Abstract
Introduction:
The trigeminal ganglion is unique among the somatosensory ganglia regarding its topography, structure, composition and possibly some functional properties of its cellular components. Being mainly responsible for the sensory innervation of the anterior regions of the head, it is a major target for headache research. One intriguing question is if the trigeminal ganglion is merely a transition site for sensory information from the periphery to the central nervous system, or if intracellular modulatory mechanisms and intercellular signaling are capable of controlling sensory information relevant for the pathophysiology of headaches.
Methods:
An online search based on PubMed was made using the keyword “trigeminal ganglion” in combination with “anatomy”, “headache”, “migraine”, “neuropeptides”, “receptors” and “signaling”. From the relevant literature, further references were selected in view of their relevance for headache mechanisms. The essential information was organized based on location and cell types of the trigeminal ganglion, neuropeptides, receptors for signaling molecules, signaling mechanisms, and their possible relevance for headache generation.
Results:
The trigeminal ganglion consists of clusters of sensory neurons and their peripheral and central axon processes, which are arranged according to the three trigeminal partitions V1–V3. The neurons are surrounded by satellite glial cells, the axons by Schwann cells. In addition, macrophage-like cells can be found in the trigeminal ganglion. Neurons express various neuropeptides, among which calcitonin gene-related peptide is the most prominent in terms of its prevalence and its role in primary headaches. The classical calcitonin gene-related peptide receptors are expressed in non-calcitonin gene-related peptide neurons and satellite glial cells, although the possibility of a second calcitonin gene-related peptide receptor in calcitonin gene-related peptide neurons remains to be investigated. A variety of other signal molecules like adenosine triphosphate, nitric oxide, cytokines, and neurotrophic factors are released from trigeminal ganglion cells and may act at receptors on adjacent neurons or satellite glial cells.
Conclusions:
The trigeminal ganglion may act as an integrative organ. The morphological and functional arrangement of trigeminal ganglion cells suggests that intercellular and possibly also autocrine signaling mechanisms interact with intracellular mechanisms, including gene expression, to modulate sensory information. Receptors and neurotrophic factors delivered to the periphery or the trigeminal brainstem can contribute to peripheral and central sensitization, as in the case of primary headaches. The trigeminal ganglion as a target of drug action outside the blood-brain barrier should therefore be taken into account.
Keywords: Trigeminal neurons, satellite glial cells, neuropeptides, signaling, neuromodulation, CGRP
Introduction
The paired trigeminal ganglion (TG), also called the semilunar or Gasserian ganglion, is unique among primary afferent ganglia both in structural and functional aspects. It is located inside the head and gives rise to three main peripheral nerves providing nearly all intra-and extracranial structures with nerve fibers of various somatosensory functions. Some of these structures, like the cornea and the cranial dura, are mainly or exclusively innervated by thin A-delta and C-fiber afferents with putative nociceptive functions (1,2,3). The so-called trigeminovascular system of the meninges, denoting the afferent fibers closely associated with intracranial blood vessels, is regarded as the structural substrate of headache generation. Notwithstanding, this system keeps amazing secrets; for example, the fact that stimulation of the densely innervated pial blood vessels does not cause any sensation (4).
Location, anatomical structure and compartments of the trigeminal ganglion
The TG is located in the Meckel’s cave, a cavern in the petrosal part of the temporal bone on the floor of the middle crania fossa, formed by a pocket of dura mater. From the rostro-lateral border of the TG, three large nerves arise, the ophthalmic (V1), the maxillary (V2), and the mandibular (V3) nerve (Figure 1(a)). In rodents, at their origin V2 and V3 form one thick bundle that divides more rostrally. On the caudal border, the trigeminal ganglion passes into the trigeminal root or so-called trigeminal nerve, which contains the central processes of trigeminal fibers that enter the brainstem at the pontine level. In addition, a thin bundle of trigeminal motor fibers passing through the ganglion accompanies the trigeminal nerve (root) medially and the mandibular nerve rostrally from the ganglion (5). After retrograde tracing of the rat spinosus nerve, an afferent nerve in the dura mater arising from the mandibular area, nerve fibers lit up in the ganglion, which obviously had no contact to any cell body. These fibers are presumably proprioceptive afferents of head muscles that pass the ganglion on their way to the mesencephalic nucleus in the brainstem, where their somata are located (6).
Figure 1.
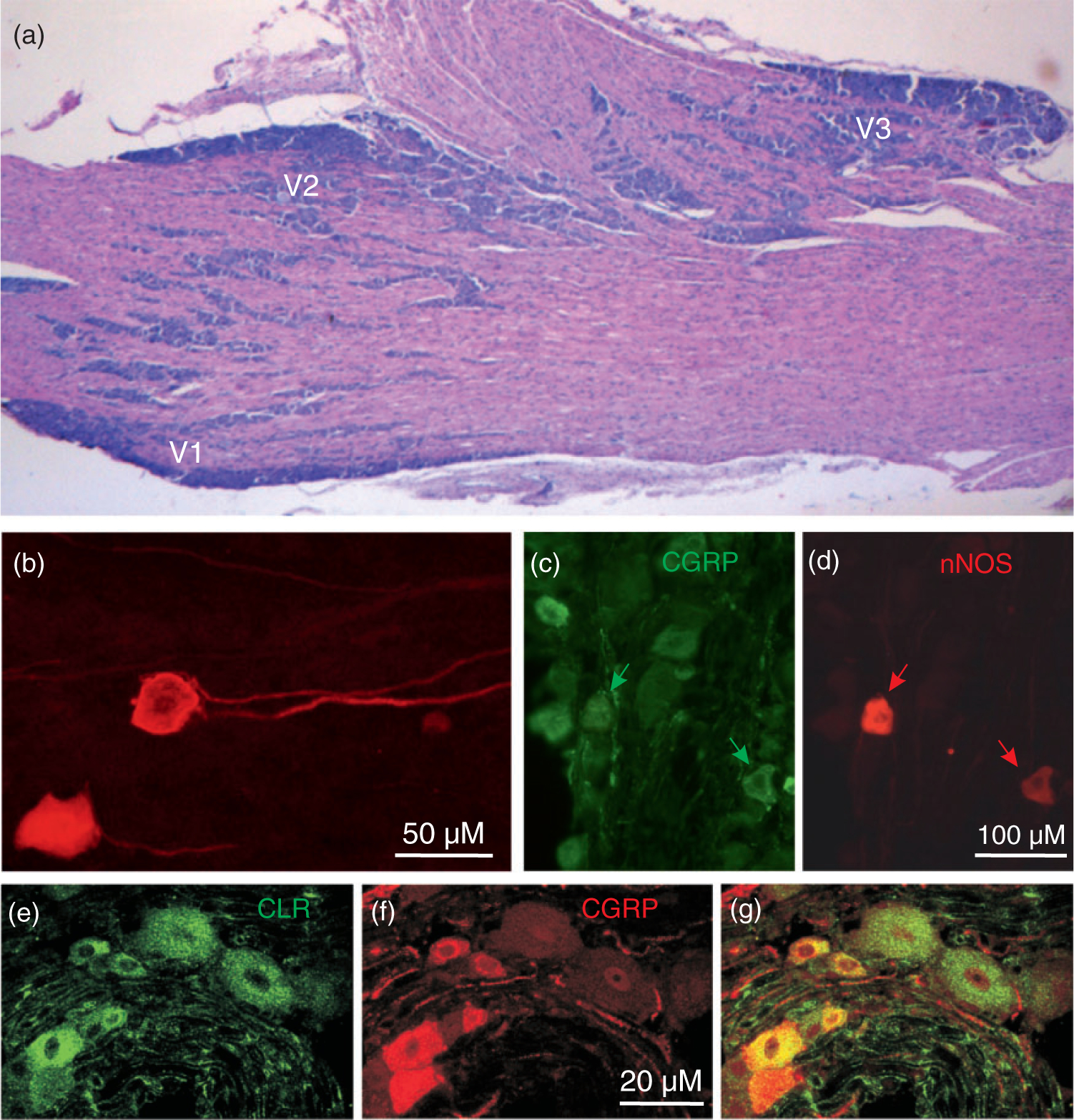
Histological characteristics of rat trigeminal ganglion. (a) Horizontal section (haematoxilin-eosin staining) showing aggregations of primary afferent somata in the ophthalmic (V1), maxillary (V2) and mandibular division (V3). In rodents, the ophthalmic and maxillary branches originate close together, while the mandibular branch is clearly separated. (b) Trigeminal ganglion neurons stained by fluorescent tracer DiI applied to the spinosus nerve near the mandibular division of the ganglion. Courtesy of Markus Schueler, Erlangen. (c), (d) Neurons immunostained for CGRP and neuronal NO synthase (nNOS), same section. Some neurons show both markers (arrows). Courtesy of Anne Dieterle, Erlangen. (e)–(g) Neurons immunostained for CGRP and the CGRP receptor component CLR, same section. Most CGRP-immunoreactive neurons are small to medium sized (red in (f)), CLR-immunoreactive neurons are mostly of middle size (green in (e)). Neurons can show both CGRP and CLR immunoreactivity (yellow in (g)) but neurons showing CGRP and all CGRP receptor components are extremely rare. Courtesy of Jochen Lennerz, Boston.
The mass of nerve fibers is thus occupying a major volume within the ganglion. The fascicles of nerve fibers separate aggregations of neurons that belong to the three partitions, V1–V3, which can be fairly distinguished according to their location within the ganglion. In horizontal sections through the rodent TG, the ophthalmic division is located rostro-medially, the maxillary division rostro-laterally, and the mandibular division forms a protrusion at the caudal lateral border (Figure 1(a)). The ganglion and the three peripheral nerves are ensheathed by a tough layer of collagenous tissue, a duplication of the dura mater, while the trigeminal root is only equipped with some sparse connective tissue, which may render it vulnerable to mechanical impact.
The functional organization of cutaneous afferents within the TG is quite well-known from lesion experiments (7) and electrophysiological recording studies in the cat (8) and the monkey (9), but there is not much information about the topographical distribution of neurons innervating intracranial structures. The problem is that the innervation territories of cutaneous afferents only roughly map the territories of meningeal afferents in humans. Furthermore, the areas of headache sensations are in no way helpful in this problem; early intraoperative experiments have shown that headache can be referred to areas that may be far from the meningeal sites of stimulation (10). Thus, it is not known which neurons are involved in headache generation and it is completely unknown if these neurons are in any morphological or functional aspect different to the neurons innervating facial skin and other cranial organs.
Morphological characteristics of cell types in the trigeminal ganglion
The TG consists mainly of primary afferent neurons of the pseudo-unipolar type and glial cells. In human trigeminal ganglia, 20,000–35,000 neurons and about 100 times more non-neuronal cells have been counted (11). The neurons can unequivocally be identified by their nearly round and centrally located nucleus, in which nucleoli and chromatin particles may be visible (12). In the rat, the diameters of neurons range from about 10 to 55 μm, with more than 90% being small to medium-sized neurons measuring 15–35 μm in diameter (13,14). The size of the somata is loosely correlated with the diameters of the nerve fibers. In some sections, one or even both processes of a neuron can be visualized but it is not entirely clear if both have the same origin, according to the classical pseudo-unipolar type, which is certainly the case in dorsal root ganglion neurons (15), or if they arise independently from the cell body close to one another (Figure 1(b)). This question is of considerable functional importance because, in the latter case, at least parts of the cell body membrane are necessarily depolarized when action potentials travel through the neuronal processes.
The TG cell bodies are surrounded by a more or less tight single layer of satellite glial cells. In the embryonic trigeminal ganglion, each neuroblast is already accompanied by 2–4 glial cells (16). The distal and central afferent processes are wrapped by Schwann cells. The Schwann cells can be either non-myelinating or can form a myelin sheath, as in the case of Aβ and Aδ fibers. In addition to glia, the TG contains fibroblasts forming collagen fibers, small blood vessels (mainly capillaries), and several types of immune cells, such as resident microglia-like macrophages (17). A functional crosstalk between neurons and macrophages via purinergic P2X3 receptors and/or satellite glial cells via P2Y receptors is assumed at least in pathological states, like in temporomandibular inflammation (18,19).
Neuropeptides in the trigeminal ganglion
The trigeminal ganglion expresses a wide range of neuropeptides. The neuropeptides calcitonin gene-related peptide (CGRP) and substance P (SP) have been localized in trigeminal ganglia of different species by immunohistochemistry (20,21,22) (Figure 1(c), (f)). These and other trigeminal neuropeptides are an increasingly important subject of investigation (23). Immunostaining studies have revealed many additional neuropeptides, such as neurokinin A (NK-A), cholecystokinin (CCK), galanin (GAL), somatostatin (SOM), and opioid peptides, in the TG; a comprehensive review has been published by E Lazarov (24). Most of the peptide-expressing neurons are small or medium-sized, which corresponds to neurons innervating intracranial (dural) structures (25,26) and are thus very likely nociceptive neurons. According to more recent data from immunostaining and RT-PCR, additional neuropeptides such as enkephalins (27), nociceptin (28), growth-associated protein GAP-43 (29), pituitary adenylate-cyclase activating polypeptide (PACAP) (30) and even angiotensin II (31) are present in the TG and can be co-localized with SP or CGRP. The ratio of neurons found to produce CGRP and SP seems to depend on the species and differences in staining; however, there is agreement that CGRP is the most prominent neuropeptide with about 40–50% of neurons being CGRP-immunoreactive (14,32). This proportion is in the same range as in dorsal root ganglia (33); however, in neurons innervating intracranial blood vessels, CGRP has been found to be enriched compared to neurons innervating facial skin (34), which seems to be a general principle regarding visceral afferent innervations (35).
Do peptidergic neurons constitute a structurally and functionally separate group of TG neurons? Nociceptive afferents are traditionally grouped into two different populations according to their expression of vanilloid-sensitive transient receptor potential (TRPV1) channels or their isolectin B4 (IB4) binding. The first group containing the peptidergic neurons is sensitive to nerve growth factor (NGF), while the second group is sensitive to glial cell-line derived neurotrophic factor (GDNF) during development (36,37). However, Price and Flores (38), using immunofluorescence, found considerable co-localization of TRPV1 and IB4 binding in primary afferent neurons of rat and mouse, particularly in the rat TG. The question whether differences in these expression types are causative for functional differences between TG and DRGs is not yet clear; after all, Price and Flores (38) found significantly more CGRP-immunoreactive neurons colocalized with TRPV1 immunoreactivity (70 %) in the TG compared with the DRGs.
On this background, CGRP release from isolated TGs or TG cell cultures has long been used as a measure for mass activation of TG neurons to examine nociceptive signaling and intracellular mechanisms (39,40,41), keeping in mind that this signal is predictive only for the peptidergic fraction of neurons. Release experiments from the intact trigeminal ganglion using microdialysis has been probed but was restricted to smaller molecules like substance P (42).
Neuropeptide receptors in the trigeminal ganglion
Similar to the large variety of neuropeptides expressed in the TG, multiple components forming receptors for neuropeptides have been found expressed on the mRNA level or by immunohistochemistry. Functional neuropeptide receptors within the ganglion may be involved in intraganglionic signaling, as will be discussed later.
CGRP receptors are heteromers composed of a seven transmembrane-spanning protein called calcitonin receptor-like receptor (CLR), a single membrane-spanning protein called receptor activity-modifying protein 1 (RAMP1), and an intracellular component, the receptor component protein (RCP) (43). RCP links the membrane components to the intracellular Gs protein that activates adenylate cyclase to increase cyclic AMP levels (44). RAMP proteins are necessary for the trafficking of CLR to the cell membrane, and specific RAMPs define the ligand specificity of the calcitonin receptor family (45). In the trigeminal ganglion of several species examined so far, neurons of mainly medium sizes and glial cells (Schwann cells and satellite cells) have been found immunopositive for both CLR and RAMP1 CGRP receptor components (14,46). Indicated by immunostaining, there is only a very small overlap of TG neurons that express both CGRP and CGRP receptor proteins (14,47) (Figure 1 (e)–(g)). In an elegant study, an antibody specifically recognizing a fusion protein of the extracellular domains of RAMP1 and CLR, which comprise the CGRP binding pocket, was used to identify the distribution of CGRP receptors in the TG of monkey and man. The study confirmed the location of CGRP receptors on neurons and satellite glial cells (48).
In addition to the canonical CLR/RAMP1 CGRP receptor, the presence of a second CGRP receptor in the trigeminal ganglion must also be considered. This second receptor is comprised of the calcitonin receptor (CTR) and RAMP1 (49). It is localized in a distinct group of trigeminal ganglion neurons different from those expressing the canonical CLR receptor (49). The CTR/RAMP1 complex was originally identified as an amylin receptor, hence it is called the AMY1 receptor. Interestingly, the expression pattern of the AMY1 receptor is suggestive of co-localization with CGRP, as discussed below, although this remains to be demonstrated. AMY1 receptors are also present in vascular smooth muscle based on immunostaining (49) and suggested by functional data from cell culture studies (50).
Thus, CGRP receptor expression in the TG is possibly involved in signaling mechanisms, including positive feedback loops that may be important for sensitization in facial pain and headache. Effects of CGRP receptor blockade in models of meningeal nociception and therapeutic effects in migraine, along with a possible site of action within the TG, are discussed below.
The existence of neurokinin-1 (NK1) receptors, receptors for substance P, has been indirectly shown by functional studies in the rat TG. The activity of spinal trigeminal neurons with afferent input from inflamed temporomandibular joint and facial skin was decreased by injection of an NK1 receptor antagonist into the TG (51). However, it is not likely that NK1 receptors in the TG are crucially involved in the generation of migraine pain, since NK1 receptor antagonists turned out to be inefficient in migraine therapy or prevention (52,53).
RT-PCR and immunohistochemistry suggested the presence of all three receptor subtypes of the VIP/PACAP receptor family, VPAC1, VPAC2 and PAC1 in small-diameter neurons in rat and human TG (54,55). Release of PACAP within the TG could thus initiate communication between neighboring trigeminal sensory neurons. Furthermore, immunohistochemistry and in situ hybridization revealed the presence of somatostatin receptors (sst2A) (56) and galanin receptors (GALR1) (57) in small to medium-sized neurons in the rat TG. Binding sites for cholecystokinin (CCK) have been localized in the TG of different species (58). Delta opioid receptors have been shown in the TG by immunohistochemistry (59) and were upregulated following experimental inflammation of the tooth pulp (60). It is unknown if somatostatin, galanin and opioid receptors, which are usually linked to an antinociceptive function, have a local role in the TG, where the respective ligand peptides could be released.
Other neuropeptides, which may have a signaling function in the TG but originate most likely from other sources, are orexins and oxytocin. Orexin receptor (OX1R and OX2R) mRNA has been detected in rat TG neurons, and inhibition of both receptors reduced the expression of downstream proteins associated with sensitization of peripheral nociception in a model of temporomandibular joint inflammation (61). Oxytocin receptor immunoreactivity has also been found in rat trigeminal neurons, the majority of which also co-expressed CGRP (62). In a recent study, oxytocin suppressed TG neuronal hyperexcitability after nerve injury, which interestingly was mediated by modulation of K+ channels through activation of vasopressin-1-receptors, immunoreactivity for which has also been found in TG neurons (63).
Receptors for other signal molecules expressed in the trigeminal ganglion
In addition to the large variety of neuropeptide receptors in the TG, multiple receptors for neurotrophic factors and other receptors involved in sensory transduction and transmission have been found expressed on the mRNA level or by immunohistochemistry (24). In addition, evidence for functional receptor types comes primarily from classical pharmacological approaches. Many of these functional studies have been performed on cultured TG cells. Hence, neuronal cell bodies are frequently regarded as a model for their peripheral or central terminals where the sensory transduction processes or presynaptic mechanisms of neurotransmission, respectively, take place. Furthermore, functional receptors within the ganglion may be involved in intercellular signaling, as will be discussed later.
Receptors for classical neurotransmitters are abundantly expressed in the TG. Using immunohistochemistry, receptor proteins for all types of glutamate receptors, AMPA, kainite, N-methyl-D-aspartate (NMDA) and metabotropic glutamate receptors (mGluR), have been localized in rat TG neurons (64,65,66); mGluR proteins have also been found in satellite glial cells (67). Besides their role in neurotransmission, NMDA receptors may functionally interact with transient receptor potential (TRPV1) receptor channels (see below) contributing to mechanical hyperalgesia (68).
Subunits of nicotinergic (nAchR) and muscarinergic acetylcholine receptors (mAchR) have been found on the mRNA level and with immunohistochemistry expressed in the rat TG (69,70). Recently, Shelukina et al. (71) measured calcium transients in cultured TG neurons and found that considerable proportions of neurons responded to carbachol and nicotine with calcium transients, suggesting that both nAchR and mAchR are functional. This finding is possibly relevant for the hypothesis of a peripheral activating effect of parasympathetic nerve fibers on trigeminal afferents promoting trigeminal autonomic cephalalgias and migraine with cranio-autonomic symptoms (72,73).
Using immunohistochemistry, about 70% of rat TG neurons and also satellite cells have been found to be GABAergic, while with RT-PCR and in situ hybridization various subtypes of GABA receptor subunits have been localized to ganglion neurons (74). GABA was released by strong depolarizing stimuli (high molar K+ solution), and Cl− currents recorded in whole cell patches showed that the subunits form functional GABA receptors. The authors speculated that GABA may modulate somatic inhibition of neurons within the TG. Glycine receptor protein was also found in rat TG using immunohistochemistry (75).
Serotonin (5-hydroxytryptamine, 5-HT) binds to several types of 5-HT receptors (5-HT1–7), which are all G-protein-coupled except the 5-HT3 receptor, which forms a cation channel. Three subtypes of the Gi-protein-coupled 5HT1 receptors, which are relevant as targets of antimigraine triptans (5HT1B/1D/1F), have been found by immunohistochemistry in the rat TG (76). Interestingly, there was no difference in receptor density compared to dorsal root ganglia, showing that the 5HT1 receptor equipment is not specific for the trigeminal system. However, one caveat is that immunohistochemistry does not necessarily reflect functional receptors. For example, the 5HT1D receptor is held in internal stores and only translocated to the cell surface of dorsal root ganglia neurons following neural stimulation (77). In human trigeminal ganglia, 5-HT1B and 5-HT1D receptor immunoreactivity was found predominantly in medium-sized neurons, colocalized with CGRP, substance P, or nitric oxide synthase, confirming a close association of 5-HT1 activation and inhibition of neuropeptide release (78).
Purinergic receptors binding ATP and other purines are either G-protein coupled (P2Y) or form cation channels (P2X). Immunohistochemically, expression of different subtypes of P2X receptors has been described in rat TG neurons of small and medium size, with a predominance of P2X2 and P2X3 receptors that are frequently co-expressed with neuropeptides (79,80,81). P2X3 receptor expression in cultured TG neurons is enhanced by CGRP and nerve growth factor (82,83) and functionally downregulated by brain natriuretic peptide (84). Functional data show that P2X3 receptors are involved in trigeminal neuropathic and inflammatory pain (85,86). Immunohistochemical and functional data suggest that P2Y receptors are expressed by glial cells in rodent TGs (87). Cell cultures imply a bidirectional signaling between neurons and glia cells via ATP (88), which seems to be enhanced in Ca(v)2.1 a1 R192Q mutant knock-in mice as a model of familial hemiplegic migraine type 1 (89). Since it has been shown that TG neurons can not only release neuropeptides, but also ATP upon noxious chemical stimulation (42), purinergic signaling within the ganglion seems possible (see below).
Significant proportions of TG neurons express receptors of the transient receptor potential (TRP) family, which in peripheral sensory endings act as transduction channels and are possibly also involved in synaptic processes at the central afferent terminals (90). Immunoreactivity for the TRP vanilloid type 1 receptor channel (TRPV1) was found colocalized with CGRP in most of the TG neurons (78). This nonspecific cation channel can be activated by exogenous substances like capsaicin or resiniferatoxin, noxious heat, acidic pH (pH < 5.3), and different endogenous compounds including membrane-derived lipid metabolites like the endocannabinoid anandamide (91). CGRP release from trigeminal ganglia or TG cell cultures induced by capsaicin is frequently used as a measure for trigeminal activation (92,93). Another member of the TRP receptor family is the transient receptor potential ankyrin 1 (TRPA1) channel, which is highly colocalized with and functionally linked to TRPV1 receptors in trigeminal neurons. TRPA1 is activated by irritating substances like mustard oil and cannabinoids (94,95). This receptor channel can also be activated by volatile constituents such as umbellulone of the “headache tree” (96). Its functional role in trigeminal nociception is controversial, since recent experimental evidence exists for both a cooperative effect with TRPV1 in meningeal afferents (97) and a dual nociceptive-antinociceptive effect in the trigeminal system (98). Immunohistochemistry showed that TRP receptor channels of the melastatin type 8 (TRPM8) are also expressed in a subset of small diameter neurons in the TG, partly co-expressed with TRPV1 or CGRP immunoreactivity (99), but very few of them seem to innervate the rodent dura mater (100). Functional data from dissociated TG neurons and a mouse corneal preparation imply that TRPM8 receptor channels can operate as osmosensors (101). Recently, TRPM8 activation was shown to reverse the increase in facial sensitivity to heat in a rat model, and in a TG cell-based assay TRPM8 activation inhibited TRPV1 effects, leading the authors to speculate that TRPM8 activation may be rather antinociceptive in migraine (102).
Apart from TRP receptor channels, acid sensing ion channels (ASICs), predominantly the ASIC3 subtype responding to low pH, have been identified in TG neurons labeled from cranial meninges and are suggested to contribute to headache under acidic or inflammatory conditions (103). Acidic metabolites released under ischemia as a consequence of cortical spreading depression during the aura phase of migraine have been speculated to contribute to the generation of migraine pain (104).
Intercellular signaling by CGRP and other factors within the trigeminal ganglion
In recent years, a close functional interplay between neurons and glia within the trigeminal ganglion involving CGRP has been elucidated. CGRP released from neurons can stimulate surrounding satellite glial cells, leading to an enhancement of ATP-gated purinergic P2Y receptors in satellite glial cells (89) and P2X3 receptors in neurons (82,83), and release of nitric oxide (105) and cytokines (92,106) (Figure 2). New immunohistochemical and functional data show that ATP-gated P2X7 receptor channels are also expressed in different types of glial cells including satellite glial cells in the TG (107). CGRP increased ATP (and ADP) levels in trigeminal cultures, which induced intracellular Ca2+ transients in neurons and glial cells, in the latter operating via P2X7 receptors (108) (Figure 2). Several of these signals can also feed back to activate CGRP synthesis and further release by paracrine mechanisms, as well as by a possible autoregulatory autocrine mechanism, as described below. Together, these intraganglionic signals could serve to sustain peripheral sensitization in migraine.
Figure 2.
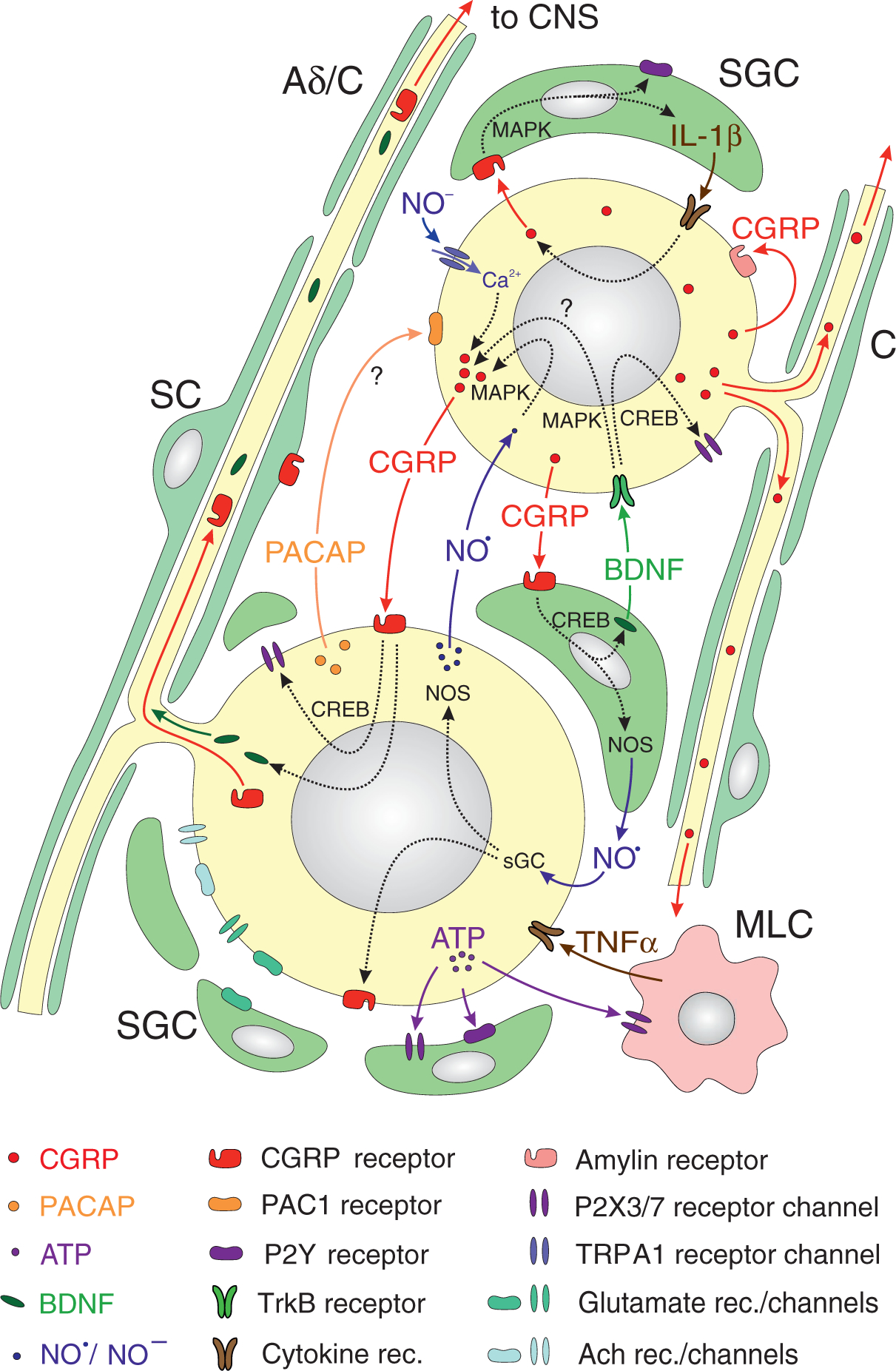
Representation of receptor expression and signaling processes in and between trigeminal ganglion cells. Coloured arrows denote diffusing or transported signal molecules or receptor proteins, inflected broken arrows through the nucleus indicate gene expression. Small neurons (mostly with unmyelinated fibers, right hand, C) expressing CGRP may signal to satellite glial cells (SGCs) and to middle-sized neurons (typically with myelinated, rarely unmyelinated fibers, left hand, Aδ/C) expressing CGRP receptors (144). CGRP release by Ca2+-dependent exocytosis can be induced by activating Ca2+-conducting ion channels like TRPA1, for example by nitroxyl (NO−) (117). Autocrine activation by CGRP may occur via CGRP-binding amylin receptors (49). CGRP and amylin receptors may activate intracellular cascades involving cAMP response-element binding protein (CREB) or mitogen-activated protein kinase (MAPK) to induce gene expression of purinergic (P2X3) receptor channels in neurons (145) and P2X7 channels (107) as well as purinergic (P2Y) receptors in SGCs (146), enzymes like nitric oxide synthase (NOS) (105), cytokines like interleukin 1β (IL-1β) (106) as well as growth factors like brain-derived neurotrophic factor (BDNF) (109). Nitric oxide (NO), cytokines and BDNF may signal back to neurons facilitating expression of purinergic receptor channels (145), CGRP (147) and CGRP receptor components like RAMP1 (118). In addition, ATP, possibly released from neurons under the influence of CGRP (108), may activate SGCs (148) and macrophage-like cells (MLC), which can signal back to neurons by cytokines like tumor necrosis factor (TNFα). Other neuropeptides like PACAP may also be involved in intercellular signaling (54). Many of the gene products like CGRP, CGRP receptor proteins and BDNF can crucially influence neuronal transduction and synaptic transmission, because they are delivered by axonal transport through the neuronal processes (surrounded by Schwann cells, SC) to the peripheral and/or central terminals of trigeminal afferents.
Regarding the purinergic P2X3 receptor, the control by CGRP is of particular interest since it involves two mechanisms, which could come into play during migraine (Figure 2). First, CGRP could directly act on neurons to initiate a cAMP-signaling cascade that activates the P2X3 gene. Second, CGRP can indirectly act via activation of the neurotrophin brain-derived neurotrophic factor (BDNF) gene and BDNF release from satellite glia (109), which can then upregulate P2X3 expression in neurons. Like CGRP, BDNF is elevated during migraine (110), suggesting the possibility of a combinatorial activation of purinergic receptors in migraine. Whether BDNF or P2X3 receptors then feed back to increase CGRP synthesis is not known, but it seems likely given that they activate pathways known to increase CGRP gene transcription (111,112,113). The end-result of this interplay would be to promote depolarization of trigeminal afferents and transmission of nociceptive stimuli (114).
Treatment of primary trigeminal ganglia cultures with CGRP has been shown to regulate the expression of multiple mitogen-activated protein (MAP) kinases and to increase the level of cytokines such as IL-1β (92,106) as well as the expression of inducible NO synthase (iNOS) in satellite glial cells with subsequent release of the gaseous transmitter nitric oxide (NO) (105). Subsequently, IL-1β as well as a NO donor stimulated the release of the inflammatory mediator pros-taglandin E2 (PGE2), mediated mainly by upregulation of the inducible form of cyclooxygenase (COX2) in satellite glial cells (115) (Figure 2). Conversely, the cytokine TNF-α was shown to increase CGRP gene transcription and CGRP secretion from TG neurons (116). Likewise, culture medium from satellite cells activated by either IL-1β or NO augmented the evoked release of CGRP from trigeminal neurons (115).
The CGRP releasing effect of NO donors on TG neurons was shown earlier to be caused by activation of the CGRP promoter activity, which, remarkably, could partly be suppressed by sumatriptan (40). In vivo, infusion into the rat of glycerol trinitrate (GTN), which mimics NO regarding the activation of the intracellular receptor for NO, soluble guanylate cyclase (sGC) (117), was followed by an increase in neurons showing CGRP immunoreactivity and, surprisingly, also immunoreactivity for the neuronal form of NOS (nNOS) (118) (Figure 2). The same treatment increased immunoreactivity for RAMP1, the rate-limiting component of the CGRP receptor, while sGC immunoreactivity in trigeminal ganglion neurons was decreased (119). Following chronic tooth pulp inflammation in rat, the number of TG neurons showing immunostaining for iNOS and nNOS increased after some days (120). The effects of NO, a radical, is probably not restricted to the activation of sGC with downstream activation of protein kinase G (PKG), possibly followed by the activation of different ion channels (121) and ERK phosphorylation (122). In addition, NO together with the gaseous transmitter H2S forms nitrosyl (NO−), a direct TRPA1 receptor-activating sibling of NO, which may play a role in the ganglia by increasing the release of CGRP (123,124).
In summary, these observations indicate that CGRP could function as a paracrine factor to stimulate nearby glial cells and neurons, which in turn could feed back to further stimulate CGRP synthesis and release. Direct autocrine regulation of the CGRP gene in trigeminal ganglia was demonstrated in primary cultures (113). Likewise, in cultured DRG cells, immunohistochemical data showed that CGRP, through cAMP increase, caused phosphorylation of cAMP response element binding (CREB) protein, suggesting that CGRP can regulate gene expression involving protein kinase A and mitogen-activated protein kinase/extracellular receptor kinase kinase (125) (Figure 2). Similar signaling mechanisms have been demonstrated in the trigeminal system (113,126). Autocrine regulation of CGRP transcription has also been speculated to occur in the cerebellar Purkinje neurons (127). While colocalization of CGRP and CGRP receptor elements was only rarely seen in the cell bodies of the ganglia (32,14,128), autocrine regulation cannot be ruled out given the discovery of a second CGRP receptor and given the reported plasticity of RAMP1 expression. With respect to the second receptor, as mentioned earlier, the AMY1 receptor is localized primarily in small and medium diameter trigeminal ganglion neurons (49) (Figure 2), which is in contrast to localization of the CLR/RAMP1 receptor primarily in large diameter neurons (32,14). However, whether CGRP and AMY1 receptors are indeed colocalized remains to be tested. With respect to receptor plasticity, this possibility is supported by the finding that the migraine trigger NO increased the number of cell bodies expressing the RAMP1 subunit in rats (129). Furthermore, dynamic regulation of CGRP receptor subunits by other migraine-relevant stimuli (e.g. stress and hypoxia) has also been reported (130). Thus, there is the possibility of increased CGRP synthesis in response to migraine triggers via paracrine and autocrine positive feedback loops.
Last, but not least, it is important to consider signaling mechanisms between primary afferent neurons, satellite glial cells (SGCs) and immune cells that does not directly involve neuropeptides. Intraganglionic signaling involving ATP has been reviewed by Goto et al. (131). Neurons may signal via ATP to other neurons and to SGCs, and these to microglia/macrophage-like cells (MLCs). SGCs and MLCs may signal back to neurons via cytokines and neurotrophic factors, thus inducing a positive loop of sensitization (Figure 2). Likewise, glutamate can be a neuro-glial transmitter within sensory ganglia. In rat trigeminal ganglia, KCl stimulation released glutamate when glutamate uptake by satellite glial cells (SGCs) was inhibited. Calcium imaging showed that neurons and SGCs respond to AMPA, NMDA, kainate and mGluR agonists, and selective antagonists blocked this response, which is indicative of functional glutamate receptors of all types (132). Inflammation by complete Freund’s adjuvant caused expression of MAPK (pERK1/2, pp38) and NF-κB in the TG involving both neurons and glia, which again points to possible neuron-glia interactions. Administration of the NMDA-receptor antagonists, kynurenic acid, inhibited these responses, indicating the importance of intraganglionic NMDA receptors (133).
Significance of the trigeminal ganglion for peripheral and central nociceptive functions
Experiments on dissociated ganglion cells or intact primary afferent ganglia are restricted systems, which lack the peripheral and central extensions of primary afferents. In these experiments, the cell bodies are often used as a model of their peripheral or central endings to study their structural and functional properties; that is, mechanisms of sensory transduction or transmission. Thus, to a certain degree, data collected from ganglia or isolated ganglion neurons can be interpreted in terms of mechanisms occurring at the peripheral or central afferent terminals, taking into account that most of the molecules expressed in the somata are packed, frequently together in the same vesicles (134) and delivered by axonal transport to the periphery and/or the central nervous system (135). For example, there is no doubt that CGRP and other neuropeptides are transported to and released from the peripheral terminals, where they induce processes of neurogenic inflammation like arterial dilatation, and to the central terminals, where they likely act as neuromodulators (Figure 2). Receptors are transported as well and integrated into the terminal cell membrane, for example 5-HT1B/D receptors, the activation of which counteracts the release of neuropeptides and thereby their peripheral and central effects. For some receptors, a more or less unidirectional transport can be assumed, as indicated by morphological and functional findings. For example, confocal immunohistochemistry has shown CGRP receptor components co-localized with axonal markers only in the central but not the peripheral processes of rat trigeminal afferents, indicating a unilateral transport into the central terminals (14), although there is conflicting data regarding this issue (136). The 5-HT1B/D agonist naratriptan suppressed evoked CGRP release from medullary slices, but not significantly, from the mouse dura mater, indicating a preferential functional presence of these receptors at the central terminals (137). Thus, changes in gene expression or transport of molecules can have consequences for peripheral and central functions. Another example is BDNF, the expression of which in cultured trigeminal neurons has been shown to be enhanced by CGRP (138) (Figure 2). BDNF can be released from central presynaptic terminals and may act on pre- and postsynaptic tyrosine kinase (TrkB) receptors to facilitate nociceptive transmission (139). Similarly, CGRP receptors integrated into the presynaptic membrane of central trigeminal terminals may be activated by CGRP released from central terminals of other trigeminal afferents to facilitate neurotransmitter release and synaptic transmission (140). This scenario is most relevant in the light of the recent discussion about big molecules like monoclonal antibodies, which are assumed to act outside the blood-brain barrier to inhibit CGRP signaling and reduce trigeminal functions involved in migraine (141), as reviewed elsewhere (142,143). Thus, substances that act within the trigeminal ganglion – which is outside the blood-brain barrier – to change signaling within or between trigeminal ganglion neurons, can by this way have considerable impact on the peripheral and central functions of nociceptive transduction and transmission.
Article highlights.
The TG is composed of primary afferent neurons and other cell types that can interact by intercellular signaling mechanisms.
Possible signal molecules are neuropeptides (CGRP), ATP, nitric oxide, cytokines and neurotrophic factors (BDNF).
Gene expression following intercellular signaling can modulate sensory information and contribute to peripheral and central sensitization.
The TG as a target of drug actions outside the blood-brain barrier should be considered regarding 5-HT mechanisms (triptans) and CGRP signaling (monoclonal antibodies).
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Footnotes
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.MacIver MB and Tanelian DL. Free nerve ending terminal morphology is fiber type specific for A delta and C fibers innervating rabbit corneal epithelium. J Neurophysiol 1993; 69: 1779–1783. [DOI] [PubMed] [Google Scholar]
- 2.Andres KH, von Düring M, Muszynski K, et al. Nerve fibres and their terminals of the dura mater encephali of the rat. Anat Embryol (Berl) 1987; 175: 289–301. [DOI] [PubMed] [Google Scholar]
- 3.Messlinger K, Hanesch U, Baumgärtel M, et al. Innervation of the dura mater encephali of cat and rat: Ultrastructure and calcitonin gene-related peptide-like and substance P-like immunoreactivity. Anat Embryol (Berl) 1993; 188: 219–237. [DOI] [PubMed] [Google Scholar]
- 4.Ray BS and Wolff HG. Experimental studies on headache: Pain sensitive structures of the head and their significance in headache. Arch Surg 1940; 1: 813–856. [Google Scholar]
- 5.Young RF and Stevens R. Unmyelinated axons in the trigeminal motor root of human and cat. J Comp Neurol 1979; 183: 205–214. [DOI] [PubMed] [Google Scholar]
- 6.Byers MR, O’Connor TA, Martin RF, et al. Mesencephalic trigeminal sensory neurons of cat: Axon pathways and structure of mechanoreceptive endings in periodontal ligament. J Comp Neurol 1986; 250: 181–191. [DOI] [PubMed] [Google Scholar]
- 7.Kerr FW and Lysak WR. Somatotopic organization of trigeminal-ganglion neurones. Arch Neurol 1964; 11: 593–602. [DOI] [PubMed] [Google Scholar]
- 8.Darian-Smith I, Mutton P and Proctor R. Functional organization of tactile cutaneous afferents within the semilunar ganglion and trigeminal spinal tract of the cat. J Neurophysiol 1965; 28: 682–694. [DOI] [PubMed] [Google Scholar]
- 9.Lende RA and Poulos DA. Functional localization in the trigeminal ganglion in the monkey. J Neurosurg 1970; 32: 336–343. [DOI] [PubMed] [Google Scholar]
- 10.Wirth FP and Van Buren JM. Referral of pain from dural stimulation in man. J Neurosurg 1971; 34: 630–642. [DOI] [PubMed] [Google Scholar]
- 11.LaGuardia JJ, Cohrs RJ and Gilden DH. Numbers of neurons and non-neuronal cells in human trigeminal ganglia. Neurol Res 2000; 22: 565–566. [DOI] [PubMed] [Google Scholar]
- 12.Wu L, Zhang H, Liao L, et al. Trigeminal ganglion morphology in human fetus. Microsc Res Tech 2013; 76: 598–605. [DOI] [PubMed] [Google Scholar]
- 13.Ambalavanar R and Morris R. The distribution of binding by isolectin I-B4 from Griffonia simplicifolia in the trigeminal ganglion and brainstem trigeminal nuclei in the rat. Neuroscience 1992; 47: 421–429. [DOI] [PubMed] [Google Scholar]
- 14.Lennerz JK,Rühle V, Ceppa EP, et al. Calcitonin receptor-like receptor (CLR), receptor activity-modifying protein 1 (RAMP1), and calcitonin gene-related peptide (CGRP) immunoreactivity in the rat trigeminovascular system: Differences between peripheral and central CGRP receptor distribution. J Comp Neurol 2008; 507: 1277–1299. [DOI] [PubMed] [Google Scholar]
- 15.Zenker W and Högl E. The prebifurcation section of the axon of the rat spinal ganglion cell. Cell Tissue Res 1976; 165: 345–363. [DOI] [PubMed] [Google Scholar]
- 16.Bruska M and Woźniak W. Ultrastructure of glial cells in the human fetal trigeminal ganglion. Folia Morphol 1991; 50: 27–48. [PubMed] [Google Scholar]
- 17.Glenn JA, Sonceau JB, Wynder HJ, et al. Histochemical evidence for microglia-like macrophages in the rat trigeminal ganglion. J Anat 1993; 183: 475–481. [PMC free article] [PubMed] [Google Scholar]
- 18.Franceschini A, Nair A, Bele T, et al. Functional crosstalk in culture between macrophages and trigeminal sensory neurons of a mouse genetic model of migraine. BMC Neurosci 2012; 13: 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Villa G, Ceruti S, Zanardelli M, et al. Temporomandibular joint inflammation activates glial and immune cells in both the trigeminal ganglia and in the spinal trigeminal nucleus. Mol Pain 2010; 6: 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee Y, Kawai Y, Shiosaka S, et al. Coexistence of calcitonin gene-related peptide and substance P-like peptide in single cells of the trigeminal ganglion of the rat: Immunohistochemical analysis. Brain Res 1985; 330: 194–196. [DOI] [PubMed] [Google Scholar]
- 21.Hanko J, Hardebo JE, Kåhrström J, et al. Existence and coexistence of calcitonin gene-related peptide (CGRP) and substance P in cerebrovascular nerves and trigeminal ganglion cells. Acta Physiol Scand Suppl 1986; 552: 29–32. [PubMed] [Google Scholar]
- 22.Edvinsson L, Hara H and Uddman R. Retrograde tracing of nerve fibers to the rat middle cerebral artery with true blue: Colocalization with different peptides. J Cereb Blood Flow Metab 1989; 9: 212–218. [DOI] [PubMed] [Google Scholar]
- 23.Eftekhari S, Salvatore CA, Johansson S, et al. Localization of CGRP, CGRP receptor, PACAP and glutamate in trigeminal ganglion. Relation to the blood-brain barrier. Brain Res 2015; 1600: 93–109. [DOI] [PubMed] [Google Scholar]
- 24.Lazarov NE. Comparative analysis of the chemical neuroanatomy of the mammalian trigeminal ganglion and mesencephalic trigeminal nucleus. Prog Neurobiol 2002; 66: 19–59. [DOI] [PubMed] [Google Scholar]
- 25.Mayberg MR, Zervas NT and Moskowitz MA. Trigeminal projections to supratentorial pial and dural blood vessels in cats demonstrated by horseradish peroxidase histochemistry. J Comp Neurol 1984; 223: 46–56. [DOI] [PubMed] [Google Scholar]
- 26.Schueler M, Neuhuber WL, De Col R, et al. Innervation of rat and human dura mater and pericranial tissues in the parieto-temporal region by meningeal afferents. Headache 2014; 54: 996–1009. [DOI] [PubMed] [Google Scholar]
- 27.Quartu M and Del Fiacco M. Enkephalins occur and colocalize with substance P in human trigeminal ganglion neurones. Neuroreport 1994; 5: 465–468. [DOI] [PubMed] [Google Scholar]
- 28.Hou M, Uddman R, Tajti J, et al. Nociceptin immunoreactivity and receptor mRNA in the human trigeminal ganglion. Brain Res 2003; 964: 179–186. [DOI] [PubMed] [Google Scholar]
- 29.Del Fiacco M, Quartu M, Priestley JV, et al. GAP-43 persists in adulthood and coexists with SP and CGRP in human trigeminal sensory neurones. Neuroreport 1994; 5: 2349–2352. [DOI] [PubMed] [Google Scholar]
- 30.Jansen-Olesen I, Baun M, Amrutkar DV, et al. PACAP-38 but not VIP induces release of CGRP from trigeminal nucleus caudalis via a receptor distinct from the PAC1 receptor. Neuropeptides 2014; 48: 53–64. [DOI] [PubMed] [Google Scholar]
- 31.Imboden H, Patil J, Nussberger J, et al. Endogenous angiotensinergic system in neurons of rat and human trigeminal ganglia. Regul Pept 2009; 154: 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eftekhari S, Salvatore CA, Calamari A, et al. Differential distribution of calcitonin gene-related peptide and its receptor components in the human trigeminal ganglion. Neuroscience 2010; 169: 683–696. [DOI] [PubMed] [Google Scholar]
- 33.Kestell GR, Anderson RL, Clarke JN, et al. Primary afferent neurons containing calcitonin gene-related peptide but not substance P in forepaw skin, dorsal root ganglia, and spinal cord of mice. J Comp Neurol 2015; 523: 2555–2569. [DOI] [PubMed] [Google Scholar]
- 34.O’Connor TP and van der Kooy D. Enrichment of a vasoactive neuropeptide (calcitonin gene related peptide) in the trigeminal sensory projection to the intracranial arteries. J Neurosci 1988; 8: 2468–2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Horgan K and van der Kooy D. Visceral targets specify calcitonin gene-related peptide and substance P enrichment in trigeminal afferent projections. J Neurosci 1992; 12: 1135–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vedder H, Affolter HU and Otten U. Nerve growth factor (NGF) regulates tachykinin gene expression and biosynthesis in rat sensory neurons during early postnatal development. Neuropeptides 1993; 24: 351–357. [DOI] [PubMed] [Google Scholar]
- 37.Price TJ, Louria MD, Candelario-Soto D, et al. Treatment of trigeminal ganglion neurons in vitro with NGF, GDNF or BDNF: Effects on neuronal survival, neuro-chemical properties and TRPV1-mediated neuropeptide secretion. BMC Neurosci 2005; 6: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Price TJ and Flores CM. Critical evaluation of the colocalization between calcitonin gene-related peptide, substance P, transient receptor potential vanilloid subfamily type 1 immunoreactivities, and isolectin B4 binding in primary afferent neurons of the rat and mouse. J Pain 2007; 8: 263–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mason RT, Peterfreund RA, Sawchenko PE, et al. Release of the predicted calcitonin gene-related peptide from cultured rat trigeminal ganglion cells. Nature 1984; 308: 653–655. [DOI] [PubMed] [Google Scholar]
- 40.Bellamy J, Bowen EJ, Russo AF, et al. Nitric oxide regulation of calcitonin gene-related peptide gene expression in rat trigeminal ganglia neurons. Eur J Neurosci 2006; 23: 2057–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eberhardt M, Hoffmann T, Sauer SK, et al. Calcitonin gene-related peptide release from intact isolated dorsal root and trigeminal ganglia. Neuropeptides 2008; 42: 311–317. [DOI] [PubMed] [Google Scholar]
- 42.Neubert JK, Matsuka Y, Maidment NT, et al. Microdialysis in trigeminal ganglia. Brain Res Brain Res Protoc 2002; 10: 102–108. [DOI] [PubMed] [Google Scholar]
- 43.Evans BN, Rosenblatt MI, Mnayer LO, et al. CGRPRCP, a novel protein required for signal transduction at calcitonin gene-related peptide and adrenomedullin receptors. J Biol Chem 2000; 275: 31438–31443. [DOI] [PubMed] [Google Scholar]
- 44.Juaneda C, Dumont Y and Quirion R. The molecular pharmacology of CGRP and related peptide receptor subtypes. Trends Pharmacol Sci 2000; 21: 432–438. [DOI] [PubMed] [Google Scholar]
- 45.McLatchie LM, Fraser NJ, Main MJ, et al. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature 1998; 393: 333–339. [DOI] [PubMed] [Google Scholar]
- 46.Eftekhari S, Gaspar RC, Roberts R, et al. Localization of CGRP receptor components and receptor binding sites in rhesus monkey brainstem: A detailed study using in situ hybridization, immunofluorescence and autoradiography. J Comp Neurol 2016; 524: 90–118. [DOI] [PubMed] [Google Scholar]
- 47.Eftekhari S, Gaspar RC, Roberts R, et al. Localization of CGRP receptor components and receptor binding sites in rhesus monkey brainstem: A detailed study using in situ hybridization, immunofluorescence, and autoradiography. J Comp Neurol 2016; 524: 90–118. [DOI] [PubMed] [Google Scholar]
- 48.Miller S, Liu H, Warfvinge K, et al. Immunohistochemical localization of the calcitonin gene-related peptide binding site in the primate trigeminovascular system using functional antagonist antibodies. Neuroscience 2016; 328: 165–183. [DOI] [PubMed] [Google Scholar]
- 49.Walker CS, Eftekhari S, Bower RL, et al. A second trigeminal CGRP receptor: Function and expression of the AMY1 receptor. Ann Clin Transl Neurol 2015; 2: 595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bohn KJ, Li B, Huang X, et al. CGRP receptor activity in mice with global expression of human receptor activity modifying protein 1. Br J Pharmacol 2017; 174: 1826–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takeda M, Takahashi M and Matsumoto S. Suppression of neurokinin-1 receptor in trigeminal ganglia attenuates central sensitization following inflammation. J Peripher Nerv Syst 2012; 17: 169–181. [DOI] [PubMed] [Google Scholar]
- 52.Goldstein DJ, Wang O, Saper JR, et al. Ineffectiveness of neurokinin-1 antagonist in acute migraine: A crossover study. Cephalalgia 1997; 17: 785–790. [DOI] [PubMed] [Google Scholar]
- 53.Goldstein DJ, Offen WW, Klein EG, et al. Lanepitant, an NK-1 antagonist, in migraine prevention. Cephalalgia 2001; 21: 102–106. [DOI] [PubMed] [Google Scholar]
- 54.Chaudhary P and Baumann TK. Expression of VPAC2 receptor and PAC1 receptor splice variants in the trigeminal ganglion of the adult rat. Brain Res Mol Brain Res 2002; 104: 137–142. [DOI] [PubMed] [Google Scholar]
- 55.Knutsson M and Edvinsson L. Distribution of mRNA for VIP and PACAP receptors in human cerebral arteries and cranial ganglia. Neuroreport 2002; 13: 507–509. [DOI] [PubMed] [Google Scholar]
- 56.Ichikawa H, Schulz S, Höllt V, et al. The somatostatin sst2A receptor in the rat trigeminal ganglion. Neuroscience 2003; 120: 807–813. [DOI] [PubMed] [Google Scholar]
- 57.Suzuki H, Iwanaga T, Yoshie H, et al. Expression of galanin receptor-1 (GALR1) in the rat trigeminal ganglia and molar teeth. Neurosci Res 2002; 42: 197–207. [DOI] [PubMed] [Google Scholar]
- 58.Ghilardi JR, Allen CJ, Vigna SR, et al. Trigeminal and dorsal root ganglion neurons express CCK receptor binding sites in the rat, rabbit, and monkey: Possible site of opiate-CCK analgesic interactions. J Neurosci 1992; 12: 4854–4866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ichikawa H, Schulz S, Höllt V, et al. Delta-opioid receptor-immunoreactive neurons in the rat cranial sensory ganglia. Brain Res 2005; 1043: 225–230. [DOI] [PubMed] [Google Scholar]
- 60.Huang J, Lv Y, Fu Y, et al. Dynamic regulation of delta-opioid receptor in rat trigeminal ganglion neurons by lipopolysaccharide-induced acute pulpitis. J Endod 2015; 41: 2014–2020. [DOI] [PubMed] [Google Scholar]
- 61.Cady RJ, Denson JE, Sullivan LQ, et al. Dual orexin receptor antagonist 12 inhibits expression of proteins in neurons and glia implicated in peripheral and central sensitization. Neuroscience 2014; 269: 79–92. [DOI] [PubMed] [Google Scholar]
- 62.Tzabazis A, Mechanic J, Miller J, et al. Oxytocin receptor: Expression in the trigeminal nociceptive system and potential role in the treatment of headache disorders. Cephalalgia 2016; 36: 943–950. [DOI] [PubMed] [Google Scholar]
- 63.Kubo A, Shinoda M, Katagiri A, et al. Oxytocin alleviates orofacial mechanical hypersensitivity associated with infraorbital nerve injury through vasopressin-1A receptors of the rat trigeminal ganglia. Pain 2017; 158: 649–659. [DOI] [PubMed] [Google Scholar]
- 64.Quartu M, Serra MP, Ambu R, et al. AMPA-type glutamate receptor subunits 2/3 in the human trigeminal sensory ganglion and subnucleus caudalis from prenatal ages to adulthood. Mech Ageing Dev 2002; 123: 463–471. [DOI] [PubMed] [Google Scholar]
- 65.Yang Z, Wang Y, Luo W, et al. Trigeminal expression of N-methyl-D-aspartate receptor subunit 1 and behavior responses to experimental tooth movement in rats. Angle Orthod 2009; 79: 951–957. [DOI] [PubMed] [Google Scholar]
- 66.Sahara Y, Noro N, Iida Y, et al. Glutamate receptor subunits GluR5 and KA-2 are coexpressed in rat trigeminal ganglion neurons. J Neurosci 1997; 17: 6611–6620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Boye Larsen D, Ingemann Kristensen G, Panchalingam V, et al. Investigating the expression of metabotropic glutamate receptors in trigeminal ganglion neurons and satellite glial cells: Implications for craniofacial pain. J Recept Signal Transduct Res 2014; 34: 261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee J, Saloman JL, Weiland G, et al. Functional interactions between NMDA receptors and TRPV1 in trigeminal sensory neurons mediate mechanical hyperalgesia in the rat masseter muscle. Pain 2012; 153: 1514–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Flores CM, DeCamp RM, Kilo S, et al. Neuronal nicotinic receptor expression in sensory neurons of the rat trigeminal ganglion: Demonstration of alpha3beta4, a novel subtype in the mammalian nervous system. J Neurosci 1996; 16: 7892–7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dussor GO, Helesic G, Hargreaves KM, et al. Cholinergic modulation of nociceptive responses in vivo and neuropeptide release in vitro at the level of the primary sensory neuron. Pain 2004; 107: 22–32. [DOI] [PubMed] [Google Scholar]
- 71.Shelukhina I, Mikhailov N, Abushik P, et al. Cholinergic nociceptive mechanisms in rat meninges and trigeminal ganglia: Potential implications for migraine pain. Front Neurol 2017; 8: 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Goadsby PJ. Trigeminal autonomic cephalalgias. Patho-physiology and classification. Rev Neurol (Paris) 2005; 161: 692–695. [DOI] [PubMed] [Google Scholar]
- 73.Barbanti P, Aurilia C, Dall’Armi V, et al. The phenotype of migraine with unilateral cranial autonomic symptoms documents increased peripheral and central trigeminal sensitization. A case series of 757 patients. Cephalalgia 2016; 36: 1334–1340. [DOI] [PubMed] [Google Scholar]
- 74.Hayasaki H, Sohma Y, Kanbara K, et al. A local GABAergic system within rat trigeminal ganglion cells. Eur J Neurosci 2006; 23: 745–757. [DOI] [PubMed] [Google Scholar]
- 75.Bae JY, Mah W, Rah J-C, et al. Expression of glycine receptor alpha 3 in the rat trigeminal neurons and central boutons in the brainstem. Brain Struct Funct 2016; 221: 4601–4613. [DOI] [PubMed] [Google Scholar]
- 76.Classey JD, Bartsch T and Goadsby PJ. Distribution of 5-HT(1B), 5-HT(1D) and 5-HT(1F) receptor expression in rat trigeminal and dorsal root ganglia neurons: Relevance to the selective anti-migraine effect of triptans. Brain Res 2010; 1361: 76–85. [DOI] [PubMed] [Google Scholar]
- 77.Ahn AH and Basbaum AI. Tissue injury regulates serotonin 1D receptor expression: Implications for the control of migraine and inflammatory pain. J Neurosci 2006; 26: 8332–8338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hou M, Kanje M, Longmore J, et al. 5-HT(1B) and 5-HT(1D) receptors in the human trigeminal ganglion: Co-localization with calcitonin gene-related peptide, substance P and nitric oxide synthase. Brain Res 2001; 909: 112–120. [DOI] [PubMed] [Google Scholar]
- 79.Xiang Z, Bo X and Burnstock G. Localization of ATP-gated P2X receptor immunoreactivity in rat sensory and sympathetic ganglia. Neurosci Lett 1998; 256: 105–108. [DOI] [PubMed] [Google Scholar]
- 80.Staikopoulos V, Sessle BJ, Furness JB, et al. Localization of P2X2 and P2X3 receptors in rat trigeminal ganglion neurons. Neuroscience 2007; 144: 208–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ambalavanar R, Moritani M and Dessem D. Trigeminal P2X3 receptor expression differs from dorsal root ganglion and is modulated by deep tissue inflammation. Pain 2005; 117: 280–291. [DOI] [PubMed] [Google Scholar]
- 82.Giniatullin R, Nistri A and Fabbretti E. Molecular mechanisms of sensitization of pain-transducing P2X3 receptors by the migraine mediators CGRP and NGF. Mol Neurobiol 2008; 37: 83–90. [DOI] [PubMed] [Google Scholar]
- 83.Simonetti M, Giniatullin R and Fabbretti E. Mechanisms mediating the enhanced gene transcription of P2X3 receptor by calcitonin gene-related peptide in trigeminal sensory neurons. J Biol Chem 2008; 283: 18743–18752. [DOI] [PubMed] [Google Scholar]
- 84.Marchenkova A, Vilotti S, Fabbretti E, et al. Brain natriuretic peptide constitutively downregulates P2X3 receptors by controlling their phosphorylation state and membrane localization. Mol Pain 2015; 11: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shinoda M, Kawashima K, Ozaki N, et al. P2X3 receptor mediates heat hyperalgesia in a rat model of trigeminal neuropathic pain. J Pain 2007; 8: 588–597. [DOI] [PubMed] [Google Scholar]
- 86.Teixeira JM, Oliveira MCG, Nociti FH, et al. Involvement of temporomandibular joint P2X3 and P2X2/3 receptors in carrageenan-induced inflammatory hyperalgesia in rats. Eur J Pharmacol 2010; 645: 79–85. [DOI] [PubMed] [Google Scholar]
- 87.Weick M, Cherkas PS, Härtig W, et al. P2 receptors in satellite glial cells in trigeminal ganglia of mice. Neuroscience 2003; 120: 969–977. [DOI] [PubMed] [Google Scholar]
- 88.Suadicani SO, Cherkas PS, Zuckerman J, et al. Bidirectional calcium signaling between satellite glial cells and neurons in cultured mouse trigeminal ganglia. Neuron Glia Biol 2010; 6: 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ceruti S, Villa G, Fumagalli M, et al. Calcitonin gene-related peptide-mediated enhancement of purinergic neuron/glia communication by the algogenic factor bradykinin in mouse trigeminal ganglia from wild-type and R192Q Cav2.1 knock-in mice: Implications for basic mechanisms of migraine pain. J Neurosci 2011; 31: 3638–3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Raisinghani M, Zhong L, Jeffry JA, et al. Activation characteristics of transient receptor potential ankyrin 1 and its role in nociception. Am J Physiol Cell Physiol 2011; 301: C587–C600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Price TJ, Patwardhan A, Akopian AN, et al. Modulation of trigeminal sensory neuron activity by the dual cannabinoid-vanilloid agonists anandamide, N-arachidonoyl-dopamine and arachidonyl-2-chloroethylamide. Br J Pharmacol 2004; 141: 1118–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Thalakoti S, Patil VV, Damodaram S, et al. Neuron-glia signaling in trigeminal ganglion: Implications for migraine pathology. Headache 2007; 47: 1008–1023, discussion 24–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Meng J, Ovsepian SV, Wang J, et al. Activation of TRPV1 mediates calcitonin gene-related peptide release, which excites trigeminal sensory neurons and is attenuated by a retargeted botulinum toxin with anti-nociceptive potential. J Neurosci 2009; 29: 4981–4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Salas MM, Hargreaves KM and Akopian AN. TRPA1-mediated responses in trigeminal sensory neurons: Interaction between TRPA1 and TRPV1. Eur J Neurosci 2009; 29: 1568–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jordt S-E, Bautista DM, Chuang H-H, et al. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 2004; 427: 260–265. [DOI] [PubMed] [Google Scholar]
- 96.Nassini R, Materazzi S, Vriens J, et al. The “headache tree” via umbellulone and TRPA1 activates the trigeminovascular system. Brain J Neurol 2012; 135: 376–390. [DOI] [PubMed] [Google Scholar]
- 97.Denner AC, Vogler B, Messlinger K, et al. Role of transient receptor potential ankyrin 1 receptors in rodent models of meningeal nociception – experiments in vitro. Eur J Pain 2017; 21: 843–854. [DOI] [PubMed] [Google Scholar]
- 98.Teicher C, De Col R and Messlinger K. Hydrogen sulfide mediating both excitatory and inhibitory effects in a rat model of meningeal nociception and headache generation. Front Neurol 2017; 8: 336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Abe J, Hosokawa H, Okazawa M, et al. TRPM8 protein localization in trigeminal ganglion and taste papillae. Brain Res Mol Brain Res 2005; 136: 91–98. [DOI] [PubMed] [Google Scholar]
- 100.Huang D, Li S, Dhaka A, et al. Expression of the transient receptor potential channels TRPV1, TRPA1 and TRPM8 in mouse trigeminal primary afferent neurons innervating the dura. Mol Pain 2012; 8: 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Quallo T, Vastani N, Horridge E, et al. TRPM8 is a neuronal osmosensor that regulates eye blinking in mice. Nat Commun 2015; 6: 7150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kayama Y, Shibata M, Takizawa T, et al. Functional interactions between transient receptor potential M8 and transient receptor potential V1 in the trigeminal system: Relevance to migraine pathophysiology. Cephalalgia 2018; 38: 833–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yan J, Wei X, Bischoff C, et al. pH-evoked dural afferent signaling is mediated by ASIC3 and is sensitized by mast cell mediators. Headache 2013; 53: 1250–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dussor G ASICs as therapeutic targets for migraine. Neuropharmacology 2015; 94: 64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li J, Vause CV and Durham PL. Calcitonin gene-related peptide stimulation of nitric oxide synthesis and release from trigeminal ganglion glial cells. Brain Res 2008; 1196: 22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vause CV and Durham PL. Calcitonin gene-related peptide differentially regulates gene and protein expression in trigeminal glia cells: Findings from array analysis. Neurosci Lett 2010; 473: 163–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nowodworska A, van den Maagdenberg AMJM, Nistri A, et al. In situ imaging reveals properties of purinergic signalling in trigeminal sensory ganglia in vitro. Purinergic Signal 2017; 13: 511–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yegutkin GG, Guerrero-Toro C, Kilinc E, et al. Nucleotide homeostasis and purinergic nociceptive signaling in rat meninges in migraine-like conditions. Purinergic Signal 2016; 12: 561–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Buldyrev I, Tanner NM, Hsieh H, et al. Calcitonin gene-related peptide enhances release of native brain-derived neurotrophic factor from trigeminal ganglion neurons. J Neurochem 2006; 99: 1338–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fischer M, Wille G, Klien S, et al. Brain-derived neurotrophic factor in primary headaches. J Headache Pain 2012; 13: 469–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Durham PL and Russo AF. Stimulation of the calcitonin gene-related peptide enhancer by mitogen-activated protein kinases and repression by an antimigraine drug in trigeminal ganglia neurons. J Neurosci 2003; 23: 807–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Viney TJ, Schmidt TW, Gierasch W, et al. Regulation of the cell-specific calcitonin/calcitonin gene-related peptide enhancer by USF and the Foxa2 forkhead protein. J Biol Chem 2004; 279: 49948–49955. [DOI] [PubMed] [Google Scholar]
- 113.Zhang Z, Winborn CS, Marquez de Prado B, et al. Sensitization of calcitonin gene-related peptide receptors by receptor activity-modifying protein-1 in the trigeminal ganglion. J Neurosci 2007; 27: 2693–2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Souslova V, Cesare P, Ding Y, et al. Warm-coding deficits and aberrant inflammatory pain in mice lacking P2X3 receptors. Nature 2000; 407: 1015–1017. [DOI] [PubMed] [Google Scholar]
- 115.Capuano A, De Corato A, Lisi L, et al. Proinflamma-tory-activated trigeminal satellite cells promote neuronal sensitization: Relevance for migraine pathology. Mol Pain 2009; 5: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bowen EJ, Schmidt TW, Firm CS, et al. Tumor necrosis factor-alpha stimulation of calcitonin gene-related peptide expression and secretion from rat trigeminal ganglion neurons. J Neurochem 2006; 96: 65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kleschyov AL, Oelze M, Daiber A, et al. Does nitric oxide mediate the vasodilator activity of nitroglycerin? Circ Res 2003; 93: e104–e112. [DOI] [PubMed] [Google Scholar]
- 118.Dieterle A, Fischer MJM, Link AS, et al. Increase in CGRP- and nNOS-immunoreactive neurons in the rat trigeminal ganglion after infusion of an NO donor. Cephalalgia 2011; 31: 31–42. [DOI] [PubMed] [Google Scholar]
- 119.Seiler K, Nusser JI, Lennerz JK, et al. Changes in calcitonin gene-related peptide (CGRP) receptor component and nitric oxide receptor (sGC) immunoreactivity in rat trigeminal ganglion following glyceroltrinitrate pretreatment. J Headache Pain 2013; 14: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liu Q, Gao Z, Zhu X, et al. Changes in nitric oxide synthase isoforms in the trigeminal ganglion of rat following chronic tooth pulp inflammation. Neurosci Lett 2016; 633: 240–245. [DOI] [PubMed] [Google Scholar]
- 121.Artinian L, Zhong L, Yang H, et al. Nitric oxide as intracellular modulator: Internal production of NO increases neuronal excitability via modulation of several ionic conductances. Eur J Neurosci 2012; 36: 3333–3343. [DOI] [PubMed] [Google Scholar]
- 122.Zhang X, Kainz V, Zhao J, et al. Vascular extracellular signal-regulated kinase mediates migraine-related sensitization of meningeal nociceptors. Ann Neurol 2013; 73: 741–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Eberhardt M, Dux M, Namer B, et al. H2S and NO cooperatively regulate vascular tone by activating a neuroendocrine HNO-TRPA1-CGRP signalling pathway. Nat Commun 2014; 5: 4381. doi: 10.1038/ncomms5381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dux M, Will C, Vogler B, et al. Meningeal blood flow is controlled by H2 S-NO crosstalk activating HNOTRPA1-CGRP signalling. Br J Pharmacol 2015; 173: 431–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Anderson LE and Seybold VS. Calcitonin gene-related peptide regulates gene transcription in primary afferent neurons. J Neurochem 2004; 91: 1417–1429. [DOI] [PubMed] [Google Scholar]
- 126.Walker CS, Raddant AC, Woolley MJ, et al. CGRP receptor antagonist activity of olcegepant depends on the signalling pathway measured. Cephalalgia 2018; 38: 437–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Edvinsson L, Eftekhari S, Salvatore CA, et al. Cerebellar distribution of calcitonin gene-related peptide (CGRP) and its receptor components calcitonin receptor-like receptor (CLR) and receptor activity modifying protein 1 (RAMP1) in rat. Mol Cell Neurosci 2011; 46: 333–339. [DOI] [PubMed] [Google Scholar]
- 128.Tajti J, Uddman R, Möller S, et al. Messenger molecules and receptor mRNA in the human trigeminal ganglion. J Auton Nerv Syst 1999; 76: 176–183. [DOI] [PubMed] [Google Scholar]
- 129.Seiler K, Nusser JI, Lennerz JK, et al. Changes in calcitonin gene-related peptide (CGRP) receptor component and nitric oxide receptor (sGC) immunoreactivity in rat trigeminal ganglion following glyceroltrinitrate pretreatment. J Headache Pain 2013; 14: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hay DL, Poyner DR and Sexton PM. GPCR modulation by RAMPs. Pharmacol Ther 2006; 109: 173–197. [DOI] [PubMed] [Google Scholar]
- 131.Goto T, Iwai H, Kuramoto E, et al. Neuropeptides and ATP signaling in the trigeminal ganglion. Jpn Dent Sci Rev 2017; 53: 117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kung L-H, Gong K, Adedoyin M, et al. Evidence for glutamate as a neuroglial transmitter within sensory ganglia. PloS One 2013; 8: e68312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Csati A, Tajti J, Tuka B, et al. Calcitonin gene-related peptide and its receptor components in the human sphenopalatine ganglion – interaction with the sensory system. Brain Res 2012; 1435: 29–39. [DOI] [PubMed] [Google Scholar]
- 134.Zhao B, Wang H-B, Lu Y-J, et al. Transport of receptors, receptor signaling complexes and ion channels via neuropeptide-secretory vesicles. Cell Res 2011; 21: 741–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Maday S, Twelvetrees AE, Moughamian AJ, et al. Axonal transport: Cargo-specific mechanisms of motility and regulation. Neuron 2014; 84: 292–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Eftekhari S, Warfvinge K, Blixt FW, et al. Differentiation of nerve fibers storing CGRP and CGRP receptors in the peripheral trigeminovascular system. J Pain 2013; 14: 1289–1303. [DOI] [PubMed] [Google Scholar]
- 137.Kageneck C, Nixdorf-Bergweiler BE, Messlinger K, et al. Release of CGRP from mouse brainstem slices indicates central inhibitory effect of triptans and kynurenate. J Headache Pain 2014; 15: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Buldyrev I, Tanner NM, Hsieh H, et al. Calcitonin gene-related peptide enhances release of native brain-derived neurotrophic factor from trigeminal ganglion neurons. J Neurochem 2006; 99: 1338–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Garraway SM and Huie JR. Spinal plasticity and behavior: BDNF-induced neuromodulation in uninjured and injured spinal cord. Neural Plast 2016; 2016: 9857201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Takhshid MA, Owji AA and Panjehshahin MR. In vitro effects of adrenomedullin and calcitonin gene related peptide on the release of serotonin and amino acids from rat dorsal spinal cord. Neurosci Lett 2007; 420: 193–197. [DOI] [PubMed] [Google Scholar]
- 141.Russo AF. Calcitonin gene-related peptide (CGRP). Annu Rev Pharmacol Toxicol 2015; 55: 533–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Edvinsson L CGRP receptor antagonists and antibodies against CGRP and its receptor in migraine treatment. Br J Clin Pharmacol 2015; 80: 193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.DosSantos MF, Holanda-Afonso RC, Lima RL, et al. The role of the blood–brain barrier in the development and treatment of migraine and other pain disorders. Front Cell Neurosci 2014; 8: 302. doi: 10.3389/fncel.2014.00302.eCollection20148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lennerz JK, Rühle V, Ceppa EP, et al. Calcitonin receptor-like receptor (CLR), receptor activity-modifying protein 1 (RAMP1), and calcitonin gene-related peptide (CGRP) immunoreactivity in the rat trigeminovascular system: Differences between peripheral and central CGRP receptor distribution. J Comp Neurol 2008; 507: 1277–1299. [DOI] [PubMed] [Google Scholar]
- 145.Simonetti M, Giniatullin R and Fabbretti E. Mechanisms mediating the enhanced gene transcription of P2X3 receptor by calcitonin gene-related peptide in trigeminal sensory neurons. J Biol Chem 2008; 283: 18743–18752. [DOI] [PubMed] [Google Scholar]
- 146.Ceruti S, Villa G, Fumagalli M, et al. Calcitonin gene-related peptide-mediated enhancement of purinergic neuron/glia communication by the algogenic factor bradykinin in mouse trigeminal ganglia from wild-type and R192Q Cav2.1 knock-in mice: Implications for basic mechanisms of migraine pain. J Neurosci 2011; 31: 3638–3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bellamy J, Bowen EJ, Russo AF, et al. Nitric oxide regulation of calcitonin gene-related peptide gene expression in rat trigeminal ganglia neurons. Eur J Neurosci 2006; 23: 2057–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Goto T, Iwai H, Kuramoto E, et al. Neuropeptides and ATP signaling in the trigeminal ganglion. Jpn Dent Sci Rev 2017; 53: 117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]


