Abstract
Background and Aims
Patients with Crohn’s disease [CD] harbour an increased number of adherent-invasive E. coli [AIEC]. The strain LF82, identified in the ileal mucosa of CD patients, has been extensively studied for pathogenic mechanisms. However, understanding of the interaction of LF82 with the intestinal mucosa of CD patients is lacking.
Methods
Here, we investigated the importance of long polar fimbriae [LPF] type 1 pili and the carcinoembryonic antigen-related cell-adhesion molecule 6 [CEACAM6] for translocation of LF82 in an in vitro model of follicle-associated epithelium [FAE], and in the FAE and villus epithelium [VE] of patients with CD and controls, using Ussing chambers.
Results
Significantly greater LF82 passage occurred in the FAE model compared with in the VE Caco-2cl1 mono-culture. Moreover, bacterial translocation was inhibited by either LPF disruption or pre-incubation with anti-CEACAM6 antibody. Tissue mounted in Ussing chambers showed significantly higher LF82 passage in FAE from patients with CD compared with control FAE, that was diminished in LF82 lacking LPF and by blocking host CEACAM6. Interestingly, addition of LF82 to the CD FAE tissues significantly increased paracellular permeability [of 51Chromium-EDTA] compared with baseline, and the increase was inhibited by anti-CEACAM6. Immunofluorescence and immunoblots showed higher expression of CEACAM6 in FAE of patients with CD compared with in FAE from controls.
Conclusions
These data suggest that the FAE of CD patients is a site of vulnerability for invasion by LF82 via a mechanism that requires both bacterial LPF and host CEACAM6. Further, LF82 has the ability to increase paracellular passage through the FAE of patients with CD. These data can help define novel therapeutic targets in CD for the prevention of clinical recurrence.
Keywords: Inflammatory bowel disease, mucosal barrier, permeability
1. Introduction
Crohn’s disease [CD] is an inflammatory bowel disease [IBD] with a multifactorial aetiology, with evidence that genetic, immunological, and environmental factors all contribute to the pathogenesis of the disease.1,2 There is a lack of defence against bacteria in CD, leading to mucosal barrier dysfunction that is a combination of increased transcellular3,4 and paracellular permeability.5,6 Postoperative recurrence of CD remains a major clinical challenge. Studies have shown alterations in the mucosal barrier of the neo-terminal ileum at the time of bowel resection,3,7,8 and CD recurrence is triggered by luminal contents in the terminal ileum proximal to the ileocolonic anastomosis,9 even during the first post-operative day.10
The earliest observable signs of CD are aphthoid lesions11 that are preceded by ultra-structural erosions in the follicle-associated epithelium [FAE] overlying the Peyer’s patches in the ileum.12 The FAE differs from the surrounding villus epithelium [VE], and the most distinguishing feature of the FAE is the presence of membranous/microfold [M] cells, which are specialized for the sampling and transport of antigens and microorganisms.13 Several microorganisms, particularly invasive bacteria such as Yersinia and Salmonella,14,15 take advantage of the transcytotic characteristics of M cells to cross the intestinal barrier. The molecular mechanism behind the ability of these bacteria to interact with and penetrate the M cells is only partially known, but the expression of long polar fimbriae [LPF] has been highlighted.15
We previously showed that human FAE is not only structurally but also functionally distinct from regular VE, with enhanced transport of antigens and bacteria into the underlying lymphoid tissue.16 In addition, we showed an enhanced bacterial uptake in CD FAE compared with that in non-IBD controls,8 with an increased internalization by pro-inflammatory dendritic cells.7 The bacterial uptake was both transcellular and paracellular in CD FAE, in contrast to that in controls, in which only transcellular uptake occurred.
Luminal enteric bacteria are considered to be the most important inflammation-driving environmental factor in CD.17,18 Studies in CD patients have shown various changes in the luminal microbiota, with a possible link to local inflammation.19,20 One potential pathogenic group of Escherichia coli has been identified that can adhere and invade the enterocytes and has been designated adherent-invasive E. coli [AIEC].21–23 AIEC have been detected in CD patients in several countries,21,22,24–26 and the concentration of bacteria increases progressively with the severity of the disease.18,27 A specific strain of AIEC, E. coli LF82, has been identified in the ileal mucosa of CD patients and has been shown to invade the lamina propria and to replicate within macrophages.18,22,23,28 Moreover, Fang et al.29 studied the abundance and properties of E. coli strains in a series of samples from a CD patient, using metagenomics-based strain-level analysis. Their results showed that the abundance and dominance of the specific E. coli strain ST1, similar to LF82, varied over time and was correlated with inflammation status.
A possible explanation for the increased numbers of AIEC associated with CD is the increased expression of the carcinoembryonic antigen-related cell-adhesion molecule 6 [CEACAM6] in the brush border of ileal enterocytes in CD patients: CEACAM6 is a receptor for AIEC, binding to the intestinal mucosa via type 1 pili.30 Interestingly, in a CEABAC10 transgenic mouse model [expressing human CEACAMs], mice infected with LF82, but not with non-pathogenic E. coli, showed a 3-fold increase in intestinal permeability and disrupted mucosal integrity with a type 1 pili–dependent mechanism.31 Moreover, we previously showed that E. coli expressing LPF translocate across an in vitro model of FAE and interact with murine and human Peyer’s patches.32
Although a number of studies have elucidated the interaction of LF82 with the intestinal epithelium, studies of mechanisms of invasion through the human intestinal mucosa of CD patients have so far been lacking. In the present study, to complement and extend findings from cell lines and mice, we sought to study LF82 translocation and the importance of LPF and CEACAM6 in vitro and ex vivo in the FAE and VE of CD patients and non-IBD controls.
2. Materials and methods
2.1. Bacterial strains
The AIEC reference strain LF82 and isogenic mutant with the lpfA gene deleted, LF82∆lpfA, were used.21,23,32 Isogenic mutants were generated with a PCR product using the method described by Datsenko et al.33 Bacteria were grown in Luria–Bertani [LB] broth overnight at 37°C without shaking. Plasmid pFPV25.1 was used to make bacterial strains expressing green fluorescent protein [GFP].34 Before experiments started, the effect of GFP labelling on bacterial growth was tested. GFP-labelled LF82 and unlabelled LF82 were plated in duplicates on LB agar plates supplemented with 100 µg/ml ampicillin, air dried for 30 min, and incubated at 37°C for 18 h. Colonies on each plate were counted and bacterial growth was evaluated by comparing the colony-forming units [CFUs] for GFP-labelled and unlabelled E. coli LF82. Results showed no difference in growth between GFP-labelled [178 CFU] and unlabelled [185 CFU] E. coli LF82. In addition, we previously showed32 that there is no difference in growth between LF82 and LF82∆lpfA. GFP labelling of Salmonella typhimurium was prepared as previously described for E. coli HB101.35
2.2. In vitro experiments
2.2.1. Cell cultures
Human colorectal epithelial clone 1 [Caco-2cl1] cells [originally obtained from Dr Maria Rescigno, Milan, Italy] were cultured in Dulbecco’s modified Eagle’s medium [DMEM] supplemented with 10% heat-inactivated foetal calf serum, 100 U/ml penicillin, 100 mg/ml streptomycin, and 2 mM L-glutamine [Gibco Invitrogen Corporation, UK] at 37°C in 5% CO2. Raji B cells [ATCC, Manasses, VA, USA] were cultured in RPMI-medium [Gibco], supplemented as DMEM, at 37°C in 5% CO2.
2.2.2. Co-culture model of human FAE
To study the translocation of AIEC LF82 in human FAE, an in vitro co-culture model was initially used, as described by Kernéis et al., showing that co-culture of Peyer’s patch lymphocytes and intestinal epithelial cells can trigger epithelial cell conversion to an M cell–like phenotype.36 We previously established a modified version of this co-culture.37
Briefly, intestinal epithelial Caco-2cl1 cells were grown for 14–17 days on Matrigel® Matrix [Corning, USA]–coated 3.0 μm polycarbonate filters [Costar, Baedvenhorp, NL] until confluent, as defined by a transepithelial resistance [TER] of 400–500 Ω × cm2, measured using a voltmeter [Millipore, Sweden]. The model FAE was obtained by adding 5 × 105 Raji B cells suspended in DMEM to the basolateral chamber of confluent Caco-2cl1 cell monolayers. Corresponding mono-cultures of Caco-2cl1 epithelial cells on matched filter supports served as controls. The co-culture was maintained for 4–6 days, until M cells were generated.37
2.2.3. Verification of the model FAE
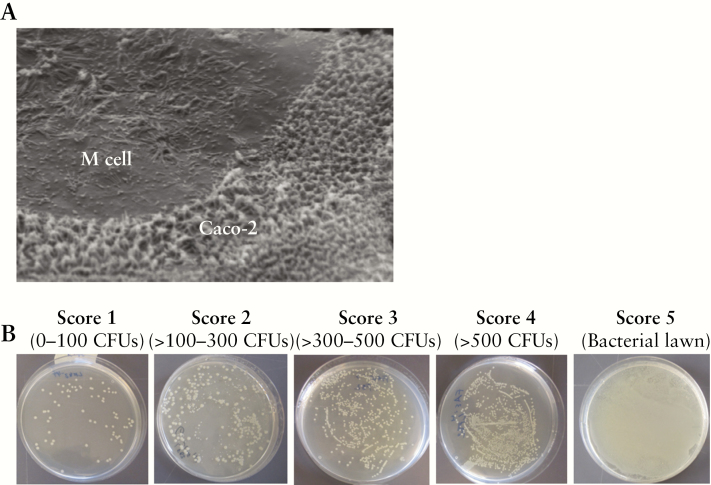
To confirm the transformation to M cells, the TER was measured daily; to be considered transformed, the TER should be at ≥10% lower in the co-culture model compared with in the Caco-2cl1.38 In the present study, we showed a median of 14.2% lower TER in the co-culture model compared with in the Caco-2cl1, confirming the transformation of epithelial cells to M cell–like cells (median [25th–75th percentiles] Ω × cm2, Caco-2cl1: 588 [542–631]; co-culture: 504 [463–551]), p < 0.05, n = 10. In addition, filters were processed for scanning electron microscopy [JSM 840, JEOL, Japan] that confirmed Caco-2cl1 cell transformation to FAE-like epithelium, as shown by areas of sparse irregular microvilli on the apical surface, indicating an M cell–like morphology [Figure 1A]. To further verify transport function in the model FAE, transcytosis of live GFP-Salmonella typhimurium was studied. Salmonella typhimurium has been shown to translocate from the apical to the basolateral side of M cell monolayers at a significantly higher rate compared with Caco-2cl1 monolayers.38 Experiments with Salmonella confirmed the transformation to model FAE by showing an increased translocation of Salmonella in the basolateral medium of co-culture monolayers compared with Caco-2cl1, measured by fluorimetry, and by plating basolateral samples containing translocated Salmonella on LB-agar overnight at 37°C, in conditions of 5% CO2, followed by assessment of CFU numbers through modification of a scoring system previously used in our group.39 Score 1 represented least CFUs and 5 represented most CFUs, and the plates in between were designated as having Scores 2, 3, or 4. Figure 1B illustrates representative agar plates categorized into the five different Scores. Fluorimetry showed an 18.5% median increased passage of Salmonella in the co-culture (118.3 fluorescence units [102.6–218.9]) compared with that in Caco-2cl1 (96.4 [86.0–112.8]), p < 0.05, and CFU counting confirmed these findings by showing a 40% median increase in Salmonella passage in the co-culture (Score 5 [4–5]) compared with in the Caco-2cl1 (3 [3–4]), p < 0.05. Exposure to Salmonella decreased the TER compared with that of unexposed cells, in both Caco-2cl1 [25.6% median decrease in TER] and the co-culture [25.9%], p < 0.05. The fluorescence intensity of the bacteria was quantified at 488 nm in a VICTORTM X3 multileader plate reader [PerkinElmer, Sweden]. All experiments were performed with 6–10 independent settings and each experiment was performed in triplicate.
Figure 1.
[A] Transformation of model follicle-associated epithelium [FAE] verified by scanning electron microscopy, as shown by areas of sparse irregular microvilli on the apical surface, indicating the presence of M cell–like cells. [B] Assessment of the number of colony-forming units [CFUs], using a scoring system. Basolateral samples containing translocated E. coli LF82 were plated on LB-agar overnight at 37°C, in 5% CO2 conditions, followed by assessment of the number of CFUs, using a scoring system in which Score 1 represented the least CFUs and Score 5 represented the most CFUs, the plates in between being scored as Scores 2, 3, or 4. This figure shows representative plates for each score. Score 4 represents plates with >500 CFUs, in which colonies were uncountable, but discernible colonies were evident.
2.2.4. Translocation of E. coli LF82 and LF82∆lpfA
Before and after transport experiments, the TER was measured to check the monolayer integrity. Upon M cell transformation, cultures were exposed in triplicate on the apical sides to 108 live GFP-labelled E. coli LF82 or E. coli LF82∆lpfA. After 3 h, the TER was measured and basolateral samples were collected in triplicate. Translocated LF82 was measured by fluorimetry and CFU counting. All experiments were performed with 6–10 independent settings and each experiment was performed in triplicate.
2.2.5. Effects of anti-CEACAM6 on LF82 translocation
It was previously shown that the Caco-2cl1 cells used in the present study express CEACAM6.30 The mono- and co-cultures were divided into two groups: [i] those infected with 108E. coli LF82; [ii] those pre-treated on the apical side with 21 ng/ml mouse monoclonal anti-human CEACAM6 [Pierce, Thermo Fisher Scientific, Sweden] for 20 min prior to administration of 108 LF82. After 3 h, the TER was measured, and basolateral samples were collected and analyzed as above. Experiments were performed with 6–10 independent settings and each experiment was performed in triplicate. Before experiments started, the concentration of the anti-CEACAM6 antibody was optimized. Ussing chamber experiments were run with concentrations 7, 21, 35, and 42 ng/ml. The highest peak in blocking of bacterial passage was achieved with 21 ng/ml. To verify the specificity of anti-CEACAM6, experiments were also performed with an irrelevant antibody [Mouse IgG1 Isotype Control, Pierce]. These experiments were performed with three of the 6–10 independent settings. Experiments were performed as described for anti-CEACAM6, but in addition, 21 ng/ml of the irrelevant antibody was added to three extra wells.
2.3. Human experiments
2.3.1. Patients and ethics
Specimens from the neo-terminal ileum, or terminal ileum next to the ileocaecal valve, were taken during surgery from 27 patients with CD and 29 patients with non-inflammatory conditions serving as non-IBD controls, at the University Hospital of Linköping.
Details of anti-inflammatory medication, indication for surgery, Montreal classification and pre-operative C-reactive protein for the CD patients (median age 51 years [range 18–81], 16 men) are given in Table 1. The non-IBD controls were colon cancer patients (70 years [range 46–88], 15 men). The patients had no generalized disease and none of them had received preoperative chemo- or radiotherapy. The Regional Ethical Review Board, Sweden, approved the study and all subjects gave their written informed consent.
Table 1.
Patient characteristics of the 27 patients with Crohn’s disease included in the study
| Age [years] | Sex | Anti-inflammatory medication | Indication for surgery | MC; age at diagnosis | MC; location of disease | MC; behaviour of disease | Pre-op P-CRP |
|---|---|---|---|---|---|---|---|
| 36 | f | prednisolone, mesalazine | stenosis | A2 | L3 | B2 | <10 |
| 61 | m | mesalazine | stenosis | A3 | L1 | B2 | <10 |
| 81 | m | none | fistula | A3 | L1 | B3 | 17 |
| 44 | f | none | stenosis | A1 | L3 | B2 | <10 |
| 39 | f | none | stenosis | A2 | L3 | B2 | <10 |
| 19 | m | budenoside | stenosis | A2 | L3 | B2 | <10 |
| 49 | m | none | stenosis | A2 | L3 | B3 | 13 |
| 51 | f | prednisolone, azathioprine | stenosis | A2 | L3 | B2 | <10 |
| 36 | f | budenoside | ileitis, stenosis | A2 | L3 | B2 | <10 |
| 71 | m | none | stenosis | A3 | L3 | B2 | <10 |
| 68 | m | prednisolone | stenosis | A3 | L1 | B2 | <10 |
| 18 | m | sulfasalazine, azathioprine | stenosis | A1 | L3 | B2 | 52 |
| 42 | f | certolizumab | stenosis | A2 | L3 | B2 | <10 |
| 39 | f | budenoside | stenosis | A2 | L1 | B2 | 10 |
| 49 | m | monoclonal antibody | stenosis | A2 | L2 | B3 | <10 |
| 61 | m | mesalazine | stenosis | A3 | L1 | B2 | <10 |
| 68 | m | prednisolone | stenosis | A3 | L1 | B2 | <10 |
| 73 | f | adalimumab | stenosis | A3 | L1 | B2 | 12 |
| 36 | f | prednisolone, mesalazine | stenosis | A2 | L3 | B2 | <10 |
| 71 | m | none | stenosis | A3 | L1 | B2 | <10 |
| 69 | m | mesalazine, prednisolone | stenosis, cecal infl. | A2 | L1 | B2 | <10 |
| 45 | m | methotrexate, mesalazine | ileocolic resection | A2 | L3 | B2 | <10 |
| 22 | m | infliximab | ileocolic resection | A2 | L1 | B3 | <10 |
| 74 | f | none | ileocolic resection | A3 | L1 | B2 | 12 |
| 53 | m | enteral nutrition | ileocolic resection | A2 | L3 | B3 | <10 |
| 51 | m | none | ileocolic resection | A2 | L1 | B2 | <10 |
| 61 | f | prednisolone | ileocolic resection | A2 | L3 | B2 | 12 |
MC= Montreal Classification; A1: <16; A2: 16–40; A3: >40; L1: ileum; L2: colon; L3: ileum and colon; B2: strictures; B3: perforations; Pre-op P-CRP: pre-operative plasma C-reactive protein.
Surgical specimens were put in ice-cold oxygenated Krebs buffer [115 mM NaCl, 1.25 mM CaCl2, 1.2 mM MgCl2, 2 mM KH2PO4, and 25 mM NaHCO3, pH 7.35] immediately after division of the ileocolic artery, and transported to the laboratory for stripping of external muscle and myenteric plexus. Microscopic identification of the VE and FAE were done as previously described.35 Segments of the FAE and VE were obtained from ileum of the same individual and run in parallel in the Ussing chamber experiments, western blot, and immunofluorescence analyses.
2.3.2. Ussing chamber experiments
Segments of the VE and FAE were mounted in modified Ussing chambers [Harvard apparatus Inc., Holliston, MA]40 as previously described.35 Briefly, mucosal compartments were filled with 1.5 ml cold 10 mM mannitol in Krebs buffer and the serosal compartments with 10 mM glucose in Krebs buffer, and continuously oxygenated at 37°C, while being mixed by gas flow. Chambers were equilibrated for 40 min to achieve steady state conditions, and barrier properties were then studied for 120 min. The experiments were performed in open circuit conditions, with the transepithelial potential difference [PD], short-circuit current [Isc], and TER being monitored every second minute using one pair of Ag/AgCl electrodes with agar–salt bridges and one pair of current-giving platinum electrodes. At the conclusion of the experiments, 10 µM of the Cl– secretagogue forskolin was added to the chambers, and the electrophysiological response was monitored to ensure continued viability of the tissues.
2.3.3. Bacterial translocation
Segments of the FAE from nine controls (79 years [55–88], 5 men) and eight patients with CD (46 years [36–78], 4 men) were mounted in Ussing chambers. For experiments on the VE, tissues from five patients with CD [LF82 and LF82∆lpfA] and eight [LF82] and six [LF82∆lpfA] non-IBD controls were mounted in Ussing chambers for analysis of bacterial passage. After equilibration, live GFP-E. coli LF82 or GFP-E. coli LF82∆lpfA were added to the mucosal side to a final concentration of 108 CFUs/ml. Two tissues received LF82, two LF82∆lpfA, and two served as Krebs-only controls. Serosal samples of 1.5 ml were collected at the start and at the end of the experiment [at time 120 min]. Bacterial translocation was determined by fluorimetry and recalculated as bacteria/chamber. In addition, basolateral samples were plated on LB-agar for CFU counting as described for in vitro experiments. Blanks and samples were run in duplicate and measured against a standard curve.
2.3.4. Paracellular permeability
Segments of the FAE and VE [from nine controls and eight patients with CD, as detailed above] were mounted in Ussing chambers, as described for the bacterial passage experiments. For paracellular studies, 34 µCi/ml of the inert probe 51Chromium-EDTA [51Cr-EDTA], mw 384 Da [Perkin Elmer, Boston, MA], was added to the mucosal side of all chambers, together with bacteria, or Krebs as a vehicle. At 0, 60, and 120 min after the start, serosal samples [300 µl] were collected and replaced with an equal volume of Krebs. 51Cr-EDTA was measured in a gamma counter [1282 Compugamma, LKB, Bromma, Sweden], and permeability was calculated during the 60–120 min period and given as Papp [apparent permeability coefficient; cm/s × 10–6]. Chambers mounted with VE and FAE from one non-IBD control, and VE from one CD patient, were excluded due to technical problems.35
2.3.5. Blocking with anti-CEACAM6
To study the influence of CEACAM6 on LF82 translocation through the FAE, segments from 10 controls (72 years [63–86], 6 men) and nine patients with CD (45 years [18–72], 4 men) were mounted in Ussing chambers. After 20 min, buffers were replaced and mouse anti-human CEACAM6 [Pierce] was added to the mucosal sides to a final concentration of 21 ng/ml. After 20 more minutes, GFP-LF82 and 51Cr-EDTA were added to the mucosal sides and samples were collected as described above.
To verify the specificity of anti-CEACAM6 in the human set-up, experiments were, as for in vitro studies, implemented with the irrelevant antibody [Pierce]. Experiments were performed on the FAE from three patients with CD (43 years [39–49] 1 man) and three controls (72 years [67–76], 3 men). Ussing chamber studies were performed as described above, but in addition, 21 ng/ml of the irrelevant antibody was added to two extra chambers.
As well as the experiments above, segments of the FAE from two controls (69 years [67–72], 2 men) and two patients with CD (53 years [51–55], 1 man) were mounted in Ussing chambers. After 20 min of pre-incubation with 21 ng/ml of anti-CEACAM6, segments were treated with 108 CFUs/ml of the non-AIEC E. coli HS and added to the mucosal sides, instead of AIEC E. coli LF82.
2.3.6. Quantification of CEACAM6 in tissue lysates by western blotting
Approximately 20–30 µg of frozen tissue [identified as VE or FAE from each patient, as described above] from eight patients with CD (49 years [22–78], 4 men) and eight controls (69 years [46–82], 4 men) was immersed in lysis buffer containing ice-cold RIPA-buffer [Pierce], Mini Protease inhibitor Cocktail [Roche, Germany], and 50 U/ml nuclease [Pierce]. Samples were homogenized in Tissue-Lyser II [Qiagen, Sweden], at a frequency of 30 movements/s, for 1 min and then centrifuged for 15 min at 18 000g at 4°C. Supernatants were redrawn, protein concentrations measured according to the Bio-Rad DC protein assay [Bio-Rad, Sweden], and lysates were diluted in Laemmli’s loading buffer [Bio-Rad] to a final concentration of 1 mg protein/ml, heated for 9 min at 95°C, and kept at -80°C until analysis.
Tissue lysates with protein concentrations of 20 µg/µl were loaded on 8–16% Criterion TGX gels [Bio-Rad], separated, and electrophoretically transferred to nitrocellulose membranes [Bio-Rad]. Membranes were blocked for 1 h in 5% non-fat milk [Bio-Rad] in PBS pH 7.6 + 0.05% Tween 20 at room temperature, followed by incubation at 4°C overnight with mouse anti-human CEACAM6 1:1400 [Pierce] and rabbit monoclonal anti-human β-actin [Cell Signaling, BioNordika, Sweden] 1:10 000 in 5% BSA. After washing, membranes were incubated with 1:20 000 secondary antibodies goat polyclonal anti-mouse Alexa Fluor 790 or goat polyclonal anti-rabbit Alexa Fluor 680 [Invitrogen, Sweden] for 1 h at room temperature. Membranes were washed and fluorescent bands were detected and quantified by Odyssey CLx and Image Studio software [LI-COR Biosciences]. Due to variations in β-actin, which probably reflects the nature of the samples [CD vs non-inflamed control samples], CEACAM6 protein levels were normalized to the β-actin loading control. Values are given in fluorescence units.
2.3.7. Immunofluorescence of epithelial CEACAM6 expression
Paired segments of the FAE and VE from eight patients with CD and eight controls [as detailed in the paragraph above] were used for studies of CEACAM6 expression. Ileal tissues were fixed in 4% PFA for 2 h and then placed in PBS with 30% sucrose until embedded in mounting medium [OCT cryomount, Histolab, Gothenburg, Sweden] and cryosectioned. Sections were blocked for 5 min with Background Sniper [Biocare Medical, USA], washed in PBS, and incubated overnight at 4°C with 1/200 primary mouse anti-CEACAM6 antibody. Sections were added to 1/200 secondary rabbit anti-mouse IgG alexa-fluor 594 [Pierce]. After 1 h of room temperature incubation, sections were mounted on slides with ProLong® Gold-DAPI [Life Technologies, Sweden]. Sections without primary antibody were used as negative controls.
Three sections per patient were evaluated in a blinded fashion for CEACAM6 expression in a Nicon-Eclipse-E800 fluorescence microscope equipped with a Nikon DS-Ri1-digital camera. In total, 20 pictures/section were obtained, and three epithelium types were identified: VE, FAE, and adjacent-VE. The adjacent-VE is defined as the VE lining the first villus next to the FAE, and differs from the VE further away from the dome; there is higher expression of the cell adhesion protein and bacterial co-receptor CD9 in adjacent-VE compared with villi further away, which expresses no, or very little, CD9.16 By using ImageJ software [NIH] the epithelial linings in each picture were encircled and a mean fluorescence intensity was calculated. From each patient/control, a mean value was calculated from the FAE, VE, and adjacent-VE, and then a median value was calculated for all patients/controls.
2.4. Statistical analysis
According to the D’Agostino–Pearson omnibus normality test, the data were judged as non-parametric, and accordingly data are given as the median [25th–75th percentiles]. Comparisons between two groups were carried out using the Mann–Whitney U test. The Wilcoxon matched-pairs signed-rank test was used for paired comparisons [in vitro TER and individual comparisons of anti-CEACAM6 effects ex vivo]. Differences with p < 0.05 were considered significant. Statistical analyses were performed using GraphPad Prism 7 Software [CA, USA].
3. Results
3.1. In vitro
3.1.1. Lower TER by E. coli LF82 and increased translocation in model FAE
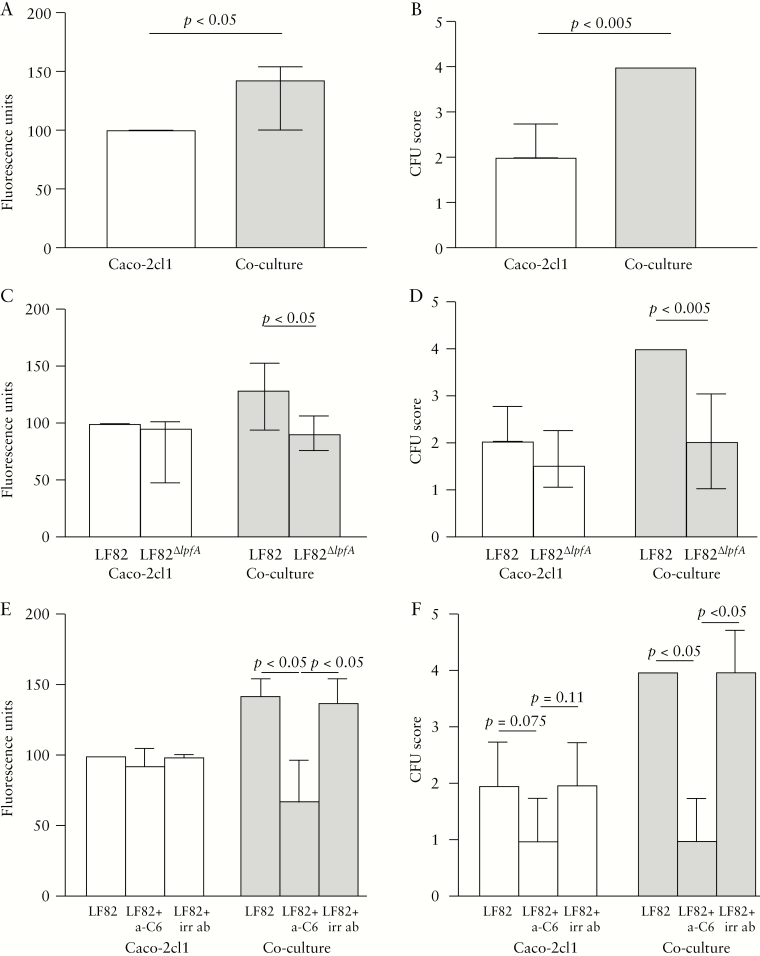
E. coli LF82 infection resulted in a 19.5% decrease in TER in the Caco-2cl1 model compared with that in control wells with no bacteria added, p < 0.05, and there was a 5.7% decrease in the co-culture model, p < 0.05 [Table 2]. Translocation of LF82 was increased by 42.9% in the co-culture compared with that in Caco-2cl1, p < 0.05 [Figure 2A]. The greater passage of LF82 was confirmed by CFU counting of the basolateral media, which showed 50% more CFUs in the co-culture compared with in Caco-2-cl1, p < 0.005 [Figure 2B].
Table 2.
Effects on transepithelial resistance [TER] by E. coli LF82, LF82ΔlpfA, and after pre-incubation with anti-CEACAM6
| Cell culture model | Time point | LF82 | LF82ΔlpfA | LF82 + a-CEACAM6 |
|---|---|---|---|---|
| Caco-2-cl1 model | 0 h | 588.0 [541.9–631.3] | 567.0 [557.0–740.0] | 612.0 [545.7–735.0] |
| Caco-2-cl1 model | 3 h | 473.3 [439.7–511.8]* | 518.3 [460.0–648.7]* | 485.0 [451.3–841.0] |
| Co-culture model | 0 h | 504.3 [463.0–551.3] | 561.8 [536.5–679.3] | 504.0 [401.7–615.0] |
| Co-culture model | 3 h | 475.5 [368.3–535.4]* | 505.5 [494.5–648.7]* | 415.0 [290.0–554.0] |
Cells were cultured as Caco-2-cl1 monocultures or co-cultures, i.e. Caco-2-cl1 co-cultured with Raji B-cells. TER was measured before [0 h] and after [3 h] infection with E. coli LF82, with the mutant E. coli LF82ΔlpfA, or after pre-incubation with anti [a]-CEACAM6 20 min prior to LF82 infection. Values are presented as the median [25th–75th percentiles] Ω × cm2. Comparisons between two groups were done with the Mann–Whitney U test, and the Wilcoxon matched-pairs signed-rank test was used for paired comparisons, *=p < 0.05 compared with time point 0 h for each treatment, respectively. All experiments were performed in 6–10 independent settings, and each experiment was performed in triplicate.
Figure 2.
Translocation of live green fluorescent protein–labelled E. coli LF82 and E. coli LF82∆lpfA, the mutant strain with the long polar fimbriae [LPF] gene deleted, and effects of anti-CEACAM6 [a-C6] in the Caco-2cl1 mono-culture model and in the co-culture model. [A] Passage of E. coli LF82 measured by fluorimetry. [B] Translocation of E. coli LF82 measured by scoring of colony-forming units [CFUs]. [C] Passage of E. coli LF82 and E. coli LF82∆lpfA measured by fluorimetry. [D] Translocation of E. coli LF82 and E. coli LF82∆lpfA measured by CFU scoring. [E] Effects of pre-incubation with a-C6 or with irrelevant antibody [irr ab] for translocation of E. coli LF82 measured by fluorimetry. [F] Effects of pre-incubation with a-C6 or with irr ab measured by CFU-scoring. Bars represents the median [25th–75th percentiles], and comparisons between the two groups were carried out using the Mann–Whitney U test.
3.1.2. Translocation of E. coli LF82 was dependent on LPF expression and CEACAM6
Transepithelial passage of LF82∆lpfA showed only a slightly decreased passage of 4.1% compared with LF82 in Caco-2cl1 epithelial monolayers; however, in the FAE co-culture there was a 29.3% decreased passage compared with in corresponding cells given LF82, p < 0.05 [Figure 2C]. Overnight culture of the basolateral media confirmed the fluorimetry results, showing a 25% [non-significantly] decreased number of LF82∆lpfA colonies compared with LF82 in Caco-2cl1, and a 50% decrease of LF82∆lpfA colonies compared with LF82 in the co-culture, p < 0.005 [Figure 2D].
Similarly to LF82, LF82∆lpfA decreased the TER, both in Caco-2cl1 [8.6%] and in the co-culture [10.9%], p < 0.05 [Table 2]. This indicated that the LF82 lacking LPF passes through the cells to a lesser extent, but still decreases the TER. Comparisons showed no significant difference in the effects on the TER between LF82 and LF82∆lpfA.
Pre-incubating the epithelial cells with anti-CEACAM6 antibodies prior to LF82 infection showed only a slightly decreased translocation of 6.9% in Caco-2cl1 monolayers; however, in the model FAE, there was a 51.7% decreased passage [p < 0.05] [Figure 2E]. Pre-incubation with an irrelevant antibody had no effect on LF82 translocation [Figure 2E]. These data were confirmed by CFU counting, which showed fewer LF82 colonies in the co-culture, p < 0.05, and close to statistically significantly fewer LF82 colonies in Caco-2cl1, p = 0.075, whereas no effect was seen with the irrelevant antibody [Figure 2F]. Pre-incubating cells with anti-CEACAM6 antibodies prior to infection with LF82 prevented the drop in TER [Table 2].
Based on the in vitro results, we set up ex vivo human experiments to further evaluate the role of CEACAM6 and LPF in LF82 uptake, and to compare tissues from patients with CD with those from controls.
3.2. Human tissues ex vivo
After equilibration, the PD was stable in all CD and control tissues, confirming their viability. Addition of LF82 had no effect on TER or Isc at any time point in the FAE or in the VE. Table 3 shows the values at 90 min as a representative time point; however, values were similar at 30, 60, and 120 min.
Table 3.
Effects of E. coli LF82 on transepithelial resistance [TER] and short-circuit current [Isc] in human ileum
| Group/treatment | TER [Ω × cm2] | Isc [μA/cm2] | ||
|---|---|---|---|---|
| Non-IBD controls | VE | FAE | VE | FAE |
| Vehicle | 74.1 [62.8–79.9] | 79.2 [65.7–82.3] | 11.9 [9.9–13.5] | 13.7 [11.2–15.3] |
| LF82 | 67.1 [58.7–72.0] | 73.3 [61.2–79.7] | 13.7 [9.9–15.8] | 15.2 [11.9–17.1] |
| Crohn’s disease | VE | FAE | VE | FAE |
| Vehicle | 69.6 [59.5–73.8] | 74.2 [63.5–77.9] | 13.9 [11.5–15.1] | 15.5 [11.2–18.5] |
| LF82 | 63.1 [52.3–71.7] | 70.2 [65.7–75.9] | 15.7 [13.4–17.5] | 18.4 [14.0–20.2] |
Segments of villus epithelium [VE] and follicle-associated epithelium [FAE] from nine non-inflammatory bowel disease [IBD] controls and eight patients with Crohn’s disease were mounted in Ussing chambers and exposed to vehicle or E. coli LF82. Comparisons were made with the Mann–Whitney U test. The values above, median [25th–75th percentiles], represent the recordings at 90 min, but there were no significant effects on TER or Isc by E. coli LF82 at any time point.
3.2.1. Increased translocation of LF82 through CD ileum
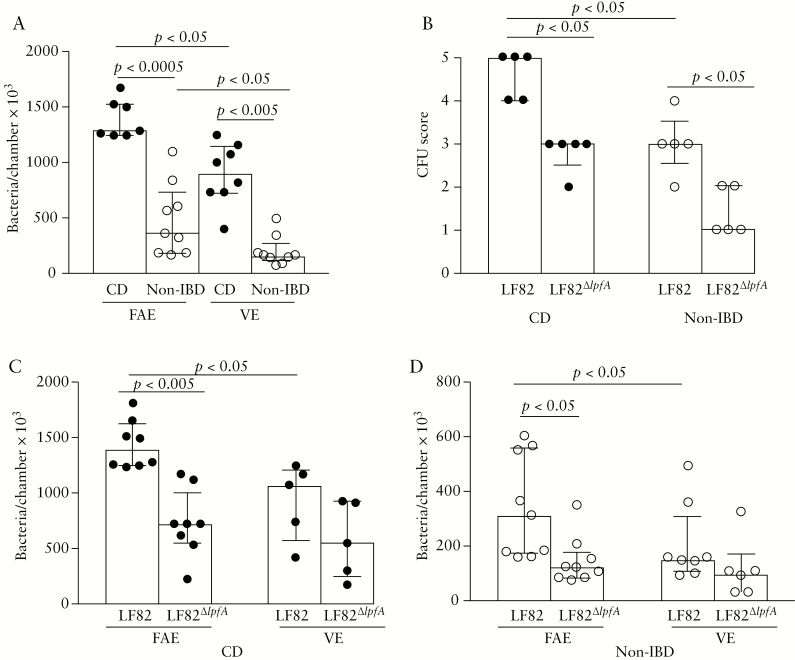
There was an increased translocation of E. coli LF82, as assessed by fluorometry, in patients with CD compared with controls, both in the FAE [p < 0.0005] and the VE [p < 0.005], and the translocation was greater across the FAE than across the VE in both groups, p < 0.05 [Figure 3A]. Overnight culture of serosal samples confirmed the increased LF82 translocation in the FAE of patients with CD compared with that of controls, p < 0.05 [Figure 3B].
Figure 3.
Translocation of live green fluorescent protein–labelled E. coli LF82 and E. coli LF82∆lpfA, the mutant strain with the long polar fimbriae [LPF] gene deleted, in the follicle-associated epithelium [FAE] and villus epithelium [VE] of patients with Crohn’s disease [CD] and of non-inflammatory bowel disease [IBD] controls. Segments of FAE and VE were obtained from ileum of the same individual and run in parallel in the Ussing chambers [two replicates/segment]. [A] Translocation of LF82 in patients with CD and in non-IBD controls. Bacterial translocation was determined at 488 nm and recalculated as bacteria/chamber × 103. [B] Scoring of colonies from overnight culture of serosal buffers on agar plates. [C] Translocation of LF82 and LF82∆lpfA in patients with CD and in controls [D]. Translocation of LF82∆lpfA was higher in patients with CD compared with in controls, both in the VE and the FAE, p < 0.0005. Bars represents the median [25th–75th percentiles], and comparisons between the two groups were carried out using the Mann–Whitney U test. One circle represents one individual.
3.2.2. Translocation of LF82 through human ileum was dependent on LPF and CEACAM6
To study the impact of LPF on LF82 translocation, experiments were performed with LF82 and its mutant lacking LPF. Results showed that there was significantly less translocation of LF82∆lpfA than of LF82 through the FAE of patients with CD, p < 0.005, and through that of controls, p < 0.05, but there was only a non-significant difference in VE, p = 0.1, in both CD patients [Figure 3C] and controls [Figure 3D]. There was no significant difference in LF82∆lpfA translocation between the epithelial types. Passage of LF82∆lpfA was higher in patients with CD compared with controls, both in VE and FAE, p < 0.0005 [Figure 3C, D]. These results indicate that LPF plays a greater role in LF82 uptake in FAE, and in patients with CD compared with controls. Overnight culture of samples from chambers mounted with FAE confirmed the increased LF82 translocation compared with LF82∆lpfA in patients with CD, and it also showed a significant difference in controls, p < 0.05 [Figure 3B].
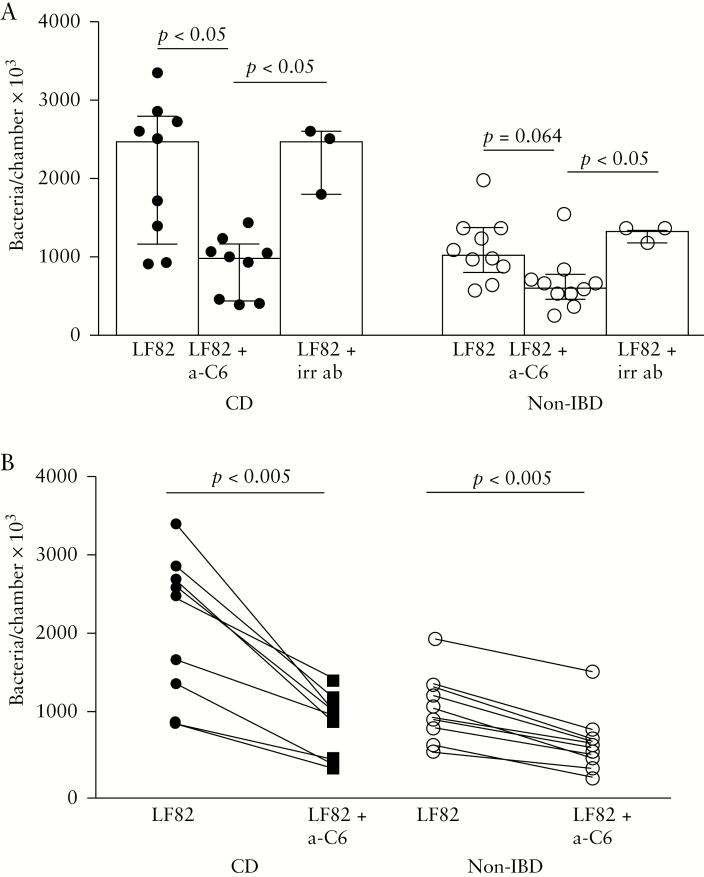
To further investigate impacts on LF82 translocation, experiments were performed with and without a blocker for CEACAM6. Results showed a 61% decrease in LF82 translocation after blocking of CEACAM6 in FAE from patients with CD, p < 0.05, and a 39% decrease in controls, p = 0.064 [Figure 4A]. Pre-incubation with the irrelevant antibody showed no effect on LF82 translocation [Figure 4A]. When comparing the effect of anti-CEACAM6 individually, using the Wilcoxon matched-pairs signed-rank test, there was a significant decrease in LF82 translocation in both patients with CD, p < 0.005, and controls, p < 0.005; however, the decrease was significantly higher in patients with CD, p < 0.05 [Figure 4B]. In FAE segments treated with the non-AIEC E. coli HS instead of AIEC E. coli LF82, no effect on translocation by pre-incubation with anti-CEACAM6 was observed, either in patients with CD {(E. coli HS: 950 [530–1370] bacteria/chamber × 103 vs E. coli HS + anti-CEACAM6 (1010 [670–1350])} or in controls (510 [360–660] vs 495 [410–525]).
Figure 4.
[A] Effects of blocking with anti-CEACAM6 [a-C6] on E. coli LF82 translocation in the follicle-associated epithelium of patients with Crohn’s disease [CD] and of non-inflammatory bowel disease [IBD] controls [two replicates/treatment]. Pre-incubation with irrelevant antibody [irr ab] instead of anti-CEACAM6 showed no inhibiting effect on LF82 translocation. [B] Individual effects on CEACAM6 blocking. Values represent the median [25th–75th percentiles], and comparisons between the two groups were carried out using the Mann–Whitney U test. Comparisons of individual effects were performed using the Wilcoxon matched-pairs signed-rank test. One circle/line represents one individual.
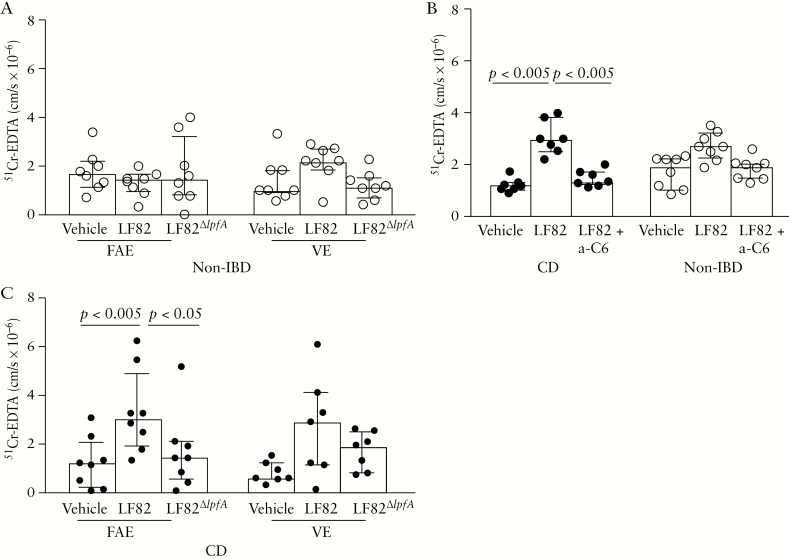
3.2.3. Increased51Cr-EDTApermeability in CD FAE by LF82 was dependent on CEACAM6
There was no significant difference when comparing baseline 51Cr-EDTA permeability between patients with CD and controls, or any significant differences between VE and FAE within the groups [Figure 5A, B]. Interestingly, when adding LF82 to the chambers, 51Cr-EDTA permeability increased compared with baseline in the FAE of patients with CD, p < 0.005. In the VE of patients with CD, there was also an increase in 51Cr-EDTA permeability, but the increase did not reach statistical significance, p = 0.078. In controls, there were no significant effects on 51Cr-EDTA permeability from LF82. The LF82-induced increase in paracellular permeability to 51Cr-EDTA through the FAE of patients with CD, p < 0.05, reverted to a normal level in tissues pre-incubated with anti-CEACAM6, p < 0.005 [Figure 5C]. There was no effect on electrophysiology by blocking of the CEACAM6 receptor [results not shown]. Addition of LF82∆lpfA to the chambers had no effect on permeability to 51Cr-EDTA in either patient group [Figure 5A, B].
Figure 5.
Effects of E. coli LF82, LF82∆lpfA, the mutant strain with the long polar fimbriae [LPF] gene deleted, and pre-incubation with anti-CEACAM6 [a-C6] on 51Cr-EDTA permeability in the follicle-associated epithelium [FAE] and villus epithelium [VE] of patients with Crohn’s disease [CD] and of non-inflammatory bowel disease [IBD] controls. Segments of FAE and VE were obtained from ileum of the same individual and run in parallel in the Ussing chambers [two replicates/segment and treatment]. [A–B] Effects on 51Cr-EDTA permeability by LF82 and LF82∆lpfA in patients with CD and non-IBD controls. When comparing the permeability in patients with CD with that in controls, it was revealed that the effect of LF82 on 51Cr-EDTA permeability was higher in patients with CD in both the VE [p < 0.005] and the FAE [p < 0.05]. The same pattern was seen for LF82∆lpfA, both in the VE [p < 0.05] and in the FAE [p < 0.005]. [C] Effects of LF82 on 51Cr-EDTA permeability with and without pre-incubation with a-C6. Bars represents the median [25th–75th percentiles], and comparisons between the two groups were made using the Mann–Whitney U test. Vehicle corresponds to chambers to which 51Cr-EDTA and Krebs buffer only were added. One circle represents one individual.
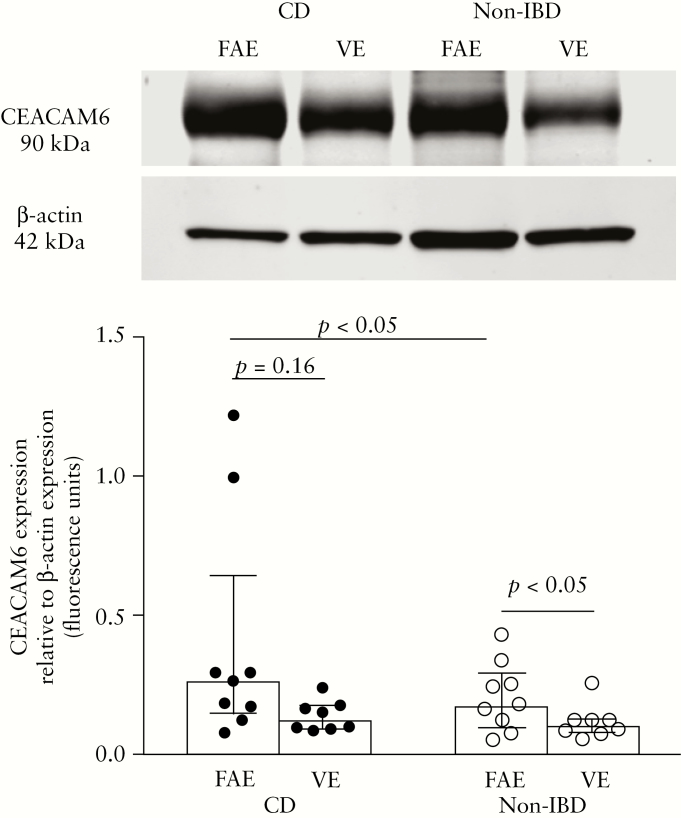
3.2.4. Increased expression of CEACAM6 in patients with CD
To define differences in CEACAM6 levels between patients with CD and controls, epithelial tissues were analyzed by western blotting. Analysis of tissue lysates revealed higher levels of CEACAM6 in the FAE of patients with CD compared with FAE from non-IBD controls, p < 0.05, but no significant difference could be observed in VE, p = 0.23 [Figure 6]. There was no significant difference between VE and FAE of patients with CD, p = 0.16; however, in controls the expression was significantly higher in FAE compared with VE, p < 0.05 [paired comparisons] [Figure 6]. In FAE from two patients, the expression of CEACAM6 was considerably higher compared with the median expression; however, these values were not judged to be outliers by the program and were therefore included in the results.
Figure 6.
Expression of CEACAM6 measured by western blotting in the follicle-associated epithelium [FAE] and villus epithelium [VE] of patients with Crohn’s disease [CD] and of non-inflammatory bowel disease [Non-IBD] controls. Segments of FAE and VE were obtained from ileum of the same individual and run in parallel. Tissue lysates from eight CD patients and eight controls were analysed in duplicate and the image shows a representative blot from one CD and one control, respectively. CEACAM6 [90 kDa] values were normalized to the loading control β-actin [mw 42 kDa]. Values represent the median [25th–75th percentiles]. Comparisons between two groups were done with the Mann–Whitney U test.
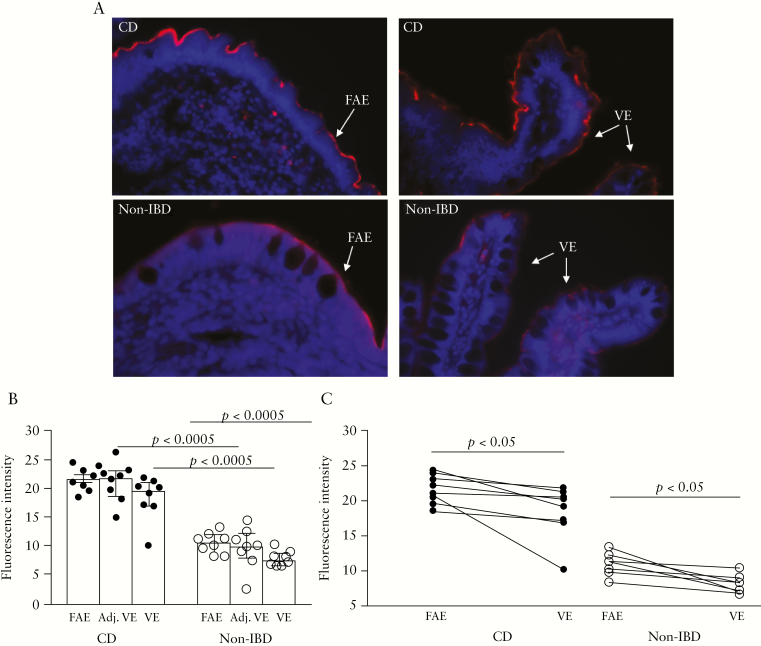
In order to confirm the western blotting results, and also to study the epithelial expression of CEACAM6 exclusively, sections of VE and FAE were stained for CEACAM6 by immunofluorescence. Representative staining is shown in Figure 7A. Analysis in ImageJ showed significantly higher apical CEACAM6 expression in FAE, VE, and adjacent-VE of patients with CD compared with controls [Figure 7B]. In both CD patients and controls, there was higher apical CEACAM6 expression in FAE compared with VE, p < 0.05 [Figure 7C].
Figure 7.
Expression of CEACAM6 revealed by immunofluorescence staining in the villus epithelium [VE], adjacent-VE, and follicle-associated epithelium [FAE] of patients with Crohn’s disease [CD] and of non-inflammatory bowel disease [IBD] controls. Segments of FAE and VE were obtained from ileum of the same individual, and three sections/segment and individual were used for immunofluorescence staining. [A] Representative photographs illustrating staining for CEACAM6 [red] and staining of the cell nuclei [blue] at ×400 magnification in FAE, adjacent-VE, and VE. [B] CEACAM6 expression was analyzed for fluorescence intensity of the epithelial cell lining using ImageJ Fiji Software. [C] CEACAM6 expression in FAE and VE of CD and controls, patient by patient. All values represent the median [25th–75th percentiles]. Comparisons between two groups were done with the Mann–Whitney U test. One circle/line represents one individual.
4. Discussion
Accumulating data indicate a central role for the intestinal microbiota in triggering inflammation in CD patients. Strains of E. coli have been associated with the occurrence of CD,41 and several isolated from CD patients have adherent-invasive properties, so are called AIEC.18,21–23,28 Here, we show for the first time that translocation of AIEC strain LF82 across the intestinal epithelium is enhanced in patients with CD compared with non-IBD controls, extending data from previous studies with epithelial cell lines. The increased uptake of wildtype LF82 in patients with CD was counteracted by anti-CEACAM6 antibodies and was not apparent in the strain lacking LPF, suggesting use of CEACAM6 or LPF antibodies could be a potential therapeutic approach to preventing AIEC-evoked recurrence in CD.
Our initial in vitro experiments confirmed previous findings,32 showing higher LF82 translocation in the FAE co-cultures compared with Caco2-cl1 mono-cultures. The increased bacterial passage in the FAE model might be due to the presence of M cell–like cells, but it could also be due to more abundant CEACAM6 expression, or both. Experiments with tissues mounted in Ussing chambers revealed a 3.5-fold higher LF82 translocation in FAE from patients with CD compared with controls, and more translocation of LF82 in FAE compared with VE, in both CD patients and controls. This confirms our previous findings with tissues from control patients,42 that LF82 targets FAE at a higher rate than VE, and uniquely illustrates the greater vulnerability of tissue from patients with CD to AIEC-evoked increases in permeability, compared with that from controls.
The next step was to study the influence of LPF on LF82 translocation. In vitro experiments showed the mutant LF82∆lpfA to pass at a lower rate compared with LF82 through model FAE, but not through Caco2-cl1. Ussing experiments on human tissues revealed higher LF82 translocation compared with LF82∆lpfA in FAE of both CD patients and controls. In VE, there was only a significant difference in patients with CD. These findings support the contention that LPF is crucial for E. coli LF82 translocation through the intestinal mucosa; moreover, LPF may be more important in VE during inflammation, since non-IBD controls showed no significant difference in passage between LF82 and its mutant. To our knowledge, there is no data supporting a direct interaction between LPF and CEACAM6, but the fact that LPF promotes translocation in the absence of CEACAM6 suggests that LPF is not using this protein as a receptor.
Our findings might be important for understanding the development of CD, since the earliest observable signs of CD are aphthoid lesions11 preceded by ultrastructural erosions in the FAE.12 With respect to CD pathogenesis or reactivation, we speculate that this could begin with dysbiosis and a bloom of [or increased] AIEC strains26 having an enhanced affinity to FAE in patients with CD and an increased rate of translocation into Peyer’s patches. These events could lead to enhanced antigen presentation in Peyer’s patches and local pro-inflammatory immune responses.7,43 Our findings could open up possibilities for new treatments targeting LF82 and other AIEC strains. Treatment with antibiotics is not optimal, because antibiotic resistance is increasing, and the risk of dysbiosis is evident.44 However, the promising results of targeting the adhesive properties of AIEC in IBD are supported by studies using synthetic mannosides,45 which have been shown to reduce LF82 in mouse gut, leading to decreased inflammatory responses.46 In addition, Galtier et al.47 recently showed that virulent bacteriophages were able to replicate in intestinal sections and faeces homogenates from murine gut samples colonized with E. coli LF82. Those authors subsequently proposed bacteriophages targeting AIEC as a new treatment for CD.
Previous in vitro studies have shown that enterocytes treated with TNF-α and IFN-γ, or infected with AIEC, express higher levels of the AIEC receptor CEACAM6.48 Moreover, there is an increased colonization of the gut by AIEC in transgenic mice expressing human CEACAMs.28,48 Studies have further shown that AIEC express type 1 pili variant to increase the binding capacity to the abnormally high CEACAM6 expression found in the ileum of CD patients.48,49 CEACAM6 is known to be involved in AIEC-adherence and has been shown to be overexpressed in the ileum of CD patients. Sasaki et al. showed that there are alterations in CD enterocytes, leading to the ability of AIEC to adhere to a greater extent.26 This was confirmed in an ex vivo study demonstrating that AIEC adhered only to enterocytes isolated from patients with CD, and not to enterocytes isolated from non-IBD patients.28,48 These results are in line with the findings of a pathological increase in CEACAM6 expression and AIEC affinity in CD patients.30 Considering this, our next step was to investigate the involvement of CEACAM6 on LF82 uptake by the FAE of CD patients, ex vivo in Ussing chambers. Our results demonstrated that once the CEACAM6 receptor was blocked by anti-CEACAM6, LF82 translocation significantly decreased in FAE from patients with CD. Our results indicate that CEACAM6 is crucial for the uptake and translocation of LF82 through the mucosa, is expressed to a higher extent in FAE, and seems to be upregulated in CD patients compared with controls. The upregulation of CEACAM6 in FAE was supported by western blot analyses showing higher expressions in CD FAE compared with in FAE from controls; however, no significant difference could be seen in VE. In contrast, immunofluorescence confirmed higher CEACAM6 expression in VE, FAE, and adjacent-VE of CD patients compared with equivalent tissues from the ileum of controls. This highlights the value of the complementary techniques; a difference would be overlooked if immunoblotting only was used. These findings support the theory provided by Darfeuille-Michaud et al. that AIEC promote the colonization of the mucosa of CD patients by inducing an increased CEACAM6 expression via the induced secretion of TNF-α by infected macrophages.48,50 Interestingly, we showed a higher CEACAM6 expression in FAE than in VE of the same individual. This suggests that FAE has a face to the lumen with increased levels of CEACAM6, facilitating LF82 uptake. An alternative explanation for upregulation of CEACAM6 is the hypothesis that CEACAM6 is a part of the innate immunity.51 In that case, it could play a protective role through trapping the invasive bacteria, and the whole complex of CEACAM6 and AIEC might then be released to the lumen from glycocalyx, hindering further invasion.52 However, our results do not support this hypothesis, since we found a decreased LF82 translocation when blocking CEACAM6.
Previous in vitro studies indicate that several pathogenic E. coli strains influence the paracellular pathway by affecting tight junctions and ion transport in intestinal epithelia cells.53,54 In the present study, exposure to LF82 was found to increase paracellular permeability in CD patients but not in controls. This points to differential vulnerability of the mucosa, and probably refers to that LF82 can more easily enter the epithelium of patients with CD, compared to controls, and affect the tight junctions due to upregulated CEACAM6 expression. Speculatively, this upregulation could be an initiating mechanism for the increased paracellular permeability seen in patients with CD. Since LF82 had an effect on 51Cr-EDTA permeability, and there was an effect on TER in vitro, one could have expected an effect on TER to also be seen in the ex vivo situation; however, TER ex vivo remained unchanged. This could relate to there being different regulation of permeability pathways, i.e. the leak and the pore pathways,55 but could also be a result of differences in TER between subjects being small and conventional TER measurements not being sensitive enough to fully detect differences between tissues.8,35
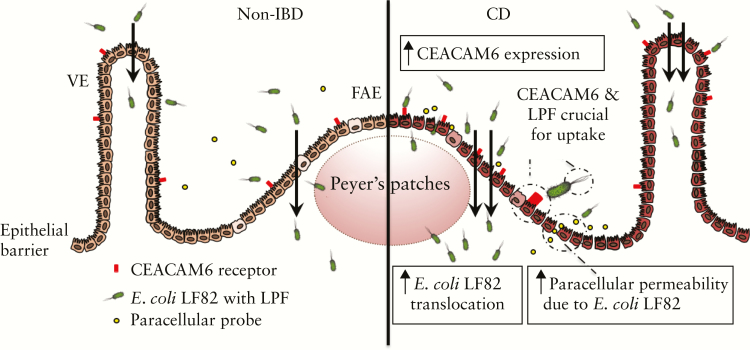
In conclusion, as illustrated in Figure 8, this study showed higher translocation of E. coli LF82 in patients with CD compared with controls, suggesting alterations in the barrier to LF82 in patients with CD. Our results further demonstrated that both bacterial LPF and host [CEACAM6] factors facilitate uptake and translocation of LF82, especially through the FAE. Our data provide insights into CD pathophysiology and might help elucidate and define novel therapeutic targets in CD to prevent recurrence before clinical symptoms and complications occur.
Figure 8.
Schematic overview of a proposed scenario based on the results. Scenario involves a higher translocation of E. coli LF82 in patients with Crohn’s disease [CD] compared with non-inflammatory bowel disease [IBD] controls, suggesting alterations in the barrier to LF82 in CD. Bacterial passage is highly dependent on both bacterial long polar fimbriae [LPF] and expression of the host receptor CEACAM6, especially in the FAE. Scenario further demonstrate that E. coli LF82 exposure increases paracellular permeability in the follicle-associated epithelium [FAE] of patients with CD. VE = villus epithelium.
This article is dedicated to Professor Arlette Darfeuille-Michaud, in loving memory.56,57
Funding
This work was supported by grants from the International Organization for the Study of Inflammatory Bowel Disease [ÅVK], Lions Clubs International Foundation [ÅVK], ALF Grants Region Östergötland [JDS], and the Swedish Research Council [VR-Medicine and Health, 2014–02537, 2017–02475 JDS, ÅVK].
Conflict of Interest
The authors disclose no competing interests.
Acknowledgments
We thank all of the patients for their participation in this study. We also thank Mrs Ylva Braaf, Mr Carlos D. L. Magana and Mr Martin E. Winberg at Linköping University for assistance in the Ussing laboratory, and Mr Felipe Meira de Faria, Linköping University, for help regarding western blot analyses. Parts of this work were presented at the Falk symposium ‘IBD: Microbiota versus the barrier’, Stuttgart, 2013 and at the Falk symposium ‘IBD 2014: Thinking out of the box’, Paris, 2014.
Glossary
Abbreviations
- AIEC
adherent-invasive E. coli
- Caco-2cl1
colorectal epithelial clone 1
- CD
Crohn’s disease
- CEACAM6
carcinoembryonic antigen-related cell-adhesion molecule 6
- 51Cr-EDTA
51Chromium-EDTA
- CFU
colony-forming unit
- DMEM
Dulbecco’s modified Eagle’s medium
- E. coli
Escherichia coli
- FAE
follicle-associated epithelium
- GFP
green fluorescent protein
- IBD
inflammatory bowel disease
- Isc
short-circuit current
- LPF
long polar fimbriae
- LB
Luria-Bertani
- M cell
membranous/microfold cell
- PD
transepithelial potential difference
- TER
transepithelial resistance
- VE
villus epithelium
Author Contributions
ÅVK: Conception and design of the study; acquisition, analysis, and interpretation of data; main responsibility for drafting the article and preparing the final version before approval. LYA: Acquisition, analysis, and interpretation of in vitro data; involvement in drafting of the article and approval of the final version. EBH: Acquisition of patients and clinical data; involvement in drafting of the article and approval of the final version. SDSH: Acquisition, analysis, and interpretation of western blot and immunofluorescence data; involvement in drafting of the article and approval of the final version. BC: Conception and design of the study; interpretation of data; involvement in drafting of the article and approval of the final version. AD-M: Conception and design of the study; interpretation of data. DMM: Interpretation of data; critical revision for important intellectual content and involvement in approval of the final version. JDS: Initiation, conception, and design of the study; acquisition of ethics approval; interpretation of data; involvement in drafting of the article and approval of the final version.
References
- 1. Vermeire S, van Assche G, Rutgeerts P. Review article: altering the natural history of Crohn’s disease—evidence for and against current therapies. Aliment Pharmacol Ther 2007;25:3–12. [DOI] [PubMed] [Google Scholar]
- 2. Podolsky DK. Inflammatory bowel disease. N Engl J Med 2002;347:417–29. [DOI] [PubMed] [Google Scholar]
- 3. Söderholm JD, Peterson KH, Olaison G, et al. Epithelial permeability to proteins in the noninflamed ileum of Crohn’s disease? Gastroenterology 1999;117:65–72. [DOI] [PubMed] [Google Scholar]
- 4. Söderholm JD, Streutker C, Yang PC, et al. Increased epithelial uptake of protein antigens in the ileum of Crohn’s disease mediated by tumour necrosis factor alpha. Gut 2004;53:1817–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Meddings JB. Review article: intestinal permeability in Crohn’s disease. Aliment Pharmacol Ther 1997;11[Suppl 3]: 47–53; discussion 53–6. [DOI] [PubMed] [Google Scholar]
- 6. Zeissig S, Bojarski C, Buergel N, et al. Downregulation of epithelial apoptosis and barrier repair in active Crohn’s disease by tumour necrosis factor alpha antibody treatment. Gut 2004;53:1295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Salim SY, Silva MA, Keita AV, et al. CD83+CCR7– dendritic cells accumulate in the subepithelial dome and internalize translocated Escherichia coli HB101 in the Peyer’s patches of ileal Crohn’s disease. Am J Pathol 2009;174:82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Keita AV, Salim SY, Jiang T, et al. Increased uptake of non-pathogenic E. coli via the follicle-associated epithelium in longstanding ileal Crohn’s disease. J Pathol 2008;215:135–44. [DOI] [PubMed] [Google Scholar]
- 9. Rutgeerts P, Goboes K, Peeters M, et al. Effect of faecal stream diversion on recurrence of Crohn’s disease in the neoterminal ileum. Lancet 1991;338:771–4. [DOI] [PubMed] [Google Scholar]
- 10. D’Haens GR, Geboes K, Peeters M, Baert F, Penninckx F, Rutgeerts P. Early lesions of recurrent Crohn’s disease caused by infusion of intestinal contents in excluded ileum. Gastroenterology 1998;114:262–7. [DOI] [PubMed] [Google Scholar]
- 11. Morson BC. The early histological lesion of Crohn’s disease. Proc R Soc Med 1972;65:71–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fujimura Y, Kamoi R, Iida M. Pathogenesis of aphthoid ulcers in Crohn’s disease: correlative findings by magnifying colonoscopy, electron microscopy, and immunohistochemistry. Gut 1996;38:724–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kraehenbuhl JP, Neutra MR. Epithelial M cells: differentiation and function. Annu Rev Cell Dev Biol 2000;16:301–32. [DOI] [PubMed] [Google Scholar]
- 14. Marra A, Isberg RR. Invasin-dependent and invasin-independent pathways for translocation of Yersinia pseudotuberculosis across the Peyer’s patch intestinal epithelium. Infect Immun 1997;65:3412–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jones BD, Ghori N, Falkow S. Salmonella typhimurium initiates murine infection by penetrating and destroying the specialized epithelial M cells of the Peyer’s patches. J Exp Med 1994;180:15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gullberg E, Keita AV, Salim SY, et al. Identification of cell adhesion molecules in the human follicle-associated epithelium that improve nanoparticle uptake into the Peyer’s patches. J Pharmacol Exp Ther 2006;319:632–9. [DOI] [PubMed] [Google Scholar]
- 17. Sartor RB. The influence of normal microbial flora on the development of chronic mucosal inflammation. Res Immunol 1997;148:567–76. [DOI] [PubMed] [Google Scholar]
- 18. Swidsinski A, Ladhoff A, Pernthaler A, et al. Mucosal flora in inflammatory bowel disease. Gastroenterology 2002;122:44–54. [DOI] [PubMed] [Google Scholar]
- 19. Neut C, Bulois P, Desreumaux P, et al. Changes in the bacterial flora of the neoterminal ileum after ileocolonic resection for Crohn’s disease. Am J Gastroenterol 2002;97:939–46. [DOI] [PubMed] [Google Scholar]
- 20. Ott SJ, Musfeldt M, Wenderoth DF, et al. Reduction in diversity of the colonic mucosa associated bacterial microflora in patients with active inflammatory bowel disease. Gut 2004;53:685–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Darfeuille-Michaud A, Boudeau J, Bulois P, et al. High prevalence of adherent-invasive Escherichia coli associated with ileal mucosa in Crohn’s disease. Gastroenterology 2004;127:412–21. [DOI] [PubMed] [Google Scholar]
- 22. Martin HM, Campbell BJ, Hart CA, et al. Enhanced Escherichia coli adherence and invasion in Crohn’s disease and colon cancer. Gastroenterology 2004;127:80–93. [DOI] [PubMed] [Google Scholar]
- 23. Darfeuille-Michaud A, Neut C, Barnich N, et al. Presence of adherent Escherichia coli strains in ileal mucosa of patients with Crohn’s disease. Gastroenterology 1998;115:1405–13. [DOI] [PubMed] [Google Scholar]
- 24. Martinez-Medina M, Aldeguer X, Lopez-Siles M, et al. Molecular diversity of Escherichia coli in the human gut: new ecological evidence supporting the role of adherent-invasive E. coli [AIEC] in Crohn’s disease. Inflamm Bowel Dis 2009;15:872–82. [DOI] [PubMed] [Google Scholar]
- 25. Baumgart M, Dogan B, Rishniw M, et al. Culture independent analysis of ileal mucosa reveals a selective increase in invasive Escherichia coli of novel phylogeny relative to depletion of Clostridiales in Crohn’s disease involving the ileum. ISME J 2007;1:403–18. [DOI] [PubMed] [Google Scholar]
- 26. Sasaki M, Sitaraman SV, Babbin BA, et al. Invasive Escherichia coli are a feature of Crohn’s disease. Lab Invest 2007;87:1042–54. [DOI] [PubMed] [Google Scholar]
- 27. Kleessen B, Kroesen AJ, Buhr HJ, Blaut M. Mucosal and invading bacteria in patients with inflammatory bowel disease compared with controls. Scand J Gastroenterol 2002;37:1034–41. [DOI] [PubMed] [Google Scholar]
- 28. Barnich N, Darfeuille-Michaud A. Abnormal CEACAM6 expression in Crohn disease patients favors gut colonization and inflammation by adherent-invasive E. coli. Virulence 2010;1:281–2. [DOI] [PubMed] [Google Scholar]
- 29. Fang X, Monk JM, Nurk S, et al. Metagenomics-based, strain-level analysis of Escherichia coli from a time-series of microbiome samples from a Crohn’s disease patient. Front Microbiol 2018;9:2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Barnich N, Carvalho FA, Glasser AL, et al. CEACAM6 acts as a receptor for adherent-invasive E. coli, supporting ileal mucosa colonization in Crohn disease. J Clin Invest 2007;117:1566–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Denizot J, Sivignon A, Barreau F, et al. Adherent-invasive Escherichia coli induce claudin-2 expression and barrier defect in CEABAC10 mice and Crohn’s disease patients. Inflamm Bowel Dis 2012;18:294–304. [DOI] [PubMed] [Google Scholar]
- 32. Chassaing B, Rolhion N, de Vallée A, et al. Crohn disease–associated adherent-invasive E. coli bacteria target mouse and human Peyer’s patches via long polar fimbriae. J Clin Invest 2011;121:966–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 2000;97:6640–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Valdivia RH, Falkow S. Bacterial genetics by flow cytometry: rapid isolation of Salmonella typhimurium acid-inducible promoters by differential fluorescence induction. Mol Microbiol 1996;22:367–78. [DOI] [PubMed] [Google Scholar]
- 35. Keita AV, Gullberg E, Ericson AC, et al. Characterization of antigen and bacterial transport in the follicle-associated epithelium of human ileum. Lab Invest 2006;86:504–16. [DOI] [PubMed] [Google Scholar]
- 36. Kernéis S, Bogdanova A, Kraehenbuhl JP, Pringault E. Conversion by Peyer’s patch lymphocytes of human enterocytes into M cells that transport bacteria. Science 1997;277:949–52. [DOI] [PubMed] [Google Scholar]
- 37. Keita AV, Carlsson AH, Cigéhn M, Ericson AC, McKay DM, Söderholm JD. Vasoactive intestinal polypeptide regulates barrier function via mast cells in human intestinal follicle-associated epithelium and during stress in rats. Neurogastroenterol Motil 2013;25:e406–17. [DOI] [PubMed] [Google Scholar]
- 38. Roberts CL, Keita AV, Duncan SH, et al. Translocation of Crohn’s disease Escherichia coli across M-cells: contrasting effects of soluble plant fibres and emulsifiers. Gut 2010;59:1331–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lewis K, Caldwell J, Phan V, et al. Decreased epithelial barrier function evoked by exposure to metabolic stress and nonpathogenic E. coli is enhanced by TNF-alpha. Am J Physiol Gastrointest Liver Physiol 2008;294:G669–78. [DOI] [PubMed] [Google Scholar]
- 40. Grass GM, Sweetana SA. In vitro measurement of gastrointestinal tissue permeability using a new diffusion cell. Pharm Res 1988;5:372–6. [DOI] [PubMed] [Google Scholar]
- 41. Chassaing B, Darfeuille-Michaud A. The commensal microbiota and enteropathogens in the pathogenesis of inflammatory bowel diseases. Gastroenterology 2011;140:1720–28. [DOI] [PubMed] [Google Scholar]
- 42. Chassaing B, Darfeuille-Michaud A. The interaction of Crohn’s disease–associated Escherichia coli to Peyer’s patches of the intestinal mucosa involves long polar fimbriae. Med Sci 2011;27:572–3. [DOI] [PubMed] [Google Scholar]
- 43. Powell JJ, Thomas-McKay E, Thoree V, et al. An endogenous nanomineral chaperones luminal antigen and peptidoglycan to intestinal immune cells. Nat Nanotechnol 2015;10:361–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bartosch S, Fite A, Macfarlane GT, McMurdo ME. Characterization of bacterial communities in feces from healthy elderly volunteers and hospitalized elderly patients by using real-time PCR and effects of antibiotic treatment on the fecal microbiota. Appl Environ Microbiol 2004;70:3575–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hartmann M, Papavlassopoulos H, Chandrasekaran V, et al. Inhibition of bacterial adhesion to live human cells: activity and cytotoxicity of synthetic mannosides. FEBS Lett 2012;586:1459–65. [DOI] [PubMed] [Google Scholar]
- 46. Sivignon A, Yan X, Alvarez Dorta D, et al. Development of heptylmannoside-based glycoconjugate antiadhesive compounds against adherent-invasive Escherichia coli bacteria associated with Crohn’s disease. MBio 2015;6:e01298–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Galtier M, De Sordi L, Sivignon A, et al. Bacteriophages targeting adherent invasive Escherichia coli strains as a promising new treatment for Crohn’s disease. J Crohns Colitis 2017;11:840–7. [DOI] [PubMed] [Google Scholar]
- 48. Barnich N, Carvalho FA, Glasser AL, et al. CEACAM6 acts as a receptor for adherent-invasive E. coli, supporting ileal mucosa colonization in Crohn disease. J Clin Invest 2007;117:1566–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Carvalho FA, Barnich N, Sivignon A, et al. Crohn’s disease adherent-invasive Escherichia coli colonize and induce strong gut inflammation in transgenic mice expressing human CEACAM. J Exp Med 2009;206:2179–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Glasser AL, Boudeau J, Barnich N, Perruchot MH, Colombel JF, Darfeuille-Michaud A. Adherent invasive Escherichia coli strains from patients with Crohn’s disease survive and replicate within macrophages without inducing host cell death. Infect Immun 2001;69:5529–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hammarström S, Baranov V. Is there a role for CEA in innate immunity in the colon? Trends Microbiol 2001;9:119–25. [DOI] [PubMed] [Google Scholar]
- 52. Keenan JI, Hooper EM, Tyrer PC, Day AS. Influences of enteral nutrition upon CEACAM6 expression by intestinal epithelial cells. Innate Immun 2014;20:848–56. [DOI] [PubMed] [Google Scholar]
- 53. Berkes J, Viswanathan VK, Savkovic SD, Hecht G. Intestinal epithelial responses to enteric pathogens: effects on the tight junction barrier, ion transport, and inflammation. Gut 2003;52:439–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yu Q, Yuan L, Deng J, Yang Q. Lactobacillus protects the integrity of intestinal epithelial barrier damaged by pathogenic bacteria. Front Cell Infect Microbiol 2015;5:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Shen L, Weber CR, Raleigh DR, Yu D, Turner JR. Tight junction pore and leak pathways: a dynamic duo. Annu Rev Physiol 2011;73:283–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Raisch J, Sivignon A, Chassaing B, et al. In memoriam, Arlette Darfeuille-Michaud, PhD. Gastroenterology 2014;147:943–4. [DOI] [PubMed] [Google Scholar]
- 57. Raisch J, Sivignon A, Chassaing B, et al. In memoriam, Arlette Darfeuille-Michaud, PhD. Gut 2014;63:1681–2. [DOI] [PubMed] [Google Scholar]