Abstract
Lung cancer is the top cause of carcinoma-associated deaths worldwide. RNA binding proteins (RBPs) dysregulation has been reported in various malignant tumors, and that dysregulation is closely associated with tumorigenesis and tumor progression. However, little is known about the roles of RBPs in lung adenocarcinoma (LUAD). In this study, we downloaded the RNA sequencing data of LUAD from The Cancer Genome Atlas (TCGA) database and determined the differently expressed RBPs between normal and cancer tissues. We then performed an integrative analysis to explore the expression and prognostic significance of these RBPs. A total of 164 differently expressed RBPs were identified, including 40 down-regulated and 124 up-regulated RBPs. Pathway and Gene ontology (GO) analysis indicated that the differently expressed RBPs were mainly related to RNA processing, RNA metabolic process, RNA degradation, RNA transport, splicing, localization, regulation of translation, RNA binding, TGF-beta signaling pathway, mRNA surveillance pathway, and aminoacyl-tRNA biosynthesis. Survival analysis revealed that the high expression of BOP1 or GNL3 or WDR12 or DCAF13 or IGF2BP3 or IGF2BP1 were associated with poor overall survival (OS). Conversely, overexpression of KHDRBS2/SMAD predicted high OS in these patients. ROC curve analysis showed that the eight hub genes with a better diagnostic accuracy to distinguish lung adenocarcinoma. The results provided novel insights into the pathogenesis of LUAD and the development of treatment targets and prognostic molecular markers.
Keywords: Lung adenocarcinoma, RNA binding proteins, Post-transcriptional regulation, Prognostic value, Integrated bioinformatics analysis
Introduction
RNA-binding proteins (RBPs) are generally recognized as proteins that bind to a variety of RNAs, such as rRNAs, miRNAs, snRNAs, ncRNAs, mRNAs, snoRNAs, and tRNAs. Currently, there are more than 1,500 experimentally validated RBP coding genes in human genome, accounting for about 7.5% of all protein-coding genes (Gerstberger, Hafner & Tuschl, 2014). They can interact with other proteins or RNAs to form ribonucleoprotein complexes that regulate mRNA stability, RNA localization, export, processing, splicing, degradation, and translation (Masuda & Kuwano, 2019). The RBP decides the function and durability of each transcript and maintains cellular homeostasis (Masuda & Kuwano, 2019). Since the RBPs play pivotal roles in post-transcriptional gene expression, it is not surprising that differentially expressed RBPs are closely associated with the pathogenesis and progression of various diseases. Mutations in RBP LIN28A cause embryonic stem cell developmental defects and a Parkinson’s disease-related phenotype (Chang et al., 2019). ZFP36L1 is an RBP protect against Osteoarthritis pathogenesis by regulating the HSP70 family members to inhibit chondrocyte apoptosis (Son et al., 2019). Besides, previous studies have shown that the RBPs such as SRSF1, HuR, Rbm38, and QKI, as critical post-transcriptional regulators, play essential roles in the occurrence and development of cardiovascular diseases (De Bruin et al., 2017; Sonnenschein et al., 2019). Although RBPs be closely related to the initiation and progression of human diseases, the general roles of RBPs in tumor are still unclear.
To date, more and more studies have revealed that RBPs were dysregulated in tumors, which influence RNA modification and protein translation, involved in cancer development and progression (Legrand, Dixon & Sobolewski, 2019; Wang et al., 2020; Chatterji & Rustgi, 2018). For instance, RBP CELF6 served as a potential tumor suppressor. It is transcriptional silencing in human breast cancer due to hypermethylation modification of its promoter (Pique et al., 2019); RBP SORBS2 inhibit liver cancer carcinogenesis and metastasis by regulating the stability of RORA (Han, Huang & Zhang, 2019); AGO2 promote tumor development by up-regulating the expression of oncogenic miR-19b (Zhang et al., 2019); RBP KHSRP promotes lung cancer cell proliferation, migration, and invasion (Yan et al., 2019); HuR by regulating related mRNA stability to induce tumor cell proliferation and metastasis of stomach carcinoma (Xie et al., 2019b). However, the functions of most RBPs have not yet been determined in cancers. A systematic functional analysis of RBPs will help us thoroughly investigate its role in tumors.
Lung cancer is a very harmful disease with an average 5-year relative survival rate of only 18% (Siegel, Miller & Jemal, 2018). In recent years, numerous diagnostic molecular markers related to lung tumor have been identified (Xie et al., 2019a; Bao et al., 2017; Tepeli et al., 2012; Jan et al., 2019; Sun et al., 2019), but it is difficult to achieve accurate early-stage detection. This is probably the most important cause of high mortality in lung cancer patients. Therefore, there is a pressing need to develop an effective means for early detection and diagnosis to improve the treatment of lung cancer. The large-scale tumor genome project provides a wealth of gene expression data, which gives us an excellent opportunity to identify potential tumor molecular markers. Herein, we obtained LUAD RNA-sequencing data with corresponding clinical information from the cancer genome atlas (TCGA) database. Then, a series of bioinformatics methods were used to identify the differential expression of RPBs in tumor and normal tissues and to analyze the potential functional and clinical significance of these RBPs. Our results have displayed a number of RBPs associated with the pathogenesis of LUAD, which might be potentially helpful for developing diagnosis and prognosis biomarkers.
Material and Methods
Data acquisition and processing
The RNA-sequencing information of 524 LUAD samples and 59 normal lung tissue samples with corresponding clinical information were obtained from TCGA database (https://portal.gdc.cancer.gov/). The DESeq2 package (http://www.bioconductor.org/packages/release/bioc/html/DESeq2. html) was used to preprocessed raw data and excluded genes with an average count value less than 1. Besides, we also used DESeq2 package to identify the differently expressed RBPs in view of —log2 fold change (FC)— ≥1 and false discovery rate (FDR)<0.05. The GSE30219 dataset were downloaded from Gene Expression Omnibus (GEO) (https://www.ncbi.nlm.nih.gov/geo/) and used as a validation cohort.
GO and pathway enrichment analysis
In order to explore the function of the differently expressed RBPs, GO enrichment and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis were performed using WEB-based Gene Set Analysis Toolkit (WebGestalt, http://www.webgestalt.org/) (Liao et al., 2019). Both P and FDR values were less than 0.05 were considered statistically significant.
Protein–protein interaction (PPI) network construction and module selecting
The PPI network is an effective means for exploring the association between proteins in various organisms. In this study, the STRING database (http://www.string-db.org/) (Szklarczyk et al., 2019) were used to identify protein-protein interaction information of the differently expressed RBPs. The PPI pairs identified by STRING database were imported into the Cytoscape 3.7.0 software to build a PPI network. The key modules and hub genes were identified by using Molecular Complex Detection (MCODE) plug-in with both MCODE score and node counts number more than 5. All P ≤ 0.05 were chosen as the significant threshold.
Survival analysis
The prognostic value of key genes screed from important modules in PPI network were analyzed by using an online database, GEPIA (http://gepia.cancer-pku.cn/), which included gene expression data and corresponding survival information of 478 LUAD patients (Tang et al., 2017b). All LUAD patient samples were divided into low or high expression group according to median expression, then analyze the overall survival (OS) by GEPIA. Log-Rank test was used to assess the prognostic value and logrank P ≤ 0.05 was considered as a significant difference.
Hub genes selection and efficacy evaluation
Given that the ten key genes from modules analysis were all up-regulated genes, we also run a survival analysis of the top 10 high expressed genes and top 20 low expressed genes, respectively. Taking together, the genes with P ≤ 0.05 in survival analysis were determined as real hub genes. Subsequently, the expression of these hub genes was verified at the translation and transcription levels by utilized the Human Protein Atlas (HPA) databases and GEPIA databases (http://www.proteinatlas.org/) (Uhlen et al., 2015). The receiver operating characteristic (ROC) curve was calculated with Graphpad prism 7.0 software to assess the capability to distinguish normal lung and LUAD tissue. Multivariate Cox regression analysis was performed on these hub genes to calculate a risk score of each patient. Subsequently, a RBPs-related prognostic signature based on the coefficient value of these hub genes was evaluated in TCGA and GSE30219 cohorts.
Mutations and copy-number alterations analysis of key genes
The mutations and copy-number alterations information of all hub genes from were determined by utilizing segmentation analysis and GISTIC algorithm in cBioPortal (https://www.cbioportal.org/). Then, we conducted the co-expression analysis and constructed the network according to the cBioPortal’s instruction. In addition, functional enrichment analysis of hub genes alterations was evaluated by WebGestalt.
Results
Identifying the differently expressed RBPs in lung adenocarcinoma patients
Total 1542 RBPs (Gerstberger, Hafner & Tuschl, 2014) were included in this study, and 164 RBPs were identified as differentially expressed genes between tumors and normal samples (P < 0.05, —log2FC)— ≥1.0), including 40 down-regulated RBPs and 124 up-regulated RBPs (Table S1). The expression distribution of these differently expressed RBPs was showed in Fig. 1.
Figure 1. The differentially expressed RBPs in lung adenocarcinoma.
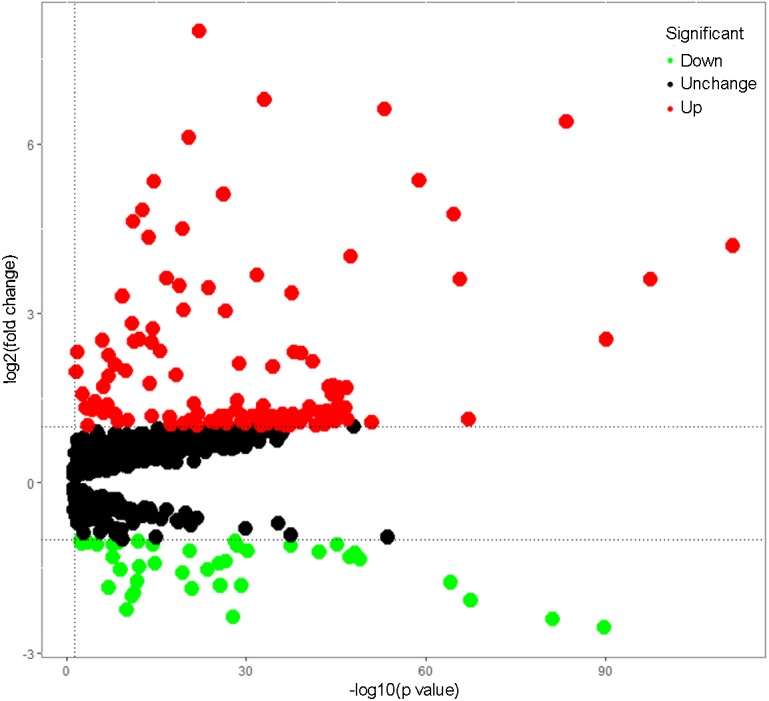
Green: down-regulation with a fold change of more than 2; black: unchanged genes; red: up-regulation with fold change of more than 2.
Functional and pathway enrichment analysis of the differently expressed RBPs
We uploaded all differently expressed RBPs to the online tool WebGestalt for GO categories and KEGG pathways analysis. GO analysis results indicated that downregulated RBPs were significantly enriched in the biological processes (BP) related to mRNA processing, regulation of translation, regulation of cellular amide metabolic processes, and metabolic processes (Table 1). The upregulated RBPs were significantly enriched in biological processes, including amide biosynthetic processes, RNA processing, translation, peptide metabolic processes, peptide biosynthetic processes, and ncRNA metabolic processes (Table 1). For molecular function (MF), the downregulated RBPs were significantly enriched in RNA binding, single-stranded RNA binding, mRNA 3′-UTR AU-rich region binding and mRNA 3′-UTR binding (Table 1), while the upregulated RBPs were significantly enriched in RNA binding, catalytic activity acting on RNA, mRNA binding, and poly-pyrimidine tract binding (Table 1). The GO cellular component (CC) analysis showed that the decreased differently expressed RBPs were enriched in RNA cap binding complex, and ribonucleoprotein complex, and upregulated RBPs were mainly enriched in ribonucleoprotein complex, ribonucleoprotein granule, cytoplasmic ribonucleoprotein granule, nucleolus, and P granule (Table 1). In addition, results of KEGG pathway analysis indicated that downregulated RBPs were mainly enriched in TGF-beta signaling pathway, while upregulated RBPs were mainly enriched in pathways of mRNA surveillance pathway, RNA degradation, RNA transport, and aminoacyl-tRNA biosynthesis (Table 1).
Table 1. GO enrichment and KEGG pathway analysis of differently expressed RBPs.
| GO term | P value | FDR | |
|---|---|---|---|
| Down-regulated RBPs | |||
| mRNA processing | 3.68E−09 | <0.0001 | |
| mRNA metabolic process | 2.43E−10 | 2.2102E−06 | |
| RNA processing | 1.86E−08 | 0.0001 | |
| regulation of translation | 2.60E−08 | 0.0001 | |
| regulation of cellular amide metabolic process | 9.26E−08 | 0.0002 | |
| RNA cap binding complex | 1.14E−06 | 0.0007 | |
| ribonucleoprotein complex | 1.19E−06 | 0.0007 | |
| RNA binding | <0.0001 | <0.0001 | |
| mRNA binding | 5.88E−11 | 5.52E−08 | |
| single-stranded RNA binding | 8.12E−10 | 5.08E−07 | |
| mRNA 3′-UTR binding | 1.65E−08 | 7.6E−06 | |
| mRNA 3′-UTR AU-rich region binding | 2.02E−08 | 7.6E−06 | |
| TGF-beta signaling pathway | 0.0004 | 0.0376 | |
| Up-regulated RBPs | |||
| RNA processing | <0.0001 | <0.0001 | |
| peptide metabolic process | <0.0001 | <0.0001 | |
| amide biosynthetic process | <0.0001 | <0.0001 | |
| peptide biosynthetic process | <0.0001 | <0.0001 | |
| translation | <0.0001 | <0.0001 | |
| ncRNA metabolic process | <0.0001 | <0.0001 | |
| ribonucleoprotein complex | <0.0001 | <0.0001 | |
| ribonucleoprotein granule | <0.0001 | <0.0001 | |
| cytoplasmic ribonucleoprotein granule | 2.22E−16 | 8.70E−14 | |
| nucleolus | 8.46E−13 | 2.45E−10 | |
| P granule | 1.46E−12 | 2.45E−10 | |
| RNA binding | <0.0001 | <0.0001 | |
| catalytic activity, acting on RNA | <0.0001 | <0.0001 | |
| mRNA binding | <0.0001 | <0.0001 | |
| poly-pyrimidine tract binding | 5.45E−08 | <0.0001 | |
| translation regulator activity | 1.39E−07 | <0.0001 | |
| mRNA surveillance pathway | <0.0001 | <0.0001 | |
| RNA degradation | <0.0001 | <0.0001 | |
| RNA transport | <0.0001 | <0.0001 | |
| Ribosome | 0.0001 | 0.0001 | |
| Aminoacyl-tRNA biosynthesis | 0.0079 | 0.0079 |
Protein-protein interaction network construction and key modules screening
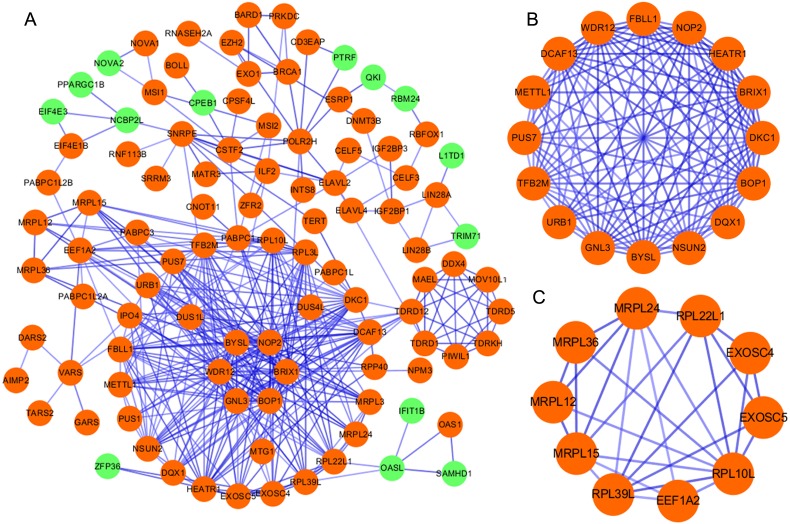
According to the information in the STRING database, we constructed a PPI network with 103 nodes and 360 edges by using Cytoscape software (Fig. 2A). Ten genes were screened as candidate hub genes by computing degree and betweenness, including BOP1, GNL3, WDR12, NOP2, BYSL, BRIX1, DCAF13, TFB2M, NSUN2 and DKC1. Furthermore, the MODE plugin was used to identify possible key modules from the co-expression network and the top 2 significant modules were screened (Figs. 2B–2C). Function enrichment analysis revealed that the RBPs involved in module 1 were related to ncRNA processing, ncRNA metabolic process, ribosome biogenesis, ribonucleoprotein complex biogenesis, RNA binding, RNA methyltransferase activity and snoRNA binding, while the RBPs in module 2 were associated with translational elongation, mitochondrial translational elongation, cellular amide metabolic process, peptide biosynthetic process, structural molecule activity, and structural constituent of ribosome.
Figure 2. Protein–protein interaction network and modules analysis.
(A) Protein–protein interaction network of RBPs; (B) critical module 1 from PPI network; (C) critical module 2 from PPI network. Green: down-regulation with a fold change of more than 2; orange: up-regulation with fold change of more than 2.
Survival analysis of candidate hub RBPs
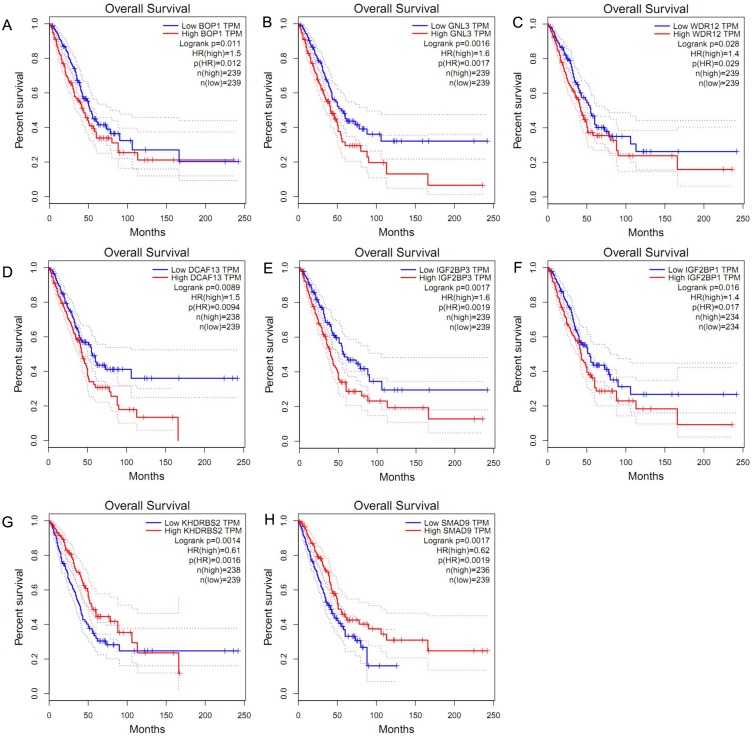
We obtained the hazard ratio (HR) of each candidate hub gene to OS by using GEPIA. The results showed that high expression of BOP1 (HR = 1.5, P = 0.011), GNL3 (HR = 1.6, P = 0.002), WDR12 (HR = 1.4, P = 0.028), and DCAF13 (HR = 1.5, P = 0.009) were significantly related to poor OS (Figs. 3A–3D). Considering that the candidate hub genes from PPI network were all up-regulated genes, we also executed survival analysis of the top ten over-expressed RBPs and top twenty down-expressed RBPs. We found that high expression of IGF2BP3 (HR = 1.6, P = 0.002) or IGF2BP1 (HR = 1.4, P = 0.016) was related to unfavorable prognosis of LUAD patients (Figs. 3E–3F), while the expression of KHDRBS2 (HR = 0.61, P = 0.001) or SMAD9 (HR = 0.62, P = 0.002) was negatively associated with OS (Figs. 3G–3H). These findings suggested that BOP1, GNL3, WDR12, DCAF13, IGF2BP3, IGF2BP1, KHDRBS2 and SMAD9 played a pivotal function in the development of lung adenocarcinoma.
Figure 3. Survival analysis of hub RBPs.
(A) BOP1, (B) GNL3, (C) WDR12, (D) DCAF13, (E) IGF2BP3, (F) IGF2BP1, (G) KHDRBS2, (H) SMAD9. The dotted lines represent the 95% confidence interval.
Hub RBPs verification and efficacy assessment
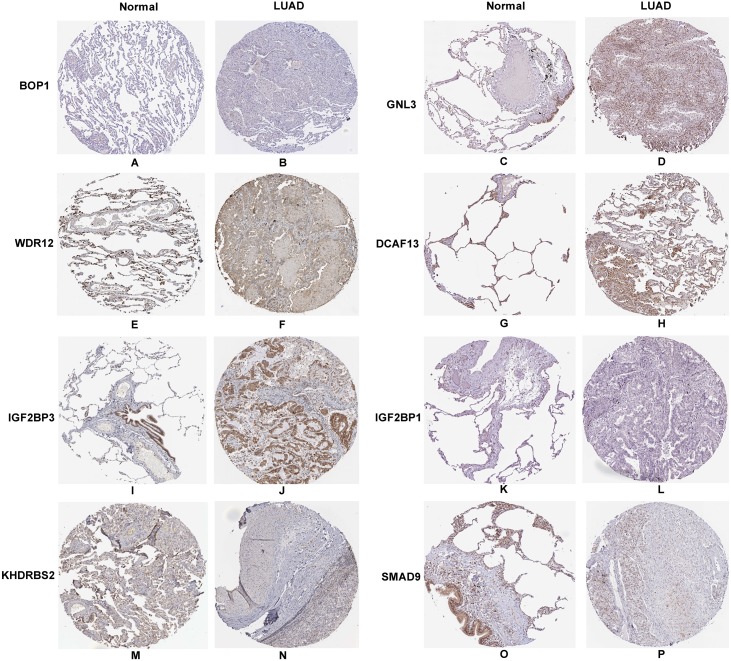
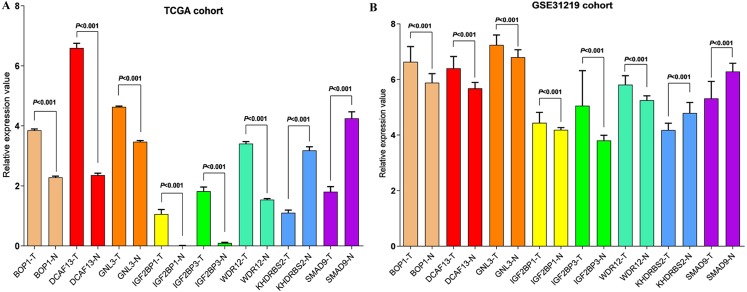
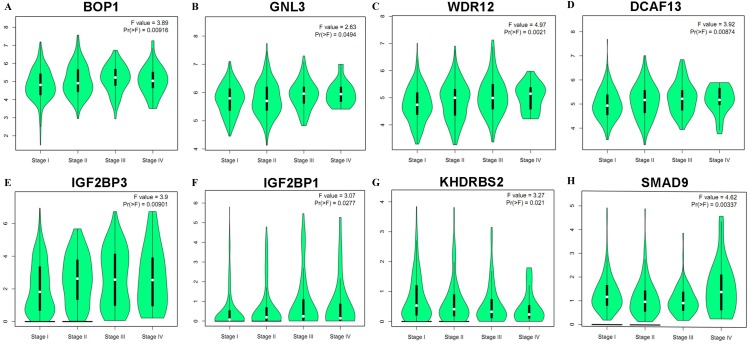
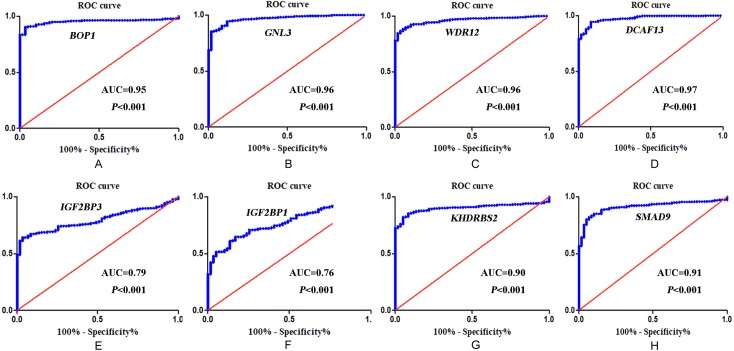
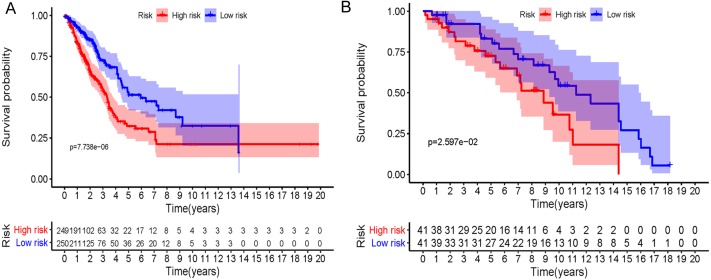
In accordance with the immunohistochemistry results from the Human Protein Atlas database, we found that the expression of hub RBPs BOP1, GNL3, WDR12, DCAF13, IGF2BP3, and IGF2BP1 were obviously elevated in lung carcinoma compared with normal lung tissues, while KHDRBS2 and SMAD9 were significantly decreased in tumor tissues (Fig. 4). Furthermore, we validated the expression level of the hub RBPs at transcription. The results indicated that the eight hub RBPs were deregulated in both TCGA and GSE30219 LUAD patient cohorts (Fig. 5). Moreover, the expression of the eight hub RBPs were significantly associated with tumor stage (Fig. 6). In addition, the ROC curve was used to assess the ability of eight hub RBPs to discriminate tumor tissue and normal lung tissue. The area under the curve (AUC) of hub genes BOP1 (AUC = 0.95, 95%CI [0.9357–0.9700], P<0.0001), GNL3 (AUC = 0.96, 95%CI [0.9509–0.9827], P<0.0001), WDR12 (AUC = 0.9614, 95%CI [0.9459–0.9769], P <0.0001), DCAF13 (AUC = 0.9747, 95%CI [0.9616–0.9879], P <0.0001), IGF2BP3 (AUC = 0.7949, 95%CI [0.7565–0.8334], P <0.0001), IGF2BP1 (AUC = 0.7754, 95%CI [0.7565–0.8334], P < 0.0001), KHDRBS2 (AUC = 0.9018, 95%CI [0.8767–0.9270], P < 0.0001), and SMAD9 (AUC = 0.9120, 95%CI [0.8855–0.9386], P < 0.0001) were greater than 0.7, suggesting that the eight hub genes with a better diagnostic accuracy for LUAD (Fig. 7). To further assess whether the eight hub genes can be used as one gene signature to predict the prognosis of LUAD patients, the risk score of every patient was calculated based on the coefficient value of the eight genes. Then, all patients were divided into high-risk and low-risk groups with the median risk score, and patients of high-risk were with poor OS compared with those of low-risk in both TCGA and GSE30219 cohorts (Fig. 8).
Figure 4. Validation the protein expression of hub genes in normal lung tissue and LUAD by the Human Protein Atlas database.
(A–B) BOP1, (C–D) GNL3, (E–F) WDR12, (G–H) DCAF13, (I–J) IGF2BP3, (K–L) IGF2BP1, (M–N) KHDRBS2, (O–P) SMAD9.
Figure 5. Validation the mRNA expression of hub RBPs in normal lung tissue and LUAD.
(A) TCGA LUAD patients cohort; (B) GSE30219 LUAD patient cohort; T: tumor tissue; N: normal tissue.
Figure 6. Correlation between eight hub RBPs expression and tumor stage in LUAD patients.
(A) BOP1, (B) GNL3, (C) WDR12, (D) DCAF13, (E) IGF2BP3, (F) IGF2BP1, (G) KHDRBS2, (H) SMAD9.
Figure 7. ROC analysis of eight hub RBPs based on TCGA database.
(A) BOP1, (B) GNL3, (C) WDR12, (D) DCAF13, (E) IGF2BP3, (F) IGF2BP1 , (G) KHDRBS2, (H) SMAD9.
Figure 8. Prognostic risk assessment of integrated eight-hub genes for LUAD patients.
(A) TCGA cohort, (B) GSE31219 cohort.
Functions and pathways analysis of the alterations in candidate hub genes and their frequently changed neighbor genes in LUAD patients
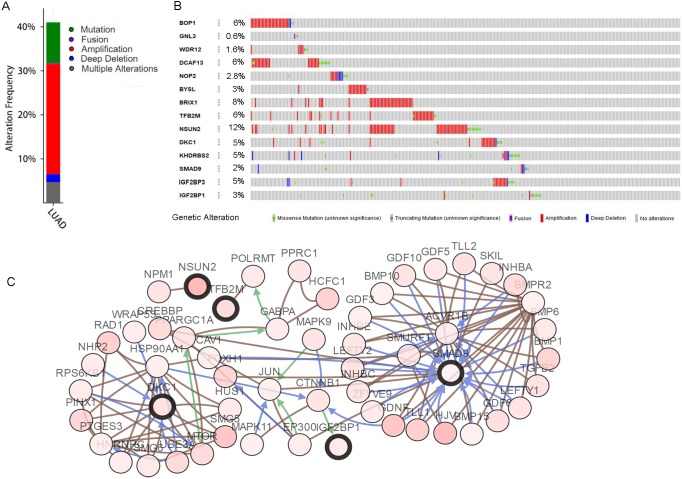
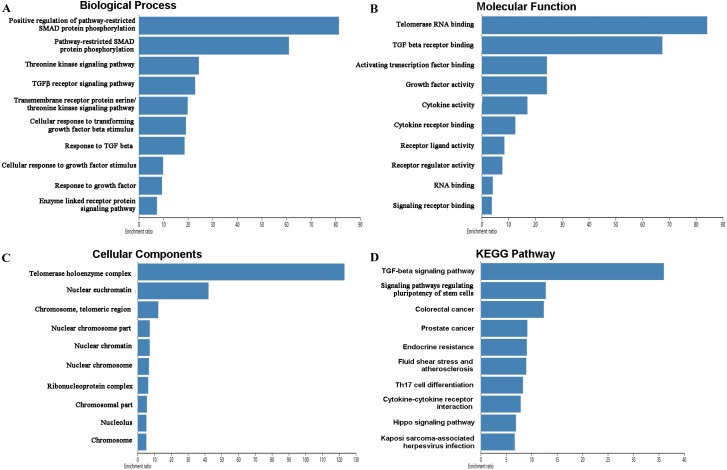
The mutations and copy-number alterations analysis of the key genes BOP1, GNL3, WDR12, NOP2, BYSL, BRIX1, DCAF13, TFB2M, NSUN2, DKC1, IGF2BP3, IGF2BP1, KHDRBS2 and SMAD9 were carried out by using cBioPortal. We found that the 14 key genes altered in 223 samples out of 507 LUAD patients (Figs. 9A–9B). Then we constructed the interaction network contains 64 nodes, including 14 key genes and the 50 most frequently altered neighbor genes (Fig. 9C). The functions and pathways analysis of candidate hub genes and the genes significantly associated with hub genes alterations were conducted by using WebGestalt. The results showed that response to growth factor, enzyme linked receptor protein signaling pathway, TGF-beta signaling pathway, telomerase RNA binding, transmembrane receptor protein serine/threonine kinase signaling pathway, Th17 cell differentiation, and Hippo signaling pathway were significantly regulated by the hub genes alterations in LUAD (Fig. 10).
Figure 9. Hub RBPs expression and alteration a nalysis in lung adenocarcinoma.
(A) Total alteration frequency; (B) genetic alteration of each hub gene; (C) co-expression network of hub RBPs and the 50 most frequently altered neighbor genes.
Figure 10. Functional enrichment analysis of key RBPs and genes significant associated with their alterations.
(A) Biological process; (B) molecular function; (C) cellular components; (D) KEGG pathway.
Discussion
Despite advances in diagnostic and treatment methods over the past few decades, the overall mortality rates of lung carcinoma remained largely unchanged. Thence, understanding the etiology and molecular mechanism of lung carcinogenesis is necessary to improve the survival rate of patients. In recent years, many studies have shown that RBPs play a pivotal role in the development and progression of various human tumors (Pereira, Billaud & Almeida, 2017; Wu et al., 2019; Lujan, Ochoa & Hartley, 2018). However, the specific functional role of most RBPs in the process of tumorigenesis remains unclear (Chen et al., 2019). In this study, a total of 164 differently expressed RBPs between LUAD and normal lung tissue were screened, consisting of 124 up-regulated RBPs and 40 down-regulated RBPs. Then, we systematically explored the potential functional pathways and constructed a PPI network of these differently expressed RBPs. Moreover, the module analysis, survival analysis, ROC analysis and copy-number alterations analysis of hub RBPs were performed to further explore their biological functions and clinical significance. These findings may contribute to develop novel biomarkers for the diagnosis and prognosis of LUAD patients.
The function enrichment analysis demonstrated that the differently expressed RBPs were significantly related to RNA processing, RNA metabolic process, RNA binding, peptide biosynthetic process, regulation of translation, regulation of cellular amide metabolic process, catalytic activity acting on RNA, single-stranded RNA binding, poly-pyrimidine tract binding, RNA cap binding complex, ribonucleoprotein complex, mRNA 3′-UTR binding, ribonucleoprotein granule, and nucleolus. Previous studies have shown that RNA metabolism and RNA processing have been increasingly recognized in various diseases (Martinez-Terroba et al., 2018; Hinkle et al., 2019; Jain et al., 2019). RBPs can bind to mRNAs to form ribonucleoprotein complexes and regulate their expression by increasing mRNA stability, which play important roles in the development of many diseases. A recent study has revealed that RNA-binding protein SRSF1 promote tumor cell proliferation and progression by increasing LIG1 mRNA stability in non-small cell lung cancer (Martinez-Terroba et al., 2018). RNA binding proteins in the nucleus play key effects in regulated mRNA alternative splicing process and lead to alter in the expression of tumor-associated genes (Shao et al., 2019). Additionally, ribonucleoprotein granule is an important area that implements protein synthesis. The mutation of ribonucleoprotein regulates the translation process and associated with tumor development (Goudarzi & Lindstrom, 2016). It has been reported that heterogeneous nuclear ribonucleoprotein A2B1 is over-expressed in tissues and blood of patients with lung cancer, and which contributes to lung tumorigenesis (Dowling et al., 2015). The KEGG pathways analysis suggested that the differently expressed RBPs regulate the occurrence and development of lung cancer by affecting RNA degradation, TGF-beta signaling pathway, RNA transport, aminoacyl-tRNA biosynthesis, and mRNA surveillance pathway.
Furthermore, we constructed a PPI network of these differently expressed RBPs and got the top ten hub genes including BOP1, GNL3, WDR12, NOP2, BYSL, BRIX1, DCAF13, TFB2M, NSUN2 and DKC1. Among these genes, NOP2, also known as p120, is found highly expressed in lung adenocarcinoma tissue and negatively associated with patients prognosis (Saijo et al., 2001; Uchiyama et al., 1997). Despite the correlation between most RBPs and LUAD still unclear, several RBPs have been reported to be closely associated with other cancers. BOP1 as Wnt/β-catenin target gene involved in induced migration, EMT, and metastasis of colorectal carcinoma (Qi et al., 2016). GNL3 is upregulated in colon carcinoma cell and tissue, and facilitate tumor cell epithelial-mesenchymal transition by activating the Wnt/β-catenin signaling pathway (Tang et al., 2017a). WDR12 as a new oncogene contributes to liver cancer spread (Yin et al., 2018). DCAF13 is upregulated in liver cancer and significantly related to poor survival (Cao et al., 2017; Qiao et al., 2019). NSUN2 as a tRNA methyltransferase, is significantly related to cancer progression in several tumors, such as esophageal squamous cell carcinoma (Li et al., 2018), breast cancer (Yi et al., 2017), and ovarian cancer (Yang et al., 2017). DKC1 is deregulated in glioma and knockdown of DKC1 restrain cancer cell proliferation, invasion and migration (Miao et al., 2019). By executing module analysis of PPI network, we found that LUAD is associated with RNA metabolic process, ribosome biogenesis, cellular amide metabolic process, peptide biosynthetic process, and translational elongation.
In addition, the survival analysis showed that 8 RBPs are obviously related to survival of LUAD patients. Increased expression of BOP1, GNL3, WDR12, DCAF13, IGF2BP3 and IGF2BP1 were related to poor overall survival, while overexpression of KHDRBS2 and SMAD were related to better overall survival. Subsequently, we verified the expression patterns of these eight hub RBPs on translation and transcription level by using the Human Protein Atlas database and GEPIA database, the results showed that BOP1, GNL3, WDR12, DCAF13, IGF2BP3 and IGF2BP1 were upregulated, and KHDRBS2 as well as SMAD were downregulated in LUAD tissues. These findings suggested that BOP1, GNL3, WDR12, DCAF13, IGF2BP3 and IGF2BP1 may have potential carcinogenic effects, KHDRBS2 and SMAD role as tumor suppressor genes. Previous study has reported that the expression IGF2BP3 or IGF2BP1 can predict poor overall survival in lung cancer patients (Shi et al., 2017), which were consistent with our results. Two other studies also showed that DCAF13 was increased in hepatocellular carcinoma or breast cancer, and obviously associated with poor overall survival (Cao et al., 2017; Wang et al., 2019). The ROC curve analysis revealed that these 8 RBPs with better diagnostic accuracy for distinguishing LUAD patients from healthy people, which could be used as potential diagnostic molecular markers in the future. Besides, we found that the eight hub genes can be used as one gene signature to predict the prognosis of LUAD patients in TCGA and GSE30219 cohorts.
Finally, we analyzed the hub genes alterations and constructed the co-expression network by using the cBioPortal online tool for LUAD. The results showed that amplification of NSUN2 was the most common alterations in all copy number variations of these RBPs. A study revealed that NSUN2 gene copy number was elevated in colorectal and oral carcinoma (Okamoto et al., 2012).We also found that DCAF13 and BOP1 have high copy number gain frequency in patients with LUAD. Previous studies have shown that DCAF13 is amplified in various tumors, such as hepatocellular carcinoma (Cao et al., 2017), breast cancer (Wang et al., 2019), and osteosarcoma (Chen et al., 2018). Another research has demonstrated that dosage increase of the BOP1 was a frequent event in colorectal carcinoma and related to cancer occurrence (Killian et al., 2006). By constructing a protein co-expression network of key 14 RBPs and the 50 most frequently altered neighbor genes, we found that the alterations of hub RBPs in LUAD were involved in regulating enzyme linked receptor protein signaling pathway, telomerase RNA binding, TGF-beta signaling pathway, Hippo signaling pathway, and transmembrane receptor protein serine/threonine kinase signaling pathway. These results indicated that mutations and copy-number alterations of RBPs were play important roles in lung carcinogenesis and progression.
In conclusion, the current study conducted a comprehensive bioinformatics analysis of differently expressed RBPs to identify the potential biomarkers and predict progression of LUAD. The results showed that the expression of BOP1, GNL3, WDR12, DCAF13, IGF2BP3, IGF2BP1, KHDRBS2 and SMAD were significantly related to prognosis of LUAD patient. These findings have the potential to provide new therapeutic targets prognostic markers for LUAD.
Supplemental Information
Funding Statement
This project was supported by the Cuiying Scientific and Technological Program of Lanzhou University Second Hospital (CY2018-MS10), the National Natural Science Foundation of China (81560343), the Natural Science Foundation of Hunan Province (2019JJ50425), and General Project of Hunan Provincial Education Department (18C1129). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Wei Li conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Na Li and Lina Gao performed the experiments, analyzed the data, prepared figures and/or tables, and approved the final draft.
Chongge You conceived and designed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Data Availability
The following information was supplied regarding data availability:
The raw data can be found at TCGA (https://portal.gdc.cancer.gov/projects/TCGA-LUAD) and GEO: GSE30219.
References
- Bao et al. (2017).Bao P, Yokobori T, Altan B, Iijima M, Azuma Y, Onozato R, Yajima T, Watanabe A, Mogi A, Shimizu K, Nagashima T, Ohtaki Y, Obayashi K, Nakazawa S, Bai T, Kawabata-Iwakawa R, Asao T, Kaira K, Nishiyama M, Kuwano H. High STMN1 expression is associated with cancer progression and chemo-resistance in lung squamous cell carcinoma. Annals of Surgical Oncology. 2017;24:4017–4024. doi: 10.1245/s10434-017-6083-0. [DOI] [PubMed] [Google Scholar]
- Cao et al. (2017).Cao J, Hou P, Chen J, Wang P, Wang W, Liu W, Liu C, He X. The overexpression and prognostic role of DCAF13 in hepatocellular carcinoma. Tumour Biology. 2017;39 doi: 10.1177/1010428317705753. Article 1010428317705753. [DOI] [PubMed] [Google Scholar]
- Chang et al. (2019).Chang MY, Oh B, Choi JE, Sulistio YA, Woo HJ, Jo A, Kim J, Kim EH, Kim SW, Hwang J, Park J, Song JJ, Kwon OC, Kim HHenry, Kim YH, Ko JY, Heo JY, Lee MJ, Lee M, Choi M, Chung SJ, Lee HS, Lee SH. LIN28A loss of function is associated with Parkinson’s disease pathogenesis. The EMBO Journal. 2019;38:e101196. doi: 10.15252/embj.2018101196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterji & Rustgi (2018).Chatterji P, Rustgi AK. RNA binding proteins in intestinal epithelial biology and colorectal cancer. Trends in Molecular Medicine. 2018;24:490–506. doi: 10.1016/j.molmed.2018.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen et al. (2019).Chen H, Liu J, Wang H, Cheng Q, Zhou C, Chen X, Ye F. Inhibition of RNA-binding protein musashi-1 suppresses malignant properties and reverses paclitaxel resistance in ovarian carcinoma. Journal of Cancer. 2019;10:1580–1592. doi: 10.7150/jca.27352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen et al. (2018).Chen Z, Zhang W, Jiang K, Chen B, Wang K, Lao L, Hou C, Wang F, Zhang C, Shen H. MicroRNA-300 regulates the ubiquitination of PTEN through the CRL4B(DCAF13) E3 ligase in osteosarcoma cells. Molecular Therapy. Nucleic Acids. 2018;10:254–268. doi: 10.1016/j.omtn.2017.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bruin et al. (2017).De Bruin RG, Rabelink TJ, Van Zonneveld AJ, Van der Veer E. Emerging roles for RNA-binding proteins as effectors and regulators of cardiovascular disease. European Heart Journal. 2017;38(18):1380–1388. doi: 10.1093/eurheartj/ehw567. [DOI] [PubMed] [Google Scholar]
- Dowling et al. (2015).Dowling P, Pollard D, Larkin A, Henry M, Meleady P, Gately K, O’Byrne K, Barr MP, Lynch V, Ballot J, Crown J, Moriarty M, O’Brien E, Morgan R, Clynes M. Abnormal levels of heterogeneous nuclear ribonucleoprotein A2B1 (hnRNPA2B1) in tumour tissue and blood samples from patients diagnosed with lung cancer. Molecular bioSystems. 2015;11:743–752. doi: 10.1039/C4MB00384E. [DOI] [PubMed] [Google Scholar]
- Gerstberger, Hafner & Tuschl (2014).Gerstberger S, Hafner M, Tuschl T. A census of human RNA-binding proteins. Nature Reviews. Genetics. 2014;15:829–845. doi: 10.1038/nrg3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudarzi & Lindstrom (2016).Goudarzi KM, Lindstrom MS. Role of ribosomal protein mutations in tumor development (Review) International Journal of Oncology. 2016;48:1313–1324. doi: 10.3892/ijo.2016.3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, Huang & Zhang (2019).Han L, Huang C, Zhang S. The RNA-binding protein SORBS2 suppresses hepatocellular carcinoma tumourigenesis and metastasis by stabilizing RORA mRNA. Liver International. 2019;39:2190–2203. doi: 10.1111/liv.14202. [DOI] [PubMed] [Google Scholar]
- Hinkle et al. (2019).Hinkle ER, Wiedner HJ, Black AJ, Giudice J. RNA processing in skeletal muscle biology and disease. Transcription. 2019;10:1–20. doi: 10.1080/21541264.2018.1558677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain et al. (2019).Jain A, Brown SZ, Thomsett HL, Londin E, Brody JR. Evaluation of post-transcriptional gene regulation in pancreatic cancer cells: studying RNA binding proteins and their mRNA targets. Methods in Molecular Biology. 2019;1882:239–252. doi: 10.1007/978-1-4939-8879-2_22. [DOI] [PubMed] [Google Scholar]
- Jan et al. (2019).Jan YH, Lai TC, Yang CJ, Huang MS, Hsiao M. A co-expressed gene status of adenylate kinase 1/4 reveals prognostic gene signature associated with prognosis and sensitivity to EGFR targeted therapy in lung adenocarcinoma. Scientific Reports. 2019;9:12329. doi: 10.1038/s41598-019-48243-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killian et al. (2006).Killian A, Sarafan-Vasseur N, Sesboue R, Le Pessot F, Blanchard F, Lamy A, Laurent M, Flaman JM, Frebourg T. Contribution of the BOP1 gene, located on 8q24, to colorectal tumorigenesis. Genes, Chromosomes & Cancer. 2006;45:874–881. doi: 10.1002/gcc.20351. [DOI] [PubMed] [Google Scholar]
- Legrand, Dixon & Sobolewski (2019).Legrand N, Dixon DA, Sobolewski C. AU-rich element-binding proteins in colorectal cancer. World Journal of Gastrointestinal Oncology. 2019;11:71–90. doi: 10.4251/wjgo.v11.i2.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2018).Li Y, Li J, Luo M, Zhou C, Shi X, Yang W, Lu Z, Chen Z, Sun N, He J. Novel long noncoding RNA NMR promotes tumor progression via NSUN2 and BPTF in esophageal squamous cell carcinoma. Cancer Letters. 2018;430:57–66. doi: 10.1016/j.canlet.2018.05.013. [DOI] [PubMed] [Google Scholar]
- Liao et al. (2019).Liao Y, Wang J, Jaehnig EJ, Shi Z, Zhang B. WebGestalt 2019: gene set analysis toolkit with revamped UIs and APIs. Nucleic Acids Research. 2019;47:W199–W205. doi: 10.1093/nar/gkz401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujan, Ochoa & Hartley (2018).Lujan DA, Ochoa JL, Hartley RS. Cold-inducible RNA binding protein in cancer and inflammation. Wiley Interdisciplinary Reviews. RNA. 2018;9:e1462. doi: 10.1002/wrna.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Terroba et al. (2018).Martinez-Terroba E, Ezponda T, Bertolo C, Sainz C, Remirez A, Agorreta J, Garmendia I, Behrens C, Pio R, Wistuba II, Montuenga LM, Pajares MJ. The oncogenic RNA-binding protein SRSF1 regulates LIG1 in non-small cell lung cancer. Laboratory Investigation. 2018;98:1562–1574. doi: 10.1038/s41374-018-0128-2. [DOI] [PubMed] [Google Scholar]
- Masuda & Kuwano (2019).Masuda K, Kuwano Y. Diverse roles of RNA-binding proteins in cancer traits and their implications in gastrointestinal cancers. Wiley Interdisciplinary Reviews. RNA. 2019;10:e1520. doi: 10.1002/wrna.1520. [DOI] [PubMed] [Google Scholar]
- Miao et al. (2019).Miao FA, Chu K, Chen HR, Zhang M, Shi PC, Bai J, You Y. Increased DKC1 expression in glioma and its significance in tumor cell proliferation, migration and invasion. Investigational New Drugs. 2019;37:1177–1186. doi: 10.1007/s10637-019-00748-w. [DOI] [PubMed] [Google Scholar]
- Okamoto et al. (2012).Okamoto M, Hirata S, Sato S, Koga S, Fujii M, Qi G, Ogawa I, Takata T, Shimamoto F, Tatsuka M. Frequent increased gene copy number and high protein expression of tRNA (cytosine-5-)-methyltransferase (NSUN2) in human cancers. DNA and Cell Biology. 2012;31:660–671. doi: 10.1089/dna.2011.1446. [DOI] [PubMed] [Google Scholar]
- Pereira, Billaud & Almeida (2017).Pereira B, Billaud M, Almeida R. RNA-binding proteins in cancer: old players and new actors. Trends in Cancer. 2017;3:506–528. doi: 10.1016/j.trecan.2017.05.003. [DOI] [PubMed] [Google Scholar]
- Pique et al. (2019).Pique L, Martinez de Paz A, Pineyro D, Martinez-Cardus A, Castro de Moura M, Llinas-Arias P, Setien F, Gomez-Miragaya J, Gonzalez-Suarez E, Sigurdsson S, Jonasson JG, Villanueva A, Vidal A, Davalos V, Esteller M. Epigenetic inactivation of the splicing RNA-binding protein CELF2 in human breast cancer. Oncogene. 2019;38:7106–7112. doi: 10.1038/s41388-019-0936-x. [DOI] [PubMed] [Google Scholar]
- Qi et al. (2016).Qi J, Yu Y, Akilli Ozturk O, Holland JD, Besser D, Fritzmann J, Wulf-Goldenberg A, Eckert K, Fichtner I, Birchmeier W. New Wnt/beta-catenin target genes promote experimental metastasis and migration of colorectal cancer cells through different signals. Gut. 2016;65:1690–1701. doi: 10.1136/gutjnl-2014-307900. [DOI] [PubMed] [Google Scholar]
- Qiao et al. (2019).Qiao GJ, Chen L, Wu JC, Li ZR. Identification of an eight-gene signature for survival prediction for patients with hepatocellular carcinoma based on integrated bioinformatics analysis. PeerJ. 2019;7:e6548. doi: 10.7717/peerj.6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saijo et al. (2001).Saijo Y, Sato G, Usui K, Sato M, Sagawa M, Kondo T, Minami Y, Nukiwa T. Expression of nucleolar protein p120 predicts poor prognosis in patients with stage I lung adenocarcinoma. Annals of Oncology. 2001;12:1121–1125. doi: 10.1023/A:1011617707999. [DOI] [PubMed] [Google Scholar]
- Shao et al. (2019).Shao Y, Chong W, Liu X, Xu Y, Zhang H, Xu Q, Guo Z, Zhao Y, Zhang M, Ma Y, Gu F. Alternative splicing-derived intersectin1-L and intersectin1-S exert opposite function in glioma progression. Cell Death & Disease. 2019;10 doi: 10.1038/s41419-019-1668-0. Article 431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi et al. (2017).Shi R, Yu X, Wang Y, Sun J, Sun Q, Xia W, Dong G, Wang A, Gao Z, Jiang F, Xu L. Expression profile, clinical significance, and biological function of insulin-like growth factor 2 messenger RNA-binding proteins in non-small cell lung cancer. Tumour biology. 2017;39 doi: 10.1177/1010428317695928. Article 1010428317695928. [DOI] [PubMed] [Google Scholar]
- Siegel, Miller & Jemal (2018).Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA: A Cancer Journal for Clinicians. 2018;68(1):7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- Son et al. (2019).Son YO, Kim HE, Choi WS, Chun CH, Chun JS. RNA-binding protein ZFP36L1 regulates osteoarthritis by modulating members of the heat shock protein 70 family. Nature Communications. 2019;10 doi: 10.1038/s41467-018-08035-7. Article 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenschein et al. (2019).Sonnenschein K, Fiedler J, Pfanne A, Just A, Mitzka S, Geffers R, Pich A, Bauersachs J, Thum T. Therapeutic modulation of RNA-binding protein Rbm38 facilitates re-endothelialization after arterial injury. Cardiovascular Research. 2019;115:1804–1810. doi: 10.1093/cvr/cvz063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun et al. (2019).Sun R, Meng X, Wang W, Liu B, Lv X, Yuan J, Zeng L, Chen Y, Yuan B, Yang S. Five genes may predict metastasis in non-small cell lung cancer using bioinformatics analysis. Oncology Letters. 2019;18:1723–1732. doi: 10.3892/ol.2019.10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szklarczyk et al. (2019).Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J, Simonovic M, Doncheva NT, Morris JH, Bork P, Jensen LJ, Mering CV. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Research. 2019;47:D607–D613. doi: 10.1093/nar/gky1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang et al. (2017a).Tang X, Zha L, Li H, Liao G, Huang Z, Peng X, Wang Z. Upregulation of GNL3 expression promotes colon cancer cell proliferation, migration, invasion and epithelial-mesenchymal transition via the Wnt/beta-catenin signaling pathway. Oncology Reports. 2017a;38:2023–2032. doi: 10.3892/or.2017.5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang et al. (2017b).Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Research. 2017b;45:W98–W102. doi: 10.1093/nar/gkx247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepeli et al. (2012).Tepeli E, Caner V, Buyukpinarbasili N, Cetin GO, Duzcan F, Elmas L, Bagci G. Expression of ERCC1 and its clinicopathological correlations in non-small cell lung cancer. Molecular Biology Reports. 2012;39:335–341. doi: 10.1007/s11033-011-0743-0. [DOI] [PubMed] [Google Scholar]
- Uchiyama et al. (1997).Uchiyama B, Saijo Y, Kumano N, Abe T, Fujimura S, Ohkuda K, Handa M, Satoh K, Nukiwa T. Expression of nucleolar protein p120 in human lung cancer: difference in histological types as a marker for proliferation. Clinical Cancer Research. 1997;3:1873–1877. [PubMed] [Google Scholar]
- Uhlen et al. (2015).Uhlen M, Fagerberg L, Hallstrom BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson A, Kampf C, Sjostedt E, Asplund A, Olsson I, Edlund K, Lundberg E, Navani S, Szigyarto CA, Odeberg J, Djureinovic D, Takanen JO, Hober S, Alm T, Edqvist PH, Berling H, Tegel H, Mulder J, Rockberg J, Nilsson P, Schwenk JM, Hamsten M, Von Feilitzen K, Forsberg M, Persson L, Johansson F, Zwahlen M, Von Heijne G, Nielsen J, Ponten F. Proteomics. Tissue-based map of the human proteome. Science. 2015;347 doi: 10.1126/science.1260419. Article 1260419. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2019).Wang K, Li L, Fu L, Yuan Y, Dai H, Zhu T, Zhou Y, Yuan F. Integrated bioinformatics analysis the function of RNA binding proteins (RBPs) and their prognostic value in breast cancer. Frontiers in Pharmacology. 2019;10 doi: 10.3389/fphar.2019.00140. Article 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2020).Wang Z, Qiu H, He J, Liu L, Xue W, Fox A, Tickner J, Xu J. The emerging roles of hnRNPK. Journal of Cellular Physiology. 2020;235:1995–2008. doi: 10.1002/jcp.29186. [DOI] [PubMed] [Google Scholar]
- Wu et al. (2019).Wu Y, Chen H, Chen Y, Qu L, Zhang E, Wang Z, Wu Y, Yang R, Mao R, Lu C, Fan Y. HPV shapes tumor transcriptome by globally modifying the pool of RNA binding protein-binding motif. Aging. 2019;11:2430–2446. doi: 10.18632/aging.101927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie et al. (2019a).Xie L, Dang Y, Guo J, Sun X, Xie T, Zhang L, Yan Z, Amin H, Guo X. High KRT8 expression independently predicts poor prognosis for lung adenocarcinoma patients. Gene. 2019a;10(1) doi: 10.3390/genes10010036. Article 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie et al. (2019b).Xie M, Ma T, Xue J, Ma H, Sun M, Zhang Z, Liu M, Liu Y, Ju S, Wang Z, De W. The long intergenic non-protein coding RNA 707 promotes proliferation and metastasis of gastric cancer by interacting with mRNA stabilizing protein HuR. Cancer Letters. 2019b;443:67–79. doi: 10.1016/j.canlet.2018.11.032. [DOI] [PubMed] [Google Scholar]
- Yan et al. (2019).Yan M, Sun L, Li J, Yu H, Lin H, Yu T, Zhao F, Zhu M, Liu L, Geng Q, Kong H, Pan H, Yao M. RNA-binding protein KHSRP promotes tumor growth and metastasis in non-small cell lung cancer. Journal of Experimental & Clinical Cancer Research. 2019;38 doi: 10.1186/s13046-019-1479-2. Article 478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang et al. (2017).Yang JC, Risch E, Zhang M, Huang C, Huang H, Lu L. Association of tRNA methyltransferase NSUN2/IGF-II molecular signature with ovarian cancer survival. Future Oncology. 2017;13:1981–1990. doi: 10.2217/fon-2017-0084. [DOI] [PubMed] [Google Scholar]
- Yi et al. (2017).Yi J, Gao R, Chen Y, Yang Z, Han P, Zhang H, Dou Y, Liu W, Wang W, Du G, Xu Y, Wang J. Overexpression of NSUN2 by DNA hypomethylation is associated with metastatic progression in human breast cancer. Oncotarget. 2017;8:20751–20765. doi: 10.18632/oncotarget.10612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin et al. (2018).Yin Y, Zhou L, Zhan R, Zhang Q, Li M. Identification of WDR12 as a novel oncogene involved in hepatocellular carcinoma propagation. Cancer Management and Research. 2018;10:3985–3993. doi: 10.2147/CMAR.S176268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al. (2019).Zhang H, Wang Y, Dou J, Guo Y, He J, Li L, Liu X, Chen R, Deng R, Huang J, Xie R, Zhao X, Yu J. Acetylation of AGO2 promotes cancer progression by increasing oncogenic miR-19b biogenesis. Oncogene. 2019;38:1410–1431. doi: 10.1038/s41388-018-0530-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
The raw data can be found at TCGA (https://portal.gdc.cancer.gov/projects/TCGA-LUAD) and GEO: GSE30219.