Introduction
Eosinophilic esophagitis (EoE) is a chronic, immune/antigen-mediated disease,1 and food elimination diets (FEDs) are an important therapeutic approach. Response rates to FEDs are well described in classically-defined EoE cases who have failed a proton pump inhibitor (PPI) trial.2,3 However, treatment with FEDs has only recently been described in patients with PPI-responsive esophageal eosinophilia (PPI-REE) who would now meet new diagnostic criteria for EoE.1,4,5 While these studies confirmed PPI-REE patients responded to FEDs, the proportion of EoE patients who respond to PPI then subsequently respond to FED in the absence of PPI treatment is unknown. This study aimed to determine FED treatment response rates in individuals with EoE who previously responded to PPIs.
Methods
In this retrospective cohort study at UNC, we identified adults with EoE, defined by the most recent diagnostic criteria, who had an initial clinical, endoscopic, and histologic response (<15 eos/hpf) to PPI treatment, and who subsequently discontinued PPI and underwent exclusive FED treatment.
Demographic, clinical, endoscopic, and histologic features were abstracted from medical records for three time-points: baseline/diagnosis (off PPI), post-PPI monotherapy (8-week course of 20–40 mg BID of any approved PPI, as per clinical protocol), and post-FED (6–8 weeks of an empiric elimination diet removing between 2 and 6 food groups). Bivariate analyses were used to analyze post-PPI and post-FED outcomes, including global patient-reported symptoms, endoscopic severity measured by the EoE Endoscopic Reference Score (EREFS), and peak eosinophil counts (eos/hpf), with histologic response defined as ≤15 eos/hpf.
Results
We identified nine patients who responded to PPIs and subsequently underwent exclusive FED while off PPI; 22% were male, 89% were white, and 89% were atopic (Supplemental Tables 1 and 3). In all cases, PPIs were stopped due to patient preference to avoid this medication class. At baseline (Supplemental Tables 1 and 3), all patients were symptomatic (89% dysphagia, 67% heartburn, 22% chest pain), median peak eosinophil count was 70 (IQR 60–86), and median total EREFS was 4 (IQR 2–5). Five (56%) subjects underwent dilation at baseline.
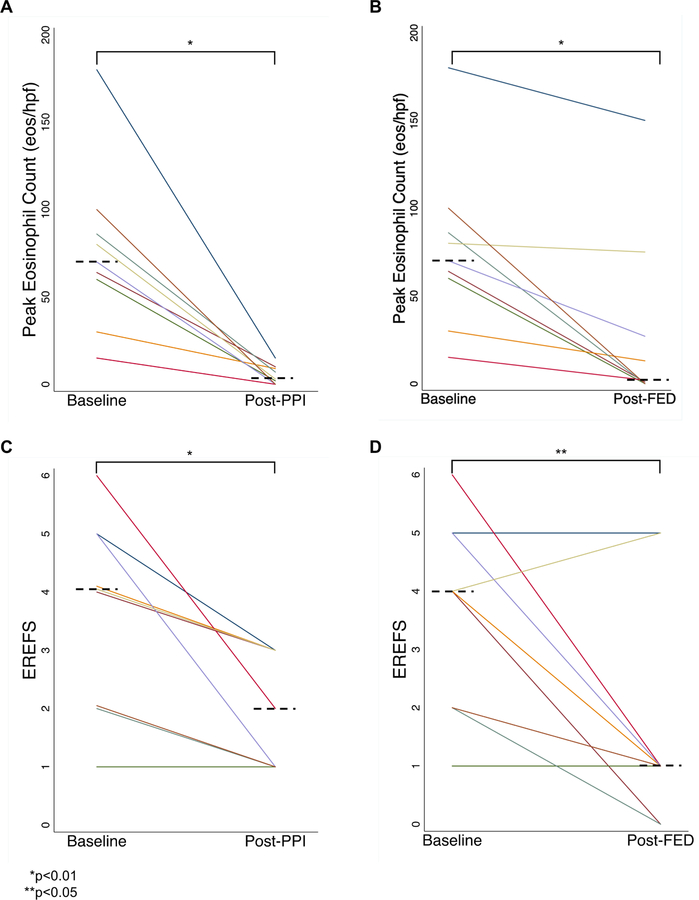
After PPI treatment, 89% had symptom improvement, all had peak eosinophil counts of ≤15 (median count 3 [IQR 0–9, p=0.008]; Fig 1A), and there was improvement in EREFS (median score 2 [IQR 1–3, p=0.008]; Fig 1C). Median PPI washout time was 84 days (IQR 63–99). For FED treatment, one-third eliminated 2 foods, one-third eliminated 3–4 foods, and one-third eliminated 6+ foods (Supplemental Tables 1 and 3). Not all patients underwent EGD post-stopping PPI and prior to started FED. There was histologic recurrence of esophageal eosinophilia in 4 patients on low-dose PPI or off PPI prior to FED initiation, and in 3 patients during sequential food re-introduction while off PPI.
Figure 1. Peak Eosinophil Counts and EREFS, Baseline and Post-Treatments.
(A) Improvement in peak eosinophil count after PPI treatment. (B) Improvement in peak eosinophil count after dietary elimination treatment. (C) Improvement in endoscopic severity as measured by the EoE Endoscopy Reference Score (EREFS) after PPI treatment. (D) Improvement in endoscopic severity after dietary elimination. For all graphs, the individual lines represent each subject, and the dotted horizontal lines represent the median values. Paired comparisons were made with the Wilcoxon Sign-Rank test.
After exclusive FED (Supplemental Table 2), 78% of subjects had symptomatic improvement and the median peak eosinophil count was 2 (IQR 0–28; p=0.008 compared to baseline; Fig 1B), with 67% having histologic response. Endoscopic features also improved (median post-diet EREFS of 1 (IQR 1–1; p=0.036; Fig 1D). Among FED-responders, egg and wheat were the most commonly identified triggers (50% each), followed by dairy (33%) (Supplemental Tables 1 and 3).
Discussion
Two previous case series demonstrated that dietary elimination in PPI-REE is potentially efficacious.4,5 Our study extends these data by reporting FED response rates in EoE patients who initially achieved clinicohistologic remission with PPIs but subsequently discontinued them. FED was successful in two-thirds of patients who discontinued PPIs, a response rate similar to those reported in classically defined EoE with PPI non-response.2,3
In light of new diagnostic guidelines for EoE and given that 40–50% of patients are PPI-responsive,1,6 our findings have therapeutic implications. PPIs are first-line treatment for EoE and are recognized to be safe and convenient.1,7 However, some patients may find the potential side effects or risks (though controversial) of PPIs to be unacceptable, preferring non-pharmacologic management. Although FEDs may be burdensome and require endoscopy to identify culprit foods, they are effective long-term.8 Step-up approaches can lessen treatment burden,3 as was the case in two-thirds of FED responders in our study.
Limitations include a retrospective design and a small, selected, tertiary care sample, which may account for the high number of strictures and dilations. The female preponderance, atypical of general EoE cohorts, is similar to that of previously reported case series and is reflective of those who pursue FED at our center.4,5,8 Despite these, the strength of this study is the reported rate of maintained remission with FEDs concurrent with PPI cessation in EoE patients who initially responded to PPIs. If confirmed in prospective studies, FEDs would be a valuable treatment option for patients who prefer PPI discontinuation.
Supplementary Material
Acknowledgments
Grant Support: This research was funded by NIH Awards T32 DK007634 (CCR) and R01 DK101856 (ESD)
Dr. Dellon is a consultant for Adare, Alivio, Allakos, AstraZeneca, Banner, Calypso, Enumeral, EsoCap, Gossamer Bio, GSK, Receptos/Celegene, Regeneron, Robarts, and Shire, receives research funding from Adare, Allakos, GSK, Meritage, Miraca, Nutricia, Receptos/Celgene, Regeneron, and Shire, and has received educational grants from Allakos, Banner, and Holoclara.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Potential competing interests: None of the other authors report and potential conflicts of interest with this study.
References
- 1.Dellon ES, Liacouras CA, Molina-Infante J, et al. Updated International Consensus Diagnostic Criteria for Eosinophilic Esophagitis: Proceedings of the AGREE Conference. Gastroenterology 2018;155:1022–1033 e1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arias A, Gonzalez-Cervera J, Tenias JM, Lucendo AJ. Efficacy of dietary interventions for inducing histologic remission in patients with eosinophilic esophagitis: a systematic review and meta-analysis. Gastroenterology 2014;146(7):1639–1648. [DOI] [PubMed] [Google Scholar]
- 3.Molina-Infante J, Arias A, Alcedo J, et al. Step-up empiric elimination diet for pediatric and adult eosinophilic esophagitis: The 2-4-6 study. J Allergy Clin Immunol 2018;141(4):1365–1372. [DOI] [PubMed] [Google Scholar]
- 4.Sodikoff J, Hirano I. Proton pump inhibitor-responsive esophageal eosinophilia does not preclude food-responsive eosinophilic esophagitis. J Allergy Clin Immunol 2016;137(2):631–633. [DOI] [PubMed] [Google Scholar]
- 5.Lucendo AJ, Arias A, Gonzalez-Cervera J, Olalla JM, Molina-Infante J. Dual response to dietary/topical steroid and proton pump inhibitor therapy in adult patients with eosinophilic esophagitis. J Allergy Clin Immunol 2016;137(3):931–934 e93. [DOI] [PubMed] [Google Scholar]
- 6.Lucendo AJ, Arias A, Molina-Infante J. Efficacy of Proton Pump Inhibitor Drugs for Inducing Clinical and Histologic Remission in Patients With Symptomatic Esophageal Eosinophilia: A Systematic Review and Meta-Analysis. Clin Gastroenterol Hepatol 2016;14(1):13–22 e11. [DOI] [PubMed] [Google Scholar]
- 7.Freedberg DE, Kim LS, Yang YX. The Risks and Benefits of Long-term Use of Proton Pump Inhibitors: Expert Review and Best Practice Advice From the American Gastroenterological Association. Gastroenterology 2017;152(4):706–715. [DOI] [PubMed] [Google Scholar]
- 8.Reed CC, Fan C, Koutlas NT, Shaheen NJ, Dellon ES. Food elimination diets are effective for long-term treatment of adults with eosinophilic oesophagitis. Aliment Pharmacol Ther 2017;46(9):836–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.