Abstract
This study was designed to evaluate the in vitro and in vivo effects of reductive acetogens isolated from ruminants on methane mitigation, and milk performance, respectively. Four acetogens, Proteiniphilum acetatigenes DA02, P. acetatigenes GA01, Alkaliphilus crotonatoxidans GA02, and P. acetatigenes GA03 strains were isolated from ruminants and used in in vitro experiment. A control (without acetogen) and a positive group (with Eubacterium limosum ATCC 8486) were also included in in vitro experiment. Based on higher acetate as well as lower methane producing ability in in vitro trial, P. acetatigenes GA03 was used as inoculum for in vivo experiment. Holstein dairy cows (n = 14) were divided into two groups viz. control (without) and GA03 group (diet supplied with P. acetatigenes GA03 at a feed rate of 1% supplementation). Milk performance and blood parameters were checked for both groups. In in vitro, the total volatile fatty acids and acetate production were higher (p < 0.05) in all 4 isolated acetogens than the control and positive treatment. Also, all acetogens significantly lowered (p < 0.05) methane production in comparison to positive and control groups however, GA03 had the lowest (p < 0.05) methane production among 4 isolates. In in vivo, the rate of milk yield reduction was higher (p < 0.05) in the control than GA03 treated group (5.07 vs 2.4 kg). Similarly, the decrease in milk fat was also higher in control (0.14% vs 0.09%) than treatment. The somatic cell counts (SCC; ×103/mL) was decreased from 128.43 to 107.00 in acetogen treated group however, increased in control from 138.14 to 395.71. In addition, GA03 increased blood glucose and decreased non-esterified fatty acids. Our results suggest that the isolated acetogens have the potential for in vitro methane reduction and P. acetatigenes GA03 strain could be a candidate probiotic strain for improving milk yield and milk fat in lactating cows with lowering SCCs.
Keywords: Methane mitigation, Milk performance, Reductive acetogens, Somatic cell count
INTRODUCTION
Volatile fatty acids (VFA), the main metabolic product of anaerobic fermentation in the rumen, especially acetic acids play a significant role in milk performance. However, the carbon dioxide (CO2) and hydrogen (H2), produced along with the acetate and butyrate, have a negative impact on the environment by contributing methane (CH4) production which has 20 to 50 times higher greenhouse effect than that of CO2 [1] and considered as dietary energy losses (2%–12%) from ruminants [2]. The ruminants produce VFA, H2, CO2, and ammonia (NH3) after fermentation of feeds by microorganisms such as bacteria, protozoa and fungi in their rumen [3]. The CH4 is produced by utilizing H2, accumulated inside the rumen, by methanogens. Therefore, it is needed to find out suitable microorganisms that are able to compete with methanogenic bacteria in the rumen for H2. Reductive acetogens, anaerobic microorganisms, produce acetic acid through the pathway of acetyl-coenzyme A (CoA), is a process which requires H2 atoms and thus competing with CH4 production by methanogens [4]. So, reductive acetogenesis is considered as an alternative pathway of hydrogen-utilization that reduce methanogenesis in the rumen and could be a probable approach to reduce greenhouse gas emissions from ruminants.
Milk performance is the prime target in dairy farmers along with the reduction of greenhouse gas from animal sources. The cows will produce larger quantities of milk with better quality only with the receiving of proper nutrition. Milk fat is synthesized by using acetate and butyrate from rumen whereas lactose is synthesized by propionate, lactate and amino acid [5]. Acetic acid accounts for 60%–70% of total VFA production which is mostly used for energy production and milk fat synthesis. In addition, various feed additives including probiotics have been used to improve the milk yield and quality of dairy cows along with blood profile [6]. Reductive acetogens, present in the rumen, are able to increase acetate production which leads to increase milk fat percentage as well as reduce enteric methane production which subsequently prevent energy loss from dairy cows. Though several reductive acetogens have been so far identified and implemented to improve in dairy production however, still needed to find more suitable strains for better performance. Therefore, reductive acetogens can be used as a good source of probiotics which will improve performance of lactating dairy cows.
Because acetogens can be the potential inhibitors to methanogenesis, this study was conducted to evaluate the effect of the ruminant’s origin reductive acetogens on in vitro rumen fermentation with emphasis on methane reduction. Consideration of lactating performance, this study targeted another objective to investigate the effects of reductive acetogens on the milking performance such as milk production, milk fat, milk protein and SCCs as well as the blood profile in dairy cattle supplemented as feed additives.
MATERIALS AND METHODS
The studies were conducted at the Sunchon National University (SCNU) animal farm and in the ruminant nutrition and anaerobe laboratory, Department of Animal Science and Technology, SCNU, Jeonnam, South Korea.
Isolation of reductive acetogens
Animals and sampling
Three Korean native goats (KNG; Capra hircus coreanae collected from Hwasun, South Korea) and 3 ruminally cannulated Holstein cows were used as experimental animal. The KNGs, having body weight of 43.93 ± 1.22 kg, were offered timothy and commercial concentrate at a rate of 2% of body weight twice daily. And, 48-months old ruminally cannulated Holstein cows, having body weight of 600 ± 47 kg, were fed concentrate feed and Italian ryegrass at a ratio of 2:8 twice a day. The rumen fluids were collected from KNGs after slaughtering and from cannulated Holstein cows 2hrs after feeding, respectively. Immediately after collection, the rumen fluids were strained through the four layers of surgical gauze and subsequently placed in amber bottles with an oxygen free headspace. Then the rumen fluid was sealed and immediately transferred to the laboratory by maintaining 39°C temperature.
Media preparation and isolation of rumen bacteria
For initial enrichment, AC-B1 medium (9.5 mL), described by Le Van et al. [7], was dispensed into 30 mL Hungate tube under oxygen-free CO2 (20%) and N2 (80%) gases, sealed with butyl-rubber septum and aluminum crimp cap. AC-B1 medium contained (per 1,000 mL) the following: KH2PO4 (0.28 g), K2HPO4 (0.94 g), NaCl (0.14 g), KCl (0.16 g), MgSO4 · 7H2O (0.02 g), NH4Cl (0.5 g), CaCl2 · 2H2O (0.001 g), trace mineral solution (10 mL), vitamin solution (10 mL), yeast extract (0.5 g), NaHCO3 (6.0 g), reducing agent [2.5% {wt/vol} each cysteine hydrochloride · H2O and Na2S · 9H2O, pH 10.0] (10 mL), clarified bovine rumen fluid (100 mL), BES [sodium salt; filter sterilized] as indicated (20 mL), and resazurin (0.001 g). After autoclaving for 15 min at 121°C, strained rumen fluid (0.5 mL) were inoculated into the medium and incubated for 7 days with horizontal shaking at 120 rpm and maintained 39°C temperature. After an initial enrichment, the culture was diluted serially from 10−2 to 10−9, inoculated into AC-B1 agar medium and incubated for another 7 days. The visible colonies were subsequently isolated and inoculated into broth medium and finally incubated for more one week with horizontal shaking at 120 rpm and 39°C temperature. The isolation step was repeated to obtain pure colonies.
PCR amplification of rumen bacterial DNA
The colonies were isolated from AC-B1 agar medium and their DNA were extracted. The 27F (5´-AGAGTTTGATCCTGGCTCAG-3´) and 1492R (5´-GGTTACCTTGTTACGACTT-3´) primers [8] were used to amplify 16S ribosomal RNA gene (16S rDNA), whereas reductive acetogenesis-related genes were used for the effective separation and identification of the target bacteria (reductive acetogens). The separation was made through the detection of the specific formyltetrahydrofolate synthetase (FTHFS) gene which was described by Leaphart and Lovell [9] using the primers FTHFSf (5´-TTY ACW GGH GAY TTC CAT GC-3´) and FTHFSr (5´-GTA TTG DGT YTT RGC CAT ACA-3´).
DNA sequencing and phylogenetic tree construction
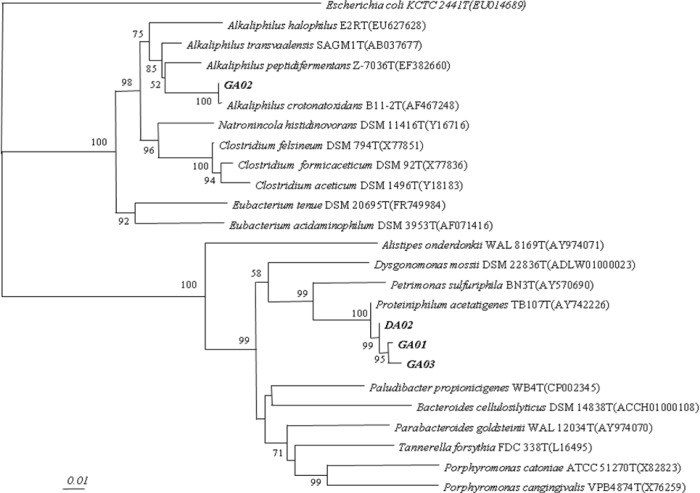
For DNA sequencing, the purified PCR products of 16S rDNA were sent to the Macrogen (Macrogen Inc. Korea, 10F, 254 Beotkkot-ro. Geumcheon-gu, Seoul 08511, Korea). The SeqMan program (DNA Star, Lasergene software, Madison, WI, USA) were used to assemble the sequenced fragments. The obtained gene sequences were compared with 16S RNA gene sequences which were available in GenBank using the program BLAST (http://www.ncbi.nlm.nih.gov/BLAST/) and EzTaxon (http://147.47.212.35:8080/index.jsp). The approximate phylogenetic affiliations were determined through aligning the gene sequences with those sequences of closely related species by using CLUSTAL W version 1.6. The neighbor-joining (NJ) method and pairwise gap removal techniques were used to construct the phylogenetic tree whereas distance matrices were calculated according to Kimura [10]. Lastly, two-parameter NJ method with in PHYLogeny Inference Package (PHYLIP package) which was employed and the bootstrap analysis was done by resampling the data (1,000 times) in order to evaluate the stability of phylogenetic tree. Only the bootstrap values larger than 50% were expressed on the internal nodes.
Cultivation of acetogens
The isolated acetogens strains from the rumen were compared to Eubacterium limosum ATCC8486 as standard acetate-producing bacteria. All acetogens, isolated from ruminant sources, were sub-cultured and inoculated in to RM02 medium in order to characterize their fermentation parameters [11]. RM02 medium contained (per 1,000 mL) the following: dH2O (950 mL), (NH4)2SO4 (0.6 g), K2HPO4 (1.4 g), L-cysteine-HCl (0.5 g), KCl (1.5 g), resazurin [0.1% solution] (4 drops), NaHCO3 (4.2 g), selenite-tungstate solution (1 mL), trace element solution (1 mL), and vitamin solution (1%). The media were dispensed under O2-free CO2 (20%) and N2 (80%) gases. The cultures were cultivated without (Con) or with the addition of mix gas (20% CO2 and 80% H2) and glucose (5 mM) and then incubated at 39°C temperature in a shaking incubator with 120 rpm for 4 days and subsequently the acetic acid production was determined.
In vitro experiment
Upon arrival of rumen fluid samples in the laboratory, rumen fluid was mixed with prepared buffer solution, recommended by Russell and Van Soest [12] at a ratio of 1:2 and adjusted pH 6.7. All serum bottles (120 mL in size), containing either soluble starch or casein powder substrates (1% DM), were dispensed with 50 mL of prepared medium anaerobically under O2-free N2 bubbling and sealed with rubber stoppers and aluminum caps immediately. The serum bottles were then incubated in a shaking incubator at 120 rpm and 39°C temperature for 24 h. The experimental groups included a negative control (without acetogens), a positive control (inoculated with 1% of the standard acetogenic bacteria, E. limosum ATCC8486), and the 4 experimental treatments (bottles inoculated with 1% of either of the isolated acetogens).
Analyses of in-vitro rumen fermentation parameters
The total gas (TG) production was measured by using a press and sensor machine (Laurel Electronics, Inc., Costa Mesa, CA) and the pH was measured by using a Pinnacle series M530p meter (Schott instruments, Mainz, Germany) just after uncapping the bottles. The method described by Chaney and Marbach [13] was followed to measure ammonia nitrogen (NH3-N) concentration in the rumen fluids. The CH4 and CO2 emitted during the incubation period were measured by using Gas chromatography (Agilent Technologies HP 5890). The CH4 production was estimated using the formula described by Orskov and McDonald [14]. VFAs production were analyzed by using high-performance liquid chromatography (HPLC; Agilent Technologies 1200 series) following the method described by Tabaru et al. [15].
In-vivo experiment
Animal management and experiment design
Holstein cows (n = 14) having initial body weight of 450 ± 50 kg, milk yield 28.63 ± 3.65 kg/d and DIM (Day in Milk) 115 ± 60 d, were blocked into 2 (two) groups by parity, DIM, and milk yield. The average parity was 2.5 and lactation stage was 2 to 6 months. Cows in each block were then randomly allocated to one of the two treatment diets (a randomized complete block design). The experiment was conducted in summer season through a period of 60 days consisting 20 days of adaptation and 40 days of sample collection. Due to higher acetate as well as lower methane producing ability of Proteiniphilum acetatigenes GA03 strain isolated through in vitro trial, which was stored in the Korea culture center of microorganisms, Seoul, Korea as SROD1 (KCCM11219F), was used as probiotics for in vivo experiment. Cows were separated into 2 groups such as control and treatment GA03. All cows were fed the same diet as a total mixed ration (TMR; Table 1) ad libitum, twice a day at 0800 h and 1900 h, allowing for 5% refusals throughout the study, and had free access to water and mineral. After 20 days feed adaptation, only the treatment group received probiotics GA03 as diet supplement daily with TMR (Table 1) and the feed intake was recorded from day 21.
Table 1. Ingredients and nutrition composition of the experimental diet.
| Items (%, DM) (unless otherwise noted) | Total |
|---|---|
| Ingredients | |
| Tall fescue | 24.7 |
| Oat straw | 19.8 |
| Timothy | 22.9 |
| Corn silage | 23.4 |
| Dry ground corn | 8.8 |
| Vitamin premix1) | 0.1 |
| Mineral premix2) | 0.1 |
| Salt | 0.1 |
| Calcium carbonate | 0.1 |
| Nutrient composition | |
| DM (%) | 65.9 |
| CP | 16.1 |
| EE | 6.5 |
| NDF | 43.09 |
| ADF | 28.54 |
Vitamin premix contained the following amount which was diluted in cellulose (g/kg premix): L-ascorbic acid, 121.2; DL-α-tocopherol acetate, 18.8; thiamin hydrochloride, 2.7; riboflavin, 9.1; pyridoxine hydrochloride, 1.8; niacin, 36.4; Ca-D-pantothenate, 12.7; myo-inositol, 181.8; D-biotin, 0.27; folic acid, 0.68; p-aminobenzoic acid, 18.2; menadione, 1.8; retinal acetate, 0.73; cholecalciferol, 0.003; cyanocobalamin, 0.003.
Mineral premix contained the following ingredients (g/kg premix): MgSO4 · 7H2O, 80.0; NaH2PO4 · 2H2O, 370.0; KCl, 130.0; ferric citrate, 40.0; ZnSO4 · 7H2O, 20.0; Ca-lactate, 356.5; CuCl, 0.2; AlCl3 · 6H2O, 0.15; KI, 0.15; Na2Se2O3, 0.01; MnSO4 · H2O, 2.0; CoCl2 · 6H2O, 1.0.
DM, dry matter; CP, crude protein; EE, ether extract; NDF, neutral detergent fiber; ADF, acid detergent fiber.
Chemical analysis of the experimental diet
The dry matter (DM), crude protein (CP) and ether extract (EE) contents of the experimental diet were analyzed according to the guidelines of the Association of Official Analytical Chemists [16]. The neutral detergent fiber (NDF) and acid detergent fiber (ADF) were measured by using an Ankom fiber analyzer (Ankom Tech. Corp, Fairport, NY, USA) following the protocol described by Van Soest et al. [17].
Preparation and supplementation of acetogen probiotics
The isolated acetogen bacterial strain GA03 (Table 2), was anaerobically cultivated at 39°C with 100 rpm for 48 h. Mixed wheat bran and corn gluten feed (6:4) supplemented with 1:1 ratio of cultivated GA03 served as treated (TRT). For the purpose of treatments with acetogens, 10% GA03 acetogen inoculum was inoculated with the basal feed and incubation for three days with after fermentation cell unit of 4.18 Log CFU/g. The GA03 acetogen treated group received the diet supplemented with GA03 as a feed rate of 1% supplementation however, control group received only 1% supplementation of wheat bran and corn gluten feed (6:4) without GA03 inoculum. The prepared probiotic mixture was supplied on the top of the basal feed for immediately feeding as early as possible.
Table 2. Standard strains and isolated reductive acetogens.
| Strains (Gen Bank accession No.) | Nearest relative (Gen Bank accession No.) | Sequence similarity (%) | Isolated from |
|---|---|---|---|
| Eubacterium limosum ATCC 8486 | Standard strain | ||
| Isolated strains | |||
| Proteiniphilum acetatigenes DA02 (MK414692) | Proteiniphilum acetatigenes TB107 (AY742226) | 99 | Holstein cow |
| Proteiniphilum acetatigenes GA01 (MK414691) | Proteiniphilum acetatigenes TB107 (AY742226) | 98 | Korean black goat |
| Alkaliphilus crotonatoxidans GA02 (MK414768) | Alkaliphilus crotonatoxidans B11-2 (AF467248) | 99 | Korean black goat |
| Proteiniphilum acetatigenes GA03 (MK414775) | Proteiniphilum acetatigenes TB107 (AY742226) | 99 | Korean black goat |
Milk production and composition
Cows were individually milked twice daily in their stalls at 0600 h and 1700 h and recorded daily milk production. The milk samples were collected on day 0 and day 60 and the milk fat, protein and somatic cells were measured by using a Milko-Scan (Foss Electric, Denmark).
Blood profile
Blood samples were collected before 30 minutes of feeding from the jugular vein on day 60. Blood samples were immediately transferred to the tube containing a solution of disodium EDTA and placed on ice. After centrifugation for 20 minutes, 1,800×g at 4°C (Labogene 1248, Korea), the plasma was transferred to a storage tube and labelled with the date and the animal identification number and subsequently analyzed the fresh sample or stored the sample at −20°C until analysis. An automatic blood analyzer (Express Plus, Ciba-Corning, CA, USA) was used to determine total protein, albumin, blood urea nitrogen (BUN) and glucose concentrations. The non-esterified fatty acid (NEFA) concentration was measured by HPLC.
Statistical analysis
All statistical analyses were carried out by using Statistical Analysis Systems (SAS version 9.1) [18]. The data were analyzed by analysis of variance (ANOVA) using the general linear model (GLM) under a randomized completely block design. Duncan’s Multiple Range Test (DMRT) was used to categorize differences among specific treatments in in vitro while t-test was used for in vivo data analysis. The significant differences were considered only when p < 0.05.
RESULTS
Isolation of acetogenic bacteria
Forty-nine colonies were isolated and identified from the rumen samples. Among them, four identified species had formyltetrahydrofolate synthetase (FTHFS) gene and considered to be acetogenic bacteria (Table 2). All these phylotype sequences showed 95%–100% similarity to the phylogenetic tree including namely DA02 (Proteiniphilum acetatigenes DA02), GA01 (P. acetatigenes GA01), GA02 (Alkaliphilus crotonatoxidans GA02), and GA03 (P. acetatigenes GA03) (Fig. 1). These four reductive acetogenic bacteria isolated from the rumen and positive control E. limosum ATCC8486 were compared for acetic acid production. The DA02, GA01 and GA03 acetogeic bacteria, except GA02 from the rumen, showed higher acetic acid production than positive strains E. limosum ATCC8486 (Table 3). With addition of glucose on the media of GA03, it had the highest (p < 0.05) acetate production (11.31 mM).
Fig. 1. Phylogenetic tree of acetate producing bacteria isolated from Holstein cows and Korean native goats constructed using the neighbor joining method based on 16S rRNA gene sequences.
Table 3. Acetic acid production (mM) of standard acetogen Eubacterium limosum and four isolated acetogens in RM02 medium.
| Culture condition | Strains | SEM | p-value | ||||
|---|---|---|---|---|---|---|---|
| E. limosum ATCC8486 | DA021) | GA012) | GA023) | GA034) | |||
| Con5) | 6.12d | 6.57c | 8.74a | 5.22e | 7.45b | 0.016 | <.0001 |
| H2-CO26) | 6.13d | 6.92c | 9.93a | 5.53e | 9.88b | 0.011 | <.0001 |
| Glucose7) | 5.18c | 5.94b | 3.3d | 5.01c | 11.31a | 0.044 | <.0001 |
SEM, Standard error of the means.
DA02, Proteiniphilum acetatigenes DA02 (MK414692).
GA01, Proteiniphilum acetatigenes GA01 (MK414691).
GA02, Alkaliphilus crotonatoxidans GA02 (MK414768).
GA03, Proteiniphilum acetatigenes GA03 (MK414775).
The cultures were cultivated without (Con) or with addition of mix gas (80% H2 and 20% CO2) and 5 mM glucose.
In vitro fermentation parameters
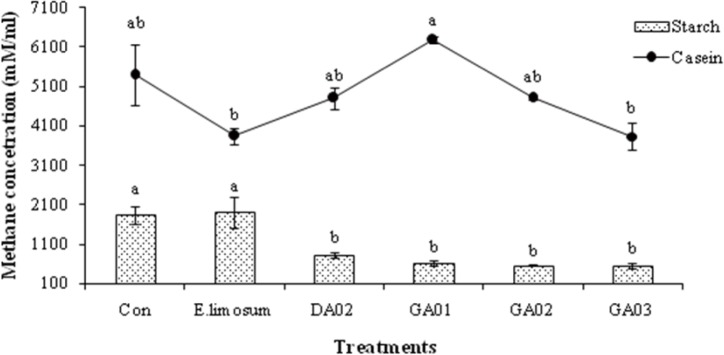
The pH, total gas, and VFA concentration of in vitro experiment are presented in Table 4. The total VFA was significantly (p < 0.05) higher in E. limosum ATCC8486 and other treatment groups than the control in both substrates, however, the pH was higher in the control than the other groups. The acetic acid production was higher (p < 0.05) in all 4 isolated acetogens in the soluble starch and casein than in the control and positive treatments. The isolated acetogens produced almost similar amount of propionate compare to positive group however higher (p < 0.05) than control (18.95 mM) while using soluble starch as substrate. In case of casien substrate, the GA03 produced lower acetic acid (17.57 mM) than other probiotic treated groups however, similar to positive group (17.59 mM) and significantly (p < 0.05) higher than the control (13.99 mM). Butyric acid was measured only in soluble starch however not detected in case of casein. The in vitro methane emission results are presented in Fig. 2. The amount of methane was lower (p < 0.05) in E. limosum ATCC8486 and GA03 groups than other probiotic groups viz. DA02, GA01, and GA02 with casein substrate. In case of starch substrate, methane production was lower (p < 0.05) in acetogens treated groups compare to control and E. limosum ATCC8486. In all experimental groups, the methane production was higher in case of casein substrate compared to starch. E. limosum ATCC8486 produced higher and lower CH4 with starch and casein, respectively. In contrast, GA01 produced higher and lower CH4 with casein and starch, respectively. However, GA03 produced lower CH4 with both starch and casein substrates.
Table 4. pH, total gas and volatile fatty acid production of in vitro fermentation using soluble starch and casein as substrates.
| Substrate | Treatment (24 h) | SEM | p-value | |||||
|---|---|---|---|---|---|---|---|---|
| Con1) | E. limosum ATCC8486 | DA022) | GA013) | GA024) | GA035) | |||
| pH | ||||||||
| Soluble starch | 5.62a | 5.20c | 5.29bc | 5.29bc | 5.31b | 5.34b | 0.914 | <.0001 |
| Casein | 6.50a | 6.35b | 6.34b | 6.37b | 6.30b | 6.35b | 1.065 | <.0001 |
| Total gas (mL) | ||||||||
| Soluble starch | 26.33d | 40.00a | 38.67a | 37.67ab | 35.33b | 32.67c | 6.072 | <.0001 |
| Casein | 16.67e | 21.33de | 32.67a | 31.00ab | 23.67dc | 27.33bc | 5.521 | <.0001 |
| Acetic acid (mM) | ||||||||
| Soluble starch | 37.65d | 48.32c | 51.24a | 50.89ab | 48.67c | 49.29bc | 8.633 | <.0001 |
| Casein | 40.63b | 46.32b | 63.36a | 70.09a | 66.87a | 65.48a | 12.419 | <.0001 |
| Propionic acid (mM) | ||||||||
| Soluble starch | 18.94b | 34.40a | 33.45a | 34.69a | 35.66a | 33.17a | 6.262 | <.0001 |
| Casein | 13.99c | 17.59b | 20.24a | 19.45a | 19.98a | 17.57b | 3.190 | <.0001 |
| Butyric acid (mM) | ||||||||
| Soluble starch | 7.37 | 9.03 | 8.97 | 8.31 | 7.94 | 7.64 | 1.686 | 0.190 |
| Casein | ND | ND | ND | ND | ND | ND | ||
| Total VFA (mM) | ||||||||
| Soluble starch | 63.97b | 91.76a | 93.66a | 93.90a | 92.26a | 90.11a | 15.950 | <.0001 |
| Casein | 54.61c | 63.90b | 83.60a | 89.55a | 86.85a | 83.04a | 15.433 | <.0001 |
| A:P | ||||||||
| Soluble starch | 2.01a | 1.41b | 1.53b | 1.47b | 1.37b | 1.48b | 0.294 | 0.0004 |
| Casein | 2.91bc | 2.63c | 3.13b | 3.60a | 3.34ab | 3.73a | 0.714 | 0.001 |
Values are the means of triplicates.
Means with different superscripts in the same row are significantly different (p < 0.05).
Con, Control.
DA02, Proteiniphilum acetatigenes DA02 (MK414692).
GA01, Proteiniphilum acetatigenes GA01 (MK414691).
GA02, Alkaliphilus crotonatoxidans GA02 (MK414768).
GA03, Proteiniphilum acetatigenes GA03 (MK414775).
SEM, standard error of the means; ND, not detected; VFA, volatile fatty acid; A:P, acetic acid:propionic acid.
Fig. 2. Methane concentration of in vitro fermentation using soluble starch and casein as substrates.
Values are the means of triplicate analyses and bars indicate the standard error. Means with different superscripts are significantly different (p < 0.05).
In vivo lactating performance and blood profile
The results of effects of reductive acetogenic bacterial strain GA03, supplemented with diet, on feed intake and lactating performance such as milk yield and milk compositions of dairy cows are presented in Table 5. The feed intake of control and treated group was 19.18 kg/d and 20.27 kg/d respectively. Initial milk production amounted to 30.90 kg in control whereas 26.37 kg in GA03 treated group (p < 0.5) which was decreased to 25.83 kg and 23.f97 kg, respectively after 60 days. The milk fat was decreased during the 60 days’ trial. The control decreased from 3.83% to 3.59% and GA03 decreased from 3.69% to 3.58%. However, the decrease (p < 0.05) was less in GA03 than control (0.11% vs 0.24%). The milk protein in control was decreased from 3.41% to 3.14% however, increased from 3.28% to 3.32% in GA03. Blood parameters of dairy cows fed with reductive acetogenic bacteria was shown in Table 6. Glucose concentration was higher (p < 0.05) in GA03 group compare to Control group (70.11 vs 67.47 mg/dL). Likewise, TP (g/d) production was higher (p < 0.05) in GA03 group, however BUN (mg/dL), and NEFA (μEq/L) production were higher (p < 0.05) in Control group.
Table 5. In vivo milk production performance of dairy cows fed with reductive acetogens.
| Items | Control | GA031) | SEM | p-value | |
|---|---|---|---|---|---|
| Feed intake (kg/d)2) | 19.18 | 20.27 | 0.450 | 0.110 | |
| Milk yield (kg/d) | 0 day | 30.90 | 26.37 | 0.620 | 0.001 |
| 60 days | 25.83 | 23.97 | 0.396 | 0.008 | |
| Differences3) | −5.07 | −2.40 | 0.306 | <.0001 | |
| Milk fat (%) | 0 day | 3.83 | 3.69 | 0.043 | 0.043 |
| 60 days | 3.59 | 3.58 | 0.029 | 0.849 | |
| Differences | −0.24 | −0.11 | 0.025 | 0.003 | |
| Milk protein (%) | 0 day | 3.41 | 3.28 | 0.029 | 0.023 |
| 60 days | 3.14 | 3.32 | 0.023 | <.0001 | |
| Differences | −0.27 | 0.04 | 0.030 | <.0001 | |
| SSC (×103/mL) | 0 day | 138.14 | 128.43 | 2.110 | 0.007 |
| 60 days | 395.71 | 107.00 | 16.750 | <.0001 | |
| Differences | 257.57 | −21.43 | 17.705 | <.0001 | |
Group treated with reductive acetogenic bacteria.
Dry matter basis.
The minus (–) symbol indicates reduction.
SEM, standard error of the means; SSC, somatic cell count.
Table 6. In vivo blood parameters of dairy cows fed with reductive acetogens.
| Items | Control | GA031) | SEM | p-value |
|---|---|---|---|---|
| ALB (g/dL) | 2.72 | 2.73 | 0.004 | 0.102 |
| BUN (mg/dL) | 18.09 | 16.37 | 0.200 | <0.001 |
| GLU (mg/dL) | 67.47 | 70.11 | 0.445 | 0.001 |
| NEFA (uEq/L) | 240.52 | 151.43 | 9.289 | <0.001 |
| TP (g/dL) | 6.59 | 7.15 | 0.060 | <0.001 |
Group treated with reductive acetogenic bacteria.
SEM, standard error of the means; ALB, albumin; BUN, blood urea nitrogen; GLU, glucose; NEFA, non-esterified fatty acid; TP, total protein.
DISCUSSION
In vitro rumen fermentation and methane mitigation
Reductive acetogens can produce acetate from hydrogen and carbon dioxides using the acetyl-CoA pathway which can compete with methangenesis in ruminants. In the present study, 4 reductive acetogens were isolated and identified from ruminants which were then used for in vitro experiments. In this study, positive control (E. limosum ATCC8486) and acetogens treated groups had significantly (p < 0.05) lower pH and higher total gas productions compared to control group with both substrates. However, the total gas production was higher with starch than casein, which may be due to the presence of higher content of easily fermentable starches viz. sugars, or hemicelluloses as substrates which was available for rumen microbes to produce more gases. This prediction is closely related to the digestibility of organic matter, CP, and ash contents of feeds [19]. In the present study, supplementation of isolated acetogens had comparable acetate production and they are significantly (p < 0.05) higher than E. limosum ATCC8486 and control while using casein as substrate and higher (p < 0.05) than control with starch substrate. Moreover, the propionate and total VFAs production were increased (p < 0.05) in acetogens treated groups compared to control either using starch or casein as substrate. A previous study showed that E. limosum ATCC8486 was able to utilize amino acid and produce acetate and butyrate, which stimulated its growth [20]. So, isolated acetogens are able to compete for amino acids which are used as a substrate for methanogen growth and methanogenesis [21]. The highest A:P ratio was observed (p < 0.05) in the control with soluble starch and GA03 with casein. Several pieces of literature from in vitro incubations of rumen contents [7] indicated that acetogens can function as hydrogenotrophs to compete with methanogenesis. Thus, reductive acetogenesis which occurs in the rumen might be an effective way for methane mitigation [22]. Lopez et al. [23] reported that E. limosum strain ATCC8486 and Ser 5 reductive acetogens were decreased 5% CH4 production after a 24 h incubation period in vitro without affecting VFA production. Likewise, this study had similar total VFAs productions among E. limosum ATCC8486 and acetogens groups using starch as substrate, however, significantly (p < 0.05) higher in acetogens groups using casein as substrate.
In the present study, higher methane production was observed in casein than soluble starch. Higher methane production in case of casein substrate in this study may be attributed to effective degradability of dry matter (EDDM). Qin et al. [24] stated that wheat had relatively higher EDDM, which was fermented more rapidly by ruminal microbes. In case of soluble starch substrate, all 4 isolated acetogens had lower (p < 0.05) CH4 production in comparison to E. limosum and the control. Among them, only the GA03 had the lower CH4 production with both substrates. This Proteiniphilum acetatigenes GA03 strain was then selected as a suitable probiotic for in vivo experiment which may contribute to lowering enteric methane emission from cows that leads to less dietary energy loss and increase hydrogen energy and ultimately improve lactation performance.
Lactating performance and blood profile
An earlier report revealed that probiotics increase milk yield at 3%–16% in dairy cows supplemented with diet [6]. In contrast, a decreasing pattern of milk yield and a tendency to decrease of feed intake in all treatments were observed in this study. This is may be due to the increase in temperature recorded during the experimental period, from day 0°C at 31°C to day 60°C at 35.5°C. This is in agreement with Mitchell and Russo [25] who reported that high temperatures in summer reduced milk yield. However, the reduction of milk production was lower in the GA03 treated group than the control (2.4 kg vs 5.07 kg) which indicated that the GA03 probiotic potentially prevents the amount of milk reduction over time. The decreased feed intake reduced overall milk production and milk fat and caused low-milk-fat syndrome which probably involves both alterations in rumen fermentation and availability of endogenous fatty acid sources [26]. However, the GA03 probiotic potentially protects the amount of milk fat reduction by producing precursor of milk fat synthesis in lactating dairy cows. This result is also supported by the in vitro result where higher acetate was produced by the acetogens treated group than the control. In terms of milk protein, our result showed that control was decreased from 3.41% to 3.14% while the GA03 treatment was increased from 3.28% to 3.32%. Usually, milk quality was valued on the basis of its fat content but at present, milk payments are based on the content of fat as well as protein [27].
The use of milk somatic cell count (SCC) in payment systems as a public health criterion for international trade is increasing. Our results revealed that the SCC (×103/mL) was decreased in GA03 treatment from 128.43 to 107.00, whereas opposite in control treatment which was increased from 138.14 to 395.71 (Table 5). The SCCs are used as a guideline for the state of health and management of the breast [28]. Dohoo et al. [29] reported that cow affects SCC when stressed, and that cow with heat stress increases SCC [30]. It is expected that at high temperature and humidity, which is a summer feature of the south of Korea, GA03 has prevented bacterial infection due to high-temperature stress and humidity. Glucose in the blood was used as an important index to determine the nutritional status of individuals [31]. In this study, the average glucose level was 70.11 mg/dL in GA03 whereas 67.47 mg/dL in control. Availability of glucose is the prerequisite for high milk production in dairy cows. NEFA resulted in 240.52 uEq/L in control with 151.43 uEq/L in GA03. The supplements of GA03 may have an impact on lactating cows. The main goal of the dairy industry is to increase milk production. So, it is important to maintain animal health. Energy requirements for milk yield in early lactation of dairy cows go beyond the available energy from feed intake resulting in a more or less severe negative energy balance, which the lactation of dairy cows tries to compensate by fat-mobilization from adipose tissue. Excessive mobilization of fatty acids can exceed the liver’s capacity for degradation and results in the elevated formation of ketone bodies and accumulation NEFA in the liver where they are converted to triglycerides and stored [32]. In this experiment, BUN tended to be as low as 16.37 mg/dL in the treatment group compared to 18.09 mg/dL in the control group. BUN has been used as a useful indicator of protein metabolism. The higher BUN was observed on control trial compared with the GA03 group, which may be due to the protein degradation at higher rates or deamination because of higher protein intake and probably a more functional rumen [33]. Overall results indicated that GA03 supplementation can be a good probiotic in lactating dairy cows.
CONCLUSION
Addition of isolated acetogens increased acetate, propionate and total VFA productions. Proteiniphilum acetatigenes GA03 strain had the lowest CH4 production than the other isolated acetogenic bacteria. The in vitro results indicated that P. acetatigenes GA03 strain can be used as a direct fed microbials in order to hinder methanogenesis. This in vivo study indicates that the P. acetatigenes GA03 strain is able to prevent the reduction of milk yield and milk fat during summer seasons by maintaining rumen fermentation in lactating dairy cows. This would be possible to reduce gross energy loss by methane.
Acknowledgements
Not applicable.
Competing interests
No potential conflict of interest relevant to this article was reported.
Funding sources
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2018R1A6A3A01012191), Korea.
Availability of data and material
Upon reasonable request, the datasets of this study can be available from the corresponding author.
Authors’ contributions
Conceptualization: Kim SH, Lee SS.
Data curation: Kim SH.
Formal analysis: Kim SH, Mamuad LL, Islam M.
Methodology: Kim SH, Lee SS.
Software: Kim SH, Mamuad LL, Islam M.
Validation: Kim SH, Lee SS.
Investigation: Kim SH, Lee SS.
Writing - original draft: Kim SH, Mamuad LL, Islam M, Lee SS.
Writing - review & editing: Kim SH, Mamuad LL, Islam M, Lee SS.
Ethics approval and consent to participate
The animals were treated according to the recommendations described in “The Guide for the Care and Use of Laboratory Animals,” published by the Institutional Animal Care and Use Committee (IACUC) of National Institute of Animal Science (2012-C-037) in Korea.
REFERENCES
- 1.Beauchemin KA, McGinn SM. Methane emissions from feedlot cattle fed barley or corn diets. J Anim Sci. 2005;83:653–61. doi: 10.2527/2005.833653x. [DOI] [PubMed] [Google Scholar]
- 2.Johnson KA, Johnson DE. Methane emissions from cattle. J Anim Sci. 1995;73:2483–92. doi: 10.2527/1995.7382483x. [DOI] [PubMed] [Google Scholar]
- 3.Lee HJ, Lee SC, Kim JD, Oh YG, Kim BK, Kim CW, et al. Methane production potential of feed ingredients as measured by in vitro gas test. Asian-Austalas J Anim Sci. 2003;16:1143–50. doi: 10.5713/ajas.2003.1143. [DOI] [Google Scholar]
- 4.Leaphart AB, Friez MJ, Lovell CR. Formyltetrahydrofolate synthetase sequences from salt marsh plant roots reveal a diversity of acetogenic bacteria and other bacterial functional groups. Appl Environ Microbiol. 2003;69:693–6. doi: 10.1128/AEM.69.1.693-696.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sawal RK, Kurar CK. Milk yield and its fat content as affected by dietary factors: a rewiew. Asian-Austalas J Anim Sci. 1998;11:217–33. doi: 10.5713/ajas.1998.217. [DOI] [Google Scholar]
- 6.Yasuda K, Hashikawa S, Sakamoto H, Tomita Y, Shibata S, Fukata T. A new synbiotic consisting of Lactobacillus casei subsp. casei and dextran improves milk production in Holstein dairy cows. J Vet Med Sci. 2007;69:205–8. doi: 10.1292/jvms.69.205. [DOI] [PubMed] [Google Scholar]
- 7.Le Van TD, Robinson JA, Ralph J, Greening RC, Smolenski WJ, Leedle JA, Schaefer DM. Assessment of reductive acetogenesis with indigenous ruminal bacterium populations and Acetitomaculum ruminis. Appl Environ Microbiol. 1998;64:3429–36. doi: 10.1128/AEM.64.9.3429-3436.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lane D. 16S/23S rRNA Sequencing. New York, NY: John Wiley and Sons; 1991. [Google Scholar]
- 9.Leaphart AB, Lovell CR. Recovery and analysis of formyltetrahydrofolate synthetase gene sequences from natural populations of acetogenic bacteria. Appl Environ Microbiol. 2001;67:1392–5. doi: 10.1128/AEM.67.3.1392-1395.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980;16:111–20. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- 11.Kenters N, Henderson G, Jeyanathan J, Kittelmann S, Janssen PH. Isolation of previously uncultured rumen bacteria by dilution to extinction using a new liquid culture medium. J Microbiol Methods. 2011;84:52–60. doi: 10.1016/j.mimet.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 12.Russell JB, Van Soest PJ. In vitro ruminal fermentation of organic acids common in forage. Appl Environ Microbiol. 1984;47:155–9. doi: 10.1128/AEM.47.1.155-159.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaney AL, Marbach EP. Modified reagents for determination of urea and ammonia. Clin Chem. 1962;8:130–2. doi: 10.1093/clinchem/8.2.130. [DOI] [PubMed] [Google Scholar]
- 14.Orskov ER, McDonald I. The estimation of protein degradability in the rumen from incubation measurements weighted according to rate of passage. J Agric Sci. 1979;92:499–503. doi: 10.1017/S0021859600063048. [DOI] [Google Scholar]
- 15.Tabaru H, Kadota E, Yamada H, Sasaki N, Takeuchi A. Determination of volatile fatty acids and lactic acid in bovine plasma and ruminal fluid by high performance liquid chromatography. Jpn J Vet Sci. 1988;50:1124–6. doi: 10.1292/jvms1939.50.1124. [DOI] [PubMed] [Google Scholar]
- 16.AOAC . Official methods of analysis of the Association of Official Analytical Chemists. Gaithersburg, MD: AOAC International; 2005. [Google Scholar]
- 17.Van Soest PJ, Robertson JB, Lewis BA. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci. 1991;74:3583–97. doi: 10.3168/jds.S0022-0302(91)78551-2. [DOI] [PubMed] [Google Scholar]
- 18.SAS . SAS/STAT: statistical analysis systems for Windows. Release 9.1. Cary, NC: SAS Institute Inc.; 2004. [Google Scholar]
- 19.Getachew G, DePeters EJ, Robinson PH. In vitro gas production provides effective method for assessing ruminant feeds. Calif Agr. 2004;58:54–8. doi: 10.3733/ca.v058n01p54. [DOI] [Google Scholar]
- 20.Pacaud S, Loubiere P, Goma G. Methanol metabolism by Eubacterium limosum B2: Effects of pH and carbon dioxide on growth and organic acid production. Curr Microbiol. 1985;12:245–50. doi: 10.1007/BF01567972. [DOI] [Google Scholar]
- 21.Mathrani IM, Boone DR. Isolation and characterization of a moderately halophilic methanogen from a solar saltern. Appl Environ Microbiol. 1985;50:140–3. doi: 10.1128/AEM.50.1.140-143.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang CL, Guan LL, Liu JX, Wang JK. Rumen fermentation and acetogen population changes in response to an exogenous acetogen TWA4 strain and Saccharomyces cerevisiae fermentation product. J Zhejiang Univ Sci B. 2015;16:709–19. doi: 10.1631/jzus.B1500013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lopez S, McIntosh FM, Wallace RJ, Newbold CJ. Effect of adding acetogenic bacteria on methane production by mixed rumen microorganisms. Anim Feed Sci Technol. 1999;78:1–9. doi: 10.1016/S0377-8401(98)00273-9. [DOI] [Google Scholar]
- 24.Qin WZ, Li CY, Kim JK, Ju JG, Song MK. Effects of defaunation on fermentation characteristics and methane production by rumen microbes in vitro when incubated with starchy feed sources. Asian-Austalas J Anim Sci. 2012;25:1381–8. doi: 10.5713/ajas.2012.12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitchell JB, Russo A. Thiols, thiol depletion, and thermosensitivity. Radiat Res. 1983;95:471–85. doi: 10.2307/3576094. [DOI] [PubMed] [Google Scholar]
- 26.Christie WW. The effects of diet and other factors on the lipid composition of ruminant tissues and milk. Prog Lipid Res. 1979;17:245–277. doi: 10.1016/0079-6832(79)90009-0. [DOI] [PubMed] [Google Scholar]
- 27.Fox PF, McSweeney PLH. Dairy chemistry and biochemistry. New York, NY: Kluwer Academic Publisher; 1998. [Google Scholar]
- 28.Rodriguez-Zas SL, Gianola D, Shook GE. Evaluation of models for somatic cell score lactation patterns in Holsteins. Livest Prod Sci. 2000;67:19–30. doi: 10.1016/S0301-6226(00)00193-7. [DOI] [Google Scholar]
- 29.Dohoo IR, Meek AH. Somatic cell counts in bovine milk. Can Vet J. 1982;23:119–25. [PMC free article] [PubMed] [Google Scholar]
- 30.Elvinger F, Hansen PJ, Natzke RP. Modulation of function of bovine polymorphonuclear leukocytes and lymphocytes by high temperature in vitro and in vivo. Am J Vet Res. 1991;52:1692–8. [PubMed] [Google Scholar]
- 31.Blowey RW, Wood DW, Davis JR. A nutritional monitoring system for dairy herds based on blood glucose, urea and albumin levels. Vet Rec. 1973;92:691–6. doi: 10.1136/vr.92.26.691. [DOI] [PubMed] [Google Scholar]
- 32.Bobe G, Young JW, Beitz DC. Pathology, etiology, prevention, and treatment of fatty liver in dairy cows. J Dairy Sci. 2004;87:3105–24. doi: 10.3168/jds.S0022-0302(04)73446-3. [DOI] [PubMed] [Google Scholar]
- 33.Hadorn U, Hammon H, Bruckmaier RM, Blum JW. Delaying colostrum intake by one day has important effects on metabolic traits and on gastrointestinal and metabolic hormones in neonatal calves. J Nutr. 1997;127:2011–23. doi: 10.1093/jn/127.10.2011. [DOI] [PubMed] [Google Scholar]