Abstract
With an aging and obese population, chronic wounds such as diabetic ulcers, pressure ulcers, and venous leg ulcers are of an increasingly relevant medical concern in the developed world. Identification of bacterial biofilm contamination as a major contributor to non-healing wounds demands biofilm-targeted strategies to treat chronic wounds. While the current standard of care has proven marginally effective, there are components of standard care that should remain part of the wound treatment regime including systemic and topical antibiotics, antiseptics, and physical debridement of biofilm and devitalized tissue. Emerging anti-biofilm strategies include novel, non-invasive means of physical debridement, chemical agent strategies, and biological agent strategies. While aging and obesity will continue to be major burdens to wound care, the emergence of wounds associated with war require investigation and biotechnology development to address biofilm strategies that manage multi-drug resistant bacteria contaminating the chronic wound. The article presents some of the recent patents related to anti-biofilm strategy in wound care.
Keywords: Bacterial biofilm, wound care, therapy
INTRODUCTION
While quality of life has vastly improved with advances in medicine and nutrition in the developed world, the related rise in obesity and the aging population threaten to reverse these advances and place a major burden on the already overwhelmed healthcare system. Chronic wounds such as diabetic foot ulcers, pressure ulcers, and venous leg ulcers have contributed to a steep rise in medical costs [1]. In the United States alone, an estimated $58 billion in medical costs is associated with chronic complications that afflict nearly 18 million diabetics [2]. Current standard of care for non-healing wounds consists of transiently effective systemic and topical antibiotic treatment often followed by amputation. An estimated 14–24% of diabetic patients in the United States will undergo amputation [1] and must suffer the resultant co-morbidities. There is clearly a need for targeted, effective therapy for the rising problem of chronic wounds.
Chronic and acute wounds initially progress through the same stages of healing [3]. In normal wound healing, the wound will progress through hemostasis, inflammation, granulation, epithelialization, and maturation; however, in the chronic wound, resolution of inflammation does not occur and the wound remains in a persistent state of “acute” inflammation [4]. Typical of unresolved inflammation, the chronic wound is characterized by prolonged expression of the inflammatory cytokines interleukin-1 [IL-1] and tumor necrosis factor-alpha [TNF-α] [5, 6]. While many factors may contribute to the abnormal condition of the chronic wound, it has become increasingly clear that bioburden is a significant barrier to normal wound healing [7]. That bioburden includes both devitalized tissue and colonizing microorganisms, including bacteria organized into biofilms (Fig. 1).
Fig. (1). The biofilm hypothesis as it applies to chronic wounds.
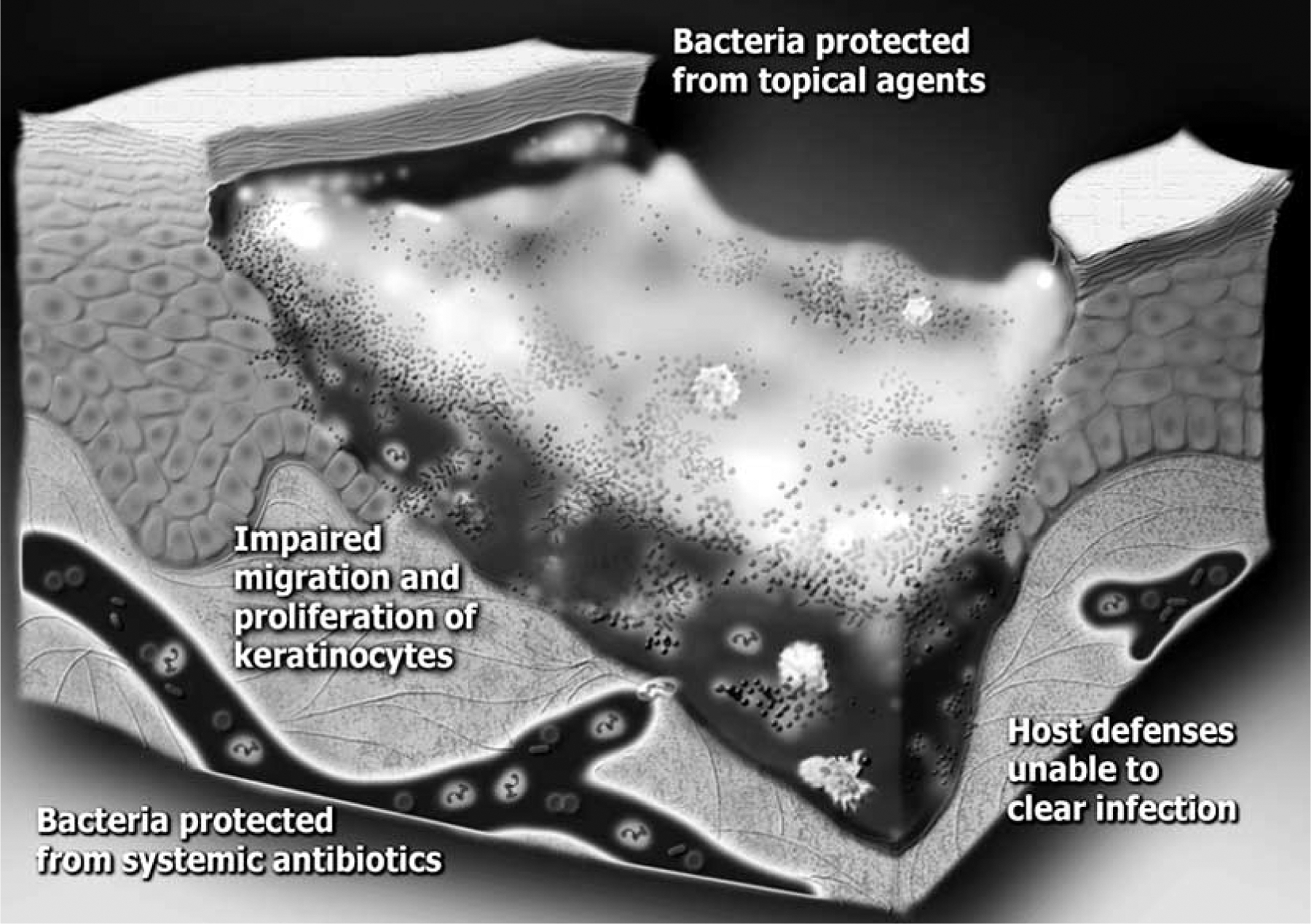
In a chronic wound, bacterial colonization of the wound bed progresses to biofilm formation. The infection persists because microorganisms in biofilms are resistant to killing by systemic antibiotics, topical antiseptics, and components of the host immune system. The presence of biofilm impairs the normal wound healing process resulting in a chronic wound.
Biofilms are structured communities of microorganisms, organized into microcolonies, adhered to a surface, and exhibiting phenotypic heterogeneity [8]. In the chronic wound, bacterial contamination develops into colonization, and devitalized tissue provides the surface to which biofilms adhere. Unlike planktonic bacteria, bacterial biofilms consist of about 80% extracellular polymeric substances [EPS] consisting of polysaccharides, proteins, and nucleic acids and about 20% bacterial cells [9]. Additionally, bacterial biofilms are typically multi-species [8]. In the chronic wound, non-healing is dependent on the bacteria successfully establishing biofilm growth [8, 10, 11]. It has been well demonstrated that the establishment of bacterial biofilm in the wound is the major reason for the failure of acute wound treatment and development of chronic, non-healing wounds [4, 12–14]. In the United States alone, the National Institutes of Health estimates that 80% of microbial inflections could be characterized as biofilms [4, 15]. In animal chronic wound models, biofilm formation in the wound is associated with unregulated inflammation and delayed or altered wound healing [16]. Clearly, therapies addressing wound healing must account for the presence of contaminating biofilm and directly incorporate strategies that target bacterial biofilm.
Treatment of bacterial biofilm in the wound is complicated by the very character of the biofilm mode of growth including increased resistance of the biofilm to traditional antimicrobial treatments and host immune defense [10, 15, 17–19]. Multiple characteristics of the biofilm contribute to this resistance. Traditional antibiotics have been designed against planktonically-grown bacteria and treat metabolically active bacteria; however, bacteria in a biofilm are metabolically different from planktonic bacteria [18] and within the biofilm, the metabolic activity of the bacteria can change significantly with highly metabolically active cells at the surface of the biofilm and metabolically inert cells deep within the biofilm [15, 20]. Furthermore, mixed species biofilms have complementary metabolic strategies for obtaining nutrients and for degradation of host immune molecules [21–24]. Further protection of the biofilm is provided by the physical barrier of the EPS surrounding the bacterial cells [9, 15] and by the presence of metabolically inactive persister cells deep within the biofilm [25]. Finally, the multispecies biofilm community creates an efficient platform for the exchange of drug resistance genes [26, 27]. In designing novel therapies for wounds, it is necessary to consider and account for the unique ability of the biofilm to resist treatment.
The present review aims to provide a perspective on the emergence of novel wound treatments designed to address the presence of bacterial biofilm contamination in the wound. There are many wound treatment strategies both in development and out on the market and it is beyond the scope of this review to comprehensively cover all these strategies, rather it is the intention of this review to provide a sampling of biofilm targeted strategies for wound healing.
CURRENT STANDARD OF CARE
Current standard of care includes, but is not limited to, the use of antiseptics and both topical and systemic antibiotics in combination with wound dressings that may or may not be designed with anti-biofilm characteristics. While topical antiseptics can be bactericidal [28], antiseptics can damage host cells such as fibroblasts and keratinocytes and may thus interfere with the normal wound healing process [29]. While not necessarily designed to be anti-microbial, wound dressings help reduce bacterial load and acute infection rates [30, 31]. The physical presence of the wound dressing, regardless of whether the dressing is designed with anti-biofilm characteristics, does appear to inhibit microbial colonization in the wound by eliminating pockets of open space at the wound surface [32, 33]; however, the physical interaction between the wound bed, the wound dressing, and colonizing bacteria remains an area of limited research.
As mentioned above, current antibiotics may have little long-term effect at preventing or treating the established biofilm as most of these antibiotics are designed to target metabolically active bacteria and dormant cells within the bacterial biofilm are unresponsive [9]. However, in the case of deep tissue wounds, systemic antibiotics are warranted to prevent systemic bacterial invasion and sepsis [28]. Conversely, when treating or preventing the establishment of bacterial biofilms within the wound, system antibiotics have been found to be only 25–32% efficacious [34, 35]. Furthermore, use of systemic antibiotics is problematic with ischemic wounds because of the lack of sufficient circulation at the wound site [28]. Although current standard of care clearly has some role in wound treatment, there is a need for more tools in the wound care toolbox.
BIOFILM CONTROL STRATEGIES IN WOUND CARE
Physical Strategies of Biofilm Management
Bioburden in the wound consists of both contaminating biofilm and devitalized tissue; removal of this bioburden not only reduces the contaminating bacteria, but also revitalizes the host immune defenses. Sharp physical debridement of the wound bed significantly reduces the microorganisms in the wound bed, removes devitalized host tissue, and is thus a vital step in biofilm control in wound care [36–38]. Standardization of physical debridement will greatly enhance the quality of wound care. To that end, recent patents on wound debridement have been submitted. For example, a method, device, and kit for lesion debridement was filed in 2005 [39]. Sharp debridement can be very painful and comprehensive debridement is dependent on the clinician; therefore, alternative methods of debridement have recently shown some interest. For example, pulsed electrical fields have been used to disaggregate bacterial biofilm [40] and use of acoustic shock waves to eradicate or prevent biofilm formation has been submitted for patent [40] as well as use of ultrasound for debriding wounds [41]. Pulsed ultrasound for biofilm disruption has been supported in vitro and its use in patients has been associated with decreased bioburden; however, a direct demonstration of the efficacy of ultrasound debridement has not yet been achieved in vivo [42, 43]. While these methods are promising as non-invasive means of debridement, their efficacy has yet to be proven in the clinic.
Chemical Strategies of Biofilm Management
*Ionic Silver
Use of ionic silver has become increasingly popular in the wound care industry and there are many wound dressings on the market that contain silver either covalently bound or as nanocrystaline particles. The large variation in silver content, silver release, and antibacterial activity between various silver containing dressings make identifying the most efficacious dressing for a wound condition difficult. Although silver dressings have been demonstrated as effective against biofilms in vitro [44, 45], there remains some debate as to whether enough ionic silver is released from silver containing dressings into the wound bed in order to treat biofilms present in the chronic wound [46]. Regardless of the variation on the market, ionic silver has been demonstrated to be bactericidal in very low concentrations and to be efficacious against multiple species of pathogenic bacteria [47, 48]. Use of silver containing materials against biofilms has been patented for use with medical devices [49]. Of recent concern, is the potential for damage to host keratinocytes with the use of high silver-containing wound dressings [50]
*Iodine
Iodine is a naturally occurring, though unstable, chemical element that has been used as a disinfectant for acute wounds for many years. While commonly used, the long-term antimicrobial efficacy of iodine remains debatable and as an anti-biofilm strategy concerns about the chemical stability of iodine remain. Of further concern is the potentially toxic effect of iodine on host cells [29, 51]. To address concerns of chemical stability, elemental iodine has been complexed with polyvinylpyrrolidone [PVP] to get providone-iodine [PVD-I]. Use of providone-iodine has been demonstrated as microbicidal on Staphylococcus epidermidis biofilms in vitro [52] and may damage the host cells less than elemental iodine [53]. Use of providone-iodine in a composition for managing bacterial biofilm has been patented [54] in addition to an older patent using providone-iodine for wound-healing preparations [55]. To make water-soluble iodine, cadexomer iodine is produced by a reaction of dextran with epichlorhydrin and iodine. While cadexomer iodine has been demonstrated as effective as part of the comprehensive treatment of venous leg ulcers [56], more recently it has been demonstrated to be directly microbicidal against Staphylococcus aureus biofilms in vitro [57]. Although iodine has been around for quite a while, the efficacy of iodine against bacterial biofilm remains to be established in vivo.
*Gallium
Gallium is a chemical element that does not occur in nature as elemental gallium, but rather as the gallium [III] salt. Because gallium is very similar to iron on an atomic scale, gallium ion can localize and interact with biological systems dependent on iron [III]. The bactericidal character of gallium is thought to result from gallium disruption of iron[III]-dependent biological processes in bacteria including respiration [58]. Gallium nitrate is FDA approved for clinical use to treat hypercalcemia associated with tumor metastasis to bone [59], and has been shown to interfere with biofilm development [28]. More recently, gallium has been investigated as an anti-biofilm strategy in cystic fibrosis and has been used effectively against biofilms comprised of the major cystic fibrosis pathogen Pseudomonas aeruginosa [60]. Recent patents have been filed claiming use of gallium against oral biofilms [61], use against antibiotic resistant pathogens [62], and use for coating medical devices to prevent biofilm formation [63]. Use of gallium as a topical wound treatment strategy hold promise; however, more research is necessary considering the pharmacokinetics of gallium [64].
*EDTA
Ethylenediaminetetraacetic acid [EDTA] is a polyamino carboxylic acid that chelates metal ions such as calcium[II] and iron[III]. EDTA has been used as an antibacterial strategy for over forty years and acts as a microbicide primarily through the ability to chelate iron and interfere with iron[III]-dependent biological pathways in bacteria [65]. While EDTA has been used extensively in the clinic to treat lead and heavy metal poisoning [66], more recently EDTA has been used therapeutically for coronary heart disease [67]. Disodium EDTA was demonstrated to inhibit Staphylococcus epidermidis attachment to medical catheters in vitro over twenty years ago [68]; however, more recently tetrasodium EDTA showed a broad spectrum inhibitory effect against in vivo generated biofilms attached to catheters [69, 70]. Finally, incorporation of EDTA into a wound gel enhanced the efficacy of the gel against Pseudomonas aeruginosa biofilms [71]. Because of its observed antimicrobial properties, use of EDTA as part of an antiseptic composition for use against biofilms has been patented [72]. Although EDTA has been used medically for years, concerns remain regarding the effect of EDTA on the host [73].
*General Biocides
While bactericidal activity cannot be disregarded in anti-biofilm strategies, the EPS characteristic of the biofilm plays an important role in biofilm resistance to antimicrobials. No matter how effective an anti-microbial is at killing bacteria, it will be virtually useless if it is unable to penetrate into the biofilm. Therefore, dispersion or disaggregation of biofilms by chemically removing the EPS renders the biofilm more susceptible to antibiotic treatment [15]. Quaternary ammonium compounds are a general biocide that increases biofilm cell susceptibility to antimicrobial peptides and antibiotics by chemically changing the biofilm structure [74]. Another general biocide is the bismuth thiols, which suppress bacterial exopolysaccharide expression in Klebsiella, Staphylococcus, and Pseudomonas sp. [75]. This ability to enhance the susceptibility of bacterial biofilm to antimicrobials makes the bismuth thiols an interesting biofilm control strategy and therefore patent submission has been made on the use of bismuth thiols for medical products [76]. Although EPS dispersal is an intriguing strategy for biofilm management in the wound, combined treatment with an antimicrobial would also be necessary to inhibit bacterial contamination into the wound.
Biological Agent Strategies of Biofilm Management
*Honey
Honey has been used medicinally by indigenous cultures for hundreds, perhaps thousands of years; however, recent investigations into the use of honey as a treatment for wounds suggest that honey may indeed have antimicrobial and pro-healing effects. Killing of Pseudomonas aeruginosa and Staphylococcus aureus biofilms in vitro was successfully demonstrated with two types of honey, Sidr and Manuka honeys. As has been observed for many antimicrobials, efficacy against planktonic culture was notably better for both honeys when compared to efficacy against biofilm culture [77]. Honey may also mediate pro-healing effects on host cells as in vitro studies with monocytic cell lines found that honey enhances release of inflammatory cytokines associated with innate immunity [78, 79]. Although it remains unclear as to how honey mediates biofilm control, it has been suggested that key mechanisms may include changes in osmotic potential and activity of phytochemicals [80]. Regardless of the mechanism of action, the recent, successful use of honey to treat wounds has lead to patent submission of a honey-based wound dressing [81].
*Lactoferrin
Lactoferrin is an iron chelating protein found in most bodily fluids, but concentrated in milk [82]. Lactoferrin has been determined to be effective at killing both planktonic and biofilm bacteria [83, 84]. Additionally, lactoferrin has been demonstrated in vitro to prevent Pseudomonas aeruginosa biofilm formation by preventing bacterial adhesion to a surface, the essential first step in biofilm formation [85]. Iron[III] binding by lactoferrin can have a bacteriostatic effect on bacteria by depriving the cells of this essential nutrient [86], but may also contribute to destabilization of the bacterial membrane [84]. In cultures with planktonic cells, lactoferrin has also been demonstrated to bind the lipopolysacchride component of the bacterial outer membrane causing membrane permeablization and cell death in Gram-negative bacteria [83]. Because of its multifaceted nature, lactoferrin is broad spectrum and has been used effectively to control in vitro biofilms consisting of periodontal pathogens [87], cystic fibrosis-associated pathogens [88], and atopic skin-associated pathogens [89]. In the clinic, lactoferrin has been used successfully as part of a comprehensive treatment regime to manage biofilm-associated chronic rhinosubusitis [90] and biofilm-associated ischemic wounds [36]. Because of the demonstrated efficacy of lactoferrin against biofilm-forming pathogens, patents describing the use of lactoferrin in treating wounds have been submitted [91, 92].
*Xylitol
Xylitol is a sugar alcohol found in a limited number of fruits and vegetables [93]. The majority of research concerning the antimicrobial properties of xylitol has been centered on oral biofilms [94]. For example, xylitol inhibited biofilm growth in a six species oral biofilm model [95] and inhibited expression of metabolizing enzymes required for surface adhesion of carriogenic streptococci [96]. Accordingly, a patent has been filed on inventions that make claims on the use of xylitol with oral products [97]. Use of xylitol to treat oral biofilms lead to the fortuitous discovery that oral use of xylitol reduces the incidence of recurrent otitis [98]. Subsequent use of xylitol in the nasal passage demonstrated that the xylitol could inhibit bacterial adhesion to the nasal mucosa and lead to the patent submission for a xylitol-based nasal spray [99]. Additionally, treatment of atopic dry skin with xylitol was effective in preventing colonization by Staphylococcus aureus [100] possibly through inhibition of bacterial glycolysis [101]. Xylitol was also recently demonstrated to be mildly efficacious against wound colonizing Pseudomonas aeruginosa biofilms in vitro [84] and patent claims have been filed on the use of xylitol to prevent or control biofilms in chronic wounds [91].
*Dispersin B
Dispersin B is a naturally occurring N-acetylglucosa-minodase produced by the periodontal pathogen Aggregatibacter actinomycetemcomitans [102]. Although the endogenous role of Dispersin B is debatable, it has been demonstrated to inhibit biofilm formation [103–105]. Principally studied for use in dentistry, Dispersin B disseminates bacterial biofilm by targeting the EPS and degrading the biofilm community structure [106]. Specifically, Dispersin B hydrolyzes glycosidic linkages in the polysaccharide of the EPS to destabilize the biofilm framework [107–111]. These investigations led to the submission of a patent for the use of Dispersin B to detach bacterial and fungal biofilms [112]. Although use of Dispersin B is unlike other biofilm strategies in that the intention is not to kill the bacteria but rather to break up the structure of the biofilm, this enzymatic disruption of the would be beneficial in combination with a microbicidal agent for managing bacterial biofilm contamination within the wound.
*Bacteriophages
Bacteriophages are viruses that infect bacteria and have recently become of renewed interest to the field of biofilm control due to their ability to lyse bacteria [113]. Bacteriophages are highly abundant and can be isolated from anywhere bacteria exist: from the soil to the human intestine [114]. Because bacteriophages only infect bacteria, therapy with bacteriophages targets sights of bacterial colonization [115]. Use of bacteriophages to control systemic bacterial infections has been successful with treatment of meningitis and septicemia [116], and bacteriophages have been demonstrated to be effective against biofilms both through disruption of the EPS and lysing of the biofilm-associated bacterial cells [117]. This suggests that use of bacteriophages to manage colonizing bacteria in the wound may prove both safe and efficacious; however, there remains concern that bacterial lysis by the bacteriophages will release endotoxin into the wound resulting in non-specific and unrestrained activation of innate immunity and the inflammatory response. Despite potential concerns regarding the patenting of living organisms, the medical use of bacteriophage to inhibit biofilm formation has been submitted as a patent [118].
*Quorum Sensing Inhibitors
Quorum sensing by bacterial species allows for coordinated gene expression based on the density of the bacterial population. Inter-and intra-species bacterial communication and genetic synchrony through quorum sensing appear to play an important role in biofilm formation [119]. Because of this apparent requirement for quorum sensing in biofilm development, inhibition of quorum sensing has developed into an area of biofilm management strategy [120]. Additionally, patents have been filed in which the invention is either a compound designed to inhibit bacterial quorum sensing [121] or a series of synthetic ligands intended to act either as agonists or antagonists to bacterial quorum sensing networks [122]. The Gram-negative bacterial quorum sensing system primarily consists of networks mediated by acylhomoserin lactone and has been identified in more than seventy species [123]. Therefore, patent filing has occurred for the use of synthetic ligands designed to modulate quorum sensing mediated through the acylhomoserin lactone pathway [124]. Other signaling molecules found in Gram-negative bacteria include autoinducers [2 and 3], diffusible signal factor, and cyclic dipeptide; however, autoinducer 2 [AI-2] has best been demonstrated as required for mixed species biofilm formation [125]. To manage bacterial biofilms, the use of halogenated furanone compounds has been investigated [126]; however there is some concern with the use of furanone compounds due to the potential for host damage [127]. Autoinducer-2 analogues have also been patented for regulating bacterial growth and pathogenesis including biofilm formation [128]. Although use of quorum sensing inhibitors may prove efficacious for management of bacterial biofilms in wounds, their efficacy and safety in vivo remains to be substantiated.
CURRENT & FUTURE DEVELOPMENTS
Although the epidemic increase in obesity and the comorbidities associated with the development of obesity-related diabetes must remain a major area of research and technology development, another area of tremendous importance in wound care has recently emerged. In modern combat, wounds that once would have been fatal are now being survived, but at a great cost to the wounded. Combat wounds are distinct from diabetic wounds and particularly challenging in part because of high contamination, the devitalized nature of the wounds, and delays to medical treatment. On the other hand, chronic war wounds are similar to chronic civilian wounds in that a major barrier to healing is mediated by the presence of bacterial biofilm, in particular multi-drug resistant Gram-negative bacteria [16, 129]. Not only are soldiers and war wounded civilians receiving wounds in the field, but are also at high risk of developing hospital-associated infections [130]. Unfortunately, war continues and regardless of the political and social complications of war, comprehensive treatment of the war wounded is an essentially moral responsibility for the biomedical discipline. Clearly, there is a need for cost effective, highly stable, biofilm targeted strategies to manage wounds received on the field and developed in hospitals.
Part of the difficulty of treating war wounds is the highly recalcitrant character of bacterial biofilm and the development of multi-drug resistance; therefore, there is a need for medical biotechnology that targets these issues. In order to manage the development of resistance, biofilm targeted strategies would best be designed with multiple targets of bacterial inhibition. A patented example of the use of combined treatment for managing bacterial biofilm in wounds is the use of lactoferrin and xylitol [91]. While both lactoferrin and xylitol are naturally occurring antimicrobials, bacteria have evolved mechanisms of resistance to the use of either of these antimicrobials alone [131–133]; however, when used in combination, lactoferrin and xylitol are significantly more efficacious against establish biofilm in vitro than when used alone [84]. Because lactoferrin and xylitol have been utilized by nature to combat bacteria, the use of these antimicrobials in combined therapy provides a cost-effective, easily obtained, biofilm-targeted strategy for treatment of both civilian chronic wounds and the war wounded. In conclusion, more strategies are needed in which integrated, biofilm-targeted therapies are used to manage the presence of bacterial biofilm in the wound.
Table 1.
Biofilm Control Strategies for Wound Care and Associated Example Patents
| Biofilm Control Stategy | Control Agent | Example Patent |
|---|---|---|
| Physical | Manual debridement | US20070135706 (2007) |
| Pulse electrical field | US20070239073 (2007) | |
| Ultrasound debridement | US20080183109 (2008) | |
| Chemical | Ionic silver | US20040131698 (2004) |
| Iodine | US20020037260 (2002) | |
| US4844898 (1989) | ||
| Gallium | US20070231406 (2007) | |
| US20080241275 (2008) | ||
| US20060018945 (2006) | ||
| EDTA | US20040110841 (2004) | |
| Bismuth thiols | US20080181950 (2008) | |
| Biological | Honey | US20056956144 (2005) |
| Lactoferrin | US20070116750 (2007) | |
| US20080318834 (2008) | ||
| Xylitol | US5536511 (1996) | |
| US5719196 (1998) | ||
| US20016258372 (2001) | ||
| Dispersin B | US20077294497 (2007) | |
| Bacteriophage | US20090191254 (2009) | |
| Quorum sensing inhibitor | EP1475092 (2004) | |
| US20060052426 (2006) | ||
| US20080312319 (2008) | ||
| US20036559176 (2003) |
ACKNOWLEDGEMENT
Dr. Ammons is a research scientist in the Center for Biofilm Engineering at Montana State University and her work is supported by Glanbia Nutritionals Inc., 523 6th Street, Monroe, WI 53566, USA and by Montana State University, Bozeman, MT 59717, USA.
Footnotes
CONFLICT OF INTEREST
The author declares no conflict of interest.
REFERENCES
- [1].James GA, Swogger E, Wolcott R, et al. Biofilms in chronic wounds. Wound Repair Regen 2008; 16(1): 37–44. [DOI] [PubMed] [Google Scholar]
- [2].Economic costs of diabetes in the U.S. in 2007. Diabetes care 2008; 31(3): 596–615. [DOI] [PubMed] [Google Scholar]
- [3].McGuckin M, Goldman R, Bolton L, Salcido R. The clinical relevance of microbiology in acute and chronic wounds. Adv Skin Wound Care 2003; 16(1): 12–23; [quiz 4–5]. [DOI] [PubMed] [Google Scholar]
- [4].Bjarnsholt T, Kirketerp-Moller K, Jensen PO, et al. Why chronic wounds will not heal: A novel hypothesis. Wound Repair Regen 2008; 16(1): 2–10. [DOI] [PubMed] [Google Scholar]
- [5].Konturek PC, Brzozowski T, Konturek SJ, Kwiecien S, Dembinski A, Hahn EG. Influence of bacterial lipopolysaccharide on healing of chronic experimental ulcer in rat. Scand J Gastroenterol 2001; 36(12): 1239–1247. [DOI] [PubMed] [Google Scholar]
- [6].Power C, Wang JH, Sookhai S, Street JT, Redmond HP. Bacterial wall products induce downregulation of vascular endothelial growth factor receptors on endothelial cells via a CD14-dependent mechanism: Implications for surgical wound healing. J Surg Res 2001; 101(2): 138–145. [DOI] [PubMed] [Google Scholar]
- [7].White RJ, Cutting KF. Critical colonization--the concept under scrutiny. Ostom/Wound Manage 2006; 52(11): 50–56. [PubMed] [Google Scholar]
- [8].Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: A common cause of persistent infections. Science 1999; 21; 284 (5418): 1318–1322. [DOI] [PubMed] [Google Scholar]
- [9].Costerton JW, Stewart PS. Battling biofilms. Sci Am 200; 285(1): 74–81. [DOI] [PubMed] [Google Scholar]
- [10].Donlan RM, Costerton JW. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev 2002; 15(2): 167–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Parsek MR, Singh PK. Bacterial biofilms: An emerging link to disease pathogenesis. Ann Rev Microbiol 2003; 57: 677–701. [DOI] [PubMed] [Google Scholar]
- [12].Davis SC, Ricotti C, Cazzaniga A, Welsh E, Eaglstein WH, Mertz PM. Microscopic and physiologic evidence for biofilm-associated wound colonization in vivo. Wound Repair Regen 2008; 16(1): 23–29. [DOI] [PubMed] [Google Scholar]
- [13].Harrison-Balestra C, Cazzaniga AL, Davis SC, Mertz PM. A wound-isolated Pseudomonas aeruginosa grows a biofilm in vitro within 10 hours and is visualized by light microscopy. Dermatol Surg 2003; 29(6): 631–635. [DOI] [PubMed] [Google Scholar]
- [14].James GA, Swogger E, Wolcott R, et al. Biofilms in chronic wounds. Wound Repair Regen 2008; 16(1): 37–44. [DOI] [PubMed] [Google Scholar]
- [15].Davies D Understanding biofilm resistance to antibacterial agents. Nat Rev Drug Discov 2003; 2(2): 114–122. [DOI] [PubMed] [Google Scholar]
- [16].Aronson NE, Sanders JW, Moran KA. In harm’s way: Infections in deployed American military forces. Clin Infect Dis 2006; 43(8): 1045–1051. [DOI] [PubMed] [Google Scholar]
- [17].Fux CA, Costerton JW, Stewart PS, Stoodley P. Survival strategies of infectious biofilms. Trends Microbiol 2005; 13(1): 34–40. [DOI] [PubMed] [Google Scholar]
- [18].Davey ME, O’Toole GA. Microbial biofilms: From ecology to molecular genetics. Microbiol Mol Biol Rev 2000; 64(4): 847–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Donlan RM. Role of biofilms in antimicrobial resistance. ASAIO J 2000; 46(6): S47–52. [DOI] [PubMed] [Google Scholar]
- [20].Sternberg C, Christensen BB, Johansen T, et al. Distribution of bacterial growth activity in flow-chamber biofilms. Appl Environ Microbiol 1999; 65(9): 4108–4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Marsh PD. Microbial ecology of dental plaque and its significance in health and disease. Adv Dent Res 1994; 8(2): 263–271. [DOI] [PubMed] [Google Scholar]
- [22].Rickard AH, Gilbert P, High NJ, Kolenbrander PE, Handley PS. Bacterial coaggregation: An integral process in the development of multi-species biofilms. Trends Microbiol 2003; 11(2): 94–100. [DOI] [PubMed] [Google Scholar]
- [23].Oggioni MR, Trappetti C, Kadioglu A, et al. Switch from planktonic to sessile life: A major event in pneumococcal pathogenesis. Mol Microbiol 2006; 61(5): 1196–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Burmolle M, Webb JS, Rao D, Hansen LH, Sorensen SJ, Kjelleberg S. Enhanced biofilm formation and increased resistance to antimicrobial agents and bacterial invasion are caused by synergistic interactions in multispecies biofilms. Appl Environ Microbiol 2006; 72(6): 3916–3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Keren I, Shah D, Spoering A, Kaldalu N, Lewis K. Specialized persister cells and the mechanism of multidrug tolerance in Escherichia coli. J Bacteriol 2004; 186(24): 8172–8180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Cookson B Clinical significance of emergence of bacterial antimicrobial resistance in the hospital environment. J Appl Microbiol 2005; 99(5): 989–996. [DOI] [PubMed] [Google Scholar]
- [27].Davies J Inactivation of antibiotics and the dissemination of resistance genes. Science 1994; 264(5157): 375–382. [DOI] [PubMed] [Google Scholar]
- [28].Rhoads DD, Wolcott RD, Percival SL. Biofilms in wounds: Management strategies. J Wound Care 2008; 17(11): 502–508. [DOI] [PubMed] [Google Scholar]
- [29].Wilson JR, Mills JG, Prather ID, Dimitrijevich SD. A toxicity index of skin and wound cleansers used on in vitro fibroblasts and keratinocytes. Adv Skin Wound Care 2005; 18(7): 373–378. [DOI] [PubMed] [Google Scholar]
- [30].Mertz PM, Eaglstein WH. The effect of a semiocclusive dressing on the microbial population in superficial wounds. Arch Surg 1984; 119(3): 287–289. [DOI] [PubMed] [Google Scholar]
- [31].White RJ, Cutting K, Kingsley A. Topical antimicrobials in the control of wound bioburden. Ostomy/Wound Manage 2006; 52(8): 26–58. [PubMed] [Google Scholar]
- [32].Percival SL, Bowler PG, Dolman J. Antimicrobial activity of silver-containing dressings on wound microorganisms using an in vitro biofilm model. Int Wound J 2007; 4(2): 186–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Newman GR, Walker M, Hobot JA, Bowler PG. Visualisation of bacterial sequestration and bactericidal activity within hydrating Hydrofiber wound dressings. Biomaterials 2006; 27(7): 1129–1139. [DOI] [PubMed] [Google Scholar]
- [34].Moss AH, Vasilakis C, Holley JL, Foulks CJ, Pillai K, McDowell DE. Use of a silicone dual-lumen catheter with a Dacron cuff as a long-term vascular access for hemodialysis patients. Am J Kidney Dis 1990; 16(3): 211–215. [DOI] [PubMed] [Google Scholar]
- [35].Marr KA, Sexton DJ, Conlon PJ, Corey GR, Schwab SJ, Kirkland KB. Catheter-related bacteremia and outcome of attempted catheter salvage in patients undergoing hemodialysis. Ann Intern Med 1997; 127(4): 275–280. [DOI] [PubMed] [Google Scholar]
- [36].Wolcott RD, Rhoads DD. A study of biofilm-based wound management in subjects with critical limb ischaemia. J Wound Care 2008; 17(4): 145–148, 50–52, 54–55. [DOI] [PubMed] [Google Scholar]
- [37].Schultz GS, Barillo DJ, Mozingo DW, Chin GA. Wound bed preparation and a brief history of time. Int Wound J 2004; 1(1): 19–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Gray M Is larval (maggot) debridement effective for removal of necrotic tissue from chronic wounds? J Wound Ostomy Continence Nurs 2008; 35(4): 378–384. [DOI] [PubMed] [Google Scholar]
- [39].Shimko DA, Olson SW Jr., Nycz JH: US20070135706 (2007).
- [40].Schaden W, Schultheiss R, Warlick J: US20070239073 (2007).
- [41].Babaev E: US20080183109 (2008).
- [42].Ensing GT, Roeder BL, Nelson JL, et al. Effect of pulsed ultrasound in combination with gentamicin on bacterial viability in biofilms on bone cements in vivo. J Appl Microbiol 2005; 99(3): 443–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ensing GT, Neut D, van Horn JR, van der Mei HC, Busscher HJ. The combination of ultrasound with antibiotics released from bone cement decreases the viability of planktonic and biofilm bacteria: an in vitro study with clinical strains. J Antimicrob Chemother 2006; 58(6): 1287–1290. [DOI] [PubMed] [Google Scholar]
- [44].Jones SA, Bowler PG, Walker M, Parsons D. Controlling wound bioburden with a novel silver-containing Hydrofiber dressing. Wound Repair Regen 2004; 12(3): 288–294. [DOI] [PubMed] [Google Scholar]
- [45].Percival SL, Bowler P, Woods EJ. Assessing the effect of an antimicrobial wound dressing on biofilms. Wound Repair Regen 2008; 16(1): 52–57. [DOI] [PubMed] [Google Scholar]
- [46].Bjarnsholt T, Kirketerp-Moller K, Kristiansen S, et al. Silver against Pseudomonas aeruginosa biofilms. APMIS 2007; 115(8): 921–928. [DOI] [PubMed] [Google Scholar]
- [47].Russell AD, Hugo WB. Antimicrobial activity and action of silver. Prog Med Chem 1994; 31: 351–370. [DOI] [PubMed] [Google Scholar]
- [48].Lansdown AB, Sampson B, Laupattarakasem P, Vuttivirojana A. Silver aids healing in the sterile skin wound: Experimental studies in the laboratory rat. Br J Dermatol 1997; 137(5): 728–735. [PubMed] [Google Scholar]
- [49].Gillis SH, Schechter P, Stiles JAR: US20040131698 (2004).
- [50].Poon VK, Burd A. in vitro Cytotoxity of silver: Implication for clinical wound care. Burns 2004; 30(2): 140–147. [DOI] [PubMed] [Google Scholar]
- [51].Kramer SA. Effect of povidone-iodine on wound healing: A review. J Vasc Nurs 1999; 17(1): 17–23. [DOI] [PubMed] [Google Scholar]
- [52].Presterl E, Suchomel M, Eder M, et al. Effects of alcohols, povidone-iodine and hydrogen peroxide on biofilms of Staphylococcus epidermidis. J Antimicrob Chemother 2007; 60(2): 417–420. [DOI] [PubMed] [Google Scholar]
- [53].Niedner R Cytotoxicity and sensitization of povidone-iodine and other frequently used anti-infective agents. Dermatology 1997; 195 (Suppl 2): 89–92. [DOI] [PubMed] [Google Scholar]
- [54].Budney JA, Budny MJ: US20020037260 (2002).
- [55].Komori S, Muramatsu T: US4844898 (1989).
- [56].Skog E, Arnesjo B, Troeng T, et al. A randomized trial comparing cadexomer iodine and standard treatment in the out-patient management of chronic venous ulcers. Br J Dermatol 1983; 109(1): 77–83. [DOI] [PubMed] [Google Scholar]
- [57].Akiyama H, Oono T, Saito M, Iwatsuki K. Assessment of cadexomer iodine against Staphylococcus aureus biofilm in vivo and in vitro using confocal laser scanning microscopy. J Dermatol 2004; 31(7): 529–534. [DOI] [PubMed] [Google Scholar]
- [58].Chitambar CR, Narasimhan J. Targeting iron-dependent DNA synthesis with gallium and transferrin-gallium. Pathobiology 1991; 59(1): 3–10. [DOI] [PubMed] [Google Scholar]
- [59].Warrell RP Jr, Bockman RS, Coonley CJ, Isaacs M, Staszewski H. Gallium nitrate inhibits calcium resorption from bone and is effective treatment for cancer-related hypercalcemia. J Clin Invest 1984; 73(5): 1487–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Kaneko Y, Thoendel M, Olakanmi O, Britigan BE, Singh PK. The transition metal gallium disrupts Pseudomonas aeruginosa iron metabolism and has antimicrobial and antibiofilm activity. J Clin Invest 2007; 117(4): 877–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Bucalo LR, Schwendner S, Wirtz UF, Sreedharan S: US20070231406 (2007).
- [62].Perl DP, Moalem S: US20080241275 (2008).
- [63].Britigan BE, Singh PK: US20060018945 (2006).
- [64].Bernstein LR, Tanner T, Godfrey C, Noll B. Chemistry and pharmacokinetics of gallium maltolate, a compound with high oral gallium bioavailability. Met Based Drugs 2000; 7(1): 33–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Gray GW, Wilkinson SG. The effect of ethylenediaminetetra-acetic acid on the cell walls of some gram-negative bacteria. J Gen Microbiol 1965; 39(3): 385–399. [DOI] [PubMed] [Google Scholar]
- [66].Aaseth J Recent advance in the therapy of metal poisonings with chelating agents. Hum Toxicol 1983; 2(2): 257–272. [DOI] [PubMed] [Google Scholar]
- [67].Ernst E Chelation therapy for coronary heart disease: An overview of all clinical investigations. Am Heart J 2000; 140(1): 139–141. [DOI] [PubMed] [Google Scholar]
- [68].Root JL, McIntyre OR, Jacobs NJ, Daghlian CP. Inhibitory effect of disodium EDTA upon the growth of Staphylococcus epidermidis in vitro: Relation to infection prophylaxis of Hickman catheters. Antimicrob Agents Chemother 1988; 32(11): 1627–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Kite P, Eastwood K, Sugden S, Percival SL. Use of in vivo-generated biofilms from hemodialysis catheters to test the efficacy of a novel antimicrobial catheter lock for biofilm eradication in vitro. J Clin Microbiol 2004; 42(7): 3073–3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Percival SL, Bowler PG, Russell D. Bacterial resistance to silver in wound care. J Hosp Infect 2005; 60(1): 1–7. [DOI] [PubMed] [Google Scholar]
- [71].Martineau L, Dosch HM. Biofilm reduction by a new burn gel that targets nociception. J Appl Microbiol 2007; 103(2): 297–304. [DOI] [PubMed] [Google Scholar]
- [72].Kite P, Hatton D: US20040110841 (2004).
- [73].Stoiber T, Bonacker D, Bohm KJ, et al. Disturbed microtubule function and induction of micronuclei by chelate complexes of mercury(II). Mutat Res 2004; 563(2): 97–106. [DOI] [PubMed] [Google Scholar]
- [74].Leung KP, Crowe TD, Abercrombie JJ, et al. Control of oral biofilm formation by an antimicrobial decapeptide. J Dental Res 2005; 84(12): 1172–1177. [DOI] [PubMed] [Google Scholar]
- [75].Domenico P, Baldassarri L, Schoch PE, Kaehler K, Sasatsu M, Cunha BA. Activities of bismuth thiols against Staphylococci and Staphylococcal biofilms. Antimicrob Agents Chemother 2001; 45(5): 1417–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Bates BL, Hiles MC, Johnson CE: US20080181950 (2008).
- [77].Alandejani T, Marsan J, Ferris W, Slinger R, Chan F. Effectiveness of honey on Staphylococcus aureus and Pseudomonas aeruginosa biofilms. Otolaryngol Head Neck Surg 2009; 141(1): 114–118. [DOI] [PubMed] [Google Scholar]
- [78].Tonks A, Cooper RA, Price AJ, Molan PC, Jones KP. Stimulation of TNF-alpha release in monocytes by honey. Cytokine 2001; 14(4): 240–242. [DOI] [PubMed] [Google Scholar]
- [79].Tonks AJ, Cooper RA, Jones KP, Blair S, Parton J, Tonks A. Honey stimulates inflammatory cytokine production from monocytes. Cytokine 2003; 21(5): 242–247. [DOI] [PubMed] [Google Scholar]
- [80].Molan PC. The evidence supporting the use of honey as a wound dressing. Int J Low Extrem Wounds 2006; 5(1): 40–54. [DOI] [PubMed] [Google Scholar]
- [81].Molan PC: US20056956144 (2005).
- [82].Valenti P, Berlutti F, Conte MP, Longhi C, Seganti L. Lactoferrin functions: Current status and perspectives. J Clin Gastroenterol 2004; 38(Suppl 6): S127–129. [DOI] [PubMed] [Google Scholar]
- [83].Brandenburg K, Jurgens G, Muller M, Fukuoka S, Koch MH. Biophysical characterization of lipopolysaccharide and lipid A inactivation by lactoferrin. Biol Chem 2001; 382(8): 1215–1225. [DOI] [PubMed] [Google Scholar]
- [84].Ammons MC, Ward LS, Fisher ST, Wolcott RD, James GA. in vitro Susceptibility of established biofilms composed of a clinical wound isolate of Pseudomonas aeruginosa treated with lactoferrin and xylitol. Int J Antimicrob Agents 2009; 33(3): 230–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Singh PK, Parsek MR, Greenberg EP, Welsh MJ. A component of innate immunity prevents bacterial biofilm development. Nature 2002; 417(6888): 552–555. [DOI] [PubMed] [Google Scholar]
- [86].Weinberg ED. Human lactoferrin: A novel therapeutic with broad spectrum potential. J Pharm Pharmacol 2001; 53(10): 1303–1310. [DOI] [PubMed] [Google Scholar]
- [87].Wakabayashi H, Yamauchi K, Kobayashi T, Yaeshima T, Iwatsuki K, Yoshie H. Inhibitory effects of lactoferrin on growth and biofilm formation of Porphyromonas gingivalis and Prevotella intermedia. Antimicrob Agents Chemother 2009; 53(8): 3308–3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].O’May CY, Sanderson K, Roddam LF, Kirov SM, Reid DW. Iron-binding compounds impair Pseudomonas aeruginosa biofilm formation, especially under anaerobic conditions. J Med Microbiol 2009; 58(Pt 6): 765–773. [DOI] [PubMed] [Google Scholar]
- [89].Leitch EC, Willcox MD. Elucidation of the antistaphylococcal action of lactoferrin and lysozyme. J Med Microbiol 1999; 48(9): 867–871. [DOI] [PubMed] [Google Scholar]
- [90].Psaltis AJ, Wormald PJ, Ha KR, Tan LW. Reduced levels of Lactoferrin in biofilm-associated chronic rhinosinusitis. Laryngoscope 2008; 118(5): 895–901. [DOI] [PubMed] [Google Scholar]
- [91].Wolcott R: US20070116750 (2007).
- [92].Cadee JA, Tips PD, Van Someren GD: US20080318834 (2008).
- [93].Granstrom TB, Izumori K, Leisola M. A rare sugar xylitol. Part II: Biotechnological production and future applications of xylitol. Appl Microbiol Biotechnol 2007; 74(2): 273–276. [DOI] [PubMed] [Google Scholar]
- [94].Burt BA. The use of sorbitol- and xylitol-sweetened chewing gum in caries control. J Am Dent Assoc 2006; 137(2): 190–196. [DOI] [PubMed] [Google Scholar]
- [95].Badet C, Furiga A, Thebaud N. Effect of xylitol on an in vitro model of oral biofilm. Oral Health Prev Dent 2008; 6(4): 337–341. [PubMed] [Google Scholar]
- [96].Shemesh M, Tam A, Feldman M, Steinberg D. Differential expression profiles of Streptococcus mutans ftf, gtf and vicR genes in the presence of dietary carbohydrates at early and late exponential growth phases. Carbohydr Res 2006; 341(12): 2090–2097. [DOI] [PubMed] [Google Scholar]
- [97].Yatka RJ: US5536511 (1996).
- [98].Uhari MK, Kontiokari TT: US5719196 (1998).
- [99].Jones AH: US20016258372 (2001).
- [100].Katsuyama M, Ichikawa H, Ogawa S, Ikezawa Z. A novel method to control the balance of skin microflora. Part 1. Attack on biofilm of Staphylococcus aureus without antibiotics. J Dermatol Sci 2005; 38(3): 197–205. [DOI] [PubMed] [Google Scholar]
- [101].Katsuyama M, Kobayashi Y, Ichikawa H, et al. A novel method to control the balance of skin microflora Part 2. A study to assess the effect of a cream containing farnesol and xylitol on atopic dry skin. J Dermatol Sci 2005; 38(3): 207–213. [DOI] [PubMed] [Google Scholar]
- [102].Ramasubbu N, Thomas LM, Ragunath C, Kaplan JB. Structural analysis of dispersin B, a biofilm-releasing glycoside hydrolase from the periodontopathogen Actinobacillus actinomycetemcomitans. J Mol Biol 2005; 349(3): 475–486. [DOI] [PubMed] [Google Scholar]
- [103].Chaignon P, Sadovskaya I, Ragunah C, Ramasubbu N, Kaplan JB, Jabbouri S. Susceptibility of Staphylococcal biofilms to enzymatic treatments depends on their chemical composition. App Microbiol Biotechnol 2007; 75(1): 125–132. [DOI] [PubMed] [Google Scholar]
- [104].Izano EA, Sadovskaya I, Vinogradov E, et al. Poly-N-acetylglucosamine mediates biofilm formation and antibiotic resistance in Actinobacillus pleuropneumoniae. Microb Pathog 2007; 43(1): 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Izano EA, Sadovskaya I, Wang H, et al. Poly-N-acetylglucosamine mediates biofilm formation and detergent resistance in Aggregatibacter actinomycetemcomitans. Microb Pathog 2008; 44(1): 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Itoh Y, Wang X, Hinnebusch BJ, Preston JF 3rd, Romeo T. Depolymerization of beta-1, 6-N-acetyl-D-glucosamine disrupts the integrity of diverse bacterial biofilms. J Bacteriol 2005; 187(1): 382–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Izano EA, Wang H, Ragunath C, Ramasubbu N, Kaplan JB. Detachment and killing of Aggregatibacter actinomycetemcomitans biofilms by dispersin B and SDS. J Dental Res 2007; 86(7): 618–622. [DOI] [PubMed] [Google Scholar]
- [108].Kaplan JB, Fine DH. Biofilm dispersal of Neisseria subflava and other phylogenetically diverse oral bacteria. Appl Environ Microbiol 2002; 68(10): 4943–4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Kaplan JB, Meyenhofer MF, Fine DH. Biofilm growth and detachment of Actinobacillus actinomycetemcomitans. J Bacteriol 2003; 185(4): 1399–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Kaplan JB, Ragunath C, Ramasubbu N, Fine DH. Detachment of Actinobacillus actinomycetemcomitans biofilm cells by an endogenous beta-hexosaminidase activity. J Bacteriol 2003; 185(16): 4693–4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Kaplan JB, Ragunath C, Velliyagounder K, Fine DH, Ramasubbu N. Enzymatic detachment of Staphylococcus epidermidis biofilms. Antimicrob Agents Chemother 2004; 48(7): 2633–2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Kaplan JB: US20077294497 (2007).
- [113].O’Flaherty S, Ross RP, Coffey A. Bacteriophage and their lysins for elimination of infectious bacteria. FEMS Microbiol Rev 2009; 33(4): 801–819. [DOI] [PubMed] [Google Scholar]
- [114].Abedon ST. Phage evolution and ecology. Adv Appl Microbiol 2009; 67: 1–45. [DOI] [PubMed] [Google Scholar]
- [115].Smith HW, Huggins MB. Successful treatment of experimental Escherichia coli infections in mice using phage: its general superiority over antibiotics. J Gen Microbiol 1982; 128(2): 307–318. [DOI] [PubMed] [Google Scholar]
- [116].Barrow P, Lovell M, Berchieri A Jr. Use of lytic bacteriophage for control of experimental Escherichia coli septicemia and meningitis in chickens and calves. Clin Diagn Lab Immunol 1998; 5(3): 294–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Hughes KA, Sutherland IW, Jones MV. Biofilm susceptibility to bacteriophage attack: The role of phage-borne polysaccharide depolymerase. Microbiol 1998; 144 (Pt 11): 3039–3047. [DOI] [PubMed] [Google Scholar]
- [118].Curtin JJ, Donlan RM: US20090191254 (2009).
- [119].Hentzer M, Wu H, Andersen JB, et al. Attenuation of Pseudomonas aeruginosa virulence by quorum sensing inhibitors. EMBO J 2003; 22(15): 3803–3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Hentzer M, Eberl L, Nielsen J, Givskov M. Quorum sensing : A novel target for the treatment of biofilm infections. BioDrugs 2003; 17(4): 241–250. [DOI] [PubMed] [Google Scholar]
- [121].Ammendola A, Aulinger-Fuchs K, Gotschlich A, Lang M, Saeb W, Sinks U, Wuzik A: EP1475092 (2004).
- [122].Despeyroux P, Frehel D, Schoentjes B, Van Dorsselaer V: US20060052426 (2006).
- [123].Federle MJ, Bassler BL. Interspecies communication in bacteria. J Clin Investig 2003; 112(9): 1291–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Blackwell HE, Geske GD, O’Neill JC: US20080312319 (2008).
- [125].Gonzalez JE, Keshavan ND. Messing with bacterial quorum sensing. Microbiol Mol Biol Rev 2006; 70(4): 859–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Hentzer M, Riedel K, Rasmussen TB, et al. Inhibition of quorum sensing in Pseudomonas aeruginosa biofilm bacteria by a halogenated furanone compound. Microbiology 2002; 148(Pt 1): 87–102. [DOI] [PubMed] [Google Scholar]
- [127].Baveja JK, Willcox MD, Hume EB, Kumar N, Odell R, Poole-Warren LA. Furanones as potential anti-bacterial coatings on biomaterials. Biomaterials 2004; 25(20): 5003–5012. [DOI] [PubMed] [Google Scholar]
- [128].Bassler BL, Dammel C, Schauder S, Shokat K, Stein J, Surette MG: US20036559176 (2003).
- [129].Yun HC, Murray CK, Roop SA, Hospenthal DR, Gourdine E, Dooley DP. Bacteria recovered from patients admitted to a deployed U.S. military hospital in Baghdad, Iraq. Mil Med 2006; 171(9): 821–825. [DOI] [PubMed] [Google Scholar]
- [130].Petersen K, Riddle MS, Danko JR, et al. Trauma-related infections in battlefield casualties from Iraq. Ann Surg 2007; 245(5): 803–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Trahan L, Mouton C. Selection for Streptococcus mutans with an altered xylitol transport capacity in chronic xylitol consumers. J Dental Res 1987; 66(5): 982–988. [DOI] [PubMed] [Google Scholar]
- [132].Trahan L, Soderling E, Drean MF, Chevrier MC, Isokangas P. Effect of xylitol consumption on the plaque-saliva distribution of mutans Streptococci and the occurrence and long-term survival of xylitol-resistant strains. J Dental Res 1992; 71(11): 1785–1791. [DOI] [PubMed] [Google Scholar]
- [133].Vogel L, Geluk F, Jansen H, Dankert J, van Alphen L. Human lactoferrin receptor activity in non-encapsulated Haemophilus influenzae. FEMS Microbiol Lett 1997; 156(1): 165–170. [DOI] [PubMed] [Google Scholar]


