Abstract
The medical impact of bacterial biofilms has increased with the recognition of biofilms as a major contributor to chronic wounds such as diabetic foot ulcers, venous leg ulcers and pressure ulcers. Traditional methods of treatment have proven ineffective, therefore this article presents in vitro evidence to support the use of novel antimicrobials in the treatment of Pseudomonas aeruginosa biofilm. An in vitro biofilm model with a clinical isolate of P. aeruginosa was subjected to treatment with either lactoferrin or xylitol alone or in combination. Combined lactoferrin and xylitol treatment disrupted the structure of the P. aeruginosa biofilm and resulted in a >2log reduction in viability. In situ analysis indicated that while xylitol treatment appeared to disrupt the biofilm structure, lactoferrin treatment resulted in a greater than two-fold increase in the number of permeabilised bacterial cells. The findings presented here indicated that combined treatment with lactoferrin and xylitol significantly decreases the viability of established P. aeruginosa biofilms in vitro and that the antimicrobial mechanism of this treatment includes both biofilm structural disruption and permeablisation of bacterial membranes.
Keywords: Pseudomonas aeruginosa, Biofilm, Lactoferrin, Xylitol
1. Introduction
In the developed world, epidemic obesity and an aging population are becoming a costly combination. As a result, expanding medical costs involve cardiovascular disease and diabetes-associated chronic wounds, including diabetic foot ulcers, pressure ulcers and venous leg ulcers [1]. Wounds are a major contributor to the estimated US$ 58 billion in medical costs in the USA related to chronic complications affecting an estimated 17.5 million diabetics [2], with an estimated 2% of the US population afflicted by non-healing wounds [3]. Current standards of treatment generally consist of transiently effective antibiotic treatment and amputation, resulting in an estimated 14–24% of diabetic patients who will undergo amputation over the course of their disease [1]. Clearly there is a need for new, cost-effective, targeted therapy to address the growing problem of chronic wounds.
Chronic and acute wounds progress through the same stages of healing, including haemostasis, inflammation, granulation, epithet-lialisation and maturation [4]; however, chronic wounds tend to be stuck in a persistent inflammatory stage [5] with chronicity initiated by persistent levels of bacteria, resulting in prolonged, elevated expression of inflammatory cytokines [6,7]. Although bacterial infection has been a known contributor to chronic wounds, recent evidence suggests that chronicity of wounds is dependent on the biofilm mode of growth of the infecting bacteria [8–10]. The biofilm mode of growth is defined as a structured community of cells, organised into microcolonies, adhered to a surface and exhibiting phenotypic heterogeneity [8]. In chronic wounds, the biofilm mode of growth is characterised by adherence to biotic or abiotic surfaces, slow development of overt symptoms, lack of resolution by the host defence and resistance to antibiotic therapy [8].
Whilst the human body co-exists daily in a symbiotic relationship with 1014 microorganisms [11] and intact skin contains microcolonies exceeding 105 bacteria [12], over one-half of the infectious diseases afflicting mildly to severely immunocompromised individuals involve bacteria that are either commensal with humans or are common in the environment, including the aquatic bacterium Pseudomonas aeruginosa [8]. More than one-half of chronic wounds are colonised by P. aeruginosa [13]; for example, in venous leg ulcers P. aeruginosa was identified as a predominate genus, and pseudomonads were shown to be a top species in diabetic foot ulcers [14]. Pseudomonas aeruginosa clearly plays an important role in the pathogenesis of chronically infected wounds.
Biofilm diseases are only transiently responsive to antimicrobial therapy [10]. The inherently defensive character of the biofilm is demonstrated by enhanced persistence of bacteria grown in the biofilm model versus bacteria grown planktonically. For example, biofilms have been shown to be more than 1000 times more resistant to antibiotics than planktonic culture [15]. With the increasing prevalence of chronic wounds infected with biofilm bacteria and the concurrent finding that antibiotics are at best transiently effective against bacterial biofilms, new tools need to be added to the medical arsenal.
Previously, Singh et al. [16] demonstrated the efficacy of lactoferrin in blocking biofilm development by P. aeruginosa. Lactoferrin is an abundant, iron-binding protein of the innate immune system (reviewed in [17]). The importance of lactoferrin for innate immune defence is demonstrated by the recurrent infections suffered by patients with inherited or acquired deficiencies in the production of lactoferrin [18].
Lactoferrin is a multifaceted protein and has a diverse role in innate immunology, from inhibition of neutrophil priming by bacterial lipopolysaccaride [19], to enhancing neutrophil adherence to endothelial cells [20] and modulating inflammation by amplifying apoptotic signals [21].
Although the mechanism of lactoferrin antimicrobial activity is also likely multifaceted [22], it has been demonstrated that lactoferrin can work synergistically [23–25]. A potentially synergistic antimicrobial partner to lactoferrin is the rare sugar alcohol xylitol. Found naturally in small quantities in fruits and vegetables [26], xylitol has been demonstrated to suppress bacterial biofilm formation and to act synergistically with farnesol, an alcohol found in many essential oils [27]. Here we examined the potentially synergistic relationship between lactoferrin and xylitol in reducing the viability of a chronic wound-derived P. aeruginosa biofilm grown in vitro.
2. Material and methods
2.1. Bacteria and media
For flow cell reactors, an overnight culture of clinical wound isolate P. aeruginosa 215 (Southwest Regional Wound Clinic, Lubbock, TX) was grown in 10% tryptic soy broth (TSB) at 37 °C and used to inoculate the reactor. 10% TSB was used for both batch mode and flow mode of growth for the flow cell reactors. Pseudomonas aeruginosa 215 overnight culture was grown in 100% brain–heart infusion (BHI) broth at 37 °C and used to inoculate 10% BHI broth at room temperature for CDC reactors. For lactoferrin and/or xylitol treatments, 2% (w/v) lactoferrin (Bioferrin®; Glanbia Nutritionals Inc., Monroe, WI) and/or 5% (w/v) xylitol (Sigma–Aldrich, St. Louis, MO) were added to the flow medium (10% BHI broth).
2.2. Biofilm growth and biofilm reactors
For the lactoferrin penetration assay, biofilms were grown with a flow cell system. Two glass flow cell chambers (model BST FC 271; BioSurface Technologies Inc., Bozeman, MT) were utilised with a microscope glass coverslip as the substratum. Flow chambers were inoculated with overnight P. aeruginosa culture in 10% TSB.
Following a 2 h incubation period with no flow to allow bacterial adherence, flow was initiated at a rate of 0.75 mL/min for 3 days using 10% TSB. After biofilm formation, fluorescently labelled lactoferrin was introduced into the flow chambers and slides were visualised by epifluorescent microscopy.
For biofilm treatment assays, P. aeruginosa was grown in CDC reactors (BioSurface Technologies Inc., Bozeman, MT) as described in ASTM Standard #E2562–07 [28], with some modifications. An overnight culture of P. aeruginosa 215 was used to inoculate the sterilised reactor containing 500 mL of 10% BHI. A 24-h batch allowed establishment of biofilms on reactor coupons before flow was initiated at 2.7 mL/min. CDC reactors were run in flow mode for 24 h with appropriate treatments. For plate count viability assays, coupons were collected in 10 mL of sterile phosphate-buffered saline (PBS) solution, vortexed for 10 s, sonicated for 2 min and then vortexed again for 10 s. Cells were serially diluted and plated on 100% tryptic soy agar. Colony-forming units were then counted and the log density of viable bacterial cells within the treated biofilm was calculated. Data are reported as log reduction normalised to the log density calculated for the control sample.
For in situ epifluorescent quantification of viability, 1 mL of disaggregated biofilm suspension was filtered onto black polycarbonate membranes and stained for viability [28]. CDC coupons were also collected, washed in sterile PBS and used to visualise biofilm in situ by epifluorescence.
2.3. Fluorescent staining
Lactoferrin (Bioferrin®) was directly labelled utilising the Alexa Fluor® 680 Protein Labeling Kit according to the manufacturer’s protocol (Molecular Probes, Eugene, OR).
Cell membrane integrity was assayed using the BacLight™ LIVE/DEAD® Bacterial Viability Kit (Molecular Probes). The nucleic acid stains in this kit enable differentiation between cells with intact versus permeablised membranes. Biofilms were either directly stained on the coupon using 2μL of each component per mL of sterile filtered water, or 1 mL of disaggregated biofilm was stained with 6 μL of each component before being adhered to the membrane. Direct staining of total biofilm mass on coupon was achieved using 10 μg/mL of 4′,6-diamidino-2-phenylindole (DAPI) stain (Pierce, Rockford, IL) in sterile filtered water. DAPI staining does not distinguish between live and dead cells, but rather allows for the visualisation of total cell mass. Stains were allowed to incubate for 15 min at room temperature before excess stain was washed off using sterile filtered water.
2.4. Imaging
Biofilm samples were imaged using a Nikon Eclipse E800 with a 60× water emersion objective for directly imaging the coupons and a 100× oil objective for imaging the bacteria adhered to the membranes. Images were collected using MetaVue and analysed using MetaMorph (Molecular Devices Corp., Downingtown, PA). For quantification of percent area of live/dead cells, at least three random images were analysed for each stain for at least three coupons per experiment. Data presented are representative of repeated experiments.
2.5. Statistical analysis
The density recorded for each coupon was log10-transformed. The log density was converted to log reduction as a measure of recovered cell viability. The log reduction is the mean log density for control coupons minus the mean log density for the corresponding treated coupons. Live/dead ratio was determined by dividing percent area threshold of the live stain by the percent area threshold of the dead stain in each sample. Relative intact versus permeabilised cells was calculated by the percent area threshold of the treated samples relative to the percent area threshold of the control samples.
Statistical analysis was performed using one-way analysis of variance (ANOVA) performed on indicated data sets. Post-test analysis used Tukey’s pairwise comparisons (GraphPad Prism Software, San Diego, CA). Pair-wise comparisons with differences at P < 0.05 were considered statistically significant.
3. Results
3.1. Lactoferrin penetrates into established Pseudomonas aeruginosa biofilms
Because lactoferrin is a large protein (ca. 80 kDa) and has an amphipathic structure similar to cationic peptides [22], it was essential to identify whether lactoferrin could even penetrate through the matrix of an established P. aeruginosa biofilm. To ascertain the ability of lactoferrin to penetrate the P. aeruginosa matrix, established biofilms were exposed to fluorescently labelled lactoferrin. Cross-sectional imaging of the biofilm after 1 h of exposure to fluorescently labelled lactoferrin demonstrated that lactoferrin was able to penetrate into the interior of the biofilm structure (Fig. 1).
Fig. 1.

Penetration of lactoferrin into established Pseudomonas aeruginosa biofilm. Left panel, transmission light microscopy imaging through a single plane indicates the microcolony structure of biofilms. Right panel, epifluorescent microscopy imaging of a single plane through the microcolonies indicates that Alexa Fluor®-labelled lactoferrin can penetrate into the interior of the biofilm within 1 h.
3.2. Lactoferrin and xylitol treatment significantly reduce viability in a biofilm-grown clinical isolate of Pseudomonas aeruginosa
Having established the ability of lactoferrin to penetrate into the interior of the P. aeruginosa biofilm, we next assayed the antimicrobial efficacy of lactoferrin and xylitol. Biofilms of a P. aeruginosa clinical wound isolate were established in vitro utilising the CDC reactor biofilm model. Biofilms were grown under standard in vitro conditions prior to 24 h treatment with either lactoferrin or xylitol alone or in combination. Direct visualisation of the total biomass of the treated biofilms is shown in Fig. 2, where the in situ biofilms have been stained with the nucleic acid dye DAPI. In the upper left panel, a distinct biofilm structure was observed in the untreated control. Whilst biofilms treated with xylitol exhibit weakened biofilm structure (upper right panel), the lactoferrin-treated sample retained a distinct biofilm structure (lower left panel). Note that the lactoferrin-treated sample appeared cloudy surrounding the biofilm structure. Combined treatment of the established biofilm with both lactoferrin and xylitol showed a nearly complete disruption of the biofilm structure with few bacterial cells still adherent to the growth surface (lower right panel).
Fig. 2.
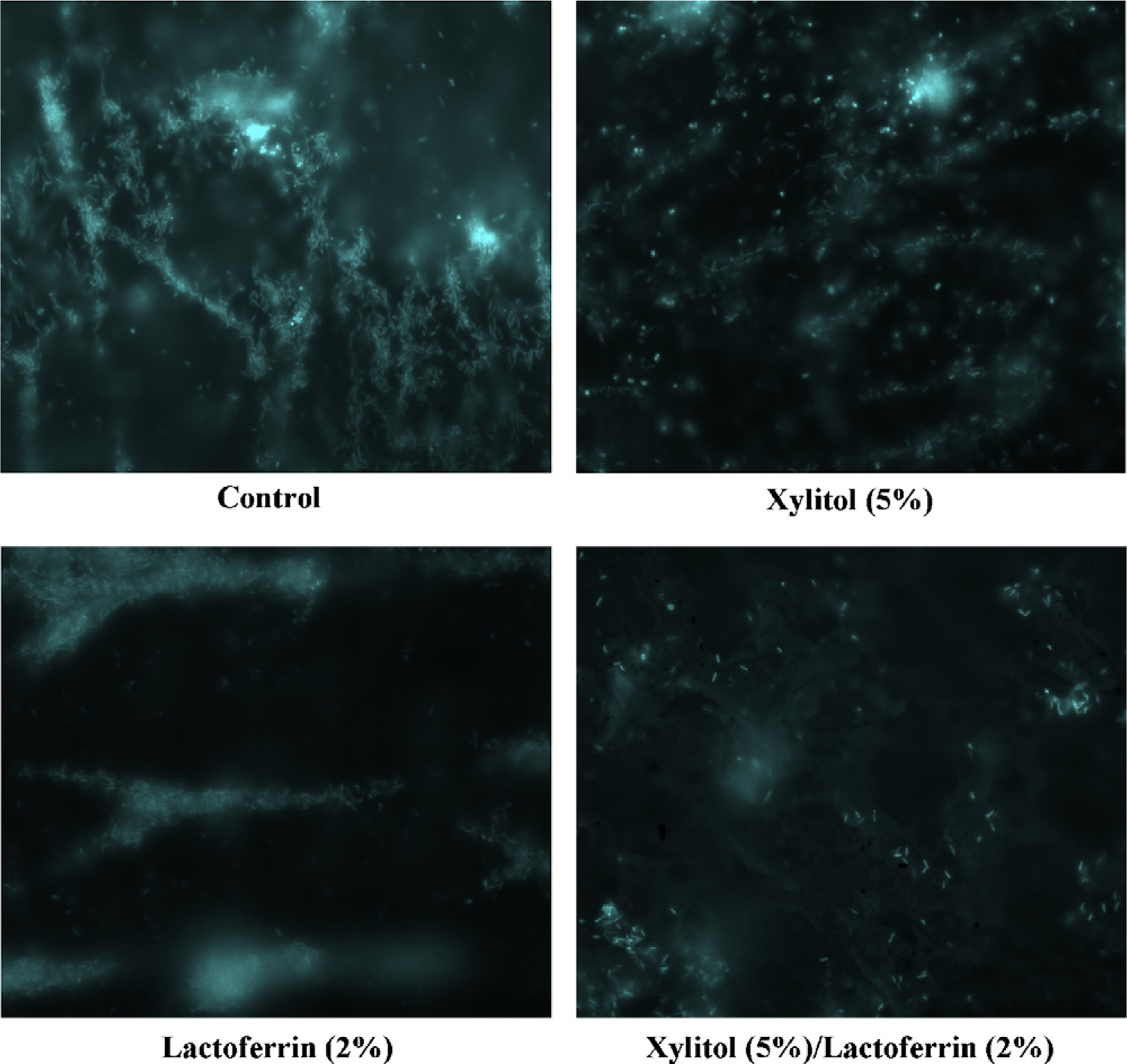
Altered macrostructure in lactoferrin- and xylitol-treated Pseudomonas aeruginosa biofilms. 4′,6-Diamidino-2-phenylindole (DAPI)-stained biofilms are imaged directly on the coupon subsurface. Images indicate structural changes in samples treated either with control medium, 5% (w/v) xylitol, 2% (w/v) lactoferrin or 5% (w/v) xylitol and 2% (w/v) lactoferrin.
To determine the viability of cells recovered from the treated biofilms, plate count assays, a standard method of measuring viability, were utilised [9]. Xylitol had a minimal effect on the viability of bacterial cells recovered from treated biofilms (Fig. 3). Although lactoferrin treatment resulted in a noteworthy reduction of viability, the combined treatment of lactoferrin and xylitol resulted in a significant inhibition of bacterial viability in cells recovered from treated biofilms. Indeed, the combined treatment appeared to have a synergistic effect, as the reduced viability with the combined treatment was greater than the reduced viability of xylitol and lactoferrin added together.
Fig. 3.

Synergistic activity of lactoferrin and xylitol treatment in reducing the viability of Pseudomonas aeruginosa bacteria. Data are presented as log reduction relative to the control, calculated from plate counts of bacteria recovered from treated biofilms. Statistically significant differences are indicated.
3.3. Lactoferrin and xylitol treatment results in significantly more permeabilised cells than intact cells compared with untreated Pseudomonas aeruginosa biofilms
Plate count analysis revealed a reduced viability of bacteria recovered from the treated biofilms (Fig. 3); however, we additionally used staining for live (intact) and dead (permeabilised) cells to provide image analysis and quantification of bacterial cells in situ. As presented in Fig. 4, direct imaging of treated biofilms indicated that while xylitol treatment resulted in few dead cells (red) within the remaining biofilm structures (upper right panel), lactoferrin treatment (lower left panel) and combined treatment (lower right panel) resulted in numerous dead cells (red) embedded within the biofilm structure along side live cells (green) in comparison with the control sample.
Fig. 4.

Live/dead imaging of treated Pseudomonas aeruginosa biofilms in situ. Epifluorescent imaging of BacLight™ LIVE/DEAD®-stained biofilms indicates cells with intact membranes (green) versus cells with permeabilised membranes (red). Samples include control, 5% (w/v) xylitol-treated cells, 2% (w/v) lactoferrin-treated cells and 5% (w/v) xylitol + 2% (w/v) lactoferrin-treated cells.
Although informative, three-dimensional (3D) images are complicated by structure, therefore to quantify the number of live cells versus dead cells in situ, treated biofilms were harvested from the growth surface, stained for intact or permeabilised cells and adhered as a single cell layer to a membrane by vacuum filtration. Relative percent of each component of the stain (live versus dead) can be quantified by detection of the pixelated intensity of the image. This allowed direct, quantifiable comparison of the actual number of live bacterial cells versus dead bacterial cells in each treatment (Fig. 5). In the control treatment, four times as many live bacterial cells were harvested from the growth surface as dead bacterial cells. Although similar ratios of live to dead bacterial cells were quantified for xylitol treatment, lactoferrin treatment and the combined treatment resulted in a significant decrease in the ratio of live versus dead bacterial cells. Importantly, our non-quantitative imaging of the growth surface in situ (Fig. 4) agreed well with the in situ quantified live/dead ratio data as shown in Fig. 5.
Fig. 5.

Reduction in the ratio of live cells relative to dead cells in Pseudomonas aeruginosa biofilms following treatment with lactoferrin or xylitol alone or in combination. Live versus dead cells were detected by BacLight™ LIVE/DEAD® staining and epifluorescent microscopy quantification. Statistically significant differences between ratios are indicated.
A comparison of intact bacterial cells in each treatment relative to the control indicated a reduced number of intact bacterial cells in the combined lactoferrin and xylitol treatment (Fig. 6). Furthermore, comparison of permeabilised bacterial cells in each treatment relative to the control indicated a noteworthy increase in cells without an intact membrane in the lactoferrin and combined treatments (Fig. 7).
Fig. 6.

Reduction in the number of intact biofilm-grown Pseudomonas aeruginosa bacterial cells following treatment with lactoferrin or xylitol alone or in combination. Intact cells were detected and quantified by SYTO® 9 staining and epifluorescent microscopy quantification. Samples were normalised to the control. Statistically significant difference is indicated.
Fig. 7.

Increase in the number of permeabilised cells in established Pseudomonas aeruginosa biofilms following treatment with lactoferrin or xylitol alone or in combination. Permeabilised cells were detected by propidium iodide staining and were quantified by epifluorescent microscopy. Quantified samples were normalised to the control sample.
4. Discussion
The biofilm mode of growth has been demonstrated as an important aspect of many bacterial diseases, including native valve endocarditis, osteomyelitis, dental caries, middle ear infections, medical device-related infections, ocular implant infection and chronic lung infections in patients with cystic fibrosis [29]. The list of bacterial biofilm-related diseases has grown to include chronic wounds associated with diabetic and cardiovascular disease [1]. As the epidemic of obesity and an aging population place an increasing burden on health care, the contribution of associated chronic wounds needs to be seriously reviewed and, because of the biofilm-associated mode of growth in these chronic wounds, innovative methods of treatment need to be incorporated into the standard of care.
A distinct characteristic of the biofilm mode of growth is the production of an extracellular matrix in which the bacterial cells are embedded. Not only does the matrix provide a scavenging system for trapping and concentrating essential minerals and nutrients from the surrounding environment [30], but it also provides a mechanism of resistance by delaying the penetration of antimicrobials [9]. Pseudomonas aeruginosa biofilms are particularly resistant to antibiotic treatment owing to the production and deposition of alginate in the matrix. For example, a 2% solution of alginate from a P. aeruginosa biofilm significantly inhibited the diffusion of gentamicin and tobramycin [31]. Alginate is a polyanionic exopolysaccaride and tends to concentrate divalent cations thus reducing the antimicrobial efficacy of aminoglycosides and tetracyclines [32]. Bacterial biofilms are composed of ca. 15% bac- terial cells and 85% matrix by volume; however, nearly 95% of the matrix is water [33]. Therefore, it is likely that the matrix only limits diffusion when a molecule interacts directly [34]. In the present study, we have demonstrated that exogenous, amphipathic lactoferrin can penetrate into the interior of established P. aeruginosa biofilms and is therefore a potential antimicrobial candidate for biofilm.
As a component of innate immunity, lactoferrin has been proven to be an effective antimicrobial. The efficacy of lactoferrin against bacterial biofilms has previously been demonstrated [16,35,36]; however, the antimicrobial mechanism of action of lactoferrin is less well defined. Whilst lactoferrin has been established as an inhibitor of bacterial growth through iron chelation [22], it has also been demonstrated to permeabilise Gram-negative bacteria by binding lipid A [37], to inhibit bacterial attachment to host cells [38] and to bind bacterial porins causing changes in bacterial membrane permeability [39]. In the present study, 3D live/dead staining of the lactoferrin-treated biofilm appeared cloudy, indicating that there may be a nucleic acid component leaching into the structure of the biofilm, suggesting either membrane leakage or bacterial lysis (Fig. 2).
Although the nucleic acid component of the extracellular matrix was not assayed, this observation was also noted with the other DNA stains SYTO® 9 and propidium iodide (Figs. 6 and 7). Furthermore, comparison of intact versus permeabilised bacterial cells imaged in situ (Figs. 6 and 7) indicated that the mechanism of action of lactoferrin is in part due to permeabilisation of the bacterial membrane.
Although use of xylitol against dental biofilms has proven efficacious, the mechanism of action of xylitol remains to be determined [40]. A potential mechanism of action includes metabolic inhibition. For example, xylitol can be accumulated as the nonmetabolisable xylitol phosphate, thus inhibiting bacterial growth[41]. Additionally, xylitol has been demonstrated to inhibit specifically DnaK-like and GroEL-like stress proteins [40]. 3D image analysis of xylitol-treated biofilms (Fig. 2) indicated that xylitol may act to disrupt the overall biofilm structure.
Although antimicrobial properties have been demonstrated for both lactoferrin and xylitol, bacteria have evolved mechanisms of protection with continued treatment of either compound alone. For example, some bacterial species are able to bind iron-saturated lactoferrin and use it as a source of iron [42]. Additionally, long-term xylitol consumption can lead to the emergence of xylitol-resistant bacterial populations in humans [41,43]. Therefore, we propose that combined treatment with lactoferrin and xylitol may more efficiently inhibit the biofilm mode of growth in the long term compared with either treatment independently.
In the current study, we demonstrated in vitro that combined treatment with lactoferrin and xylitol effectively reduces the viability of a clinical wound isolate of P. aeruginosa grown as a biofilm. Whilst standard plate count analysis (Fig. 3) provided quantification of the viability of bacterial cells recovered from the treated biofilms, staining for live (intact) versus dead (permeabilised) cells (Fig. 5) provided quantification of the viability of cells harvested directly from the biofilm. Although image analysis of the growth surface in situ (Fig. 4) only provided one image of a vastly larger surface, the quantified live/dead ratio (Fig. 5) agreed well with what is observed of the biofilms in situ and, importantly, the quantified live/dead ratio agreed well with the quantified plate counts (Fig. 3). In conclusion, the data presented here suggest that combined xylitol and lactoferrin treatment effectively inhibits bacterial viability in part by disrupting biofilm structure and by permeablising the membrane of bacterial cells within the biofilm structure. The potential efficacy of this combined treatment against biofilm-associated chronic wounds has recently been demonstrated clinically [44], validating the need for further investigation to elucidate more clearly the mechanism of action of these antimicrobial treatments.
Acknowledgment
Dr. Wolcott kindly supplied the clinical wound isolates.
Funding: This work was supported by Glanbia Nutritionals Inc., 523, 6th Street, Monroe, WI 53566, USA.
Footnotes
Competing interests: L.S.W. is employed by Glanbia Nutritionals Inc. and provided the Bioferrin® used in these experiments. R.D.W. and Glanbia Nutritionals Inc. have applied to patent the use of lactoferrin and xylitol in combination in the treatment of chronic wounds.
Ethical approval: Not required.
References
- [1].James GA, Swogger E, Wolcott R, Pulcini ED, Secor P, Sestrich J, et al. Biofilms in chronic wounds. Wound Repair Regen 2008;16:37–44. [DOI] [PubMed] [Google Scholar]
- [2].American Diabetes Association. Economic costs of diabetes in the U.S. in 2007. Diab Care 2008;31:596–615. Erratum in: Diab Care 2008;31:1271. [DOI] [PubMed] [Google Scholar]
- [3].Gottrup F A specialized wound-healing center concept: importance of a multidisciplinary department structure and surgical treatment facilities in the treatment of chronic wounds. Am J Surg 2004;187(5A):38S–43S. [DOI] [PubMed] [Google Scholar]
- [4].McGuckin M, Goldman R, Bolton L, Salcido R. The clinical relevance of microbiology in acute and chronic wounds. Adv Skin Wound Care 2003;16:12–23, quiz 24–5. [DOI] [PubMed] [Google Scholar]
- [5].Bjarnsholt T, Kirketerp-Moller K, Jensen PO, Madsen KG, Phipps R, Krogfelt K, et al. Why chronic wounds will not heal: a novel hypothesis. Wound Repair Regen 2008;16:2–10. [DOI] [PubMed] [Google Scholar]
- [6].Konturek PC, Brzozowski T, Konturek SJ, Kwiecien S, Dembinski A, Hahn EG. Influence of bacterial lipopolysaccharide on healing of chronic experimental ulcer in rat. Scand J Gastroenterol 2001;36:1239–47. [DOI] [PubMed] [Google Scholar]
- [7].Power C, Wang JH, Sookhai S, Street JT, Redmond HP. Bacterial wall products induce downregulation of vascular endothelial growth factor receptors on endothelial cells via a CD14-dependent mechanism: implications for surgical wound healing. J Surg Res 2001;101:138–45. [DOI] [PubMed] [Google Scholar]
- [8].Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science 1999;284:1318–22. [DOI] [PubMed] [Google Scholar]
- [9].Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev 2002;15:167–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Parsek MR, Singh PK. Bacterial biofilms: an emerging link to disease pathogenesis. Ann Rev Microbiol 2003;57:677–701. [DOI] [PubMed] [Google Scholar]
- [11].Saye DE. Recurring and antimicrobial-resistant infections: considering the potential role of biofilms in clinical practice. Ostomy Wound Manage 2007;53:46–8, 50, 52 passim. [PubMed] [Google Scholar]
- [12].Edwards R, Harding KG. Bacteria and wound healing. Curr Opin Infect Dis 2004;17:91–6. [DOI] [PubMed] [Google Scholar]
- [13].Gjodsbol K, Christensen JJ, Karlsmark T, Jorgensen B, Klein BM, Krogfelt KA. Multiple bacterial species reside in chronic wounds: a longitudinal study. Int Wound J 2006;3:225–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Dowd SE, Sun Y, Secor PR, Rhoads DD, Wolcott BM, James GA, et al. Survey of bacterial diversity in chronic wounds using pyrosequencing, DGGE, and full ribosome shotgun sequencing. BMC Microbiol 2008;8:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ceri H, Olson ME, Stremick C, Read RR, Morck D, Buret A. The Calgary Biofilm Device: new technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J Clin Microbiol 1999;37:1771–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Singh PK, Parsek MR, Greenberg EP, Welsh MJ. A component of innate immunity prevents bacterial biofilm development. Nature 2002;417:552–5. [DOI] [PubMed] [Google Scholar]
- [17].Valenti P, Berlutti F, Conte MP, Longhi C, Seganti L. Lactoferrin functions: current status and perspectives. J Clin Gastroenterol 2004;38(6 Suppl):S127–9. [DOI] [PubMed] [Google Scholar]
- [18].Weinberg ED. Human lactoferrin: a novel therapeutic with broad spectrum potential. J Pharm Pharmacol 2001;53:1303–10. [DOI] [PubMed] [Google Scholar]
- [19].Cohen MS, Mao J, Rasmussen GT, Serody JS, Britigan BE. Interaction of lactoferrin and lipopolysaccharide (LPS): effects on the antioxidant property of lactoferrin and the ability of LPS to prime human neutrophils for enhanced superoxide formation. J Infect Dis 1992;166:1375–8. [DOI] [PubMed] [Google Scholar]
- [20].Oseas R, Yang HH, Baehner RL, Boxer LA. Lactoferrin: a promoter of polymor-phonuclear leukocyte adhesiveness. Blood 1981;57:939–45. [PubMed] [Google Scholar]
- [21].Valenti P, Greco R, Pitari G, Rossi P, Ajello M, Melino G, et al. Apoptosis of Caco-2 intestinal cells invaded by Listeria monocytogenes: protective effect of lactoferrin. Exp Cell Res 1999;250:197–202. [DOI] [PubMed] [Google Scholar]
- [22].Orsi N The antimicrobial activity of lactoferrin: current status and perspectives. Biometals 2004;17:189–96. [DOI] [PubMed] [Google Scholar]
- [23].Stephens S, Dolby JM, Montreuil J, Spik G. Differences in inhibition of the growth of commensal and enteropathogenic strains of Escherichia coli by lactotransferrin and secretory immunoglobulin A isolated from human milk. Immunology 1980;41:597–603. [PMC free article] [PubMed] [Google Scholar]
- [24].Dolby JM, Stephens S. Antibodies to Escherichia coli O antigens and the in-vitro bacteriostatic properties of human milk and its IgA. Acta Paediatr Scand 1983;72:577–82. [DOI] [PubMed] [Google Scholar]
- [25].Rainard P Activation of the classical pathway of complement by binding of bovine lactoferrin to unencapsulated Streptococcus agalactiae. Immunology 1993;79:648–52. [PMC free article] [PubMed] [Google Scholar]
- [26].Granstrom TB, Izumori K, Leisola M. A rare sugar xylitol. Part II: biotechnological production and future applications of xylitol. Appl Microbiol Biotechnol 2007;74:273–6. [DOI] [PubMed] [Google Scholar]
- [27].Katsuyama M, Ichikawa H, Ogawa S, Ikezawa Z. A novel method to control the balance of skin microflora. Part 1. Attack on biofilm of Staphylococcus aureus without antibiotics. J Dermatol Sci 2005;38:197–205. [DOI] [PubMed] [Google Scholar]
- [28].Kim J, Pitts B, Stewart PS, Camper A, Yoon J. Comparison of the antimicrobial effects of chlorine, silver ion, and tobramycin on biofilm. Antimicrob Agents Chemother 2008;52:1446–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Jefferson KK. What drives bacteria to produce a biofilm? FEMS Microbiol Lett 2004;236:163–73. [DOI] [PubMed] [Google Scholar]
- [30].Carpentier B, Cerf O. Biofilms and their consequences, with particular reference to hygiene in the food industry. J Appl Bacteriol 1993;75:499–511. [DOI] [PubMed] [Google Scholar]
- [31].Hatch RA, Schiller NL. Alginate lyase promotes diffusion of aminoglycosides through the extracellular polysaccharide of mucoid Pseudomonas aeruginosa. Antimicrob Agents Chemother 1998;42:974–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Dunne WM Jr. Bacterial adhesion: seen any good biofilms lately? Clin Microbiol Rev 2002;15:155–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lawrence JR, Korber DR, Hoyle BD, Costerton JW, Caldwell DE. Optical sectioning of microbial biofilms. J Bacteriol 1991;173:6558–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Stewart JA, Silimperi D, Harris P, Wise NK, Fraker TD Jr, Kisslo JA. Echocardio-graphic documentation of vegetative lesions in infective endocarditis: clinical implications. Circulation 1980;61:374–80. [DOI] [PubMed] [Google Scholar]
- [35].Singh PK. Iron sequestration by human lactoferrin stimulates P. aeruginosa surface motility and blocks biofilm formation. Biometals 2004;17: 267–70. [DOI] [PubMed] [Google Scholar]
- [36].Psaltis AJ, Wormald PJ, Ha KR, Tan LW. Reduced levels of lactoferrin in biofilm-associated chronic rhinosinusitis. Laryngoscope 2008;118:895–901. [DOI] [PubMed] [Google Scholar]
- [37].Ellison RT 3rd, Giehl TJ, LaForce FM. Damage of the outer membrane of enteric gram-negative bacteria by lactoferrin and transferrin. Infect Immun 1988;56:2774–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Dial EJ, Lichtenberger LM. Effect of lactoferrin on Helicobacter felis induced gastritis. Biochem Cell Biol 2002;80:113–7. [DOI] [PubMed] [Google Scholar]
- [39].Erdei J, Forsgren A, Naidu AS. Lactoferrin binds to porins OmpF and OmpC in Escherichia coli. Infect Immun 1994;62:1236–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hrimech M, Mayrand D, Grenier D, Trahan L. Xylitol disturbs protein synthesis, including the expression of HSP-70 and HSP-60, in Streptococcus mutans. Oral Microbiol Immunol 2000;15:249–57. [DOI] [PubMed] [Google Scholar]
- [41].Trahan L, Mouton C. Selection for Streptococcus mutans with an altered xylitol transport capacity in chronic xylitol consumers. J Dent Res 1987;66:982–8. [DOI] [PubMed] [Google Scholar]
- [42].Vogel L, Geluk F, Jansen H, Dankert J, van Alphen L. Human lactoferrin receptor activity in non-encapsulated Haemophilus influenzae. FEMS Microbiol Lett 1997;156:165–70. [DOI] [PubMed] [Google Scholar]
- [43].Trahan L, Soderling E, Drean MF, Chevrier MC, Isokangas P. Effect of xylitol consumption on the plaque–saliva distribution of mutans streptococci and the occurrence and long-term survival of xylitol-resistant strains. J Dent Res 1992;71:1785–91. [DOI] [PubMed] [Google Scholar]
- [44].Wolcott RD, Rhoads DD. A study of biofilm-based wound management in subjects with critical limb ischaemia. J Wound Care 2008;17:145–8, 150–2, 154–5. [DOI] [PubMed] [Google Scholar]


