)/synonymous rate (KS) ratio (ω = KA/KS) provides a measure of selective pressure at the amino acid level. As suggested by Makalowski & Boguski (1998), the ω values less than one (KA/KS <1) indicate that the gene is under negative selection and vice versa (Wojciech & Mark, 1998; Meng et al., 2018). Recent studies have detected many positively selected chloroplast genes (KA/KS >1), such as the ndhC, ndhJ, psbK, psbN, rpl14, rpl16, rps4, rps15, rps18, rps19, infA, and rpoB genes in Echinacanthus and the petA, psbD, psbE, ycf3, psaI, rps4, psbM, ndhE, ndhG and rpoC1 genes in Allium (Gao, Wang & Deng, 2018; Xie et al., 2019).
The cp genomes of five Dicliptera species were sequenced, compared, and reported for the first time in this study. The five most variable regions were identified through genome comparison analysis and nucleotide variability; these were chosen as candidate molecular markers for taxonomic identification and systematic analysis in the future. Codon usage analysis was conducted to find the codon bias in the genus Dicliptera. 285 simple sequence repeats (SSRs), 21 polymorphic SSRs, and 229 long repeats were detected and described. The phylogenetic relationships of the five species and other members of the family Acanthaceae were analyzed. Finally, the orthologous protein-coding genes were identified in the family Acanthaceae and the selective pressure for these genes was analyzed. This work may contribute to future adaptive evolution analysis of the Acanthaceae species.
Material and Methods
Plant materials and DNA extraction
Fresh leaf tissues were collected during several botanical surveys conducted by South China Botanical Gardens, Chinese Academy of Sciences and Facultad de Ciencias Biológicas y Museo de Historia Natural, Universidad Nacional Mayor de San Marcos in Peru. The samples were dried in silica gel immediately after collection. Voucher specimens were deposited at the Museo de Historia Natural, Universidad Nacional Mayor de San Marcos (USM) and the herbarium of South China Botanical Garden, Chinese Academy of Sciences (IBSC) (Table 2). The specimens were visually identified by Deng Yunfei and the total genomic DNA was extracted using a modified CTAB method (Doyle & Doyle, 1987) that included 4% CTAB with 2% polyvinyl polypyrrolidone (PVP) (Yang, Li & Li, 2014).
Table 2. Species information and genome features of the chloroplast genomes of five Dicliptera species.
| Species | D. acuminata | D. peruviana | D. montana | D. ruiziana | D. mucronata |
|---|---|---|---|---|---|
| Location | 9.05°S, 77.81°W | 11.41°S, 77.23°W | 11.79°S, 77.05°W | 15.87°S, 74.15°W | 12.21°S, 76.82°W |
| Geographic region | Caraz, Paron, Peru | Lomas de lguanil, Huaral province, Peru | Lomas de Carabayllo, Lima province, Peru | Lomas de Cháparra, Caravelí province, Peru | Santuario del Amancay, Lima Province, Peru |
| Voucher specimens No. | P10-091 | P170099 | P170177 | P170209 | P170492 |
| Assemblied reads | 3745800 | 2103300 | 2272793 | 2108293 | 1927500 |
| Mean coverage | 3727.5 | 2092.0 | 2262.4 | 2097.8 | 1918.3 |
| Size (bp) | 150738 | 150811 | 150689 | 150750 | 150720 |
| LSC length (bp) | 82844 | 82919 | 82796 | 82843 | 82834 |
| SSC length (bp) | 17092 | 17090 | 17091 | 17091 | 17084 |
| IR length (bp) | 25401 | 25401 | 25401 | 25408 | 25401 |
| CDSs total length | 78714 | 78714 | 78714 | 78747 | 78717 |
| Number of total genes | 114 | 114 | 114 | 114 | 114 |
| Number of Protein-coding genes | 80 | 80 | 80 | 80 | 80 |
| Number of tRNA genes | 30 | 30 | 30 | 30 | 30 |
| Number of rRNA genes | 4 | 4 | 4 | 4 | 4 |
| Overall GC content (%) | 38.0% | 38.0% | 38.0% | 38.0% | 38.0% |
| GC content in LSC (%) | 36.0% | 36.0% | 36.0% | 36.0% | 36.0% |
| GC content in SSC (%) | 31.9% | 31.7% | 31.9% | 31.9% | 31.6% |
| GC content in IR (%) | 43.3% | 43.4% | 43.3% | 43.3% | 43.4% |
| Genbank accession number | MK830556 | MK833945 | MK833946 | MK833947 | MK848596 |
Genome sequencing, assembly, and annotation
Short-insert (300–500 bp) libraries were constructed using the Nextera XT DNA Library Prep Kit (Illumina) following the manufacturer’s instructions. Illumina × Ten instruments at BGI-Wuhan were used to perform paired-end (PE) sequencing for each sample. GetOrganelle v. 1.6.2 (Jin et al., 2018) was used to assemble the sequenced PE reads. Andrographis paniculata (GenBank accession no. NC_022451) served as a reference and the sequenced reads were filtered using Bowtie2 v. 2.3.5.1 (Langmead & Salzberg, 2012); SPAdes v. 3.13.1 (Bankevich et al., 2012) was used to assemble the filtered plastid reads and the final “fastg” files were reduced using the “slim_fastg.py” script in GetOrganelle to retain the pure plastid contigs; the filtered De Brujin graph files were transferred to Bandage v. 0.8.1 (Wick et al., 2015) for visualization and to obtain the paths of the final “fasta” files of the cp genomes; finally, the genome structures of all five species were compared to the reference genome using Mauve v. 1.1.1 software (Darling, Mau & Perna, 2010) to determine the accuracy of the final genome. The assembled cp genome was annotated using PGA v. 2019 (Qu et al., 2019) using the annotated A. paniculata as a reference. The boundaries of the annotated genes were manually modified and coupled with CDSs in Geneious v. 2019.0.3 (Kearse et al., 2012). All transfer RNA (tRNA) genes were determined using tRNAscan-SE v. 2.0 (Schattner, Brooks & Lowe, 2005). The annotated cp genome files were submitted to OGDRAW v. 1.3.1 (https://chlorobox.mpimp-golm.mpg.de/OGDraw.html) (Greiner, Lehwark & Bock, 2019) to create a circular cp genome map for each species. The five cp genomes (D. acuminata, MK830556; D. pruviana, MK833945; D. montana, MK833946; D. ruiziana, MK833947; D. mucronata, MK848596) were submitted to Genbank.
Genome comparison and structural analysis
Five cp genomes were compared using mVISTA v. 2.0 (Frazer et al., 2004) with Shuffle-LAGAN mode and the annotation of D. acuminata as a reference (Brudno et al., 2003). The conserved regions were visualized on an mVISTA plot. DnaSP v. 5.1 (Librado & Rozas, 2009) was used to calculate the nucleotide variance (Pi) within the five Dicliptera species. The SC and IR boundaries were compared with A. paniculata as a reference. The Relative Synonymous Codon Usage (RSCU) of all protein-coding genes was analyzed for each species using CondoW v. 1.4.2 (Sharp & Li, 1987). MISA v. 1.0 (Thiel et al., 2003) was used to identify the simple sequence repeats (SSRs). The locations and lengths of long repeats (including forward, palindrome, complement, and reverse repeats) were analyzed using REPuter v. 2.74 (Kurtz et al., 2001) with the minimum repeat size set to 20 bp. Tandem repeats were identified using Tandem Repeats Finder v. 4.09 (Benson, 1999).
Phylogenetic analyses
Phylogenetic analysis was conducted for all of the sequenced cp genomes (each cp genome included only one IR), including the five species reported in this study and ten previously reported species of Acanthaceae (Table S1). Sesamum indicum (NC_016433) (Pedaliaceae) and Mentha spicata (NC_037247) (Lamiaceae) were used as outgroups. The complete cp genomes were aligned using MAFFT v. 1.3.7 (Katoh & Standley, 2013) and were adjusted manually as needed. The substitution models with the best fit were chosen by MrModeltest v. 2.3 (Nylander, 2004) based on the Akaike Information Criterion (AIC). RAxML v. 8.0.0 (Stamatakis, 2014) was used to reconstruct the phylogenetic relationship with the maximum likelihood (ML) method. Maximum parsimony (MP) analysis was run in Paup v. 4.0a (Swofford, 2003). Bootstrap values exceeding 50% were shown next to the corresponding branches. Bayesian inference (BI) analysis was conducted using MrBayes 3.2.7 (Ronquist & Huelsenbeck, 2003) with posterior probabilities (PP) obtained for each branch.
Selective pressure analysis
OrthoMCL v. 2.0 (Li, Stoeckert & Roos, 2003) was used to find the orthologous genes for the family Acanthaceae. The sequences for each orthologous gene were aligned separately using MAFFT v. 1.3.7 (Katoh & Standley, 2013). The nonsynonymous (KA) and synonymous (K S) substitution rates were calculated using PAML v. 4.9 with the codeml program to analyze the selective pressures of every orthologous gene sequence (Yang, 2007). The ω value (ω = KA/KS) was estimated using the method reported by Yang & Nielsen (2000). The genes under positive selection were confirmed by computing the likelihood ratio tests (LRTs).
Results
Chloroplast genome features
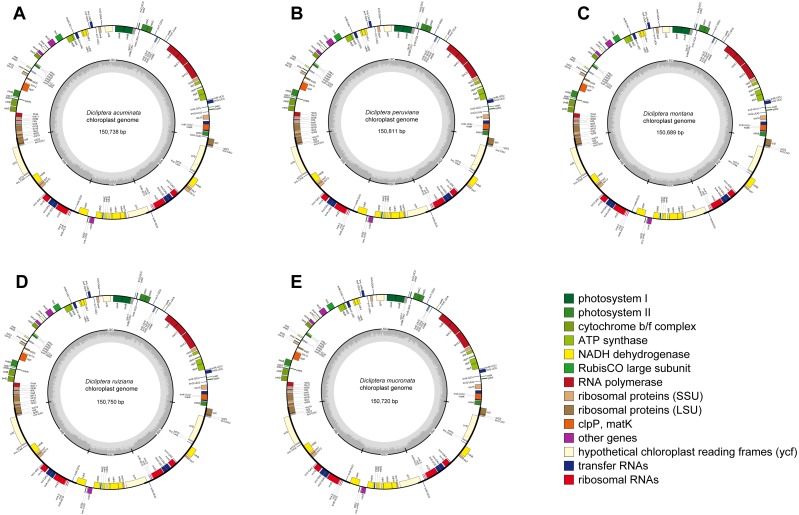
The average assemblies of the five cp genomes varied from 1918.3 to 3727.5 bp. The cp genome sequences were 150,738 bp (Dicliptera acuminata), 150,811 bp (D. peruviana), 150,689 bp (D. montana), 150,750 bp (D. ruiziana), and 150,720 bp (D. mucronata) in length (Table 2). Each of the sequences encoded 80 protein-coding genes, 30 transfer RNA (tRNA) genes, and 4 ribosomal RNA (rRNA) genes (Table 2 and Table S2; Fig. 1), of which three protein-coding genes had two introns and nine had one intron. The Rps12 gene was trans-spliced because of the locations of the first exon at the LSC and the other two exons at the IRs. Six protein-coding genes (rpl2, rpl23, ycf2, ycf15, ndhB, and rps7), seven tRNA (trnI-CAU, trnI-GAU, trnL-CAA, trnN-GUU, trnR-ACG, trnV-GAC, and trnA-UGC), and all four rRNA (rrn4.5, rrn5, rrn16 and rrn23) had two copies because of their location at the IR regions. The rps12 gene was identified as the pseudogene in D. mucronata by the existence of internal stop codons.
Figure 1. Gene maps of chloroplast genomes.
(A) Dicliptera acuminata; (B) D. peruviana; (C) D. montana; (D) D. ruiziana; (E) D. mucronata. Genes shown outside of the circle are transcribed clockwise, whereas genes inside of the circle are transcribed counterclockwise. The colored bars indicate known protein-coding genes, tRNA and rRNA. The dark gray area in the inner circle indicates GC content, while the light gray area indicates AT content. LSC, large single copy; SSC, small single copy; IR, inverted repeats.
The five cp genomes displayed a typical quadripartite structure, including a large single copy (LSC) region (range from 82,796–82,919 bp), a small single copy (SSC) region (range from 17,084–17,092 bp), and a pair of inverted repeat (IR) regions (25,401–25,408 bp; Table 2). The Guanine-Cytosine (GC) content of the cp genomes of the five Dicliptera was approximately 38.0%. The GC content in the IR regions (43.3–43.4%) was noticeably above that of the LSC (36.0%) and SSC (31.6–31.9%) regions in each cp genome.
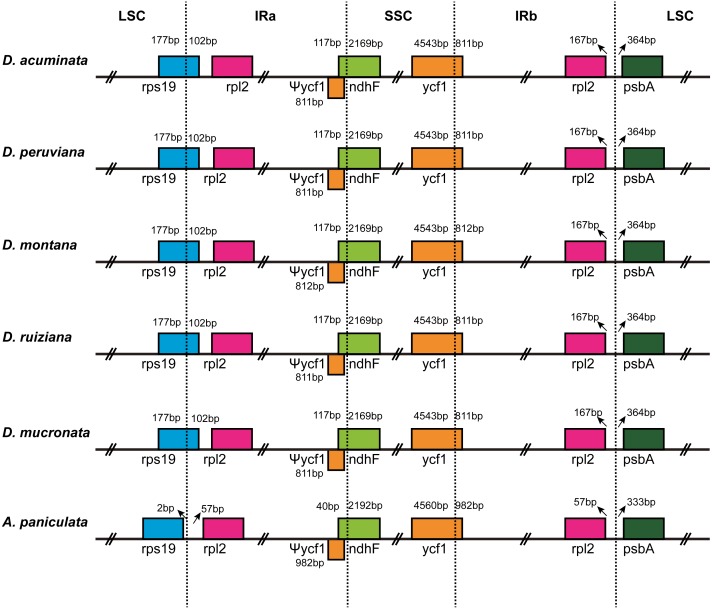
IR contraction and expansion
The IR/LSC and IR/SSC borders of the cp genomes of the five Dicliptera species and the A. paniculata were compared to identify the expansion or contraction of the IR (Fig. 2). The genes, rps19, rpl2, ndhF, ycf1, and psbA were present at the juncture of the LSC/IRa, IRa/SSC, SSC/IRb, and IRb/LSC borders. The five Dicliptera species have identical IR/SC borders with the exception of the ycf1 gene at the SSC/IRb border in D. montana, which varies from those in A. paniculata. There are 167 bp from the border of rpl2 to the juncture of LSC/IRa of Dicliptera, while this distance is just 57 bp in A. paniculata. The rps19 gene is on the LSC/IRa border in all five Dicliptera species, indicating that this border has moved toward the LSC region when compared to A. paniculata. The ndhF gene in Dicliptera is situated at the junction of the IRa/SSC region and has 117 bp sequences located at IRa, however, the comparable region in A. paniculata is 40 bp long, indicating that the IRa/SSC boundary has moved toward the SSC region. The ycf1 gene is located at the IRb/SSC junction and the border has moved toward the SSC region because there are 4,543 bp and 4,560 bp sequences situated at SSC in Dicliptera and A. paniculata, respectively. The ycf1 gene duplications located in IRa are 811–812 bp in Dicliptera and 982 bp in A. paniculata, indicating a slight expansion of the IR regions. Likewise, the space in Dicliptera from psbA to the IRb/LSC boundary (364 bp) is enlarged compared to that of A. paniculata (333 bp). These findings reveal that the IR regions in the cp genome of Dicliptera have expanded compared to those of A. paniculata.
Figure 2. Comparison of the border regions of the LSC, SSC and IR among six Acanthaceae chloroplast genomes.
The IRb/SSC junction extended into the ycf1 genes creating various lengths of ycf1 pseudogenes (Ψycf1) among the six cp genomes. The number above, below or adjacent to genes shows the distance between the ends of genes and the boundary sites. The figure features are not to scale.
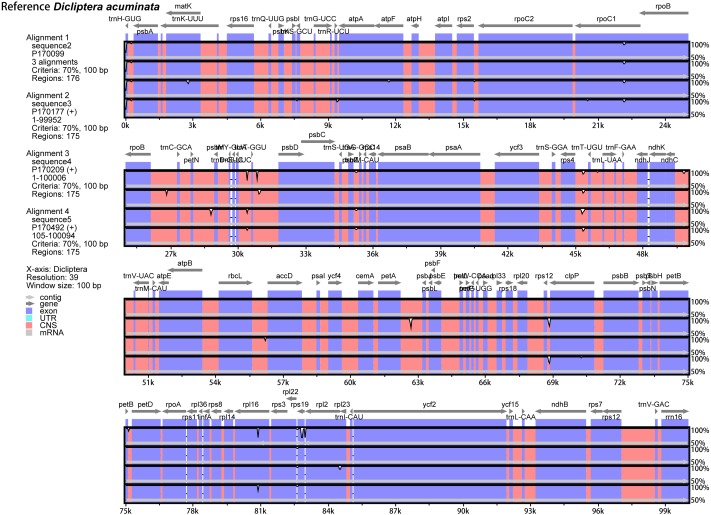
Comparative chloroplast genome analysis
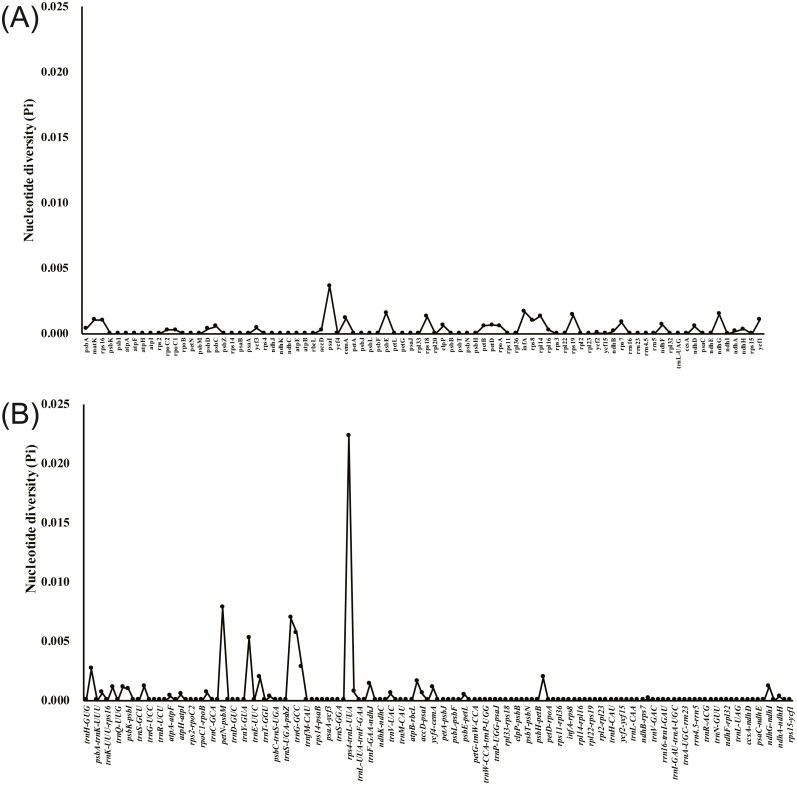
The annotated D. acuminata cp genome was used as a reference in mVISTA for the alignment of the cp genome among the five Dicliptera species (Fig. 3). The size and gene order of the chloroplast genomes of the Dicliptera species are conserved, but some divergent regions were identified, including the trnH-GUG, rpl16, petN-psbM, trnS-trnG, trnT-trnF, ndhC-trnV, petA-psbJ, and rps12-clpP genes. The nucleotide variability (Pi) was calculated for the coding and non-coding regions, respectively, in order to further confirm the sequence variations (Table S3, Fig. 4). However, the Pi values are rather low among the five species (0 to 0.02230) and a total of 5 hotspot regions were identified with Pi >0.005 (trnY-GUA-trnE-UUC, trnG-GCC, psbZ-trnG-GCC, petN-psbM, and rps4-trnL-UUA).
Figure 3. Comparison of the five Dicliptera chloroplast genomes using mVISTA.
CNS indicates conserved noncoding sequences. The Y-scale represents the percent identity between 50% and 100%.
Figure 4. Comparative analysis of the nucleotide diversity (Pi) value among five Dicliptera chloroplast genomes.
(A) Coding regions. (B) Non-coding regions.
Codon usage
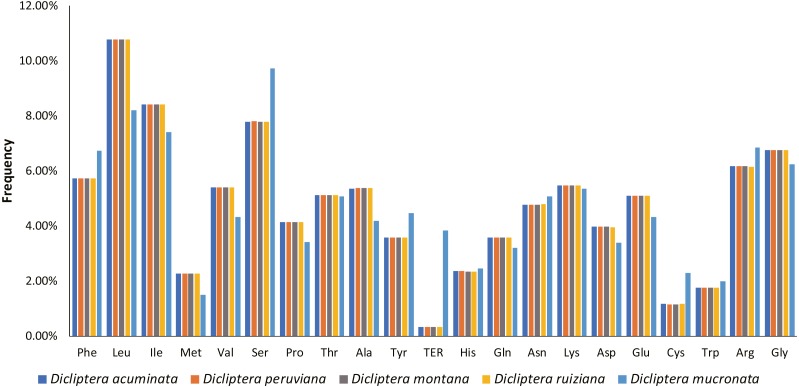
A total of 78714-78747 bp protein-coding genes were identified in the five Dicliptera cp genomes, accounting for 52.19%-52.24% of the entire genome sequence. These genes are encoded in 26238-26249 codons. Leucine (Leu, encoded by UUA, UUG, CUU, CUC, CUA and CUG) was the most frequent amino acid encoded by these codons, comprising 2,825–2,828 (10.8%) of the total number of codons; cysteine (Cys, encoded by UGU and UGC) was the least frequently encoded amino acid, with 303–305 codons (1.2%) (Fig. 5). For all codons, from the first to third position, the AU contents are 55.8%–60.2%, 60.6%-64.1%, and 64.9%-65.8%, respectively. The majority of the preferred codons (RSCU >1) ended with A or U, with the exception of UUG (RSCU = 1.25). This phenomenon is congruent with the results from other plant studies (Table S4) (Lu et al., 2018; Yang et al., 2014; Yi & Kim, 2012).
Figure 5. Amino acid frequencies in five Dicliptera species protein-coding sequences.
As shown in the column diagram, Leucine was the most frequent amino acid (10.8%), Cysteine was the least (1.2%).
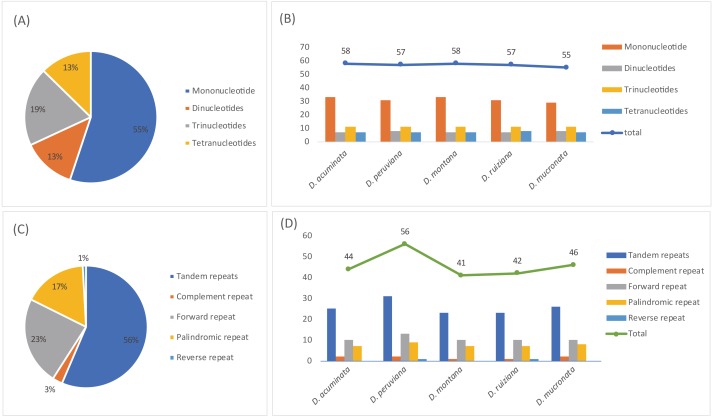
Simple Sequence Repeats (SSRs) and long repeat analysis
A total of 285 SSRs were identified in this study. The numbers of mono-, di-, tri-, and tetranucleotides were 157, 37, 55, and 36, respectively (Tables S5 and S7). Mononucleotide repeats were the most common repeats, accounting for 55.1% of the total repeats, while dinucleotides repeats accounted for 13.0%, and other SSRs occurred less frequently (Fig. 6A). The SSR varied in number and type depending on the species; D. acuminata and D. montana (58) had the most SSRs and D. mucronata (55) had the least (Fig. 6B, Table 3). Five categories of long repeats (tandem, complement, forward, palindromic and reverse repeats) were detected and analyzed in the five Dicliptera cp genomes (Tables S6 and S7, Fig. 6C). 229 long repeats were identified and were composed of 128 tandem repeats, 8 complement repeats, 53 forward repeats, 38 palindromic repeats, and 2 reverse repeats (Fig. 6C). The number of repeats was highest in D. peruviana (56) and lowest in D. montana (41) (Fig. 6D).
Figure 6. The type and presence of simple sequence repeats (SSRs) and long repeated sequences in the chloroplast genomes of five Dicliptera species.
(A) Percentage of SSR types; (B) number of SSRs and their types; (C) percentage of five repeat types; (D) number of five repeats types.
Table 3. The polymorphic SSRs among five Dicliptera species.
LSC, large single copy; SSC, small single copy.
| Type | D. acuminata/D. peruviana/ D. montana/D. ruiziana/D. mucronata | Location | Regions |
|---|---|---|---|
| A | 11/11/10/11/13 | psbI-trnS-GCU | LSC |
| T | 0/0/0/10/0 | trnS-GCU-trnG-UCC | LSC |
| T | 13/13/14/13/13 | trnR-UCU-atpA | LSC |
| A | 12/11/11/10/11 | aptF | LSC |
| A | 10/10/10/0/0 | atpI-rps2 | LSC |
| TA | 0/0/0/0/8 | trnE-UUC-trnT-GGU | LSC |
| AT | 0/9/0/0/0 | trnE-UUC-trnT-GGU | LSC |
| T | 13/10/12/16/14 | psbZ-trnG-GCC | LSC |
| G | 11/11/12/9/8 | psbZ-trnG-GCC | LSC |
| T | 10/10/10/10/11 | rps4-trnT-UGU | LSC |
| ATAA | 3/3/3/6/3 | rps4-trnT-UGU | LSC |
| TA | 6/7/7/7/7 | rps4-trnT-UGU | LSC |
| T | 12/11/13/13/11 | ndhC-trnV-UAC | LSC |
| T | 12/11/11/12/11 | psaI-ycf4 | LSC |
| T | 11/10/11/11/10 | petG-trnW-CCA | LSC |
| T | 11/10/10/10/0 | clpP intron | LSC |
| TA | 7/6/6/6/6 | rpl22-rps19 | LSC |
| T | 10/10/10/10/11 | ndhF-rpl32 | SSC |
| A | 10/11/10/10/10 | ndhD-psaC | SSC |
| G | 11/0/11/11/10 | ndhG-ndhI | SSC |
| A | 11/10/10/10/0 | ndhA intron | SSC |
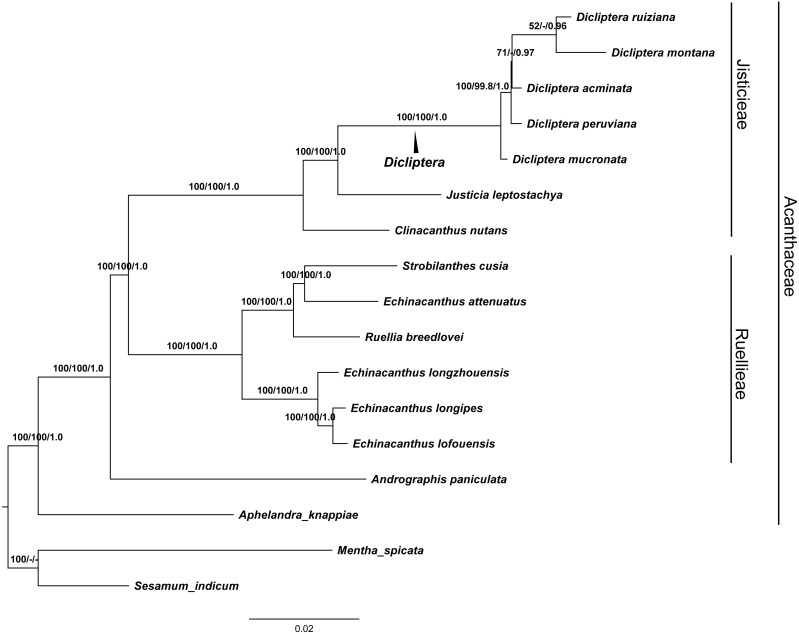
Phylogenetic analyses
GTR and SYM+G were the best fit models used for the ML and BI trees to display the completed cp genomes. The data matrix for all of the MP, ML, and BI analyses revealed trees with highly congruent topologies. The phylogenetic relationships within the 17 cp genomes sequences analyzed were well-resolved (Fig. 7). Our phylogenetic analyses strongly support the monophyly of the Dicliptera species [BP(MP) = 100%, BP(ML) = 100%, PP = 1.0], in which D. ruiziana has the closest relationship with D. montana [BP(ML) = 52%, PP = 0.96], followed by D. acuminata [BP(ML)=69%, PP = 0.97], D. peruviana [BP(MP) = 99.8%, BP(ML) = 100%, PP = 1.0], and D. mucronata (BP[MP] = 100%, BP[ML] = 100%, PP = 1.0).
Figure 7. The maximum Likelihood (ML) tree of Acanthaceae.
Numbers associated with branches are ML bootstrap values, MP bootstrap values and Bayesian posterior probabilities, respecively. Hyphens indicate the bootstrap support or posterior probability lower than 50% or 0.5. Mentha spicata (NC_037247) and Sesamum indicum (NC_016433) were used as outgroups.
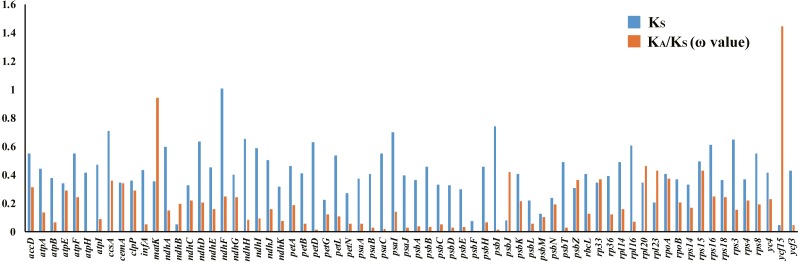
Selective pressure events
There were 68 orthologous protein-coding genes found in this study. The ω values of most genes were low (ω <1), approaching zero, except for the ycf15 gene found in the SSC region, which had a ratio of 1.4453. The ω value of the matK gene was 0.9418, indicating a relaxed selection (Table S8, Fig. 8).
Figure 8. Synonymous (KS) substitution rates and ω values (ω= KA/KS) among all Acanthaceae species.
As shown in the column diagram, the order of genes is alphabetical. The ω value of the ycf15 gene (1.4453) is clearly higher than other genes.
Discussion
Sequence variation among five Dicliptera species
The results of our study showed that the cp genomes of five Peruvian Dicliptera species were similar in structure, content, and order (Table 2, Fig. 1). The cp genomes ranged in size from 150,689 bp to 150,811 bp in D. montana and D. peruviana, respectively. These structures are longer than the cp genome of A. paniculata (15,249 bp) (Ding et al., 2016). The genome size of all Dicilptera is relevant to LSC variation (Table 2) and this phenomenon has also been identified in other species (Zhao et al., 2018; Li, Zhao & Liu, 2018; Meng et al., 2018). mVISTA revealed a low divergence between the genomes of the five Dicliptera species, suggesting that the cp genomes were conserved. The IR regions were more highly conserved than the SC regions and the coding regions were less variable than the non-coding regions, which is also found in other angiosperms (Gao, Wang & Deng, 2018; Meng et al., 2018; Li, Zhao & Liu, 2018; Yan et al., 2019). Khakhlova & Bock (2006) suggested that gene divergence with less variability in the IR and coding regions may be a result of copy corrections during gene conversion, which can correct or delete the mutation. Codons were shown to have a strong tendency toward A or U at the third codon position, which is similar to the expression of an A/U ending in other plants (Gao et al., 2017; Clegg et al., 1994; Mader et al., 2018; Meng et al., 2018). This phenomenon may explain why the Adenine-Thymine (AT) content is slightly higher than the GC content in the cp genome of Dicliptera.
IR expansion analysis
IR regions are the most conserved regions in the cp genomes. Frequent expansions and contractions at the junctions of SSR and LSC with IRs illustrate the relationships among taxa and have been recognized as evolutionary signals (Khakhlova & Bock, 2006; Inkyu et al., 2018; Lu et al., 2018; Raubeson et al., 2007; Wang et al., 2008). In this study, only a few variations were found among the five Dicliptera species. When compared with the cp genome of A. paniculata, the IR regions of the cp genome of Dicliptera revealed a slight expansion. The size differences among the cp genome of the five Dicliptera species (150,689–150,811 bp) and Andrographis paniculata (150,249 bp) are congruent with the results of previous studies. The contractions and expansions at the LSC/IRs and SSC/IRs junctions contribute to the size variations of the cp genomes (Kim & Lee, 2004; Raubeson et al., 2007). Gene conversion during speciation is thought to be responsible for small IR expansions or contractions. (Wang et al., 2008; Goulding et al., 1996; Khakhlova & Bock, 2006; Meng et al., 2018; Choi, Jansen & Ruhlman, 2019).
Molecular markers
Simple sequence repeats (SSRs), known as microsatellites, are short stretches of DNA which consist of only one, or a few, tandemly repeated nucleotides. Polymorphic SSRs are the same units with different unit numbers located in the homologous regions; these are frequently used to identify variable species complexes (Diethard & Manfred, 1984; Jerzy & Charit, 1995; George et al., 2015; Gao, Wang & Deng, 2018). 21 SSRs were identified as polymorphic SSRs among the five Dicliptera species; these may be used as candidate genetic markers for further phylogenetic studies in the genus Dicliptera (Table 3). The presence of these repeats indicates that these regions are important hotspots for genome recombination. All polymorphic SSRs are located in LSC/SSC regions. Polymorphic SSRs are mainly distributed in non-coding regions, which are also highly variable regions in the chloroplast genomes (Asaf et al., 2017). The presence of long sequence repeats are indicators of mutational hotspots (Borsch & Quandt, 2009; Jiang et al., 2018).
The ycf1 gene was previously reported for its use in DNA barcodes due to its abundance of variable sites (Kurt et al., 2008; Gernandt et al., 2009; Dong et al., 2012; Drew & Sytsma, 2013; Dong et al., 2015). Shingo et al. (2013) concluded that the ycf1 gene is crucial for plant viability because it encodes the Arabidopsis protein, Tic214, which is essential for photosynthetic protein import. A substantial size difference was noted between the ycf1 gene of the five Dilicptera species (5,354–5,355 bp) and A. paniculata (5,542 bp). The nucleotide variability of the ycf1 gene (Pi = 0.0109) was slightly higher than that of the regions matK (Pi = 0.00107) and rpl16 (Pi=0.00103). The two regions are currently used in the DNA barcodes for the tribe Justicieae and other angiosperms (Kiel et al., 2017; Särkinen & George, 2013). Therefore, the ycf1 gene should be a potential molecular marker for the Diciptera species as well. The most divergent regions among the Dicliptera species, as determined by a comparison of nucleotide variability, are rps4-trnL-UUA (Pi = 0.02230), petN-psbM (Pi = 0.00783), psbZ-trnG-GCC (Pi=0.00697), trnG-GCC (Pi = 0.00571), and trnY-GUA-trnE-UUC (Pi = 0.00526). The variability in these regions was much higher than that in the coding regions and the highly variable regions identified here could be validated and used as molecular markers in future species delimitation and phylogenetic studies.
Phylogenetic analyses
The phylogenetic trees (MP, ML and BI) demonstrated a significant relationship among Acanthaceae with high bootstrap values and posterior probabilities (Fig. 7). The genus Aphelandra was found to be the earliest diverging lineage; tribes Justicieae and Ruellieae are strongly supported as monophyletic groups [BP(MP) = 100%, BP(ML) = 100%, PP = 1.0] that form sister groups with each other. The results are consistent with previous studies (Mcdade, Daniel & Kiel, 2008; Huang, Deng & Ge, 2019). Phylogenetic analysis strongly supports Dicliptera as a monophyletic group. The clade formed by all five Dicliptera species is a sister to the species Justicia leptostachya [BP(MP) = 100%, BP(ML) = 100%, PP = 1.0], which supports the conclusion by Kiel et al. (2017) that the genus Dicliptera should be placed in the justicioid lineage. D. mucronata and D. peruviana are the first and second diverging clades among the five Dicliptera species; D. acuminata, D. ruiziana, and D. montana are species that can confidently be assigned to one clade. Trees with the same topology were retrieved from the ML and BI analyses. D. ruiziana was most closely related to D. montana, followed by D. acuminata. However, the relationships among D. acuminata, D. ruiziana and D. montana were not resolved using MP analysis. The sister relationship between D. ruiziana and D. montana is supported by their shared morphological characteristics, including a lanceolate calyx and ovate leaves of 1.0–1.5 × 0.8–1.0 cm versus the has subulate calyx and oblong-lanceolate leaves of 3.5–7.0 × 1.5–2.5 cm of D. acuminata (Table 1).
Adaptive evolution analysis
Positively selected genes are known to play a key role in adapting to different environments (Wang et al., 2016; Ma et al., 2017; Fan et al., 2018; Gao, Wang & Deng, 2018; Wu et al., 2018) and it is important to understand the adaptive evolutionary history of Acanthaceae. Orthologous genes are a particular class of homologous genes that diverged following the speciation of their host species; they are ideal markers for analyzing evolutionary history (Gargaud et al., 2015). 68 protein-coding genes were found to be orthologous in the family Acanthaceae and the selective pressure of these genes was measured. The resulting measurements found that most genes in the family Acanthaceae were under negative selection (ω <1) except for ycf15 (ω = 1.4453). According to previous studies, the ycf15 gene is a member of the PFAM protein family accession PF10705 (Sara et al., 2018) and was not considered to be a protein-coding gene because of its unknown function (Steane, 2005; Feng et al., 2018). The ycf15 gene acts as a pseudogene in some species because of its premature stop codons (Chen et al., 2018; Jiang et al., 2018). The ycf15 gene should be further investigated for its role in adaptive evolution and gene function.
Conclusions
Our study sequenced and analyzed the complete cp genomes of five Peruvian Dicliptera species (D. acuminata, D. peruviana, D. montana, D. ruiziana, and D. mucronata) for the first time. The identification of the chloroplast genomes and the new molecular markers of these five species contributes to the genetic resources available for future identification and phylogenetic studies. The goal of this study was to determine the appropriate DNA barcode for the identification of the Diciptera species, especially those found in Peru. The genes ycf1, rps4-trnL-UUA, petN-psbM, psbZ-trnG-GCC, trnG-GCC, and trnY-GUA-trnE-UUC were found to be the most suitable DNA barcode for the species Dicliptera. The interspecies relationships among the five species were resolved. However, further phylogenetic analysis using additional genes from the nucleus will have to be conducted in order to understand how gene introgression and hybridization affects the phylogeny of Dicliptera (Birky, 1995; Meng et al., 2018; Lu et al., 2018). A single gene, ycf15, was found to be positively selected among all of the protein coding genes that were identified. This gene may play an important role in the adaptive evolution of the Acanthaceae species and its function should to be further studied. Our genome data enhances the cp genome resources for the family Acanthaceae and our understanding of its species identification, phylogeny, and evolutionary history.
Supplemental Information
Funding Statement
This work was financially supported by the International Partnership Program of Chinese Academy of Sciences (Grant No. 151644KYSB20160005, GJHZ1620), the National Natural Science Foundation of China (Grant no. 31470302, 31670191), and “One-Three-Five” Strategic Planning of SCBG, CAS to Yunfei Deng and Xuejun Ge. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Sunan Huang conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Xuejun Ge conceived and designed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Asunción Cano and Betty Gaby Millán Salazar performed the experiments, prepared figures and/or tables, apply the collecting permission, and approved the final draft.
Yunfei Deng conceived and designed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
DNA Deposition
Data Availability
The following information was supplied regarding data availability:
Data is available at NCBI SRA: PRJNA531624, PRJNA531625, PRJNA531630, PRJNA531639, PRJNA531641.
References
- Antonio Galán et al. (2009).Antonio Galán DM, Eliana Linares P, José CDLC, José Alfredo Vicente O. Nuevas observaciones sobre la vegetación del sur del Perú. Del Desierto Pacífico al Altiplano. Acta Botanica Malacitana. 2009;34:107–144. [Google Scholar]
- Asaf et al. (2017).Asaf S, Khan AL, Khan MA, Waqas M, Kang SM, Yun BW, Lee IJ. Chloroplast genomes of Arabidopsis halleri ssp. gemmifera and Arabidopsis lyrata ssp. petraea: Structures and comparative analysis. Scientific Reports. 2017;7(1) doi: 10.1038/s41598-017-07891-5. Article 7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkwill, Norris & Balkwill (1996).Balkwill K, Norris FG, Balkwill M-J. Systematic studies in the Acanthaceae; Dicliptera in southern Africa. Kew Bulletin. 1996;51:1–61. doi: 10.2307/4118744. [DOI] [Google Scholar]
- Bankevich et al. (2012).Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. Journal of Computational Biology. 2012;19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson (1999).Benson G. Tandem repeats finder: a program to analyze DNA sequences. Nucleic Acids Research. 1999;27:573–580. doi: 10.1093/nar/27.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birky (1995).Birky CW. Uniparental inheritance of mitochondrial and chloroplast genes: mechanisms and evolution. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:11331–11338. doi: 10.1073/pnas.92.25.11331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsch & Quandt (2009).Borsch T, Quandt D. Mutational dynamics and phylogenetic utility of noncoding chloroplast DNA. Plant Systematics and Evolution. 2009;282:169–199. doi: 10.1007/s00606-009-0210-8. [DOI] [Google Scholar]
- Brako & Zarucchi (1993).Brako L, Zarucchi J. Catálogo de las Angiospermas y Gimnospermas del Perú. Monograph in Systematic Botany from Missouri Botanic Gardens. 1993;45:1–1286. [Google Scholar]
- Brudno et al. (2003).Brudno M, Malde S, Poliakov A, Do CB, Couronne O, Dubchak I, Batzoglou S. Glocal alignment: finding rearrangements during alignment. Bioinformatics. 2003;19S1:i54–i62. doi: 10.1093/bioinformatics/btg1005. [DOI] [PubMed] [Google Scholar]
- Bussmann & Glenn (2010).Bussmann RW, Glenn A. A medicinal plants used in Northern Peru for reproductive problems and female health. Journal of Ethnobiology and Ethnomedicine. 2010;6 doi: 10.1186/1746-4269-6-30. Article 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen et al. (2018).Chen HM, Shao JJ, Zhang H, Jiang M, Huang LF, Zhang Z, Yang D, He M, Mostafa R, Luo X, Botao S, Wu WW, Liu C. Sequencing and analysis of strobilanthes cusia (nees) kuntze chloroplast genome revealed the rare simultaneous contraction and expansion of the inverted repeat region in angiosperm. Frontiers in Plant Science. 2018;9 doi: 10.3389/fpls.2018.00324. Article 324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, Jansen & Ruhlman (2019).Choi IS, Jansen R, Ruhlman T. Lost and found: return of the inverted repeat in the legume clade defined by its absence. Genome Biology Evolution. 2019;11:1321–1333. doi: 10.1093/gbe/evz076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg et al. (1994).Clegg MT, Gaut BS, Learn GH, Morton BR. Rates and patterns of chloroplast DNA evolution. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:6795–6801. doi: 10.1073/pnas.91.15.6795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darbyshire (2008).Darbyshire I. Notes on the genus Dicliptera (Acanthaceae) in eastern Africa. Kew Bulletin. 2008;63:361–383. doi: 10.1007/s12225-008-9053-7. [DOI] [Google Scholar]
- Darling, Mau & Perna (2010).Darling AE, Mau B, Perna NT. progressiveMauve: multiple genome alignment with gene gain, loss and rearrangement. PLOS ONE. 2010;5:e11147. doi: 10.1371/journal.pone.0011147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diethard & Manfred (1984).Diethard T, Manfred R. Simple sequences are ubiquitous repetitive components of eukaryotic genomes. Nucleic Acids Research. 1984;12:4127–4138. doi: 10.1093/nar/12.10.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding et al. (2016).Ding P, Shao YH, Li Q, Gao JL, Zhang RJ, Lai X. The chloroplast genome sequence of the medicinal plant Andrographis paniculata. Mitochondria DNA. 2016;27:2347–2348. doi: 10.3109/19401736.2015.1025258. [DOI] [PubMed] [Google Scholar]
- Dong et al. (2012).Dong WP, Liu J, Yu J, Wang L, Zhou SL. Highly variable chloroplast markers for evaluating plant phylogeny at low taxonomic levels and for DNA barcoding. PLOS ONE. 2012;7:e35071. doi: 10.1371/journal.pone.0035071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong et al. (2015).Dong WP, Xu C, Li CH, Sun JH, Zuo YJ, Shi S, Cheng T, Guo JJ, Zhou SL. ycf1, the most promising plastid DNA barcode of land plants. Scientific Report. 2015;5:8348. doi: 10.1038/srep08348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle & Doyle (1987).Doyle JJ, Doyle JL. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochemical Bulletin. 1987;19:11–15. [Google Scholar]
- Drew & Sytsma (2013).Drew BT, Sytsma KJ. The South American radiation of Lepechinia (Lamiaceae): phylogenetics, divergence times and evolution of dioecy. Botanical Journal of the Linnean Society. 2013;171:171–190. doi: 10.1111/j.1095-8339.2012.01325.x. [DOI] [Google Scholar]
- Fan et al. (2018).Fan WB, Wu Y, Yang J, Shahzad K, Li ZH. Comparative chloroplast genomics of Dipsacales species: Insights into sequence variation, adaptive evolution, and phylogenetic relationships. Frontiers in Plant Science. 2018;9 doi: 10.3389/fpls.2018.00689. Article 689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng et al. (2018).Feng XJ, Yuan XY, Sun YW, Hu YH, Zulfiqar S, Ouyang XH, Dang M, Zhou HJ, Keith W, Zhao P. Resources for studies of iron walnut (Juglans sigillata) gene expression, genetic diversity, and evolution. Tree Genetics & Genomes. 2018;14 doi: 10.1007/s11295-018-1263-z. Article 51. [DOI] [Google Scholar]
- Frazer et al. (2004).Frazer KA, Pachter L, Poliakov A, Rubin EM, Dubchak I. VISTA: computational tools for comparative genomics. Nucleic Acids Research. 2004;32:W273–W279. doi: 10.1093/nar/gkh458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, Wang & Deng (2018).Gao CM, Wang J, Deng YF. The complete chloroplast genomes of Echinacanthus species (acanthaceae): phylogenetic relationships, adaptive evolution, and screening of molecular markers. Frontiers in Plant Science. 2018;9 doi: 10.3389/fpls.2018.01989. Article 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao et al. (2017).Gao QB, Li Y, Gengji ZM, Gornall RJ, Wang JL, Hai-Rui Liu HR, Jia LK, Chen SL. Population genetic differentiation and taxonomy of three closely related species of Saxifraga (saxifragaceae) from Southern Tibet and the Hengduan Mountains. Frontiers in Plant Science. 2017;8:1325. doi: 10.3389/fpls.2017.01325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargaud et al. (2015).Gargaud M, Irvine W, Amils R, Cleaves HJ, Pinti Daniele L, Quintanilla Jose C, Rouan D, Spohn T, Tirard S, Viso M, editors. Encyclopedia of astrobiology. Springer Berlin Heidelberg; Berlin, Heidelberg: 2015. pp. 1803–1803. [Google Scholar]
- George et al. (2015).George B, Bhatt BS, Awasthi M, George B, Singh AK. Comparative analysis of microsatellites in chloroplast genomes of lower and higher plants. Current Genetics. 2015;61:665–677. doi: 10.1007/s00294-015-0495-9. [DOI] [PubMed] [Google Scholar]
- Gernandt et al. (2009).Gernandt DS, Hernández-León S, Salgado-Hernández E, Pérez de la Rosa JA. Phylogenetic relationships of pinus subsection ponderosae inferred from rapidly evolving cpDNA regions. Systematic Botany. 2009;34:481–491. doi: 10.1600/036364409789271290. [DOI] [Google Scholar]
- Goulding et al. (1996).Goulding SE, Wolfe K, Olmstead R, Morden C. Ebb and flow of the chloroplast inverted repeat. Molecular and General Genetics. 1996;252:195–206. doi: 10.1007/BF02173220. [DOI] [PubMed] [Google Scholar]
- Greiner, Lehwark & Bock (2019).Greiner S, Lehwark P, Bock R. OrganellarGenomeDRAW (OGDRAW) version 1.3.1: expanded toolkit for the graphical visualization of organellar genomes. Nucleic Acids Research. 2019;47:W59–W64. doi: 10.1093/nar/gkz238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall & Strickberger (2008).Hall B, Strickberger BW. Strickberger’s evolution. Burlington: Jones & Bartlett Learning; 2008. [Google Scholar]
- Horacio, Graciela & Percy (2007).Horacio DLC, Graciela V, Percy AZ. Ethnobotanical study of medicinal plants used by the Andean people of Canta, Lima, Peru. Journal of Ethnopharmacology. 2007;111:284–294. doi: 10.1016/j.jep.2006.11.018. [DOI] [PubMed] [Google Scholar]
- Hu et al. (2011).Hu JQ, Deng YF, John RIW, Thomas FD. St. Louis: Science Press, Beijing & Missouri Botanical Garden PressDicliptera in Flora of China, vol. 19. 2011
- Huang, Deng & Ge (2019).Huang SN, Deng YF, Ge XJ. The complete chloroplast genome of Aphelandra knappiae (Acanthaceae) Mitochondrial DNA Part B-Resources. 2019;4:273–274. doi: 10.1080/23802359.2018.1541718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inkyu et al. (2018).Inkyu P, Sungyu Y, Wook JM, Pureum N, Hyun OL, Byeong CM. The complete chloroplast genomes of Six Ipomoea species and indel marker development for the discrimination of authentic pharbitidis semen (seeds of I. nil or I. purpurea) Frontiers in Plant Science. 2018;9 doi: 10.3389/fpls.2018.00965. Article 965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerzy & Charit (1995).Jerzy J, Charit P. Simple repetitive DNA sequences from primates: compilation and analysis. Journal of Molecular Evolution. 1995;40:120–126. doi: 10.1007/BF00167107. [DOI] [PubMed] [Google Scholar]
- Jiang et al. (2018).Jiang M, Chen HM, He SB, Wang LQ, Chen A, Liu C. Sequencing, characterization, and comparative analyses of the plastome of Caragana rosea var. rosea. International Journal of Molecular Sciences. 2018;19 doi: 10.3390/ijms19051419. Article 1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin et al. (2018).Jin JJ, Yu WB, Yang JB, Song Y, Yi TS, Li DZ. GetOrganelle: a simple and fast pipeline for de novo assembly of a complete circular chloroplast genome using genome skimming data. bioRxiv. 2018:256479. [Google Scholar]
- Katoh & Standley (2013).Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearse et al. (2012).Kearse M, Moir R, Wilson A, Stoneshavas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28:1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakhlova & Bock (2006).Khakhlova O, Bock R. Elimination of deleterious mutations in plastid genomes by gene conversion. The Plant Journal. 2006;46:85–94. doi: 10.1111/j.1365-313X.2006.02673.x. [DOI] [PubMed] [Google Scholar]
- Kiel et al. (2017).Kiel CA, Daniel TF, Darbyshire I, McDade LA. Unraveling relationships in the morphologically diverse and taxonomically challenging. Taxon. 2017;66:645–674. doi: 10.12705/663.8. [DOI] [Google Scholar]
- Kim & Lee (2004).Kim KJ, Lee HL. Complete chloroplast genome sequences from Korean ginseng (Panax schinseng Nees) and comparative analysis of sequence evolution among 17 vascular plants. DNA Research. 2004;11:247–261. doi: 10.1093/dnares/11.4.247. [DOI] [PubMed] [Google Scholar]
- Kurt et al. (2008).Kurt M, Mark Whiten NW, Carlsward BS, Blanco MA, Endara L, Williams NH, Moore M. Phylogenetic utility of ycf1 in orchids: a plastid gene more variable than matK. Faculty Research & Creative Activity. 2008;277:75–84. [Google Scholar]
- Kurtz et al. (2001).Kurtz S, Choudhuri JV, Ohlebusch E, Schleiermacher C, Stoye J, Giegerich R. REPuter: the manifold applications of repeat analysis on a genomic scale. Nucleic Acids Research. 2001;29:4633–4642. doi: 10.1093/nar/29.22.4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead & Salzberg (2012).Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nature Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- León (2006).León B. Acanthaceae endémicas del Perú (en línea) Revista peruana de Biología. 2006;13(2):23–29. [Google Scholar]
- Li, Zhao & Liu (2018).Li D, Zhao CH, Liu X. Complete chloroplast genome sequences of Kaempferia galanga and Kaempferia elegans: molecular structures and comparative analysis. Molecules. 2018;24 doi: 10.3390/molecules24030474. Article 474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Stoeckert & Roos (2003).Li L, Stoeckert CJ, Roos DS. OrthoMCL: identification of ortholog groups for eukaryotic genomes. Genome Research. 2003;13:2178–2189. doi: 10.1101/gr.1224503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Librado & Rozas (2009).Librado P, Rozas J. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics. 2009;25:1451–1452. doi: 10.1093/bioinformatics/btp187. [DOI] [PubMed] [Google Scholar]
- Lu et al. (2018).Lu QX, Ye WQ, Lu RS, Xu WQ, Qiu YX. Phylogenomic and comparative analyses of complete plastomes of Croomia and Stemona (stemonaceae) International Journal of Molecular Sciences. 2018;19:2383. doi: 10.3390/ijms19082383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma et al. (2017).Ma QY, Li SX, Bi CW, Hao ZD, Sun CR, Ye N. Complete chloroplast genome sequence of a major economic species, Ziziphus jujuba (Rhamnaceae) Current Genetics. 2017;63:117–129. doi: 10.1007/s00294-016-0612-4. [DOI] [PubMed] [Google Scholar]
- Mabberley (2017).Mabberley D. Mabberley’s plant-book. A portable dictionary of plants, their classifications and uses. forth edition Cambridge University Press; Cambridge: 2017. [Google Scholar]
- Mader et al. (2018).Mader M, Pakull B, Blanc-Jolivet C, Paulini-Drewes M, Bouda Z, Degen B, Small I, Kersten B. Complete chloroplast genome sequences of four Meliaceae species and comparative analyses. International Journal of Molecular Sciences. 2018;19 doi: 10.3390/ijms19030701. Article 701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makalowski & Boguski (1998).Makalowski W, Boguski MS. Evolutionary parameters of the transcribed mammalian genome: an analysis of 2,820 orthologous rodent and human sequences. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:9407–9412. doi: 10.1073/pnas.95.16.9407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcdade, Daniel & Kiel (2008).Mcdade L, Daniel T, Kiel C. Toward a comprehensive understanding of phylogenetic relationships among lineages of Acanthaceae S.L. (Lamiales) American Journal of Botany. 2008;95:1136–1152. doi: 10.3732/ajb.0800096. [DOI] [PubMed] [Google Scholar]
- Meng et al. (2018).Meng XX, Xian YF, Xiang L, Zhang D, Shi YH, Wu ML, Dong GQ, Ip SP, Lin ZX, Wu L. Complete chloroplast genomes from Sanguisorba: identity and variation among four species. Molecules. 2018;23 doi: 10.3390/molecules23092137. Article 2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nylander (2004).Nylander JAA. Evolutionary Biology Centre, Uppsala Universityhttps://github.com/nylander/MrModeltest2 MrModeltest v2 Program distributed by the author. 2004
- Olmstead & Palmer (1994).Olmstead RG, Palmer JD. Chloroplast DNA systematics: a review of methods and data analysis. American Journal of Botany. 1994;81:1205–1224. doi: 10.1002/j.1537-2197.1994.tb15615.x. [DOI] [Google Scholar]
- Qu et al. (2019).Qu XJ, Moore MJ, Li DZ, Yi TS. PGA: a software package for rapid, accurate, and fexible batch annotation of plastomes. Plant Methods. 2019;15:1. doi: 10.1186/s13007-018-0385-5. Article 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raubeson et al. (2007).Raubeson LA, Peery R, Chumley TW, Dziubek C, Fourcade HM, Boore JL, Jansen RK. Comparative chloroplast genomics: analyses including new sequences from the angiosperms Nuphar advena and Ranunculus macranthus. BMC Genomics. 2007;8:174. doi: 10.1186/1471-2164-8-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronquist & Huelsenbeck (2003).Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- Sara et al. (2018).Sara EG, Jaina M, Alex B, Sean RE, Aurelien L, Simon CP, Matloob Q, Lorna JR, Gustavo AS, Alfredo S, Sonnhammer ELL, Layla H, Lisanna P, Damiano P, Tosatto SCE, Finn RD. The Pfam protein families database in 2019. Nucleic Acid Research. 2018;D1:D427–D432. doi: 10.1093/nar/gky995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Särkinen & George (2013).Särkinen T, George M. Predicting plastid marker variation: can complete plastid genomes from closely related species help? PLOS ONE. 2013;8:e82266. doi: 10.1371/journal.pone.0082266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schattner, Brooks & Lowe (2005).Schattner P, Brooks AN, Lowe TM. The tRNAscan-SE, snoscan and snoGPS web servers for the detection of tRNAs and snoRNAs, snoscan and snoGPS web servers for the detection of tRNAs and snoRNAs. Nucleic Acids Research. 2005;33:W686–W689. doi: 10.1093/nar/gki366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz et al. (2015).Schwarz EN, Ruhlman TA, Sabir JSM, Hajrah NH, Alharbi NS, Almalki AL, Bailey CD, Jansen RK. Plastid genome sequences of legumes reveal parallel inversions and multiple losses of rps16 in papilionoids. Journal of Systematics and Evolution. 2015;53:458–468. doi: 10.1111/jse.12179. [DOI] [Google Scholar]
- Scotland & Vollesen (2000).Scotland R, Vollesen K. Classification of Acanthaceae. Kew Bulletin. 2000;55:513–589. [Google Scholar]
- Scottphillips et al. (2013).Scottphillips TC, Laland KN, Shuker DM, Dickins TE, West ST. The niche construction perspective: a critical appraisal. Evolution. 2013;68:1231–1243. doi: 10.1111/evo.12332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp & Li (1987).Sharp PM, Li WH. The codon adaptation index-a measure of directional synonymous codon usage bias, and its potential applications. Nucleic Acids Research. 1987;15:1281–1295. doi: 10.1093/nar/15.3.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shingo et al. (2013).Shingo K, Jocelyn B, Minako H, Yoshino H, Maya O, Midori I, Mai T, Toru I, Masato N. Uncoverign the protein translocon at the chloroplast inner envelope membrane. Science. 2013;339:571–574. doi: 10.1126/science.1229262. [DOI] [PubMed] [Google Scholar]
- Stamatakis (2014).Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steane (2005).Steane DA. Complete nucleotide sequence of the chloroplast genome from the Tasmanian blue gum, Eucalyptus globulus (Myrtaceae) DNA Research. 2005;12:215–220. doi: 10.1093/dnares/dsi006. [DOI] [PubMed] [Google Scholar]
- Swofford (2003).Swofford DL. Sinauer Associates Press; Sunderland: 2003. [Google Scholar]
- Telefo, Moundipa & Tchouanguep (2002).Telefo P, Moundipa P, Tchouanguep F. Oestrogenicity and effect on hepatic metabolism of the aqueous extract of the leaf mixture of Aloe buettneri, Dicliptera verticillata, Hibiscus macranthus and Justicia insularis. Fitoterapia. 2002;73:472–478. doi: 10.1016/S0367-326X(02)00177-6. [DOI] [PubMed] [Google Scholar]
- Thiel et al. (2003).Thiel T, Michalek W, Varshney R, Graner A. Exploiting EST databases for the development and characterization of gene-derived SSR-markers in barley (Hordeum vulgare L.) Theoretical and Applied Genetics. 2003;106:411–422. doi: 10.1007/s00122-002-1031-0. [DOI] [PubMed] [Google Scholar]
- Victor, Jhan & Raúl (2017).Victor A, Jhan C-C, Raúl S. Inventory of plant species of La Libertad (Peru) and analysis of its agro-industrial potential. Agroindustrial Science. 2017;7:87–104. doi: 10.17268/agroind.sci.2017.02.05. [DOI] [Google Scholar]
- Wang et al. (2016).Wang L, Wuyun TN, Du HY, Wang DP, Cao DM. Complete chloroplast genome sequences of Eucommia ulmoides: genome structure and evolution. Tree Genetics & Genomes. 2016;12 doi: 10.1007/s11295-016-0970-6. Article 12. [DOI] [Google Scholar]
- Wang et al. (2008).Wang RJ, Cheng CL, Chang CC, Wu CL, Su TM, Chaw SM. Dynamics and evolution of the inverted repeat-large single copy junctions in the chloroplast genomes of monocots. BMC Evolutionary Biology. 2008;8:36. doi: 10.1186/1471-2148-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng et al. (2013).Weng ML, Blazier JC, Govindu M, Jansen RK. Reconstruction of the ancestral plastid genome in Geraniaceae reveals a correlation between genome rearrangements, repeats, and nucleotide substitution rates. Molecular Biology and Evolution. 2013;31:645–659. doi: 10.1093/molbev/mst257. [DOI] [PubMed] [Google Scholar]
- Wick et al. (2015).Wick RR, Schultz MB, Zobel J, Holt KE. Bandage: interactive visualization of de novo genome assemblies. Bioinformatics. 2015;31:3350–3352. doi: 10.1093/bioinformatics/btv383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicke et al. (2011).Wicke S, Schneeweiss GM, Müller KF, Quandt D. The evolution of the plastid chromosome in land plants: gene content, gene order, gene function. Plant Molecular Biology. 2011;76:273–297. doi: 10.1007/s11103-011-9762-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojciech & Mark (1998).Wojciech M, Mark SB. Evolutionary parameters of the transcribed mammalian genome: An analysis of 2,820 orthologous rodent and human sequences. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:9407–9412. doi: 10.1073/pnas.95.16.9407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu et al. (2018).Wu Y, Liu F, Yang DG, Li W, Zhou XJ, Pei XY, Liu YG, He KL, Zhang WS, Ren ZY. Comparative chloroplast genomics of Gossypium species: insights into repeat sequence variations and phylogeny. Frontiers in Plant Science. 2018;9 doi: 10.3389/fpls.2018.00376. Article 376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie et al. (2019).Xie DF, Yu HX, Price M, Xie C, Deng YQ, Chen PJ, Yu Y, Zhou SD, He XJ. Phylogeny of Chinese Allium species in section Daghestanica and adaptive evolution of Allium (Amaryllidaceae, Allioideae) species revealed by the chloroplast complete genome. Frontiers in Plant Science. 2019;10 doi: 10.3389/fpls.2019.00460. Article 460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan et al. (2019).Yan C, Du JC, Gao L, Li Y, Hou XL. The complete chloroplast genome sequence of watercress (Nasturtium officinale R. Br.): Genome organization, adaptive evolution and phylogenetic relationships in Cardamineae. Gene. 2019;699:24–36. doi: 10.1016/j.gene.2019.02.075. [DOI] [PubMed] [Google Scholar]
- Yang, Li & Li (2014).Yang JB, Li DZ, Li HT. Highly effective sequencing whole chloroplast genomes of angiosperms by nine novel universal primer pairs. Molecular Ecology Resources. 2014;14:1024–1031. doi: 10.1111/1755-0998.12251. [DOI] [PubMed] [Google Scholar]
- Yang et al. (2014).Yang Y, Dang YY, Li Q, Lu JJ, Li XW, Wang YT. Complete chloroplast genome sequence of poisonous and medicinal plant Datura stramonium: organizations and implications for genetic engineering. PLOS ONE. 2014;9:e110656. doi: 10.1371/journal.pone.0110656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang (2007).Yang ZH. PAML 4: phylogenetic analysis by maximum likelihood. Molecular Biology and Evolution. 2007;24:1586–1591. doi: 10.1093/molbev/msm088. [DOI] [PubMed] [Google Scholar]
- Yang & Nielsen (2000).Yang ZH, Nielsen R. Estimating synonymous and nonsynonymous substitution rates under realistic evolutionary models. Molecular Biology and Evolution. 2000;17:32–43. doi: 10.1093/oxfordjournals.molbev.a026236. [DOI] [PubMed] [Google Scholar]
- Yang & Swanson (2002).Yang ZH, Swanson WJ. Codon-substitution models to detect adaptive evolution that account for heterogeneous selective pressures among site classes. Molecular Biology and Evolution. 2002;19:49–57. doi: 10.1093/oxfordjournals.molbev.a003981. [DOI] [PubMed] [Google Scholar]
- Yi & Kim (2012).Yi DK, Kim KJ. Complete chloroplast genome sequences of important oilseed crop Sesamum indicum L. PLOS ONE. 2012;7:e35872. doi: 10.1371/journal.pone.0035872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Zhu & Gao (2010).Zhang KF, Zhu H, Gao Y. Research on active extracts of Dicliptera chinensis on liver protection. China Journal of Chinese Materia Medica. 2010;35:497–498. doi: 10.4268/cjcmm20100420. [DOI] [PubMed] [Google Scholar]
- Zhao et al. (2018).Zhao ZY, Wang X, Yu Y, Yuan SB, Jiang D, Zhang YJ, Zhang T, Zhong WH, Yuan QJ, Huang LQ. Complete chloroplast genome sequences of Dioscorea: characterization, genomic resources, and phylogenetic analyses. PeerJ. 2018;6:e6032. doi: 10.7717/peerj.6032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
Data is available at NCBI SRA: PRJNA531624, PRJNA531625, PRJNA531630, PRJNA531639, PRJNA531641.