Abstract
Despite recent advances in treatment of non-small cell lung cancer(NSCLC), prognosis still remains poor and new therapeutic approaches are needed. Studies demonstrate the importance of the EGFR/HER-receptor family in NSCLC growth, as well as that of other tumors. Recently, HER3 is receiving increased attention because of its role in drug resistance and aggressive growth. Activation of overexpressed G-protein-coupled receptors (GPCR) can also initiate growth by transactivating EGFR/HER-family members. GPCR transactivation of EGFR has been extensively studied, but little is known of its ability to transactivate other EGFR/HER-members, especially HER3. To address this, we studied the ability of bombesin receptor(BnR) activation to transactivates all EGFR/HER-family members and their principal downstream signaling cascades, the PI3K/Akt- and MAPK/ERK-pathways, in human NSCLC cell-lines. In all three cell-lines studied, which possessed EGFR, HER2 and HER3, Bn rapidly transactivated EGFR, HER2 and HER3, as well as Akt and ERK. Immunoprecipitation studies revealed Bn-induced formation of both HER3/EGFR- and HER3/HER2-heterodimers. Specific EGFR/HER3 antibodies or siRNA-knockdown of EGFR and HER3, demonstrated Bn-stimulated activation of EGFR/HER members is initially through HER3, not EGFR. In addition, specific inhibition of HER3, HER2 or MAPK, abolished Bn-stimulated cell-growth, while neither EGFR nor Akt inhibition had an effect. These results show HER3 transactivation mediates all growth effects of BnR activation through MAPK. These results raise the possibility that targeting HER3 alone or with GPCR activation and its signal cascades, may be a novel therapeutic approach in NSCLC. This is especially relevant with the recent development of HER3-blocking antibodies.
Keywords: Non-small cell lung cancer (NSCLC), Lung cancer, HER3, EGF receptor family, Transactivation, Bombesin, G protein-coupled receptors (GPCRs)
1. Introduction
Lung cancer is the leading cause of cancer mortality in United States[1]. Despite the recent advances in its diagnosis and treatment, more than one-half of patients with lung cancer are diagnosed with advanced disease, and the prognosis remains poor with 5-year survival rates of 5% and 19% for those in advanced and all stages, respectively[1]. The epidermal growth factor (EGFR)/HER-receptor family including EGFR, HER2, HER3, and HER4, are widely overexpressed and significantly involved in the tumorigenesis of lung cancer, as well as a number of other various malignancies[2,3]. Recent studies have shown the significant antitumor activity of receptor tyrosine kinase (RTK) inhibitors targeting EGFR in patients with non-small cell lung cancer (NSCLC) possessing EGFR mutations[4–6], however, most patients become refractory to these agents limiting prolongation of their survival[7,8]. Thus, although these studies establish the importance of the EGFR receptor family in lung cancer progression, because many patients only transiently respond, there is an urgent need for other novel therapeutic strategies in this area.
Among the EGFR/HER-receptor family, HER3 is a kinase dead RTK[9,10], whereas HER2 is an orphan (ligand-less) RTK[11]. The activation of HER3 particularly has been receiving increased attention, with studies showing it has an important role in the initial and subsequent development of therapeutic escape from other antitumor agents including EGFR inhibitors [12–15] as well as tumor aggressiveness[16,16–19] and because of its ability to activate other EGFR/HER-receptor family members[ 10,11,20]. Despite HER3’s lack of intrinsic kinase activity, with its ability to form heterodimers with other EGFR/HER family members, particularly HER2[11,21], HER3 can subsequently activate numerous downstream cascades, particularly important for growth/resistance being the PI3K/Akt and MAPK/ERK pathways[22,23]. Overexpression of HER3 is reported in many cancers and has been particularly well studied in NSCLC, where it is reported to be very frequently overexpressed[24,25]. For example, in 7 representative studies[25–32] of EGFR/HER family member overexpression involving 1346 NSCLC patients, HER3 overexpression was seen in 51%[20-83%](mean ±SEM) of all patient’s NSCLCs, which was as frequent as overexpression of EGFR/HER1-52%[39-72%], and more frequent than overexpression of HER2-28%[13-45%] .The overexpression of HER3 in is associated with worse survival[16,18,19], as well as more aggressive malignant behavior[16,17]. Therefore, potentially targeting HER3, is becoming an increasingly attractive therapeutic target, both for initial treatment and for overcoming drug resistance in lung cancer as well as other tumors.
The mammalian bombesin receptor (BnR) family are G-protein coupled-receptors(GPCRs), comprising the neuromedin B receptor (NMBR), the gastrin-releasing peptide receptor (GRPR) and the orphan receptor bombesin-receptor subtype-3 (BRS-3)[33]. Each receptor is frequently overexpressed in numerous common tumors, including NSCLC[33–37]. This family of receptors has received particular attention in lung cancer because they act as autocrine growth factors and have potent growth effects[33,35,38,39]. Activation of BnR, as well as other GPCRs, can transactivate the EGFR/HER-receptor family in the absence of native EGFR/HER-receptor family ligands, such as EGF and neuregulin-1 (NRG-1)[11]. The crosstalk between GPCRs and EGFR/HER-receptor family has been widely studied in a number of cancers, primarily focusing on EGFR, which show a synergistic effect on tumor growth[40–42], as well as participating as one of the mechanisms underlying resistance to EGFR inhibitors[43,44]. Whereas the transactivation of EGFR/HER1 by GPCRs has been well-studied, particularly in NSCLCs[45–47], little is known about the ability of GPCR’s to transactivate HER3, and its role in tumor growth, as well as its effect on tumorigenesis. Recent studies in other tumors demonstrate that EGFR can either be activated directly by a stimulant or it can be due to the initial activation of HER3, which then heterodimerizes with EGFR and activates it[45,48–50]. Therefore, in previous studies in which only the activation of EGFR/HER1 by stimulation of a GRPR was examined, the possible importance of HER3 in the process cannot be appreciated. Therefore, the possible ability of GPCRs to transactivate HER3 is becoming increasingly important potential novel area of therapy, especially with the recent development of anti-HER3 agents[11,51].
Therefore, to provide insight into the possible role of GPCR transactivation of HER3, as well as the other EGFR/HER-family members, we have studied the interaction of BnR activation and EGFR/HER-receptor family transactivation in human NSCLC cells, and defined the roles of each member, as well as their downstream signaling cascades, in mediating effects on tumoral growth.
2. Materials and Methods
2.1. Materials
All cell-lines were obtained from the American Type Culture Collection (Rockville, MD). Dulbecco’s minimum-essential medium (DMEM), Roswell Park Institute medium1X (RPMI 1640), phosphate-buffered saline (PBS), fetal bovine serum (FBS), trypsin-EDTA, penicillin/streptomycin, Novex® 4-20% Tris-Glycine gel, Lipofectamine™ RNAiMAX, OPTI-MEM and ethidium bromide solution were from Invitrogen/Gibco (Carlsbad, CA); bombesin, gefitinib (Tocris Bioscience, Bristol, UK), 2-mercaptoethanol, sodium lauryl sulfate (SDS), Tris/Glycine/SDS (10×), nitrocellulose membranes, and SDS Laemmli sample buffer were from Bio-Rad Laboratories (Hercules, CA); TWEEN® 20, NP-40, phenylmethanesulfonylfluoride (PMSF), sodium orthovanadate, sodium deoxycholate, BCA protein assay kit and Super Signal West (Dura) chemiluminescent substrate were from Thermo Fisher scientific (Rockford, IL); Tris/HCl (pH 7.6) was from Mediatech Inc. (Herndon, VA); bovine serum-albumin (BSA) fraction V was from ICN Pharmaceutical Inc. (Aurora, OH); protease inhibitor tablet was from Roche (Basel, Switzerland); non-fat dry milk was from American Bio-analytical (Natick, MA); MAB3481, NRG-1 and EGF were from R&D systems (Minneapolis, MN); PD98059 was from Apexbio (Houston, TX); U0126, wortmannin and LY294002 was from Sigma (St. Louis, MO); immobilized protein A/G PLUS agarose was from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA); horseradish peroxidase-linked anti-rabbit secondary antibody, monoclonal rabbit anti-α/β-tubulin, rabbit polyclonal EGFR, HER2, HER3 and HER4 antibodies, rabbit polyclonal anti-phosphorylated forms p44/42-MAP-Kinase (Thr202/Tyr204), EGFR (Tyr1068), HER2 (Tyr1248) and HER3 (Tyr1289) antibodies were from Cell Signaling Technology (Beverly, MA); cell counting kit-8 (CCK-8) was from Dojindo (Rockville, MD); CellTiter 96® AQueous One Solution Cell-proliferation (MTS) assay was from Promega (Madison, WI); Small interfering RNA oligo (siRNA) pools for EGFR (M-003114-01), HER3 (M-003127-03) and non-targeting control (D-001206-14-20) were purchased from Dharmacon (Lafayette, CO).
2.2. Cell culture
Human NSCLC cell-lines were maintained in a humidified atmosphere of 5%CO2/95% air at 37 °C. H157, Calu-3 and PC9 were cultured in DMEM supplemented with 10% FBS and 1% penicillin/streptomycin. All other cells were cultured in RPMI1640 containing 10% FBS and 1% penicillin/streptomycin. The cells were mycoplasma free and were used when they were in exponential growth phase.
2.3. RNA extraction and reverse transcription polymerase chain reaction (RT-PCR)
Total RNA was isolated from 17 human lung cancer cells using a RNeasy Mini Kit (Qiagen, Valencia, CA, USA) as described previously[41,52]. After treatment with DNase Digestion (Qiagen) to remove contaminating DNA. Total RNA (1 μg) was reverse transcribed using a Superscript™ III First-Strand Synthesis SuperMix for qRT-PCR (Invitrogen) for complementary DNA (cDNA) synthesis. PCR amplifications for EGFR/HER3-receptor family were performed using the HotStarTaq® Master Mix Kit (Qiagen) following the manufacturer’s instructions. Amplification conditions for PCR-reactions included an initial cycle of 95 °C for 15 min, followed by 35-cycles of denaturation at 94 °C for 30s, annealing at 60 °C for 30 s and extension at 72 °C for 1 min. After the final-cycle, all PCR-reactions concluded with a 10 min extension at 72 °C. The PCR products were analyzed on a 3% agarose gel and visualized by ethidium bromide staining. The primer sequences used in this study were listed in Table 1. β-actin was used as internal control.
Table 1.
Primers used in RT-PCR
| Gene | Forward (5’-3’) | Reverse (5’-3’) | Size (bp) |
|---|---|---|---|
| EGFR | TCTTCGGGGAGCAGCGAT | TCGTGCCTTGGCAAACTTTC | 119 |
| HER2 | AGCCGCAGTGAGCACCATGG | GTGCCGGTGCACACTTGGGT | 102 |
| HER3 | CCTATGCAGGGCTACGATTGG | GTTGGGCTCAGCAGGTAACT | 131 |
| HER4 | CATTTGACCATGACCATGTAAACGTC | GGAACTGATGACCTTTGGAGGAA | 135 |
| β-actin | CCTCGCCTTTGCCGATCC | GGAATCCTTCTGACCCATGC | 205 |
2.4. Western blotting and immunoprecipitation
The expression and phosphorylation levels of EGFR/HER-receptor family, p44/42 and Akt were investigated by Western blotting as described previously[41,52]. Briefly, cells were placed in 6-well plate and when 80% confluent they were serum starved overnight. After incubation in the presence or absence of Gefitinib (30 min) or MAB3481 (1 hr), cells were treated with either Bn (0.1-100 nM), NRG-1 (0.1-100 ng/mL), EGF (10 nM) or no addition with 3-60 min and washed twice ice-cold PBS. Cells were then disrupted with Radio-Immunoprecipitation Assay (RIPA) buffer containing 50 mM Tris-HCl (pH 7.6), 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS, 1 mM phenylmethylsulfonylfluoride, 0.2 mM sodium vanadate, and one tablet of EDTA-free protease inhibitor per 10 mL, and the lysate was sonicated for 5 s at 4 °C. The insoluble fraction was eliminated by centrifugation (13000 rpm, 10 min) and protein concentration in cell lysates was determined by the BCA assay.
Equal amounts of protein for each lysate were electrophoretically separated on 4–20% Tris-glycin gel and transferred onto nitrocellulose membranes. After blocking in TBS containing 5% BSA and 0.05% Tween-20, membranes were incubated with primary antibodies (1:1000) overnight at 4°C, followed by incubation with secondary antibody (1:10000) for 1 hr at room temperature. The blots were developed with SuperSignal West Dura chemiluminescent substrate and proteins bands were measured using GeneTools software from Syngene (Bangalore, India), which were assessed in the linear detection range. Densitometric values were background subtracted.
For immunoprecipitation, 800 μg of protein was incubated with 10 μL of the anti-HER3 antibody (NeoMarkers, Fremont, CA) at 4°C for 1 hr, followed by overnight incubation with 20 μl of protein A/G agarose beads at 4°C under agitation. Samples were washed five times with RIPA buffer, re-suspended in 40 μL of 2X SDS Laemmli buffer and boiled for 5 min before electrophoresis.
2.5. Transfection with siRNA
For siRNA-mediated knockdown, 5 × 105 H441 or Calu3 cells were seeded in 6-well plates with complete medium without antibiotics. When they reached 50-70% confluence (about 24 hr), cells were then transfected with 10 nM of siRNAs against human EGFR or HER3, or non-targeting Control-siRNA in Opti-MEM medium using Lipofectamine according to the manufacture’s instruction. After 48 hr of transfection, cells were serum starved for another 24 hr before the following experiments.
2.6. Cell-proliferation assay
Cell-proliferation was measured by CCK-8 and MTS assay according to the manufacture’s instruction. In brief, H441 or Calu3 cells with or without EGFR/HER3-knockdown were seeded in quadruplicate in 96-well plates at a density of 5000 cells/well. The next day, cells were treated with indicated stimulants with/without inhibitors for 24 hr. Subsequently, 20μL of MTS reagent or 10μL of CCK-8 solution were added to each well at the end of each time point and incubated for 2 hr at 37°C. Optical density was measured at 490 nm for MTS assay and 450 nm for CCK-8 assay, respectively.
2.7. Statistical analysis
All results are expressed as mean ± SEM from at least 3 experiments, and results were considered statistically significant if p value was < 0.05 in student’s t test or one-way ANOVA (Dunnett’s multiple tests, as a posttest). All statistical analyses were performed using the GraphPad PRISM software (GraphPad Software Inc., La Jolla, CA).
3. Results
3.1. Expression of HER-family mRNA and protein in human NSCLC cell-lines
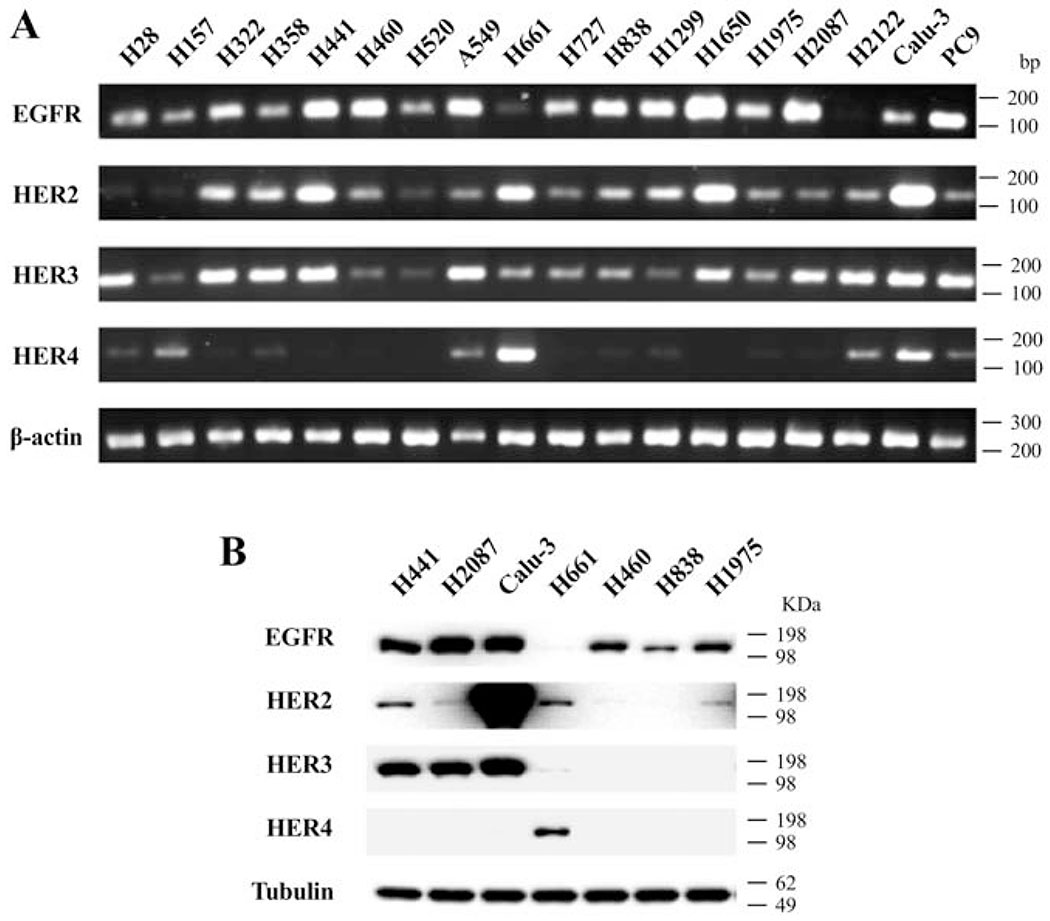
The expression of EGFR, HER2, HER3 and HER4 was initially assessed by PCR in 18 lung cancer cell-lines, including 16 NSCLC cells, one mesothelioma cell-line(H28) and one neuroendocrine tumor cell-line (H727). In the cell-lines, EGFR (94%), HER2 (88%) and HER3 (100%) mRNA were frequently expressed, while HER4 mRNA was detected only in 47% of cell-lines (Fig. 1A). We next performed Western blotting to assess the protein expression of HER-family in 7 NSCLC cell-lines (Fig. 1B). Consistent with the findings from PCR, EGFR protein was frequently detected (86%), whereas the detection rate of HER2 and HER3 protein in these 7 cell-lines was 71% and 43%, respectively (Fig. 1B). In addition, HER4 protein was detected only in H661 cells (Fig. 1B).
Figure 1.
Expression of the EGFR/HER-receptor family in human lung cancer cell-lines. (A) RT-PCR was performed with 17 human lung cancer cell-lines to evaluate the expression of EGFR/HER-receptor mRNA. β-actin was used as loading control. Primers used are shown in Table 1 and experimental conditions are as described in METHODS. (B) Whole cell lysate from 7 human NSCLC cell-lines were analyzed for the EGFR/HER-receptor expression by Western blotting. Tubulin was used as loading control. These results are representative of 2 others.
3.2. Time course of Bn- and NRG-1-induced activation of HER-family in human NSCLC cell-lines
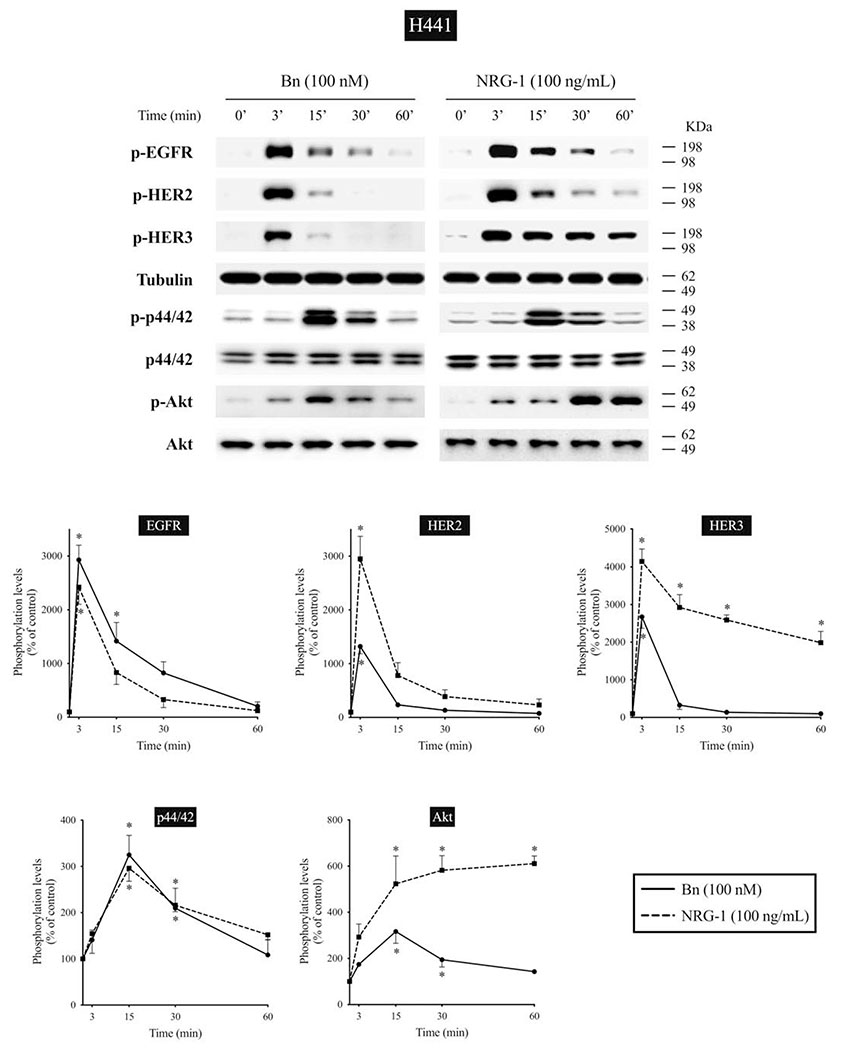
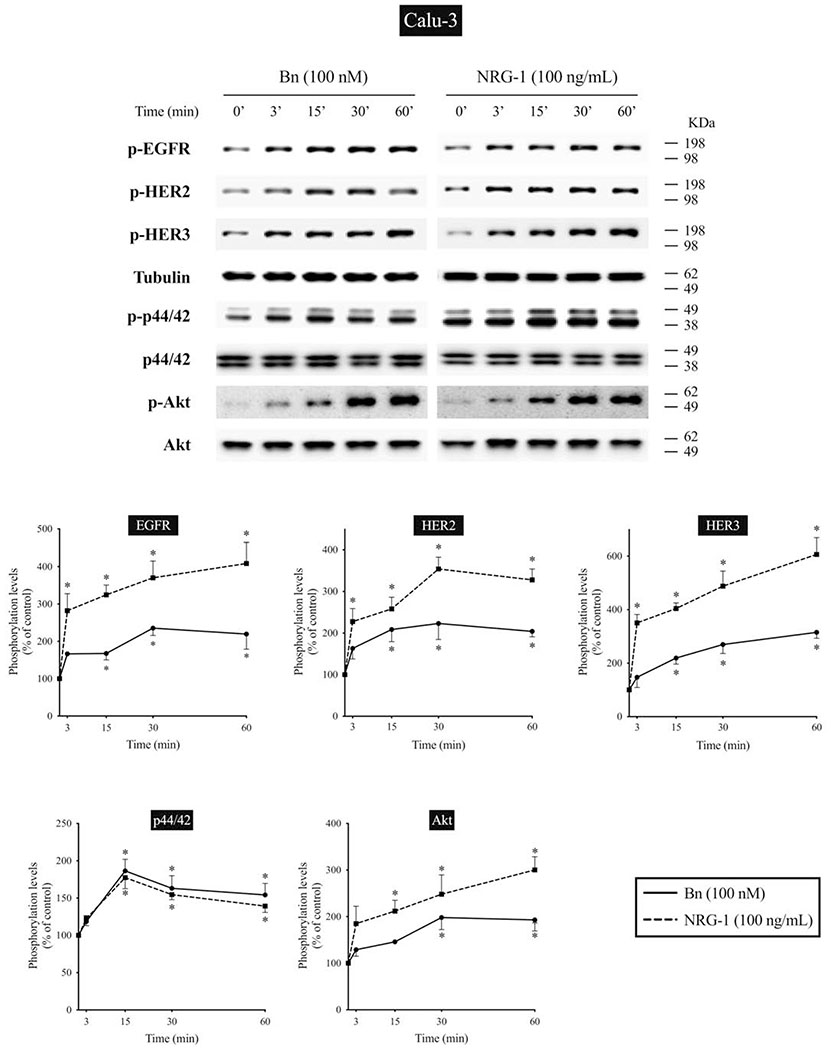
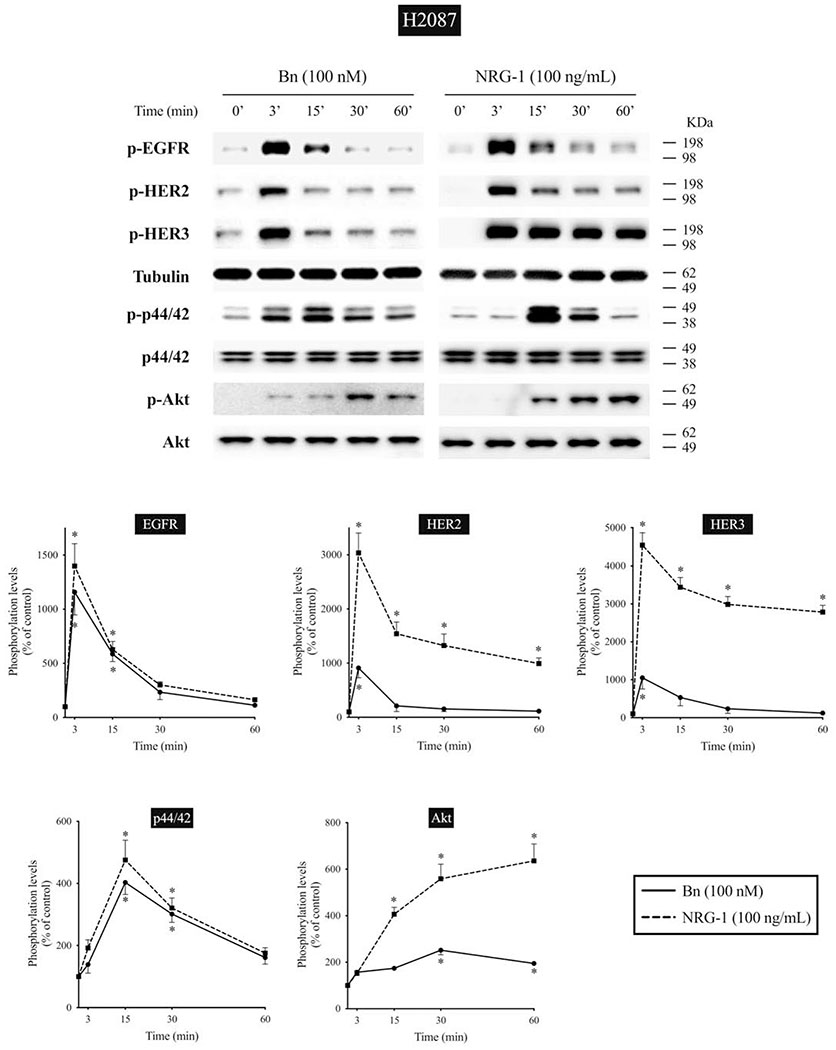
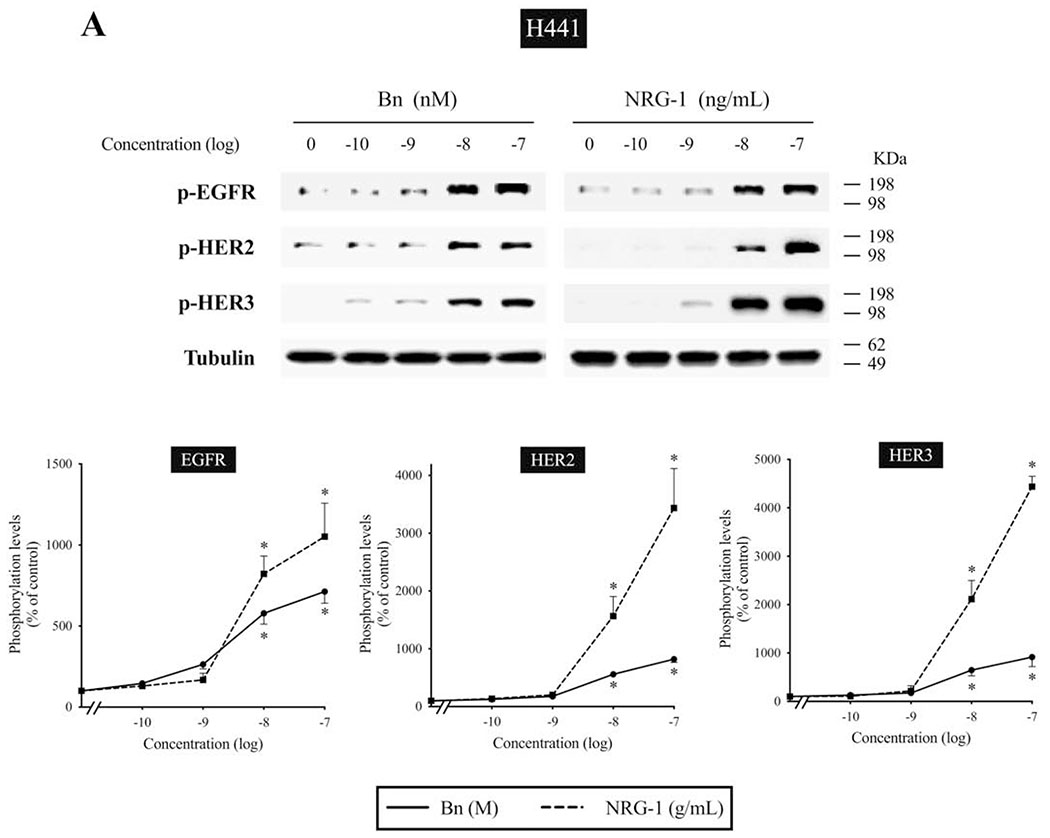
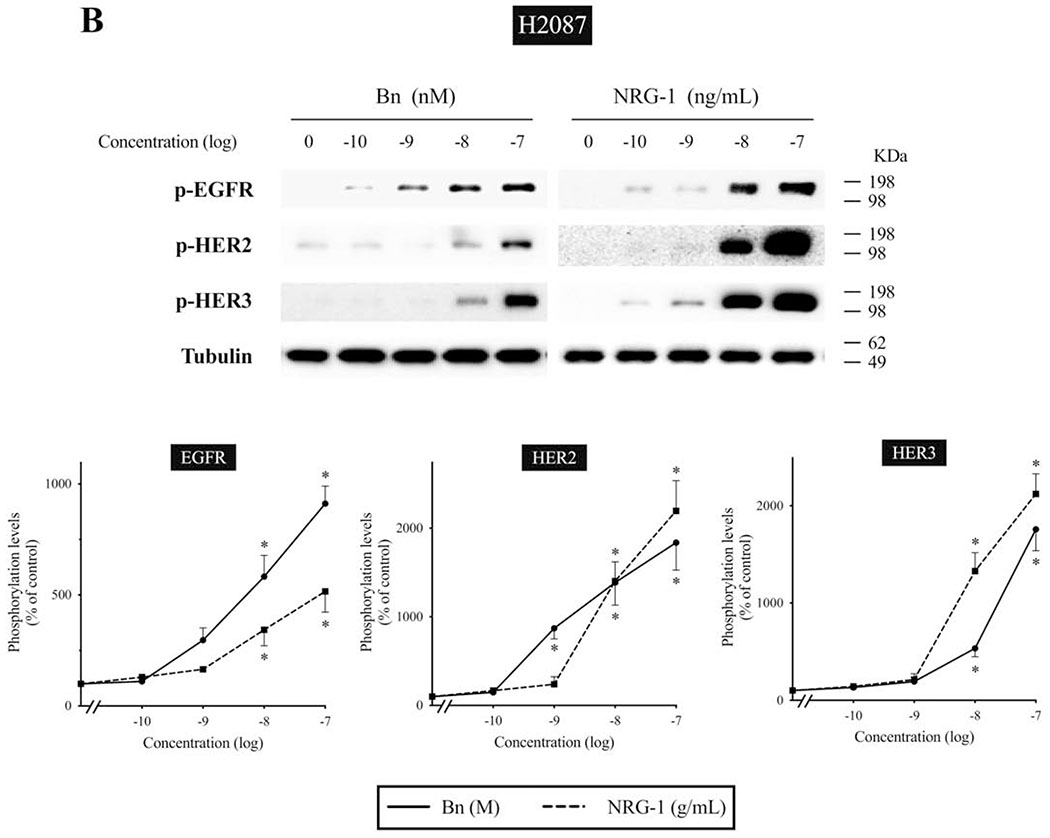
We next studied the time-dependent ability of Bn or NRG-1 to activate the EGF/HER-receptor family in 3 human NSCLC cell-lines that express EGFR, HER2, and HER3, but do not express detectible HER4 protein assessed by Western blotting(i.e. H441, H2087, and Calu-3 cells) (Fig. 2–4). In H441 cells, Bn caused a rapid and maximum phosphorylation of EGFR, HER2, and HER3 at 3 minutes (13- to 29-fold increase, p < 0.05 vs. control), which then fell off and was not present after 30-60 minutes (Fig. 2). NRG-1 also caused rapid phosphorylation of EGFR, HER2 and HER3 peaking at 3 minutes (24- to 42-fold increase, p < 0.05 vs. control), which then decreased with time (Fig. 2). However, in contrast to Bn-stimulation, NRG-1-stimulated phosphorylation of HER3 was still maintained at 60 minutes (20-fold increase, p < 0.05 vs. control). Similar time-dependent activation patterns were observed in H2087 cells, however, with NRG-1, prolonged phosphorylation of HER2 and HER3 were seen (Fig. 3). Conversely, Bn and NRG-1 induced a slower and more prolonged time-dependent EGFR, HER2 and HER3 phosphorylation in Calu-3 cells (Fig. 4), reaching a maximum after 30-60 min stimulation time. In each of these three cell-lines, NRG-1 maximal stimulation was greater than that seen with Bn (Fig. 2–4).
Figure 2.
Time course of Bn- and NRG-1-induced activation of the EGFR/HER-receptor family, MAPK (p44/42) and Akt in H441 NSCLC cells. Cells were treated Bn (100 nM) or NRG-1 (100 ng/mL) for the indicated times. Phosphorylation levels of EGFR, HER2, HER3, p44/42, and Akt were analyzed by Western blotting. Tubulin was used as loading control for EGFR, HER2, and HER3. Top panel shows a representative blot and bottom graphs show means ± SEM of three independent experiments, respectively. Results are expressed as % of basal phosphorylation. * P < 0.05 vs. control.
Figure 4.
Time course of Bn- and NRG-1-induced activation of the EGFR/HER-receptor family, MAPK (p44/42) and Akt in Calu-3 NSCLC cells. Phosphorylation levels of Bn- and NRG-1-stimulated EGFR, HER2, HER3, p44/42, and Akt were analyzed by Western blotting as described in Figure 2 legend. * P < 0.05 vs. control.
Figure 3.
Time course of Bn- and NRG-1-induced activation of the EGFR/HER-receptor family, MAPK (p44/42) and Akt in H2087 NSCLC cells. Phosphorylation levels of Bn- and NRG-1-stimulated EGFR, HER2, HER3, p44/42, and Akt were analyzed by Western blotting as described in Figure 2 legend. * P < 0.05 vs. control.
3.3. Time course of Bn- and NRG-1-induced stimulation of p44/42 MAPK and Akt in human NSCLC cell-lines
In previous studies in lung cancer cells, activation of the EGFR/HER-receptor family resulted in activation of MAPK and Akt[13,14,23,53]. Therefore, we studied the time course of activation of MAPK and Akt to compare it with EGFR, HER2, and HER3 activation (Fig. 2–4). Both Bn and NRG-1 rapid activated both p44/42 and Akt in each of the 3 NSCLC cell-lines with maximum phosphorylation of p44/42 after 15-min stimulation time in the 3 cell-lines (1.9- to 4.0-fold increase, p < 0.05 vs. control) (Fig. 2–4). In the different cell-lines, the time-course for activation of p44/42 varied, whereas it was similar in each cell-line for Akt. Specifically, in H441 and H2087 cells, phosphorylation of p44/42 was not maintained at 60 minutes, whereas it was still present in Calu-3 cells (Fig. 2–4). For each NSCLC cell-line with Akt, Bn showed maximum phosphorylation at 15 minutes (3.2-fold increase, p < 0.05 vs. control) and then decreased with further time in H441 cells, while Bn-induced phosphorylation reached a plateau at 30 minutes (2.0- to 2.5-fold increase, p < 0.05 vs. control) and was still maintained at 60 minutes (1.9-fold increase, p < 0.05 vs. control) in H2087 and Calu-3 cells (Fig. 2–4). NRG-1 showed detectible increases in phosphorylation of Akt at 15 minutes in all 3 cells (2.1- to 5.2-fold increase, p < 0.05 vs. control), and continued to increase in a time-dependent manner reaching maximum at 60 minutes (3.0- to 6.4-fold increase, p < 0.05 vs. control) (Fig. 2–4). A comparison of the time course of activation of EGFR, HER2 and HER3 by Bn- and NRG-1 with that for p44/42 and Akt demonstrated a similar pattern with the 3 cell-lines (Fig. 2–4).
3.4. Dose-response effect of Bn- and NRG-1-induced phosphorylation of HER-family in human NSCLC cell-lines
We examined the dose-response curve for EGFR, HER2, and HER3 activation in response to increasing concentrations of Bn and NRG-1 (Fig. 5A, B). In H441 cells, Bn produced a detectible increase in EGFR, HER2 and HER3 phosphorylation at 10 nM, maximal stimulation at 100 nM (EGFR, 7.1-fold; HER2, 8.2-fold; HER3, 9.1-fold increase), with a half-maximal effect (EC50) of 3.5±0.4 nM, 7.2±1.2 nM, 6.5±1.0 nM in EGFR, HER2 and HER3 phosphorylation, respectively (Fig. 5A). NRG-1 also induced dose-dependent increases in EGFR, HER2, and HER3 phosphorylation, reaching a 2.0-fold increase in EGFR (EC50 = 4.9±0.9 ng/mL), 34-fold increase in HER2 (EC50 = 16.3±3.4 ng/mL), and a 44-fold increase in HER3 (EC50 = 14.3±1.7 ng/mL), respectively. Similarly, EGFR, HER2 and HER3 phosphorylation increased with increasing Bn and NRG-1 concentration in H2087 cells (Fig. 5B). The maximum EGFR phosphorylation reached a 9.1-fold increase with 100 nM Bn (EC50 = 7.6±1.3 nM) and a 5.2-fold increase with 100 ng/mL NRG-1 (EC50 = 9.7±1.3 ng/mL). HER2 phosphorylation reached an 18-fold increase with 100 nM Bn (EC50 = 1.2±0.2 nM) and a 22-fold increase with 100 ng/mL NRG-1 (EC50 = 7.8±2.0 ng/mL). HER3 phosphorylation reached a 17-fold increase with 100 nM Bn (EC50 = 51.4±7.3 nM) and a 21-fold increase with 100 ng/mL NRG-1 (EC50 = 8.1±1.7 ng/mL).
Figure 5.
Dose-response effect of Bn- and NRG-1-stimulated activation of the EGFR/HER-receptor family in H441 and H2087 NSCLC cells. H441 (A) and H2087 (B) cells were treated with the indicated concentrations of Bn and NRG-1 for 3 min and then lysed. Phosphorylation levels of EGFR, HER2 and HER3 were analyzed by Western blotting. Tubulin was used as loading control. Top panel shows a representative blot and bottom graphs show means ± SEM of three independent experiments, respectively. Results are expressed as % of basal phosphorylation. * P < 0.05 vs. control.
3.5. Bn- and NRG-1-induced dimerization of HER3 in human NSCLC cell-lines
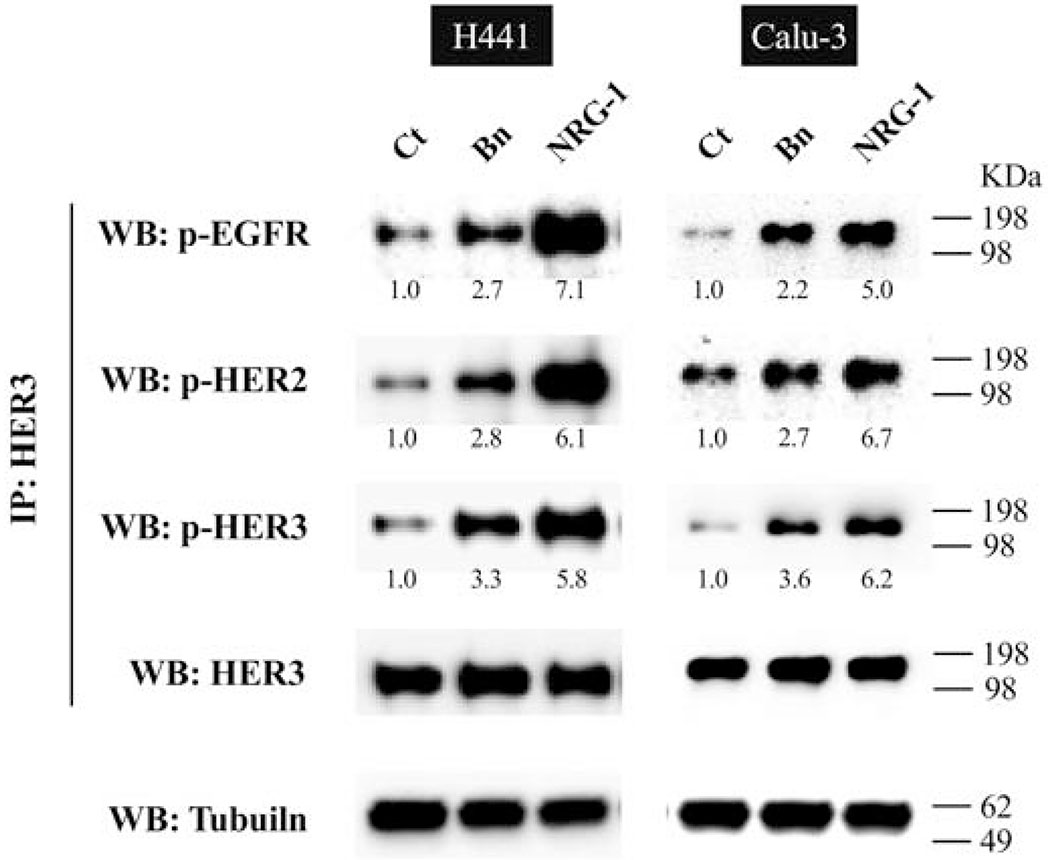
Because of its impaired catalytic kinase activity, activation of HER3 requires heterodimerization with other EGFR/HER-receptor members (EGFR, HER2, and HER4), with a marked preference for HER2 as a dimer partner[11,21,54]. To investigate in more detail the partner of the dimerization caused by Bn and NRG-1 with HER3 activation, we carried out an immunoprecipitation study in H441 and Calu-3 cells (Fig. 6). Both Bn and NRG-1 induced HER3/EGFR and HER3/HER2 heterodimerization in both cell-lines. The ability to form activated heterodimers was 1.7- to 2.6-fold higher with NRG-1 than with Bn (Fig. 6).
Figure 6.
Dimerization of HER3 with other EGFR/HER-receptor members on stimulation with Bn or NRG-1. Immunoprecipitation experiments were performed to detect the Bn- and NRG-1-induced dimerization of HER3 in two NSCLC cell-lines. H441 and Calu-3 cells were treated with no addition, 100 nM Bn, or 100 ng/mL NRG-1. The lysates were immunoprecipitated with anti-HER3 antibody and proteins were detected by Western blotting with the indicated antibodies. In parallel, whole cell extracts were immunoblotted to detect tubulin for loading control. Relative abundance is represented as a ratio of the control below the blot. This experiment is representative of 2 others.
3.6. Effect of HER3 inhibitor on the activation of HER-family in human NSCLC cell-lines
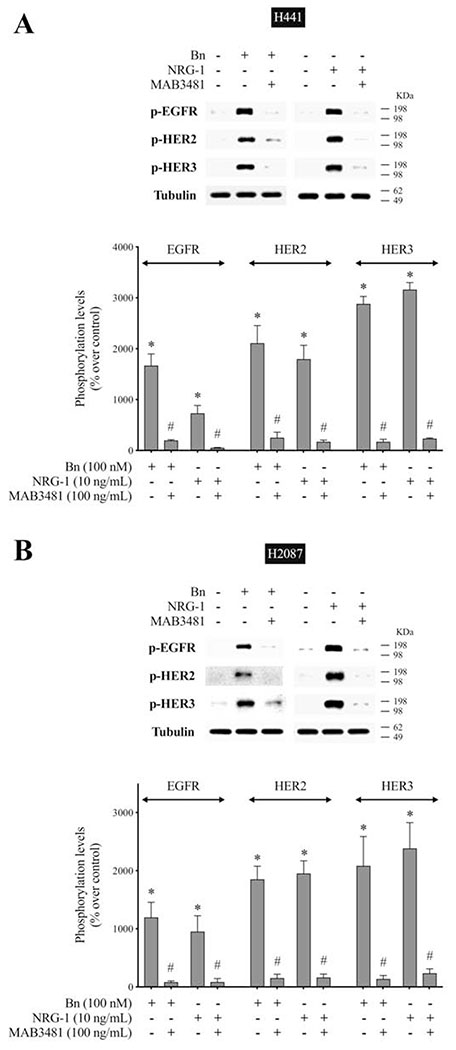
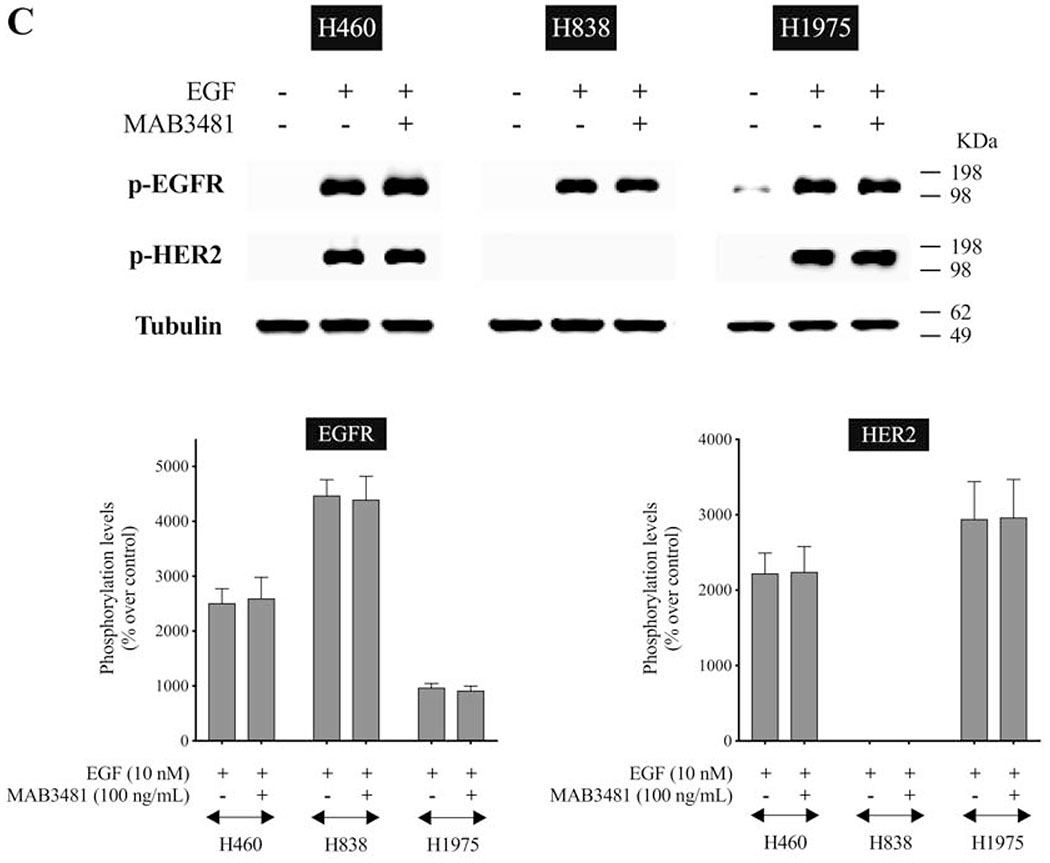
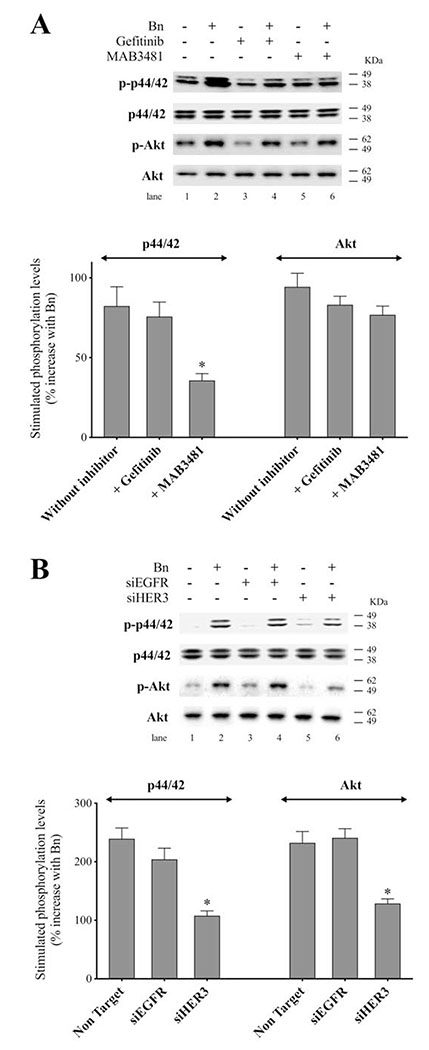
To examine further the role of HER3 heterodimerization in the activation of EGFR/HER-receptor family, we used MAB3481, a specific antibody against HER3 which inhibits HER3 activation[20,55]. In H441 cells, pre-incubation with MAB3481 significantly inhibited Bn- and NRG-1-induced phosphorylation of HER3 (92.6%-94.1%, p < 0.05), as well as EGFR (88.3%-92.8%, p < 0.05) and HER2 (88.1%-90.5%, p < 0.05) (Fig. 7A). Similar results were obtained in H2087 lung cancer cells (Fig. 7B), which showed that MAB3481 significantly decreased Bn- and NRG-1-stimulated phosphorylation of EGFR (91.0%-93.2%, p < 0.05), HER2 (91.7%-92.6%, p < 0.05), and HER3 (90.1%-93.4%, p < 0.05). To test the specificity of MAB3481 to inhibit HER3 but not EGFR and HER2, three lung cancer cell-lines in which no HER3 protein (H460, H838 and H1975) was detectible on Western blotting, were stimulated with EGF in the presence or absence of MAB3481 (Fig. 7C). In these three cell-lines MAB3481 had no inhibitory effect on EGF-induced phosphorylation of EGFR or HER2. These results support the conclusion that Bn-induced transactivation of EGFR and HER2 in the NSCLC H441 and H2087 cancer cells was mediated through the initial activation of HER3.
Figure 7.
Effect of HER3 inhibition on Bn- or NRG-1-induced activation of the EGFR/HER-receptor family in two NSCLC cell-lines. H441 (A) and H2087 (B) cells were preincubated for 1 hr in the presence or absence of the HER3 inhibitor, MAB3481 (100 ng/mL) and then treated with Bn (100 nM) or NRG-1 (10 ng/mL) for 3 min. (C) To test the specificity of the inhibition effect on HER3 by MAB3481, three cell-lines that had no HER3 protein detected were treated with EGF (10 nM) in the presence or absence of MAB3481. Phosphorylation levels of EGFR, HER2 and HER3 were analyzed by Western blotting. Tubulin was used as loading control. Top panel shows a representative blot and bottom graphs show means ± SEM of three independent experiments, respectively. Results are expressed as % increase over basal phosphorylation. * P < 0.05 vs. control, # P < 0.05 vs. no inhibitor (i.e. stimulant alone).
3.7. Effect of EGFR or HER3 knockdown on the activation of the EGFR/HER-family in human NSCLC cell-lines
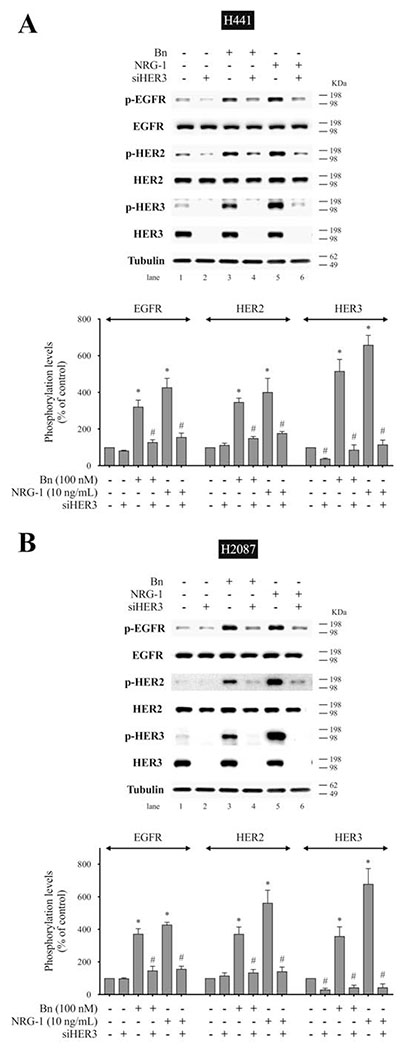
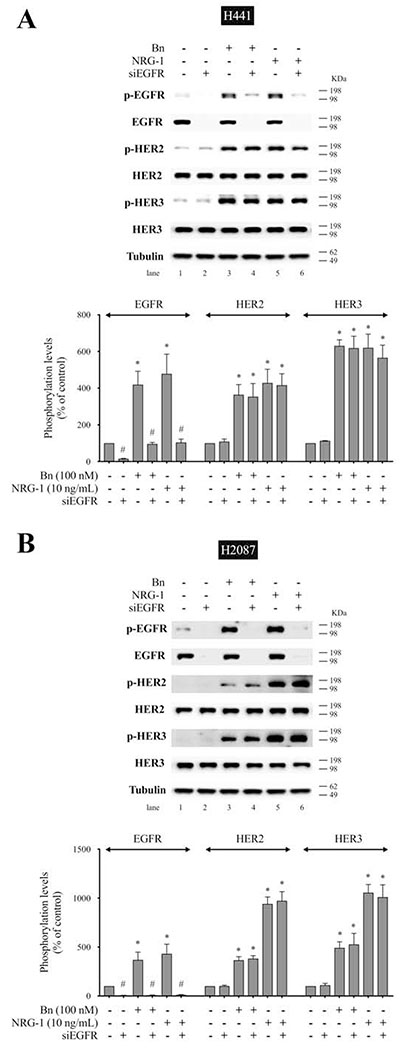
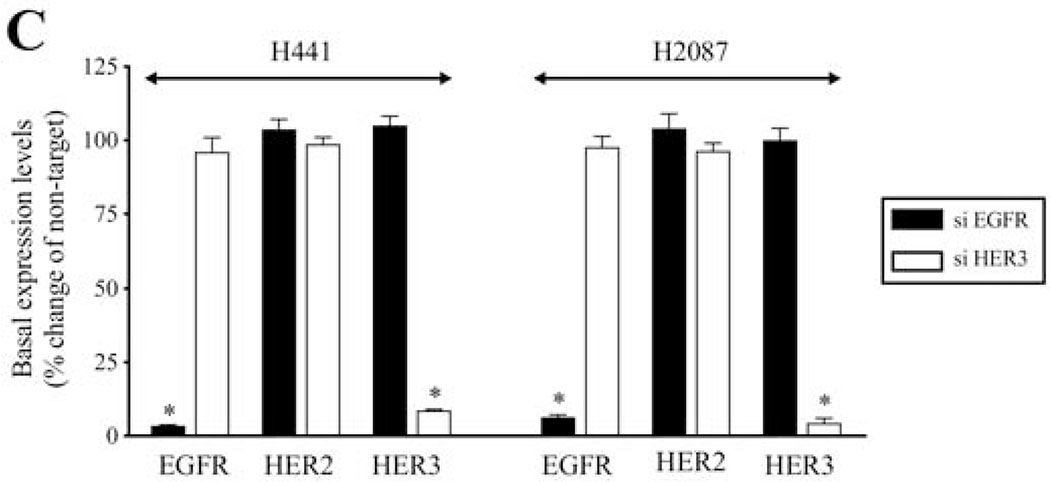
To confirm the above results using the specific HER3 antibody, MAB3481, we studied the effect of EGFR and HER3 knockdown using siRNA on Bn-induced activation of the EGFR/HER-receptor family. In H441 cells, anti-HER3 siRNA treatment significantly inhibited Bn- and NRG-1-induced phosphorylation of EGFR (83.0%-87.5%, p < 0.05), HER2 (74.4%-79.9%, p < 0.05), and HER3 (97.3%-103.3%, p < 0.05) (Fig. 8A). Similarly, HER3 knockdown inhibited Bn- and NRG-1-induced phosphorylation of EGFR (82.7%-82.8%, p < 0.05), HER2 (87.5%-90.9%, p < 0.05), and HER3 (109.9%-122.1%, p < 0.05) in H2087 cells (Fig. 8B). In contrast, siRNA treatment against EGFR markedly inhibited Bn- and NRG-1-induced phosphorylation of EGFR in H441 (Fig. 9A) and H2087 cells (Fig. 9B), while it did not affect either HER2 or HER3 phosphorylation induced by Bn and NRG-1 in these cells (Fig. 9A, B). In both cell-lines, the siRNAs were specific for their targets with siEGFR only effecting EGFR levels and siHER3 effecting HER3 levels (Fig. 9C). The siRNA results provide additional support for the conclusion that Bn transactivates EGFR and HER2 via initial activation of HER3.
Figure 8.
Effect of siRNA-mediated HER3 knock down on Bn- or NRG-1-induced activation of the EGFR/HER-receptor family in two NSCLC cell-lines. H441 (A) and H2087 (B) cells were transfected with either non-targeting (lane 1, 3, 5) or anti-HER3 (lane 2, 4, 6) siRNA for 48 hr, and then treated with no additions (lane 1, 2), 100 nM Bn (lane 3, 4) or 10 ng/mL NRG-1 (lane 5, 6) for 3 min and then lysed. Phosphorylation levels of EGFR, HER2 and HER3 were analyzed by Western blotting. Tubulin was used as loading control. Top panel shows a representative blot and bottom graphs show means ± SEM of three independent experiments, respectively. Results are expressed as % of basal phosphorylation with control. * P < 0.05 vs. control, # P < 0.05 vs. non-targeting siRNA (i.e. stimulants alone).
Figure 9.
Effect of siRNA-mediated EGFR knock down on Bn- or NRG-1-induced activation of the EGFR/HER-receptor family in NSCLC cell-lines. H441 (A) and H2087 (B) cells were transfected with either non-targeting (lane 1, 3, 5) or anti-EGFR (lane 2, 4, 6) siRNA for 48 hr, and then treated with no additions (lane 1, 2), 100 nM Bn (lane 3, 4) or 10 ng/mL NRG-1 (lane 5, 6) for 3 min and then lysed. Phosphorylation levels of EGFR, HER2 and HER3 were analyzed by Western blotting. Tubulin was used as loading control. Top panel shows a representative blot and bottom graphs show means ± SEM of three independent experiments, respectively. Results are expressed as % of basal phosphorylation with control. * P < 0.05 vs. control, # P < 0.05 vs. non-targeting siRNA (i.e. stimulants alone). (C) siRNA-mediated knock down effect of HER3 or EGFR was confirmed by detecting basal expression levels of EGFR/HER-receptor family by Western blotting. Data was presented as means ± SEM of three independent experiments. Tubulin was used as loading control. * P < 0.05 vs. non-targeting siRNA.
3.8. Effect of EGFR or HER3 inhibition on Bn-induced cell-proliferation in human NSCLC cell-lines
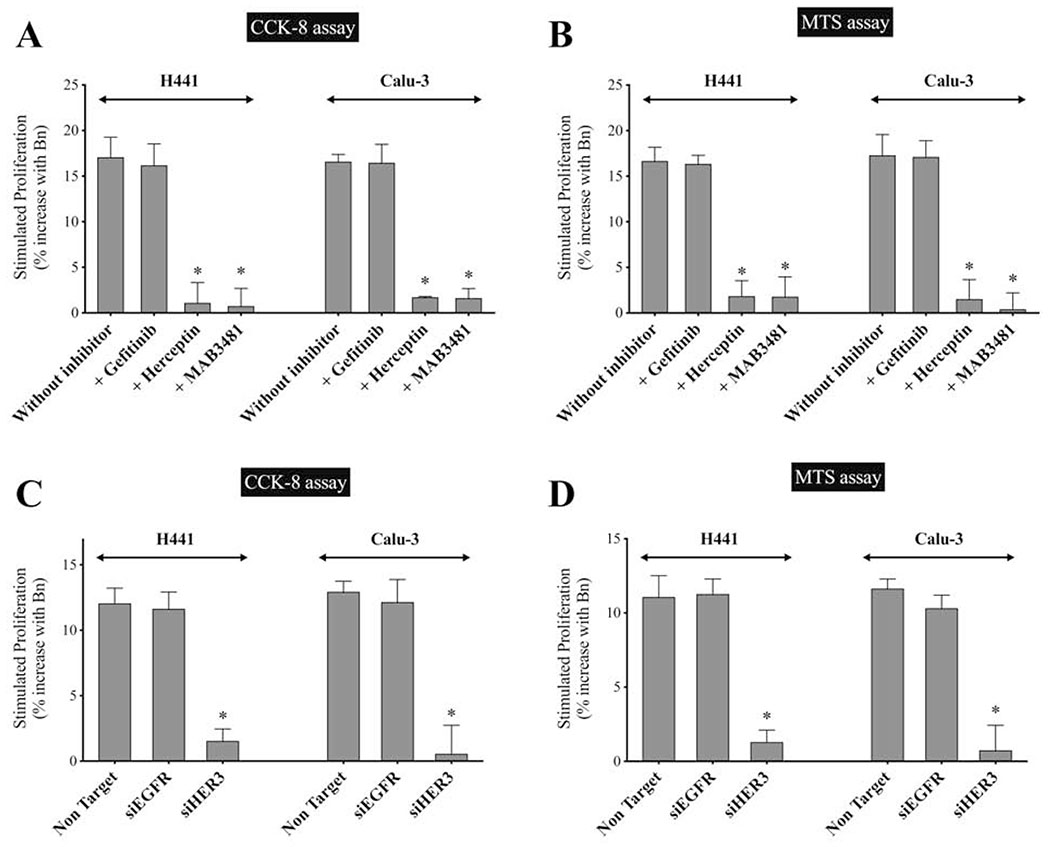
To assess the effect of Bn on cell-proliferation mediated by EGFR, HER2 and HER3, we carried both a CCK-8 assay and an MTS assay in the presence or absence of gefitinib (an EGFR inhibitor), Herceptin(a HER2 inhibitor), or MAB3481(a HER3 inhibitor) in H441 and Calu-3 cells (Fig. 10A, B). Results with both proliferative assays showed similar results (Fig. 10A, B). Gefitinib had no effect on Bn-stimulated increase in cell-proliferation in H441 and Calu-3 cells (Fig. 10A, B). However, proliferation was significantly inhibited by pretreatment with either herceptin (88.9%-93.6%, p < 0.05 vs. without inhibitor) or MAB3481 (89.2%-97.6%, p < 0.05) (Fig. 10A, B).
Figure 10.
Effect of EGFR/HER3 inhibition on Bn-induced cell-proliferation in human two NSCLC cell-lines. (A, B) H441 and Calu-3 cells were incubated with or without Bn (1 μM) in the presence or absence of gefitinib (10 μg/mL), herceptin (10 μg/mL) or MAB3481 (100 ng/mL) for 24 hr. (C, D, ) H441 and Calu-3 cells transfected with either anti-EGFR, anti-HER3 or non-targeting siRNA were incubated with Bn (1 μM) for 24 hr. Cell-proliferation was assessed by both a CCK-8 assay (A, C), and by a MTS assay (B, D). Data was presented as % increase with Bn stimulation against basal proliferation. Basal stimulation for the two cell-lines were 100% for control, 36%-45% of control with gefitinib, 81%-83% for herceptin, and 87-90% for MAB3481, respectively, and maximal stimulation with Bn for the two cell-lines were 116–117% for control, 52%-61% for gefitinib, 82%-84% for herceptin, and 88–93% for MAB3481, respectively. The mean value ± SEM of 4 experiments is indicated. * P < 0.05 vs. no inhibitor (A, B); * P < 0.05 vs. non-targeting siRNA (C, D). These results are representative of 2 others.
We further examined the effect of EGFR or HER3 knockdown on Bn-stimulated cell-proliferation in these cell-lines (Fig. 10C, D). HER3 knockdown significantly reduced Bn-stimulated increment in cell-proliferation compared to the cells treated with non-targeting siRNA in H441 (87.1%-88.3%, p < 0.05) and Calu-3 (93.6%-95.6%, p < 0.05) (Fig. 10C, D). However, similar to the results obtained with gefitinib, EGFR knockdown did not have a significant effect on Bn-stimulated proliferation in either NSCLC cell-line (Fig. 10C, D). These results demonstrated that stimulation of Bn induced cell-proliferation in these NSCLC cells is mediated by initial HER3 activation with formation of heterodimers with HER2, not with EGFR.
3.9. Effect of EGFR or HER3 inhibition on Bn-stimulated activation of p44/42 and Akt in human NSCLC cell-lines
In various tumor cells, the MAPK/ERK and PI3K/AKT signaling pathways are the major downstream cascades that play important roles in HER3-mediated effects on the cells[13,14,23,53]. Therefore, we next examined whether these pathways are involved in the Bn-stimulated activation of HER3 signaling by using inhibitors and siRNA against EGFR and HER3. In H441 cells, when stimulated with Bn (Fig. 11A), gefitinib did not affect the Bn-stimulated phosphorylation of p44/42, while addition of MAB3481 significantly decreased the Bn-induced increment of p44/42 phosphorylation (56.6%, p < 0.05 vs. no inhibitor). The results from siRNA treatment also showed that HER3 knockdown significantly reduced Bn-induced increment of p44/42 phosphorylation (55.0%, p < 0.05 vs. non-targeting), while no inhibitory effect was observed with EGFR knockdown (Fig. 11B). With Bn stimulation of Akt, HER3 knockdown significantly reduced Bn-induced increment of Akt phosphorylation (46.5%, p < 0.05 vs. non-targeting), while no significant inhibitory effect was observed with either EGFR/HER3 inhibitors or EGFR knockdown (Fig. 11A, B). These results show that HER3 activation plays an important role in both the Bn-activation p44/42 and Akt signaling system.
Figure 11.
Effect of EGFR/HER3 inhibition on Bn-induced activation of p44/42 or Akt in H441 NSCLC cells. (A) H441 cells were preincubated in the presence or absence of gefitinib (10 μg/mL, lane 3, 4) or MAB3481 (100 ng/mL, lane 5, 6) and then treated with no addition (lane 1, 3, 5) or Bn (100 nM, lane 2, 4, 6) for 15 min. Basal and maximal phosphorylation of p44/42 were 100% and 182% for control, 75% and 149% of control with gefitinib, and 101% and 137% for MAB3481, respectively. Basal and maximal phosphorylation of Akt were 100% and 194% for control, 79% and 162% for gefitinib, and 96% and 173% for MAB3481, respectively. (B) H441 cells were transfected with either non-targeting (lane 1, 2), anti-EGFR (lane 3, 4) or anti-HER3 (lane 5, 6) siRNA for 48 hr, and then treated with no addition (lane 1, 3, 5) or Bn (100 nM, lane 2, 4, 6). Phosphorylation levels of p44/42 and Akt were analyzed by Western blotting. Top panel shows the representative blot and bottom graphs shows means ± SEM of three independent experiments, respectively. Results are expressed as % increase with Bn stimulation against basal phosphorylation. * P < 0.05 vs. no inhibitor (A); * P < 0.05 vs. non-targeting siRNA (B).
3.10. Effect of p44/42 or Akt inhibition on Bn-stimulated cell-proliferation in human NSCLC cell-lines
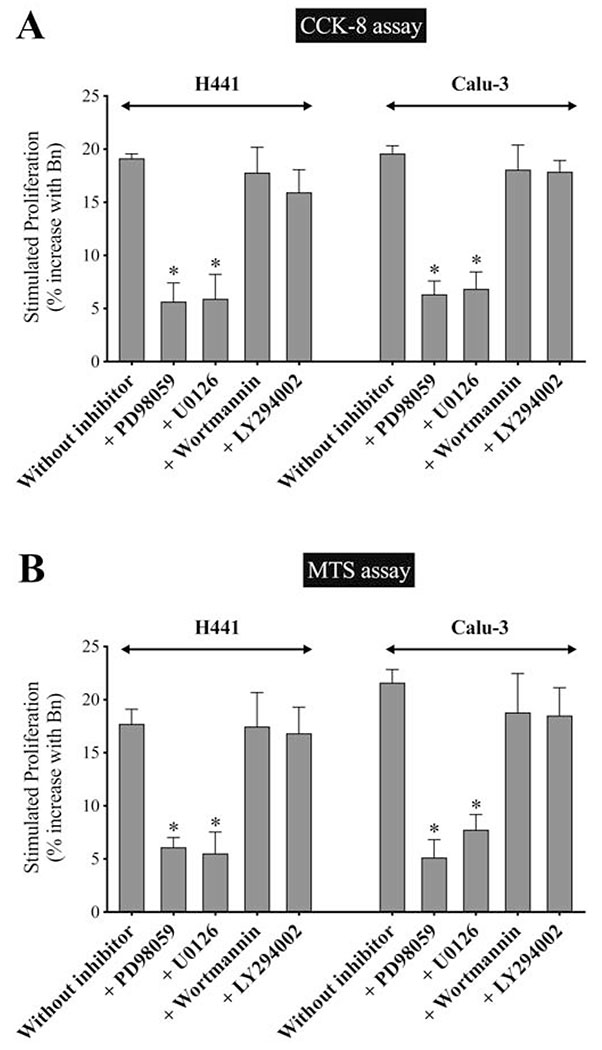
To further evaluate the role of p44/42 and Akt in mediating Bn-stimulated cell-proliferation of the NSCLC cells, a CCK-8 assay and MTS assay were performed with or without specific inhibitors of MEK, resulting in specific MAPK inhibition(PD98059 and U0126) or PI3K (Wortmannin and LY294002) resulting in AKT inhibition, in both H441 and Calu-3 cells (Fig. 12A, B). In both proliferation assays, in the presence of the p44/42 inhibition, Bn-stimulated proliferation was significantly inhibited by 65.5%-76.2% with PD98059 (p < 0.05 vs. no inhibitor) and 64.1%-69.1% with U0126 (p < 0.05 vs. no inhibitor) in both cell-lines (Fig. 12A, B). In contrast, no significant effect was observed with PI3K inhibition with either Wortmannin or LY294002 on Bn-stimulated increase in cell-proliferation (Fig. 12A, B). These results demonstrated that Bn-induced activation of HER3 signaling increasing cell-proliferation was mediated primarily by activation of the p44/42 pathway, not Akt.
Figure 12.
Effect of p44/42 or Akt inhibition on Bn-stimulated cell-growth in NSCLC cells. (A, B) H441 and Calu-3 cells were incubated with/without Bn (1 μM), in the presence or absence of PD98059 (10 μM), U0126 (10 μM), Wortmannin (1 μM) or LY294002 (10 μM) for 24 hr. Cell-proliferation was assessed by CCK-8 assay (A), and the results were confirmed by MTS assay (B). Data was presented as % increase with Bn stimulation against basal proliferation. Basal stimulation for the two cell-lines were 100% for control, 72%-83% for PD98059, 65%-80% for U0126, 68%-80% for Wortmannin, and 56%-73% for LY294002, respectively, and maximal stimulation with Bn for the two cell-lines were 116%-121% for control, 72%-86% of control with PD98059, 69%-85% for U0126, 83%-94% for Wortmannin, and 69%-87% for LY294002, respectively. The mean value ± SEM of 4 experiments is indicated. * P < 0.05 vs. no inhibitor (E, F). These results are representative of 2 others.
4. Discussion
The EGFR/HER-receptor family are highly expressed in many malignancies, particularly cancers of the lung, breast, colorectum, pancreas, and head/neck (squamous[HNSCC])[3,11,18,51]. The activation of the EGFR/HER-receptor family is important not only in the pathogenesis of these tumors, but also an important target in their treatment, the development of treatment resistance, as a determinant of their aggressiveness and for prognosis[12–14,16,18,19,38,56]. The EGFR/HER-receptors in these cancers are stimulated by growth factors, activating mutations, amplification, as well as transactivation by various cellular receptors including GPCRs stimulated by various peptides, hormones, and bioactive lipids[3,11,54]. In many cancers, inhibition of the EGFR/HER-receptor family has led to significant therapeutic responses; however, drug resistance and therapeutic escape frequently develop[11,54]. Thus, there is a role for new treatment approaches and increased understanding of EGFR/HER-receptor family’s role in the tumor’s pathogenesis, treatment and therapeutic response.
Mammalian BnRs (GRPR, NMBR, BRS-3), as well as a number of other GPCRs, are widely overexpressed in numbers of common tumors, including lung, breast, colon, prostate, CNS, and HNSCC[33–36]. Numerous studies have shown that EGFR transactivated by GPCRs can be particularly important in these cancers, not only because the GPCRs can stimulate tumor growth and invasiveness, but also because dual-inhibition of the GPCRs and EGFR/HER-receptor family can have a synergistic cytotoxic effect[38,40–42,56], raising the possibility of novel treatment approaches. Furthermore, activation of the GPCRs resulting in EGFR’s transactivation can play an important role in the resistance against EGFR-targeted therapies[43,44]. Most studies of the transactivation of EGFR/HER-receptor family by GPCRs have only investigated the transactivation of EGFR, thus, the possibility that one of the other EGFR/HER-receptor family (HER3, HER4) is, in fact, the principal initiator in the transactivation process, with resultant heterodimerization with one of the other EGFR/HER members including EGFR, has not been well studied. This distinction is important with the recent development of specific inhibitors for the different EGFR/HER-receptor family members[11].
Recent studies have emphasized the importance of the kinase deficient HER3 through heterodimerization with the other EGFR/HER members in mediating treatment failure[12–14], promoting cancer metastasis[16,17], and as an important prognostic factor for worse survival[16,18,19]. Furthermore, a number of studies suggest HER3 activation can be an important mediator of EGFR/HER2 transactivation by different agents contributing to enhanced tumor growth as well as development of therapeutic escape[40,57]. The importance of HER3 transactivation by GPCR’s and other-receptors in cells/tumors (affecting cell/tumor behavior, growth, aggressiveness, migration) is reported for neurotensin receptors in breast and lung cancer cells[40,57]; estrogen, progesterone and transforming growth factor (TGF)-β receptors in breast cancer cells[49,58,59]; macrophage inhibitory cytokine-1 receptor in both breast and gastric cancer[60]; activation of P2Y2 nucleotide receptors in salivary gland cells[48]; and activation of angiotensin 1/2 receptors in both normal cells and disease states[61–63]. Furthermore, in numerous cancers (prostate, lung, and hepatocellular), activation of GPCR’s and growth factor receptors can have a marked effect on expression of HER3[57,64–66]. These results strongly suggest that activation of GPCRs by a number of tumor growth stimulants can transactivate HER3 and thus targeting the HER3/GPCR interaction could be a novel therapeutic strategy among these diseases. Hence, elucidating in detail the mechanisms of transactivation of HER3 signaling cascades and its effect on cell-growth would provide important mechanistic insights which could reveal novel strategies with possible therapeutic potential. Therefore, the aim of this study was to study in detail in lung cancer cells(concentrating on NSCLC ), in which the EGFR/HER-receptor family has been extensively studied[42–44,47,52,67], the specific role of the EGFR/HER-receptor family in transactivation caused by GPCRs. Because the Bn receptor family is very frequently overexpressed in lung cancer cells[33,52] and has been shown to frequently stimulate EGFR transactivation in this cancer[41,42,44,47,52], the ability of Bn to transactivate the different EGFR/HER-family members in lung cancer cells was concentrated on.
A number of our results support the conclusion that both Bn-transactivation and the HER3 agonist, NRG-1, stimulate each of the EGFR/HER-receptor family members in each of the NSCLC cells examined (H441, H2087 and Calu-3). This conclusion was supported by our finding that both Bn and NRG-1 caused increased phosphorylation of each of the three EGFR/HER-receptor family members in each NSCLC cell-line. Furthermore, a co-immunoprecipitation study showed both Bn and NRG-1 stimulated heterodimerization of HER3/HER2 and HER3/EGFR. In general, the patterns of activation of the different EGFR/HER-receptor members by NRG-1 and Bn-transactivation were similar, however, the kinetics and magnitude of activation varied in the different NSCLC cells, as well as the kinetics and magnitudes of the activation of the different EGFR/HER members in a given NSCLC cell. Although no previous study in the same cells has compared in detail the kinetics and magnitudes of activation of all EGFR/HER members by a native EGFR/HER-receptor ligand to that seen with transactivation by GPCRs, as we performed in the current study, our results show both differences and similarities from previous studies examining only EGFR(HER1)-receptor transactivation. First, the magnitude of stimulation of HER2 and HER3, but not EGFR, differed in each of the cell-lines for BnR transactivation compared with NRG-1 stimulation, with NRG-1 showing greater stimulation. Our results are similar to studies showing greater stimulation of EGFR by the native EGFR ligand, EGF, than by transactivation by GPCR ligands in several tumor cells [neuroendocrine tumor[68], NSCLC[44,47] and prostate cancer[69]], as well as in normal fibroblasts[70]. Our results are also similar to one detailed study involving different normal cells transfected with numerous GPCRs, which showed greater activation of EGFR by EGF than by transactivation of a member of GPCR’s[62]. Second, in a given NSCLC cell-line, the kinetics of activation of each of the three EGFR/HER members was similar with BnR transactivation. In contrast, with NRG-1 stimulation, the relative kinetics of EGFR/HER-receptor activation in a given cell were more variable and more prolonged for HER2/HER3 activation. In terms of BnR transactivation, our results are different from a previous study demonstrating distinct kinetics of different members of the EGFR/HER-receptor family’s transactivation induced by angiotensin II in fibroblasts[62]. However, our kinetic results with Bn-transactivation are similar to different EGFR/HER-receptor’s transactivation by progesterone in ovarian cancer[59] and by growth hormone releasing hormone in prostate cancer cells[71]. Our results with NRG-1 stimulation are different from the previous studies reporting similar kinetics of different EGFR/HER-receptor’s activation, especially between EGFR and HER3, by their native ligands in ovarian cancer[72] and breast cancer cells[73] as well as in normal fibroblasts[74]. Third, in the different NSCLC cell-lines, for a given EGFR/HER member, the kinetics and/or magnitude of stimulation by either NRG-1 or Bn varied markedly. Similar to our results, a number of studies in various tumor cell-lines reported the similar rapid activation of HER3 induced by NRG-1 peaking at early stimulation time, including breast cancer[73,75,76], prostate cancer[76] melanoma[77], as well as in the normal cells (fibroblast)[74]. In terms of transactivation of EGFR/HER-receptors by GPCRs activation, results resembling the kinetic patterns we find, are reported with a slow stimulated time course in neuroendocrine tumors[68], prostate cancer[69] and breast cancers[59], while other studies reported the opposite results in NSCLC[42,44] and prostate cancer cells[78]. Fourth, in our study, both Bn- and NRG-1-induced activation of the EGFR/HER-family members were dose-dependent with similar EC50’s in a nanomolar range in the different cells. These are comparable with reports with GPCR ligands transactivating EGFR in neuroendocrine tumors[68], lung cancer[41,52] and prostate cancer cells[69], as well as for NRG-1 activating HER3 in ovarian cancer[72], hepatocellular carcinoma[65], and pancreatic cancer cells[55]. Our finding that Bn-transactivation was more potent than NRG-1 for activating the EGFR/HER-family members is in contrast to their comparable magnitude of activation which shows the reverse pattern. These results, combined with the previous findings, suggest that the mechanisms involved in the kinetics and magnitude of response, and potency of the stimulation for EGFR/HER-receptor family’s activation, can vary for specific native ligands and GPCRs transactivation within the same cell, as well as in different cell-lines for different EGFR/HER members.
A number of our results support the conclusion that in each of the NSCLC cells, Bn-transactivation as well as NRG-1 stimulation of each of the three EGFR/HER-family members (EGFR, HER2 and HER3) present, is mediated by heterodimerization with HER3, not EGFR. NRG-1, which only stimulates HER3 and HER4[79], stimulated activation of the three members of the EGFR/HER-family present in the each of the cells studied(HER1,HER2,HER3). Therefore, NRG-1 is causing these effects by activating HER3, because there is no HER4 present. Furthermore, because HER3 is kinase-deficient, it must heterodimerize with EGFR or HER2 to be active[9]. In many cells, HER2 is the preferred partner[21]. However, our immunoprecipitation studies show HER3 with NRG-1 stimulation heterodimerizes with both EGFR and HER2. Similarly, HER2 requires heterodimerization to be activated because no known specific native ligands for it have been identified[11]. Therefore, the activation of all three EGFR/HER members by NRG-1 strongly suggests initial HER3 activation is mediated activation of EGFR and HER2. Second, a specific HER3 antibody, MAB3481[20,55], completely inhibited both Bn-transactivation, as well as NRG-1 activation of each of the EGFR/HER-members. This was not due to nonspecific inhibition of EGFR or HER2, because this antibody had no effect on EGF activation of EGFR and HER2 in cells not possessing HER3, but only EGFR and HER2. Third, HER3 siRNA which only resulted in knockdown of HER3, had a similar effect to the HER3 antibody, blocked both Bn-transactivation and NRG-1 stimulation of each of the three EGFR/HER members present in each cell. In contrast, siRNA against EGFR, which specifically downregulated only EGFR, only inhibited EGFR activation. Although there are only limited studies of the ability of GPCR’s to transactivate all of the EGFR/HER-family members in a given cell, our results have both differences and similarities to the results in these studies. Our results demonstrating that in the NSCLC cells studied, BnR-stimulation caused activation of HER2 through a HER3-mediated process, differs from a number of studies reporting activation of various GPCR’s in different tumor cells stimulate HER2 via activation of EGFR[41,80–85]. These studies include EGFR/HER2 transactivation by GRP or lysophosphatidic acid in HNSCC[80,83]; EGFR/HER2 transactivation by leptin receptors stimulation in both breast cancer and vascular cells[81,85]; by endothelin receptor and Pituitary adenylate cyclase activating polypeptide receptor transactivation of EGFR/HER2 in lung cancer cells[41,82]; and protease-activated receptor transactivation of EGFR/HER2 in breast cancer cells[84]. Our results demonstrating with BnR transactivation that both EGFR and HER2 is activated by a HER3-mediated mechanism is similar to studies of estrogen receptor transactivation in breast cancer cells[49], macrophage inhibitory cytokine-1 receptor transactivation of EGFR/HER-receptors in gastric and breast cancer cells[60] and transactivation of EGFR/HER-family members in salivary grand cells by stimulation of the P2X2 nucleotide receptor[48].
Numerous studies demonstrate that not only are the family of BnR receptors(especially GRPR) present in the majority of NSCLC cells[33,35,46,52], but also, they are potent growth factors for these cells[33,35]. A number of our results support the conclusion that BnR-mediated transactivation of EGFR/HER-family members is a major determinant of their growth effect, and that this is affected initially by a HER3-dependent process. First, a specific inhibitor of HER3, but not EGFR, completely inhibited Bn-stimulated cell-growth in NSCLC cells. Second, specific knockdown of HER3 with siRNA showed a similar inhibitory effect to the HER3 antibody, whereas EGFR specific knockdown had no effect. These results differ from the studies reporting complete inhibition of various GPCR agonist-stimulated cell-growth by EGFR blockade in some lung cancers[42], HNSCC[80,83,86] and neuroendocrine tumor[68], as well as prostaglandin- and bradykinin-stimulated cell-growth in EGFR-knockout fibroblasts[87]. In contrast, our results are similar to the study showing that downregulation of HER3 by siRNA, as well as HER2 antibody, completely inhibit estrogen-stimulated cell-growth in mammary tumors[49].
Numerous studies in both lung cancer cells and other cells demonstrate activation of the EGFR/HER-family is coupled to stimulation of the MAPK-cascade as well as activation of the PI3K/Akt pathway, each of which have can have an important effect on cell behavior (growth, differentiation, migration, apoptosis, inflammation, and biological response)[22,23]. Our results demonstrate both NRG-1 and Bn-transactivation stimulate each of these signaling cascades in each NSCLC cell-line, and they show both similarities and differences from results in other cells. Our results which show a rapid activation of ERK and Akt in each NSCLC cells with Bn-transactivation are similar to studies reporting endothelin-transactivation of EGFR in fibroblasts[70] and GRP-transactivation of the EGFR/HER-family in other lung cancer cells[42,44]. Similarly, our results demonstrating sustained activation of Akt with NRG-1 stimulation agree with results in breast cancer cells[23,75] and prostate cancer cells[76]. In contrast to our results, NRG-1 causes a transient activation of Akt in ovarian cancer cells[72] and melanoma cells[77] and sustained activation of ERK in breast cancer cells[73]. These results demonstrate that the kinetics of activation of the ERK and PI3K/Akt can differ markedly in different cells both when the EGFR/HER-family are transactivated by GPCRs or by direct stimulation with a native ligand.
While our results demonstrate that Bn-transactivation of HER3 stimulates both the PI3K- and MAPK-pathways, only activation of the MAPK/ERK-pathway, not the PI3K/Akt, mediates Bn-stimulated cell-proliferation in the NSCLC cells studied. Our results differ from a number of studies in both tumor/nontumor cells reporting PI3K activation is the principal downstream effector of HER2/HER3-signaling on cellular growth[13,14,22,67], with EGFR homodimers as well as EGFR/HER2 heterodimer complexes preferentially activating MAPK signaling and HER2/HER3 heterodimer to a lesser extent[15,21,22]. Conversely, similar to our results, the superiority of MAPK inhibitors over PI3K inhibitors on HER3-mediated growth activity is reported in several studies with breast cancer[88,89] and HNSCC[15,22]. Our results are also similar to a study in gastric cancer which reported that ERK, but not Akt, was involved in HER3-transactivation caused by histone deacetylase inhibitors[90]. These results demonstrate that prominence of MAPK-activation or PI3K-activation in mediating HER3 downstream events including growth, can vary markedly in different tumor cells.
In conclusion, we have demonstrated that with Bn-transactivation as well as NRG-1 stimulation of NSCLC-cells, the stimulation of HER3-signaling by forming heterodimers with HER2 is essential in mediating cell-proliferation. Furthermore, the activation of MAPK, not PI3K, through HER2/HER3 heterodimers is the main downstream growth stimulating cascade. Previous studies demonstrate that not only do Bn-peptides act as tumor autocrine growth factors in lung cancer and a number of other common cancers, their receptor activation can stimulate EGFR/HER-family transactivation causing tumor growth, but its inhibition also increases the sensitivity of EGFR tyrosine kinase inhibitors[33,35,39,91]. With the increasing development of HER3 antagonists, as well as the availability of BnR receptor antagonists [11,51,91–96], our results support the conclusion that targeting the crosstalk between GPCR and HER3 transactivation, together with its downstream cascades, could be a novel antitumor therapeutic target among various malignancies expressing these receptors, especially in NSCLC.
Supplementary Material
Research highlights:
NSCLC cell lines all express all EGFR/HER family members except HER4
Activation BnR or NRG1 in NSCLC cells activates HER1,HER2,HER3 but kinetics/magnitude varies
Activation BnR or NRG1 in NSCLC cells activates MAPK and PI3K/Akt pathways
BnR transactivation of EGFR/HER family initially mediated by HER3 activation
BnR stimulated NSCLC growth mediated via HER3 transactivation and MAPK signaling
Acknowledgments
This work is partially supported by the Intramural Research Program of the NIDDK and NCI, NIH.
Abbreviations:
- EGFR
epidermal growth factor receptor
- RTK
receptor tyrosine kinase
- NSCLC
non-small cell lung cancer
- PI3K
phosphatidylinositol-3 kinase
- MAPK
Mitogen-activated protein kinase
- ERK
extracellular-signal-regulated kinase
- Bn
bombesin
- BnR
bombesin receptor
- GPCR
G protein-coupled receptors
- NMBR
neuromedin B receptor
- GRPR
gastrin-releasing peptide receptor
- BRS-3
bombesin receptor subtype 3
- NRG-1
neuregulin-1
- siRNA
small interfering RNA
- EC50
half-maximal effect
- HNSCC
head and neck squamous cell cancer
- CNS
central nerve system
- TGF
transforming growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- [1].Siegel RL, Miller KD, Jemal A, Cancer statistics, 2019, CA Cancer J Clin 69 (2019) 7–34. [DOI] [PubMed] [Google Scholar]
- [2].Hynes NE and Lane HA, ERBB receptors and cancer: the complexity of targeted inhibitors, Nat. Rev Cancer 5 (2005) 341–354. [DOI] [PubMed] [Google Scholar]
- [3].Baselga J and Swain SM, Novel anticancer targets: revisiting ERBB2 and discovering ERBB3, Nat. Rev Cancer 9 (2009) 463–475. [DOI] [PubMed] [Google Scholar]
- [4].Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R, Pallares C, Sanchez JM, Porta R, Cobo M, Garrido P, Longo F, Moran T, Insa A, De Marinis F, Corre R, Bover I, Illiano A, Dansin E, de Castro J, Milella M, Reguart N, Altavilla G, Jimenez U, Provencio M, Moreno MA, Terrasa J, Munoz-Langa J, Valdivia J, Isla D, Domine M, Molinier O, Mazieres J, Baize N, Garcia-Campelo R, Robinet G, Rodriguez-Abreu D, Lopez-Vivanco G, Gebbia V, Ferrera-Delgado L, Bombaron P, Bernabe R, Bearz A, Artal A, Cortesi E, Rolfo C, Sanchez-Ronco M, Drozdowskyj A, Queralt C, A.de I, Ramirez JL, Sanchez JJ, Molina MA, Taron M, Paz-Ares L, Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial, Lancet Oncol. 13 (2012) 239–246. [DOI] [PubMed] [Google Scholar]
- [5].Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, Louis DN, Christiani DC, Settleman J, D A.Haber, Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib, N. Engl. J Med 350 (2004) 2129–2139. [DOI] [PubMed] [Google Scholar]
- [6].Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, Sunpaweravong P, Han B, B Margono Ichinose Y, Nishiwaki Y, Ohe Y, Yang JJ, Chewaskulyong B, Jiang H, Duffield EL, Watkins CL, Armour AA, Fukuoka M, Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma, N. Engl. J Med 361 (2009) 947–957. [DOI] [PubMed] [Google Scholar]
- [7].Pao W and Chmielecki J, Rational, biologically based treatment of EGFR-mutant non-small-cell lung cancer, Nat. Rev Cancer 10 (2010) 760–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kosaka T, Yatabe Y, Endoh H, Yoshida K, Hida T, Tsuboi M, Tada H, Kuwano H, Mitsudomi T, Analysis of epidermal growth factor receptor gene mutation in patients with non-small cell lung cancer and acquired resistance to gefitinib, Clin Cancer Res 12 (2006) 5764–5769. [DOI] [PubMed] [Google Scholar]
- [9].Berger MB, Mendrola JM, Lemmon MA, ErbB3/HER3 does not homodimerize upon neuregulin binding at the cell surface, FEBS Lett. 569 (2004) 332–336. [DOI] [PubMed] [Google Scholar]
- [10].Black LE, Longo JF, Carroll SL, Mechanisms of Receptor Tyrosine-Protein Kinase ErbB-3 (ERBB3) Action in Human Neoplasia, Am J Pathol. 189 (2019) 1898–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Yarden Y and Sliwkowski MX, Untangling the ErbB signalling network, Nat. Rev Mol Cell Biol 2 (2001) 127–137. [DOI] [PubMed] [Google Scholar]
- [12].Engelman JA, Janne PA, Mermel C, Pearlberg J, Mukohara T, Fleet C, Cichowski K, Johnson BE, Cantley LC, ErbB-3 mediates phosphoinositide 3-kinase activity in gefitinib-sensitive non-small cell lung cancer cell lines, Proc Natl Acad. Sci U. S. A 102 (2005) 3788–3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Garrett JT, Olivares MG, Rinehart C, Granja-Ingram ND, Sanchez V, Chakrabarty A, Dave B, Cook RS, Pao W, McKinely E, Manning HC, Chang J, Arteaga CL, Transcriptional and posttranslational up-regulation of HER3 (ErbB3) compensates for inhibition of the HER2 tyrosine kinase, Proc Natl Acad. Sci U. S. A 108 (2011) 5021–5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sergina NV, Rausch M, Wang D, Blair J, Hann B, Shokat KM, Moasser MM, Escape from HER-family tyrosine kinase inhibitor therapy by the kinase-inactive HER3, Nature 445 (2007) 437–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zhang L, Castanaro C, Luan B, Yang K, Fan L, Fairhurst JL, Rafique A, Potocky TB, Shan J, Delfino FJ, Shi E, Huang T, Martin JH, Chen G, Macdonald D, Rudge JS, Thurston G, Daly C, ERBB3/HER2 signaling promotes resistance to EGFR blockade in head and neck and colorectal cancer models, Mol Cancer Ther. 13 (2014) 1345–1355. [DOI] [PubMed] [Google Scholar]
- [16].Travis A, Pinder SE, Robertson JF, Bell JA, Wencyk P, Gullick WJ, Nicholson RI, Poller DN, Blamey RW, Elsto CW, Ellis IO, C-erbB-3 in human breast carcinoma: expression and relation to prognosis and established prognostic indicators, Br. J. Cancer 74 (1996) 229–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sithanandam G, Fornwald LW, Fields J, Anderson LM, Inactivation of ErbB3 by siRNA promotes apoptosis and attenuates growth and invasiveness of human lung adenocarcinoma cell line A549, Oncogene 24 (2005) 1847–1859. [DOI] [PubMed] [Google Scholar]
- [18].Li Q, Zhang L, Li X, Yan H, Yang L, Li Y, Li T, Wang J, Cao B, The prognostic significance of human epidermal growth factor receptor family protein expression in operable pancreatic cancer : HER1–4 protein expression and prognosis in pancreatic cancer, BMC. Cancer 16 (2016)910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Yi ES, Harclerode D, Gondo M, Stephenson M, Brown RW, Younes M, Cagle PT, High c-erbB-3 protein expression is associated with shorter survival in advanced non-small cell lung carcinomas, Mod. Pathol 10 (1997) 142–148. [PubMed] [Google Scholar]
- [20].Garner AP, Bialucha CU, Sprague ER, Garrett JT, Sheng Q, Li S, Sineshchekova O, Saxena P, Sutton CR, Chen D, Chen Y, Wang H, Liang J, Das R, Mosher R, Gu J, Huang A, Haubst N, Zehetmeier C, Haberl M, Elis W, Kunz C, Heidt AB, Herlihy K, Murtie J, Schuller A, Arteaga CL, Sellers WR, Ettenberg SA, An antibody that locks HER3 in the inactive conformation inhibits tumor growth driven by HER2 or neuregulin, Cancer Res 73 (2013) 6024–6035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Alvarado D, Ligon GF, Lillquist JS, Seibel SB, Wallweber G, Neumeister VM, Rimm DL, McMahon G, LaVallee TM, ErbB activation signatures as potential biomarkers for anti-ErbB3 treatment in HNSCC, PLoS. ONE 12 (2017) e0181356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kirouac DC, Du J, Lahdenranta J, Onsum MD, Nielsen UB, Schoeberl B, McDonagh CF, HER2+ Cancer Cell Dependence on PI3K vs. MAPK Signaling Axes Is Determined by Expression of EGFR, ERBB3 and CDKN1B, PLoS. Comput. Biol 12 (2016) e1004827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Yen L, Cao Z, Wu X, Ingalla ER, Baron C, Young LJ, Gregg JP, Cardiff RD, Borowsky AD, Sweeney C, Carraway KL III, Loss of Nrdp1 enhances ErbB2/ErbB3-dependent breast tumor cell growth, Cancer Res 66 (2006) 11279–11286. [DOI] [PubMed] [Google Scholar]
- [24].Poller DN, Spendlove I, Baker C, Church R, Ellis IO, Plowman GD, Mayer RJ, Production and characterization of a polyclonal antibody to the c-erbB-3 protein: examination of c-erbB-3 protein expression in adenocarcinomas, J Pathol. 168 (1992) 275–280. [DOI] [PubMed] [Google Scholar]
- [25].Yi ES, Harclerode D, Gondo M, Stephenson M, Brown RW, Younes M, Cagle PT, High c-erbB-3 protein expression is associated with shorter survival in advanced non-small cell lung carcinomas, Mod. Pathol 10 (1997) 142–148. [PubMed] [Google Scholar]
- [26].Al-Saad S, Al-Shibli K, Donnem T, Andersen S, Bremnes RM, Busund LT, Clinical significance of epidermal growth factor receptors in non-small cell lung cancer and a prognostic role for HER2 gene copy number in female patients, J Thorac. Oncol 5 (2010) 1536–1543. [DOI] [PubMed] [Google Scholar]
- [27].Berghoff AS, Magerle M, Ilhan-Mutlu A, Dinhof C, Widhalm G, Dieckman K, Marosi C, Wohrer A, Hackl M, Zochbauer-Muller S, Preusser M, Birner P, Frequent overexpression of ErbB--receptor family members in brain metastases of non-small cell lung cancer patients, APMIS 121 (2013) 1144–1152. [DOI] [PubMed] [Google Scholar]
- [28].Reinmuth N, Jauch A, Xu EC, Muley T, Granzow M, Hoffmann H, Dienemann H, Herpel E, Schnabel PA, Herth FJ, Gottschling S, Lahm H, Steins M, Thomas M, Meister M, Correlation of EGFR mutations with chromosomal alterations and expression of EGFR, ErbB3 and VEGF in tumor samples of lung adenocarcinoma patients, Lung Cancer 62 (2008) 193–201. [DOI] [PubMed] [Google Scholar]
- [29].Renouf DJ, Wood-Baker R, Ionescu DN, Leung S, Masoudi H, Gilks CB, Laskin J, BCL-2 expression is prognostic for improved survival in non-small cell lung cancer, J Thorac. Oncol 4 (2009) 486–491. [DOI] [PubMed] [Google Scholar]
- [30].Scharpenseel H, Hanssen A, Loges S, Mohme M, Bernreuther C, Peine S, Lamszus K, Goy Y, Petersen C, Westphal M, Glatzel M, Riethdorf S, Pantel K, Wikman H, EGFR and HER3 expression in circulating tumor cells and tumor tissue from non-small cell lung cancer patients, Sci Rep. 9 (2019) 7406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Siegfried JM, Lin Y, Diergaarde B, Lin HM, Dacic S, Pennathur A, Weissfeld JL, Romkes M, Nukui T, Stabile LP, Expression of PAM50 Genes in Lung Cancer: Evidence that Interactions between Hormone Receptors and HER2/HER3 Contribute to Poor Outcome, Neoplasia. 17 (2015)817–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Xu JM, Han Y, Duan HQ, Gao EM, Zhang Y, Liu XQ, Zhang JS, Toschi L, Galetta D, Azzariti A, Paradiso A, EGFR mutations and HER2/3 protein expression and clinical outcome in Chinese advanced non-small cell lung cancer patients treated with gefitinib, J Cancer Res Clin Oncol. 135 (2009) 771–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Jensen RT, Battey JF, Spindel ER, Benya RV, International Union of Pharmacology. LVIII. Mammalian Bombesin Receptors: Nomenclature, distribution, pharmacology, signaling and functions in normal and disease states., Pharmacol. Rev 60 (2008) 1–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Mattei J, Achcar RD, Cano CH, Macedo BR, Meurer L, Batlle BS, Groshong SD, Kulczynski JM, Roesler R, Dal LL, Brunetto AT, Schwartsmann G, Gastrin-releasing peptide receptor expression in lung cancer, Arch. Pathol. Lab Med 138 (2014) 98–104. [DOI] [PubMed] [Google Scholar]
- [35].Moreno P, Ramos-Alvarez I, Moody TW, Jensen RT, Bombesin related peptides/receptors and their promising therapeutic roles in cancer imaging, targeting and treatment, Expert Opin. Ther. Targets 20 (2016) 1055–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Weber HC, Regulation and signaling of human bombesin receptors and their biological effects, Curr Opin. Endocrinol Diabetes Obes 16 (2009) 66–71. [DOI] [PubMed] [Google Scholar]
- [37].Sancho V, Di Florio A, Moody TW, Jensen RT, Bombesin receptor-mediated imaging and cytotoxicity: review and current status, Curr Drug Deliv. 8 (2011) 79–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Cornelio D, Roesler R, Schwartsmann G, Gastrin-releasing peptide receptor as a molecular target in experimental anticancer therapy, Ann. Oncol 18 (2007) 1457–1466. [DOI] [PubMed] [Google Scholar]
- [39].Moody TW, Chan D, Fahrenkrug J, Jensen RT, Neuropeptides as autocrine growth factors in cancer cells, Curr. Pharm. Des 9 (2003) 495–509. [DOI] [PubMed] [Google Scholar]
- [40].Dupouy S, Doan VK, Wu Z, Mourra N, Liu J, De Wever O, Llorca FP, Cayre A, Kouchkar A, Gompel A, Forgez P, Activation of EGFR, HER2 and HER3 by neurotensin/neurotensin receptor 1 renders breast tumors aggressive yet highly responsive to lapatinib and metformin in mice, Oncotarget. 5 (2014) 8235–8251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Moody TW, Ramos-Alvarez I, Moreno P, Mantey SA, Ridnour L, Wink D, Jensen RT, Endothelin causes transactivation of the EGFR and HER2 in non-small cell lung cancer cells, Peptides 90 (2017) 90–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Thomas SM, Grandis JR, Wentzel AL, Gooding WE, Lui VW, Siegfried JM, Gastrin-releasing peptide receptor mediates activation of the epidermal growth factor receptor in lung cancer cells, Neoplasia. 7 (2005) 426–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kuzumaki N, Suzuki A, Narita M, Hosoya T, Nagasawa A, Imai S, Yamamizu K, Morita H, Suzuki T, Okada Y, Okano HJ, Yamashita JK, Okano H, Narita M, Multiple analyses of G-protein coupled receptor (GPCR) expression in the development of gefitinib-resistance in transforming non-small-cell lung cancer, PLoS. ONE 7 (2012) e44–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Liu X, Carlisle DL, Swick MC, Gaither-Davis A, Grandis JR, Siegfried JM, Gastrin-releasing peptide activates Akt through the epidermal growth factor receptor pathway and abrogates the effect of gefitinib, Exp. Cell Res 313 (2007) 1361–1372. [DOI] [PubMed] [Google Scholar]
- [45].Moody TW, Nuche-Berenguer B, Nakamura T, Jensen RT, EGFR Transactivation by Peptide G Protein-Coupled Receptors in Cancer, Curr Drug Targets. 17 (2016) 520–528. [DOI] [PubMed] [Google Scholar]
- [46].Jensen RT and Moody TW, Bombesin-Related Peptides and Neurotensin: Effects on Cancer Growth/Proliferation and Cellular Signaling in Cancer, in: Kastin AJ (Ed.), Handbook of Biologically active peptides, Elsevier, Amsterdam, 2006, pp.429–434. [Google Scholar]
- [47].Moody TW, Berna MJ, Mantey S, Sancho V, Ridnour L, Wink DA, Chan D, Giaccone G, Jensen RT, Neuromedin B receptors regulate EGF receptor tyrosine phosphorylation in lung cancer cells, Eur J Pharmacol 637 (2010) 38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Ratchford AM, Baker OJ, Camden JM, Rikka S, Petris MJ, Seye CI, Erb L, Weisman GA, P2Y2 nucleotide receptors mediate metalloprotease-dependent phosphorylation of epidermal growth factor receptor and ErbB3 in human salivary gland cells, J Biol Chem 285 (2010) 7545–7555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Liu B, Ordonez-Ercan D, Fan Z, Huang X, Edgerton SM, Yang X, Thor AD, Estrogenic promotion of ErbB2 tyrosine kinase activity in mammary tumor cells requires activation of ErbB3 signaling, Mol Cancer Res 7 (2009) 1882–1892. [DOI] [PubMed] [Google Scholar]
- [50].Macdonald-Obermann JL, Adak S, Landgraf R, Piwnica-Worms D, Pike LJ, Dynamic analysis of the epidermal growth factor (EGF) receptor-ErbB2-ErbB3 protein network by luciferase fragment complementation imaging, J Biol Chem 288 (2013) 30773–30784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Beji A, Horst D, Engel J, Kirchner T, Ullrich A, Toward the prognostic significance and therapeutic potential of HER3 receptor tyrosine kinase in human colon cancer, Clin Cancer Res 18 (2012) 956–968. [DOI] [PubMed] [Google Scholar]
- [52].Moreno P, Mantey SA, Lee SH, Ramos-Alvarez I, Moody TW, Jensen RT, A possible new target in lung-cancer cells: The orphan receptor, bombesin receptor subtype-3, Peptides 101 (2018) 213–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Hemi R, Paz K, Wertheim N, Karasik A, Zick Y, Kanety H, Transactivation of ErbB2 and ErbB3 by tumor necrosis factor-alpha and anisomycin leads to impaired insulin signaling through serine/threonine phosphorylation of IRS proteins, J Biol Chem 277 (2002) 8961–8969. [DOI] [PubMed] [Google Scholar]
- [54].Yarden Y and Pines G, The ERBB network: at last, cancer therapy meets systems biology, Nat. Rev Cancer 12 (2012) 553–563. [DOI] [PubMed] [Google Scholar]
- [55].Le Clorennec C, Bazin H, Dubreuil O, Larbouret C, Ogier C, Lazrek Y, Garambois V, Poul MA, Mondon P, Barret JM, Mathis G, Prost JF, Pelegrin A, Chardes T, Neuregulin 1 Allosterically Enhances the Antitumor Effects of the Noncompeting Anti-HER3 Antibody 9F7-F11 by Increasing Its Binding to HER3, Mol Cancer Ther. 16 (2017) 1312–1323. [DOI] [PubMed] [Google Scholar]
- [56].Jaeger M, Nor C, de Farias CB, Abujamra AL, Schwartsmann G, Brunetto AL, Roesler R, Anti-EGFR therapy combined with neuromedin B receptor blockade induces the death of DAOY medulloblastoma cells, Childs Nerv. Syst 29 (2013) 2145–2150. [DOI] [PubMed] [Google Scholar]
- [57].Younes M, Wu Z, Dupouy S, Lupo AM, Mourra N, Takahashi T, Flejou JF, Tredaniel J, Regnard JF, Damotte D, Alifano M, Forgez P, Neurotensin (NTS) and its receptor (NTSR1) causes EGFR, HER2 and HER3 over-expression and their autocrine/paracrine activation in lung tumors, confirming responsiveness to erlotinib, Oncotarget. 5 (2014) 8252–8269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].St-Laurent V, Sanchez M, Charbonneau C, Tremblay A, Selective hormone-dependent repression of estrogen receptor beta by a p38-activated ErbB2/ErbB3 pathway, J Steroid Biochem Mol Biol 94 (2005) 23–37. [DOI] [PubMed] [Google Scholar]
- [59].Kitowska K, Kowalska A, Mieszkowska M, Piasecka D, Skladanowski AC, Romanska HM, Sadej R, Progesterone impairs Herceptin effect on breast cancer cells, Oncol. Lett 15 (2018) 1817–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Kim KK, Lee JJ, Yang Y, You KH, Lee JH, Macrophage inhibitory cytokine-1 activates AKT and ERK-1/2 via the transactivation of ErbB2 in human breast and gastric cancer cells, Carcinogenesis 29 (2008) 704–712. [DOI] [PubMed] [Google Scholar]
- [61].Knowle D, Ahmed S, Pulakat L, Identification of an interaction between the angiotensin II receptor sub-type AT2 and the ErbB3 receptor, a member of the epidermal growth factor receptor family, Regul. Pept 87 (2000) 73–82. [DOI] [PubMed] [Google Scholar]
- [62].O’Brien SL, Johnstone EKM, Devost D, Conroy J, Reichelt ME, Purdue BW, Ayoub MA, Kawai T, Inoue A, Eguchi S, Hebert TE, Pfleger KDG, Thomas WG, BRET-based assay to monitor EGFR transactivation by the AT1R reveals Gq/11 protein-independent activation and AT1R-EGFR complexes, Biochem Pharmacol 158 (2018) 232–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Akhtar S, Chandrasekhar B, Attur S, Dhaunsi GS, Yousif MH, Benter IF, Transactivation of ErbB Family of Receptor Tyrosine Kinases Is Inhibited by Angiotensin-(1-7) via Its Mas Receptor, PLoS. ONE 10 (2015) e0141657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Sotomayor S, Carmena MJ, Schally AV, Varga JL, Sanchez-Chapado M, Prieto JC, Bajo AM, Transactivation of HER2 by vasoactive intestinal peptide in experimental prostate cancer: Antagonistic action of an analog of growth-hormone-releasing hormone, Int. J. Oncol 31 (2007) 1223–1230. [PubMed] [Google Scholar]
- [65].Buta C, Benabou E, Lequoy M, Regnault H, Wendum D, Meratbene F, Chettouh H, Aoudjehane L, Conti F, Chretien Y, Scatton O, Rosmorduc O, Praz F, Fartoux L, Desbois-Mouthon C, Heregulin-1B and HER3 in hepatocellular carcinoma: status and regulation by insulin, J Exp. Clin Cancer Res 35 (2016) 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Kanashiro CA, Schally AV, Varga JL, Hammann B, Halmos G, Zarandi M, Antagonists of growth hormone releasing hormone and bombesin inhibit the expression of EGF/HER receptor family in H-69 small cell lung carcinoma, Cancer Lett. 226 (2005) 123–131. [DOI] [PubMed] [Google Scholar]
- [67].Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, Lindeman N, Gale CM, Zhao X, Christensen J, Kosaka T, Holmes AJ, Rogers AM, Cappuzzo F, Mok T, Lee C, Johnson BE, Cantley LC, Janne PA, MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling, Science 316 (2007) 1039–1043. [DOI] [PubMed] [Google Scholar]
- [68].Di Florio A, Sancho V, Moreno P, Delle Fave GF, Jensen RT, Gastrointestinal hormones stimulate growth of Foregut Neuroendocrine Tumors by transactivating the EGF receptor, Biochim. Biophys Acta 1833 (2013) 573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Amorino GP, Deeble PD, Parsons SJ, Neurotensin stimulates mitogenesis of prostate cancer cells through a novel c-Src/Stat5b pathway, Oncogene 26 (2007) 745–756. [DOI] [PubMed] [Google Scholar]
- [70].Daub H, Weiss FU, Wallasch C, Ullrich A, Role of transactivation of the EGF receptor in signalling by G-protein-coupled receptors, Nature 379 (1996) 557–560. [DOI] [PubMed] [Google Scholar]
- [71].Munoz-Moreno L, Arenas MI, Carmena MJ, Schally AV, Prieto JC, Bajo AM, Growth hormone-releasing hormone antagonists abolish the transactivation of human epidermal growth factor receptors in advanced prostate cancer models, Invest New Drugs 32 (2014) 871–882. [DOI] [PubMed] [Google Scholar]
- [72].Schoeberl B, Pace EA, Fitzgerald JB, Harms BD, Xu L, Nie L, Linggi B, Kalra A, Paragas V, Bukhalid R, Grantcharova V, Kohli N, West KA, Leszczyniecka M, Feldhaus MJ, Kudla AJ, Nielsen UB, Therapeutically targeting ErbB3: a key node in ligand-induced activation of the ErbB receptor-PI3K axis, Sci Signal 2 (2009) ra31. [DOI] [PubMed] [Google Scholar]
- [73].Yang C, Liu Y, Lemmon MA, Kazanietz MG, Essential role for Rac in heregulin beta1 mitogenic signaling: a mechanism that involves epidermal growth factor receptor and is independent of ErbB4, Mol Cell Biol 26 (2006) 831–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].van Lengerich B, Agnew C, Puchner EM, Huang B, Jura N, EGF and NRG induce phosphorylation of HER3/ERBB3 by EGFR using distinct oligomeric mechanisms, Proc Natl Acad. Sci U. S. A 114 (2017) E2836–E2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Okita R, Mougiakakos D, Ando T, Mao Y, Sarhan D, Wennerberg E, Seliger B, Lundqvist A, Mimura K, Kiessling R, HER2/HER3 signaling regulates NK cell-mediated cytotoxicity via MHC class I chain-related molecule A and B expression in human breast cancer cell lines, J Immunol 188 (2012) 2136–2145. [DOI] [PubMed] [Google Scholar]
- [76].Soler M, Mancini F, Meca-Cortes O, Sanchez-Cid L, Rubio N, Lopez-Fernandez S, Lozano JJ, Blanco J, Fernandez PL, Thomson TM, HER3 is required for the maintenance of neuregulin-dependent and -independent attributes of malignant progression in prostate cancer cells, Int. J Cancer 125 (2009) 2565–2575. [DOI] [PubMed] [Google Scholar]
- [77].Kugel CH III, Hartsough EJ, Davies MA, Setiady YY, Aplin AE, Function-blocking ERBB3 antibody inhibits the adaptive response to RAF inhibitor, Cancer Res 74 (2014) 4122–4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Munoz-Moreno L, Bajo AM, Prieto JC, Carmena MJ, Growth hormone-releasing hormone (GHRH) promotes metastatic phenotypes through EGFR/HER2 transactivation in prostate cancer cells, Mol Cell Endocrinol 446 (2017) 59–69. [DOI] [PubMed] [Google Scholar]
- [79].Meyer D and Birchmeier C, Multiple essential functions of neuregulin in development, Nature 378 (1995) 386–390. [DOI] [PubMed] [Google Scholar]
- [80].Lui VW, Thomas SM, Zhang Q, Wentzel AL, Siegfried JM, Li JY, Grandis JR, Mitogenic effects of gastrin-releasing peptide in head and neck squamous cancer cells are mediated by activation of the epidermal growth factor receptor, Oncogene 22 (2003) 6183–6193. [DOI] [PubMed] [Google Scholar]
- [81].Beltowski J and Jazmroz-Wisniewska A, Transactivation of ErbB receptors by leptin in the cardiovascular system: mechanisms, consequences and target for therapy, Curr Pharm. Des 20 (2014) 616–624. [DOI] [PubMed] [Google Scholar]
- [82].Moody TW, Lee L, Iordanskaia T, Ramos-Alvarez I, Moreno P, Boudreau HE, Leto TL, Jensen RT, PAC1 regulates receptor tyrosine kinase transactivation in a reactive oxygen species-dependent manner, Peptides (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Gschwind A, Prenzel N, Ullrich A, Lysophosphatidic acid-induced squamous cell carcinoma cell proliferation and motility involves epidermal growth factor receptor signal transactivation, Cancer Res 62 (2002) 6329–6336. [PubMed] [Google Scholar]
- [84].Arora P, Cuevas BD, Russo A, Johnson GL, Trejo J, Persistent transactivation of EGFR and ErbB2/HER2 by protease-activated receptor-1 promotes breast carcinoma cell invasion, Oncogene 27 (2008) 4434–4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Soma D, Kitayama J, Yamashita H, Miyato H, Ishikawa M, Nagawa H, Leptin augments proliferation of breast cancer cells via transactivation of HER2, J Surg. Res 149 (2008) 9–14. [DOI] [PubMed] [Google Scholar]
- [86].Lui VW, Thomas SM, Wentzel AM, Siegfried JM, Li JY, Grandis JR, Mitogenic effects of gastrin-releasing peptide in head and neck squamous cancer cells are mediated by activation of the epidermal growth factor receptor, Oncogene 22 (2003) 6183–6193. [DOI] [PubMed] [Google Scholar]
- [87].Thomas SM, Bhola NE, Zhang Q, Contrucci SC, Wentzel AL, Freilino ML, Gooding WE, Siegfried JM, Chan DC, Grandis JR, Cross-talk between G protein-coupled receptor and epidermal growth factor receptor signaling pathways contributes to growth and invasion of head and neck squamous cell carcinoma, Cancer Res. 66 (2006) 11831–11839. [DOI] [PubMed] [Google Scholar]
- [88].Fiddes RJ, Janes PW, Sivertsen SP, Sutherland RL, Musgrove EA, Daly RJ, Inhibition of the MAP kinase cascade blocks heregulin-induced cell cycle progression in T-47D human breast cancer cells, Oncogene 16 (1998) 2803–2813. [DOI] [PubMed] [Google Scholar]
- [89].Kim S, Han J, Shin I, Kil WH, Lee JE, Nam SJ, A functional comparison between the HER2(high)/HER3 and the HER2(low)/HER3 dimers on heregulin-beta1-induced MMP-1 and MMP-9 expression in breast cancer cells, Exp. Mol Med 44 (2012) 473–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Shi X, zheng C, Li C, Hou K, Wang X, Yang Z, Liu C, Liu Y, Che X, Qu X, 4-Phenybutyric acid promotes gastric cancer cell migration via histone deacetylase inhibition-mediated HER3/HER4 up-regulation, Cell Biol Int. 42 (2018) 53–62. [DOI] [PubMed] [Google Scholar]
- [91].Ramos-Alvarez I, Moreno P, Mantey SA, Nakamura T, Nuche-Berenguer B, Moody TW, Coy DH, Jensen RT, Insights into bombesin receptors and ligands: highlighting recent advances, Peptides 72 (2015) 128–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Wang LH, Coy DH, Taylor JE, Jiang NY, Kim SH, Moreau JP, Huang SC, Mantey SA, Frucht H, Jensen RT, Desmethionine alkylamide bombesin analogues: a new class of bombesin receptor antagonists with a potent antisecretory activity in pancreatic acini and antimitotic activity in Swiss 3T3 cells, Biochemistry (Mosc). 29(3) (1990) 616–622. [DOI] [PubMed] [Google Scholar]
- [93].Wang LH, Coy DH, Taylor JE, Jiang NY, Moreau JP, Huang SC, Frucht H, Haffar BM, Jensen RT, Des-Met carboxyl-terminally modified analogues of bombesin function as potent bombesin receptor antagonists, partial agonists, or agonists, J. Biol. Chem 265(26) (1990) 15695–15703. [PubMed] [Google Scholar]
- [94].von Schrenck T, Wang LH, Coy DH, Villanueva ML, Mantey S, Jensen RT, Potent bombesin receptor antagonists distinguish receptor subtypes, Am. J. Physiol 259 (1990) G468–G473. [DOI] [PubMed] [Google Scholar]
- [95].Coy DH, Taylor JE, Jiang NY, Kim SH, Wang LH, Huang SC, Moreau JP, Gardner JD, Jensen RT, Short-chain pseudopeptide bombesin receptor antagonists with enhanced binding affinities for pancreatic acinar and Swiss 3T3 cells display strong antimitotic activity, J. Biol. Chem 264 (1989) 14691–14697. [PubMed] [Google Scholar]
- [96].Heinz-Erian P, Coy DH, Tamura M, Jones SW, Gardner JD, Jensen RT, [D-Phe12]bombesin analogues: a new class of bombesin receptor antagonists, Am. J. Physiol 252 (1987) G439–G442. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.