Abstract
Background
On high-resolution computed tomography (HRCT), pulmonary artery (PA) dimensions may hint at the presence of pulmonary hypertension. We aimed to determine how accurately various measures of the PA, as viewed on HRCT, predict right heart catheterisation (RHC)-confirmed pulmonary hypertension.
Methods
We retrospectively reviewed patients who had HRCT and RHC between 2010 and 2018. Analyses considered respiratory cycle, pulmonary hypertension diagnostic criteria, time between HRCT and RHC, and subgroup analysis in interstitial lung disease (ILD) and chronic obstructive pulmonary disease (COPD).
Results
Of 620 patients, 375 had pulmonary hypertension. For pulmonary hypertension (defined as mean PA pressure (mPAP) ≥25 mmHg) and from HRCT performed within 60 days of RHC, main PA diameter (MPAD) ≥29 mm had a sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of 88%, 42%, 0.70 and 0.70, respectively, while ratio of the diameter of the PA to the diameter of the ascending aorta (PA:Ao) ≥1.0 showed 53%, 85%, 0.84 and 0.54, respectively. In general, results were similar when the interval between HRCT and RHC varied from 7 to 60 days and when measured on expiratory images. In ILD, the sensitivity of MPAD was higher; in COPD, the specificity of PA:Ao was higher. There was moderately positive correlation between mPAP and inspiratory MPAD, PA:Ao, right PA diameter (RPAD), left PA diameter (LPAD) and (RPAD+LPAD)/2 (r=0.48, 0.51, 0.34, 0.34 and 0.36, respectively), whereas there was weak negative correlation between mPAP and PA angle (r= −0.24).
Conclusions
Findings on HRCT may assist in the diagnosis of RHC-confirmed pulmonary hypertension. MPAD ≥29 mm had high sensitivity and PA:Ao ≥1.0 had high specificity. Compared with the entire cohort, MPAD had greater sensitivity in ILD and PA:Ao had higher specificity in COPD.
Short abstract
Findings on HRCT may assist in the diagnosis of RHC-confirmed pulmonary hypertension. MPAD ≥29 mm has high sensitivity, whereas PA:Ao ≥1.0 has high specificity. MPAD has greater sensitivity in ILD and PA:Ao has higher specificity in COPD. http://bit.ly/2EcrEUY
Introduction
Pulmonary hypertension is a potentially debilitating condition that may be idiopathic or develop as a consequence of any number of underlying diseases, including those that primarily affect the lung, heart or liver. Pulmonary hypertension may also be a manifestation of systemic autoimmune disease or recurrent thromboembolism. Despite the development of a growing number of targeted therapies, pulmonary hypertension-related morbidity and mortality remain high [1, 2]. As in other progressive, life-shortening but potentially treatable conditions, in pulmonary hypertension, early detection and implementation of appropriate treatment is crucial to maintaining or improving patients’ quality of life and prolonging survival.
Although right heart catheterisation (RHC) remains the gold standard investigation to confirm pulmonary hypertension, it is invasive and unavailable in many places around the world [3]. Several clinical variables, including those derived from the clinical history, physical examination or pulmonary function tests, hint at the presence of pulmonary hypertension. Measurements taken on chest computed tomography (CT) scan images, including the absolute main pulmonary artery (PA) diameter (MPAD) (e.g. >29 mm) or its diameter relative to the ascending aorta (Ao) (PA:Ao ratio) (e.g. ≥1.0), do the same [4–12]. Results from a meta-analysis revealed that MPAD measured on chest CT scan was 79% sensitive and 83% specific for pulmonary hypertension diagnosed by RHC. Results were similar for PA:Ao, which was 74% sensitive and 81% specific [5]. In the overwhelming majority of included studies, MPAD was measured in inspiration on standard chest CT scans or CT angiography (CTA).
High-resolution CT (HRCT) protocols often (and at our institution, always) include expiratory images. The diameter of the PA (a capacitance vessel) likely changes in response to alterations in intrathoracic pressure and volume, as occurs in a normal respiratory cycle. We hypothesised that, on HRCT, PA dimensions measured in expiration would be more specific for RHC-confirmed pulmonary hypertension than dimensions taken in inspiration. Our goal was to build on and extend previously published results by assessing the performance of various metrics of the PA measured (in inspiration or expiration) on HRCT for identifying RHC-confirmed pulmonary hypertension in a cohort of patients with various cardiopulmonary conditions. We also hypothesised that we could identify specific cut-off values for these metrics for predicting RHC-confirmed pulmonary hypertension in patients with interstitial lung disease (ILD) or chronic obstructive pulmonary disease (COPD).
Methods
Subjects
We included adult (age ≥18 years) patients who had HRCT and RHC within 60 days of each other at National Jewish Health (Denver, CO, USA) between 2010 and 2018. We excluded patients who had abnormal anatomy of great vessels in the mediastinum (e.g. thoracic aortic aneurysm, PA aneurysm), mediastinal tumour, known intracardiac shunt, or those who had undergone heart, lung or heart–lung transplant. The study was approved by the National Jewish Health Institutional Review Board (HS#3162).
Radiographic evaluation
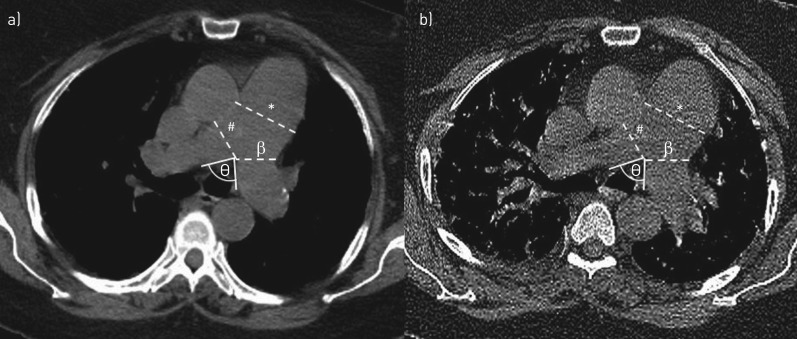
All patients underwent volumetric CT imaging on a multidetector row helical CT scanner (Siemens Definition AS+ or Siemens Sensation 64; Siemens, Forchheim, Germany) at full-inspiration and end-expiration. CT scans were reconstructed with a slice thickness of 0.75 mm. Scans were acquired at 50–200 mAs and 120 kV peak. Vessel dimensions were measured using mediastinal windows. A radiologist (J.C.R.) and pulmonologist (P.R.) decided on, reviewed and practised the protocol for measurements: MPAD was measured at the widest portion of the main PA perpendicular to the wall abutting the Ao. The Ao was also measured at this level to establish PA:Ao. Left and right PA diameters (LPAD and RPAD, respectively) were measured at their widest points after the bifurcation. The angle between the main PA at the bifurcation (PA angle) was also measured (figure 1). We used various cut-offs, including the conventional values MPAD ≥29 mm and PA:Ao ≥1.0, to assess diagnostic accuracy of HRCT measures for RHC-confirmed pulmonary hypertension [4, 7, 13]. All HRCT scans were reviewed by a pulmonologist (P.R.) blinded to the presence of pulmonary hypertension and results of the RHC. To assess the validity of measurements made by the pulmonologist, 50 randomly selected scans were also reviewed by a chest radiologist (A.O.) who was also blinded to the presence of pulmonary hypertension and results of the RHC.
FIGURE 1.
Pulmonary artery (PA) measurements from a) inspiratory and b) expiratory high-resolution computed tomography scan images. *: main PA diameter; #: right PA diameter; β: left PA diameter; θ: PA angle.
RHC haemodynamics
All pressure measurements were performed at end-expiration while patients were in the supine position and breathing spontaneously. We defined pulmonary hypertension as mean PA pressure (mPAP) ≥25 mmHg [14]. In certain analyses, we used the newly proposed criterion (mPAP ≥20 mmHg) from the 6th World Symposia on Pulmonary Hypertension [3].
Statistical analysis
Because data were not normally distributed, we report median values (interquartile range) for continuous variables. Differences between groups were evaluated using Chi-squared or Wilcoxon rank sum tests as appropriate. We used intraclass correlation and Bland–Altman analyses to assess inter-rater reliability and agreement between measurements made by the radiologist and pulmonologist. To assess the diagnostic performance of HRCT-derived measures for RHC-derived measures, we generated 2×2 contingency tables and calculated the sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV). We conducted separate analyses for subjects who had HRCT and RHC within 7, 14, 30 or 60 days of each other. Spearman correlation was used to examine associations between HRCT- and RHC-derived measures. We considered p<0.05 to represent statistical significance. All analyses were conducted using Stata version 11 (StataCorp, College Station, TX, USA).
Results
Study populations
We included 620 scans from 620 patients; 375 (60.48%) had RHC-confirmed pulmonary hypertension, with mPAP ≥25 mmHg. Demographic and clinical characteristics are presented in table 1. Most patients were non-Hispanic Whites and over half had heart disease; one-fifth had COPD and over one-third had ILD. Compared with the non-pulmonary hypertension group, the group with pulmonary hypertension had greater MPAD, RPAD, LPAD and PA:Ao in both respiratory cycles, whereas the PA angle was greater in the non-pulmonary hypertension group. In the subgroup with pulmonary hypertension, the median MPAD was 34.60 mm in inspiration and 34.65 mm in expiration, while in the non-pulmonary hypertension group it was 30.00 mm in inspiration and 30.50 mm in expiration.
TABLE 1.
Demographic and clinical characteristics of the cohort
| Total | Pulmonary hypertension group# | Non-pulmonary hypertension group | p-value | |
| Subjects | 620 | 375 | 245 | |
| Age years | 70.01±15.15 | 71.09±15.67 | 70.96±14.54 | 0.779 |
| Male | 302 (48.71) | 179 (47.73) | 123 (50.20) | 0.547 |
| Height cm | 168.00±14.50 | 167.10±14.20 | 169.00±14.99 | 0.184 |
| Weight kg | 83.00±30.71 | 84.37±30.46 | 78.47±29.99 | 0.002 |
| BMI kg·m−2 | 29.31±9.42 | 30.03±9.97 | 28.20±8.46 | <0.001 |
| Race/ethnicity | ||||
| Non-Hispanic White | 458 (73.87) | 284 (75.73) | 174 (71.02) | 0.010 |
| Non-Hispanic Black | 20 (3.23) | 14 (3.73) | 6 (2.45) | |
| Hispanic | 26 (4.19) | 20 (5.33) | 6 (2.45) | |
| Other | 6 (0.97) | 1 (0.27) | 5 (2.04) | |
| Unknown | 110 (17.74) | 56 (14.93) | 54 (22.04) | |
| Smoking history | ||||
| Nonsmoker | 261 (42.10) | 150 (40.00) | 11 (45.31) | 0.159 |
| Current smoker | 15 (2.42) | 12 (3.20) | 3 (1.22) | |
| Ex-smoker | 344 (55.48) | 213 (56.80) | 131 (53.47) | |
| Underlying disease | ||||
| COPD | 136 (21.94) | 93 (24.80) | 43 (17.55) | 0.033 |
| ILD | 235 (37.90) | 137 (36.53) | 98 (40.00) | 0.384 |
| Embolism | 42 (6.77) | 23 (6.13) | 19 (7.76) | 0.432 |
| Heart disease | 353 (56.94) | 229 (61.07) | 124 (50.61) | 0.010 |
| Haemodynamics | ||||
| mPAP mmHg | 27±13 | 33±12 | 20±5 | <0.001 |
| CO (TD) L·min−1 | 4.69±1.93 | 4.60±2.06 | 4.80±1.80 | 0.065 |
| CO (Fick) L·min−1 | 4.60±1.75 | 4.59±1.81 | 4.65±1.63 | 0.458 |
| PVR (TD) WU | 3.02±2.69 | 4.09±3.28 | 2.11±1.11 | <0.001 |
| PVR (Fick) WU | 3.10±2.64 | 4.00±3.26 | 2.18±1.31 | <0.001 |
| RAP mmHg | 7±5 | 9±6 | 5±4 | <0.001 |
| PCWP mmHg | 12±6 | 14±7 | 10±5 | <0.001 |
| Inspiratory HRCT median (IQR) | ||||
| MPAD mm | 32.75 (7.35) | 34.60 (7.10) | 30.00 (6.50) | <0.001 |
| PA:Ao | 0.95 (0.22) | 1.01 (0.23) | 0.87 (0.16) | <0.001 |
| RPAD mm | 25.95 (5.35) | 27.00 (4.90) | 24.30 (5.10) | <0.001 |
| LPAD mm | 25.20 (4.80) | 26.15 (4.50) | 24.10 (4.70) | <0.001 |
| PA angle deg | 84.45 (29.57) | 80.17 (28.83) | 90.67 (30.62) | <0.001 |
| Expiratory HRCT median (IQR) | ||||
| MPAD mm | 32.80 (7.20) | 34.65 (7.05) | 30.50 (6.00) | <0.001 |
| PA:Ao | 0.94 (0.21) | 0.99 (0.22) | 0.86 (0.16) | <0.001 |
| RPAD mm | 25.50 (5.35) | 26.60 (5.00) | 23.80 (4.70) | <0.001 |
| LPAD mm | 25.60 (4.90) | 26.10 (4.90) | 24.20 (4.70) | <0.001 |
| PA angle deg | 94.44 (23.02) | 92.99 (22.46) | 98.56 (25.96) | 0.001 |
Data are presented as n, mean±sd or n (%), unless otherwise stated. BMI: body mass index; COPD: chronic obstructive pulmonary disease; ILD: interstitial lung disease; PA: pulmonary artery; mPAP: mean PA pressure; CO: cardiac output; TD: thermodilution technique; PVR: pulmonary vascular resistance; WU: Wood Units; RAP: right atrial pressure; PCWP: pulmonary capillary wedge pressure; HRCT: high-resolution computed tomography; IQR: interquartile range; MPAD: main PA diameter; PA:Ao: PA to ascending aorta ratio; RPAD: right PA diameter; LPAD: left PA diameter. #: mPAP ≥25 mmHg on right heart catheterisation.
Inter-rater reliability
For the 50 scans assessed by both the radiologist and pulmonologist, intraclass correlation coefficients (ICCs) suggested very strong correlation between ratings for most variables, including for the MPAD in which the ICC was 0.95 (supplementary table S1). The Bland–Altman analysis showed a mean inter-rater difference of 0.044 (95% CI −0.476–0.564) mm for MPAD and 0.016 (95% CI 0.010–0.042) for PA:Ao (supplementary figures S1 and S2).
Diagnostic performance of HRCT measures
Table 2 shows the diagnostic performance of HRCT scan parameters for pulmonary hypertension defined as mPAP ≥25 mmHg. For MPAD ≥29 mm measured in inspiration, sensitivity was 88% and specificity was 42%, whereas for PA:Ao ≥1.0 measured in inspiration, sensitivity was 53% and specificity was 84%. When we considered the combination of MPAD ≥29 mm and PA:Ao ≥1.0, specificity was slightly higher (87%), but sensitivity was lower (51%). If either criterion were met, sensitivity improved (90%) but specificity decreased (40%). In general, results were similar when we considered data from subjects whose HRCT and RHC were performed within 60, 30, 14 or 7 days and when PA dimensions were measured on expiratory images. In general, diagnostic performance of HRCT parameters was similar when we defined pulmonary hypertension as mPAP ≥20 mmHg (supplementary table S2).
TABLE 2.
Diagnostic performance of various high-resolution computed tomography (HRCT)-derived measures for pulmonary hypertension defined as mean pulmonary artery (PA) pressure ≥25 mmHg on right heart catheterisation (RHC)
| Interval days# | Subjects n | Sensitivity % | Specificity % | PPV | NPV | |
| Inspiration | ||||||
| MPAD ≥29 mm | 7 | 214 | 86.82 | 45.88 | 0.71 | 0.70 |
| 14 | 295 | 86.34 | 43.75 | 0.71 | 0.66 | |
| 30 | 422 | 87.60 | 43.29 | 0.71 | 0.69 | |
| 60 | 620 | 88.00 | 42.04 | 0.70 | 0.70 | |
| PA:Ao ≥1.0 | 7 | 214 | 48.06 | 85.88 | 0.84 | 0.52 |
| 14 | 295 | 49.18 | 84.82 | 0.84 | 0.51 | |
| 30 | 422 | 52.33 | 82.93 | 0.83 | 0.53 | |
| 60 | 620 | 52.80 | 84.49 | 0.84 | 0.54 | |
| MPAD and PA:Ao | 7 | 214 | 47.29 | 88.24 | 0.86 | 0.52 |
| 14 | 295 | 48.09 | 87.50 | 0.86 | 0.51 | |
| 30 | 422 | 51.16 | 85.98 | 0.85 | 0.53 | |
| 60 | 620 | 51.20 | 87.35 | 0.86 | 0.54 | |
| MPAD or PA:Ao | 7 | 214 | 87.60 | 43.54 | 0.70 | 0.70 |
| 14 | 295 | 87.43 | 41.07 | 0.71 | 0.67 | |
| 30 | 422 | 88.76 | 40.24 | 0.70 | 0.69 | |
| 60 | 620 | 89.60 | 39.18 | 0.69 | 0.71 | |
| Expiration | ||||||
| MPAD ≥29 mm | 7 | 207 | 88.00 | 39.02 | 0.69 | 0.68 |
| 14 | 286 | 88.70 | 35.78 | 0.69 | 0.66 | |
| 30 | 410 | 87.70 | 39.24 | 0.70 | 0.67 | |
| 60 | 600 | 88.59 | 36.21 | 0.69 | 0.67 | |
| PA:Ao ≥1.0 | 7 | 207 | 41.60 | 79.27 | 0.75 | 0.47 |
| 14 | 286 | 42.94 | 79.82 | 0.78 | 0.46 | |
| 30 | 410 | 46.83 | 81.65 | 0.80 | 0.49 | |
| 60 | 600 | 48.37 | 80.60 | 0.80 | 0.50 | |
| MPAD and PA:Ao | 7 | 207 | 41.60 | 80.49 | 0.76 | 0.47 |
| 14 | 286 | 42.94 | 80.73 | 0.78 | 0.47 | |
| 30 | 410 | 46.03 | 82.28 | 0.81 | 0.49 | |
| 60 | 600 | 47.55 | 81.47 | 0.80 | 0.49 | |
| MPAD or PA:Ao | 7 | 207 | 88.00 | 37.80 | 0.68 | 0.67 |
| 14 | 286 | 88.70 | 34.86 | 0.69 | 0.66 | |
| 30 | 410 | 88.49 | 38.61 | 0.70 | 0.68 | |
| 60 | 600 | 89.40 | 35.34 | 0.69 | 0.68 |
PPV: positive predictive value; NPV: negative predictive value; MPAD: main PA diameter; PA:Ao: PA to ascending aorta ratio. #: time between HRCT and RHC.
ILD and COPD subgroups
Among patients with COPD, the inspiratory MPAD ≥29 mm criterion had a sensitivity and specificity of 88% and 42%, respectively, while for patients with ILD, sensitivity and specificity were 91% and 38%, respectively (table 3). For the inspiratory PA:Ao ≥1.0 criterion, sensitivity and specificity were 51% and 88%, respectively, for the COPD subgroup and 52% and 81%, respectively, for the ILD subgroup. Among patients with ILD, 74 (31.5%) had connective tissue-related ILD, including 33 (44.6%) with systemic sclerosis, 21 (28.4%) with rheumatoid arthritis, seven (9.5%) with dermatomyositis/polymyositis, five (6.8%) with Sjögren's syndrome and five (6.8%) with undifferentiated connective tissue disease. The diagnostic performance of HRCT in these connective tissue-related ILD subgroups was generally similar to the entire ILD group.
TABLE 3.
Diagnostic performance of various high-resolution computed tomography-derived measures for pulmonary hypertension defined as mean pulmonary artery (PA) pressure ≥25 mmHg on right heart catheterisation for subgroups with chronic obstructive pulmonary disease (COPD) or interstitial lung disease (ILD)
| Subjects n | Sensitivity % | Specificity % | PPV | NPV | |
| COPD | |||||
| Inspiration | |||||
| MPAD ≥29 mm | 136 | 88.17 | 41.86 | 0.77 | 0.62 |
| PA:Ao ≥1.0 | 136 | 50.54 | 88.37 | 0.90 | 0.45 |
| MPAD and PA:Ao | 136 | 50.54 | 88.37 | 0.90 | 0.45 |
| MPAD or PA:Ao | 136 | 88.17 | 41.86 | 0.77 | 0.62 |
| Expiration | |||||
| MPAD ≥29 mm | 131 | 90.11 | 37.50 | 0.77 | 0.63 |
| PA:Ao ≥1.0 | 131 | 50.55 | 85.00 | 0.88 | 0.43 |
| MPAD and PA:Ao | 131 | 49.45 | 85.00 | 0.88 | 0.43 |
| MPAD or PA:Ao | 131 | 91.21 | 37.50 | 0.77 | 0.65 |
| ILD | |||||
| Inspiration | |||||
| MPAD ≥29 mm | 235 | 91.24 | 37.76 | 0.67 | 0.76 |
| PA:Ao ≥1.0 | 235 | 52.55 | 80.61 | 0.79 | 0.55 |
| MPAD and PA:Ao | 235 | 50.36 | 84.69 | 0.82 | 0.55 |
| MPAD or PA:Ao | 235 | 93.43 | 33.67 | 0.66 | 0.79 |
| Expiration | |||||
| MPAD ≥29 mm | 231 | 91.97 | 32.98 | 0.67 | 0.74 |
| PA:Ao ≥1.0 | 231 | 45.99 | 78.72 | 0.76 | 0.50 |
| MPAD and PA:Ao | 231 | 45.99 | 79.79 | 0.77 | 0.50 |
| MPAD or PA:Ao | 231 | 91.97 | 31.91 | 0.66 | 0.73 |
PPV: positive predictive value; NPV: negative predictive value; MPAD: main PA diameter; PA:Ao: PA to ascending aorta ratio.
Correlation
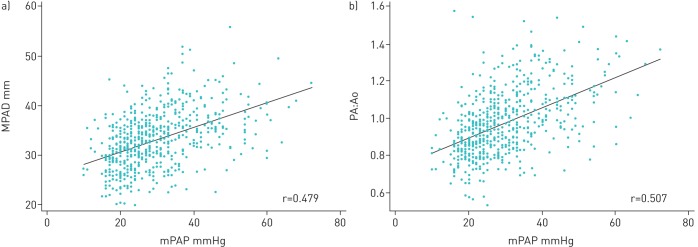
There were weak positive correlations between RHC-measured mPAP and inspiratory MPAD, RPAD, LPAD and (RPAD+LPAD)/2 (table 4 and figure 2). There was moderate positive correlation between mPAP and PA:Ao. There was weak negative correlation between mPAP and the PA angle. In general, compared with inspiration, correlations were not as strong between mPAP and HRCT measurements taken in expiration. Compared with the ILD subgroup, in the subgroup with COPD, correlations were generally stronger between HRCT measures and mPAP.
TABLE 4.
Spearman correlation coefficients showing the relationship between various high-resolution computed tomography-derived measures for pulmonary hypertension defined as mean pulmonary artery (PA) pressure ≥25 mmHg on right heart catheterisation
| Inspiration | Expiration | |||||
| All | ILD | COPD | All | ILD | COPD | |
| MPAD | 0.479 | 0.456 | 0.574 | 0.444 | 0.415 | 0.534 |
| RPAD | 0.335 | 0.295 | 0.462 | 0.327 | 0.327 | 0.327 |
| LPAD | 0.339 | 0.323 | 0.405 | 0.273 | 0.254 | 0.183ns |
| (RPAD+LPAD)/2 | 0.360 | 0.329 | 0.463 | 0.297 | 0.269 | 0.265 |
| PA:Ao | 0.507 | 0.489 | 0.579 | 0.488 | 0.461 | 0.564 |
| PA angle | −0.241 | −0.212 | −0.246 | −0.241 | −0.327 | −0.316 |
ILD: interstitial lung disease; COPD: chronic obstructive pulmonary disease; MPAD: main PA diameter; RPAD: right PA diameter; LPAD: left PA diameter; PA:Ao: PA to ascending aorta ratio. All values statistically significant unless indicated; ns: nonsignificant.
FIGURE 2.
Scatterplot and regression lines for the relationship between a) inspiratory main pulmonary artery (PA) diameter (MPAD) and mean PA pressure (mPAP) from right heart catheterisation and b) inspiratory ratio of the diameter of the PA to the diameter of the ascending aorta (PA:Ao) as measured on high-resolution computed tomography scan and mPAP.
Cut-offs and receiver operating characteristic curves
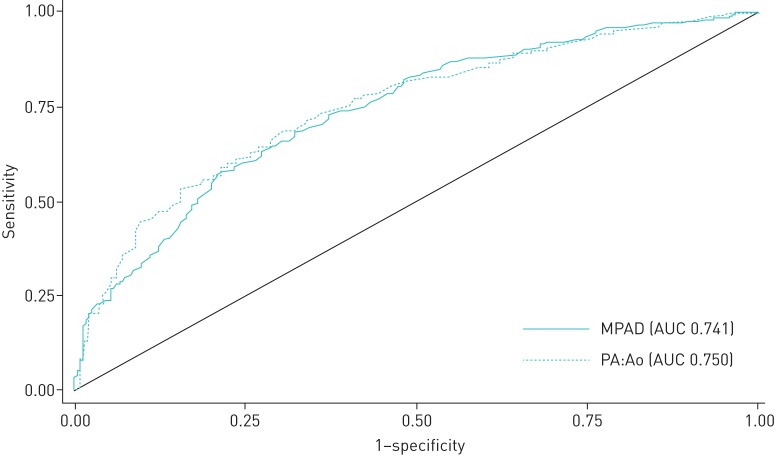
For the cohort as a whole, the areas under the receiver operating characteristic curves (AUCs) for inspiratory MPAD and inspiratory PA:Ao (for RHC-confirmed pulmonary hypertension defined as mPAP ≥25 mmHg) were 0.741 and 0.750, respectively (figure 3). Sensitivity, specificity and AUC at different cut-offs values for MPAD and PA:Ao are shown in supplementary table S3. For the cohort as a whole, the cut-offs MPAD ≥32.5 mm and PA:Ao ≥0.94 yielded the most favourable diagnostic profiles. Sensitivity, specificity, AUC and balance accuracy at different cut-offs values for MPAD and PA:Ao in ILD and COPD subgroups are also shown in supplementary table S4 and S5. For pulmonary hypertension (defined as mPAP ≥25 mmHg), the cut-offs MPAD ≥32.5 mm and PA:Ao ≥0.92 yielded the most favourable diagnostic profiles in the ILD subgroup, while the cut-offs MPAD ≥32.5 mm and PA:Ao ≥0.90 yielded the most favourable diagnostic profiles in COPD subgroup.
FIGURE 3.
Receiver operating characteristic (ROC) curves showing the sensitivity and specificity of main pulmonary artery (PA) diameter (MPAD) and ratio of the diameter of the PA to the diameter of the ascending aorta (PA:Ao) as predictors of pulmonary hypertension when mean PA pressure ≥25 mmHg. AUC: area under the ROC curve.
Discussion
We evaluated over 600 HRCT scans from patients with various cardiopulmonary conditions who had RHC within 60 days of the scan and derived diagnostic performance estimates for various HRCT measurements of the PA to identify RHC-confirmed pulmonary hypertension. In this, the largest study of HRCT for this purpose, we found MPAD ≥29 mm to be highly sensitive (but not specific) and PA:Ao ≥1.0 to be highly specific (but not sensitive). Our hypothesis that expiration would improve specificity was incorrect: on balance, diagnostic performance of HRCT measurements in exhalation was no better (and frequently worse) than measurements in inhalation. This likely stems from the far fewer expiratory than inspiratory images generated for HRCT scans at our institution. With fewer images, we were occasionally unable to take measurements at the exact level we desired. In addition, volume averaging effects created inaccuracies in measurements.
To date, in the vast majority of studies of the performance of CT measures to identify RHC-confirmed pulmonary hypertension, investigators have used either CT pulmonary angiograms or standard chest CT scans, not HRCT. In fact, performance of HRCT measures in identifying RHC-confirmed pulmonary hypertension has been assessed in only a few other published studies. In a meta-analysis of 20 publications, CT-measured MPAD had a mean sensitivity of 79% and a mean specificity of 83% for identifying RHC-confirmed pulmonary hypertension, and PA:Ao had a mean sensitivity of 74% and a mean specificity of 81% [5]. On balance, in each study of HRCT included in the meta-analysis, diagnostic performance of HRCT was not as good as standard CT or CTA, and our results suggest the same [6, 12].
A wide range of cut-off values for identifying RHC-confirmed pulmonary hypertension have been proposed for MPAD (from 25 to 38 mm) and PA:Ao (from 0.84 to 1.4) [4, 6, 7, 9, 10, 15–28]. We elected to use the cut-offs proposed in the European Society of Cardiology/European Respiratory Society pulmonary hypertension guideline for our main analyses [13, 14], but also ran analyses using a range of values, and found that alternative cut-off values performed better. We also conducted analyses for a lower threshold for mPAP (20 mmHg), as that may be adopted as the threshold for pulmonary hypertension in the future [3]. Reassuringly, on balance, results were similar whether we considered scans within 1 week or out to within 2 months of the RHC.
Results for the subgroups with COPD or ILD were similar to those for the cohort as a whole: MPAD was highly sensitive (>90% for the ILD subgroup) but poorly specific and PA:Ao was poorly sensitive but highly specific for each of the two subgroups. For subjects with COPD, cut-offs of MPAD 32.5 mm and PA:Ao 0.90 yielded the most favourable diagnostic profile. For subjects with ILD, cut-offs of MPAD 32.5 mm and PA:Ao 0.92 yielded the most favourable diagnostic profiles. Because HRCT is used most often in patients with ILD, we propose these as cut-offs values for these patients. Of course, our work will require validation. Alhamad et al. [8] found MPAD ≥25 mm had a sensitivity of 86%, a specificity of 41% and yielded the largest AUC (0.65) among 100 subjects with various forms of ILD. Among 34 subjects without ILD, including eight with COPD, they found MPAD ≥31.6 mm had a sensitivity of 47%, a specificity of 93% and yielded the largest AUC (0.73) [8].
Our study has limitations. Being a retrospective study, there could be significant bias in results, as HRCT scans and RHC were performed for clinical purposes and at the discretion of treating physicians, not the investigators. However, the reasons these investigations were performed in our subjects are likely the same as for patients in the general population, suggesting the results can likely be extrapolated to the general population of patients who get HRCT. Only 50 scans were reviewed by two investigators; however, inter-rater correlation was very high and agreement was very strong. Measurements taken in expiration were imprecise because of volumetric averaging and the fewer number of slices obtained limited optimal slice selection for measurements. Despite these limitations, we believe our study has merit and builds another layer on the foundation of previously published data.
Conclusions
Measurements of the PA taken on HRCT scan may suggest the presence or absence of pulmonary hypertension; these measures may be highly sensitive (MPAD) or specific (PA:Ao), but not both. In ILD, the sensitivity of MPAD was higher, while in COPD, the specificity of PA:Ao was higher. Among patients in whom HRCT is performed, inspiratory measures for MPAD and PA:Ao may raise or lower the level of concern for pulmonary hypertension. A MPAD ≥32.5 mm in a patient with ILD or COPD has high sensitivity for RHC-confirmed pulmonary hypertension.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00232-2019.SUPPLEMENT (490.4KB, pdf)
Footnotes
This article has supplementary material available from openres.ersjournals.com.
Author contributions: J.J. Swigris initiated the study concept. P. Ratanawatkul, J.C. Richards and J.J. Swigris designed the study. The data were collected by P. Ratanawatkul, A. Oh and J.C. Richards. The data were analysed by P. Ratanawatkul and J.J. Swigris. The study results were interpreted by P. Ratanawatkul, A. Oh, J.C. Richards and J.J. Swigris. The manuscript was written by P. Ratanawatkul, A. Oh and J.J. Swigris. All authors critically proofread and approved the final version of the manuscript.
Support statement: J.J. Swigris was supported in part by generous gifts from the Munn and Clarence V. LaGuardia Foundations.
Conflict of interest: P. Ratanawatkul has nothing to disclose.
Conflict of interest: A. Oh has nothing to disclose.
Conflict of interest: J.C. Richards has nothing to disclose.
Conflict of interest: J.J. Swigris reports grant support for investigator-initiated studies, and nonbranded, disease-state speaker's bureau fees from Boehringer Ingelheim and Genentech for work outside the scope of the submitted work.
References
- 1.Benza RL, Miller DP, Barst RJ, et al. . An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL Registry. Chest 2012; 142: 448–456. doi: 10.1378/chest.11-1460 [DOI] [PubMed] [Google Scholar]
- 2.D'Alonzo GE, Barst RJ, Ayres SM, et al. . Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med 1991; 115: 343–349. doi: 10.7326/0003-4819-115-5-343 [DOI] [PubMed] [Google Scholar]
- 3.Galiè N, McLaughlin VV, Rubin LJ, et al. . An overview of the 6th World Symposium on Pulmonary Hypertension. Eur Respir J 2019; 53: 1802148. doi: 10.1183/13993003.02148-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuriyama K, Gamsu G, Stern R, et al. . CT-determined pulmonary artery diameters in predicting pulmonary hypertension. Invest Radiol 1984; 19: 16–22. doi: 10.1097/00004424-198401000-00005 [DOI] [PubMed] [Google Scholar]
- 5.Shen Y, Wan C, Tian P, et al. . CT-base pulmonary artery measurement in the detection of pulmonary hypertension: a meta-analysis and systematic review. Medicine 2014; 93: e256. doi: 10.1097/MD.0000000000000256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahammedi A, Oshmyansky A, Hassoun PM, et al. . Pulmonary artery measurements in pulmonary hypertension: the role of computed tomography. J Thorac Imaging 2013; 28: 96–103. doi: 10.1097/RTI.0b013e318271c2eb [DOI] [PubMed] [Google Scholar]
- 7.Tan RT, Kuzo R, Goodman LR, et al. . Utility of CT scan evaluation for predicting pulmonary hypertension in patients with parenchymal lung disease. Medical College of Wisconsin Lung Transplant Group. Chest 1998; 113: 1250–1256. doi: 10.1378/chest.113.5.1250 [DOI] [PubMed] [Google Scholar]
- 8.Alhamad EH, Al-Boukai AA, Al-Kassimi FA, et al. . Prediction of pulmonary hypertension in patients with or without interstitial lung disease: reliability of CT findings. Radiology 2011; 260: 875–883. doi: 10.1148/radiol.11103532 [DOI] [PubMed] [Google Scholar]
- 9.Dornia C, Lange T, Behrens G. Multidetector computed tomography for detection and characterization of pulmonary hypertension in consideration of WHO classification. J Comput Assist Tomogr 2012; 36: 175–180. doi: 10.1097/RCT.0b013e31824afbdf [DOI] [PubMed] [Google Scholar]
- 10.Lange TJ, Dornia C, Stiefel J, et al. . Increased pulmonary artery diameter on chest computed tomography can predict borderline pulmonary hypertension. Pulm Circ 2013; 3: 363–368. doi: 10.4103/2045-8932.113175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corson N, Armato SG, Labby ZE, et al. . CT-based pulmonary artery measurements for the assessment of pulmonary hypertension. Acad Radiol 2014; 21: 523–530. doi: 10.1016/j.acra.2013.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pérez-Enguix D, Morales P, Tomás JM, et al. . Computed tomographic screening of pulmonary arterial hypertension in candidates for lung transplantation. Transplant Proc 2007; 39: 2405–2408. doi: 10.1016/j.transproceed.2007.07.055 [DOI] [PubMed] [Google Scholar]
- 13.Galiè N, Humbert M, Vachiery J-L, et al. . 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J 2015; 46: 903. doi: 10.1183/13993003.01032-2015 [DOI] [PubMed] [Google Scholar]
- 14.Hoeper MM, Bogaard HJ, Condliffe R, et al. . Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol 2013; 62: D42–D50. doi: 10.1016/j.jacc.2013.10.032 [DOI] [PubMed] [Google Scholar]
- 15.Ng C, Wells A, Padley S. A CT sign of chronic pulmonary arterial hypertension: the ratio of main pulmonary artery to aortic diameter. J Thorac Imaging 1999; 14: 270–278. doi: 10.1097/00005382-199910000-00007 [DOI] [PubMed] [Google Scholar]
- 16.Truong QA, Bhatia HS, Szymonifka J, et al. . A four-tier classification system of pulmonary artery metrics on computed tomography for the diagnosis and prognosis of pulmonary hypertension. J Cardiovasc Comput Tomogr 2018; 12: 60–66. doi: 10.1016/j.jcct.2017.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan A, Juarez M, Shelton D. Novel computed tomographic chest metrics to detect pulmonary hypertension. BMC Med Imaging 2011; 11: 7. doi: 10.1186/1471-2342-11-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guthaner DF, Wexler L, Harell G. CT demonstration of cardiac structures. AJR Am J Roentgenol 1979; 133: 75–81. doi: 10.2214/ajr.133.1.75 [DOI] [PubMed] [Google Scholar]
- 19.Kam JC, Pi J, Doraiswamy V, et al. . CT scanning in the evaluation of pulmonary hypertension. Lung 2013; 191: 321–326. doi: 10.1007/s00408-013-9464-6 [DOI] [PubMed] [Google Scholar]
- 20.Shin S, King CS, Brown AW, et al. . Pulmonary artery size as a predictor of pulmonary hypertension and outcomes in patients with chronic obstructive pulmonary disease. Respir Med 2014; 108: 1626–1632. doi: 10.1016/j.rmed.2014.08.009 [DOI] [PubMed] [Google Scholar]
- 21.Burger IA, Husmann L, Herzog BA, et al. . Main pulmonary artery diameter from attenuation correction CT scans in cardiac SPECT accurately predicts pulmonary hypertension. J Nucl Cardiol 2011; 18: 634–641. doi: 10.1007/s12350-011-9413-9 [DOI] [PubMed] [Google Scholar]
- 22.Sanal S, Aronow WS, Ravipati G, et al. . Prediction of moderate or severe pulmonary hypertension by main pulmonary artery diameter and main pulmonary artery diameter/ascending aorta diameter in pulmonary embolism. Cardiol Rev 2006; 14: 213–214. doi: 10.1097/01.crd.0000181619.87084.8b [DOI] [PubMed] [Google Scholar]
- 23.Ussavarungsi K, Whitlock J, Lundy T, et al. . The significance of pulmonary artery size in pulmonary hypertension. Diseases 2014; 2: 243. doi: 10.3390/diseases2030243 [DOI] [Google Scholar]
- 24.Zisman DA, Karlamangla AS, Ross DJ, et al. . High-resolution chest CT findings do not predict the presence of pulmonary hypertension in advanced idiopathic pulmonary fibrosis. Chest 2007; 132: 773–779. doi: 10.1378/chest.07-0116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shin S, King CS, Puri N, et al. . Pulmonary artery size as a predictor of outcomes in idiopathic pulmonary fibrosis. Eur Respir J 2016; 47: 1445–1451. doi: 10.1183/13993003.01532-2015 [DOI] [PubMed] [Google Scholar]
- 26.Iyer AS, Wells JM, Vishin S, et al. . CT scan-measured pulmonary artery to aorta ratio and echocardiography for detecting pulmonary hypertension in severe COPD. Chest 2014; 145: 824–832. doi: 10.1378/chest.13-1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wells JM, Washko GR, Han MK, et al. . Pulmonary arterial enlargement and acute exacerbations of COPD. N Engl J Med 2012; 367: 913–921. doi: 10.1056/NEJMoa1203830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Devaraj A, Wells A, Meister M, et al. . Detection of pulmonary hypertension with multidetector CT and echocardiography alone and in combination. Radiology 2010; 254: 609–616. doi: 10.1148/radiol.09090548 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00232-2019.SUPPLEMENT (490.4KB, pdf)