Abstract
Objective
The revised Standards of Medical Care in Diabetes-2018 recommend a less-intensive HbA1c target for elderly individuals than for younger ones. This study aimed to investigate the development and progression of chronic kidney disease (CKD) according to HbA1c levels separately for elderly and middle-aged individuals in a general Japanese population.
Methods
This was a retrospective cohort study using health checkup data in Iki City, Japan. The participants of the study were 5,554 residents who attended health checkups more than 2 times over 8 years. This study consists of two sets of analyses to determine (1) the effects of HbA1c on the development of CKD among 4,570 subjects who did not have CKD at baseline and (2) the effects of HbA1c on the progression of CKD in 953 subjects with existing CKD at baseline.
Results
After adjusting for various risk factors, the multivariable-adjusted hazard ratios for development of CKD increased with the HbA1c level: 1.43 for 7-9% and 1.67 for >9% compared with the reference of <7% (p<0.306 for trend). Similar findings were also observed for the progression of CKD: hazard ratios of 2.48 for 7-9% and 2.46 for >9% compared with the reference of <7% (p<0.077 for trend). No significant differences in the effects of HbA1c level on the development or progression of CKD were observed between elderly and middle-aged individuals (p>0.3 for interaction).
Conclusion
The risks of the development and progression of CKD increased from HbA1c levels of 7% in a general Japanese population. Similar associations were observed for both elderly and middle-aged individuals.
Keywords: CKD, diabetes
Introduction
Chronic kidney disease (CKD) is a leading cause of cardiovascular comorbidities and premature death as well as end-stage renal disease (ESRD) and cardiovascular disease (1). Diabetic nephropathy (DN) is a major cause of CKD (2), and it is essential to prevent the development and progression of DN in order to reduce the enormous burden of ESRD in Japan as well as other countries worldwide.
A number of randomized controlled trials (RCTs) have investigated the effects of intensive glucose control on DN among diabetic patients. In the Diabetes Control and Complications Trial (DCCT) (3) for patients with type 1 diabetes, the UK Prospective Diabetes Study (UKPDS) (4), and the Kumamoto Study (5) for patients with type 2 diabetes, it was demonstrated that intensive glucose control with a target hemoglobin A1c (HbA1c) <7.0% significantly reduced the development and progression of DN, compared with the cases with a target HbA1c <9.0%. In addition, the ADVANCE (6), ACCORD (7), and VADT (8) trials also showed that intensive glucose control clearly reduced the rate of development and progression of DN, as evaluated by quantitative urinary albumin. A sub-analysis for the ADVANCE trial (9) suggested a significant benefit of intensive glucose control on the prevention of ESRD. Based on these previous findings, many guidelines concerning the management of patients with diabetes mellitus recommend intensive glucose control with a target HbA1c level of 6.5-7% (10) with the aim of preventing the development and progression of DN.
The revised Standards of Medical Care in Diabetes-2018 recommend a less-intensive HbA1c target for elderly diabetic patients than for younger individuals in order to facilitate the prevention of CVD morbidity (11), because the harms of glucose control with a target HbA1c level outweigh the benefits in this population (9). In the ACCORD study for diabetic patients who had either established CVD or additional cardiovascular risk factors, intensive therapy that targeted normal HbA1c levels for 3.5 years increased the mortality compared with standard therapy and did not significantly reduce major cardiovascular events (5). The VADT study for military veterans with poorly controlled type 2 diabetes also showed that intensive therapy had no significant effect on the rates of major cardiovascular events and death (6). Detailed investigations on the associations between HbA1c levels and the development and progression of CKD associated with diabetes have been limited.
Therefore, we conducted a population-based retrospective cohort study for residents of Iki Island. The rate of dialysis for ESRD on this island is the highest by far in Nagasaki Prefecture, and the prevention of development and progression of CKD is the most urgent challenge facing public health officials. We felt that investigations concerning CKD stratified by age were urgently needed, so we evaluated the development and progression of CKD according to HbA1c levels separately for elderly and middle-aged individuals from the general population of this island.
Materials and Methods
Study design and participants
The Iki Epidemiological Study of Atherosclerosis and Chronic Kidney Disease (ISSA-CKD) Project is a population-based retrospective cohort study of residents in Iki City, Nagasaki Prefecture, Japan. Iki Island is located in the north of Nagasaki Prefecture and has approximately 27,000 residents. From 2008 to 2016, a total of 7,745 residents underwent annual health checkups conducted in Iki City. After the exclusion of 2,219 residents who attended only once or had missing serum creatinine values, a total of 5,526 residents were retrospectively included in this study.
This study aimed to investigate the following: the effects of diabetes and HbA1c on development of CKD among 4570 subjects who did not have CKD at baseline, and the effects of diabetes and HbA1c on the progression of CKD among 953 subjects with existing CKD at baseline. This study was approved by the Fukuoka University Clinical Research and Ethics Center.
Definitions of outcomes and independent variables
The outcomes of the present analysis were the development of CKD and progression of CKD. At each health checkup, random blood and urine samples were collected. The serum creatinine level was determined using an enzymatic method. The estimated glomerular filtration rate (eGFR) was determined using the formula of the Japanese Society of Nephrology, as follows: eGFR (mL/min/1.73 m2) =194×serum creatinine (-1.094) × age (-0.287) (×0.739, if female) (12). The eGFR was classified into 5 stages: G1-2 (eGFR≥60), G3a (45-59), G3b (30-44), G4 (15-29), and G5 (<15). Urinary protein was examined using the dipstick method and classified into three stages: A0 (- or ±), A1 (1+), and A2 (≥2+) or higher. The development of CKD was defined as reduction of eGFR down to <60 mL/min/1.73 m2 (stage G3-5) and/or new-onset proteinuria (stage A1 or A2) at a follow-up examination, which was confirmed at the final follow-up examination, among subjects without CKD at baseline. The progression of CKD was defined as any progression of the eGFR stage and/or proteinuria stage at a follow-up examination with confirmation at the final follow-up examination among subjects with CKD at baseline.
Plasma glucose levels and HbA1c levels were determined by the enzyme method. Diabetes was defined as a fasting glucose level ≥7.0 mmol/L, non-fasting glucose level ≥11.1 mmol/L, HbA1c (National Glycohemoglobin Standardization Program) level ≥6.5%, and/or the use of glucose-lowering treatments. Participants were divided into 3 groups according to their HbA1c levels (<7%, 7-9%, and >9%), as was done in a previous study (13).
The height and body mass were measured with the participant wearing light clothes without shoes, and the body mass index (BMI; kg/m2) was calculated. Obesity was defined by a BMI ≥25 kg/m2 (14). Blood pressure (BP) was measured at the right upper arm using mercury, automated, or aneroid sphygmomanometers with appropriately sized cuffs after at least 5 min of rest in a sitting position, by trained staff, according to a standardized guideline (15). Hypertension was classified as systolic BP >140 mmHg, diastolic BP >90 mmHg, and/or the use of blood pressure-lowering medications. Serum low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, and triglyceride levels were determined enzymatically. Dyslipidemia was defined as LDL cholesterol level ≥3.62 mmol/L, HDL cholesterol level <1.03 mmol/L, and/or triglyceride level ≥1.69 mmol/L (16) or the use of lipid-lowering medications. The serum uric acid level was determined using the enzyme method, and hyperuricemia was defined as a uric acid level ≥420 μmol/L (17). Information on smoking habits was obtained using a standardized questionnaire. Current smokers were defined as participants who had smoked ≥100 cigarettes or who had smoked continuously for >6 months at the time of the baseline examination.
Statistical analyses
Baseline characteristics were compared among individuals with and those without diabetes mellitus using Wilcoxon's test for continuous variables and Pearson's chi-squared test for categorical variables. The incidence was calculated using a person-year approach. Effects of diabetes and HbA1c levels on outcomes were estimated using univariable and multivariable Cox's proportional-hazards models. In the multivariable analysis, sex, age, hypertension, dyslipidemia, hyperuricemia, obesity, and smoking were used for adjustment. Subgroup analyses were performed to determine the effects of HbA1c levels on the outcomes separately for subgroups defined by age (<65 vs. ≥65 years). Differences in the effects of diabetes and HbA1c levels between subgroups were tested by adding interaction terms to the statistical models. The software program STATA Statistical Software, Release 14 (StataCorp, College Station, USA) was used for statistical analyses. All reported p values were two-tailed, and the level of significance was set at p<0.05.
Results
Baseline characteristics
Table 1 shows the baseline characteristics of participants stratified by diabetes and HbA1c levels. Participants with high HbA1c levels were more likely to be male and obese; however, there was no trend for age.
Table 1.
Baseline Characteristics of the Participants Stratified by Diabetes Status and HbA1c Levels.
| Diabetes | p value | HbA1c | p value | ||||
|---|---|---|---|---|---|---|---|
| Absent (n=4,935) | Present (n=588) | <7% (n=5,056) | 7%-9% (n=94) | >9% (n=38) | |||
| Male | 2,191 (44.3) | 352 (59.8) | <0.001 | 2,301 (45.5) | 105 (54.1) | 21 (55.2) | 0.031 |
| Age, years | 60.0 (10.5) | 64.0 (6.5) | <0.001 | 60.1 (10.4) | 64.1 (6.2) | 61.3 (7.8) | <0.001 |
| Body mass index, kg/m2 | 23.6 (3.4) | 25.2 (3.8) | <0.001 | 23.6 (3.4) | 25.7 (3.6) | 25.8 (5.2) | <0.001 |
| Obesity | 1,535 (31.1) | 296 (50.4) | <0.001 | 1,601 (31.6) | 110 (56.7) | 19 (50.0) | <0.001 |
| Systolic BP, mmHg | 129.2 (18.5) | 135.2 (17.6) | <0.001 | 129.3 (18.5) | 136.1 (17.7) | 133.6 (17.0) | 0.547 |
| Diastolic BP, mmHg | 74.9 (11.0) | 74.8 (10.2) | 0.881 | 74.8 (11.0) | 75.3 (10.4) | 75.3 (10.1) | 0.484 |
| Hypertension | 1,414 (28.6) | 235 (39.9) | <0.001 | 1,460 (28.8) | 81 (41.7) | 12 (31.5) | <0.001 |
| Triglyceride, mmol/L | 1.3 (0.8) | 1.5 (1.0) | <0.001 | 1.3 (0.9) | 1.6 (1.1) | 1.8 (1.2) | <0.001 |
| HDL cholesterol, mmol/L | 1.6 (0.4) | 1.5 (0.5) | 0.054 | 1.5 (0.4) | 1.5 (0.4) | 1.5 (0.4) | 0.396 |
| LDL cholesterol, mmol/L | 3.1 (0.8) | 3.0 (0.8) | <0.001 | 3.1 (0.8) | 3.0 (0.8) | 3.3 (1.1) | 0.006 |
| Dyslipidemia | 2,287 (46.3) | 326 (55.4) | 0.357 | 2,361 (46.6) | 104 (53.6) | 23 (60.5) | 0.041 |
| Uric acid, mmol/L | 303.4 (87.6) | 313.8 (135.4) | 0.011 | 303.5 (81.4) | 291.7 (71.9) | 284.8 (95.2) | 0.027 |
| Hyperuricemia, mmol/L | 470 (9.5) | 69 (11.8) | 0.078 | 490 (9.6) | 13 (6.8) | 51 (13.5) | 0.298 |
| Current smoking | 1,167 (23.6) | 165 (28.0) | 0.018 | 1,228 (24.2) | 54 (27.8) | 9 (23.6) | 0.526 |
Values are presented as the mean (SD), median (IQR), or n (%). P values were estimated using Wilcoxon’s test or the chi-squared test. BP: blood pressure, HDL: high-density lipoprotein, LDL: low-density lipoprotein, IQR: interquartile range
Analysis 1: The development of CKD
Over a mean follow-up period of 4.6 years, development of CKD was confirmed in 746 patients. The CKD incidence (per 1,000 person-years) in diabetic individuals was 48.5, which was higher than that in non-diabetic individuals (34.2) (Table 2). The relationship between diabetes and the development of CKD was significant even after adjusting for other risk factors [multivariable-adjusted hazard ratio (HR) 1.61, 95% CI 1.28-2.02]. The CKD incidence was 34.1 for HbA1c level <7%, 54.8 for 7-9%, and 37.1 for >9%. The relationship between the HbA1c level and the development of CKD was also significant in a multivariable analysis: HR 1.43 (95% CI 0.99-2.08) for HbA1c 7-9%, and HR 1.67 (95% CI 0.84-4.48) for >9%, compared with the reference group of <7% (p=0.306 for trend). The relationship between the HbA1c level and new-onset proteinuria was also significant in the multivariable analysis: HR 2.33 (95% CI 1.34-4.05) for HbA1c 7-9% and HR 4.01 (95% CI 1.27%-12.5) for >9%, compared with the reference group of <7% (p=0.017 for trend). Furthermore, the HbA1c levels were associated with a larger annual reduction in eGFR in the multivariable analysis: annual reduction in eGFR per year (mL/min/1.73 m2/year) was -0.15 (95% CI -0.80-0.49) for HbA1c 7-9% and -1.09 (95% CI -2.68-0.48) for >9%, compared with the reference group of <7% (p=0.175 for trend).
Table 2.
Effects of Diabetes and HbA1c Levels on New-onset and Progression of CKD.
| Number of events | Incidence* | Crude | Multivariable-adjusted** | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Hazard ratio (95% CI) | p value | Hazard ratio (95% CI) | p value | ||||||
| New-onset CKD | |||||||||
| Diabetes | |||||||||
| Absent (n=4,541) | 675 | 34.2 | Reference | Reference | |||||
| Present (n=759) | 71 | 48.5 | 1.91 (1.53-2.38) | <0.001 | 1.61 (1.28-2.02) | <0.001 | |||
| HbA1c | |||||||||
| <7% (n=4,270) | 685 | 34.1 | Reference | Reference | |||||
| 7-9% (n=105) | 23 | 54.8 | 1.87 (1.29-2.70) | 0.001 | 1.43 (0.99-2.08) | 0.054 | |||
| >9% (n=19) | 3 | 37.1 | 1.49 (0.55-3.99) | 0.422 | 1.67 (0.84-4.48) | 0.306 | |||
| p value for trend | 0.007 | 0.306 | |||||||
| CKD progression | |||||||||
| Diabetes | |||||||||
| Absent (n=982) | 144 | 37.7 | Reference | Reference | |||||
| Present (n=194) | 45 | 83.7 | 2.27 (1.64-3.12) | <0.001 | 2.17 (1.55-3.42) | <0.001 | |||
| HbA1c | |||||||||
| <7% (n=856) | 151 | 38.5 | Reference | Reference | |||||
| 7-9% (n=44) | 16 | 95.4 | 2.47 (1.55-3.96) | <0.001 | 2.48 (1.52-4.07) | <0.001 | |||
| >9% (n=13) | 5 | 94.4 | 2.19 (0.81-5.94) | 0.121 | 2.46 (0.91-6.71) | 0.077 | |||
| p for trend | 0.001 | 0.077 | |||||||
*Per 1,000 person-years. **Adjusted for age, sex, hypertension, dyslipidemia, hyperuricemia, obesity, and smoking.
CKD: chronic kidney disease, CI: confidence interval
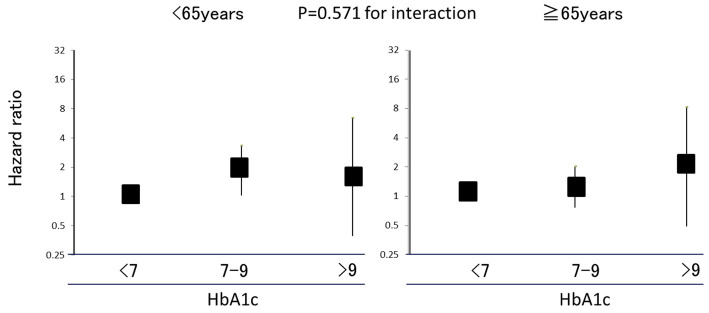
Fig. 1 shows the effects of HbA1c on the development of CKD stratified by age. Comparable associations between HbA1c and the development CKD were observed for participants <65 years old and those ≥65 years old (p=0.571 for interaction). Even after excluding participants without diabetes, there were no clear differences in the associations of HbA1c levels with new-onset CKD between participants <65 years old [HR 1.60 (95% CI 0.71-3.58) for HbA1c 7-9% and HR 1.34 (95% CI 0.29-6.23) for >9%, compared with the reference group of 6.5-7%] and those ≥65 years old [HR 0.75 (95% CI 0.39-1.41) for HbA1c 7-9% and HR 0.69 (95% CI 0.153-3.182) for >9%, compared with the reference group of 6.5-7%] (p=0.397 for interaction).
Figure 1.
The effects of HbA1c on new-onset chronic kidney disease (CKD) stratified by age.
Analysis 2: The progression of CKD
Over a mean follow-up period of 4.6 years, CKD progression was confirmed in 189 patients. The incidence rate of CKD progression (per 1,000 person-years) in diabetic individuals was 83.7, which was higher than that in non-diabetic individuals (37.7) (Table 2). The relationship between diabetes and the progression of CKD was significant even after adjusting for other risk factors (multivariable-adjusted HR 2.17, 95% CI 1.55-3.42). The incidence rates of CKD progression were 38.5 for an HbA1c level of <7%, 95.4 for 7-9% and 94.4 for >9%. The relationship between the HbA1c level and the progression of CKD was also significant in the multivariable analysis: HR 2.48 (95% CI 1.52-4.07) for HbA1c 7-9% and HR 2.46 (95% CI 0.91-6.71) for >9% compared with the reference group of <7% (p=0.077 for trend). The HbA1c levels were not clearly associated with progression of urinary proteinuria stages [HR 2.08 (95% CI 1.17-3.71) for HbA1c 7-9% and HR 1.41 (95% CI 0.34-5.77) for >9%, compared with the reference group of <7%, p=0.627 for trend] or annual reduction in eGFR per year [-1.42 mL/min/1.73 m2/year (95% CI -2.45-0.38) for HbA1c 7-9% and -2.09 mL/min/1.73 m2/year (95% CI -3.98-0.20) for >9%, compared with the reference group of <7%, p=0.030 for trend].
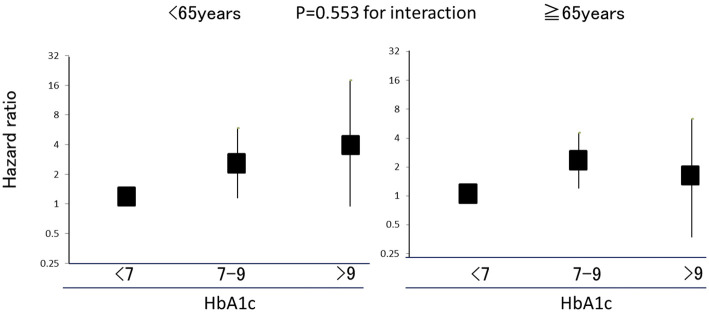
Fig. 2 shows the effects of HbA1c on CKD progression stratified by age. Comparable associations between HbA1c levels and CKD progression were observed for participants <65 years old and those ≥65 years old (p=0.553 for interaction). Even after excluding participants without diabetes, there were no clear differences in the associations of HbA1c levels with the progression of CKD between participants <65 years old [HR 1.82 (95% CI 0.41-7.99) for HbA1c 7-9% and HR 7.60 (95% CI 0.83-69.2) for >9%, compared with the reference group of 6.5-7%] and those ≥65 years old [HR 1.66 (95% CI 0.73-3.76) for HbA1c 7-9%, and HR 1.29 (95% CI .267-6.29) for >9%, compared with the reference group of 6.5-7%] (p=0.868 for interaction).
Figure 2.
The effects of HbA1c on the exacerbation of chronic kidney disease (CKD) stratified by age.
Discussion
In the present study, the HbA1c level was linearly associated with the development and progression of CKD, even after controlling for the effects of sex, age, hypertension, dyslipidemia, hyperuricemia, obesity, and smoking in a general population of Japanese individuals. Both the development and progression of CKD increased significantly from an HbA1c level of 7.0%. Similar associations were observed for participants <65 years old as well as for those ≥65 years old.
In the present analysis, HbA1c levels <7% were associated with a reduced incidence of new-onset CKD among participants who did not have CKD at baseline. With regard to randomized evidence, ADVANCE, ACCORD, and VADT demonstrated that intensive glucose control suppressed the development of DN; however, their evaluations were not based on the eGFR but rather albuminuria. Therefore, these studies did not clarify whether or not intensive glucose control can attenuate the decline in the eGFR. However, a sub-analysis of the ADVANCE study demonstrated that intensive glucose control with a target HbA1c ≤7.0% significantly suppressed the development of ESRD. Based on the above evidence, strict glucose control with a target HbA1c level <7% appears to suppress the eGFR decline as well as the development and progression of albuminuria in diabetic patients with a preserved renal function.
In this study, HbA1c levels of <7% were also associated with reduced risks of CKD progression among participants with existing CKD at baseline. Although there has been limited randomized evidence on the effects of intensive glucose control on kidney outcomes among patients with a decreased eGFR, an observational study of patients with diabetes mellitus who have a decreased eGFR between 25 and 59 [units?] demonstrated lower risks of ESRD among patients who achieved HbA1c levels <7% than among those with HbA1c levels ≥9% (13). These findings support the theory that intensive glucose control with a target HbA1c level ≤7.0% appears to be protective against progression of CKD.
Despite an early loss of glycemic differences in the intensive therapy group compared with the conventional therapy group, a continued reduction in microvascular risk from any cause was observed during 10 years of post-trial follow-up (18). The revised Standards of Medical Care in Diabetes-2018 released by the American Diabetes Association recommend less-intensive glucose control with a target HbA1c ≤7.5% for elderly people (e.g., ≥65 years old). In this study, however, linear increases in the risks of the development and progression of CKD were observed from an HbA1c level of 7.0% for elderly participants as well as those <65 years old. It is possible that, even among elderly people, strict glucose control with a target HbA1c <7.0% can prevent the development and progression of CKD. However, as a matter of course, it was possible to avoid adverse effects, such as hypoglycemia.
Although this is a large-scale observational study in a general Japanese population, there are some associated limitations. Because of the retrospective design, the findings of the present analysis may be affected by selection bias. A second limitation is that our findings are based on one-time measurements of serum glucose and HbA1c, which may not accurately reflect the glucose tolerance status of the study participants. However, this source of variability could not account for the relationship observed in the present study, as a random misclassification of such nature would tend to underestimate study findings and bias the results toward the null hypothesis. Therefore, the true association may be stronger than that observed in our study. A third limitation is the slight inaccuracy of the assessment of urinary protein due to our using the dipstick method.
In conclusion, the risks of development and progression of CKD increased from HbA1c levels of 7% in elderly as well as in middle-aged individuals in a general Japanese population.
Written informed consent was obtained from the patients before the commencement of the data analyses.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Levey AS, de Jong PE, Coresh J, et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int 80: 17-28, 2011. [DOI] [PubMed] [Google Scholar]
- 2. Coresh J. Update on the burden of CKD. J Am Soc Nephrol 28: 1020-1022, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Levey AS, Greene T, Schluchter MD, et al. Glomerular filtration rate measurements in clinical trials. Modification of Diet in Renal Disease Study Group and the Diabetes Control and Complications Trial Research Group. J Am Soc Nephrol 4: 1159-1171, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Watkins P. The UKPDS. A model for gathering the evidence for the management of chronic diseases. UK Prospective Diabetes Study Group. J R Coll Physicians Lond 32: 510-511, 1998. [PubMed] [Google Scholar]
- 5. Ohkubo Y, Kishikawa H, Araki E, et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract 28: 103-117, 1995. [DOI] [PubMed] [Google Scholar]
- 6. ADVANCE Collaborative Group, Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 358: 2560-2572, 2008. [DOI] [PubMed] [Google Scholar]
- 7. Ismail-Beigi F, Craven T, Banerji MA, et al. ; ACCORD trial group Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet 376: 419-430, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Duckworth W, Abraira C, Moritz T, et al. ; VADT Investigators Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 360: 129-139, 2009. [DOI] [PubMed] [Google Scholar]
- 9. Perkovic V, Heerspink HL, Chalmers J, et al. ; ADVANCE Collaborative Group Intensive glucose control improves kidneyoutcomes in patients with type 2 diabetes. Kidney Int 83: 517-523, 2013. [DOI] [PubMed] [Google Scholar]
- 10. Handelsman Y, Bloomgarden ZT, Grunberger G, et al. American association of clinical endocrinologists and american college of endocrinology - clinical practice guidelines for developing a diabetes mellitus comprehensive care plan - 2015. Endocr Pract 21 (Suppl): 1-87, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Qaseem A, Wilt TJ, Kansagara D, Horwitch C, Barry MJ, Forciea MA; Clinical Guidelines Committee of the American College of Physicians Hemoglobin A1c targets for glycemic control with pharmacologic therapy for nonpregnant adults with type 2 diabetes mellitus: a guidance statement update from the American College of Physicians. Ann Intern Med 168: 569-576, 2018. [DOI] [PubMed] [Google Scholar]
- 12. Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 53: 982-992, 2009. [DOI] [PubMed] [Google Scholar]
- 13. Shurraw S, Hemmelgarn B, Lin M, et al. ; Alberta Kidney Disease Network Association between glycemic control and adverse outcomes in people with diabetes mellitus and chronic kidney disease: a population-based cohort study. Arch Intern Med 171: 1920-1927, 2011. [DOI] [PubMed] [Google Scholar]
- 14. Examination Committee of Criteria for ‘Obesity Disease’ in Japan; Japan Society for the Study of Obesity New Criteria for “Obesity Disease” in Japan. Circ J 66: 987-992, 2002. [DOI] [PubMed] [Google Scholar]
- 15. The Japanese Society of Cardiovascular Disease Prevention. In: Handbook for Cardiovascular Prevention. Hokendojinsha, Tokyo, 2014. [Google Scholar]
- 16. Kinoshita M, Yokote K, Arai H, et al. ; Committee for Epidemiology and Clinical Management of Atherosclerosis Japan Atherosclerosis Society (JAS) guidelines for prevention of atherosclerotic cardiovascular disease 2017. J Atheroscler Thromb 25: 846-984, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yamanaka H; Japanese Society of Gout and Nucleic Acid Metabolism Japanese guideline for the management of hyperuricemia and gout: second edition. Nucleosides Nucleotides Nucleic Acids 30: 1018-1029, 2011. [DOI] [PubMed] [Google Scholar]
- 18. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 359: 1577-1589, 2008. [DOI] [PubMed] [Google Scholar]