Abstract
Constitutive activation of the Janus kinase/signal transduction and activator of transcription (JAK-STAT) signaling pathway plays a central role in the pathogenesis of myelofibrosis (MF) and pulmonary hypertension (PH) is a known complication of MF. On the other hand, it has been proposed that the JAK-STAT pathway, especially signal transducer and activation of transcription (STAT) 3 activation, protects cardiomyocytes from various stresses. We describe the case of a patient with MF-associated PH who developed left ventricular dysfunction after five years of treatment with the JAK 1/2 inhibitor, ruxolitinib. This is the first report with histopathological findings that demonstrate possible contradictory effects of a JAK 1/2 inhibitor: improvement of MF-associated PH and cardiotoxicity.
Keywords: onco-cardiology, pulmonary hypertension, tyrosine kinase inhibitor, cardiotoxicity, myelofibrosis
Introduction
Myelofibrosis (MF) is a chronic myeloproliferative disorder characterized by aberrant clonal proliferation of myeloid precursors accompanied by stromal fibrosis of the bone marrow. The discovery of the Janus kinase (JAK)2 V617F mutation, a gain-of-function driver mutation detected in more than half of patients with MF, greatly improved the understanding of the molecular pathogenesis of MF (1). Constitutive JAK2 activation increases the level of phosphorylated signal transducer and activation of transcription (STAT), particularly STAT3 and STAT5, which causes splenomegaly, progressive anemia, and extramedullary hematopoiesis (2). A JAK 1/2 inhibitor, ruxolitinib, is the first drug approved for the treatment of MF (3). Pulmonary hypertension (PH) is a known complication of MF that occurs in approximately 30% of patients. Ruxolitinib has been reported to improve MF-associated PH (4). On the other hand, STAT3 activation plays a protective role against various stresses on the heart (5). We herein report the first case in which ruxolitinib reduced the cardiac function despite improving MF-associated PH and demonstrate the histopathological findings.
Case Report
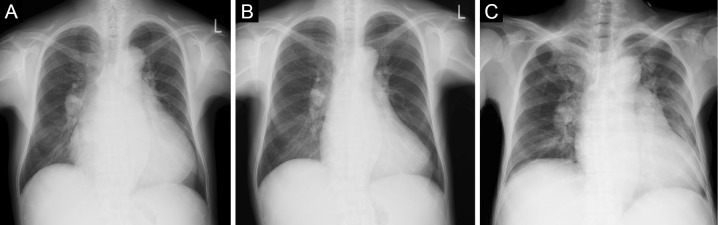
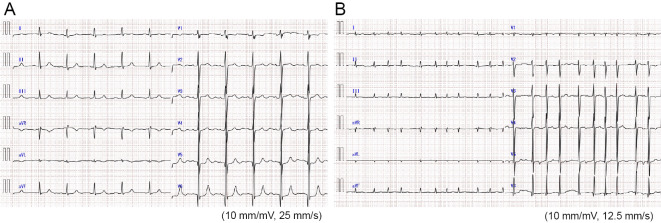
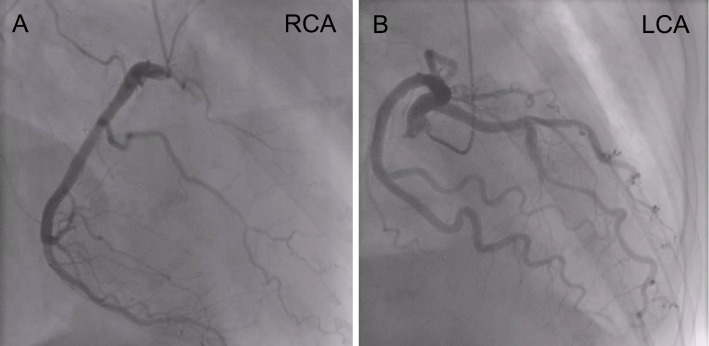
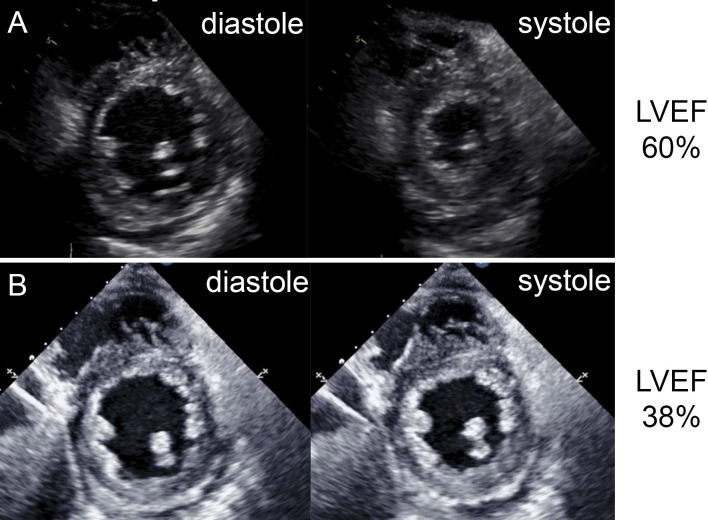
A 51-year-old woman was referred to our hospital for asymptomatic thrombocytosis. She was diagnosed with MF based on bone marrow biopsy in 1996. She had been followed as an outpatient for 15 years without medication. In 2009, paroxysmal atrial fibrillation (PAF) was detected and warfarin was started. In 2011, she gradually developed dyspnea with New York Heart Association (NYHA) functional class III and was admitted to our hospital for further evaluation. Chest radiography revealed cardiomegaly and a cardiothoracic ratio (CTR) of 67% (Fig. 1A). Electrocardiography showed sinus rhythm and incomplete right bundle branch block with no ST-segment change (Fig. 2A). She had stage 2 chronic kidney disease but no risk factors for coronary artery disease. Coronary angiography reveled no significant stenosis (Fig. 3). Echocardiography revealed the following findings: left ventricular ejection fraction (LVEF), 60% (Fig. 4A); left ventricular diameter at end-diastole (LVDd), 55 mm; left ventricular diameter at end-systole (LVDs), 36 mm; left atrial internal diameter at end-systole (LADs), 58 mm; ratio of mitral peak velocity of early filling (E wave) to that of late filling (A wave), 1.5; deceleration time, 170 msec; ratio of E wave to mitral annular early diastolic velocity (E/E'), 12.5; and tricuspid regurgitation pressure gradient (TRPG), 56 mmHg. The serum brain natriuretic peptide (BNP) level was 659.2 pg/mL. Right heart catheterization (RHC) revealed the following: systolic pulmonary artery pressure (sPAP), 61 mmHg; diastolic PAP (dPAP), 18 mmHg; mean PAP (mPAP), 32 mmHg; mean pulmonary arterial wedge pressure (PAWP), 10 mmHg; mean right atrium pressure (mRAP), 8 mmHg; diastolic pressure gradient (DPG), 8 mmHg; cardiac index (CI), 3.0 L/min/m2; and pulmonary vascular resistance (PVR), 4.7 Wood units. Pulmonary function studies showed mild restriction (vital capacity, 78% of predicted) with a reduced diffusing capacity of the lungs for carbon monoxide (DLCO, 50% of predicted). An arterial blood gas analysis showed hypoxemia [partial pressure of arterial oxygen (PaO2), 61.9 mmHg on room air]. Serological examinations were negative for connective tissue disease and liver disease. Chest computed tomography and lung perfusion scintigraphy revealed no evidence of lung disease or chronic thromboembolic pulmonary hypertension. After these diagnostic tests, she was diagnosed with MF-associated PH which was categorized into WHO group 5 PH, although diastolic dysfunction of the heart might partially contribute to the development of PH. Because anemia, progressive splenomegaly, an increased number of blasts in the peripheral blood, and symptoms associated with MF collectively suggested the progression of MF, treatment with furosemide (40 mg/day) and ruxolitinib (10 mg/day) was initiated instead of PAH-specific therapy.
Figure 1.
Chest radiography (A, B; taken in the upright position. C; taken in the supine position). (A) Chest radiography on the first admission before ruxolitinib treatment. (B) Chest radiography 5 months after the initiation of ruxolitinib treatment. (C) Chest radiography on the last admission for heart failure.
Figure 2.
Electrocardiograms. (A) Electrocardiogram before ruxolitinib treatment shows sinus rhythm and no significant ST-segment change. (B) Electrocardiogram on the last admission for heart failure shows atrial fibrillation and no ST-segment change.
Figure 3.
Coronary angiograms. (A) Coronary angiogram shows no significant stenosis in right coronary artery. (B) Coronary angiogram shows no significant stenosis in left coronary artery.
Figure 4.
Transthoracic echocardiography images. (A) Parasternal short-axis views (left, end-diastolic; right, end-systolic) show the preserved systolic function of the left ventricle before ruxolitinib treatment. (B) Parasternal short-axis views (left, end-diastolic; right, end-systolic) show the decreased systolic function of the left ventricle after ruxolitinib treatment.
Five months later, chest radiography showed improvement of cardiomegaly (CTR, 55%) (Fig. 1B) and her TRPG decreased from 56 mmHg to 21 mmHg. The BNP level also decreased to 229.1 pg/mL. Subsequently, the patient was stable with NYHA functional class I, although PAF progressed to chronic AF from 2013. Five years after the initiation of ruxolitinib, she gradually developed lower extremity edema, dyspnea and fatigue with NYHA functional class III. She was rehospitalized in 2016. On admission, her blood pressure was 89/60 mmHg and heart rate was 112 beats/min. Chest radiography revealed cardiomegaly and a CTR of 65% with pulmonary congestion (Fig. 1C). Electrocardiography showed atrial fibrillation and non-specific ST-T abnormalities (Fig. 2B). No myocardial necrosis biomarkers were positive. Her BNP level was 444.4 pg/mL. Echocardiography showed the following: diffuse left ventricular hypokinesis with a decreased LVEF of 38% (Fig. 4B); LVDd, 49 mm; LVDs, 40 mm; LADs, 52 mm; and E/E', 10.2. RHC, performed after the medical treatment of congestive heart failure with diuretics and dobutamine, revealed the following : sPAP, 32 mmHg; dPAP, 16 mmHg; mPAP, 24 mmHg; PAWP, 7 mmHg; mRAP, 7 mmHg; CI, 3.9 L/min/m2; and PVR, 2.7 Wood units, suggesting the improvement of precapillary PH. Unfortunately, she died of gastrointestinal bleeding 2 weeks later and an autopsy was performed.
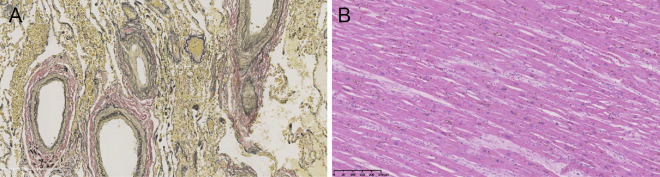
Macroscopic findings showed no myocardial infarction and no obstructed coronary artery. The pulmonary artery histopathology (Fig. 5A) showed thickening of the intimal layer of the muscular pulmonary arteries (Heath-Edwards classification grade I). On the other hand, histological examination of the left ventricle (Fig. 5B) showed mild basophilic changes in cardiomyocytes and congestive changes between cardiomyocytes, indicating myocardial injury and congestive heart failure. There was no evidence of fibrosis or cardiomyocyte hypertrophy.
Figure 5.
Histopathological examinations. (A) Histopathology of the pulmonary artery at high magnification. Thickening of the intimal layer of the muscular pulmonary arteries was identified on Elastica van Gieson staining. (B) Histopathology of the left ventricle of the heart at low magnification. Mild basophilic changes in cardiomyocytes and congestive changes between cardiomyocytes were identified on Hematoxylin and Eosin staining.
Discussion
The present case highlights the possible conflicting effects of long-term treatment with the JAK 1/2 inhibitor on MF-associated PH and the cardiac function. Myocardial infarction, atrial fibrillation, chronic kidney disease and secondary cardiomyopathies may become other causes of LV dysfunction, in addition to the cardiotoxicity of ruxolitinib. However, autopsy revealed no evidence of myocardial infarction, obstruction of the epicardial coronary artery or secondary cardiomyopathy. Although atrial fibrillation and chronic kidney disease may partially contribute to LV dysfunction, we still consider the possibility that ruxolitinib was pathogenically involved in the development of LV dysfunction.
Most molecular therapies target dysregulated signaling pathways. However, signaling pathways are ubiquitous and many are shared by different cell types in the same organism, although a cell-type-specific modification at the receptor, effector, or epigenetic level might exist (6). Importantly, ruxolitinib inhibits wild-type JAK2 as well as JAK2 with gain-of-function mutations, which means that ruxolitinib can inhibit JAK2 in all cell types, including hematopoietic cells, pulmonary artery smooth muscle cells (PASMCs), and cardiomyocytes. Our case cautions clinicians to be aware of possible side effects based on the cell-type-specific functional role of the signaling pathway.
MF originates clonally from abnormal hematopoietic progenitors in which JAK family kinases, specifically JAK1 and JAK2, are dysregulated. The constitutive activation of the JAK-STAT pathway in hematopoietic cells is a general feature of MF (7). This pathway is also involved in the pulmonary vascular remodeling observed in pulmonary arterial hypertension; STAT3 activation upregulates mediators that lead to the proliferation and anti-apoptosis of PASMCs (8). Animal experiments show that circulating hematopoietic myeloid progenitors released from bone marrow can participate in pathological pulmonary vascular remodeling (9). This finding suggests that abnormal hematopoietic progenitors in patients with MF might contribute to the development of MF-associated PH. Thus, ruxolitinib might resolve MF-associated PH by acting on both PASMCs and myeloid progenitors. In fact, ruxolitinib was reported to resolve MF-associated PH (4) although a conflicting case has also been reported (10). In the present case, MF-associated PH improved after ruxolitinib therapy.
On the other hand, several animal experiments have shown that STAT3 activation in response to stress plays a cardio-protective role in various murine models of heart failure (11-13). Notably, the administration of a selective JAK2 inhibitor after acute myocardial infarction results in deterioration of myocardial viability along with a reduction of STAT3 phosphorylation (14). These findings suggest that inhibition of JAK1/2 might worsen the cardiac function under various stresses. Indeed, the COMFORT-I trial demonstrated that long-term ruxolitinib treatment is associated with an increased incidence of congestive heart failure in patients with MF (0.7% at <12 months vs. 6.2% at >48 months) (15).
The present case was associated with several limitations. First, RHC was not performed in the acute phase of heart failure. Second, coronary angiography was not performed after the development of LV dysfunction. Despite these limitations, we consider that this is an important case report that suggests possible contradictory effects of JAK 1/2 inhibitor: improvement of MF-associated PH and cardiotoxicity.
In conclusion, we observed the improvement of MF-associated PH after the initiation of ruxolitinib; however, left ventricular systolic dysfunction developed after long-term treatment.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Tefferi A. Myeloproliferative neoplasms: a decade of discoveries and treatment advances. Am J Hematol 91: 50-58, 2016. [DOI] [PubMed] [Google Scholar]
- 2. Tefferi A. Primary myelofibrosis: 2017 update on diagnosis, risk-stratification, and management. Am J Hematol 91: 1262-1271, 2016. [DOI] [PubMed] [Google Scholar]
- 3. Harrison C, Kiladjian JJ, Al-Ali HK, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med 366: 787-798, 2012. [DOI] [PubMed] [Google Scholar]
- 4. Tabarroki A, Lindner DJ, Visconte V, et al. Ruxolitinib leads to improvement of pulmonary hypertension in patients with myelofibrosis. Leukemia 28: 1486-1493, 2014. [DOI] [PubMed] [Google Scholar]
- 5. Yamauchi-Takihara K, Kishimoto T. A novel role for STAT3 in cardiac remodeling. Trends Cardiovasc Med 10: 298-303, 2000. [DOI] [PubMed] [Google Scholar]
- 6. Schaefer MH, Yang JS, Serrano L, Kiel C. Protein conservation and variation suggest mechanisms of cell type-specific modulation of signaling pathways. PLoS Comput Biol 10: e1003659, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rampal R, Al-Shahrour F, Abdel-Wahab O, et al. Integrated genomic analysis illustrates the central role of JAK-STAT pathway activation in myeloproliferative neoplasm pathogenesis. Blood 123: e123-e133, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Paulin R, Courboulin A, Meloche J, et al. Signal transducers and activators of transcription-3/pim1 axis plays a critical role in the pathogenesis of human pulmonary arterial hypertension. Circulation 123: 1205-1215, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yan L, Chen X, Talati M, et al. Bone marrow-derived cells contribute to the pathogenesis of pulmonary arterial hypertension. Am J Respir Crit Care Med 193: 898-909, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Low AT, Howard L, Harrison C, Tulloh RM. Pulmonary arterial hypertension exacerbated by ruxolitinib. Haematologica 100: e244-e245, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Harada M, Qin Y, Takano H, et al. G-CSF prevents cardiac remodeling after myocardial infarction by activating the Jak-Stat pathway in cardiomyocytes. Nat Med 11: 305-311, 2005. [DOI] [PubMed] [Google Scholar]
- 12. Kunisada K, Negoro S, Tone E, et al. Signal transducer and activator of transcription 3 in the heart transduces not only a hypertrophic signal but a protective signal against doxorubicin-induced cardiomyopathy. Proc Natl Acad Sci U S A 97: 315-319, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hikoso S, Yamaguchi O, Higuchi Y, et al. Pressure overload induces cardiac dysfunction and dilation in signal transducer and activator of transcription 6-deficient mice. Circulation 110: 2631-2637, 2004. [DOI] [PubMed] [Google Scholar]
- 14. Negoro S, Kunisada K, Tone E, et al. Activation of JAK/STAT pathway transduces cytoprotective signal in rat acute myocardial infarction. Cardiovasc Res 47: 797-805, 2000. [DOI] [PubMed] [Google Scholar]
- 15. Verstovsek S, Mesa RA, Gotlib J, et al. Long-term treatment with ruxolitinib for patients with myelofibrosis: 5-year update from the randomized, double-blind, placebo-controlled, phase 3 COMFORT-I trial. J Hematol Oncol 10: 55, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]