Abstract
Comorbid Lewy body pathology is very common in Alzheimer’s disease and may confound clinical trial design, yet there is no in vivo test to identify patients with this. Tissue (and/or radioligand imaging) studies have shown cardiac sympathetic denervation in Parkinson’s disease and dementia with Lewy bodies, but this has not been explored in Alzheimer’s subjects with Lewy bodies not meeting dementia with Lewy bodies clinicopathological criteria. To determine if Alzheimer’s disease with Lewy bodies subjects show sympathetic cardiac denervation, we analysed epicardial and myocardial tissue from autopsy-confirmed cases using tyrosine hydroxylase and neurofilament immunostaining. Comparison of tyrosine hydroxylase fibre density in 19 subjects with Alzheimer’s disease/dementia with Lewy bodies, 20 Alzheimer’s disease with Lewy bodies, 12 Alzheimer’s disease subjects without Lewy body disease, 19 Parkinson’s disease, 30 incidental Lewy body disease and 22 cognitively normal without Alzheimer’s disease or Lewy body disease indicated a significant group difference (P < 0.01; Kruskal–Wallis analysis of variance) and subsequent pair-wise Mann–Whitney U tests showed that Parkinson’s disease (P < 0.05) and Alzheimer’s disease/dementia with Lewy bodies (P < 0.01) subjects, but not Alzheimer’s disease with Lewy bodies subjects, had significantly reduced tyrosine hydroxylase fibre density as compared with cognitively normal. Both Parkinson’s disease and Alzheimer’s disease/dementia with Lewy bodies subjects also showed significant epicardial losses of neurofilament protein-immunoreactive nerve fibre densities within the fibre bundles as compared with cognitively normal subjects (P < 0.01) and both groups showed high pathologic alpha-synuclein densities (P < 0.0001). Cardiac alpha-synuclein densities correlated significantly with brain alpha-synuclein (P < 0.001), while cardiac tyrosine hydroxylase and neurofilament immunoreactive nerve fibre densities were negatively correlated with the densities of both brain and cardiac alpha-synuclein, as well as Unified Parkinson’s Disease Rating Scale scores (P < 0.05). The clear separation of Alzheimer’s disease/dementia with Lewy bodies subjects from Alzheimer’s disease and cognitively normal, based on cardiac tyrosine hydroxylase fibre density, is the first report of a statistically significant difference between these groups. Our data do not show significant sympathetic cardiac denervation in Alzheimer’s disease with Lewy bodies, but strongly confirm that cardiac nuclear imaging with a noradrenergic radioligand is worthy of further study as a potential means to separate Alzheimer’s disease from Alzheimer’s disease/dementia with Lewy bodies during life.
Keywords: peripheral nervous system, Lewy body, Parkinson’s disease, autopsy, dementia with Lewy bodies
In this study, we strengthen the rationale that sympathetic cardiac denervation might be driven by alpha-synuclein pathology, which suggests that an in vivo test for such denervation would allow clinical differentiation between subjects with Alzheimer’s disease and subjects with dementia with Lewy bodies with comorbid Alzheimer’s disease.
Graphical Abstract
Graphical Abstract.
Introduction
The Dementia with Lewy Bodies (DLB) Consortium consensus clinical diagnostic criteria have high specificity (Jolly-Tornetta and Wolf, 2000) for diagnosis of patients with the fully developed clinical syndrome, but low sensitivity (McKeith et al., 1996, 2017; Litvan et al., 1998; Nelson et al., 2010; Huang and Halliday, 2013; Malek-Ahmadi et al., 2019). Even in specialist research settings, only 10–30% of subjects neuropathologically confirmed as DLB are diagnosed during life, with the most common misdiagnosis being Alzheimer’s disease (Alzheimer’s disease). Furthermore, comorbid Lewy body (LB) disease is very common in Alzheimer’s disease (Dickson et al., 1991); up to 60% of Alzheimer’s disease subjects also have pathologic alpha-synuclein (a-syn) at autopsy (Hamilton, 2000; Tsuang et al., 2006; Uchikado et al., 2006). Some of these are subjects that meet clinicopathological criteria for both diagnoses, Alzheimer’s disease and DLB, but most have a-syn that does not meet density and distribution criteria for DLB and hence have been termed Alzheimer’s disease with Lewy bodies (ADLB) (McKeith et al., 2005, 2017; Beach et al., 2009). This is a critical concern for Alzheimer’s disease clinical trials, as subjects with both Alzheimer’s disease and a-syn may have a different clinical course (Malek-Ahmadi et al., 2019) and may not respond well to therapeutic agents targeting only Alzheimer’s disease pathology. In the USA, sympathetic neuroimaging is rarely used for the diagnosis of autonomic or movement disorders, but this has been commonly used in Europe and Japan. Tissue studies have shown cardiac sympathetic denervation in both clinical and neuropathologically diagnosed Parkinson’s disease (PD) and DLB, but this has not been explored in ADLB (Yoshita et al., 1997, 2001, 2015; Yoshita, 1998; Goldstein, 2001; Orimo et al., 2002, 2008; Fujishiro et al., 2008; Goldstein et al., 2009; Takahashi et al., 2015; Manabe et al., 2017). In this study, we tested the hypothesis that ADLB will be distinguishable from Alzheimer’s disease without LB by having lower densities of cardiac noradrenergic nerve fibres.
Materials and methods
Human subjects
Human hearts came from subjects who were volunteers in the Arizona Study of Aging and Neurodegenerative Disorders, a longitudinal clinicopathological study of normal aging, cognition and movement in the elderly since 1996 in Sun City, Arizona (Beach et al., 2008a, 2015). Autopsies were performed by the Banner Sun Health Research Institute’s Brain and Body Donation Program (www.brainandbodydonationprogram.org). All subjects signed Institutional Review Board-approved informed consents allowing both clinical assessments during life and several options for brain and/or bodily organ donation after death. All subjects were clinically characterized by expert clinicians and most of them had annual standardized test batteries consisting of general neurological, cognitive and movement disorders components, including the Unified Parkinson’s Disease Rating Scale (UPDRS), Hoehn and Yahr staging and the Scales for Outcomes in Parkinson’s disease questionnaire autonomic (Damian et al., 2012). Subjects for the current study had a complete pathological evaluation by medically licensed pathologists (Table 1; N = 121) and were chosen by searching the Banner Sun Health Research Institute’s Brain and Body Donation Program database for cases with a whole-body autopsy and specific clinicopathological diagnoses including controls who were defined as non-demented individuals without parkinsonism and without LB pathology in the brain or examined peripheral tissue (CN, n = 22) and non-demented, non-Parkinsonian individuals without any neurodegenerative disorder diagnosis who also had incidental Lewy bodies (ILBD) at autopsy (n = 30), Parkinson's disease (n = 19), Alzheimer’s disease (n = 12), AD/DLB (n = 19) and ADLB (n = 19).
Table 1.
Patient demographics
| DX (n) | Age (SD) | Gender (M:F) | UPDRS OFF (SD) | Hoehn and Yahr (SD) | SCOPA-Aut total (SD) | PMI (SD) | a-syn sum Brain (SD) |
|---|---|---|---|---|---|---|---|
| CN (22) | 82 (14) | 13:9 | 6.1 (5.5) | 0.2 (0.8) | 19.8 (15.7) | 6.0 (14.8) | 0 |
| PD (19) | 81 (6) | 16:3 | 39.4 (18.8)* | 2.9 (1.5)* | 25.1 (8.5) | 3.4 (1.3) | 27.2 (5.9)* |
| AD/DLB (19) | 82 (8) | 12:7 | 34.9 (18.6)* | 1.8 (1.8)* | 27.5 (12.9) | 3.6 (1.5) | 32.8 (5.6)* |
| ADLB (20) | 80 (8) | 13:7 | 23.9 (23.1)* | 1.5 (2.2) | 29.3 (10.5) | 4.4 (5.9) | 13.7 (6.3)* |
| AD (12) | 77 (9) | 8:4 | 17.5 (20.9) | 0.6 (1.5) | 15.3 (6.0) | 3.7 (0.8) | 0 |
| ILBD (30) | 86 (9) | 18:12 | 8.2 (6.3) | 0.0 (0.0) | 17.7 (9.8) | 4.6 (4.4) | 8.0 (8.0)* |
AD = Alzheimer’s disease; AD/DLB = Alzheimer’s disease and dementia with Lewy bodies; ADLB = Alzheimer’s disease with Lewy bodies; CN = non-demented movement control; F = female; ILBD = incidental Lewy bodies; M = male; Parkinson's disease = Parkinson’s disease; PMI = post-mortem interval; SCOPA-Aut = SCales for Outcomes in Parkinson’s disease questionnaire autonomic; SD = standard deviation; UPDRS = Unified Parkinson’s Disease Rating Scale.
P < 0.05 post-test when compared with CN (CN).
Pathological examination
Complete gross and microscopic pathological examination was performed using standard Arizona Study of Aging and Neurodegenerative Disorders methods and included pathologist assessment of both brain and peripheral organs (Beach et al., 2008a, 2015). Cardiac samples included epicardial and adjacent myocardial tissue collected at the left circumflex coronary artery, lateral to the pulmonary trunk and inferior to the auricle of the left atrium. Tissue blocks were fixed in neutral-buffered formalin and embedded in paraffin. All sections were stained with haematoxylin and eosin for general pathological assessment. Immunohistochemical staining was used to document the presence of a-syn pathology in brain and cardiac nerve fibres. The antibody used for p-synuclein (raised against alpha-synuclein phosphorylated at serine 129) was privately developed and its characterization has been previously described (Fujiwara et al., 2002; Walker et al., 2013). The signal development steps have been described in previous publications (Beach et al., 2008b). Tyrosine hydroxylase (TH; Sigma Catalogue # T2928) and neurofilament (NF; ABCAM Catalogue # AB8135) antibodies were used to localize noradrenergic sympathetic nerve terminals and all nerve fibres, respectively. Immunohistochemical procedures were identical for all three methods, except for differing epitope exposure: 20 min proteinase K pre-treatment for p-synuclein; 20 min in boiling citrate buffer for TH and no antigen retrieval step for NF. Primary antibody concentrations were 1:10 000 for p-synuclein and 1:3000 for TH and NF. Stained epicardial nerve bundles were semi-quantitatively analysed blinded to the final clinicopathological diagnoses. We counted the numbers of NF-positive bundles to ensure a good sample size and sections were blindly graded for TH, NF and p-synuclein using templates analogous to those recommended by CERAD (Mirra et al., 1991) with separate semi-quantitative density estimates of either absent (0), sparse (1), moderate (2) or numerous (3) densities within nerve bundles. The neuropathological examination was performed in a standardized manner and consisted of gross and microscopic observations, the latter including assessment of frontal, parietal, temporal and occipital lobes, all major diencephalic nuclei and major subdivisions of the brainstem, cerebellum and spinal cord. Following fresh brain slicing and subsequent fixation in cold 10% neutral-buffered formalin for 36–60 h, histological preparations included paraffin-embedded 6 µm sections, as well as large-format (3 × 5 cm), 40–80 µm-thick, cryoprotected frozen sections. Both sets were stained with haematoxylin and eosin and the former set was also immunohistochemically stained for phosphorylated p-synuclein in 10 standard brain regions including olfactory bulb, anterior medulla, anterior and mid-pons, midbrain with substantia nigra, amygdala, anterior cingulate gyrus and three neocortical regions (middle frontal gyrus, middle temporal gyrus, inferior parietal lobule). Each region was graded as 0–4 for p-synuclein density using the template provided by McKeith et al. (2005). A summary brain score of all 10 regions is recorded to give an overall brain load estimate, with the highest possible score being 40. Senile plaques, neurofibrillary changes and other neuronal and glial tauopathies were assessed using thioflavin S, Gallyas and Campbell-Switzer methods and were graded blindly as recommended by CERAD with separate semi-quantitative density estimates of none, sparse, moderate or frequent. All scores were converted to a 0–3 scale for statistical purposes. Regions scored included cortical grey matter from frontal (F), temporal (T), parietal (P), hippocampal CA1 (H) and entorhinal (E) regions, with the sum of all brain regions giving a maximum score of 15.
Statistical analysis
One-way ANOVA was used to analyse group differences in demographics; the Kruskal–Wallis test with subsequent pair-wise Mann–Whitney U tests were used to analyse group differences in brain and heart a-syn and TH density. Spearman’s correlation was used to test for relationships between TH fibre density and a-syn, in both brain and heart.
Data availability
The authors confirm that the data supporting the findings of this study are available at the Banner Sun Health Research Institute’s Brain and Body Donation Program (https://www.brainandbodydonationregistration.org) and upon request to the corresponding author.
Results
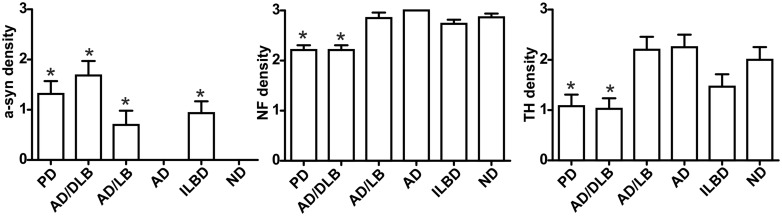
There were no significant differences in group mean age or post-mortem intervals (Supplementary Table 1). UPDRS scores were significantly higher in Parkinson's disease, AD/DLB and ADLB when compared with CN, while Hoehn and Yahr scores were only significantly higher in Parkinson's disease and AD/DLB. Furthermore, SCOPA-Aut total scores were significantly different between the groups, but neither neurodegenerative group was significantly different when compared with CN (Table 1). NF staining confirmed the presence of numerous nerve fibre bundles in the epicardium around coronary artery branches in the cardiac tissue blocks (Fig. 1), with an average of 85 nerve fibre bundles per sample. Both Parkinson's disease and AD/DLB subjects showed significant losses of NF protein-immunoreactive nerve fibres within bundles as compared with CN (P < 0.01) and both groups showed higher a-syn densities within bundles when compared with CN (Fig. 2; P < 0.0001). Cardiac a-syn was also present in ILBD and ADLB, but the mean from each group was not significantly different than CN. Cardiac a-syn densities correlated significantly with medulla, amygdala and brain summation a-syn densities, with medulla showing the strongest correlation (Table 2; P < 0.001). Furthermore, cardiac TH-immunoreactive nerve fibre densities were significantly different between the groups (P < 0.01), and subsequent pair-wise analysis showed that Parkinson's disease and AD/DLB subjects had significantly reduced TH fibre densities when compared with CN (P < 0.01) and Alzheimer’s disease (P < 0.05). Neither NF nor TH fibre densities in Alzheimer's disease, ADLB or ILBD were significantly different from those of CN. Cardiac TH- and NF-immunoreactive nerve fibre densities were negatively correlated with the densities of both brain and cardiac a-syn, as well as UPDRS scores (Gau et al., 2002).
Figure 1.
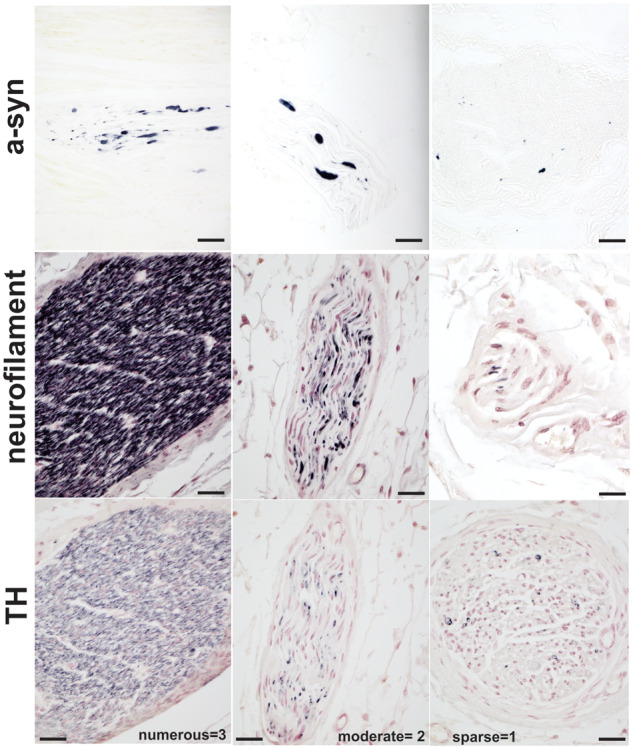
Myocardial immunostaining with p-αsynuclein, NF and TH. Numerous, moderate, sparse or absent densities scores were used to analyse nerve denervation and a-syn pathology in autopsy collected myocardia.
Figure 2.
Densities of myocardial nerve immunostained fibres for p-αsynuclein, NF and TH. Both Parkinson's disease and AD/DLB subjects showed high a-syn densities in myocardial nerve bundles and significant losses of NF and TH protein-immunoreactive nerve fibres as compared with CN (P < 0.01).
Table 2.
Statistical correlation of heart pathology with brain pathology and UPDRS score
| Correlation | Correlation coefficient | P-value |
|---|---|---|
| a-syn heart versus a-syn sum brain | 0.588 | <0.0001 |
| a-syn heart versus a-syn medulla | 0.694 | <0.000001 |
| a-syn heart versus a-syn amygdala | 0.420 | <0.000001 |
| a-syn heart versus TH heart | −0.201 | <0.05 |
| a-syn sum brain versus TH heart | −0.279 | <0.01 |
| a-syn medulla versus TH heart | −0.0323 | <0.001 |
| a-syn amygdala versus TH heart | −0.155 | NS |
| a-syn heart versus NF heart | −0.331 | <0.0001 |
| a-syn sum brain versus NF heart | −0.547 | <0.0001 |
| a-syn sum medulla versus NF heart | −0.612 | <0.000001 |
| a-syn sum amygdala versus NF heart | −0.445 | <0.000001 |
| UPDRS versus a-syn heart | 0.284 | <0.001 |
| UPDRS versus TH heart | −0.178 | 0.08 |
| UPDRS versus NF heart | −0.430 | <0.0001 |
a-syn = pathologic alpha-synuclein; NF = neurofilament; sum = summation; TH = tyrosine hydroxylase; UPDRS = Unified Parkinson’s Disease Rating Scale.
Discussion
This study strengthens the rationale that sympathetic cardiac denervation might be driven by a-syn pathology, such that an in vivo test for such denervation would allow clinical differentiation between pure Alzheimer’s disease and AD/DLB while patients are still alive. While we found no evidence that cardiac denervation could be used to distinguish between Alzheimer’s disease and those Alzheimer’s disease patients that have a-syn pathology that does not meet DLB criteria, being able to separate cases of AD/DLB from Alzheimer’s disease during life would be a huge advance for dementia epidemiologic and treatment studies (McKeith et al., 1996, 2017; Litvan et al., 1998; Nelson et al., 2010; Huang and Halliday, 2013). For instance, close to 50% of the AD/DLB cases used, in this study, were clinically diagnosed during life as Alzheimer’s disease alone. The symptoms of these subjects did not manifest like typical DLB and may have a different clinical course (Malek-Ahmadi et al., 2019). Furthermore, it is also known that up to 60% of Alzheimer’s disease subjects have comorbid a-syn at autopsy (Hamilton, 2000; Uchikado et al., 2006; Malek-Ahmadi et al., 2019). Some of these subjects received a final autopsy diagnosis of Alzheimer’s disease and DLB, while others had a-syn that did not meet density and distribution criteria for DLB. This is a critical concern for Alzheimer’s disease clinical trials, since subjects with both Alzheimer’s disease and a-syn may be less responsive or even resistant to Alzheimer's disease-specific therapeutic agents. Those DLB subjects who do not have at least two core clinical features (cognitive fluctuations, Parkinsonism, dream enactment behaviour and visual hallucinations) are more likely to be misdiagnosed as Alzheimer’s disease or dementia NOS (McKeith et al., 2017). In the USA, only two of the three recommended biomarkers, dopamine transporter single positron emission tomography (DaTscan) or polysomnography to document presence of rapid eye movement sleep behaviour disorder are available to support a DLB diagnosis (in combination with one core clinical feature). 123I-MIBG (123I-metaiodobenzylguanidine) scanning has been approved by the United States Food and Drug Administration for the evaluation of pheochromocytoma and some forms of heart failure and has been recently incorporated as a supportive biomarker for clinical DLB diagnoses in the most recent revised DLB Consortium consensus criteria (McKeith et al., 2017; Uyama et al., 2017; Goldstein and Cheshire, 2018). Such a noradrenergic radioligand would be worthy of study to validate its use for diagnosing DLB, particularly in demented patients who do not have either cognitive fluctuations or visual hallucinations, and also lack parkinsonism or dream enactment behaviour.
Multiple studies have shown sympathetic cardiac denervation in subjects with Parkinson's disease, and similar trends in DLB, by the use of MIBG analysis or post-mortem cardiac analysis (Yoshita et al., 1997; Yoshita, 1998; Goldstein, 2001; Orimo et al., 2002, 2007; Fujishiro et al., 2008; Orimo et al., 2008). However, studies to date in DLB cases either lacked pathological confirmation or used limited numbers of post-mortem samples with no statistical analysis (Yoshita et al., 2001, 2015; Orimo et al., 2005; Takahashi et al., 2015; Manabe et al., 2017). To our knowledge, this is the first report with a substantial number of autopsied cases, and nerve bundles analysed, showing a statistically significant separation of AD/DLB cases from CN and Alzheimer’s disease cases based on cardiac TH- and NF-immunoreactive nerve fibre density. Our results provide the physiological basis to justify further research on validation of cardiac nuclear imaging ligands for diagnosing DLB but it would not be likely to clinically separate ADLB from Alzheimer’s disease subjects without a-syn. This is probably because, as for ILBD subjects, the peripheral spread and severity of a-syn pathology in these individuals is not yet as burdensome as it is in Parkinson's disease and DLB (Beach et al., 2010). We also showed a significant negative correlation of TH fibre densities with a-syn densities in both brain and heart. This supports previous observations and provides further evidence for a disease process wherein spread of a-syn pathology directly causes depletion of sympathetic nerve fibres from the myocardium (Yoshita et al., 2001, 2015; Orimo et al., 2002, 2008; Fujishiro et al., 2008; Takahashi et al., 2015; Manabe et al., 2017). Importantly, our study provides autopsy evidence for sympathetic denervation of the heart in individuals with AD/DLB who were not formally diagnosed during life. Since Alzheimer’s disease pathology in these subjects may lead to masking of core DLB features, and previous work using 123I-MIBG has shown sympathetic denervation in clinically diagnosed DLB subjects, our data suggest that sympathetic nuclear imaging ligands are worthy of further study to better identify these mixed AD/DLB cases during life.
Supplementary material
Supplementary material is available at Brain Communications online.
Supplementary Material
Acknowledgements
We are grateful to the Banner Sun Health Research Institute Brain and Body Donation Program’s donors and supportive staff.
Funding
The Brain and Body Donation Program is supported by the National Institute on Aging (P30 AG19610 Arizona Alzheimer’s Disease Core Center), the Arizona Department of Health Services (contract 211002, Arizona Alzheimer’s Research Center), the Arizona Biomedical Research Commission (contracts 4001, 0011, 05-901 and 1001 to the Arizona Parkinson’s Disease Consortium) and the Michael J. Fox Foundation for Parkinson’s Research.
Competing interest
G.E.S., M.C., B.C., M.G., J.W., A.I., N.Z., L.I.S., D.V. and C.M.B. have nothing to disclose. D.S. received research support from the Arizona Alzheimer’s Consortium, Abbvie, Acadia, Axovant, Biogen, Eli Lilly, Neurocrine, Michael J Fox Foundation, NIH and Teva; consultant fees from Abbvie, Teva, Lundbeck, Merz and Neurocrine; speaker fees from Acadia, Lundbeck, Sunovion, Teva and US World Meds. C.H.A. received funding from MJFF, Consulting: Jazz, Neurocrine, Scion, Sunovion. H.A.S. received support from Biogen, Dong-A ST Co., Ltd., MagQu. Intec Pharma, Ltd, US World Meds, Sunovion/Cynapsus Therapeutics, Inc and consulting honoraria for advisory boards from Abbvie and Sunovion. E.D.-D. received support from Biogen and Abbvie. S.H.M. has had consulting relationships with Abbvie and Sunovion. E.Z. received research support from Biogen, Lilly, Eisai, Novartis, Janssen, Merck, Avid, Neurocrine, AbbVie, PPMI, Neurocrine Biosciences, Roche, Navidea, Axovant, Takeda, and Genentech. T.G.B. received research funding from the National Institutes of Health (P30 AG19610), the Michael J. Fox Foundation for Parkinson’s Research, Department of Health and Human Services of the State of Arizona, Avid Radiopharmaceuticals, Navidea Biopharmaceuticals and Aprionoia Therapeutics, and consulted for Vivid Genomics and Prothena Biosciences.
Glossary
- DLB =
dementia with Lewy bodies
- AD =
Alzheimer’s disease
- LB =
Lewy body
- a-syn
alpha-synuclein
- ADLB =
Alzheimer’s disease with Lewy bodies
- PD =
Parkinson’s disease
- UPDRS =
Unified Parkinson’s Disease Rating Scale
- CN =
non-demented movement control
- ILBD =
incidental Lewy bodies
- SCOPA-Aut =
SCales for Outcomes in Parkinson’s disease questionnaire autonomic
- SD =
standard deviation
- M =
male
- F =
female
- PMI =
post-mortem interval
- TH =
tyrosine hydroxylase
- NF =
neurofilament
- Sum =
Summation
- DaTscan =
dopamine transporter single positron emission tomography
- 123I-MIBG =
123I-metaiodobenzylguanidine
References
- Beach TG, Adler CH, Lue L, Sue LI, Bachalakuri J, Henry-Watson J, et al. ; the Arizona Parkinson’s Disease Consortium. Unified staging system for Lewy body disorders: correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta Neuropathol 2009; 117: 613–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach TG, Adler CH, Sue LI, Serrano G, Shill HA, Walker DG, et al. . Arizona study of aging and neurodegenerative disorders and brain and body donation program. Neuropathology 2015; 35: 354–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach TG, Adler CH, Sue LI, Vedders L, Lue L, White Iii CL, et al. ; Arizona Parkinson’s Disease Consortium. Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol 2010; 119: 689–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach TG, Sue LI, Walker DG, Roher AE, Lue L, Vedders L, et al. . The Sun Health Research Institute Brain Donation Program: description and experience, 1987–2007. Cell Tissue Banking 2008a; 9: 229–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach TG, White CL, Hamilton RL, Duda JE, Iwatsubo T, Dickson DW, et al. . Evaluation of alpha-synuclein immunohistochemical methods used by invited experts. Acta Neuropathol 2008b; 116: 277–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damian A, Adler CH, Hentz JG, Shill HA, Caviness JN, Sabbagh MN, et al. . Autonomic function, as self-reported on the SCOPA-autonomic questionnaire, is normal in essential tremor but not in Parkinson’s disease. Parkinsonism Relat Disord 2012; 18: 1089–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson DW, Ruan D, Crystal H, Mark MH, Davies P, Kress Y, et al. . Hippocampal degeneration differentiates diffuse Lewy body disease (DLBD) from Alzheimer’s disease: light and electron microscopic immunocytochemistry of CA2-3 neurites specific to DLBD. Neurology 1991; 41: 1402–9. [DOI] [PubMed] [Google Scholar]
- Fujishiro H, Frigerio R, Burnett M, Klos KJ, Josephs KA, Delledonne A, et al. . Cardiac sympathetic denervation correlates with clinical and pathologic stages of Parkinson’s disease. Mov Disord 2008; 23: 1085–92. [DOI] [PubMed] [Google Scholar]
- Fujiwara H, Hasegawa M, Dohmae N, Kawashima A, Masliah E, Goldberg MS, et al. . Alpha-synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol 2002; 4: 160–4. [DOI] [PubMed] [Google Scholar]
- Gau J-T, Steinhilb ML, Kao T-C, D'Amato CJ, Gaut JR, Frey KA, et al. . Stable beta-secretase activity and presynaptic cholinergic markers during progressive central nervous system amyloidogenesis in Tg2576 mice. Am J Pathol 2002; 160: 731–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DS. Cardiac sympathetic neuroimaging to distinguish multiple system atrophy from Parkinson disease. Clin Auton Res 2001; 11: 341–2. [DOI] [PubMed] [Google Scholar]
- Goldstein DS, Cheshire WP Jr.. Roles of cardiac sympathetic neuroimaging in autonomic medicine. Clin Auton Res 2018; 28: 397–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DS, Sharabi Y, Karp BI, Bentho O, Saleem A, Pacak K, et al. . Cardiac sympathetic denervation preceding motor signs in Parkinson disease. Cleve Clin J Med 2009; 76 Suppl 2: S47–S50. [DOI] [PubMed] [Google Scholar]
- Hamilton RL. Lewy bodies in Alzheimer’s disease: a neuropathological review of 145 cases using alpha-synuclein immunohistochemistry. Brain Pathol 2000; 10: 378–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Halliday G.. Can we clinically diagnose dementia with Lewy bodies yet? Transl Neurodegener 2013; 2: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly-Tornetta C, Wolf BA.. Protein kinase C regulation of intracellular and cell surface amyloid precursor protein (APP) cleavage in CHO695 cells. Biochemistry 2000; 39: 15282–90. [DOI] [PubMed] [Google Scholar]
- Litvan I, MacIntyre A, Goetz CG, Wenning GK, Jellinger K, Verny M, et al. . Accuracy of the clinical diagnoses of Lewy body disease, Parkinson disease, and dementia with Lewy bodies: a clinicopathologic study. Arch Neurol 1998; 55: 969–78. [DOI] [PubMed] [Google Scholar]
- Malek-Ahmadi M, Beach TG, Zamrini E, Adler CH, Sabbagh MN, Shill HA, et al. . Faster cognitive decline in dementia due to Alzheimer disease with clinically undiagnosed Lewy body disease. PLoS.One 2019; 14: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manabe Y, Inui Y, Toyama H, Kosaka K.. 123I-metaiodobenzylguanidine myocardial scintigraphy with early images alone is useful for the differential diagnosis of dementia with Lewy bodies. Psychiatry Res Neuroimaging 2017; 261: 75–9. [DOI] [PubMed] [Google Scholar]
- McKeith IG, Boeve BF, Dickson DW, Halliday G, Taylor JP, Weintraub D, et al. . Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology 2017; 89: 88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeith IG, Dickson DW, Lowe J, Emre M, O'Brien JT, Feldman H, et al. ; Consortium on DLB. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 2005; 65: 1863–72. [DOI] [PubMed] [Google Scholar]
- McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, et al. . Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology 1996; 47: 1113–24. [DOI] [PubMed] [Google Scholar]
- Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, et al. ; Participating CERAD Neuropathologists. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology 1991; 41: 479–86. [DOI] [PubMed] [Google Scholar]
- Nelson PT, Jicha GA, Kryscio RJ, Abner EL, Schmitt FA, Cooper G, et al. . Low sensitivity in clinical diagnoses of dementia with Lewy bodies. J Neurol 2010; 257: 359–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orimo S, Amino T, Itoh Y, Takahashi A, Kojo T, Uchihara T, et al. . Cardiac sympathetic denervation precedes neuronal loss in the sympathetic ganglia in Lewy body disease. Acta Neuropathol 2005; 109: 583–8. [DOI] [PubMed] [Google Scholar]
- Orimo S, Oka T, Miura H, Tsuchiya K, Mori F, Wakabayashi K, et al. . Sympathetic cardiac denervation in Parkinson’s disease and pure autonomic failure but not in multiple system atrophy. J Neurol Neurosurg Psychiatry 2002; 73: 776–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orimo S, Takahashi A, Uchihara T, Mori F, Kakita A, Wakabayashi K, et al. . Degeneration of cardiac sympathetic nerve begins in the early disease process of Parkinson’s disease. Brain Pathol 2007; 17: 24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orimo S, Uchihara T, Nakamura A, Mori F, Kakita A, Wakabayashi K, et al. . Axonal alpha-synuclein aggregates herald centripetal degeneration of cardiac sympathetic nerve in Parkinson’s disease. Brain 2008; 131: 642–50. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Ikemura M, Oka T, Uchihara T, Wakabayashi K, Kakita A, et al. . Quantitative correlation between cardiac MIBG uptake and remaining axons in the cardiac sympathetic nerve in Lewy body disease. J Neurol Neurosurg Psychiatry 2015; 86: 939–44. [DOI] [PubMed] [Google Scholar]
- Tsuang DW, Riekse RG, Purganan KM, David AC, Montine TJ, Schellenberg GD, et al. . Lewy body pathology in late-onset familial Alzheimer’s disease: a clinicopathological case series. J Alzheimers Dis 2006; 9: 235–42. [DOI] [PubMed] [Google Scholar]
- Uchikado H, Lin WL, DeLucia MW, Dickson DW.. Alzheimer disease with amygdala Lewy bodies: a distinct form of alpha-synucleinopathy. J Neuropathol Exp Neurol 2006; 65: 685–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyama N, Otsuka H, Shinya T, Otomi Y, Harada M, Sako W, et al. . The utility of the combination of a SPECT study with [123I]-FP-CIT of dopamine transporters and [123I]-MIBG myocardial scintigraphy in differentiating Parkinson disease from other degenerative parkinsonian syndromes. Nucl Med Commun 2017; 38: 487–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DG, Lue LF, Adler CH, Shill HA, Caviness JN, Sabbagh MN, et al. . Changes in properties of serine 129 phosphorylated alpha-synuclein with progression of Lewy-type histopathology in human brains. Exp Neurol 2013; 240: 190–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshita M. Differentiation of idiopathic Parkinson’s disease from striatonigral degeneration and progressive supranuclear palsy using iodine-123 meta-iodobenzylguanidine myocardial scintigraphy. J Neurol Sci 1998; 155: 60–7. [DOI] [PubMed] [Google Scholar]
- Yoshita M, Arai H, Arai H, Arai T, Asada T, Fujishiro H, et al. . Diagnostic accuracy of 123I-meta-iodobenzylguanidine myocardial scintigraphy in dementia with Lewy bodies: a multicenter study. PLoS One 2015; 10: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshita M, Hayashi M, Hirai S. [Iodine 123-labeled meta-iodobenzylguanidine myocardial scintigraphy in the cases of idiopathic Parkinson’s disease, multiple system atrophy, and progressive supranuclear palsy]. Rinsho Shinkeigaku 1997; 37: 476–82. [PubMed] [Google Scholar]
- Yoshita M, Taki J, Yamada M.. A clinical role for [(123)I]MIBG myocardial scintigraphy in the distinction between dementia of the Alzheimer’s-type and dementia with Lewy bodies. J Neurol Neurosurg Psychiatry 2001; 71: 583–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available at the Banner Sun Health Research Institute’s Brain and Body Donation Program (https://www.brainandbodydonationregistration.org) and upon request to the corresponding author.