Abstract
Background:
Cardiac surgery induces hemodynamic stress on the myocardium, and this process can be associated with significant post-operative morbidity and mortality. Soluble suppression of tumorigenicity 2 (sST2) and galectin-3 (gal-3) are biomarkers of myocardial remodeling and fibrosis; however, their potential association with post-operative changes is unknown.
Methods:
We measured peri-operative plasma sST2 and gal-3 levels in two prospective cohorts (TRIBE-AKI and NNE) of over 1800 patients who underwent cardiac surgery. sST2 and gal-3 levels were evaluated for association with a composite primary outcome of cardiovascular event or mortality over median follow-up periods of 3.4 and 6.0 years, respectively, for the two cohorts. Meta-analysis of hazard ratio estimates from the cohorts was performed using random effects models.
Results:
Cohorts demonstrated event rates of 70.2 and 66.8 per 1000 person-years for the primary composite outcome. After adjustment for clinical covariates, higher post-operative sST2 and gal-3 levels were significantly associated with cardiovascular event or mortality [pooled estimate HRs: sST2 1.29 (95% CI 1.16, 1.44); gal-3 1.26 (95% CI 1.09, 1.46)]. These associations were not significantly modified by pre-operative congestive heart failure or AKI.
Conclusions:
Higher post-operative sST2 and gal-3 values were associated with increased incidence of cardiovascular event or mortality. These two biomarkers should be further studied for potential clinical utility for patients undergoing cardiac surgery.
Keywords: sST2, gal-3, biomarker, cardiac surgery, peri-operative, post-operative mortality, post-operative cardiovascular event, Meso assay
Introduction
Over the past four decades, there has been a decline in death from cardiovascular disease in the United States1-3. From 1980-2000, it is estimated that 47% of this decline was attributable to evidence-based medical and surgical treatments4. However, despite advances in technology and pre-surgical optimization, cardiac surgery continues to be associated with significant post-operative morbidity and mortality5. Clinicians may benefit from additional tools to predict post-operative outcomes or to identify patients for whom the benefit of surgery outweighs associated risk.
Blood biomarkers are ideally easily measurable, inexpensive, accurate, and indicative of a specific pathophysiologic finding6. Cardiac troponins and natriuretic peptides (brain natriuretic peptide, or BNP, and its N-terminal fragment, NT-proBNP) are among the most well-known biomarkers used in diagnosis and assessment of patients presenting with acute coronary syndromes and heart failure7-11. They associate with mortality in acute coronary syndromes and after cardiac surgery12, 13. More recently, soluble ST2 (sST2) and galectin-3 (gal-3) have been described as novel cardiac biomarkers with FDA-approved assays for clinical use in patients with heart failure. sST2 is a member of the interleukin-1 (IL-1) receptor family that serves as a decoy for IL-33, interfering with IL-33’s ability to protect against hypertrophy and fibrosis14-16. Gal-3 is released from activated macrophages and is a global marker of inflammation and fibrosis17-19. These biomarkers are included in the American College of Cardiology Foundation/American Heart Association guidelines (class IIb) to risk stratify patients with heart failure6, 11, 20-22. Since they are mechanistically involved in remodeling and fibrosis, we sought to explore the applicability of sST2 and gal-3 as biomarkers for patients undergoing cardiac surgery.
Cardiac surgery often entails clamping of major blood vessels and exposure of blood to the extracorporeal circuit, and is expected to initiate an inflammatory cascade. In some cases, there may be dysregulation or overactivation of the immune system, which could contribute to post-operative morbidity and mortality. We hypothesized that higher levels of sST2 and gal-3 following cardiac surgery would indicate fibrosis and/or maladaptive remodeling, and would be associated with post-operative cardiovascular event (CVD) or mortality. We conducted an ancillary study from a large adult population undergoing cardiac surgery across six academic medical centers in North America [the TRIBE-AKI cohort23] to study this hypothesis, and then performed a validation of the study results in a second cohort [the Northern New England Cardiovascular Disease Study Group, or NNE Biomarker Study24]. We performed meta-analysis of the results from the two cohorts to summarize the strengths of association of peri-operative biomarker levels with clinical outcomes.
Methods
Patient Cohorts
TRIBE-AKI:
Detailed methods of the Translational Research Investigating Biomarker Endpoints in AKI (TRIBE-AKI) study have been previously described23. A total of 1601 patients undergoing cardiac surgery [coronary artery bypass grafting (CABG) or valve surgery] who were at high risk for developing AKI were prospectively enrolled at six academic medical centers in North America between July 2007 and December 2009. Ethylenediaminetetraacetic acid (EDTA) plasma specimens from these participants were collected pre-operatively (up to two months prior to surgery) and 0-6 hours post-operatively. Participants for whom long-term administrative data was not available (n=304) or for whom all biomarker measurements were not available (n=104) were excluded, leaving a total of 1193 participants in final analyses (Supplemental Figure 1a).
NNE:
To evaluate the validity of our observed associations, we studied a second cardiac surgery cohort, the Northern New England Cardiovascular Disease Study Group (NNE Biomarker Study). Detailed information on this cohort has been described24-27. A total of 1690 patients undergoing cardiac surgery (primarily isolated CABG) across 8 hospitals in New Hampshire, Maine, and Vermont were included in the prospective observational cohort. Plasma specimens were collected pre-operatively (prior to incision) and approximately 24 hours after surgery. Patients for whom administrative data was not available (n=387), for whom other exclusion criteria were met (n=54), or for whom biomarker measurements were not available (n=589) were excluded, leaving 660 participants for the final analyses (Supplemental Figure 1b).
Outcome Definitions
TRIBE-AKI:
The primary outcome for this study was a composite of cardiovascular event or all-cause mortality after discharge, with discharge being used as a follow-up time of zero. Mortality was assessed by calling participants’ homes, reviewing hospital records, and utilizing the National Death Index for participants in the United States or the Institute for Clinical Evaluative Sciences (ICES) for participants in Canada. A cardiovascular event was defined as hospitalization for acute coronary syndrome, myocardial infarction, congestive heart failure (CHF), coronary bypass, or percutaneous coronary intervention. Outcomes for American participants were obtained through linkages with the Center for Medicare and Medicaid Services databases, and for Canadian participants through data holdings at ICES. Cardiovascular events were identified using coding from the International Classification of Diseases (revisions 9 and 10) and the Canadian Classification of Health Interventions. Datasets were linked using unique, encoded identifiers, and analyzed at ICES. Accuracies of diagnostic codes have been previously published28.
NNE:
Cardiovascular events and all-cause mortality were obtained using Medicare in-patient claims, state all-payer in-patient claims using name, gender, social security number, date of birth, and zip-code of residence at the time of surgery, and the National Death Index. Maine and Vermont completed links internally. Probabilistic linking was used for New Hampshire all-payer in-patient claims. Complete ascertainment was achieved for Medicare, Vermont, and Maine. Five percent of New Hampshire patients were not matched in the New Hampshire in-patient claims24, 25.
Biomarker Assays
A multiplex Meso Scale assay (Meso Scale Diagnostics, Rockville, MD) was used to measure sST2 and gal-3. Assay characteristics are provided in Supplemental Table 1. Meso Scale assays had excellent characteristics with linearity of dilution and spike and recovery experiments, though the gal-3 assay had lower correlation to its respective FDA-approved assay (sST2, r = 0.98; gal-3, r = 0.78).
Statistical Analyses
Descriptive statistics were reported as mean (95% confidence interval) or median (interquartile range) for continuous variables, and as frequency (percentage) for categorical variables. Biomarker concentrations were modeled continuously as log-transformed (base e) variables, and categorically as tertiles with the lowest tertile serving as the reference group. Cox proportional hazards regression models were used to estimate the associations between the studied biomarkers and time to the primary composite outcome. Kaplan Meier product limit curves were produced to graphically examine the association between biomarkers and the primary composite endpoint. The Kolmogorov-type supremum test was used to evaluate the proportional hazards assumption.
We adjusted models for the following covariates: Society of Thoracic Surgery (STS) score, sex, cardiopulmonary bypass time, non-elective surgery, hypertension, and CHF (any diagnostic coding for CHF). The STS score is a previously published score used to estimate the risk of post-operative morbidity and mortality in patients undergoing cardiac surgery, and is composed of pre-operative serum creatinine, age, surgery type, diabetes, chronic lung disease, recent myocardial infarction, race, reoperation, New York Heart Association class, and cardiogenic shock29. Chronic lung disease was not captured in the TRIBE-AKI study, and we therefore calculated the STS score with all participants coded as not having this condition. Where specified, data from the TRIBE-AKI cohort was also adjusted for NT-proBNP and cardiac troponin T (NT-proBNP and troponin T values were not available from the NNE cohort).
After exploring associations between biomarkers and outcomes in each cohort, we combined results of the two cohorts. We used Cochran’s Q test statistic to test for heterogeneity between studies, and the I-squared statistic to quantify the magnitude of heterogeneity. A pooled estimate was not presented in cases where the Q test was statistically significant. Pooled hazard ratio estimates were calculated using the random effects meta-analysis method.
To determine if the associations between biomarkers and the primary outcome were modified by pre-operative CHF, interaction terms between each biomarker and CHF were included in the model. This modeling approach was also used to examine if AKI was an effect modifier.
Analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC) and R version 3.1.2 (R foundation for Statistical Computing, Vienna, Austria). Tests of significance were two-sided, with p < 0.05 considered significant.
Study Approval
Institutional review boards from each participating site approved this study and its protocols, and all participants or their surrogates provided written informed consent.
Funding
The research reported in this article was supported by the American Heart Association Clinical Development Award, as well as by grant R01HL-085757 from the National Heart, Lung, and Blood Institute. C.R.P. is also supported by an NIH grant (K24DK090203). S.G.C., A.X.G., and C.R.P. are members of the NIH-sponsored Assess, Serial Evaluation, and Subsequent Sequelae in Acute Kidney Injury (ASSESSAKI) Consortium (U01DK082185). S.G.C. M.G.S, and C.R.P. are members and are supported in part by the Chronic Kidney Disease Biomarker Consortium (1U01DK106962-01). The study was also supported by CTSA Grant Number UL1 RR024139 from the National Center for Research Resources (NCRR). This study was supported by the Institute for Clinical Evaluative Sciences (ICES), which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC); Dr. Amit Garg is supported by the Dr. Adam Linton Chair in Kidney Health Analytics; there are no other relationships or activities that could appear to have influenced the submitted work. Dr. Brown and the NNE Biomarker Study were supported in part by the American Heart Association (0625950T), NHLBI (R01 HL119664), and the Northern New England Cardiovascular Disease Study Group. D.G.M. is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (K23DK117065) and the American Heart Association (18CDA34060118). S.G.M. is supported by the American Heart Association (18CDA34110151). The opinions, results, and conclusions reported in this paper are those of the authors and are independent from the funding sources. No endorsement by ICES or the Ontario MOHLTC is intended or should be inferred. Parts of this material are based on data and information compiled and provided by the Canadian Institute for Health Information (CIHI). However, the analyses, conclusions, opinions, and statements expressed herein are those of the authors, and not necessarily those of CIHI.
The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents.
Results
Baseline characteristics
The TRIBE-AKI and NNE cohorts respectively included 1193 and 660 patients, with median follow-up periods of 3.4 (2.4, 4.2) and 6.0 (3.9, 6.0) years. Table 1 a-b lists patient characteristics by tertile of post-operative sST2 and gal-3. Compared to the TRIBE-AKI cohort, patients of the NNE cohort had fewer pre-operative comorbidities (diabetes, hypertension, and CHF) but higher prevalence of prior myocardial infarction, and were less likely to have an elective surgery (28% in NNE versus 86% in TRIBE-AKI). The majority of NNE patients underwent isolated CABG, with only 5% receiving CABG with valve repair compared to 24% of TRIBE-AKI patients. Post-operative gal-3 levels were lower in men, and sST2 and gal-3 levels were higher in patients with pre-existing CHF. Post-operative sST2 and gal-3 levels were higher in patients who underwent CABG with valve repair, and in those with longer perfusion times or hospitalizations. Biomarkers were lower in patients who had been scheduled for elective surgery. Baseline and clinical characteristics by pre-operative biomarker levels were similar to post-operative findings and are presented in Supplemental Table 2 a-b.
Table 1 a-b. Characteristics of patient cohorts by post-operative sST2 and gal-3.
Numbers are provided as percentages or interquartile ranges. a) Tertiles are defined for sST2 as follows. For TRIBE-AKI: tertile 1 (T1) 0.6 – 4.2, tertile 2 (T2) 4.2 – 7.3, tertile 3 (T3) 7.3 – 357.3 in ng/ml. For NNE: tertile 1 (T1) 0.2 – 34.7, tertile 2 (T2) 34.8 – 67.1, tertile 3 (T3) 67.5 – 408.4 in ng/ml. b) Tertiles are defined for gal-3 as follows. For TRIBE-AKI: tertile 1 (T1) 0.4 – 9.7, tertile 2 (T2) 9.7 – 16.1, tertile 3 (T3) 16.1 – 81.5 in ng/ml. For NNE: tertile 1 (T1) 0.5 – 8.7, tertile 2 (T2) 8.7 – 14.4, tertile 3 (T3) 14.4 – 390.2 in ng/ml.
| a: Characteristics of patient cohorts by post-operative sST2. | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient characteristics | Post-operative sST2 (TRIBE-AKI) | Post-operative sST2 (NNE) | ||||||||
| All (N=1193) | T1 (n=397) | T2 (n=398) | T3 (n=398) | p-value | All (N=660) | T1 (n=217) | T2 (n=221) | T3 (n=222) | p-value | |
| Age at surgery (SD) | 73 (8.4) | 74 (8.2) | 73 (8.2) | 73 (8.8) | 0.08 | 71 (7.9) | 70 (8.4) | 71 (7.2) | 72 (8.0) | 0.02 |
| Male | 837 (70%) | 263 (66%) | 288 (72%) | 286 (72%) | 0.1 | 501 (76%) | 171 (79%) | 164 (74%) | 166 (75%) | 0.5 |
| Preoperative comorbidities | ||||||||||
| Diabetes | 444 (37%) | 130 (33%) | 171 (43%) | 143 (36%) | 0.01 | 273 (41%) | 80 (37%) | 93 (42%) | 100 (45%) | 0.2 |
| Hypertension | 955 (80%) | 308 (78%) | 321 (81%) | 326 (82%) | 0.3 | 555 (84%) | 181 (83%) | 188 (85%) | 186 (84%) | 0.9 |
| Myocardial infarction | 302 (26%) | 112 (28%) | 101 (26%) | 90 (23%) | 0.2 | 309 (47%) | 87 (40%) | 104 (47%) | 118 (53%) | 0.02 |
| Congestive heart failure | 244 (20%) | 62 (16%) | 62 (16%) | 120 (30%) | <0.001 | 86 (13%) | 22 (10%) | 20 (9%) | 44 (20%) | 0.001 |
| Preoperative renal function | ||||||||||
| Serum creatinine | 1.1 (0.3) | 1.1 (0.3) | 1.1 (0.3) | 1.1 (0.4) | 0.2 | 1.2 (0.7) | 1.2 (0.9) | 1.1 (0.5) | 1.3 (0.8) | 0.1 |
| ≥ 90 | 129 (11%) | 41 (10%) | 42 (11%) | 46 (12%) | 95 (14%) | 31 (14%) | 38 (17%) | 26 (12%) | ||
| eGFR (ml/min per 1.73 m2) | 661 (55%) | 224 (56%) | 228 (57%) | 209 (53%) | 0.6 | 358 (54%) | 123 (57%) | 115 (52%) | 120 (54%) | 0.05 |
| ≤ 60 | 403 (34%) | 132 (33%) | 128 (32%) | 143 (36%) | 207 (31%) | 63 (29%) | 68 (31%) | 76 (34%) | ||
| Characteristics of surgery and hospitalization | ||||||||||
| Elective surgery | 1023 (86%) | 350 (88%) | 346 (87%) | 326 (82%) | 0.03 | 183 (28%) | 72 (33%) | 66 (30%) | 45 (20%) | 0.007 |
| CABG with valve repair | 285 (24%) | 59 (15%) | 100 (25%) | 126 (32%) | <0.001 | 34 (5%) | ** | ** | ** | ** |
| CABG | 576 (48%) | 234 (59%) | 211 (53%) | 131 (33%) | 618 (94%) | 209 (96%) | 203 (92%) | 206 (93%) | ||
| Valve | 331 (28%) | 105 (26%) | 87 (22%) | 139 (35%) | 8 (1%) | ** | ** | ** | ** | |
| On-Pump | 1074 (90%) | 342 (86%) | 359 (90%) | 373 (94%) | 0.001 | 630 (95%) | 204 (94%) | 208 (94%) | 218 (98%) | 0.9 |
| Perfusion time (min) | 104 (80, 140)* | 88 (70, 108) | 100 (79, 129) | 134 (104, 175) | <0.001 | 104 (87, 122) | 96 (78, 113) | 103 (89, 122) | 111 (96, 130) | <0.001 |
| Days hospitalized | 6 (5, 9) | 6 (5, 8) | 6 (5, 8) | 7 (6, 10) | <0.001 | 8 (6, 12) | 7 (6, 10) | 8 (7, 12) | 10 (7, 15) | <0.001 |
| Acute Kidney Injury | 419 (35%) | 96 (24%) | 140 (35%) | 183 (46%) | <0.001 | 273 (41%) | 60 (27%) | 87 (39.3%) | 126 (56%) | <0.001 |
| Post-operative complications | ||||||||||
| Stroke | 7 (1%) | 2 (1%) | 2 (1%) | 3 (1%) | 0.87 | ** | ** | ** | ** | ** |
| Re-operation | 57 (5%) | 9 (2%) | 10 (3%) | 38 (10%) | <.001 | 26 (4%) | ** | ** | 15 (2%) | ** |
| Prolonged Ventilation | 39 (3%) | 3 (1%) | 6 (2%) | 30 (8%) | <.001 | 38 (6%) | ** | 12 (1%) | 16 (2%) | ** |
| DSWI | 9 (1%) | 3 (1%) | 0 | 6 (2%) | 0.05 | 4 (<%1) | ** | ** | ** | ** |
| b: Characteristics of patient cohorts by post-operative gal-3. | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient characteristics | Post-operative gal-3 (TRIBE-AKI) | Post-operative gal-3 (NNE) | |||||||||
| All (N=1193) | T1 (n=397) | T2 (n=398) | T3 (n=398) | p-value | All (N=660) | T1 (n=215) | T2 (n=222) | T3 (n=223) | p-value | ||
| Age at surgery (SD) | 73 (8.4) | 73 (8.3) | 74 (8.6) | 73 (8.3) | 0.8 | 71 (7.9) | 70 (7.2) | 72 (7.2) | 71 (9.0) | <0.001 | |
| Male | 837 (70%) | 296 (75%) | 289 (73%) | 252 (63%) | 0.001 | 501 (76%) | 185 (86%) | 164 (74%) | 152 (68%) | <0.001 | |
| Preoperative comorbidities | |||||||||||
| Diabetes | 444 (37%) | 139 (35%) | 153 (38%) | 152 (38%) | 0.5 | 273 (41%) | 78 (36%) | 91 (41%) | 104 (47%) | 0.09 | |
| Hypertension | 955 (80%) | 314 (79%) | 313 (79%) | 328 (82%) | 0.3 | 555 (84%) | 174 (81%) | 190 (86%) | 191 (86%) | 0.3 | |
| Myocardial infarction | 302 (26%) | 103 (26%) | 108 (27%) | 92 (23%) | 0.5 | 309 (47%) | 103 (48%) | 98 (44%) | 108 (48%) | 0.6 | |
| Congestive heart failure | 244 (20%) | 64 (16%) | 73 (18%) | 107 (27%) | <0.001 | 86 (13%) | 18 (8%) | 27 (12%) | 41 (18%) | 0.007 | |
| Preoperative renal function | |||||||||||
| Serum creatinine | 1.1 (0.3) | 1.0 (0.3) | 1.1 (0.3) | 1.1 (0.4) | 0.3 | 1.2 (0.7) | 1.1 (0.5) | 1.1 (0.4) | 1.4 (1.1) | <0.001 | |
| ≥90 | 129 (11%) | 46 (12%) | 43 (11%) | 40 (10%) | 95 (14%) | 39 (18%) | 29 (13%) | 27 (12%) | |||
| eGFR (ml/min per 1.73 m2) | 60-90 | 661 (55%) | 225 (57%) | 226 (57%) | 210 (53%) | 0.5 | 358 (54%) | 139 (65%) | 118 (53%) | 101 (45%) | <0.001 |
| ≤60 | 403 (34%) | 126 (32%) | 129 (32%) | 148 (37%) | 207 (31%) | 37 (17%) | 75 (34%) | 95 (43%) | |||
| Characteristics of surgery and hospitalization | |||||||||||
| Elective surgery | 1023 (86%) | 345 (87%) | 351 (88%) | 326 (82%) | 0.03 | 183 (28%) | 68 (32%) | 61 (27%) | 54 (24%) | 0.2 | |
| CABG with valve repair | 285 (24%) | 78 (20%) | 85 (21%) | 122 (31%) | <0.001 | 34 (5%) | ** | ** | ** | ** | |
| CABG | 576 (48%) | 226 (57%) | 209 (53%) | 141 (36%) | 618 (94%) | 198 (92%) | 221 (>99%) | 199 (84%) | |||
| Valve | 331 (28%) | 93 (23%) | 104 (26%) | 134 (34%) | ** | ** | ** | ** | ** | ||
| On-Pump | 1074 (90%) | 342 (86%) | 351 (88%) | 381 (96%) | 630 (95%) | 206 (96%) | 212 (95%) | 212 (95%) | |||
| Perfusion time (min) | 104 (80, 140)* | 93 (71, 119) | 99 (74, 130) | 123 (98, 158) | <0.001 | 104 (87, 122) | 98 (81, 117) | 105 (88, 124) | 108 (93, 123) | 0.01 | |
| Days hospitalized | 6 (5, 9) | 6 (5, 8) | 6 (5, 8) | 7 (6, 10) | <0.001 | 8 (6, 12) | 8 (6, 10) | 8 (6, 11) | 10 (7, 14) | 0.01 | |
| Acute Kidney Injury | 419 (35%) | 106 (27%) | 131 (33%) | 182 (46%) | 273 (41%) | 70 (33%) | 83 (37%) | 120 (53%) | <0.001 | ||
| Post-operative complication | |||||||||||
| Stroke | 7 (1%) | 2 (1%) | 5 (1%) | 0.07 | ** | ** | ** | ** | ** | ||
| Re-operation | 57 (5%) | 17 (4%) | 13 (3%) | 27 (7%) | 0.06 | 26 (4%) | ** | 14 (2%) | ** | ** | |
| Prolonged Ventilation | 39 (3%) | 8 (2%) | 8 (2%) | 23 (6%) | 0.003 | 38 (6%) | ** | 17 (2%) | 14 (2%) | ** | |
| DSWI | 9 (1%) | 4 (1%) | 2 (1%) | 3 (1%) | 0.70 | ** | ** | ** | ** | ** | |
Information was not available for a small number of patients (less than 50).
n < 11 with inability to pull data from the Medicare data server. SD = standard deviation; eGFR = estimated glomerular filtration rate; ICU = intensive care unit; DSWI = deep sternal wound infection.
Association of biomarkers with the primary composite outcome
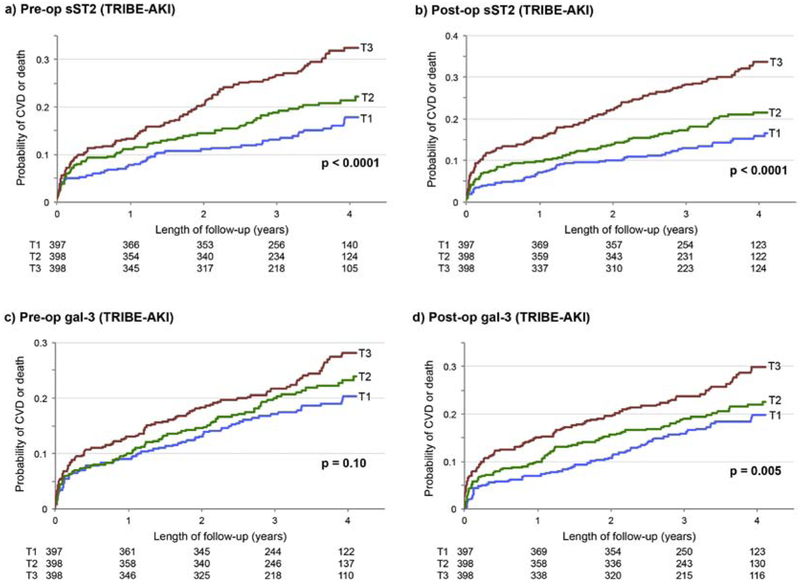
Event rates for the primary composite outcome were 70.2 and 66.8 per 1000 person-years for the TRIBE-AKI and NNE cohorts, respectively, with approximately half of the events coming from cardiovascular events and half from deaths (Supplemental Table 3a). Biomarker values were approximately 1.5-fold higher following surgery (Supplemental Figure 2a-b). Event curves of the primary outcome for TRIBE-AKI participants per year are shown in Figure 1 a-d, demonstrating increased probability of CVD or death for participants with higher values of sST2 pre- and post-operatively, and for higher values of gal-3 post-operatively.
Figure 1.
a-d. Event curves by tertiles of pre- and post- operative sST2 and gal-3 for the TRIBE-AKI cohort. Higher tertiles of pre- and post- operative sST2 levels (a, b) and gal-3 levels (c, d) demonstrate association with higher event frequency, though this trend was not significant for pre-operative gal-3. Numbers of patients at risk per year for each tertile are listed below each graph.
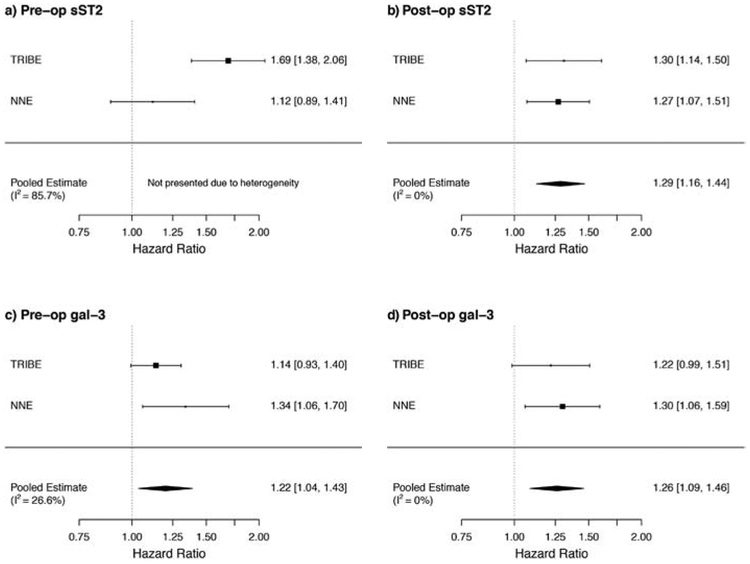
Hazard ratios for the primary outcome using log-transformed sST2 and gal-3 values and tertiles are shown in Table 2. After adjustment for clinical covariates, there was a 69% higher risk of the primary outcome for each log-unit increase of pre-operative sST2 in the TRIBE-AKI cohort, and the highest tertile of pre-operative sST2 had a HR of 1.91 (95% CI 1.40, 2.60) for the primary outcome compared with the lowest tertile. However, the significance of this association was not confirmed by NNE cohort data. Post-operatively, TRIBE-AKI patients with the highest tertile of sST2 values had a two-fold risk (HR 2.0; 95% CI 1.44, 2.78) for the primary outcome compared to patients with the lowest tertile of sST2 values, with NNE patients demonstrating similar outcomes. Results for both biomarkers were comparable when looking at cardiovascular events alone, suggesting that cardiovascular events (largely driven by heart failure events) and death events likely contributed similarly to the reported association (Supplemental Table 3b). Post-operative associations remained unchanged after adjustment for pre-operative biomarker values (data not shown). In a subset analysis of the TRIBE-AKI cohort only, the highest tertile of sST2 remained significantly associated with the primary outcome even after added adjustments for NT-proBNP or troponin T (Supplemental Table 4). Combining data from both cohorts in a meta-analysis, pooled HR estimates demonstrated a 1.29-fold risk (95% CI 1.16, 1.44) for the primary outcome for each log-unit increase in post-operative sST2 (Figure 2 a-b). Spearman correlations between sST2 and gal-3 were 0.056 for pre-op values and 0.251 for post-op values, supporting the idea that these biomarkers do not always correlate in expression and may not be able to be used interchangeably.
Table 2. Hazard ratios for the primary outcome by biomarker value.
Definitions of tertiles with n for each tertile are as listed in Table 1 and Supplemental Table 2.
| Biomarker | Hazard Ratio (95% CI) of cardiovascular event or mortality |
|||||||
|---|---|---|---|---|---|---|---|---|
| TRIBE-AKI | NNE | |||||||
| Event rate per 1000py |
Unadjusted | Adjusteda | Event rate per 1000py |
Unadjusted | Adjusteda | |||
| sST2 | Pre-op | Log | 1.85 (1.52, 2.25) | 1.69 (1.38, 2.06) | 1.33 (1.07, 1.66) | 1.12 (0.89, 1.41) | ||
| T1 | 48.5 | 1.0 (referent) | 1.0 (referent) | 52.11 | 1.0 (referent) | 1.0 (referent) | ||
| T2 | 64.8 | 1.31 (0.95, 1.82) | 1.38 (0.99, 1.91) | 70.22 | 1.34 (0.95, 1.89) | 1.28 (0.91, 1.81) | ||
| T3 | 100.2 | 2.00 (1.48, 2.70) | 1.91 (1.40, 2.60) | 78.51 | 1.49 (1.06, 2.10) | 1.16 (0.82, 1.65) | ||
| Post-op | Log | 1.39 (1.23, 1.56) | 1.31 (1.14, 1.50) | 1.49 (1.24, 1.78) | 1.27 (1.07, 1.51) | |||
| T1 | 47.0 | 1.0 (referent) | 1.0 (referent) | 53.93 | 1.0 (referent) | 1.0 (referent) | ||
| T2 | 62.0 | 1.31 (0.94, 1.83) | 1.32 (0.94, 1.85) | 54.24 | 1.01 (0.70, 1.45) | 0.98 (0.68, 1.40) | ||
| T3 | 104.2 | 2.18 (1.61, 2.95) | 2.00 (1.44, 2.78) | 95.65 | 1.74 (1.26, 2.41) | 1.43 (1.03, 1.99) | ||
| Gal-3 | Pre-op | Log | 1.24 (1.01, 1.53) | 1.14 (0.93, 1.40) | 1.52 (1.20, 1.91) | 1.34 (1.06, 1.69) | ||
| T1 | 59.4 | 1.0 (referent) | 1.0 (referent) | 45.41 | 1.0 (referent) | 1.0 (referent) | ||
| T2 | 69.1 | 1.17 (0.87, 1.59) | 1.10 (0.81, 1.49) | 74.87 | 1.62 (1.14, 2.30) | 1.55 (1.09, 2.20) | ||
| T3 | 82.7 | 1.39 (1.03, 1.87) | 1.20 (0.89, 1.63) | 83.44 | 1.79 (1.26, 2.54) | 1.50 (1.05, 2.13) | ||
| Post-op | Log | 1.37 (1.11, 1.68) | 1.22 (0.99, 1.51) | 1.53 (1.26, 1.87) | 1.30 (1.06, 1.58) | |||
| T1 | 55.7 | 1.0 (referent) | 1.0 (referent) | 49.10 | 1.0 (referent) | 1.0 (referent) | ||
| T2 | 66.2 | 1.19 (0.87, 1.63) | 1.15 (0.84, 1.57) | 59.85 | 1.22 (0.85, 1.74) | 1.03 (0.72, 1.49) | ||
| T3 | 90.2 | 1.61 (1.20, 2.16) | 1.35 (0.99, 1.85) | 94.27 | 1.87 (1.34, 2.62) | 1.45 (1.03, 2.06) | ||
Adjusted model included Society of Thoracic Surgery (STS) score, sex, cardiopulmonary bypass time, non-elective surgery, hypertension, and congestive heart failure.
Figure 2.
a-d. Pooled hazard ratios for the primary composite outcome. HRs by pre- and post-operative (a, b) sST2 and (c, d) gal-3 values are shown, with pooled estimates and I2 values. TRIBE-AKI: n=1193 with 70.2 events per 1000 person-years. NNE: n=660 with 66.8 events per 1000 person-years.
In the TRIBE-AKI cohort, each log higher value of pre- or post-operative gal-3 was respectively associated with 14% or 22% higher risk of incidence CV event or mortality (Table 2). While the significance of this association was not maintained after adjustment for clinical covariates in the TRIBE-AKI cohort, the NNE cohort supported higher incidence of the primary outcome for the highest tertiles of pre- and post-operative gal-3 compared to the lowest tertiles [pre-op HR 1.50 (95% CI 1.05, 2.13), post-op HR 1.45 (95% CI 1.03, 2.06)]. Meta-analyses demonstrated a pooled 1.26-fold risk (95% CI 1.09, 1.46) for the primary outcome for each log-unit increase in post-operative gal-3 (Figure 2 c-d).
To evaluate the prognostic utility of pre-operative sST2 or gal-3 compared to STS scores, we then quantified c-indices (represented as areas under the curve or AUC), integrated discrimination index (IDI) scores, net reclassification index (NRI) scores, and reclassification proportions (Table 3). Use of pre-operative sST2, gal3, or both did not improve the prognostic utility of the STS score for 1-year or 3-year CVD or death, as demonstrated by similar AUC numbers and low IDI and NRI scores. However, use of biomarker values did capture some events that would not have been noted by STS score alone (events correctly reclassified), and did correctly reclassify non-events that would have otherwise been noted by a high STS score.
Table 3. Prognostic utility of sST2 and gal-3 compared to STS scores.
Due to the low event rate at 30 days, values were quantified for 1 and 3 years post-operatively. NRI calculations used thresholds to define low, medium and high risk. For 1-year outcomes the categories were defined as <7.5%, 7.5–15% and >15% and for 3-years <15%, 15–30% and >30%.
| Outcome | Model | AUC | AUC difference |
IDI (95% CI) |
NRI (95% CI) |
% Events correctly reclassified |
% Non- events correctly reclassified |
|---|---|---|---|---|---|---|---|
| 1-year CVD or death | STS score | 0.60 | |||||
| STS + log pre-op sST2 | 0.63 | 0.03 (−0.003, 0.06) | 0.0054 (0.001, 0.010) | 0.086 (−0.003, 0.175) | 7% | 2% | |
| STS + log pre-op gal-3 | 0.61 | 0.002 (−0.01, 0.02) | 0.0012 (−0.0005, 0.003) | 0.008 (−0.045, 0.062) | 2% | −2% | |
| STS + sST2 and gal-3 | 0.63 | 0.03 (−0.004, 0.06) | 0.0064 (0.001, 0.012) | 0.073 (−0.020, 0.167) | 5% | 3% | |
| 3-year CVD or death | STS score | 0.61 | |||||
| STS + log pre-op sST2 | 0.66 | 0.05 (0.02, 0.08) | 0.0222 (0.013, 0.033) | 0.098 (0.015, 0.181) | 1% | 8% | |
| STS + log pre-op gal-3 | 0.61 | −0.001 (−0.007, 0.006) | 0.001 (−0.0005, 0.002) | −0.012 (−0.040, 0.015) | −2% | 1% | |
| STS + sST2 and gal-3 | 0.66 | 0.05 (0.02, 0.08) | 0.0226 (0.013, 0.032) | 0.105 (0.023, 0.188) | 1% | 9% |
Association of biomarkers stratified by clinically important subgroups
We evaluated if our observed associations of sST2 and gal-3 with the primary outcome were modified by the prevalence of pre-operative CHF. There was no significant interaction between prevalence of CHF and the primary outcome for sST2 in either cohort (Supplemental Table 5). However, there was a significant interaction between pre-operative gal-3 and CHF status for the primary outcome, with a trend towards incidence of the primary outcome for patients with pre-operative CHF.
We also evaluated potential effect modification of AKI on the association of sST2 and gal-3 with the primary outcome. In the TRIBE-AKI cohort, there was an interaction between pre-operative gal-3 and AKI status for the primary outcome, as gal-3 appeared to associate with the primary outcome in patients without pre-operative AKI. However, this observation was not confirmed in the NNE cohort (Supplemental Table 6). There was no significant interaction between prevalence of AKI and the primary outcome for post-operative sST2 or gal-3 for the primary outcome in either cohort.
Discussion
In this study of adults undergoing cardiac surgery, we evaluated association of two cardiac biomarkers, plasma sST2 and gal-3, with a primary composite outcome of long-term cardiovascular event or all-cause mortality. We conclude that post-operative sST2 and gal-3 associate with the primary composite outcome, even after adjustment for clinical covariates, NT-proBNP, or troponin T. These findings were independent of pre-existing CHF or AKI.
Interestingly, higher pre-operative sST2 and gal-3 levels also demonstrated association with the primary outcome, though results had high heterogeneity. This finding raises questions regarding mechanisms and timing of biomarker expression and activity in cardiac tissue. Both sST2 and gal-3 have been studied in a variety of cardiac diseases including heart failure, atrial fibrillation, myocardial infarction, hypertrophy, and hypertension30-35, and higher baseline levels could indicate more severe underlying cardiac dysfunction, which could contribute to future morbidity and mortality.
Though we studied associations between biomarker levels and clinical outcomes, our study did not address mechanisms by which these biomarkers influence the myocardium. Both biomarkers were first discovered to play a role in cardiac remodeling through large microarray analyses, with sST2 linked to myocardial injury in response to myocardial infarction36, and gal-3 to cardiac dysfunction in hypertrophied rat hearts19. The specifics of their cellular actions, however, are yet to be fully understood and are complicated by multiple isoforms and protein structures, and by heterogeneous expression in tissues.
sST2, for instance, is one of three isoforms of the ST2 gene (the other two being a membrane-bound ST2L receptor and a variant ST2)16, 37, 38. IL-33 is a functional ligand for ST2L and promotes release of inflammatory cytokines and chemokines. Abundance of sST2, which serves as a decoy for active IL-33 in the extracellular space, could aid in avoiding damage caused by excessive inflammation. In fact, sST2 reduces inflammation in models of AKI and renal ischemia reperfusion39, 40. However, in cardiac tissue, the IL-33/ST2L axis is cardioprotective in shielding against cardiomyocyte apoptosis and maladaptive hypertrophy, and sST2 is therefore considered to be harmful in blocking antihypertrophic effects of IL-3314, 15. Gal-3 has an even wider variety of expression patterns and functions17, 18. It is ubiquitously expressed in the digestive tract, kidneys, lungs, and heart, and has different effects in the cytoplasm, cell surfaces, and extracellular environment. Gal-3 levels are low in cardiac tissue but are upregulated during processes such as left ventricular hypertrophy and heart failure19. One proposed mechanism for gal-3 activity during cardiac hypertrophy is that macrophages release gal-3 to induce fibrosis via TGF-beta pathways19, 35, 41-43.
The possibility of therapeutics targeting these biomarkers in the setting of cardiac surgery is also unclear. While blockade of IL-33 is studied in asthma45, 46 and blockade of sST2 in graft-versus-host disease47, 48, sST2 inhibition has not been studied in cardiac disease. Gal-3 inhibition is studied in cancer and nonalcoholic steatohepatitis49-51, and the gal-3 inhibitor TD139 was FDA-approved for patients with idiopathic pulmonary fibrosis50. Gal-3 blockade mitigates cardiac remodeling in animal models42, 43, 52, but there are not yet studies of gal-3 inhibition in patients with cardiac disease. The expression patterns and multifaceted roles of these biomarkers will make it challenging to ensure specificity of therapeutic targets, and studies of upstream and downstream modulators may help elucidate therapeutics targets to reduce the incidence of cardiovascular events and mortality.
Our study has several strengths, including that it is based on two multicenter, prospective studies, with broad participation of adults and high-quality data, sample collection, handling, and processing across study sites. It is the first study with a validation cohort to analyze the association of sST2 and gal-3 with future cardiac events and mortality in patients undergoing cardiac surgery. We demonstrated significant association of these biomarkers with the primary composite outcome even after adjustment for clinical covariates and NT-proBNP or troponin. Measurement of sST2 and gal-3 could provide additional prognostic value to these more traditional methods of assessment. Future studies could address the clinical applicability of sST2 and gal-3 measurement in patients undergoing cardiac surgery.
Our study does have several limitations. Most notably, while there are FDA-approved assays for sST2 and gal-3 for use in patients with heart failure, our study used Meso Scale assays and did not provide parameters for clinical use in patients undergoing cardiac surgery. Ultimately, clinical use would necessitate re-measurement of biomarker values using FDA approved assays. The studied biomarkers have been shown to vary by certain patient characteristics. Baseline sST2 levels are higher in men, elderly patients, and in patients with diabetes53. Gal-3 independently associates with age, gender (higher gal-3 values in women), eGFR, urinary albumin excretion, body mass index, NT-proBNP, serum cholesterol, and systolic blood pressure54-58. The majority of participants in the study cohorts were Caucasian males, and the original enrollment criteria for the TRIBE-AKI cohort were limited to patients at high risk for AKI23. A significant portion of the cohort populations was excluded from the final analyses (25% for TRIBE-AKI and 61% for NNE). Participants in the study cohorts had an average age over 70, and our data may not be applicable to younger patients undergoing surgery. Though withdrawal from Medicare or emigration from Canadian provinces is rare, it is possible that 100% administrative follow-up was not achieved.59, 60 Noted differences in patient population and characteristics between TRIBE-AKI and NNE (including type of surgery) limit direct comparison of these cohorts, and differences in sampling times as detailed in the methods section may limit the precision of reported associations. Furthermore, troponin T and NT-proBNP values were not measured in patients from the NNE cohort, limiting our ability to conclude that sST2 and gal-3 definitively remain associated with the primary outcome after adjustment for these traditional markers of cardiac risk.
In summary, we provide the first evidence for association of post-operative sST2 and gal-3 measurement in patients undergoing cardiac surgery, demonstrating that expression of these biomarkers is linked to cardiovascular event or mortality. We suggest that the prognostic value of sST2 and gal-3 is additive to risk associated with traditional covariates, NT-proBNP, and cardiac troponin T. However, further data from a large clinical trial would be necessary to assess clinical utility of these biomarkers. We propose that post-operative sST2 and gal-3 levels be evaluated as a tool to guide risk assessment for patients undergoing cardiac surgery.
Supplementary Material
Highlights:
Higher sST2 and gal-3 values associate with post-op mortality
Association was shown in two independent patient cohorts
Association is independent of heart failure or acute kidney injury
sST2 and gal-3 measurement may supplement traditional markers of injury such as troponin T and NT-proBNP
Acknowledgements:
Plasma cardiac biomarker assays were provided by Roche Diagnostics. This agency did not participate in protocol development, analysis, or interpretation of results.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: S.G.C. and C.R.P. are on the Advisory Board of RenalytixAI, and both own equity in the same. S.G.C. has served as a consultant for AKI therapeutics for Quark Biopharma and CHF Solutions. The remaining authors have no disclosures.
References:
- 1.In: Health, United States, 2016: With Chartbook on Long-term Trends in Health. Hyattsville (MD); 2017. [PubMed] [Google Scholar]
- 2.Mensah GA, Wei GS, Sorlie PD, Fine LJ, Rosenberg Y, Kaufmann PG, et al. Decline in Cardiovascular Mortality: Possible Causes and Implications. Circ Res 2017;120(2):366–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease C, Prevention. Decline in deaths from heart disease and stroke--United States, 1900-1999. MMWR Morb Mortal Wkly Rep 1999;48(30):649–56. [PubMed] [Google Scholar]
- 4.Ford ES, Ajani UA, Croft JB, Critchley JA, Labarthe DR, Kottke TE, et al. Explaining the decrease in U.S. deaths from coronary disease, 1980-2000. N Engl J Med 2007;356(23):2388–98. [DOI] [PubMed] [Google Scholar]
- 5.Ball L, Costantino F, Pelosi P. Postoperative complications of patients undergoing cardiac surgery. Curr Opin Crit Care 2016;22(4):386–92. [DOI] [PubMed] [Google Scholar]
- 6.Thomas MR, Lip GY. Novel Risk Markers and Risk Assessments for Cardiovascular Disease. Circ Res 2017;120(1):133–149. [DOI] [PubMed] [Google Scholar]
- 7.Beattie WS, Wijeysundera DN. Perioperative cardiac biomarkers: the utility and timing. Curr Opin Crit Care 2013;19(4):334–41. [DOI] [PubMed] [Google Scholar]
- 8.Ghashghaei R, Arbit B, Maisel AS. Current and novel biomarkers in heart failure: bench to bedside. Curr Opin Cardiol 2016;31(2):191–5. [DOI] [PubMed] [Google Scholar]
- 9.Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth universal definition of myocardial infarction (2018). Eur Heart J 2018. [DOI] [PubMed] [Google Scholar]
- 10.Wettersten N, Maisel AS. Biomarkers for Heart Failure: An Update for Practitioners of Internal Medicine. Am J Med 2016;129(6):560–7. [DOI] [PubMed] [Google Scholar]
- 11.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Colvin MM, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Card Fail 2017;23(8):628–651. [DOI] [PubMed] [Google Scholar]
- 12.Antman EM, Tanasijevic MJ, Thompson B, Schactman M, McCabe CH, Cannon CP, et al. Cardiac-specific troponin I levels to predict the risk of mortality in patients with acute coronary syndromes. N Engl J Med 1996;335(18):1342–9. [DOI] [PubMed] [Google Scholar]
- 13.Belley-Cote EP, Parikh CR, Shortt CR, Coca SG, Garg AX, Eikelboom JW, et al. Association of cardiac biomarkers with acute kidney injury after cardiac surgery: A multicenter cohort study. J Thorac Cardiovasc Surg 2016;152(1):245–251 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seki K, Sanada S, Kudinova AY, Steinhauser ML, Handa V, Gannon J, et al. Interleukin-33 prevents apoptosis and improves survival after experimental myocardial infarction through ST2 signaling. Circ Heart Fail 2009;2(6):684–91. [DOI] [PubMed] [Google Scholar]
- 15.Sanada S, Hakuno D, Higgins LJ, Schreiter ER, McKenzie AN, Lee RT. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J Clin Invest 2007;117(6):1538–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mueller T, Dieplinger B. The Presage((R)) ST2 Assay: analytical considerations and clinical applications for a high-sensitivity assay for measurement of soluble ST2. Expert Rev Mol Diagn 2013;13(1):13–30. [DOI] [PubMed] [Google Scholar]
- 17.Diaz-Alvarez L, Ortega E. The Many Roles of Galectin-3, a Multifaceted Molecule, in Innate Immune Responses against Pathogens. Mediators Inflamm 2017;2017:9247574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sciacchitano S, Lavra L, Morgante A, Ulivieri A, Magi F, De Francesco GP, et al. Galectin-3: One Molecule for an Alphabet of Diseases, from A to Z. Int J Mol Sci 2018;19(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharma UC, Pokharel S, van Brakel TJ, van Berlo JH, Cleutjens JP, Schroen B, et al. Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation 2004;110(19):3121–8. [DOI] [PubMed] [Google Scholar]
- 20.Bayes-Genis A, de Antonio M, Vila J, Penafiel J, Galan A, Barallat J, et al. Head-to-head comparison of 2 myocardial fibrosis biomarkers for long-term heart failure risk stratification: ST2 versus galectin-3. J Am Coll Cardiol 2014;63(2):158–66. [DOI] [PubMed] [Google Scholar]
- 21.Meijers WC, van der Velde AR, de Boer RA. ST2 and Galectin-3: Ready for Prime Time? EJIFCC 2016;27(3):238–52. [PMC free article] [PubMed] [Google Scholar]
- 22.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;62(16):e147–239. [DOI] [PubMed] [Google Scholar]
- 23.Parikh CR, Coca SG, Thiessen-Philbrook H, Shlipak MG, Koyner JL, Wang Z, et al. Postoperative biomarkers predict acute kidney injury and poor outcomes after adult cardiac surgery. J Am Soc Nephrol 2011;22(9):1748–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacobs JPA SS; Owens SL; Parker DM; Razaee M; Likosky DS; Shahian DM; Jacobs ML; Thiessen-Philbrook H; Wyler von Ballmoos M; Lobdell K; MacKenzie T; Everett AD; Parikh CR; Brown JR. The Association between Novel Biomarkers and 1-year Readmission or Mortality After Cardiac Surgery. Ann Thorac Surg 2018;(in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown JR, Jacobs JP, Alam SS, Thiessen-Philbrook H, Everett A, Likosky DS, et al. Utility of Biomarkers to Improve Prediction of Readmission or Mortality after Cardiac Surgery. Ann Thorac Surg 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown JR, MacKenzie TA, Dacey LJ, Leavitt BJ, Braxton JH, Westbrook BM, et al. Using biomarkers to improve the preoperative prediction of death in coronary artery bypass graft patients. J Extra Corpor Technol 2010;42(4):293–300. [PMC free article] [PubMed] [Google Scholar]
- 27.Polineni S, Parker DM, Alam SS, Thiessen-Philbrook H, McArthur E, DiScipio AW, et al. Predictive Ability of Novel Cardiac Biomarkers ST2, Galectin-3, and NT-ProBNP Before Cardiac Surgery. J Am Heart Assoc 2018;7(14). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parikh CR, Puthumana J, Shlipak MG, Koyner JL, Thiessen-Philbrook H, McArthur E, et al. Relationship of Kidney Injury Biomarkers with Long-Term Cardiovascular Outcomes after Cardiac Surgery. J Am Soc Nephrol 2017;28(12):3699–3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mehta RH, Grab JD, O'Brien SM, Bridges CR, Gammie JS, Haan CK, et al. Bedside tool for predicting the risk of postoperative dialysis in patients undergoing cardiac surgery. Circulation 2006;114(21):2208–16; quiz 2208. [DOI] [PubMed] [Google Scholar]
- 30.Chang KW, Hsu JC, Toomu A, Fox S, Maisel AS. Clinical Applications of Biomarkers in Atrial Fibrillation. Am J Med 2017;130(12):1351–1357. [DOI] [PubMed] [Google Scholar]
- 31.Dong R, Zhang M, Hu Q, Zheng S, Soh A, Zheng Y, et al. Galectin-3 as a novel biomarker for disease diagnosis and a target for therapy (Review). Int J Mol Med 2018;41(2):599–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hernandez-Romero D, Vilchez JA, Lahoz A, Romero-Aniorte AI, Jover E, Garcia-Alberola A, et al. Galectin-3 as a marker of interstitial atrial remodelling involved in atrial fibrillation. Sci Rep 2017;7:40378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kortekaas KA, Hoogslag GE, de Boer RA, Dokter MM, Versteegh MI, Braun J, et al. Galectin-3 and left ventricular reverse remodelling after surgical mitral valve repair. Eur J Heart Fail 2013;15(9):1011–8. [DOI] [PubMed] [Google Scholar]
- 34.Pascual-Figal DA, Lax A, Perez-Martinez MT, del Carmen Asensio-Lopez M, Sanchez-Mas J, Network G. Clinical relevance of sST2 in cardiac diseases. Clin Chem Lab Med 2016;54(1):29–35. [DOI] [PubMed] [Google Scholar]
- 35.Suthahar N, Meijers WC, Sillje HHW, Ho JE, Liu FT, de Boer RA. Galectin-3 Activation and Inhibition in Heart Failure and Cardiovascular Disease: An Update. Theranostics 2018;8(3):593–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weinberg EO, Shimpo M, De Keulenaer GW, MacGillivray C, Tominaga S, Solomon SD, et al. Expression and regulation of ST2, an interleukin-1 receptor family member, in cardiomyocytes and myocardial infarction. Circulation 2002;106(23):2961–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mueller T, Dieplinger B. Soluble ST2 and Galectin-3: What We Know and Don't Know Analytically. EJIFCC 2016;27(3):224–37. [PMC free article] [PubMed] [Google Scholar]
- 38.Mildner M, Storka A, Lichtenauer M, Mlitz V, Ghannadan M, Hoetzenecker K, et al. Primary sources and immunological prerequisites for sST2 secretion in humans. Cardiovasc Res 2010;87(4):769–77. [DOI] [PubMed] [Google Scholar]
- 39.Liang H, Xu F, Wen XJ, Liu HZ, Wang HB, Zhong JY, et al. Interleukin-33 signaling contributes to renal fibrosis following ischemia reperfusion. Eur J Pharmacol 2017;812:18–27. [DOI] [PubMed] [Google Scholar]
- 40.Akcay A, Nguyen Q, He Z, Turkmen K, Won Lee D, Hernando AA, et al. IL-33 exacerbates acute kidney injury. J Am Soc Nephrol 2011;22(11):2057–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gehlken C, Suthahar N, Meijers WC, de Boer RA. Galectin-3 in Heart Failure: An Update of the Last 3 Years. Heart Fail Clin 2018;14(1):75–92. [DOI] [PubMed] [Google Scholar]
- 42.Liu YH, D'Ambrosio M, Liao TD, Peng H, Rhaleb NE, Sharma U, et al. N-acetylseryl-aspartyl-lysyl-proline prevents cardiac remodeling and dysfunction induced by galectin-3, a mammalian adhesion/growth-regulatory lectin. Am J Physiol Heart Circ Physiol 2009;296(2):H404–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sharma U, Rhaleb NE, Pokharel S, Harding P, Rasoul S, Peng H, et al. Novel anti-inflammatory mechanisms of N-Acetyl-Ser-Asp-Lys-Pro in hypertension-induced target organ damage. Am J Physiol Heart Circ Physiol 2008;294(3):H1226–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu CK, Su MY, Lee JK, Chiang FT, Hwang JJ, Lin JL, et al. Galectin-3 level and the severity of cardiac diastolic dysfunction using cellular and animal models and clinical indices. Sci Rep 2015;5:17007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee HY, Rhee CK, Kang JY, Byun JH, Choi JY, Kim SJ, et al. Blockade of IL-33/ST2 ameliorates airway inflammation in a murine model of allergic asthma. Exp Lung Res 2014;40(2):66–76. [DOI] [PubMed] [Google Scholar]
- 46.Lei Y, Boinapally V, Zoltowska A, Adner M, Hellman L, Nilsson G. Vaccination against IL-33 Inhibits Airway Hyperresponsiveness and Inflammation in a House Dust Mite Model of Asthma. PLoS One 2015;10(7):e0133774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reichenbach DK, Schwarze V, Matta BM, Tkachev V, Lieberknecht E, Liu Q, et al. The IL-33/ST2 axis augments effector T-cell responses during acute GVHD. Blood 2015;125(20):3183–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang J, Ramadan AM, Griesenauer B, Li W, Turner MJ, Liu C, et al. ST2 blockade reduces sST2-producing T cells while maintaining protective mST2-expressing T cells during graft-versus-host disease. Sci Transl Med 2015;7(308):308ra160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Traber PG, Zomer E. Therapy of experimental NASH and fibrosis with galectin inhibitors. PLoS One 2013;8(12):e83481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mackinnon AC, Gibbons MA, Farnworth SL, Leffler H, Nilsson UJ, Delaine T, et al. Regulation of transforming growth factor-beta1-driven lung fibrosis by galectin-3. Am J Respir Crit Care Med 2012;185(5):537–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clark MC, Pang M, Hsu DK, Liu FT, de Vos S, Gascoyne RD, et al. Galectin-3 binds to CD45 on diffuse large B-cell lymphoma cells to regulate susceptibility to cell death. Blood 2012;120(23):4635–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu L, Ruifrok WP, Meissner M, Bos EM, van Goor H, Sanjabi B, et al. Genetic and pharmacological inhibition of galectin-3 prevents cardiac remodeling by interfering with myocardial fibrogenesis. Circ Heart Fail 2013;6(1):107–17. [DOI] [PubMed] [Google Scholar]
- 53.Coglianese EE, Larson MG, Vasan RS, Ho JE, Ghorbani A, McCabe EL, et al. Distribution and clinical correlates of the interleukin receptor family member soluble ST2 in the Framingham Heart Study. Clin Chem 2012;58(12):1673–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.de Boer RA, van Veldhuisen DJ, Gansevoort RT, Muller Kobold AC, van Gilst WH, Hillege HL, et al. The fibrosis marker galectin-3 and outcome in the general population. J Intern Med 2012;272(1):55–64. [DOI] [PubMed] [Google Scholar]
- 55.Nishiyama J, Kobayashi S, Ishida A, Nakabayashi I, Tajima O, Miura S, et al. Up-regulation of galectin-3 in acute renal failure of the rat. Am J Pathol 2000;157(3):815–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.O'Seaghdha CM, Hwang SJ, Ho JE, Vasan RS, Levy D, Fox CS. Elevated galectin-3 precedes the development of CKD. J Am Soc Nephrol 2013;24(9):1470–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tang WH, Shrestha K, Shao Z, Borowski AG, Troughton RW, Thomas JD, et al. Usefulness of plasma galectin-3 levels in systolic heart failure to predict renal insufficiency and survival. Am J Cardiol 2011;108(3):385–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van der Velde AR, Meijers WC, van den Heuvel ER, Bakker SJ, van Gilst WH, van der Harst P, et al. Determinants of temporal changes in galectin-3 level in the general population: Data of PREVEND. Int J Cardiol 2016;222:385–90. [DOI] [PubMed] [Google Scholar]
- 59.Ontario Population Projections Update 2012-2036. . In. Toronto, Ontario: Ontario Ministry of Finance; 2013. [Google Scholar]
- 60.Mues KE, Liede A, Liu J, Wetmore JB, Zaha R, Bradbury BD, et al. Use of the Medicare database in epidemiologic and health services research: a valuable source of real-world evidence on the older and disabled populations in the US. Clin Epidemiol 2017;9:267–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.