Abstract
With the approval of calcitonin gene-related peptide (CGRP) and CGRP receptor monoclonal antibodies by the Federal Drug Administration, a new era in the treatment of migraine patients is beginning. However, there are still many unknowns in terms of CGRP mechanisms of action that need to be elucidated to allow new advances in migraine therapies. CGRP has been studied both clinically and preclinically since its discovery. Here we review some of the preclinical data regarding CGRP in animal models of migraine.
Keywords: Animal model, Antibody, CGRP, Migraine
1. Introduction
Migraine is the third most common medical condition in the world (Vos et al. 2012) and a highly debilitating neurological disease (Lipton et al. 2007). Calcitonin gene-related peptide (CGRP) has been in the forefront of migraine research for years, both clinically and in animal models. With the arrival of CGRP monoclonal antibodies for the treatment of migraine headaches, patients are hoping to find better relief for a disorder that highly impairs their quality of life. Here we will review some of the preclinical evidence that led to the realization that CGRP is a key player in migraine pathophysiology (Edvinsson et al. 2018; Ong et al. 2018; Russo 2015a).
After the initial discovery of CGRP (Amara et al. 1982; Rosenfeld et al. 1983), it was suggested that the neuropeptide was linked to nociception and cardiovascular regulation due to its distribution in small trigeminal and spinal sensory ganglion cells and in sensory fibers surrounding the blood vessels (Rosenfeld et al. 1983). Functional studies soon demonstrated that CGRP is the most potent vasodilatory peptide (Brain et al. 1985, 1986), a record that still stands today (Brain and Grant 2004; Russell et al. 2014). In the following years, more systematic studies of CGRP distribution (Skofitsch and Jacobowitz 1985), as well as its colocalization with substance P (Lee et al. 1985; Uddman et al. 1985; Wiesenfeld-Hallin et al. 1984), provided hints that CGRP might play a role in migraine pathophysiology (Edvinsson 1985). Studies in humans and animal models soon afterward laid the foundation for the field as we know it today (Edvinsson 2017).
2. Involvement of CGRP in Animal Migraine Models
Over the years there have been a variety of animal models developed to study migraine. Many, which are described below, were shown to involve CGRP and its receptor in some way. Since CGRP has a multitude of actions in the body (Russell et al. 2014), it is hard to predict which of these may be key for migraine. Nonetheless, evidence from animal models suggests there are both peripheral and central sensitization mechanisms that may be relevant to migraine (Russo 2015b). In the periphery, CGRP is released from primary afferents of the trigeminal nerve into the perivascular space of the meninges, as well as within the ganglia. Receptors have been identified on arterioles, primary afferents that do not express CGRP, glia, and mast cells (Fig. 1). Actions at some or all of these sites can lead to sensitization of trigeminal nociceptive fibers that could contribute to the headache of migraine. In the central nervous system, CGRP released from neurons can act as a neuromodulator to increase glutamatergic signaling. This enhanced neurotransmission could in turn lead to central sensitization that could contribute to headache and other heightened sensory perceptions, such as photophobia. These models are outlined in Fig. 1 and are described below.
Fig. 1.
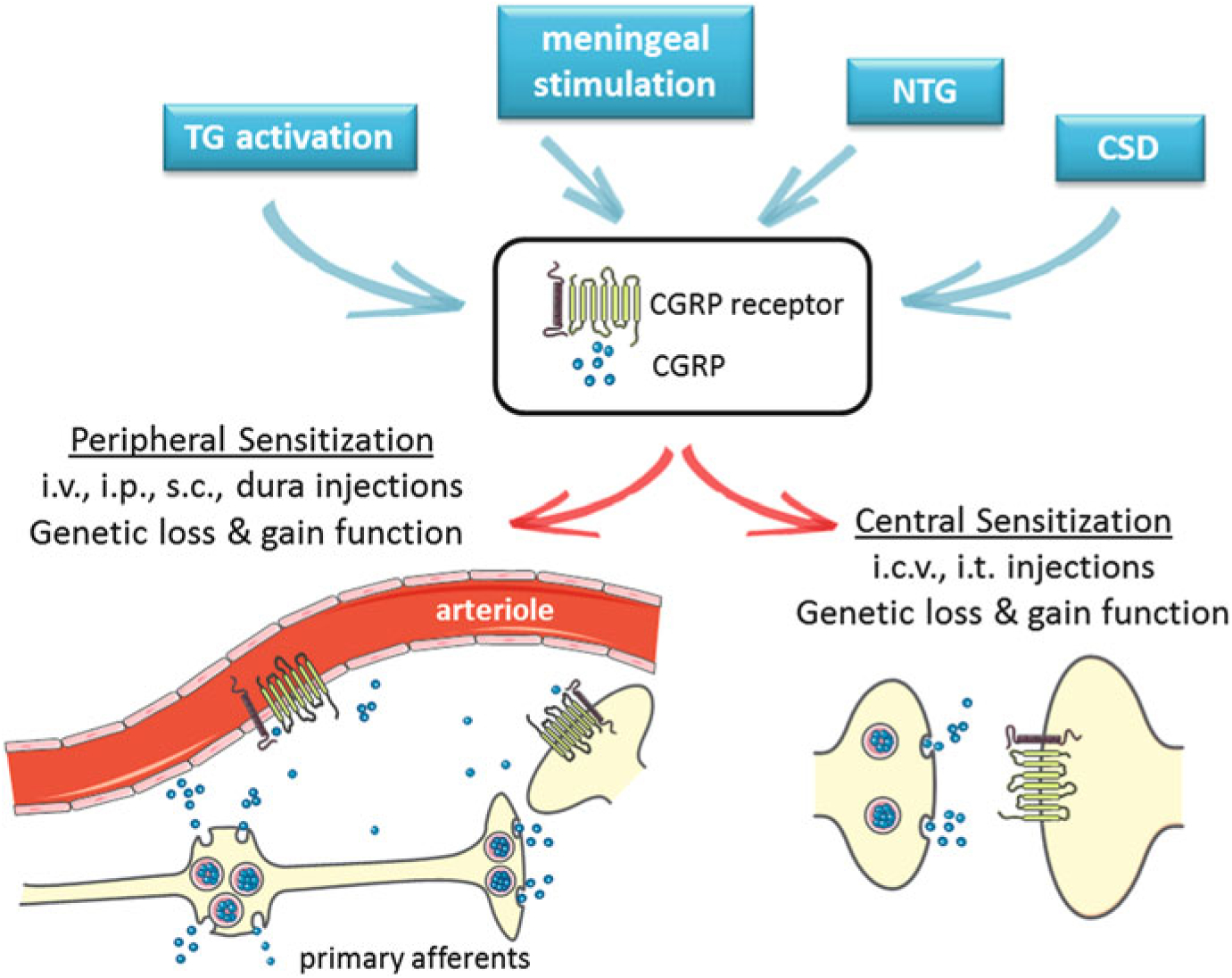
CGRP in animal models of migraine. Animal models of migraine induced by activation of the trigeminal ganglia (TG), meningeal stimulation, infusion of nitroglycerin (NTG), or cortical spreading depression (CSD) have been shown to involve CGRP and its receptor. A schematic of the calcitonin-like receptor and RAMP1 complex is shown on vessels and neurons. Not shown are CGRP receptors on other cells, including mast cells and glia. While the exact sites and actions of CGRP that are important for migraine are not known, evidence from animal models suggests there are both peripheral and central sensitization mechanisms. Likewise, administration of CGRP by peripheral and central routes is believed to induce migraine-like phenotypes through these sensitization mechanisms. Peripheral delivery routes include intravenous (i.v.), intraperitoneal (i.p.), subcutaneous (s.c.), and directly onto the dura. Central delivery includes intracerebroventricular (i.c.v.) and intrathecal (i.t.) routes. Genetic models involving loss or gain of CGRP and/or receptor subunits can also modulate peripheral and central CGRP actions
2.1. Trigeminal Ganglion Activation Model
The subjective nature of headaches often precludes proper diagnosis and treatment but also makes this pathology hard to model in animals, which cannot orally report their pain. Based on the idea that migraine involves the activation of the trigeminovascular system, one of the first animal models for migraine headache was stimulation of the trigeminal ganglion. This model helped improve our understanding of the anatomy and pharmacology of the trigeminovascular system (Akerman et al. 2013). In particular, Goadsby et al. showed that the electrical stimulation of the trigeminal ganglion in cats induced an elevation of CGRP-like immunoreactivity in blood samples taken from the external jugular vein (Goadsby et al. 1988) and increased the release of CGRP into the cranial circulation on the side of the stimulation (Goadsby and Edvinsson 1993). In rats, stimulation of the trigeminal ganglion caused an increase in blood flow ipsilateral to the side of stimulation that was reduced by intravenous injection of the CGRP antagonist CGRP8–37 (Escott et al. 1995).
2.2. Meningeal Stimulation Model
One of the most widely used models of migraine headache to date is stimulation of the dura mater of the meninges that line the brain. Meningeal stimulation can be achieved by application of inflammatory compounds or by electrical stimulation. In anesthetized cats, electrical stimulation of the superior sagittal sinus increased the levels of CGRP in jugular vein blood by 85%, which provided the first evidence that activation of craniovascular afferents causes release of vasodilatory peptides (Zagami et al. 1990). Using immunohistochemistry, Messlinger and colleagues later showed that the parietal dura mater of the rat was densely innervated by CGRP nerve fibers (Messlinger et al. 1995). Furthermore, it was shown that electrical stimulation of the dural surface caused a depletion of CGRP-immunopositive fibers, suggesting a release of CGRP, and an increase of the dural blood flow around branches of the medial meningeal artery (Messlinger et al. 1995). It was concluded that the stimulation of trigeminal afferents innervating the dura mater induced the release of CGRP from peptidergic afferent terminals, which in turn caused vasodilation and increased meningeal blood flow.
This increase in meningeal blood flow was inhibited in a dose-dependent manner by topical application of the CGRP antagonist CGRP8–37 (Kurosawa et al. 1995). In separate studies, Williamson et al. showed that CGRP8–37 and two different 5HT1B/1D agonists (sumatriptan and rizatriptan) were able to reduce the dilation of dural vessels induced by electrical stimulation in rats and guinea pigs (Williamson et al. 1997, 2001), which mimicked for the first time clinical findings that triptans were able to normalize CGRP levels during a migraine attack (Goadsby et al. 1990). Intravenous injection of another CGRP antagonist, BIBN4096BS, was able to prevent the vasodilatory actions of endogenous CGRP released following transcranial electrical stimulation in rats (Petersen et al. 2004; Troltzsch et al. 2007), as well as inhibit trigeminocervical superior sagittal sinus-evoked activity in cats (Storer et al. 2004). Taken together, those studies show that across species, there are CGRP receptors in the trigeminocervical complex. These data supported the hypothesis that blocking CGRP would be an effective treatment of migraine.
Using isolated rat middle cerebral arteries, CGRP was shown to induce a concentration-dependent dilation with abluminal application, but not by luminal application (Edvinsson et al. 2007). This suggested that CGRP could act on smooth muscle cell CGRP receptors, but could not cross the endothelial barrier. CGRP blockers such as CGRP8–37, BIBN4096BS, and CGRP antibody were able to inhibit CGRP-induced relaxation (Edvinsson et al. 2007).
Meningeal stimulation can also be achieved by application of substances directly on the dura. A recognized symptom of migraine is heightened sensitivity to stimuli. The perception of touch as a painful stimulus is reported by nearly half of migraineurs (LoPinto et al. 2006; Mathew et al. 2004). In animal models, this mechanical allodynia can be measured using von Frey filaments. Application of inflammatory mediators directly onto the dura elicits both facial and plantar allodynia that can be reversed by sumatriptan and CGRP8–37 (Edelmayer et al. 2009), once again showing that targeting CGRP is a valid strategy to treat pain associated with migraine.
More recently, a study investigated sex differences in behavioral responses after application of inflammatory soup (IS) on the dura (Stucky et al. 2011). While both male and female rats showed behavioral responses (activity measures as well as withdrawal responses for periorbital and perimasseter mechanical testing) to IS application compared to saline, females showed effects at lower doses than males and for longer duration. However, males required fewer applications of IS to exhibit responses (Stucky et al. 2011). In the same study, levels of transcripts for CGRP and the different subunits of its principal receptor (RAMP1, receptor activity-modifying protein 1; CLR, calcitonin-like receptor; and RCP, receptor component unit) were assessed in different areas of the CNS, at baseline or after application of IS to the dura. At baseline, females had lower levels of the receptor components in the trigeminal ganglion and in the medulla, while their CGRP mRNA levels were higher in the medulla than males. After IS and saline application to the dura, CGRP transcript levels were upregulated in all groups (Stucky et al. 2011). This suggests that the CGRP pathway responds to changes in intracranial pressure or meningeal stretch, while migraine-like behaviors occur after meningeal inflammation.
2.3. Nitroglycerin-Induced Model
Nitric oxide (NO) is a regulator of cerebral blood flow and vessel diameter. Nitroglycerin (NTG), a NO donor, can be used to trigger migraine in migraineurs (Christiansen et al. 1999; Thomsen et al. 1994), and response to nitroglycerin is a diagnostic test for migraine (Ferrari et al. 2015). This has led to the use of NTG administration as a trigger for sensory hypersensitivity associated with migraine in laboratory animals. Considering that NO is an important signaling molecule involved in the synthesis and release of CGRP from trigeminal ganglion neurons (Bowen et al. 2006), many of the drugs designed to block CGRP to treat migraine symptoms are also effective in NTG-induced migraine models.
NTG administration induces thermal and mechanical allodynia as well as thermal hyperalgesia in rodents (Bates et al. 2010; Tassorelli et al. 2003). Sumatriptan, the gold-standard anti-migraine drug is a 5-HT1B and 5-HT1D agonist that can prevent the release of CGRP in plasma (Goadsby and Edvinsson 1994). Administered centrally (i.t.) and peripherally (i.p.), sumatriptan was able to reduce NTG-induced thermal hypersensitivity; only the central injection of sumatriptan was able to reduce mechanical hypersensitivity (Bates et al. 2010). Similarly, NO-induced increase in spinal trigeminal activity can be reduced by the CGRP receptor antagonists BIBN4096BS (later called olcegepant) and MK-8825 (Feistel et al. 2013; Koulchitsky et al. 2009). Subsequently, it was found that nitroglycerin (i.p.) administration to rats increased CGRP levels in the brainstem and trigeminal ganglia (Capuano et al. 2014). Additionally, those authors showed that an injection of CGRP in the whisker pads of rats only increased the time the rats spent in face rubbing when they were pre-treated with NTG (Capuano et al. 2014). This suggests that NTG can sensitize the trigeminal system for CGRP to induce a painful behavior in rats (Capuano et al. 2014). In order to study the progression from acute to chronic migraine, Pradhan and colleagues used chronic injection of NTG every other day for 9 days, which induced progressive and sustained hyperalgesia (Pradhan et al. 2014). This time however, systemic or central sumatriptan did not ameliorate NTG-induced chronic hyperalgesia (Pradhan et al. 2014).
2.4. Cortical Spreading Depression Model
Cortical spreading depression (CSD) is hypothesized to cause migraine auras (Cutrer and Huerter 2007). There is some evidence pointing toward an interaction between CSD events and CGRP actions (Close et al. 2018). A recent study showed that BIBN4096BS could decrease the amplitude and propagation rate of repeated retinal spreading depression episodes induced by potassium in chick retinal preparation (Wang et al. 2016). Blocking CGRP receptors with CGRP8–37 attenuated CSD-associated hyperperfusion in the rat (Reuter et al. 1998) and reduced CSD-induced pial dilatation (Colonna et al. 1994; Wahl et al. 1994), suggesting that the release of CGRP by trigeminal sensory neurons is responsible, at least in part, for some of the vascular changes associated with CSD. In a recent study, MK-8825 (CGRP receptor antagonist) did not alter CSD waves or CSD-induced change in regional cerebral blood flow (Filiz et al. 2017). It did however attenuate CSD-induced trigeminal nerve-mediated freezing and spontaneous responses (both body and head grooming, wet dog shakes, and head shakes). Other behaviors such as eating/drinking, rearing, and turning that are impaired after induction of CSD were not changed after administration of MK-8825 (Filiz et al. 2017). Finally, CSD-induced periorbital allodynia is reversed by administration of MK-8825 (Filiz et al. 2017). Taken together, those studies show that the blockade of CGRP can decrease the impact of CSD and seem to indicate that CGRP may be involved in the propagation of CSD (but not its initiation). Interestingly, a recent study showed that induction of CSD by KCl in rats resulted in increased CGRP protein expression in the trigeminal ganglia, although there was no change in CGRP transcript levels (Yisarakun et al. 2015), which points to a possible positive feedback loop between CSD and CGRP (Close et al. 2018).
3. Animal Models of Migraine Induced by Injection of CGRP
After it was reported that (1) CGRP levels are elevated during spontaneous migraine and in between attacks in patients with chronic migraine (Bellamy et al. 2006; Goadsby et al. 1990; van Dongen et al. 2017), and (2) an intravenous infusion of CGRP could induce a delayed migraine-like headache in migraineurs (Asghar et al. 2011; Hansen et al. 2010; Lassen et al. 2002), animal models of migraine induced by injection of CGRP were developed. Administration of CGRP by peripheral and central routes is believed to induce migraine-like phenotypes through peripheral and central sensitization mechanisms, respectively (Fig. 1). However, it must be emphasized that the mechanisms are not mutually exclusive. For example, peripheral sensitization can lead to central sensitization. In this section, we describe animal models based on the peripheral and central delivery routes, which are outlined in Fig. 1 and summarized in Table 1.
Table 1.
Potential migraine-related effects of CGRP administered by different routes in rodents
| CGRP delivery | Phenotype | Species and reference |
|---|---|---|
| Intravenous |
|
Rat Bhatt et al. (2015) |
|
Rat Lappe et al. (1987) |
|
|
Rat Siren and Feuerstein 1988 |
|
|
Rat Cumberbatch et al. (1999) |
|
|
Rat Petersen et al. (2004) |
|
|
Rat Petersen et al. 2005a, b) |
|
|
Rat Levy et al. (2005) |
|
| Intracerebroventricular |
|
Rat Bhatt et al. (2014) |
|
Mouse Kaiser et al. (2012) |
|
|
Mouse Mason et al. (2017) |
|
|
Rat Huang et al. (2000) |
|
|
Rat Pecile et al. (1987) |
|
|
Mouse Schorscher-Petcu et al. (2009) |
|
| Intrathecal |
|
Mouse Mogil et al. (2005) |
|
Rat Oku et al. (1987) |
|
|
Mouse Marquez de Prado et al. (2009) |
|
| Intraperitoneal |
|
Mouse Mason et al. (2017) |
|
Mouse Rea et al. (2018) |
|
|
Mouse Kaiser et al. (2017) |
|
| Dural and epidural |
|
Rat Levy et al. 2005 |
|
Rat Yao et al. (2017) |
|
|
Mouse Burgos Vega et al. (2017) |
|
| Subcutaneous and intradermal |
|
Rat Chu et al. (2000) |
|
Rat Nakamura-Craig and Gill (1991) |
|
|
Mouse Mogil et al. (2005) |
|
|
Mouse Marquez de Prado et al. (2009) |
3.1. Intravenous CGRP Delivery
Intravenous infusion of CGRP at a dose able to induce vasodilation is sufficient to induce a migraine-like headache in 66% of migraineurs (Asghar et al. 2011; Guo et al. 2016; Hansen et al. 2010; Lassen et al. 2002). In contrast, it only provokes a mild headache in non-migraineurs (Petersen et al. 2005a), suggesting that migraineurs are more sensitive to CGRP (Russo et al. 2009). Based on these clinical observations, the effects of i.v. CGRP have been studied in animals (Table 1).
Up until the discovery of one unique wild-type rat displaying spontaneous episodic trigeminal allodynia (Munro et al. 2018; Oshinsky et al. 2012), scientists had not been able to witness any occurrence of spontaneous migraine symptoms in laboratory animals. It was therefore not possible to discriminate a migraineur vs. non-migraineur population in animals without evoking symptoms. Nevertheless, scientists have studied the effect of i.v. CGRP in preclinical settings. Considering that migraine has historically been considered a vascular disorder, vascular actions of CGRP are important to take into account. In animals, an infusion of CGRP induced a dose-dependent decrease in blood pressure and increase in heart rate (Bhatt et al. 2015; Lappe et al. 1987; Siren and Feuerstein 1988). IV CGRP administration in rats caused a dilation of the cortical pial arteries and arterioles and of the middle meningeal artery and increased local cortical cerebral blood flow, all of which could be inhibited by the CGRP receptor antagonist BIBN4096BS (Cumberbatch et al. 1999; Petersen et al. 2004, 2005b). Surprisingly, and to our knowledge, there is very little in the literature about nociceptive actions of i.v. CGRP. IV CGRP facilitated vibrissal responses, which seemed to indicate that CGRP-induced vasodilation was activating primary afferent meningeal nociceptors (Cumberbatch et al. 1999). However, electrophysiological studies later showed that it was not the case (Levy et al. 2005). Additionally, a recent study showed that CGRP infusion in awake rats failed to increase c-Fos and Zif268 (neuronal pain markers) expression in the trigeminal nucleus caudalis (Bhatt et al. 2015).
Although i.v. CGRP is the most translational approach for CGRP administration, the inherent difficulty and stress from performing an i.v. injection in rodents, especially in mice, led to the use of other routes of injections in preclinical studies.
3.2. Intraperitoneal CGRP Delivery
A relatively easy method to deliver CGRP to the peripheral tissues of rodents to allow assessment of migraine-like symptoms is by intraperitoneal (i.p.) injections (Table 1). Notably, i.p. CGRP induced light aversion both in CD1 and C57BL/6J mice, which was attenuated by both sumatriptan and an anti-CGRP antibody (Mason et al. 2017). These results, coupled with results obtained centrally, suggest that CGRP actions to induce migraine-like behavior are mediated by both peripheral and central mechanisms (Mason et al. 2017).
Recently, we also described i.p. CGRP-induced spontaneous pain in mice. The mice showed increased facial signs of discomfort (grimace and squint) (Rea et al. 2018). Those phenotypes were also reversed by anti-CGRP antibody. Interestingly, sumatriptan partially inhibited CGRP-induced spontaneous pain in males but not females (Rea et al. 2018). Of importance, the dose of 0.1 mg/kg i.p. used in all of our studies is able to induce vasodilation visible as redness of the ears (Rea et al. 2018).
Additionally, migraine symptomatology includes gastrointestinal problems, which occur in 22% of migraineurs (Kelman 2004). Our team reported that i.p. CGRP administration induced diarrhea in C57BL/6J mice and that olcegepant (previously called BIBN4096BS), a CGRP receptor antagonist, was able to attenuate this symptom (Kaiser et al. 2017).
The use of triptans in the previously mentioned studies validates the symptoms as being migraine-related but also provides some clues about triptan mechanisms of action. It is known that triptans are vasoconstrictors that can also inhibit endogenous neuropeptide release via 5-HT1D receptors (Durham and Russo 2002; Loder 2010). Importantly, clinical studies demonstrated that triptans can reverse CGRP-induced vasodilation and headache in normal subjects and migraine patients (Asghar et al. 2010, 2011). Thus, in both mice and humans, triptans are able to override exogenous CGRP, suggesting that their mechanism of action must be more than just inhibition of CGRP release. Moreover, colocalization of 5-HT1D and CGRP in the spinal trigeminal nucleus and other areas in the brainstem such as the parabrachial nucleus (Noseda et al. 2008) and the fact that triptans can downregulate nociceptive signal transmission in the spinal trigeminal nucleus (Levy et al. 2005; Mitsikostas et al. 1999) support the hypothesis that triptans can mask the effect of a bolus injection of CGRP injected either centrally or peripherally. It is very likely that triptan mechanism of action to relieve migraine-like symptoms involves actions at multiple sites (Ahn and Basbaum 2005; Kaiser et al. 2012).
3.3. Subcutaneous and Intradermal CGRP Delivery
In accordance to the results obtained with other routes of administration, intradermal CGRP (in rats and in rabbits) induced an increase in blood flow (Brain et al. 1985; Chu et al. 2000) (Table 1). However, intradermal CGRP did not induce any thermal hyperalgesia in rats (Chu et al. 2000). Early studies also showed a lack of effect of subplantar CGRP compared to that of substance P and neurokinin A in exacerbating the response to paw pressure (mechanical hyperalgesia) in Wistar rats (Nakamura-Craig and Gill 1991). This is consistent with early results in the human skin where CGRP was proposed to have a role in blood flow regulation and in mediating flare response but most likely had no direct role in nociception since the concentrations at which it induced histamine release exceeded normal physiologic concentrations (Brain et al. 1986; Weidner et al. 2000). In a later study, Mogil and colleagues reported a strain difference in the development of thermal hyperalgesia after subcutaneous (s.c.) CGRP injection into the plantar hindpaw. AKR mice but not in C57BL/6J mice seemed to become hypersensitive (Mogil et al. 2005). In our hands, C57BL/6J mice did not develop tactile allodynia assessed by von Frey filaments after intraplantar injection of CGRP (Marquez de Prado et al. 2009). Since we have shown that CD1 mice are more sensitive to CGRP-induced light aversion (Mason et al. 2017), this lack of effect may be strain specific.
3.4. Dural and Epidural CGRP Delivery
Similar to previously described models of meningeal stimulation, and because the activation of the trigeminal nerve leads to release of CGRP from perivascular nerve endings at meningeal blood vessels, dural/epidural delivery of CGRP was used as a model for migraine pathophysiology (Table 1). Dural delivery of CGRP induced a significant increase in dural blood flow, although it reportedly did not activate or sensitize meningeal nociceptors (Levy et al. 2005). Recently however, Yao and colleagues described a reduction in climbing and face-grooming behaviors accompanied by increased immobile behavior after epidural CGRP administration in rats (Yao et al. 2017). Very interestingly, Dussor and colleagues have recently reported that CGRP can directly stimulate the dura of female but not male rodents to induce periorbital hypersensitivity (Burgos Vega et al. 2017). Additionally, CGRP was able to prime female rodents to a usually innocuous dural application of a pH 7.0 solution.
3.5. Intracerebroventricular CGRP Delivery
Since studies pointed towards a central mechanism of CGRP in migraine pathophysiology, intracerebroventricular (i.c.v.) injections of CGRP were studied (Table 1). Although i.c.v. injection in awake rats did not increase c-Fos expression in the trigeminal nucleus caudalis (Bhatt et al. 2014), a similar injection showed migraine-like behavioral effects. An important trigger and/or symptom experienced by migraineurs is photophobia or photosensitivity, which is an altered perception of light that elicits discomfort (Boulloche et al. 2010; Kelman 2007; Martin and Behbehani 2001; Mulleners et al. 2001; Rasmussen 1993; Spierings et al. 2001). In rodents, light aversion represents a surrogate for photophobia and can be measured using a conflict assay between a light and a dark chamber. Using this test, our lab has shown that i.c.v. injection of CGRP in mice induced light-aversive behavior when exposed to bright light (27,000 lux) but not to dim light (55 lux), which was attenuated by rizatriptan, a 5-HT1B/D agonist anti-migraine drug (Kaiser et al. 2012; Mason et al. 2017). Those animals also showed an increased time spent resting in the dark compartment, which is a behavior similar to migraineurs who tend to seek a dark place to rest during attacks (Kaiser et al. 2012; Mason et al. 2017). These findings indicate that CGRP can act in the CNS to cause light aversion.
Other studies assessed the role of i.c.v. CGRP on nociception. Antinociceptive effects of CGRP administered intracerebroventricularly into the nucleus raphe magnus, amygdala, nucleus accumbens, or the periaqueductal gray were reported in rats submitted to thermal and mechanical stimulations (Huang et al. 2000; Li et al. 2001; Pecile et al. 1987; Xu et al. 2003; Yu et al. 2003; Zhou et al. 2003). Additionally, it was reported that i.c.v. CGRP increased paw withdrawal latencies to thermal stimuli in C57BL/6 mice but not in AKR mice while decreasing depression-like behaviors in both strains in the forced swim test (Schorscher-Petcu et al. 2009). In the same study, i.c.v. CGRP and CGRP receptor antagonists failed to modulate activity in the elevated plus maze, a model of anxiety (Schorscher-Petcu et al. 2009).
3.6. Intrathecal CGRP Delivery
Studies showed that CGRP is located in small diameter dorsal root ganglion neurons (Hokfelt et al. 1992), dorsal horns (Hokfelt et al. 1992; Ishida-Yamamoto and Tohyama 1989), and intermediolateral and ventral horns of the spinal cord (Bennett et al. 2000; Marti et al. 1987; Senba and Tohyama 1988). Spinal cord central sensitization following intradermal capsaicin injection has been shown to be mediated by CGRP and its receptors (Carlton et al. 1990; Sun et al. 2004). Thus, researchers explored the effects of intrathecal (i.t.) CGRP administration on pain responses (Table 1). Administration of i.t. CGRP induced mechanical and thermal hyperalgesia in rats (Mogil et al. 2005; Oku et al. 1987). Our team showed that a low dose of CGRP injected into the lumbar spinal region did not exacerbate the response to an innocuous mechanical stimulus (von Frey filaments), while a higher dose evoked mechanical allodynia (Marquez de Prado et al. 2009). These data indicate that CGRP can act centrally to sensitize mice to thermal and mechanical stimuli.
4. Genetic Manipulation of CGRP in Migraine Models
Genetic manipulations have been used to directly investigate CGRP signaling. Transgenic and knockout mice have been generated that have either a loss or gain of function of the CGRP ligand or receptor subunits. These genetic models allow modulation of peripheral and central CGRP actions (Fig. 1). A thorough review of all CGRP and receptor subunit mutant mice and their phenotypes can be found elsewhere (Sowers et al. 2017). We will focus on the few cases where migraine-like phenotypes were assessed.
4.1. Overexpression of Human RAMP1
With the goal to study migraine, our laboratory developed a subset of genetic constructs revolving around the overexpression of the human receptor activity-modifying protein 1 (RAMP1) subunit of the CGRP receptor. Using RAMP1, which has been shown to be functionally rate-limiting (Zhang et al. 2006, 2007), allowed the development of CGRP-sensitized mice. Overexpressing the human version of the gene provided the advantage that it can be targeted by drugs designed for clinical application and therefore allow more translatable models (Russo 2015b). To generate those models, the approach was to use double-transgenic mice that express hRAMP1 in a tissue-specific Cre-dependent manner or in all tissues.
Global overexpression of hRAMP1 in all tissues was achieved using mice expressing Cre recombinase under the control of the ubiquitous adenovirus EIIa promoter (Bohn et al. 2017). Cultures obtained from vascular smooth muscle and trigeminal ganglia from those global mice showed an increased CGRP receptor activity that could be blocked by drugs such as CGRP receptor antagonists telcagepant and CGRP8–37 (Bohn et al. 2017). Mice with global hRAMP1 overexpression display increased vasodilation of the carotid and basilar arteries, and cerebral arterioles after CGRP application (Chrissobolis et al. 2010), and decreased angiotensin II-induced hypertension (Sabharwal et al. 2010).
In order to study more specifically the role of CGRP and its receptor in the nervous system, double-transgenic mice were developed using nestin-Cre to drive expression in neurons and some glia cells (Zhang et al. 2007). The injection of CGRP in the whisker pads of those animals increased neurogenic inflammation by doubling plasma extravasation (Zhang et al. 2007). Those animals also display behaviors consistent with migraine such as mechanical allodynia and photosensitivity. While the nestin/hRAMP1 mice have similar hindpaw withdrawal thresholds to von Frey filament stimulation than control littermates, their response frequency drastically increased after intrathecal CGRP injection, while the same dose of CGRP did not elicit a response in control animals (Marquez de Prado et al. 2009). Nestin/hRAMP1 mice also show an increased sensitivity to tactile stimulation after capsaicin injection which extended to the contralateral hindpaw, suggesting central sensitization (Marquez de Prado et al. 2009). The transgenic nestin/hRAMP1 mice display light-aversive behavior when confronted to bright light (Recober et al. 2009). This light aversion is enhanced after i.c.v. injection of CGRP even when exposed to very dim light (55 lux) (Recober et al. 2010). In the same conditions, those mice also display a decrease in motility behaviors once in the dark, such as rearing, distance travelled, time spent moving, and ambulatory velocity (Recober et al. 2010), which resembles the behavior of migraineurs who will seek out a dark room to rest during an attack.
As mentioned earlier, i.p. injection of CGRP in wild-type mice induced light aversion when exposed to very bright light (Mason et al. 2017). Interestingly, and contrasting to the results obtained with i.c.v. CGRP, nestin/hRAMP1 transgenic mice were not sensitized to i.p. CGRP when exposed to dim lights (Mason et al. 2017). In conclusion, the hRAMP1 double transgenic mice enabled the understanding that CGRP is a key player in migraine both centrally through action on neurons and peripherally on receptors that are not located in the nervous system. Experiments are currently underway to assess the role of CGRP receptors on smooth muscle and the endothelium in the periphery.
4.2. Other Transgenic Models
A few other transgenic models affecting CGRP signaling assessed nociceptive and vascular changes that can have implications for migraine pathophysiology.
In terms of nociception, different lines of CGRP knockout mice have been developed that show maladaptation to pain. In contrast to wild-type mice, Zhang and colleagues reported a CT/αCGRP knockout mouse that showed no sign of secondary hyperalgesia after development of carrageenan-induced inflammation in the knee joint (Zhang et al. 2001). Another strain of αCGRP knockout showed an attenuated licking response to capsaicin and formalin injections as well as a reduction of the edema produced by carrageenan injection in the hindpaw (Salmon et al. 2001). This transgenic mouse also displayed no sign of thermal hyperalgesia after ATP-induced TRPV1 potentiation (Devesa et al. 2014) and reduced morphine analgesia (Salmon et al. 1999). CGRP knockout mice also present a reduced vestibule-ocular reflex (Luebke et al. 2014) and abnormal cochlear response (Maison et al. 2003) which can be of importance in the pathophysiology of migraine. Keeping in mind that migraine has a vascular component, the effect of CGRP gene deletion on the cardiovascular system was assessed but remains controversial, with reports of a lack of effect (Lu et al. 1999) and reports of increased blood pressure (Gangula et al. 2000; Oh-hashi et al. 2001). In one study, RAMP1 knockout mice also had elevated blood pressure (Tsujikawa et al. 2007).
5. CGRP Antibodies: New Era in Migraine Treatment
Monoclonal antibodies that target either CGRP or its receptor have now been approved by the Federal Drug Administration for the preventive treatment of migraine. Erenumab (Amgen/Novartis) blocks CGRP receptors. Fremanezumab (Teva Pharmaceuticals) and galcanezumab (Eli Lilly) bind to CGRP and block its binding to the receptors. A fourth antibody, eptinezumab (Alder Biopharmaceuticals), also blocks CGRP and is on track for approval.
In the 1980s and 1990s, it was found that intrathecal injection of CGRP antisera could block the pain induced by thermal (Kawamura et al. 1989) and mechanical (Kawamura et al. 1989; Kuraishi et al. 1988) noxious stimuli in rats receiving injections of adjuvant arthritis or carrageenin in the paw. In addition, CGRP antiserum partially rescued the reduced nociceptive threshold evoked by repeated cold stress (Satoh et al. 1992). However, antibody studies that are more relevant to migraine have only been pursued in the past few years.
Several studies have examined the effect of CGRP-blocking antibodies on migraine-like symptoms in mice. Mason et al. studied the effect of one monoclonal anti-CGRP antibody (ALD405) in light aversion in mice (Mason et al. 2017). These mice were first treated with CGRP (i.p.) to establish the degree of their responsiveness to CGRP. The mice were then given anti-CGRP antibody (i.p.) 24 h before given CGRP (i.p.) a second time. The amount of anti-CGRP antibody injected was ~eightfold excess over exogenous CGRP. The results showed that CGRP antibody attenuated CGRP (i.p.)-induced light-aversive behavior (details about CGRP-induced light aversion in Sect. 3.2). The results suggest a peripheral action of CGRP in the induction of light aversion. Likewise, Rea et al. showed that ALD405 administration (i.p.) prevented CGRP (i.p.)-induced spontaneous grimace (indicator of facial discomfort) in CD1 mice both in males and females. ALD405 administration also prevented the grimace in restrained C57BL/6J mice independent of the light. Another measurement of facial discomfort is squint, which was the principle component of the grimace, accounting for 77% of the total variation of grimace scale. CGRP-induced squint in restrained CD1 mice and C57BL/6J mice was prevented by ALD405 administration (i.p.). This suggests that CGRP can act in the periphery to induce a pain response. Gastrointestinal issues are one of the most common symptoms of migraine including diarrhea (Kelman 2004) (see Sect. 3.4). CGRP injection induced diarrhea in C57BL/6J mice, while anti-CGRP antibodies blocked CGRP-induced diarrhea (Kaiser et al. 2017). Moreover, it has been reported that a different CGRP antibody suppressed CSD as indicated by increased latency of CSD, and this effect was blocked by exogenous CGRP (Jiang et al. 2018).
In a series of experiments in rats, i.v. administration of the CGRP-blocking antibody fremanezumab was shown to inhibit the activation of high-threshold trigeminovascular neurons that were responsive to mechanical stimulation of the dura, but not to either innocuous or noxious stimulation of the skin or cornea. Fremanezumab also prevented the activation of trigeminovascular high-threshold neurons by CSD induced mechanically by inserting a glass micropipette into the visual cortex (Melo-Carrillo et al. 2017a). Moreover, fremanezumab pretreatment inhibited the response of Aδ, but not C-fiber, neurons in response to CSD (Melo-Carrillo et al. 2017b). These results provide a mechanism by which fremanezumab could reduce the intracranial pain of migraine. In addition, it was demonstrated that fremanezumab can treat medication overuse headache symptoms in rats. For these experiments, rats were primed with repeated sumatriptan or morphine treatments. Fremanezumab significantly inhibited bright-light stress or NO donor-induced cutaneous allodynia (Kopruszinski et al. 2017). The data suggest that medication overuse headache may be CGRP-dependent and that the anti-CGRP antibody may be a potential therapeutic.
6. Conclusion
In conclusion, many animal models of migraine involve CGRP to some degree. The importance of CGRP in those models is confirmed by the ability of direct injection of CGRP to induce several migraine-like symptoms in rodents. Further, these preclinical observations are in full alignment with the recent success of CGRP-based migraine therapeutics in patients. Thus, CGRP in animal models of migraine is an excellent example of successful translation of science from the lab to the patient.
Acknowledgments
The authors thank members of the lab for their comments and support from Dept. Defense W81XWH-16-1-0071, W81XWH-16-1-0211; VA-ORD (RR&D) 1IO1RX002101; C6810-C and NIH NS075599.
Contributor Information
Anne-Sophie Wattiez, Department of Molecular Physiology and Biophysics, University of Iowa, Iowa City, IA, USA; Center for the Prevention and Treatment of Visual Loss, Iowa VA Health Care System, Iowa City, IA, USA.
Mengya Wang, Department of Pharmacology, University of Iowa, Iowa City, IA, USA.
Andrew F. Russo, Department of Molecular Physiology and Biophysics, University of Iowa, Iowa City, IA, USA Center for the Prevention and Treatment of Visual Loss, Iowa VA Health Care System, Iowa City, IA, USA; Department of Neurology, University of Iowa, Iowa City, IA, USA.
References
- Ahn AH, Basbaum AI (2005) Where do triptans act in the treatment of migraine? Pain 115:1–4. 10.1016/j.pain.2005.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akerman S, Holland PR, Hoffmann J (2013) Pearls and pitfalls in experimental in vivo models of migraine: dural trigeminovascular nociception. Cephalalgia 33:577–592. 10.1177/0333102412472071 [DOI] [PubMed] [Google Scholar]
- Amara SG, Jonas V, Rosenfeld MG, Ong ES, Evans RM (1982) Alternative RNA processing in calcitonin gene expression generates mRNAs encoding different polypeptide products. Nature 298:240–244 [DOI] [PubMed] [Google Scholar]
- Asghar MS et al. (2010) Dilation by CGRP of middle meningeal artery and reversal by sumatriptan in normal volunteers. Neurology 75:1520–1526. 10.1212/WNL.0b013e3181f9626a [DOI] [PubMed] [Google Scholar]
- Asghar MS et al. (2011) Evidence for a vascular factor in migraine. Ann Neurol 69:635–645. 10.1002/ana.22292 [DOI] [PubMed] [Google Scholar]
- Bates EA et al. (2010) Sumatriptan alleviates nitroglycerin-induced mechanical and thermal allodynia in mice. Cephalalgia 30:170–178. 10.1111/j.1468-2982.2009.01864.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellamy JL, Cady RK, Durham PL (2006) Salivary levels of CGRP and VIP in rhinosinusitis and migraine patients. Headache 46:24–33. 10.1111/j.1526-4610.2006.00294.x [DOI] [PubMed] [Google Scholar]
- Bennett AD, Chastain KM, Hulsebosch CE (2000) Alleviation of mechanical and thermal allodynia by CGRP(8–37) in a rodent model of chronic central pain. Pain 86:163–175 [DOI] [PubMed] [Google Scholar]
- Bhatt DK, Gupta S, Ploug KB, Jansen-Olesen I, Olesen J (2014) mRNA distribution of CGRP and its receptor components in the trigeminovascular system and other pain related structures in rat brain, and effect of intracerebroventricular administration of CGRP on Fos expression in the TNC. Neurosci Lett 559:99–104. 10.1016/j.neulet.2013.11.057 [DOI] [PubMed] [Google Scholar]
- Bhatt DK, Ramachandran R, Christensen SL, Gupta S, Jansen-Olesen I, Olesen J (2015) CGRP infusion in unanesthetized rats increases expression of c-Fos in the nucleus tractus solitarius and caudal ventrolateral medulla, but not in the trigeminal nucleus caudalis. Cephalalgia 35:220–233. 10.1177/0333102414535995 [DOI] [PubMed] [Google Scholar]
- Bohn KJ et al. (2017) CGRP receptor activity in mice with global expression of human receptor activity modifying protein 1. Br J Pharmacol 174:1826–1840. 10.1111/bph.13783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulloche N, Denuelle M, Payoux P, Fabre N, Trotter Y, Geraud G (2010) Photophobia in migraine: an interictal PET study of cortical hyperexcitability and its modulation by pain. J Neurol Neurosurg Psychiatry 81:978–984. 10.1136/jnnp.2009.190223 [DOI] [PubMed] [Google Scholar]
- Bowen EJ, Schmidt TW, Firm CS, Russo AF, Durham PL (2006) Tumor necrosis factor-alpha stimulation of calcitonin gene-related peptide expression and secretion from rat trigeminal ganglion neurons. J Neurochem 96:65–77. 10.1111/j.1471-4159.2005.03524.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brain SD, Grant AD (2004) Vascular actions of calcitonin gene-related peptide and adrenomedullin. Physiol Rev 84:903–934. 10.1152/physrev.00037.2003 [DOI] [PubMed] [Google Scholar]
- Brain SD, Williams TJ, Tippins JR, Morris HR, MacIntyre I (1985) Calcitonin gene-related peptide is a potent vasodilator. Nature 313:54–56 [DOI] [PubMed] [Google Scholar]
- Brain SD, Tippins JR, Morris HR, MacIntyre I, Williams TJ (1986) Potent vasodilator activity of calcitonin gene-related peptide in human skin. J Invest Dermatol 87:533–536 [DOI] [PubMed] [Google Scholar]
- Burgos Vega C, Quigley L, Patel M, Price T, Arkopian A, Dussor G (2017) Meningeal application of prolactin and CGRP produces female specific migraine-related behavior in rodents. J Pain 18:S11 [Google Scholar]
- Capuano A, Greco MC, Navarra P, Tringali G (2014) Correlation between algogenic effects of calcitonin-gene-related peptide (CGRP) and activation of trigeminal vascular system, in an in vivo experimental model of nitroglycerin-induced sensitization. Eur J Pharmacol 740:97–102. 10.1016/j.ejphar.2014.06.046 [DOI] [PubMed] [Google Scholar]
- Carlton SM, Westlund KN, Zhang DX, Sorkin LS, Willis WD (1990) Calcitonin gene-related peptide containing primary afferent fibers synapse on primate spinothalamic tract cells. Neurosci Lett 109:76–81 [DOI] [PubMed] [Google Scholar]
- Chrissobolis S, Zhang Z, Kinzenbaw DA, Lynch CM, Russo AF, Faraci FM (2010) Receptor activity-modifying protein-1 augments cerebrovascular responses to calcitonin gene-related peptide and inhibits angiotensin II-induced vascular dysfunction. Stroke 41:2329–2334. 10.1161/STROKEAHA.110.589648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen I, Thomsen LL, Daugaard D, Ulrich V, Olesen J (1999). Glyceryl trinitrate induces attacks of migraine without aura in sufferers of migraine with aura. Cephalalgia 19:660–667. Discussion 626. 10.1046/j.1468-2982.1999.019007660.x [DOI] [PubMed] [Google Scholar]
- Chu DQ, Choy M, Foster P, Cao T, Brain SD (2000) A comparative study of the ability of calcitonin gene-related peptide and adrenomedullin(13–52) to modulate microvascular but not thermal hyperalgesia responses. Br J Pharmacol 130:1589–1596. 10.1038/sj.bjp.0703502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close LN, Eftekhari S, Wang M, Charles AC, Russo AF (2018) Cortical spreading depression as a site of origin for migraine: role of CGRP. Cephalalgia 333102418774299. 10.1177/0333102418774299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonna DM, Meng W, Deal DD, Busija DW (1994) Calcitonin gene-related peptide promotes cerebrovascular dilation during cortical spreading depression in rabbits. Am J Physiol 266: H1095–H1102. 10.1152/ajpheart.1994.266.3.H1095 [DOI] [PubMed] [Google Scholar]
- Cumberbatch MJ, Williamson DJ, Mason GS, Hill RG, Hargreaves RJ (1999) Dural vasodilation causes a sensitization of rat caudal trigeminal neurones in vivo that is blocked by a 5-HT1B/1D agonist. Br J Pharmacol 126:1478–1486. 10.1038/sj.bjp.0702444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutrer FM, Huerter K (2007) Migraine aura. Neurologist 13:118–125. 10.1097/01.nrl.0000252943.82792.38 [DOI] [PubMed] [Google Scholar]
- Devesa I, Ferrandiz-Huertas C, Mathivanan S, Wolf C, Lujan R, Changeux JP, Ferrer-Montiel A (2014) alphaCGRP is essential for algesic exocytotic mobilization of TRPV1 channels in peptidergic nociceptors. Proc Natl Acad Sci U S A 111:18345–18350. 10.1073/pnas.1420252111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham PL, Russo AF (2002) New insights into the molecular actions of serotonergic antimigraine drugs. Pharmacol Ther 94:77–92 [DOI] [PubMed] [Google Scholar]
- Edelmayer RM et al. (2009) Medullary pain facilitating neurons mediate allodynia in headache-related pain. Ann Neurol 65:184–193. 10.1002/ana.21537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edvinsson L (1985) Functional role of perivascular peptides in the control of cerebral circulation. Trends Neurosci 8:126–131 [Google Scholar]
- Edvinsson L (2017) The trigeminovascular pathway: role of CGRP and CGRP receptors in migraine. Headache 57(Suppl 2):47–55. 10.1111/head.13081 [DOI] [PubMed] [Google Scholar]
- Edvinsson L, Nilsson E, Jansen-Olesen I (2007) Inhibitory effect of BIBN4096BS, CGRP(8–37), a CGRP antibody and an RNA-Spiegelmer on CGRP induced vasodilatation in the perfused and non-perfused rat middle cerebral artery. Br J Pharmacol 150:633–640. 10.1038/sj.bjp.0707134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edvinsson L, Haanes KA, Warfvinge K, Krause DN (2018) CGRP as the target of new migraine therapies – successful translation from bench to clinic. Nat Rev Neurol 14:338–350. 10.1038/s41582-018-0003-1 [DOI] [PubMed] [Google Scholar]
- Escott KJ, Beattie DT, Connor HE, Brain SD (1995) Trigeminal ganglion stimulation increases facial skin blood flow in the rat: a major role for calcitonin gene-related peptide. Brain Res 669:93–99 [DOI] [PubMed] [Google Scholar]
- Feistel S, Albrecht S, Messlinger K (2013) The calcitonin gene-related peptide receptor antagonist MK-8825 decreases spinal trigeminal activity during nitroglycerin infusion. J Headache Pain 14:93 10.1186/1129-2377-14-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari MD, Klever RR, Terwindt GM, Ayata C, van den Maagdenberg AM (2015) Migraine pathophysiology: lessons from mouse models and human genetics. Lancet Neurol 14:65–80. 10.1016/S1474-4422(14)70220-0 [DOI] [PubMed] [Google Scholar]
- Filiz A, Tepe N, Eftekhari S, Boran HE, Dilekoz E, Edvinsson L, Bolay H (2017) CGRP receptor antagonist MK-8825 attenuates cortical spreading depression induced pain behavior. Cephalalgia 333102417735845. 10.1177/0333102417735845 [DOI] [PubMed] [Google Scholar]
- Gangula PR et al. (2000) Increased blood pressure in alpha-calcitonin gene-related peptide/calcitonin gene knockout mice. Hypertension 35:470–475 [DOI] [PubMed] [Google Scholar]
- Goadsby PJ, Edvinsson L (1993) The trigeminovascular system and migraine: studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann Neurol 33:48–56. 10.1002/ana.410330109 [DOI] [PubMed] [Google Scholar]
- Goadsby PJ, Edvinsson L (1994) Human in vivo evidence for trigeminovascular activation in cluster headache. Neuropeptide changes and effects of acute attacks therapies. Brain 117 (Pt 3):427–434 [DOI] [PubMed] [Google Scholar]
- Goadsby PJ, Edvinsson L, Ekman R (1988) Release of vasoactive peptides in the extracerebral circulation of humans and the cat during activation of the trigeminovascular system. Ann Neurol 23:193–196. 10.1002/ana.410230214 [DOI] [PubMed] [Google Scholar]
- Goadsby PJ, Edvinsson L, Ekman R (1990) Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann Neurol 28:183–187. 10.1002/ana.410280213 [DOI] [PubMed] [Google Scholar]
- Guo S, Vollesen ALH, Olesen J, Ashina M (2016) Premonitory and non-headache symptoms induced by CGRP and PACAP38 in patients with migraine. Pain 157:2773–2781. 10.1097/j.pain.0000000000000702 [DOI] [PubMed] [Google Scholar]
- Hansen JM, Hauge AW, Olesen J, Ashina M (2010) Calcitonin gene-related peptide triggers migraine-like attacks in patients with migraine with aura. Cephalalgia 30:1179–1186. 10.1177/0333102410368444 [DOI] [PubMed] [Google Scholar]
- Hokfelt T et al. (1992) Calcitonin gene-related peptide in the brain, spinal cord, and some peripheral systems. Ann N Y Acad Sci 657:119–134 [DOI] [PubMed] [Google Scholar]
- Huang Y, Brodda-Jansen G, Lundeberg T, Yu LC (2000) Anti-nociceptive effects of calcitonin gene-related peptide in nucleus raphe magnus of rats: an effect attenuated by naloxone. Brain Res 873:54–59 [DOI] [PubMed] [Google Scholar]
- Ishida-Yamamoto A, Tohyama M (1989) Calcitonin gene-related peptide in the nervous tissue. Prog Neurobiol 33:335–386 [DOI] [PubMed] [Google Scholar]
- Jiang L, Wang Y, Xu Y, Ma D, Wang M (2018) The transient receptor potential ankyrin type 1 plays a critical role in cortical spreading depression. Neuroscience 382:23–34. 10.1016/j.neuroscience.2018.04.025 [DOI] [PubMed] [Google Scholar]
- Kaiser EA, Kuburas A, Recober A, Russo AF (2012) Modulation of CGRP-induced light aversion in wild-type mice by a 5-HT(1B/D) agonist. J Neurosci 32:15439–15449. 10.1523/JNEUROSCI.3265-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser EA, Rea BJ, Kuburas A, Kovacevich BR, Garcia-Martinez LF, Recober A, Russo AF (2017) Anti-CGRP antibodies block CGRP-induced diarrhea in mice. Neuropeptides 64:95–99. 10.1016/j.npep.2016.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura M, Kuraishi Y, Minami M, Satoh M (1989) Antinociceptive effect of intrathecally administered antiserum against calcitonin gene-related peptide on thermal and mechanical noxious stimuli in experimental hyperalgesic rats. Brain Res 497:199–203 [DOI] [PubMed] [Google Scholar]
- Kelman L (2004) The premonitory symptoms (prodrome): a tertiary care study of 893 migraineurs. Headache 44:865–872. 10.1111/j.1526-4610.2004.04168.x [DOI] [PubMed] [Google Scholar]
- Kelman L (2007) The triggers or precipitants of the acute migraine attack. Cephalalgia 27:394–402. 10.1111/j.1468-2982.2007.01303.x [DOI] [PubMed] [Google Scholar]
- Kopruszinski CM et al. (2017) Prevention of stress- or nitric oxide donor-induced medication overuse headache by a calcitonin gene-related peptide antibody in rodents. Cephalalgia 37:560–570. 10.1177/0333102416650702 [DOI] [PubMed] [Google Scholar]
- Koulchitsky S, Fischer MJ, Messlinger K (2009) Calcitonin gene-related peptide receptor inhibition reduces neuronal activity induced by prolonged increase in nitric oxide in the rat spinal trigeminal nucleus. Cephalalgia 29:408–417. 10.1111/j.1468-2982.2008.01745.x [DOI] [PubMed] [Google Scholar]
- Kuraishi Y, Nanayama T, Ohno H, Minami M, Satoh M (1988) Antinociception induced in rats by intrathecal administration of antiserum against calcitonin gene-related peptide. Neurosci Lett 92:325–329 [DOI] [PubMed] [Google Scholar]
- Kurosawa M, Messlinger K, Pawlak M, Schmidt RF (1995) Increase of meningeal blood flow after electrical stimulation of rat dura mater encephali: mediation by calcitonin gene-related peptide. Br J Pharmacol 114:1397–1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappe RW, Slivjak MJ, Todt JA, Wendt RL (1987) Hemodynamic effects of calcitonin gene-related peptide in conscious rats. Regul Pept 19:307–312 [DOI] [PubMed] [Google Scholar]
- Lassen LH, Haderslev PA, Jacobsen VB, Iversen HK, Sperling B, Olesen J (2002) CGRP may play a causative role in migraine. Cephalalgia 22:54–61. 10.1046/j.1468-2982.2002.00310.x [DOI] [PubMed] [Google Scholar]
- Lee Y et al. (1985) Coexistence of calcitonin gene-related peptide and substance P-like peptide in single cells of the trigeminal ganglion of the rat: immunohistochemical analysis. Brain Res 330:194–196 [DOI] [PubMed] [Google Scholar]
- Levy D, Burstein R, Strassman AM (2005) Calcitonin gene-related peptide does not excite or sensitize meningeal nociceptors: implications for the pathophysiology of migraine. Ann Neurol 58:698–705. 10.1002/ana.20619 [DOI] [PubMed] [Google Scholar]
- Li N, Lundeberg T, Yu LC (2001) Involvement of CGRP and CGRP1 receptor in nociception in the nucleus accumbens of rats. Brain Res 901:161–166 [DOI] [PubMed] [Google Scholar]
- Lipton RB, Bigal ME, Diamond M, Freitag F, Reed ML, Stewart WF, Group AA (2007) Migraine prevalence, disease burden, and the need for preventive therapy. Neurology 68:343–349. 10.1212/01.wnl.0000252808.97649.21 [DOI] [PubMed] [Google Scholar]
- Loder E (2010) Triptan therapy in migraine. N Engl J Med 363:63–70. 10.1056/NEJMct0910887 [DOI] [PubMed] [Google Scholar]
- LoPinto C, Young WB, Ashkenazi A (2006) Comparison of dynamic (brush) and static (pressure) mechanical allodynia in migraine. Cephalalgia 26:852–856. 10.1111/j.1468-2982.2006.01121.x [DOI] [PubMed] [Google Scholar]
- Lu JT et al. (1999) Mice lacking alpha-calcitonin gene-related peptide exhibit normal cardiovascular regulation and neuromuscular development. Mol Cell Neurosci 14:99–120. 10.1006/mcne.1999.0767 [DOI] [PubMed] [Google Scholar]
- Luebke AE, Holt JC, Jordan PM, Wong YS, Caldwell JS, Cullen KE (2014) Loss of alpha-calcitonin gene-related peptide (alphaCGRP) reduces the efficacy of the Vestibulo-ocular Reflex (VOR). J Neurosci 34:10453–10458. 10.1523/JNEUROSCI.3336-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maison SF, Emeson RB, Adams JC, Luebke AE, Liberman MC (2003) Loss of alpha CGRP reduces sound-evoked activity in the cochlear nerve. J Neurophysiol 90:2941–2949. 10.1152/jn.00596.2003 [DOI] [PubMed] [Google Scholar]
- Marquez de Prado B, Hammond DL, Russo AF (2009) Genetic enhancement of calcitonin gene-related Peptide-induced central sensitization to mechanical stimuli in mice. J Pain 10:992–1000. 10.1016/j.jpain.2009.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti E et al. (1987) Ontogeny of peptide- and amine-containing neurones in motor, sensory, and autonomic regions of rat and human spinal cord, dorsal root ganglia, and rat skin. J Comp Neurol 266:332–359. 10.1002/cne.902660304 [DOI] [PubMed] [Google Scholar]
- Martin VT, Behbehani MM (2001) Toward a rational understanding of migraine trigger factors. Med Clin North Am 85:911–941 [DOI] [PubMed] [Google Scholar]
- Mason BN, Kaiser EA, Kuburas A, Loomis MM, Latham JA, Garcia-Martinez LF, Russo AF (2017) Induction of migraine-like photophobic behavior in mice by both peripheral and central cgrp mechanisms. J Neurosci 37:204–216. 10.1523/JNEUROSCI.2967-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew NT, Kailasam J, Seifert T (2004) Clinical recognition of allodynia in migraine. Neurology 63:848–852 [DOI] [PubMed] [Google Scholar]
- Melo-Carrillo A, Noseda R, Nir RR, Schain AJ, Stratton J, Strassman AM, Burstein R (2017a) Selective inhibition of trigeminovascular neurons by fremanezumab: a humanized monoclonal anti-CGRP. Antibody J Neurosci 37:7149–7163. 10.1523/JNEUROSCI.0576-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo-Carrillo A, Strassman AM, Nir RR, Schain AJ, Noseda R, Stratton J, Burstein R (2017b) Fremanezumab-A humanized monoclonal anti-CGRP antibody-inhibits thinly myelinated (adelta) but not unmyelinated (C) meningeal nociceptors. J Neurosci 37:10587–10596. 10.1523/JNEUROSCI.2211-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messlinger K, Hanesch U, Kurosawa M, Pawlak M, Schmidt RF (1995) Calcitonin gene related peptide released from dural nerve fibers mediates increase of meningeal blood flow in the rat. Can J Physiol Pharmacol 73:1020–1024 [DOI] [PubMed] [Google Scholar]
- Mitsikostas DD, Sanchez del Rio M, Moskowitz MA, Waeber C (1999) Both 5-HT1B and 5-HT1F receptors modulate c-fos expression within rat trigeminal nucleus caudalis. Eur J Pharmacol 369:271–277 [DOI] [PubMed] [Google Scholar]
- Mogil JS et al. (2005) Variable sensitivity to noxious heat is mediated by differential expression of the CGRP gene. Proc Natl Acad Sci U S A 102:12938–12943. 10.1073/pnas.0503264102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulleners WM, Aurora SK, Chronicle EP, Stewart R, Gopal S, Koehler PJ (2001) Self-reported photophobic symptoms in migraineurs and controls are reliable and predict diagnostic category accurately. Headache 41:31–39 [DOI] [PubMed] [Google Scholar]
- Munro G, Petersen S, Jansen-Olesen I, Olesen J (2018) A unique inbred rat strain with sustained cephalic hypersensitivity as a model of chronic migraine-like pain. Sci Rep 8:1836 10.1038/s41598-018-19901-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura-Craig M, Gill BK (1991) Effect of neurokinin A, substance P and calcitonin gene related peptide in peripheral hyperalgesia in the rat paw. Neurosci Lett 124:49–51 [DOI] [PubMed] [Google Scholar]
- Noseda R, Monconduit L, Constandil L, Chalus M, Villanueva L (2008) Central nervous system networks involved in the processing of meningeal and cutaneous inputs from the ophthalmic branch of the trigeminal nerve in the rat. Cephalalgia 28:813–824. 10.1111/j.1468-2982.2008.01588.x [DOI] [PubMed] [Google Scholar]
- Oh-hashi Y et al. (2001) Elevated sympathetic nervous activity in mice deficient in alphaCGRP. Circ Res 89:983–990 [DOI] [PubMed] [Google Scholar]
- Oku R, Satoh M, Fujii N, Otaka A, Yajima H, Takagi H (1987) Calcitonin gene-related peptide promotes mechanical nociception by potentiating release of substance P from the spinal dorsal horn in rats. Brain Res 403:350–354 [DOI] [PubMed] [Google Scholar]
- Ong JJY, Wei DY, Goadsby PJ (2018) Recent advances in pharmacotherapy for migraine prevention: from pathophysiology to new drugs. Drugs 78:411–437. 10.1007/s40265-018-0865-y [DOI] [PubMed] [Google Scholar]
- Oshinsky ML, Sanghvi MM, Maxwell CR, Gonzalez D, Spangenberg RJ, Cooper M, Silberstein SD (2012) Spontaneous trigeminal allodynia in rats: a model of primary headache. Headache 52:1336–1349. 10.1111/j.1526-4610.2012.02247.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecile A, Guidobono F, Netti C, Sibilia V, Biella G, Braga PC (1987) Calcitonin gene-related peptide: antinociceptive activity in rats, comparison with calcitonin. Regul Pept 18:189–199 [DOI] [PubMed] [Google Scholar]
- Petersen KA, Birk S, Doods H, Edvinsson L, Olesen J (2004) Inhibitory effect of BIBN4096BS on cephalic vasodilatation induced by CGRP or transcranial electrical stimulation in the rat. Br J Pharmacol 143:697–704. 10.1038/sj.bjp.0705966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen KA, Lassen LH, Birk S, Lesko L, Olesen J (2005a) BIBN4096BS antagonizes human alpha-calcitonin gene related peptide-induced headache and extracerebral artery dilatation. Clin Pharmacol Ther 77:202–213 [DOI] [PubMed] [Google Scholar]
- Petersen KA, Nilsson E, Olesen J, Edvinsson L (2005b) Presence and function of the calcitonin gene-related peptide receptor on rat pial arteries investigated in vitro and in vivo. Cephalalgia 25:424–432. 10.1111/j.1468-2982.2005.00869.x [DOI] [PubMed] [Google Scholar]
- Pradhan AA, Smith ML, McGuire B, Tarash I, Evans CJ, Charles A (2014) Characterization of a novel model of chronic migraine. Pain 155:269–274. 10.1016/j.pain.2013.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen BK (1993) Migraine and tension-type headache in a general population: precipitating factors, female hormones, sleep pattern and relation to lifestyle. Pain 53:65–72 [DOI] [PubMed] [Google Scholar]
- Rea BJ et al. (2018) Peripherally administered calcitonin gene-related peptide induces spontaneous pain in mice: implications for migraine. Pain. 10.1097/j.pain.0000000000001337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recober A, Kuburas A, Zhang Z, Wemmie JA, Anderson MG, Russo AF (2009) Role of calcitonin gene-related peptide in light-aversive behavior: implications for migraine. J Neurosci 29:8798–8804. 10.1523/JNEUROSCI.1727-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recober A, Kaiser EA, Kuburas A, Russo AF (2010) Induction of multiple photophobic behaviors in a transgenic mouse sensitized to CGRP. Neuropharmacology 58:156–165. 10.1016/j.neuropharm.2009.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter U et al. (1998) Perivascular nerves contribute to cortical spreading depression-associated hyperemia in rats. Am J Physiol 274:H1979–H1987 [DOI] [PubMed] [Google Scholar]
- Rosenfeld MG et al. (1983) Production of a novel neuropeptide encoded by the calcitonin gene via tissue-specific RNA processing. Nature 304:129–135 [DOI] [PubMed] [Google Scholar]
- Russell FA, King R, Smillie SJ, Kodji X, Brain SD (2014) Calcitonin gene-related peptide: physiology and pathophysiology. Physiol Rev 94:1099–1142. 10.1152/physrev.00034.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo AF (2015a) Calcitonin gene-related peptide (CGRP): a new target for migraine. Annu Rev Pharmacol Toxicol 55:533–552. 10.1146/annurev-pharmtox-010814-124701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo AF (2015b) CGRP as a neuropeptide in migraine: lessons from mice. Br J Clin Pharmacol 80:403–414. 10.1111/bcp.12686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo AF, Kuburas A, Kaiser EA, Raddant AC, Recober A (2009) A potential preclinical migraine model: CGRP-sensitized mice. Mol Cell Pharmacol 1:264–270 [PMC free article] [PubMed] [Google Scholar]
- Sabharwal R, Zhang Z, Lu Y, Abboud FM, Russo AF, Chapleau MW (2010) Receptor activity-modifying protein 1 increases baroreflex sensitivity and attenuates angiotensin-induced hypertension. Hypertension 55:627–635. 10.1161/HYPERTENSIONAHA.109.148171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon AM, Damaj I, Sekine S, Picciotto MR, Marubio L, Changeux JP (1999) Modulation of morphine analgesia in alphaCGRP mutant mice. Neuroreport 10:849–854 [DOI] [PubMed] [Google Scholar]
- Salmon AM, Damaj MI, Marubio LM, Epping-Jordan MP, Merlo-Pich E, Changeux JP (2001) Altered neuroadaptation in opiate dependence and neurogenic inflammatory nociception in alpha CGRP-deficient mice. Nat Neurosci 4:357–358. 10.1038/86001 [DOI] [PubMed] [Google Scholar]
- Satoh M, Kuraishi Y, Kawamura M (1992) Effects of intrathecal antibodies to substance P, calcitonin gene-related peptide and galanin on repeated cold stress-induced hyperalgesia: comparison with carrageenan-induced hyperalgesia. Pain 49:273–278 [DOI] [PubMed] [Google Scholar]
- Schorscher-Petcu A, Austin JS, Mogil JS, Quirion R (2009) Role of central calcitonin gene-related peptide (CGRP) in locomotor and anxiety- and depression-like behaviors in two mouse strains exhibiting a CGRP-dependent difference in thermal pain sensitivity. J Mol Neurosci 39:125–136. 10.1007/s12031-009-9201-z [DOI] [PubMed] [Google Scholar]
- Senba E, Tohyama M (1988) Calcitonin gene-related peptide containing autonomic efferent pathways to the pelvic ganglia of the rat. Brain Res 449:386–390 [DOI] [PubMed] [Google Scholar]
- Siren AL, Feuerstein G (1988) Cardiovascular effects of rat calcitonin gene-related peptide in the conscious rat. J Pharmacol Exp Ther 247:69–78 [PubMed] [Google Scholar]
- Skofitsch G, Jacobowitz DM (1985) Calcitonin gene-related peptide: detailed immunohistochemical distribution in the central nervous system. Peptides 6:721–745 [DOI] [PubMed] [Google Scholar]
- Sowers LP, Tye AE, Russo AF (2017) Lessons learned from CGRP mutant mice In: Dalkara T, Moskowitz MA (eds) Neurobiological basis of migraine, 1st edn. Wiley, Hoboken [Google Scholar]
- Spierings EL, Ranke AH, Honkoop PC (2001) Precipitating and aggravating factors of migraine versus tension-type headache. Headache 41:554–558 [DOI] [PubMed] [Google Scholar]
- Storer RJ, Akerman S, Goadsby PJ (2004) Calcitonin gene-related peptide (CGRP) modulates nociceptive trigeminovascular transmission in the cat. Br J Pharmacol 142:1171–1181. 10.1038/sj.bjp.0705807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stucky NL, Gregory E, Winter MK, He YY, Hamilton ES, McCarson KE, Berman NE (2011) Sex differences in behavior and expression of CGRP-related genes in a rodent model of chronic migraine. Headache 51:674–692. 10.1111/j.1526-4610.2011.01882.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun RQ, Lawand NB, Lin Q, Willis WD (2004) Role of calcitonin gene-related peptide in the sensitization of dorsal horn neurons to mechanical stimulation after intradermal injection of capsaicin. J Neurophysiol 92:320–326. 10.1152/jn.00086.2004 [DOI] [PubMed] [Google Scholar]
- Tassorelli C, Greco R, Wang D, Sandrini M, Sandrini G, Nappi G (2003) Nitroglycerin induces hyperalgesia in rats – a time-course study. Eur J Pharmacol 464:159–162 [DOI] [PubMed] [Google Scholar]
- Thomsen LL, Kruuse C, Iversen HK, Olesen J (1994) A nitric oxide donor (nitroglycerin) triggers genuine migraine attacks. Eur J Neurol 1:73–80. 10.1111/j.1468-1331.1994.tb00053.x [DOI] [PubMed] [Google Scholar]
- Troltzsch M, Denekas T, Messlinger K (2007) The calcitonin gene-related peptide (CGRP) receptor antagonist BIBN4096BS reduces neurogenic increases in dural blood flow. Eur J Pharmacol 562:103–110. 10.1016/j.ejphar.2007.01.058 [DOI] [PubMed] [Google Scholar]
- Tsujikawa K et al. (2007) Hypertension and dysregulated proinflammatory cytokine production in receptor activity-modifying protein 1-deficient mice. Proc Natl Acad Sci U S A 104:16702–16707. 10.1073/pnas.0705974104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddman R, Edvinsson L, Ekman R, Kingman T, McCulloch J (1985) Innervation of the feline cerebral vasculature by nerve fibers containing calcitonin gene-related peptide: trigeminal origin and co-existence with substance P. Neurosci Lett 62:131–136 [DOI] [PubMed] [Google Scholar]
- van Dongen RM et al. (2017) Migraine biomarkers in cerebrospinal fluid: a systematic review and meta-analysis. Cephalalgia 37:49–63. 10.1177/0333102415625614 [DOI] [PubMed] [Google Scholar]
- Vos T et al. (2012) Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the global burden of disease study 2010. Lancet 380:2163–2196. 10.1016/S0140-6736(12)61729-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl M, Schilling L, Parsons AA, Kaumann A (1994) Involvement of calcitonin gene-related peptide (CGRP) and nitric oxide (NO) in the pial artery dilatation elicited by cortical spreading depression. Brain Res 637:204–210 [DOI] [PubMed] [Google Scholar]
- Wang Y, Li Y, Wang M (2016) Involvement of CGRP receptors in retinal spreading depression. Pharmacol Rep 68:935–938. 10.1016/j.pharep.2016.05.001 [DOI] [PubMed] [Google Scholar]
- Weidner C et al. (2000) Acute effects of substance P and calcitonin gene-related peptide in human skin – a microdialysis study. J Invest Dermatol 115:1015–1020. 10.1046/j.1523-1747.2000.00142.x [DOI] [PubMed] [Google Scholar]
- Wiesenfeld-Hallin Z, Hokfelt T, Lundberg JM, Forssmann WG, Reinecke M, Tschopp FA, Fischer JA (1984) Immunoreactive calcitonin gene-related peptide and substance P coexist in sensory neurons to the spinal cord and interact in spinal behavioral responses of the rat. Neurosci Lett 52:199–204 [DOI] [PubMed] [Google Scholar]
- Williamson DJ, Hargreaves RJ, Hill RG, Shepheard SL (1997) Sumatriptan inhibits neurogenic vasodilation of dural blood vessels in the anaesthetized rat – intravital microscope studies. Cephalalgia 17:525–531. 10.1046/j.1468-2982.1997.1704525.x [DOI] [PubMed] [Google Scholar]
- Williamson DJ, Hill RG, Shepheard SL, Hargreaves RJ (2001) The anti-migraine 5-HT(1B/1D) agonist rizatriptan inhibits neurogenic dural vasodilation in anaesthetized guinea-pigs. Br J Pharmacol 133:1029–1034. 10.1038/sj.bjp.0704162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Lundeberg T, Wang YT, Li Y, Yu LC (2003) Antinociceptive effect of calcitonin gene-related peptide in the central nucleus of amygdala: activating opioid receptors through amygdala-periaqueductal gray pathway. Neuroscience 118:1015–1022 [DOI] [PubMed] [Google Scholar]
- Yao G, Huang Q, Wang M, Yang CL, Liu CF, Yu TM (2017) Behavioral study of a rat model of migraine induced by CGRP. Neurosci Lett 651:134–139. 10.1016/j.neulet.2017.04.059 [DOI] [PubMed] [Google Scholar]
- Yisarakun W, Chantong C, Supornsilpchai W, Thongtan T, Srikiatkhachorn A, Reuangwechvorachai P, Maneesri-le Grand S (2015) Up-regulation of calcitonin gene-related peptide in trigeminal ganglion following chronic exposure to paracetamol in a CSD migraine animal model. Neuropeptides 51:9–16. 10.1016/j.npep.2015.03.008 [DOI] [PubMed] [Google Scholar]
- Yu LC, Weng XH, Wang JW, Lundeberg T (2003) Involvement of calcitonin gene-related peptide and its receptor in anti-nociception in the periaqueductal grey of rats. Neurosci Lett 349:1–4 [DOI] [PubMed] [Google Scholar]
- Zagami AS, Goadsby PJ, Edvinsson L (1990) Stimulation of the superior sagittal sinus in the cat causes release of vasoactive peptides. Neuropeptides 16:69–75 [DOI] [PubMed] [Google Scholar]
- Zhang L, Hoff AO, Wimalawansa SJ, Cote GJ, Gagel RF, Westlund KN (2001) Arthritic calcitonin/alpha calcitonin gene-related peptide knockout mice have reduced nociceptive hypersensitivity. Pain 89:265–273 [DOI] [PubMed] [Google Scholar]
- Zhang Z, Dickerson IM, Russo AF (2006) Calcitonin gene-related peptide receptor activation by receptor activity-modifying protein-1 gene transfer to vascular smooth muscle cells. Endocrinology 147:1932–1940. 10.1210/en.2005-0918 [DOI] [PubMed] [Google Scholar]
- Zhang Z, Winborn CS, Marquez de Prado B, Russo AF (2007) Sensitization of calcitonin gene-related peptide receptors by receptor activity-modifying protein-1 in the trigeminal ganglion. J Neurosci 27:2693–2703. 10.1523/JNEUROSCI.4542-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Li JJ, Yu LC (2003) Plastic changes of calcitonin gene-related peptide in morphine tolerance: behavioral and immunohistochemical study in rats. J Neurosci Res 74:622–629. 10.1002/jnr.10770 [DOI] [PubMed] [Google Scholar]


