Abstract
Objective
Pneumonia develops in bedridden patients, even in those receiving oral care, and malnutrition is associated with the development of pneumonia. We examined the effects of nutritional treatment on the prevention of pneumonia.
Patients and Methods
We retrospectively examined the effects of nutritional treatment on the prevention of pneumonia by analyzing the records of bedridden patients (n=68; mean age: 68.0 years) who stayed in a hospital for 2 years or longer.
Results
Among the analyzed patients, pneumonia developed in 52 (76%) patients, and the mean frequency of pneumonia was 1.6 times per year during the first year of stay. In a multivariate analysis, the serum albumin level at admission in the pneumonia group was lower than that in the non-pneumonia group. The frequency of pneumonia during the second year of stay was lower than that during the first year of stay. Serum levels of albumin and total protein (TP) at one year after admission were higher than those at admission in all analyzed patients, and in all patients (n=52) and elderly (≥65 years) patients (n=31) in the pneumonia group. The proportions of patients with hypoalbuminemia (<3.5 g/dL) and hypoproteinemia (<6.5 g/dL) at one year after admission were lower than those at admission. The increases in the proportions of patients presenting a reduced frequency of pneumonia were correlated with increases in the proportions of patients presenting increased levels of albumin and/or TP.
Conclusion
Nutritional treatment may reduce the frequency of pneumonia by improving malnutrition in bedridden patients receiving oral care.
Keywords: bedridden patients, malnutrition, nutritional treatment, oral care, prevention of pneumonia
Introduction
The number of patients with pneumonia has been increasing in Japan, and pneumonia is the third leading cause of death. Aspiration pneumonia is frequent among elderly adults (1). In particular, nerve dysfunction, which can be caused by various diseases, such as cerebral infarction, affects both swallowing and the cough reflex and is associated with the aspiration of oropharyngeal secretions (2), which contain bacteria (3). Bedridden patients with severely reduced activities of daily living (ADL) may lose the ability to brush their teeth or perform good oral hygiene (4), which may increase colonization by oral bacteria and induce aspiration pneumonia (2). In addition, aspiration pneumonia is one of the most common causes of death in bedridden patients receiving percutaneous endoscopic gastrostomy (PEG) feeding (5), and pneumonia develops even when patients receive appropriate oral care (6).
Bedridden patients exhibit several factors that are associated with the development of pneumonia and high mortality, including limitations in ADL, older age and/or the presence of pre-existing lung and heart disease (7). Langmore et al. identified several predictors of aspiration pneumonia, including feeding and oral care dependency, the number of decayed teeth and reliance on tube feeding, and highlighted the importance of malnutrition in the development of aspiration pneumonia (8). Low body mass index (BMI), hypoalbuminemia and limitations in ADL are also associated with mortality in patients with senile pneumonia (9). We reported that low serum levels of albumin are independent risk factors for pneumonia in elderly bedridden adults (10); these results are consistent with a report indicating that malnutrition reduces immune responses against infection (11). Takenoshita et al. reported that tube feeding decreases the incidence of pneumonia in patients with severe dementia (12). However, the relationship between the improvement of malnutrition by nutritional treatment and the reduction in pneumonia frequency in bedridden adults has not been studied.
In the present study, we retrospectively examined, by analyzing patient records, whether nutritional treatment may improve malnutrition and whether the improvement of malnutrition may be associated with a reduction in the frequency of pneumonia in bedridden adults receiving oral care.
Materials and Methods
Design, Settings and Participants
We enrolled patients according to the order of the discharge date and analyzed patient records. The inclusion criteria were as follows: patients who were bedridden, received oral care and were admitted to Hachinohe National Hospital to receive rehabilitation and nursing care for two years or longer after being treated for an acute disease or after receiving mechanical ventilation between October 2001 and September 2016.
All patients underwent suctioning of nasopharyngeal secretions, repositioning of the bed, changing of position for feeding, and changing of urinary catheters, as previously described (10). Hachinohe National Hospital Ethics Committee approved this retrospective study.
Measurements
The observation period, age, sex, type of oral care, causes of hospitalization, comorbidities, number of deaths during stay, treatment with drugs to improve the swallowing reflex, treatment with proton pump inhibitors (PPIs), type of tube feeding used, use of tracheostomy and mechanical ventilation, use of urinary catheterization, presence of pressure ulcers, total energy of nutrition (energy expenditure) and consciousness level were recorded. Consciousness levels were defined using the Glasgow Coma Scale (13).
We also checked the following daily hospital data recorded at the time of patient admission and at one year after admission, as previously reported (10): BMI; the white blood cell (WBC) and lymphocyte counts in the peripheral venous blood; hemoglobin (Hb); and the serum levels of total protein (TP), albumin, total cholesterol, blood urea nitrogen (BUN), creatinine, creatine phosphokinase (CPK), iron, uric acid and C-reactive protein (CRP).
Pathogens in sputum or aspirates from the tracheostomy tube were investigated using standard microbiological procedures. These examinations were ordered when pneumonia developed in each patient.
The frequency of pneumonia was measured and analyzed by counting the frequency of pneumonia development during the first and second years of stay. As we previously reported (10), the low level of albumin at admission was correlated with the frequency of pneumonia during the 9 months of stay following admission. Thus, to examine the relationship between the nutritional condition at one year and two years after admission and the frequency of pneumonia during the first and second years after admission, the laboratory data and BMI obtained at admission and at one year after admission were analyzed.
Definition of pneumonia
Pneumonia was diagnosed based on the following standard criteria: fever (body temperature ≥37.8°C), high CRP level and infiltrate shadows on chest X-ray and/or computed tomography (14).
Grouping of patients
The patients were divided into pneumonia (n=52) and non-pneumonia (n=16) groups. The pneumonia group included patients who developed pneumonia during the first year of stay, and the non-pneumonia group included patients who did not develop pneumonia during the first year of stay.
Furthermore, the frequency of pneumonia and biochemical properties during nutritional treatment were analyzed in all patients (n=68), all pneumonia group patients (n=52) and all elderly pneumonia group patients (≥65 years) (n=31).
Additionally, the data for all patients (n=68), all pneumonia group patients (n=52), all elderly patients (n=44), and all elderly pneumonia patients (n=31) were analyzed to examine the relationship between nutritional improvement and the reduction in the frequency of pneumonia.
The relationship between malnutrition improvement and decreases in the frequency of pneumonia
To examine the relationship between malnutrition improvement and decreases in the frequency of pneumonia, we analyzed the distribution of the patients. The patients were divided into nine subgroups according to changes in the values of serum albumin, serum TP, or BMI (increase, no change, or decrease) by comparing the values at one year after admission to the values at the time of admission and according to changes in the frequency of pneumonia (increase, no change, or decrease) by comparing the frequency during the second year of stay to the frequency during the first year of stay.
Nutritional management
The calorific values of the nutritional treatment using tube feeding, intravenous hyperalimentation and meal ingestion were defined by calculating energy expenditure by the Harris-Benedict equation (basal metabolism) multiplied by an activity factor and a stress factor (15-17) as follows.
Men: The value of energy expenditure = Harris-Benedict equation [66.4730+13.7516×body weight (kg)+5.0033×height (cm)-6.7550×age (year)]×activity factor×stress factor.
Women: The value of energy expenditure = Harris-Benedict equation [655.0955+9.5643×body weight (kg)+1.8496×height (cm)-4.6756×age (year)]×activity factor×stress factor.
In the equation used in the present study, the activity factor was 1.0 because all patients were bedridden. Furthermore, the stress factor was 1.1-1.5 in patients with pressure ulcers according to the grade of the ulcer or 1.0 in patients without pressure ulcers.
Statistical analysis
The results are expressed as the mean±SD. Student's t-test, Pearson's chi-squared test or Fisher's exact test was used for the comparison of continuous variables between the two groups. An analysis of covariance (ANCOVA) was performed to examine the association between pneumonia and the albumin level. We adjusted for potential confounders, including age (continuous variable) (1, 8), consciousness level (continuous variable) (18), tube feeding (no or yes) (5, 8), mechanical ventilation (no or yes) (19), and pulmonary disease (no or yes) (7, 20, 21). These variables were additionally entered into models (Models 1-5) (22). A paired t-test and McNemar's test were performed to compare variables between the first and second one-year observation periods (frequency of pneumonia) and between admission and one year after admission (BMI and laboratory data). A chi-square test was performed to examine the relationship between the improvement in malnutrition (the albumin and TP levels and BMI) and the reduced frequency of pneumonia. An ANCOVA was also used when the abovementioned confounding factors were considered.
Results
Characteristics of subjects
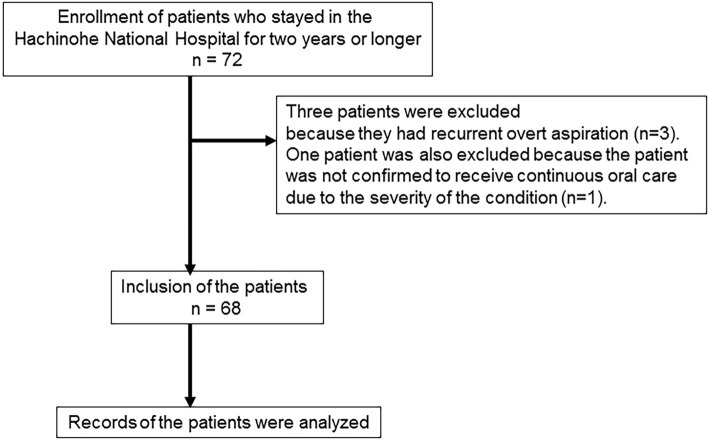
In the present study, 72 patients were initially enrolled. The patients were admitted to Hachinohe National Hospital between October 2001 and September 2016. Of the enrolled patients, three were excluded because they had recurrent overt aspiration (n=3), and one was excluded because we could not confirm whether the patient had received continuous oral care due to a severe condition (n=1) (Figure).
Figure.
A schematic diagram outlining the patient selection process.
The characteristics of the 68 patients examined in the study are shown in Table 1. The mean age of all analyzed patients, patients in the pneumonia group and patients in the non-pneumonia group was 68.0 years, 66.9 years and 71.6 years, respectively (Table 1). Patients who were admitted to the hospital between October 2001 and September 2006 received oral care using wet gauze (6). In contrast, patients who were admitted to the hospital between October 2006 and September 2016 received oral hygiene using a toothbrush (6). However, the proportions of patients receiving oral care using wet gauze or a toothbrush in the pneumonia group did not differ from the proportions in the non-pneumonia group (Table 1).
Table 1.
Patient Characteristics and Nutritional Supply.
| Characteristics, interventions, and effects | Analyzed patients | Pneumonia group | Non-pneumonia group | p value |
|---|---|---|---|---|
| Characteristics | ||||
| Number of patients, n | 68 | 52 | 16 | |
| Age (years, mean±SD) | 68.0±14.7 | 66.9±14.6 | 71.6±14.7 | 0.263 |
| Males, n (%)1) | 32 (47.1) | 26 (50.0) | 6 (37.5) | 0.381 |
| Oral care using a toothbrush, n (%)2) | 60 (88.2) | 46 (88.5) | 14 (87.5) | 0.609 |
| Oral care using wet gauze, n (%)2) | 7 (10.3) | 6 (11.5) | 1 (6.3) | 0.474 |
| Cause of hospitalization, n (%) | ||||
| ALS1) | 25 (36.8) | 20 (38.5) | 5 (31.3) | 0.601 |
| Cerebral infarction2) | 9 (13.2) | 7 (13.5) | 2 (12.5) | 0.645 |
| Muscular dystrophy2) | 9 (13.2) | 9 (17.3) | 0 (0.0) | 0.075 |
| COPD2) | 4 (4.4) | 2 (3.8) | 2 (12.5) | 0.233 |
| Others2,3) | 21 (30.9) | 16 (30.8) | 5 (31.3) | 0.599 |
| Comorbidities, n | ||||
| Pulmonary diseases1) | 23 (33.8) | 20 (38.5) | 3 (18.8) | 0.145 |
| Cardiovascular diseases1) | 22 (32.4) | 14 (26.9) | 8 (50.0) | 0.084 |
| Diabetes mellitus2) | 16 (23.5) | 13 (25.0) | 3 (18.8) | 0.442 |
| Hypertension2) | 12 (17.6) | 8 (15.4) | 4 (25.0) | 0.295 |
| Atrial fibrillation2) | 11 (16.2) | 7 (13.5) | 4 (25.0) | 0.233 |
| Cerebral infarction2) | 9 (13.2) | 9 (17.3) | 0 (0.0) | 0.075 |
| Epilepsy2) | 8 (11.8) | 7 (13.5) | 1 (6.3) | 0.391 |
| Others1,4) | 31 (45.6) | 24 (46.2) | 7 (43.8) | 0.866 |
| Number of death, n2) | 2 (2.9) | 2 (3.8) | 0 (0.0) | 1.000 |
| Treatment with drugs to improve the swallowing reflex, n (%)2) | 9 (13.2) | 9 (17.3) | 0 (0.0) | 0.075 |
| Treatment with ACEIs2) | 1 (1.5) | 1 (1.9) | 0 (0.0) | 0.765 |
| Treatment with dopamine analogs2) | 7 (10.3) | 7 (13.5) | 0 (0.0) | 0.138 |
| Treatment with cilostazol2) | 1 (1.5) | 1 (1.9) | 0 (0.0) | 0.765 |
| Treatment with PPIs2) | 7 (10.3) | 6 (11.5) | 1 (6.3) | 0.474 |
| Tube feeding, n (%)2) | 61 (89.7) | 48 (92.3) | 13 (81.3) | 0.204 |
| PEG tube feeding2) | 47 (69.1) | 37 (71.2) | 10 (62.5) | 0.358 |
| Nasogastric tube feeding2) | 14 (20.6) | 11 (21.2) | 3 (18.8) | 0.572 |
| IVH, n (%)2) | 2 (2.9) | 1 (1.9) | 1 (6.3) | 0.418 |
| Oral intake with assistance, n (%)2) | 5 (7.4) | 3 (5.8) | 2 (12.5) | 0.335 |
| Tracheostomy, n (%)2) | 50 (73.5) | 38 (73.1) | 12 (75.0) | 0.578 |
| Mechanical ventilation, n (%)1) | 41 (60.3) | 30 (57.7) | 11 (68.8) | 0.429 |
| Urinary catheterization, n (%)1) | 34 (50.0) | 24 (46.2) | 10 (62.5) | 0.253 |
| Pressure ulcer, n (%)1) | 28 (41.2) | 22 (42.3) | 6 (37.5) | 0.733 |
| Nutrition, K cal, mean±SD | 1,066±228 | 1,021±203 | 1,214±249 | 0.002 |
| Consciousness level, mean±SD | 12.9±3.5 | 12.6±3.7 | 13.8±2.7 | 0.215 |
For the comparison of continuous variables between the two groups, Student’s t-test, Pearson’s chi-squared test or Fisher’s exact test was used.
1)Pearson’s chi-squared test.
2)Fisher’s exact test.
3)The "Others" category includes cerebral palsy (n=3), Parkinson’s disease (n=3), tuberculous meningitis (n=3), cerebral bleeding (n=2), hypoxic encephalopathy (n=2), unresponsive wakefulness syndrome (n=1), brain contusion (n=1), Guillain-Barré syndrome (n=1), multiple system atrophy (n=1), syringomyelia (n=1), symptomatic epilepsy (n=1), traumatic cervical spinal cord injury (n=1), and brain tumor (n=1).
4)The "Others" category includes bladder stone, cancer, cholecystitis, cholelithiasis, chronic hepatitis, gastroduodenal ulcer, hydrocephalus, hyperthyroidism, hypothyroidism, hypoxic encephalopathy, intestinal obstruction, Parkinson’s disease, renal stone, schizophrenia and subdural hematoma.
ACEI: angiotensin-converting enzyme inhibitor, ALS: amyotrophic lateral sclerosis, IVH: intravenous hyperalimentation, PEG: percutaneous endoscopic gastrostomy, PPI: proton pump inhibitor
The causes of hospitalization among bedridden patients are shown in Table 1. The proportions of the causes of hospitalization did not differ between the pneumonia and non-pneumonia groups.
In addition to treatments for these diseases, the patients were treated with drugs for comorbid conditions (Table 1). The proportions of the comorbidities did not differ between the two groups. Two patients in the pneumonia group died of pneumonia at 20 and 23 months of observation; however the mortality rate did not differ between the two groups (Table 1).
The proportions of patients receiving treatment with drugs to improve their swallowing reflex or treatment with PPIs, tube feeding, intravenous hyperalimentation (IVH), tracheostomy, mechanical ventilation and urinary catheterization as well as the proportion of patients with pressure ulcers and the consciousness levels of the patients did not differ between the two groups (Table 1). The proportion of patients who could self-feed (defined as meal ingestion) did not differ between the two groups. In contrast, the calorific values of the nutritional treatment for the patients in the non-pneumonia group were higher than those for the patients in the pneumonia group.
Physical examination and biochemical properties at baseline
The BMI of patients in the pneumonia group tended to be lower than that of patients in the non-pneumonia group; however, the difference was not statistically significant (Table 2). The serum levels of albumin and creatinine in patients in the pneumonia group were lower than those in patients in the non-pneumonia group (Table 2). The serum levels of TP and total cholesterol in the patients in the pneumonia group also tended to be lower than those in the patients in the non-pneumonia group; however, the difference was not statistically significant (Table 2). The serum CRP levels in the pneumonia group tended to be higher than those in the non-pneumonia group (Table 2).
Table 2.
Physical Examination and Laboratory Findings at Baseline.
| Physical examination and laboratory data | All analyzed patients (n=68) | Pneumonia group (n=52) | Non- pneumonia group (n=16) | p value |
|---|---|---|---|---|
| Physical examinations and laboratory data at admission | ||||
| Physical examination | ||||
| BMI (mean±SD) | 17.8±3.6 | 17.6±3.6 | 18.6±3.6 | 0.324 |
| Laboratory data | ||||
| White blood cells (/μL, mean±SD) | 8,084±3,788 | 8,315±4,188 | 7,331±1,908 | 0.367 |
| Lymphocytes (/μL, mean±SD) | 1,665±657 | 1,704±662 | 1,538±642 | 0.382 |
| CRP (mg/dL, mean±SD) | 1.9±4.0 | 2.3±4.5 | 0.7±0.8 | 0.176 |
| Hb (g/dL, mean±SD) | 11.7±1.9 | 11.7±1.9 | 11.8±1.8 | 0.812 |
| Serum iron (μg/dL, mean±SD) | 50.6±23.8 (n=36) |
53.5±24.8 (n=27) |
42.0±19.1 (n=9) |
0.214 |
| Total protein (g/dL, mean±SD) | 6.5±0.7 | 6.4±0.7 | 6.7±0.7 | 0.071 |
| Albumin (g/dL, mean±SD) | 3.2±0.5 | 3.1±0.5 | 3.4±0.6 | 0.028 |
| Total cholesterol (mg/dL mean±SD) | 160±38 | 157±40 | 172±31 | 0.179 |
| BUN (mg/dL, mean±SD) | 17.6±12.5 | 17.7±13.7 | 17.2±7.3 | 0.898 |
| Creatinine (mg/dL, mean±SD) | 0.41±0.28 | 0.38±0.26 | 0.54±0.32 | 0.045 |
| CPK (mg/dL, mean±SD) | 57.8±68.3 (n=63) |
54.7±51.7 (n=47) |
66.8±104.9 (n=16) |
0.545 |
| Uric acid (mg/dL, mean±SD) | 3.6±1.9 (n=67) |
3.4±1.7 (n=51) |
4.3±2.4 (n=16) |
0.108 |
| Proportion of patients, n (%) | ||||
| with>9,000 /μL WBC1) | 16 (23.5) | 14 (26.9) | 2 (12.5) | 0.200 |
| with<1,000 /μL lymphocytes1) | 8 (11.8) | 6 (11.5) | 2 (12.5) | 0.609 |
| with<6.5 g/dL total protein2) | 33 (48.5) | 29 (55.8) | 4 (25.0) | 0.031 |
| with<3.5 g/dL albumin1) | 48 (70.6) | 42 (80.8) | 6 (37.5) | 0.002 |
| with anemia2,3) | 44 (64.7) | 34 (65.4) | 10 (62.5) | 0.833 |
| with iron deficiency1,3) | 17 (47.2) (n=36) |
12 (44.4) (n=27) |
5 (55.6) (n=9) |
0.423 |
| with high CRP1,3) | 51 (75.0) | 39 (75.0) | 12 (75.0) | 0.639 |
| with low uric acid2,3) | 25 (37.3) (n=67) |
20 (39.2) (n=51) |
5 (31.3) (n=16) |
0.565 |
For the comparison of continuous variables between the two groups, Student’s t-test, Pearson’s chi-squared test or Fisher’s exact test was used.
Serum iron was measured in 26 patients in the pneumonia group and 9 patients in the nonpneumonia group. CPK was measured in 47 patients in the pneumonia group and 16 patients in the nonpneumonia group. Uric acid was measured in 51 patients in the pneumonia group and 16 patients in the nonpneumonia group.
1)Fisher’s exact test.
2)Pearson’s chi-squared test.
3)Anemia: <11.3 g/dL Hb in women, <13.5 g/dL Hb in men; iron deficiency: <43 μg/dL Fe in women, <54 μg/dL Fe in men; high CRP: >0.2 mg/dL; low uric acid: <2.3 μg/dL uric acid in women, <3.6 mg/dL uric acid in men.
BMI: body mass index, BUN: blood urea nitrogen, CPK: creatine phosphokinase, CRP: C-reactive protein, Hb: hemoglobin, WBC: white blood cells
The proportions of patients with low TP (<6.5 g/dL) and albumin (<3.5 g/dL) values in the pneumonia group were significantly higher than those in the non-pneumonia group (Table 2).
Association between serum albumin and pneumonia
In the multivariate analysis, the serum albumin level among individuals in the pneumonia group was lower than that among those in the non- pneumonia group. This association was confirmed, even when potential confounding factors were included (Model 5, p=0.038) (Table 3).
Table 3.
Adjusted Mean (95% Confidence Intervals) Serum Albumin Levels among the Pneumonia and Non-pneumonia Groups.
| Pneumonia group | Non-pneumonia group | p for trend | |
|---|---|---|---|
| Crude | 3.10 (2.95, 3.24) | 3.43 (3.17, 3.69) | 0.028 |
| Model 1a) | 3.08 (2.95, 3.22) | 3.48 (3.23, 3.72) | 0.007 |
| Model 2b) | 3.09 (2.96, 3.23) | 3.45 (3.21, 3.70) | 0.012 |
| Model 3c) | 3.10 (2.96, 3.23) | 3.44 (3.19, 3.68) | 0.018 |
| Model 4d) | 3.10 (2.96, 3.23) | 3.44 (3.19, 3.69) | 0.018 |
| Model 5e) | 3.10 (2.97, 3.24) | 3.41 (3.16, 3.66) | 0.038 |
Values are presented as the estimated mean (95% CI).
a) Adjusted for age (continuous variable).
b) Additionally adjusted for consciousness level (continuous variable).
c) Additionally adjusted for tube feeding (no or yes).
d) Additionally adjusted for mechanical ventilation (no or yes).
e) Additionally adjusted for pulmonary diseases (no or yes).
Identified pathogens
Pathogens were identified in 47 of 49 patients (96%) in whom microbiological evaluation was performed. Pseudomonas aeruginosa was the most frequently identified pathogen in patients in the pneumonia group (n=41) (Table 4). Furthermore, Acinetobacter species, methicillin-susceptible Staphylococcus aureus (MSSA), Klebsiella pneumoniae and Escherichia coli were identified in 14, 12, 10 and 10 patients, respectively. Thus, Gram-negative bacteria were identified more frequently than Gram-positive bacteria. In addition, two or more bacteria were identified in more than half of the patients (n=28, 57%) (Table 4).
Table 4.
Pathogens Identified in Patients in the Pneumonia Group.
| Number of patients | |
|---|---|
| Pathogen isolated | 471) |
| No pathogen isolated | 2 |
| No evaluated | 3 |
| Gram-positive pathogens | |
| Streptococcus pneumoniae | 3 |
| Staphylo coccus aureus | |
| MSSA | 12 |
| MRSA | 7 |
| Gram-negative pathogens | |
| Hemophilus Influenzae | 4 |
| Klebsiella pneumoniae | 10 |
| Pseudomonasaeru ginosa | 41 |
| Escherichia coli | 10 |
| Acinetobacter species | 14 |
| Moraxella catarrhalis | 7 |
| Other Gram-negative pathogens | 2 |
1)Two or more species of bacteria were identified in 28 patients.
Frequency of pneumonia and biochemical properties during nutritional treatment
In all analyzed patients, the mean frequency of pneumonia was 1.6 times per year during the first year of stay (Table 5). Pneumonia developed in 52 (pneumonia group patients) of the 68 patients (76%, 52/68) analyzed during the first year of stay and in 31 patients in the pneumonia group during the second year of stay. In addition, in the non-pneumonia group, one patient developed pneumonia during the second year of stay, although no patients in the group developed pneumonia during the first year of stay. In all analyzed patients, the frequency of pneumonia during the second year of stay was significantly lower than that during the first year of stay (Table 5).
Table 5.
Frequency of Pneumonia and Physical Examination and Laboratory Findings for All Analyzed Patients at the First and Second Years of Stay.
| Physical examination and laboratory data | First year (n=68) |
Second year (n=68) |
p value |
|---|---|---|---|
| Frequency of pneumonia1,2) (/year, mean±SD) | 1.6±1.5 | 0.7±0.9 | <0.001 |
| Physical examination and laboratory data2,3) | |||
| Physical examination | |||
| BMI (mean±SD) | 17.8±3.6 | 18.0±3.2 | 0.291 |
| Laboratory data | |||
| Total protein (g/dL, mean±SD) | 6.5±0.7 | 7.0±0.6 | <0.001 |
| Albumin (g/dL, mean±SD) | 3.2±0.5 | 3.4±0.5 | <0.001 |
| Total cholesterol (mg/dL mean±SD) | 160±38 (n=68) |
160±27 (n=66) |
0.990 |
| Hb (g/dL, mean±SD) | 11.7±1.9 | 11.5±1.9 | 0.235 |
| Serum iron (μg/dL, mean±SD) | 50.6±23.8 (n=37) |
48.9±23.1 (n=11) |
0.489 |
| Uric acid (mg/dL, mean±SD) | 3.6±1.9 (n=67) |
3.7±1.8 (n=61) |
0.635 |
| White blood cells (/μL, mean±SD) | 8,083±3,788 | 6,938±2,650 | 0.011 |
| Lymphocytes (/μL, mean±SD) | 1,665±657 | 1,798±719 | 0.094 |
| CRP (mg/dL, mean±SD) | 1.9±4.0 | 1.5±2.1 | 0.488 |
| Proportion of patients, n (%) | |||
| with <6.5 g/dL total protein | 33 (48.5) | 12 (17.6) | <0.001 |
| with <3.5 g/dL albumin | 48 (70.6) | 32 (47.1) | 0.001 |
| with anemia | 44 (64.7) | 43 (63.2) | 1.000 |
| with iron deficiency | 17 (47.2) (n=36) |
7 (63.6) (n=11) |
1.000 |
| with low uric acid | 25 (37.3) (n=67) |
22 (36.1) (n=61) |
0.804 |
| with >9,000 /μL WBC | 16 (23.5) | 12 (17.6) | 0.424 |
| with <1,000 /μL lymphocytes | 8 (11.8) | 9 (13.2) | 1.000 |
| with high CRP | 51 (75.0) | 51 (75.0) | 1.000 |
1)The frequency of pneumonia was measured by counting the frequency of pneumonia development during the first and second years of stay, separately.
2)For the comparison of variables between the first and second years of stay (frequency of pneumonia) and between the time of admission and one year after admission (BMI and laboratory data), paired t-tests and McNemar’s tests were used.
3)Laboratory data and BMI at admission and one year after admission are reported.
Furthermore, the serum TP and albumin values at one year after admission were significantly higher than those at the time of admission (Table 5). The proportions of patients with low TP and albumin values at one year after admission were lower than those of patients with these characteristics at the time of admission (Table 5).
Similarly, in the patients in the pneumonia group, the frequency of pneumonia during the second year of stay was significantly lower than the frequency during the first year of stay (Table 6). Furthermore, the serum TP and albumin values at one year after admission were higher than those at the time of admission (Table 6). The proportions of patients with low TP and albumin values at one year after admission were lower than those of patients with these characteristics at the time of admission (Table 6).
Table 6.
Characteristics, Frequency of Pneumonia, Physical Examination or Laboratory Findings Related to the Nutritional Condition of All or Elderly Patients in the Pneumonia Group during the First and Second Years of Stay.
| All patients in the pneumonia group (n=52) | First year | Second year | p value |
|---|---|---|---|
| Frequency of pneumonia1) (/year, mean±SD) | 2.1±1.3 | 0.9±1.0 | <0.001 |
| Physical examination and laboratory data2,3) | |||
| Physical examination | |||
| BMI (mean±SD) | 17.6±3.6 | 17.7±3.0 | 0.551 |
| Laboratory data | |||
| Total protein (g/dL, mean±SD) | 6.4±0.7 | 7.0±0.6 | <0.001 |
| Albumin (g/dL, mean±SD) | 3.1±0.5 | 3.4±0.5 | <0.001 |
| Total cholesterol (mg/dL mean±SD) | 157±40 (n=52) |
161±27 (n=50) |
0.459 |
| Proportion of patients, n (%) | |||
| with <6.5 g/dL total protein | 29 (55.8) | 10 (19.2) | <0.001 |
| with <3.5 g/dL albumin | 42 (80.8) | 27 (51.9) | 0.001 |
| Elderly patients in the pneumonia group (n=31) | First year | Second year | p value |
| Characteristics | |||
| Age (years, mean±SD) | 76.6±8.1 | - | - |
| Males, n (%) | 12 (38.7) | - | - |
| Frequency of pneumonia1,3) (/year, mean±SD) | 2.0±1.5 | 0.9±1.0 | <0.001 |
| Physical examinations and laboratory data2,3) | |||
| Physical examination | |||
| BMI (mean±SD) | 17.6±3.2 | 17.9±3.0 | 0.299 |
| Laboratory data | |||
| Total protein (g/dL, mean±SD) | 6.2±0.6 | 6.8±0.7 | <0.001 |
| Albumin (g/dL, mean±SD) | 3.0±0.5 | 3.3±0.5 | <0.001 |
| Total cholesterol (mg/dL, mean±SD) | 156±39 (n=31) |
165±27 (n=29) |
0.257 |
| Proportion of patients, n (%) | |||
| with <6.5 g/dL total protein | 21 (67.7) | 9 (29.0) | 0.004 |
| with <3.5 g/dL albumin | 28 (90.3) | 18 (58.1) | 0.006 |
1)The frequency of pneumonia was measured by counting the frequency of pneumonia development during the first and second years of stay, separately.
2)Laboratory data and BMI at admission and one year after admission are reported.
3)For the comparison of variables between the first and second years of stay (frequency of pneumonia) and between admission and one year after admission (BMI and laboratory data), paired t-tests and McNemar’s tests were used.
Among the elderly patients in the pneumonia group, the frequency of pneumonia during the second year of stay was significantly lower than the frequency during the first year of stay (Table 6). Furthermore, the serum TP and albumin values at one year after admission were higher than the values at the time of admission (Table 6). The proportions of patients with low TP and albumin values at one year after admission were lower than those at the time of admission (Table 6).
Nutritional condition in dead patients
In one of the two patients who died of pneumonia, the patient's BMI markedly decreased from 19.0 kg/m2 at the time of admission to 14.7 kg/m2 at one year after admission, and the serum albumin levels continued to be extremely low (2.2 g/dL at admission and 2.1 g/dL at one year after admission). In contrast, the serum TP level increased from 5.9 g/dL at admission to 6.3 g/dL at one year after admission, although the TP levels remained low.
In the other patient who died of pneumonia, the patient's BMI decreased from 23.2 kg/m2 at admission to 21.6 kg/m2 at one year after admission, and the serum albumin level was extremely low at both time points (2.9 g/dL at the admission and 2.7 g/dL at one year after admission). Furthermore, the serum level of TP decreased from 8.6 g/dL at admission to 5.6 g/dL at one year after admission.
Relationship between nutritional improvement and the reduction in the frequency of pneumonia
In the patients in the pneumonia group, there was a significant relationship between improvement in the serum level of albumin measured at one year after admission and a decreased frequency of pneumonia during the second year of stay (Table 7). A high proportion of patients presenting both an increased serum albumin level and a decreased frequency of pneumonia was observed in the pneumonia group (Table 7).
Table 7.
Relationship between Improvement in the Serum Levels of Albumin and Total Protein and the Decreased Frequency of Pneumonia in Patients in the Pneumonia Group.
| Number (%) of patients | p value3) | |||
|---|---|---|---|---|
| Decreased ALB1,2) | No change in ALB2) | Increased ALB2) | ||
| All pneumonia group patients (n=52) | ||||
| Changes in the frequency of pneumonia | ||||
| Increase | 1 (1.9) | 2 (3.8) | 1 (1.9) | 0.003 |
| No change | 2 (3.8) | 1 (1.9) | 5 (9.6) | |
| Decrease | 2 (3.8) | 1 (1.9) | 36 (69.2) | |
| Elderly pneumonia group patients (n=31) | ||||
| Changes in the frequency of pneumonia | ||||
| Increase | 1 (3.2) | 1 (3.2) | 1 (3.2) | 0.008 |
| No change | 1 (3.2) | 1 (3.2) | 2 (6.5) | |
| Decrease | 1 (3.2) | 1 (3.2) | 22 (71.0) | |
| Number (%) of patients | p value3) | |||
| Decreased TP2,4) | No change in TP2) | Increased TP2) | ||
| All pneumonia group patients (n=52) | ||||
| Changes in the frequency of pneumonia | ||||
| Increase | 2 (3.8) | 0 (0.0) | 2 (3.8) | 0.016 |
| No change | 2 (3.8) | 1 (1.9) | 5 (9.6) | |
| Decrease | 3 (5.8) | 3 (5.8) | 34 (65.4) | |
| Elderly pneumonia group patients (n=31) | ||||
| Changes in the frequency of pneumonia | ||||
| Increase | 1 (3.2) | 0 (0.0) | 2 (6.5) | 0.269 |
| No change | 1 (3.2) | 0 (0.0) | 3 (9.7) | |
| Decrease | 2 (6.5) | 2 (6.5) | 20 (64.5) | |
1)ALB: serum albumin levels.
2)The term "Decreased" means that the values of TP and ALB at one year after admission were lower than those measured at the time of admission. The term "No change" means that the values of TP and ALB at one year after admission did not differ from those measured at the time of admission. Similarly, the term "Increased" means that the values of TP and ALB at one year after admission were higher than those measured at the time of admission.
3)Statistical analysis was performed using the chi-square test.
4)TP: serum total protein levels.
Similarly, among the elderly patients in the pneumonia group, there was a significant relationship between improvements in the albumin level and a decreased frequency of pneumonia (Table 7).
In addition, in the patients in the pneumonia group, there was a significant relationship between improvement in the TP level measured at one year after admission and a decreased frequency of pneumonia during the second year of stay (Table 7). In elderly patients in the pneumonia group, a relationship between improvement in the TP level and a decreased frequency of pneumonia was observed; however, this difference was not statistically significant, probably due to the small sample size (Table 7).
In all analyzed patients and in all elderly patients in the two groups (the pneumonia and non-pneumonia groups), there was a significant relationship between improvement in the serum level of albumin measured at one year after admission and a decreased frequency of pneumonia during the second year of stay (Table 8).
Table 8.
Relationship between Improvement in the Serum Levels of Albumin and Total Protein and the Decreased Frequency of Pneumonia in All Patients and All Elderly Patients in the Pneumonia and Non-pneumonia Groups.
| Number (%) of patients | p value3) | |||
|---|---|---|---|---|
| Decreased ALB1,2) | No change in ALB1,2) | Increased ALB1,2) | ||
| All patients (n=68) | ||||
| Changes in the frequency of pneumonia | ||||
| Increase | 2 (2.9) | 2 (2.9) | 1 (1.5) | <0.001 |
| No change | 7 (10.3) | 3 (4.4) | 13 (19.1) | |
| Decrease | 2 (2.9) | 2 (2.9) | 36 (52.9) | |
| Elderly patients (n=44) | ||||
| Changes in the frequency of pneumonia | ||||
| Increase | 2 (4.5) | 1 (2.3) | 1 (2.3) | 0.001 |
| No change | 5 (11.4) | 2 (4.5) | 9 (20.5) | |
| Decrease | 1 (2.3) | 1 (2.3) | 22 (50.0) | |
| Number (%) of patients | p value3) | |||
| Decreased TP4) | No change in TP | Increased TP | ||
| All patients (n=68) | ||||
| Changes in the frequency of pneumonia | ||||
| Increase | 3 (4.4) | 0 (0.0) | 2 (2.9) | 0.004 |
| No change | 5 (7.4) | 3 (4.4) | 15 (22.1) | |
| Decrease | 3 (4.4) | 3 (4.4) | 34 (50.0) | |
| Elderly patients (n=44) | ||||
| Changes in the frequency of pneumonia | ||||
| Increase | 2 (4.5) | 0 (0.0) | 2 (4.5) | 0.053 |
| No change | 4 (9.1) | 1 (2.3) | 11 (25.0) | |
| Decrease | 2 (4.5) | 2 (4.5) | 20 (45.5) | |
1)ALB: serum albumin levels.
2)The term "Decreased" means that the values of TP and ALB at one year after admission were lower than those measured at the time of admission. The term "No change" means that the values of TP and ALB at one year after admission did not differ from those measured at the time of admission. Similarly, the term "Increased" means that the values of TP and ALB at one year after admission were higher than those measured at the time of admission.
3)Statistical analysis was performed using the chi-square test.
4)TP: serum total protein levels.
Furthermore, in all patients in the two groups, there was a significant relationship between improvement in the level of TP and a decreased frequency of pneumonia (Table 8).
In all elderly patients in the two groups, a relationship was observed between improvement in the levels of TP and a decreased frequency of pneumonia; however, this difference was not statistically significant (Table 8).
In addition, in the multivariate analysis, an increased albumin level measured at one year after admission was associated with a decreased frequency of pneumonia during the second year of stay in all analyzed patients (Table 9).
Table 9.
Multivariate Analysis between the Changes in Serum Albumin Level and the Frequency of Pneumonia in All Patients.
| Albumin (ALB) | ||||
|---|---|---|---|---|
| Decrease of ALB | No change of ALB | Increase of ALB | p for trend | |
| Crude | 0.09 (-0.64, 0.82) | -0.14 (-1.05, 0.77) | -1.16 (-1.50, -0.82) | 0.003 |
| Model 1a) | 0.16 (-0.60, 0.92) | -0.09 (-1.05, 0.87) | -1.18 (-1.53, -0.83) | 0.003 |
Values are presented as the estimated mean (95% CI).
a)Adjusted for age (continuous variable), consciousness level (continuous variable), tube feeding (no or yes), mechanical ventilation (no or yes), pulmonary diseases (no or yes).
The relationship between the improvement in BMI and reduction in the frequency of pneumonia
We examined the relationship between improvement in the BMI and a decreased frequency of pneumonia because BMI is an important factor that represents the nutritional status (23) and because we found that the BMI values of the pneumonia group tended to be lower in comparison to the non-pneumonia group. However, in patients in the pneumonia group, there was no association between improvement in BMI measured at one year after admission and a decreased frequency of pneumonia during the second year of stay (p=0.632, Fisher's test).
Discussion
We demonstrated that 76% of all analyzed bedridden patients receiving oral care developed pneumonia with a mean frequency of 1.6 times per year during the first year of stay. Gram-negative bacteria were identified more frequently than Gram-positive bacteria. The frequency of pneumonia during the second year of stay was lower than that during the first year of stay, and the serum levels of TP and albumin at one year after admission were higher than those at admission in all analyzed patients, and in the patients in the pneumonia group. The proportions of patients with low TP and albumin levels at one year after admission were lower in comparison to the time of admission. The increase in the proportion of patients presenting a reduced frequency of pneumonia was correlated with the increases in the proportion of patients presenting increased albumin and/or TP levels. These findings suggest that nutritional treatment may improve malnutrition in patients and may prevent pneumonia in bedridden patients receiving oral care.
We demonstrated that the serum level of albumin in the pneumonia group was lower in comparison to the non-pneumonia group. The proportions of patients with low albumin and TP values in the pneumonia group were significantly higher than those in the non-pneumonia group. Furthermore, in the multivariate analysis, the serum albumin level among individuals in the pneumonia group was lower than that among individuals in the non-pneumonia group. These findings are consistent with those in our previous study (10).
We found that the increase in the proportions of patients presenting a reduced frequency of pneumonia was correlated with the increase in the proportions of patients presenting increased albumin and/or TP levels. In contrast, we could not determine the relationship between the proportions of patients presenting a reduced frequency of pneumonia and the increase in the proportion of patients presenting increased BMI values, which is also an important marker of the nutritional status (23). These findings suggest that albumin and TP may be more sensitive than BMI for showing the relationship between the nutritional status and pneumonia development.
In the two patients who died of pneumonia, the BMI and serum albumin values were decreased during the observation time, and the serum albumin level remained extremely low in both patients (2.1 g/dL and 2.7 g/dL, respectively) at one year after admission. The serum TP levels of these patients were also low (<6.5 g/dL). Thus, severe malnutrition was observed in patients who died of pneumonia.
In the present study, the calorific values of the nutritional treatment were defined by the energy expenditure (calculated by the Harris-Benedict equation; basal metabolism) multiplied by an activity factor and a stress factor (15-17). We found that the calorific values in patients in the non-pneumonia group were higher than those in the pneumonia group. The precise reasons are uncertain; however, in the non-pneumonia group, the BMI values, which were used to calculate basal metabolism, were increased by 1 kg/m2 in comparison to those in the pneumonia group. In contrast, the activity and stress factors in both groups might be similar because all patients were bedridden and the proportion of patients with pressure ulcers did not differ between the two groups. Furthermore, age reduces the energy expenditure by reducing basal metabolism (15); however, the patients in the non-pneumonia group tended to be older than those in the pneumonia group. These findings suggest that higher body weight might have been associated with higher calorific values in the patients in the non-pneumonia group.
Carbohydrates, proteins and lipids were contained in the nutritional formulations used in tube feeding, the infusion solution used in IVH, and in the food. Thus, continuous nutritional treatment based on the patient conditions might be effective in improving the serum levels of albumin and TP.
The patients were enrolled according to the order of discharge date to prevent any selection bias. Patients are usually discharged from the hospital within 6 months of admission; however, patients with some diseases, such as amyotrophic lateral sclerosis (ALS) or muscular dystrophy, especially those receiving mechanical ventilation, stayed for more than two years. The enrolled patients were admitted to receive rehabilitation and nursing care after being treated for an acute disease or after receiving mechanical ventilation. Thus, the stable conditions of the analyzed patients might have been associated with the low mortality in the present study.
The patients who were analyzed in the present study had several types of disease that caused hospitalization as well as comorbidities, including ALS, cerebral infarction and diabetes mellitus. The patients also had characteristics, such as hypoalbuminemia, limitations in ADL, oral care dependency and tube feeding. Furthermore, more than 60% of the patients received mechanical ventilation. The diseases and factors observed in the patients are associated with the development of pneumonia (1-8, 18-21, 24). Thus, the combination of these diseases and factors might be associated with the high frequency of pneumonia development (76%) in the present study in comparison to the frequency observed in elderly subjects without dementia and disability with silent cerebral infarction (20%) or without silent cerebral infarction (5%) (25).
In the present study, the proportion of the elderly patients (≥65 years) was 65% among all analyzed patients and 60% among the pneumonia group patients. In the elderly patients in the pneumonia group, we observed a reduction in the frequency of pneumonia and increases in the levels of albumin and TP, and we found a significant relationship between improvement in the albumin levels and a decreased frequency of pneumonia. Thus, nutritional treatment may also be effective for preventing pneumonia in elderly patients.
Malnutrition, exhibited by characteristics such as hypoalbuminemia and body weight loss, is an important risk factor for infection, including pneumonia and wound infection (26) as it reduces the immune response against infection (11). Furthermore, low BMI and hypoalbuminemia are associated with mortality in patients with senile pneumonia (9). Albumin has effects on host defense mechanisms against bacterial infection through the complement function and the production of defensin (27, 28). Thus, nutritional treatment might partially reduce the malnutrition-related risk factors for pneumonia development by improving defense mechanisms against bacterial infection.
Chronic inflammation has also been suggested to reduce serum albumin levels (10, 29). In the present study, we observed that the patients' CRP values tended to be decreased at one year after admission. Thus, the improvement of inflammation might also be associated with the elevation of serum albumin levels.
In addition, deficiencies in other substrates, including vitamin D and zinc (30, 31), have been associated with the development of respiratory tract infections and pneumonia; however, we did not measure the serum levels of these components. Further studies are required.
We previously reported that the proportion of patients with hypoalbuminemia was higher among bedridden patients with pneumonia than among those without pneumonia (10). However, Sergi et al. reported that hypoalbuminemia was observed in 55% of elderly outpatients with low BMI, although the frequency of pneumonia was not examined (32). These findings suggest that malnutrition treatment may also be important for preventing pneumonia in elderly people with better ADLs because a high proportion of elderly outpatients may have hypoalbuminemia.
The present study was associated with some limitations. We performed a retrospective, single center study, and the sample size was relatively small. Thus, prospective multicenter studies are required to confirm the preventive effects of malnutrition treatment. In addition, the size of the non-pneumonia group was very different from that of the pneumonia group. Patients who stayed in the hospital for two years or longer were enrolled according to the order of the discharge date, and after analyzing the patient records, we found that pneumonia developed in more than 76% of the patients. As a result, the size of the non-pneumonia group decreased in comparison to that of the pneumonia group.
In conclusion, nutritional treatment may improve malnutrition and may prevent pneumonia in bedridden patients receiving oral care.
The authors state that they have no Conflict of Interest (COI).
Financial Support
This work was supported by grants of research support from Astellas Pharma (RS2017A000223 and RS2018A000301) and Teijin Pharma (TJNS20170731009 and TJNS20180506002).
Acknowledgement
We are also grateful to Mr. Yutaka Takisawa for providing information regarding nutritional treatment.
References
- 1. Teramoto S, Fukuchi Y, Sasaki H, et al. ; Japanese Study Group on Aspiration Pulmonary Disease High incidence of aspiration pneumonia in community- and hospital-acquired pneumonia in hospitalized patients: a multicenter, prospective study in Japan. J Am Geriatr Soc 56: 577-579, 2008. [DOI] [PubMed] [Google Scholar]
- 2. Yamaya M, Yanai M, Ohrui T, et al. Progress in geriatrics: interventions to prevent pneumonia among older adults. J Am Geriatr Soc 49: 1-6, 2001. [DOI] [PubMed] [Google Scholar]
- 3. Kikuchi Y, Watabe N, Konno T, et al. High incidence of silent aspiration in elderly patients with community-acquired pneumonia. Am J Respir Crit Care Med 150: 231-253, 1994. [DOI] [PubMed] [Google Scholar]
- 4. Scannapieco FA, Papandonatos GD, Dunford RG. Association between oral conditions and respiratory disease in a national sample survey population. Ann Periodontol 3: 251-256, 1998. [DOI] [PubMed] [Google Scholar]
- 5. Grant MD, Rudberg MA, Brody JA. Gastrostomy placement and mortality among hospitalized medicine beneficiaries. JAMA 279: 1973-1976, 1998. [DOI] [PubMed] [Google Scholar]
- 6. Matsusaka K, Ohi A, Tahata K, et al. Addition of oral cavity brusing and rehabilitation reduces fever in tube-fed patients. Geriatr Gerontol Int 13: 1082-1084, 2013. [DOI] [PubMed] [Google Scholar]
- 7. Sasaki H. Single pathogenesis of geriatric syndrome. Geriatr Gerontol Int 8: 1-4, 2008. [DOI] [PubMed] [Google Scholar]
- 8. Langmore SE, Terpenning MS, Schork A, et al. Predictors of aspiration pneumonia: how important is dysphagia? Dysphagia 13: 69-81, 1998. [DOI] [PubMed] [Google Scholar]
- 9. Kosai K, Izumikawa K, Imamura Y, et al. Importance of functional assessment in the management of community-acquired and healthcare-associated pneumonia. Intern Med 53: 1613-1620, 2014. [DOI] [PubMed] [Google Scholar]
- 10. Matsusaka K, Kawakami G, Kamekawa H, et al. Pneumonia risks in bedridden patients receiving oral care and their screening tool: Malnutrition and urinary tract infection-induced inflammation. Geriatr Gerontol Int 18: 714-722, 2018. [DOI] [PubMed] [Google Scholar]
- 11. Lesourd BM. Nutrition and immunity in the elderly: modification of immune responses with nutritional treatments. Am J Clin Nutr 66: 478S-484S, 1997. [DOI] [PubMed] [Google Scholar]
- 12. Takenoshita S, Kondo K, Okazaki K, et al. Tube feeding decreases pneumonia rate in patients with severe dementia: comparison between pre- and post-intervention. BMC Geriatr 17: 267, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Teasdale G, Jennett B. Assessment of coma and impaired consciousness. Lancet 13: 81-84, 1974. [DOI] [PubMed] [Google Scholar]
- 14. Jackson ML, Nelson JC, Jackson LA. Risk factors for community-acquired pneumonia in immunocompetent seniors. J Am Geriatr Soc 57: 882-888, 2009. [DOI] [PubMed] [Google Scholar]
- 15. Harris JA, Benedict FG. A biometric study of human basal metabolism. Proc Natl Acad Sci USA 4: 370-373, 1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Inoue Y. Estimation of energy need-should stress factor and activity factor be considered? Jomyaku Keicho Eiyo 25: 573-579, 2010. [Google Scholar]
- 17. Hwang TL, Huang SL, Chen MF. The use of indirect calorimetry in critically ill patients--the relationship of measured energy expenditure to Injury Severity Score, Septic Severity Score, and APACHE II Score. J Trauma 34: 247-251, 1993. [DOI] [PubMed] [Google Scholar]
- 18. Hu X, Lee JS, Pianosi PT, et al. Aspiration-related pulmonary syndromes. Chest 147: 815-823, 2015. [DOI] [PubMed] [Google Scholar]
- 19. Damas P, Frippiat F, Ancion A, et al. Prevention of ventilator-associated pneumonia and ventilator-associated conditions: a randomized controlled trial with subglottic secretion suctioning. Crit Care Med 43: 22-30, 2015. [DOI] [PubMed] [Google Scholar]
- 20. Heck S, Al-Shobash S, Rapp D, et al. High probability of comorbidities in bronchial asthma in Germany. NPJ Prim Care Respir Med 27: 28, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Braeken DC, Rohde GG, Franssen FM, et al. Risk of community-acquired pneumonia in chronic obstructive pulmonary disease stratified by smoking status: a population-based cohort study in the United Kingdom. Int J Chron Obstruct Pulmon Dis 12: 2425-2432, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huang C, Niu K, Momma H, et al. Breakfast consumption frequency is associated with grip strength in a population of healthy Japanese adults. Nutr Metab Cardiovasc Dis 24: 648-655, 2014. [DOI] [PubMed] [Google Scholar]
- 23. Ignacio de Ulıbarri J, González-Madrono A, de Villar NG, et al. CONUT: a tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp 20: 38-45, 2005. [PubMed] [Google Scholar]
- 24. Hardiman O. Management of respiratory symptoms in ALS. J Neurol 258: 359-365, 2011. [DOI] [PubMed] [Google Scholar]
- 25. Nakagawa T, Sekizawa K, Kosaka Y, et al. Silent cerebral infarction: a potential risk for pneumonia in the elderly. J Int Med 247: 255-259, 2000. [DOI] [PubMed] [Google Scholar]
- 26. Gorse GJ, Messner RL, Stephens ND. Association of malnutrition with nosocomial infection. Infect Control Hosp Epidemiol 10: 194-203, 1989. [DOI] [PubMed] [Google Scholar]
- 27. Sakamoto M, Fujisawa Y, Nishioka K. Physiologic role of the complement system in host defense, disease, and malnutrition. Nutrition 14: 391-398, 1998. [DOI] [PubMed] [Google Scholar]
- 28. Sherman H, Chapnik N, Froy O. Albumin and amino acids upregulate the expression of human beta-defensin 1. Mol Immunol 43: 1617-1623, 2006. [DOI] [PubMed] [Google Scholar]
- 29. Kim JH, Lee CT, Yoon HI, et al. Relation of ghrelin, leptin and inflammatory markers to nutritional status in active pulmonary tuberculosis. Clin Nutr 29: 512-518, 2010. [DOI] [PubMed] [Google Scholar]
- 30. Laaksi I, Ruohola JP, Tuohimaa P, et al. An association of serum vitamin D concentrations <40 nmol/L with acute respiratory tract infection in young Finnish men. Am J Clin Nutr 86: 714-717, 2007. [DOI] [PubMed] [Google Scholar]
- 31. Saleh NY, Abo El, Fotoh WMM. Low serum zinc level: the relationship with severe pneumonia and survival in critically ill children. Int J Clin Pract 72: e13211, 2018. [DOI] [PubMed] [Google Scholar]
- 32. Sergi G, Coin A, Enzi G, et al. Role of visceral proteins in detecting malnutrition in the elderly. Eur J Clin Nutr 60: 203-209, 2006. [DOI] [PubMed] [Google Scholar]