Abstract
Non-typhoidal Salmonella (NTS) infection is a major pathogen causing gastroenteritis among immunocompetent adults. NTS infection is mainly transmitted by contaminated food and water, but some cases are transmitted by animal contact. Salmonella enterica subsp. enterica serovar Poona (S. Poona) is an NTS usually transmitted by reptiles, and cases including outbreaks of gastroenteritis have been reported previously. However, invasive infections due to this organism among immunocompetent adults are rare. We herein report a case of a 39-year-old man who was admitted to our hospital for a fever and headache. Blood cultures were positive for S. Poona, although he did not recall any exposure to reptiles. He was treated successfully with intravenous ceftriaxone without any subsequent complications. This case implies that NTS bacteremia can occur in immunocompetent adults, and the diagnosis may be challenging since there may be no clear exposure or focal physical signs.
Keywords: non-typhoidal Salmonella, Salmonella Poona, bacteremia, immunocompetent adult
Introduction
Salmonella species are classified into over 2,500 serotypes of which about 50 subtypes cause infections in humans (1). Clinically, Salmonella species can be classified into 2 types: typhoidal Salmonella and non-typhoidal Salmonella (NTS). NTS is a major pathogen causing gastroenteritis in immunocompetent patients (2). However, it can also cause invasive infections, such as bacteremia, meningitis, osteomyelitis, and aortitis in children under five years of age, pregnant women, elderly people, and those with impaired cellular immunity (3). Humans can acquire NTS by either consuming contaminated food and water or by direct/indirect contact with animals (2).
Salmonella enterica subsp. enterica serovar Poona (S. Poona) is an NTS typically transmitted by reptiles. To date, the majority of S. Poona infection cases reported have been gastroenteritis among children, and invasive infectious cases among adults are limited. We experienced a rare case of S. Poona bacteremia in an immunocompetent adult without any clear exposure to reptiles.
Case Report
A 39-year-old Japanese man visited the emergency room for a fever and headache that had persisted for the past 2 days. These symptoms were exacerbated at night, with the body temperature reaching a maximum of 38.9℃, but resolved spontaneously in the morning. In addition, he had felt pain in the left upper quadrant of the abdomen four days earlier, although it had been alleviated by taking oral acetaminophen. He had no remarkable medical history and was taking no medications on a regular basis. He did not have allergies to any medicines or foods. He was a non-smoker but consumed alcohol occasionally. He did not recall any exposure to contaminated animals, plants, or water. He had traveled to the United States two months earlier.
In the emergency room, his vitals were as follows: body temperature: 36.6℃, blood pressure: 111/78 mmHg, heart rate: 96 /min, respiratory rate: 16/min, and oxygen saturation: 98% on ambient air. He was alert and oriented. A physical examination revealed tenderness in the left hypochondrium. No other remarkable findings, such as meningeal signs or skin rash, were noted. Laboratory data revealed the following: white blood cell (WBC) count: 7,300 /μL, C-reactive protein: 4.0 mg/dL, aspartate aminotransferase (AST): 81 U/L, and lactate dehydrogenase (LDH): 339 U/L. Abdominal ultrasound revealed a slightly enlarged spleen. Oral acetaminophen was prescribed, and he was scheduled for a follow-up three days later.
However, he returned to the emergency room after two days because of symptom exacerbation. He complained that the fever had not only continued to worsen at night but now persisted throughout the day. A physical examination showed no apparent change, but his body temperature was now 38.5℃. The WBC count as well as the AST, and LDH levels were found to be elevated to 8,700 /μL, 141 I/U, and 399 U/L, respectively. Abdominal computed tomography revealed thickening of the colon wall and swelling of the mesenteric lymph nodes at the ileocecum (Fig. 1). Blood cultures at this point became positive for Gram-negative rods (Fig. 2). Based on these findings, he was tentatively diagnosed with bacteremia, and intravenous ceftriaxone was initiated. On the first day of admission, his maximum body temperature was 38.6℃, but by day 4, he was afebrile. The isolate from blood cultures was classified as Salmonella spp. which had the polysaccharide O13 antigen according to the Kauffman-White scheme. Antimicrobial susceptibility testing showed that the organism was sensitive to ceftriaxone (Table). Since our initial results raised high suspicion of a rare organism, the isolate was sent to an external laboratory at the Department of Bacteriology 1, National Institute of Infectious Disease, for further identification. It was serotyped as Salmonella enterica, subsp. enterica serovar Poona (S. Poona) with multilocus sequence typing (MLST) Sequence Type (ST) 1,069 (4). Pulsed-field gel electrophoresis (PFGE) analyzed by XbaI and BlnI revealed patterns that had not been reported previously in Japan (5). His symptoms ameliorated promptly after intiating intervenous ceftriaxone, and he was subsequently switched to oral levofloxacin a week later (Fig. 3). The total duration of antibiotics was 14 days, and there were no signs of relapse during the follow-up.
Figure 1.
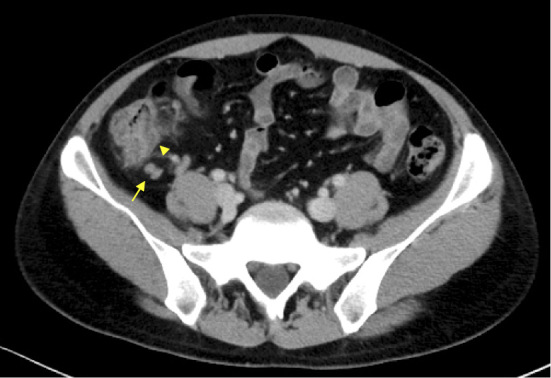
Initial abdominal computed tomography revealed mesenteric lymph node swelling and colitis at the ileocecal area. The arrow with the line shows lymph node swelling. The arrow without the line shows colitis at the ascending colon.
Figure 2.
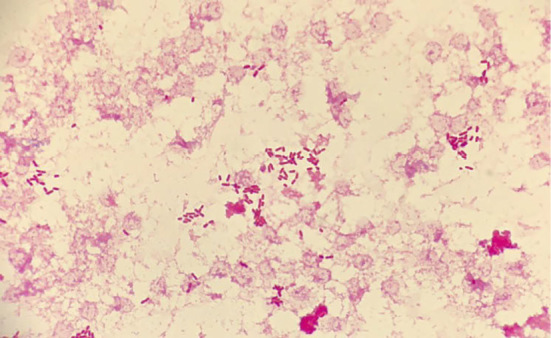
Gram stain obtained from blood culture shows Gram-negative bacilli.
Table.
Antimicrobial Susceptibility Profile of Salmonella Poona Isolates.
| Drug | Sensitivity | MIC |
|---|---|---|
| Ampicillin (ABPC) | S | <8 |
| Piperacillin (PIPC) | S | <8 |
| Piperacillin/Tazobactam (PIPC/TAZ) | S | <16 |
| Ampicillin/Sulbactam (ABPC/SBT) | S | <8 |
| Cefcapene (CFPN) | S | 1 |
| Cefotaxime (CTX) | S | <1 |
| Cefoperazone/Sulbactam (CPZ/SBT) | S | <16 |
| Flomoxef (FMOX) | S | <8 |
| Cefepime (CFPM) | S | <2 |
| Ceftazidime (CAZ) | S | <4 |
| Ceftriaxone (CTRX) | S | <1 |
| Imipenem (IPM) | S | <1 |
| Meropenem (MEPM) | S | <1 |
| Aztreonam (AZT) | S | <4 |
| Minocycline (MINO) | S | <2 |
| Fosfomycin (FOM) | S | <4 |
| Nalidixic acid (NA) | S | |
| Levofloxacin (LVFX) | S | <0.5 |
| Sulfamethoxazole-Trimethoprim (ST) | S | <2 |
Figure 3.
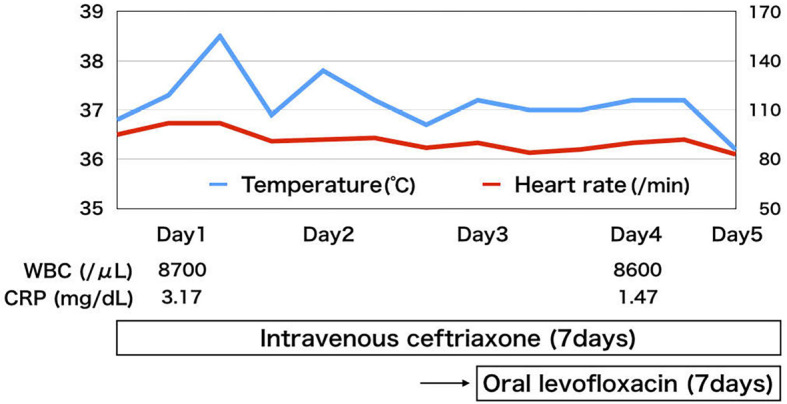
Clinical course of the patient.
Discussion
We herein report a case of S. Poona bacteremia in an immunocompetent adult who had no exposure to contaminated food, water, or animals. The diagnosis was challenging because of the lack of any clear exposure, an intermittent fever, and the absence of any other symptoms relevant to enterocolitis besides mild abdominal pain.
The proportion of enteric illnesses caused by NTS attributable to animal contact is estimated to be about 10% in the United States (6). Notably, pet turtles hold NTS in their intestinal tracts as normal gut flora, secreting them intermittently. In 1975, turtle-associated salmonellosis spread among children in the United States (7). Consequently, having or selling a pet turtle <4 inches in shell length was banned by federal law. However, from 2006 to 2014, 15 multi-state outbreaks of salmonellosis caused by turtle-associated NTS occurred in the United States (8).
S. Poona is an NTS species associated with exotic pets such as turtles, iguanas, and lizards (9). In Japan, all reported S. Poona cases have been in children. The first case of S. Poona-associated bacteremia was reported in a seven-month-old child in 2006 (10). While there was an African spurred tortoise in his house, the same pathogen was not detected in specimens from the pet and the house. However, the same S. Poona was detected in stool samples obtained from his brother and mother. In 2009, three pediatric patients in a nursery school presented with diarrhea, and S. Poona was detected in their stool samples (11). The antimicrobial susceptibility and PFGE profiles using XbaI and BlnI were identical in all of the specimens. Although an outbreak from an African spurred tortoise was suspected in the nursery school, S. Poona was not detected in the specimens obtained from the tortoise. Two children contracted turtle-associated Salmonellosis in Kanagawa prefecture in 2015 (12). One of them was a five-year-old boy who presented with diarrhea for two days and was found to have S. Poona in his stool. Both families of the infected children reported that they had an aquatic turtle as a pet, but additional examinations could not be performed. In general, healthy adults needs to acquire 106-108 NTS organisms in order for salmonellosis to develop (3); however, children may require much less to develop an infection. Furthermore, there have been reports of person-to-person transmission among children (13). S. Poona infection may not tend to affect adults since the amont of organisms received through contact with reptiles may be too small to spur an infection.
Among the cases of salmonellosis studied previously (14), bacteremia has been reported in only about 8% of them. Multi-center research on Salmonella enterica bacteremia in northwestern America, Europe, and Australia has shown that the incidence of total, typhoidal and non-typhoidal infections per 10 million cases is 1.02, 0.21, and 0.81, respectively (15), with the incidence being higher among the elderly than in other populations. Risk factors affecting the incidence of NTS bacteremia include Salmonella serotype and host factors. A 2008-2015 study of Salmonella-associated bacteremia in children in Nigeria revealed that S. typhimurium (45%) and S. enteritidis (39%) were the major pathogens, while S. Poona was rarely (about 3%) the causative agent (16). Likewise, a study in Malaysia revealed that S. enteriditis was the most frequent cause of bacteremia (17). Host factors include old age and an immunocompromised condition, such as malignancy, rheumatological disease, transplantation, and HIV infection (18). In addition, gastric acid suppression, malnutrition, recent antibiotic use, and concomitant rotavirus infection are factors that may increase the risk of NTS bacteremia (19). Our case is rare since the patient was an immunocompetent adult with no risk factors for bacteremia, and the causative organism was S. Poona.
Our patient did not recall having any direct exposure to turtles. However, initial computed tomography revealed inflammation and lymph node adenopathy in this region that disappeared four months later. Therefore, the entry site for the S. Poona might have been the ileocecal area. He had stayed in the United States until two months before his presentation, but this two-month gap is much longer than the usual incubation period for most NTS infections. Salmonella species often colonizes the human intestinal tract, and the median duration of excretion is five weeks (20). Excretion is prolonged in children under five years old and those with symptomatic infections or infected with S. typhimurium (20); however, persistent or convalescent excretion is uncommon in immunocompetent adults. Consequently, we presume that he had received indirect exposure to food or water that was contaminated with S. Poona after returning to Japan.
One study in Japan examined the differences in the clinical manifestations between cases with and without bacteremia (21). The incidence of a fever, diarrhea, and abdominal pain was higher in the bacteremia group than in the non-bacteremia group. In addition, the percentage of cases wherein a fever or diarrhea lasted for more than four days was higher in the bacteremia group than in the non-bacteria group. Our patient presented with a fever, and abdominal pain but no diarrhea, and these symptoms lasted for more than four days. A blood culture was useful for his diagnosis. Since such cases in adults are rare, the relationship between the duration of symptoms and bacteremia remains to be elucidated. Although the patient did not have any diarrhea, the duration of his symptoms was longer than usual, and other characteristics suggested bacteremia.
We reported an adult case of S. Poona bacteremia, which to our knowledge is the first report in an immunocompetent adult in Japan. Our report indicates that NTS bacteremia can be difficult to diagnose if the exposure is not clear and there are no focal physical signs. Although NTS usually causes gastroenteritis in immunocompetent patients, clinicians should consider bacteremia and perform a blood culture even in adults if their fever persists.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Jackson BR, Griffin PM, Cole D, Walsh KA, Chai SJ. Outbreak-associated Salmonella enterica serotypes and food Commodities, Unites States, 1998-2008. Emerg Infect Dis 19: 1239-1244, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pegues DA, Miller SI. Salmonella species. In: Principles and Practice of Infectious Diseases. 8th ed John EB, Raphael D, Martin JB et al. , Eds. Elsevier Saunders, Philadelphia, 2015: 2559-2568. [Google Scholar]
- 3. Chen HM, Wang Y, Su LH, Chiu CH. Nontyphoid Salmonella infection: microbiology, clinical features, and antimicrobial therapy. Pediatr Neonatol 54: 147-152, 2013. [DOI] [PubMed] [Google Scholar]
- 4. Achtman M, Wain J, Weill FX, et al. Multilocus sequence typing as a replacement for serotyping in Salmonella enterica. PLoS Pathog 8: e1002776, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ribot EM, Fair MA, Gautom R, et al. Standardization of pulsed-field gel electrophoresis protocols for the subtyping of Escherichia coli O157:H7, Salmonella, and Shigella for PulseNet. Foodborne Pathog Dis 3: 59-67, 2006. [DOI] [PubMed] [Google Scholar]
- 6. Hale CR, Scallan E, Cronquist AB, et al. Estimates of enteric illness attributable to contact with animals and their environments in the United States. Clin Infect Dis 54: S472-S479, 2012. [DOI] [PubMed] [Google Scholar]
- 7. Lamm SH, Taylor A, Gangarosa EJ, et al. Turtle-associated salmonellosis. I. An estimation of the magnitude of the problem in the United States, 1970-1971. Am J Epidemiol 95: 511-517, 1972. [DOI] [PubMed] [Google Scholar]
- 8. Bosch S, Tauxe RV, Behravesh CB. Turtle-associated salmonellosis, United States, 2006-2014. Emerg Infect Dis 22: 1149-1155, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Woodward DL, Khakhria R, Johnson WM. Human salmonellosis associated with exotic pets. J Clin Microbiol 35: 2786-2790, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nishiwaki K, Iizuka T, Watanabe O, et al. An infant case of sepsis due to S. Poona infected presumably from a tortoise, May 2006 - Niigata. Infectious Agents Surveillance Report 27: 203-204, 2006. [Google Scholar]
- 11. Tsuchiya Y, Hata N, Kato K, Yamamoto Y, Koizumi I, Shirahata H. Sporadic Salmonella Poona infection in nursery schools in Hamamatsu City, June 2009. Infectious Agents Surveillance Report 31: 105-107, 2010. [Google Scholar]
- 12. Kuroki T, Ito K, Ishihara T, et al. Turtle-associated Salmonella infections in Kanagawa, Japan. Jpn J Infect Dis 68: 333-337, 2015. [DOI] [PubMed] [Google Scholar]
- 13. Olsen SJ, Bishop R, Brenner FW, et al. The changing epidemiology of Salmonella: trends in serotypes isolated from humans in the United States, 1987-1997. J Infect Dis 183: 753-761, 2002. [DOI] [PubMed] [Google Scholar]
- 14. Mandal BK, Brennand J. Bacteraemia in salmonellosis: A 15 year retrospective study from a regional infectious diseases unit. Br Med J 297: 1242-1243, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Laupland K, Schønheyder H, Kennedy K, et al. Salmonella enterica bacteraemia: a multi-national population-based cohort study. BMC Infect Dis 10: 10-15, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Obaro SK, Hassan-Hanga F, Olateju EK, et al. Salmonella bacteremia among children in central and Northwest Nigeria, 2008-2015. Clin Infect Dis 61: S325-S331, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dhanoa A, Fatt QK. Non-typhoidal Salmonella bacteraemia: epidemiology, clinical characteristics and its' association with severe immunosuppression. Ann Clin Microbiol Antimicrob 8: 15, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gordon MA. Salmonella infections in immunocompromised adults. J Infect 56: 413-422, 2008. [DOI] [PubMed] [Google Scholar]
- 19. Hung T-Y, Liu M-C, Hsu C-F, Lin Y-C. Rotavirus infection increases the risk of bacteremia in children with nontyphoid Salmonella gastroenteritis. Eur J Clin Microbiol Infect Dis 28: 425-428, 2009. [DOI] [PubMed] [Google Scholar]
- 20. Buchwald DS, Blaser MJ. A review of human salmonellosis: II. Duration of excretion following infection with nontyphi Salmonella. Rev Infect Dis 6: 345-356, 1984. [DOI] [PubMed] [Google Scholar]
- 21. Aoki Y, Kitazawa K, Kobayashi H, et al. Clinical features of children with nontyphoidal Salmonella bacteremia: a single institution survey in rural Japan. PLoS One 12: 1-9, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]


