Abstract
Atrial fibrillation (AF) is a very common arrhythmia in clinical practice. Its incidence and prevalence are age-related and are growing in the last years. Age is a risk factor also for coronary artery disease (CAD), and with the evolution of preventive care, the first event (acute coronary syndrome (ACS) or percutaneous coronary intervention (PCI)) takes place at a later age. If elderly patients with AF and CAD undergo ACS or PCI, they have indication to assume triple therapy. Triple therapy (oral anticoagulation (OAC) plus dual antiplatelet therapy (DAPT)) exposes patients to high bleeding risk. In the last 10 years, several clinical trials have tested dual therapy (OAC plus single antiplatelet therapy) in AF patients who undergo ACS or elective PCI. WOEST trial has tested warfarin + clopidogrel against triple therapy. PIONEER AF-PCI trial has tested low-dose rivaroxaban + P2Y12 inhibitor or very low-dose rivaroxaban + DAPT against standard triple therapy with warfarin. RE-DUAL PCI trial has tested two doses of dabigatran + P2Y12 inhibitor against standard triple therapy with Warfarin. AUGUSTUS trial has tested apixaban against warfarin both in dual therapy with P2Y12 inhibitor and in triple therapy with a P2Y12 inhibitor and aspirin. ENTRUST-AF PCI, last published study, has tested edoxaban + P2Y12 inhibitor against triple therapy. All these trials show dual therapy reduces significantly bleeding risk than triple therapy. In this paper, we analyze these clinical trials to understand if dual therapy results can be applied to elderly patients and what is probably the better approach in elderly AF patients undergo to ACS or PCI.
Keywords: Acute coronary syndrome, Atrial fibrillation, Dual therapy, Oral anticoagulation, The elderly
1. Introduction
Atrial fibrillation (AF) is the most common arrhythmia in elderly people and it is one of the principal cause of heart failure, stroke, and cardiovascular morbidity. AF prevalence and incidence are age-related and are growing year by year. It is estimated that by 2030 will be 14–17 million AF patients only in Europe.[1]
Also, coronary artery disease (CAD) has an age-related prevalence and in the last years, the success of cardiovascular prevention politics has delayed the age of the first ischemic event. This implies that CAD patients are getting older and with many comorbidities.[2] Frailty is then an important parameter to consider when we approach elderly patients with CAD and especially with acute coronary syndromes (ACS).[3]
Often AF and CAD are coexisting in the same patient. About 30%–60% of AF patients have a CAD, and between 5%–15% of them will undergo percutaneous coronary intervention (PCI) during their lives. AF is also one of the most common arrhythmias during acute myocardial infarction (AMI).[4] Considering AF and CAD age-related prevalence is obvious that the majority of these patients are older adults.[4],[5] As a consequence, patients usually have to follow triple therapy, consisted of oral anticoagulant (OAC) associated with dual antiplatelet therapy (DAPT).[5] Is this therapy the better for elderly, and probably frail, patients? In this article, we discussed available information about triple therapy aimed to find a better approach to balance risk-benefit ratio in antithrombotic and antiplatelet therapy in elderly people, which could differ from this kind of triple therapy applied in adults.
2. Standard of care, ischemic vs. bleeding risk
It is known that OAC is more effective than single antiplatelet therapy or DAPT in stroke prevention in AF patients with CHA2DS2VASc score of more than two in men (or more than 3 in women).[1] This positive effect of OAC is more evident in elderly people who have a higher ischemic risk.[6] Therefore, in patients with high ischemic risk (CHA2DS2VASc score ≥ 2 in men and ≥ 3 in women), OAC is recommended. OAC therapy involves the use of vitamin K antagonist (VKA) or direct oral anticoagulant (DOAC). VKA therapy has difficult management because it has a narrow therapeutic range (INR: 2.0–3.0), food interaction and dose adjustments. DOAC therapy that includes direct Xa factor inhibitors (apixaban, edoxaban, and rivaroxaban) or thrombin inhibitor (dabigatran), has more simple management but it is possible to use it only in non-valvular AF and in non-severe renal failure.[1]
In ACS and after a PCI with stent implantation, DAPT has demonstrated to be the best therapy to prevent stent thrombosis and major adverse cardiac events (MACE).[7] The last European Society of Cardiology (ESC) DAPT guidelines recommended DAPT therapy (aspirin plus clopidogrel) for 6 months after a PCI in stable CAD (1–3 months is high bleeding risk patients) and DAPT therapy (aspirin plus ticagrerol or prasugrel or clopidogrel) for 1 year after ACS (6 month in high bleeding risk patients).[8]
In patients with both AF and ACS or PCI, combination therapy with OAC and DAPT is indicated to prevent both thromboembolic complications and MACE or stent thrombosis. Unfortunately, the weak point of this triple therapy is that it could cause bleeding in patients. In a cohort study on 82,854 AF Danish people (mean age 73.9 years), Hansen, et al.[9] demonstrated that triple therapy increases fatal and non-fatal bleeding risk by three times compared with OAC mono-therapy. In the work of Hess, et al.,[10] no differences are shown in MACE (combined of death, AMI, stroke) between triple therapy and DAPT in 4959 patients (age ≥ 65 years) with AMI and AF (32.6% vs. 32.7%, P = 0.99). The superiority of triple therapy over DAPT is only in stroke prevention (3.2% vs. 4.7%, P = 0.02). On the other hand, triple therapy increases significantly bleeding (17.6% vs. 11.0%, P < 0.0001) than DAPT and in particular doubles intracranial bleeding (3.4% vs. 1.5%, P = 0.001). The correct balance between ischemic and bleeding risk is not usually simple to do. For the estimation of ischemic/thromboembolic risk, it is in use CHA2DS2VASc score (Congestive heart failure, Hypertension, Age ≥ 75 years, Diabetes, Stroke, Vascular disease, Age ≥ 65 years, female Sex) in AF patients. Acute presentation and coronary anatomical type of lesion are parameters used for the estimation of ischemic risk in CAD patients. Scores available for evaluation of bleeding risk in AF patients are: HASBLED (Hypertension, Abnormal renal/liver function, Stroke, Bleeding history, Labile INR, Elderly > 65 years, Drugs/Alcohol), HEMORR2HAGES (Hepatic/renal dysfunction, Ethanol abuse, Malignancy, Older age > 75 years, Reduced platelet function, Rebleeding risk, Hypertension, Anaemia, Genetic factor, Excessive falls, Stroke) and ATRIA (anaemia, severe renal disease, age ≥ 75 years, prior bleed, hypertension). In CAD, short DAPT rather than standard/long DAPT is recommended when PRECISE-DAPT score ≥ 25. PRECISE-DAPT score is a bleeding risk score calculated by an algorithm that considers hemoglobin, white blood cells, age, creatinine clearance and prior bleeding.[1],[5] How it is possible to observe, most of the parameters are both ischemic/thromboembolic and bleeding risk factors. In elderly people, in addition to older age, per se an ischemic and bleeding risk factor, coexist several comorbidities such as hypertension, diabetes, previous stroke, anemia and chronic renal failure that increase both ischemic and hemorrhagic risk. All these important considerations in clinical practice make difficulty in choosing the right therapy.
3. Can dual therapy be the solution? WOEST, PIONEER AF-PCI, RE-DUAL PCI, AUGUSTUS and ENTRUST-AF PCI trials
Triple therapy bleeding problem has encouraged scientific research in the last decade (Table 1). The first is WOEST trial, which has compared, in 573 patients (mean age 68.7 years) undergoing PCI and receiving OAC, dual therapy (clopidogrel plus warfarin) against triple therapy (aspirin, clopidogrel and warfarin). WOEST trial has demonstrated dual therapy is more safety than triple therapy with a significant reduction in total bleeding (19.4% vs. 44.4%, P < 0.0001) and more effective, with decrease in composite events (stroke, death, myocardial infarction, re-PCI/CABG, stent thrombosis) (11.1% vs. 17.6%, P = 0.025).[11] The small sample size is one of the main limitations of WOEST trial, in fact it has not enough powerful to evaluate efficacy endpoint. Moreover, dual therapy bleeding reduction is principal due to reduction in TIMI minimal (6.5% vs. 16.7%, P < 0.001) or minor (11.2% vs. 27.25, P < 0.001) bleeding events, without any differences in TIMI major bleeding between dual and triple therapy (3.2% vs. 5.6%, P = 0.159).[12]
Table 1. Clinical trials on antithrombotic and antiplatelet regiment in patients with AF and PCI.
| WOEST | PIONEER AF-PCI | RE-DUAL PCI | AUGUSTUS | ENTRUST-AF PCI | |
| Population | 573 (PCI + OAC indication) | 2124 (AF+PCI) | 2725 (AF+PCI) | 4600 (AF+ACS or PCI) | 1506 (AF+PCI) |
| Mean age, yrs | 68.7 | 70.1 | 70.8 | 70.7 | 70 |
| Objective | Compare dual therapy (VKA plus clopidogrel) vs. standard triple therapy | Two RIV treatment strategies vs standard triple therapy through 12 months | Two dose DAB regimens plus P2Y12 inhibitor vs. standard triple therapy up to 30 months | Assess the non-inferiority safety of API vs VKA (both combined with P2Y12 receptor antagonist) up to 6 months | Evaluate the safety of EDO plus P2Y12 Inhibitor vs standard triple therapy through 12 months |
| Primary endpoint | All bleeding | TIMI clinically relevant bleeding | ISTH clinically relevant bleeding | ISTH clinically relevant bleeding | ISTH clinically relevant bleeding |
| Results | Dual therapy causes less bleeding than standard triple therapy | RIV-based therapy is associated with a lower rate of clinically significant bleeding than standard triple therapy | Dual therapy with both DAB doses is safer than standard triple therapy. | API plus P2Y12 inhibitor causes less bleeding than standard triple therapy without increasing in ischemic events | EDO plus P2Y12 inhibitor is non-inferior for bleeding compared with standard triple therapy, without significant differences in ischaemic events |
AF: atrial fibrillation; API: apixaban; DAB: dabigatran; EDO: edoxaban; ISTH: International Society of Thrombosis and Haemostasis; PCI: percutaneous coronary intervention; RIV: rivaroxban; TIMI: thrombolysis in myocardial infarction; VKA: vitamin K antagonist.
The second trial that has evaluated dual vs. triple therapy is PIONEER AF-PCI. 2124 patients (mean age 70.1 years) with non-valvular AF and undergoing PCI were randomized in three groups. Group 1 (709 patients, mean age 70.4 years) was treated with rivaroxaban 15 mg OD (with reduction to 10 mg in moderate renal impairment, glomerular filtration rate (GFR): 30–50 mL/min) plus P2Y12 inhibitor (clopidogrel, ticagrelor or prasugrel) for 12 months. Group 2 (709 patients, mean age 70.0 years) was treated with rivaroxaban 2.5 mg (b.i.d) plus DAPT and then rivaroxaban 15 mg OD plus low-dose aspirin till to 12 months. Group 3 (706 patients, mean age 69.9 years), was treated with VKA (INR: 2.0–3.0) plus DAPT then VKA plus low-dose aspirin till to 12 months. DAPT duration (1, 6 or 12 months) and type of P2Y12 inhibitor have been chosen by the investigator before randomization. It has been shown that bleeding events are lesser in group 1 (16.8%) and group 2 (18.0%) than in group 3 (26.7%). Rivaroxaban groups are more safety than standard therapy with VKA [bleeding reduction hazard ratio (HR) for group 1 vs. group 3 is 0.59 P < 0.001; HR for group 2 vs. group 3 is 0.63; P < 0.001]. Rivaroxaban groups bleeding reduction compared to VKA group was independent of INR stability. For efficacy endpoint (composite for death from cardiovascular causes, myocardial infarction or stroke), there were no differences between three groups. A post-hoc analysis has demonstrated a reduction in all-cause mortality and in recurrent hospitalization for rivaroxaban groups. PIONEER AF-PCI trial has some limits: it is not powered for efficacy outcome analysis; low-dose of rivaroxaban (15/10 mg OD) is tested for stroke prevention in AF people only in a small trial on 639 Japanese patients (J-ROCKET) where non-inferiority against warfarin is not demonstrated; very low-dose of rivaroxaban (2.5 mg, b.i.d) is not approved for stroke prevention in AF.[13]–[15]
In RE-DUAL PCI trial, 2725 AF patients who received PCI (mean age 70.8 years) were randomized 1: 1: 1 to receive triple therapy with VKA plus aspirin plus P2Y12 inhibitor (clopidogrel or ticagrelor) or to receive dual therapy with dabigatran 150 mg (b.i.d) plus P2Y12 inhibitor (clopidogrel or ticagrelor) or to receive dual treatment with dabigatran 110 mg (b.i.d) plus P2Y12 inhibitor (clopidogrel or ticagrelor). The minimum treatment was 6 months. Outside United States, elderly people (> 80 years or > 70 years in Japan) were assigned only to dabigatran 110 mg (b.i.d) group or to VKA group. Both dabigatran 150 mg (b.i.d) group and dabigatran 110 mg (b.i.d) group have resulted in less bleeding than triple therapy group. The incidence of primary endpoint was 20.2% in 150 mg dual therapy group against 25.7% in triple therapy group (HR = 0.72, P < 0.001 for non-inferiority) and 15.4% in 110 mg dual therapy compared to 26.9% in corresponding triple therapy group (HR = 0.52, P < 0.001 for non-inferiority; P < 0.001 for superiority). There were no differences in the incidence of composite efficacy endpoint (all-cause death, thrombotic events or unplanned revascularization) between the union of two dual therapy groups compared to the triple therapy group (13.7% vs. 13.4%, HR = 1.04, P = 0.005 for non-inferiority). The power of the study was not permitted to compare a single dose of dabigatran groups with triple therapy for efficacy endpoint.[16]
In AUGUSTUS trial, 4614 AF patients (mean age: 70.7 years) with ACS or PCI who were treated with P2Y12 inhibitor (clopidogrel, ticagrelor or prasugrel) first were randomized to VKA or apixaban (5 mg (b.i.d) or 2.5 mg (b.i.d) if reduction dose criteria was present) and then each group were randomized to aspirin or placebo. Incidence of primary safety outcome (major bleeding or clinically relevant non-major bleeding) is 10.5% in apixaban group compared to 14.7% in VKA group (HR = 0.69, P < 0.001 for non-inferiority and for superiority) and 16.1% in patients receiving aspirin compared to 9.0% in patients receiving placebo (HR = 1.89, P < 0.001). The highest number of bleeding was reported in a triple therapy group with P2Y12 inhibitor plus aspirin plus VKA (18.7%). The lowest number of bleeding was reported in dual therapy with P2Y12 inhibitor plus apixaban (7.3%). AUGUSTUS trial confirms that dual therapy (apixaban+P2Y12 or VKA+P2Y12) reduce bleeding as compared to triple therapy (apixaban+P2Y12 aspirin or VKA+P2Y12+aspirin). Inside each therapy, regimen (dual or triple therapy) use of apixaban is safer than the use of VKA as OAC. Incidence of ischemic events (myocardial infarction, stent thrombosis, stroke or urgent revascularization) was similar in all groups. Apixaban administration, instead, has reduced hospitalization compared to VKA group.[17]
The last published trial is ENTRUST-AF PCI. 1506 AF patients (mean age 70 years) who received PCI for stable CAD (48%) or for ACS (52%) were randomized 1: 1 to receive edoxaban regimen or VKA regimen. Edoxaban regimen group (751 patients; mean age 69 years) was treated with edoxaban 60 mg OD (with reduction to 30 mg OD in renal impairment with GFR 15–50 mL/min or in bodyweight less or equal to 60 kg or in patients using potent P-glycoprotein inhibitors) plus clopidogrel (or prasugrel or ticagrelor at discretion of the investigator) for 12 months. VKA regimen group (755 patients; mean age 70 years) was treated with VKA plus clopidogrel (or prasugrel or ticagrelor at discretion of the investigator) for 12 months plus low-dose aspirin for a minimum of 1 month up to 12 months. P2Y12 inhibitor choice and triple therapy duration was made before randomization. Incidence of primary safety outcome (ISTH major bleeding or clinically relevant non-major bleeding) is 17% in edoxaban regimen compared to 20% in VKA group (HR = 0.83, P < 0.001 for non-inferiority and P = 0.115 for superiority). After 12 months of follow-up, the incidence of efficacy outcome (the composite of cardiovascular death, stroke, systemic embolic events, AMI and stent thrombosis) is 7% in edoxaban regimen and 6% in VKA regimen (HR = 1.06). The rates for each component of the efficacy outcome were similar between regimens. ENTRUST-AF PCI trial has some limits: it is not powered for efficacy outcome analysis; bleeding reduction in edoxaban regimen group is due to reduction in clinically relevant non-major bleeding with same incidence of major bleeding between groups (6% in edoxaban regimen and 6% in VKA regimen).[18]
4. Discussion
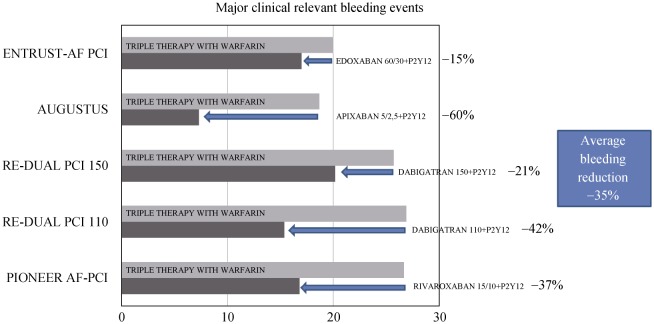
All these trials have shown that dual therapy reduces significantly bleeding risks as compared to triple therapy without an increase in ischemic events. RE-DUAL PCI and AUGUSTUS, the only two trials with enough statistical power, have demonstrated that removing aspirin from triple therapy does not increase ischemic events. Data on dual therapy with DOACs plus P2Y12 inhibitor has been showed an average reduction in major bleeding about 35%. Trials demonstrated 21% of reduction in safety endpoint with dabigatran 150 mg (b.i.d), 37% of reduction with rivaroxaban 15/10 mg OD (the real dual therapy arm in trial), 42% of reduction with dabigatran 110 mg (b.i.d), 60% of reduction with apixaban and 15% of reduction with edoxaban (Figure 1). In all these trials, there is a little representation of elderly population. In PIONEER AF-PCI, about one third of patients (34%) were more than 75 years old, in RE-DUAL PCI elderly patients (age ≥ 80 years or age ≥ 70 years old in Japanese people) were 22.9% of total trial population and this data is not known in WOEST, in AUGUSTUS and in ENTRUST-AF PCI trials where mean age was respectively 69.9, 70.7 and 70 years. Analyzing trials patients here we can find a population poor in comorbidity, thus it can not reflect typical frail elderly patient. Mean CHA2DS2VASc score was 3.8 in PIONEER AF-PCI, 3.6 in RE DUAL-PCI, 3.9 in AUGUSTUS and 4 in ENTRUST-AF PCI. Mean HASBLED score was 3.0 in PIONEER AF-PCI, 2.7 in RE DUAL-PCI, 2.9 in AUGUSTUS and 3.0 in ENTRUST-AF PCI. For renal function, only 18% of WOEST patients have history of renal failure, in PIONEER AF-PCI mean creatinine clearance was 78.8 mL/min with 27.9% of patients with a moderate renal impairment and only 0.8% of patients with a severe renal impairment, in RE DUAL-PCI mean creatinine clearance was 78.5 mL/min, in AUGUSTUS, 91.6% of patients have a serum creatinine < 1.5 mg/dL and in ENTRUST-AF PCI mean creatinine clearance was 71.8 mL/min. No data were available about hemoglobin values or anemia history and about previous bleeding events in trials population (except in WOEST trail where only 5% of patients have a history of gastrointestinal bleeding and in ENTRUST-AF PCI trial where only 6%–7% of patients have a history of bleeding).[11],[14],[16]–[18]
Figure 1. Major bleeding reduction with dual therapy with DOACs plus P2Y12 inhibitor as compared with standard triple therapy with VKA plus Clopidogrel plus aspirin.
AF: atrial fibrillation; DOACs: direct oral anticoagulants; PCI: percutaneous coronary intervention; VKA: vitamin K antagonist.
Elderly people with AF are at higher risk of both stroke and bleeding events than younger patients. Although the bleeding risk is increased, OAC benefits to prevent stroke and death are more evident in elderly than in younger patients, thus OAC is an essential part of AF therapy in elderly patients.[19],[20] Van Rein, et al.,[21] find a very high rate of bleeding events in very old patients (age > 90 years) or in patients with a CHA2DS2VASc score ≥ 6 or with previous major bleeding when they are under triple therapy. Frailty, per se is an independent predictor of major bleeding in elderly patients with ACS.[22] In clinical practice, often AF elderly patients in particular, coexist frailty and comorbidity are undertreat without the administration of OAC for bleeding prevention. Medical doctors, when treating elderly patients, are more afraid of bleeding events than of ischemic events.[20] DOAC has shown to reduce bleeding events as compared with VKA and this safety profile is present also in elderly patients.[23] In RELY trial, a sub-analysis in elderly patients (> 75 years), dabigatran 110 mg has the same rate of stroke and ischemic events of VKA (1.89% vs. 2.14%) but less intracranial hemorrhages (ICH) (0.37% vs. 1.00%). Dabigatran 150 mg reduce stroke rate (1.43% vs. 2.14%) and ICH (0.41% vs. 1.00%) as compared with VKA. There are no significant differences between dabigatran 110/150 and VKA in the elderly.[23],[24] In elderly (> 75 years) subgroup analysis of ROCKET-AF trial, rates of stroke or systemic embolism (2.29% vs. 2.85%), major bleeding (4.86% vs. 4.40%) and ICH (0.66% vs. 0.83%) were similar with rivaroxaban versus warfarin.[23],[25] Apixaban, in ARISTOTLE trial subgroup of elderly patients, significantly reduce the rate of stroke and systemic embolism (1.56% vs. 2.19%), major bleeding (3.33% vs. 5.19%) and ICH (0.43% vs. 1.29%).[23],[26] Edoxaban reduce significantly ICH as compared with VKA in a subgroup of elderly (> 75 years) patients of ENGAGE AF-TIMI 48 trial (0.5% vs. 1.2%) without significant differences in stroke and systemic embolism (1.9% vs. 2.3%) and in major bleeding (4.0% vs. 4.8%).[27],[28] These data could induce medical doctors to use DOAC to prevent stroke or systemic embolism also in elderly patients.
Often, real-world elderly patients are frail, with many comorbidities, and do not resemble those of the trials (“fit” elderly). Anemia, polypharmacy, falls, cognitive status impairment, renal failure and nutritional status are conditions often present in elderly people and frequently need to be considered when analyzing bleeding risk. In population of frail, elderly patients bleeding events are much more present than in fit elderly population because comorbidity and frailty are important bleeding risk factors. If we do not consider dabigratran 150 mg (b.i.d.), which is not indicated in frail elderly patients, an average reduction of dual therapy (DOACs plus P2Y12 inhibitor) in major bleeding risk rise to about 39%. If we assume that this surprising results, obtained in trials where there is a small representation of elderly population (represented by “fit elderly”), are also maintained in real-world frail elderly population, it is simple to understand that this result is the key to treating elderly patients with AF and ACS/PCI. In fact, in a population with higher absolute bleeding events, the same relative reduction means a more significant absolute reduction in event numbers.
5. Conclusions
It is known that bleeding events, in particular, major bleeding events, are associated with worsening of prognosis, with rehospitalization, and with death in frail elderly people.[29],[30] As already mentioned, triple therapy increases risk of bleeding in AF patients with ACS or PCI and in particularly in frail-elderly patients, it is essential to balance between benefits of antithrombotic and antiplatelet therapy and bleeding prevention.
Although frail elderly patients are not well studied in these trials, we think that results of dual therapy trials in the reduction of bleeding events without increasing ischemic events are relevant, especially in this setting of patients with very high bleeding risk.
In order to reduce bleeding risk in elderly patients it is possible to do: (1) DOACs have shown to reduced bleeding event as compared with VKA.[23]–[28] DOACs using over VKA to stroke prevention in AF could be recommended. (2) When antiplatelet therapy is indicated for ACS or PCI in AF patients, the possibility of treat elderly patients with FA and ACS/PCI only with dual therapy (without aspirin) is an important opportunity. Dual therapy with DOACs should be preferred to dual with VKA to reduce major bleeding. Clopidogrel is P2Y12 inhibitor more used in dual therapy trials (> 90%) and in frail elderly patients it is probably the safest.[11]–[18] (3) Use triple therapy should be only when ischemic risk is very high (STEMI, complex PCI, main left PCI) and for the shortest possible time (one month).[8]
References
- 1.Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. doi: 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 2.Padilla IM, Martìn-Asenjo R, Bueno H. Management of acute coronary syndromes in geriatric patients. Heart Lung Circ. 2017;26:107–113. doi: 10.1016/j.hlc.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 3.Tonet E, Pavasini R, Biscaglia S, et al. Frailty in patients admitted to hospital for acute coronary syndrome: when, how and why? J Geriatr Cardiol. 2019;16:129–137. doi: 10.11909/j.issn.1671-5411.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Bari M, Pratesi A, Nigro FM, et al. DAPT plus anticoagulant therapy: The difficult coexistence post-ACS in older patients with atrial fibrillation. Monaldi Arch Chest Dis. 2018;88:957. doi: 10.4081/monaldi.2018.957. [DOI] [PubMed] [Google Scholar]
- 5.Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS. Eur J Cardiothorac Surg. 2018;53:34–78. doi: 10.1093/ejcts/ezx334. [DOI] [PubMed] [Google Scholar]
- 6.Bencivenga L, Komici K, Corbi G, et al. The management of combined antithrombotic therapy in patients with atrial fibrillation undergoing percutaneous coronary intervention: a particularly complex challenge, especially in the elderly. Front Physiol. 2018;9:876. doi: 10.3389/fphys.2018.00876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leon MB, Baim DS, Popma JJ, et al. A clinical trial comparing three antithrombotic-drug regimens after coronary-artery stenting. N Engl J Med. 1998;339:1665–1671. doi: 10.1056/NEJM199812033392303. [DOI] [PubMed] [Google Scholar]
- 8.Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. EuroIntervention. 2019;20; 14:1435–1534. doi: 10.4244/EIJY19M01_01. [DOI] [PubMed] [Google Scholar]
- 9.Hansen ML, Sørensen R, Clausen MT, et al. Risk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with atrial fibrillation. Arch Intern Med. 2010;13; 170:1433–1441. doi: 10.1001/archinternmed.2010.271. [DOI] [PubMed] [Google Scholar]
- 10.Hess CN, Peterson ED, Peng SA, et al. Use and outcomes of triple therapy among older patients with acute myocardial infarction and atrial fibrillation. J Am Coll Cardiol. 2015;66:616–627. doi: 10.1016/j.jacc.2015.05.062. [DOI] [PubMed] [Google Scholar]
- 11.Dewilde WJ, Oirbans T, Verheugt FW, et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet. 2013;30; 381:1107–1115. doi: 10.1016/S0140-6736(12)62177-1. [DOI] [PubMed] [Google Scholar]
- 12.Rubboli A, De Caterina R. The WOEST study: critical considerations and applicability. Cor Vasa. 2014;56:e254–e258. [Google Scholar]
- 13.Gibson CM, Mehran R, Bode C, et al. An open-label, randomized, controlled, multicentre study exploring two treatment strategies of rivaroxaban and a dose-adjusted oral vitamin k antagonist treatment strategy in subjectswith atrial fibrillation who undergo percutaneous coronary intervention (PIONEER AF-PCI) Am Heart J. 2015;169:472–478. doi: 10.1016/j.ahj.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 14.Gibson CM, Mehran R, Bode C, et al. Prevention of bleeding in patients with atrial fibrillation undergoing PCI. N Engl J Med. 2016;375:2423–2434. doi: 10.1056/NEJMoa1611594. [DOI] [PubMed] [Google Scholar]
- 15.Kerneis M, Yee MK, Mehran R, et al. Association of international normalized ratio stability and bleeding outcomes among atrial fibrillation patients undergoing percutaneous coronary intervention insights from the PIONEER AF-PCI Trial. Circ Cardiovasc Interv. 2019;12:e007124. doi: 10.1161/CIRCINTERVENTIONS.118.007124. [DOI] [PubMed] [Google Scholar]
- 16.Cannon CP, Bhatt DL, Oldgren J, et al. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med. 2017;377:1513–1524. doi: 10.1056/NEJMoa1708454. [DOI] [PubMed] [Google Scholar]
- 17.Lopes RD, Heizer G, Aronson R, et al. Antithrombotic therapy after acute coronary syndrome or PCI in atrial fibrillation. N Engl J Med. 2019;380:1509–1524. doi: 10.1056/NEJMoa1817083. [DOI] [PubMed] [Google Scholar]
- 18.Vranckx P, Valgimigli M, Eckardt L, et al. Edoxaban-based versus vitamin K antagonist-based antithrombotic regimen after successful coronary stenting in patients with atrial fibrillation (ENTRUST-AF PCI): a randomised, open-label, phase 3b trial. Lancet. 2019;394:1335–1343. doi: 10.1016/S0140-6736(19)31872-0. [DOI] [PubMed] [Google Scholar]
- 19.Patti G, Lucerna M, Pecen L, et al. Thromboembolic risk, bleeding outcomes and effect of different antithrombotic strategies in very elderly patients with atrial fibrillation: a sub-analysis from the PREFER in AF (Prevention of thromboembolic events–European registry in a trial fibrillation) J Am Heart Assoc. 2017;6:e005657. doi: 10.1161/JAHA.117.005657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Díez-Villanueva P, Alfonso F. Atrial fibrillation in the elderly. J Geriatric Cardiol. 2019;16:49–53. doi: 10.11909/j.issn.1671-5411.2019.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Rein N, Heide-Jørgensen U, Lijfering WM, et al. Major bleeding rates in atrial fibrillation patients on single, dual, or triple antithrombotic therapy: results from a nationwide danish cohort study. Circulation. 2019;139:775–786. doi: 10.1161/CIRCULATIONAHA.118.036248. [DOI] [PubMed] [Google Scholar]
- 22.Salinas GLA, Fernández MS, Izco MP, et al. Frailty predicts major bleeding within 30 days in elderly patients with acute coronary syndrome. Int J Cardiol. 2016;222:590–593. doi: 10.1016/j.ijcard.2016.07.268. [DOI] [PubMed] [Google Scholar]
- 23.Capranzano P, Miccichè E, D'Urso L, et al. Personalizing oral anticoagulant treatment in patients with atrial fibrillation. Expert Rev Cardiovasc Ther. 2013;11:959–973. doi: 10.1586/14779072.2013.818819. [DOI] [PubMed] [Google Scholar]
- 24.Lauw MN, Eikelboom JW, Coppens M, et al. Effects of dabigatran according to age in atrial fibrillation. Heart. 2013;103:1015–1023. doi: 10.1136/heartjnl-2016-310358. [DOI] [PubMed] [Google Scholar]
- 25.Halperin JL, Hankey GJ, Wojdyla DM, et al. Efficacy and safety of rivaroxaban compared with warfarin among elderly patients with nonvalvular atrial fibrillation in the rivaroxaban once daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation (ROCKET AF) Circulation. 2014;130:138–146. doi: 10.1161/CIRCULATIONAHA.113.005008. [DOI] [PubMed] [Google Scholar]
- 26.Halvorsen S, Atar D, Yang H, et al. Efficacy and safety of apixaban compared with warfarin according to age for stroke prevention in atrial fibrillation: observations from the ARISTOTLE trial. Eur Heart J. 2014;35:1864–1872. doi: 10.1093/eurheartj/ehu046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruff CT, Giugliano RP, Antman EM, et al. Evaluation of the novel factor Xa inhibitor edoxaban compared with warfarin in patients with atrial fibrillation: design and rationale for the effective anticoagulation with factor Xa next generation in atrial fibrillation–thrombolysis in myocardial infarction study 48 (ENGAGE AF–TIMI 48) Am Heart J. 2010;160:635–641. doi: 10.1016/j.ahj.2010.06.042. [DOI] [PubMed] [Google Scholar]
- 28.Kato ED, Giugliano RP, Ruff CT, et al. Efficacy and safety of edoxaban in elderly patients with atrial fibrillation in the ENGAGE AF–TIMI 48 Trial. J Am Heart Assoc. 2016;5:e003432. doi: 10.1161/JAHA.116.003432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lopes RD, Rao M, Simon DN, et al. Triple vs. dual antithrombotic therapy in patients with atrial fibrillation and coronary artery disease. Am J Med. 2016;129:592–599. doi: 10.1016/j.amjmed.2015.12.026. [DOI] [PubMed] [Google Scholar]
- 30.Chhatriwalla AK, Amin AP, Kennedy KF, et al. Association between bleeding events and in-hospital mortality after percutaneous coronary intervention. JAMA. 2013;309:1022–1029. doi: 10.1001/jama.2013.1556. [DOI] [PubMed] [Google Scholar]