Abstract
Purpose
Dietary inflammatory index (DII) is a new tool for assessing the inflammatory potential of diet. Since there is no study that has investigated the association of DII and benign breast diseases (BBD), the aim of our study was to compare DII scores in patients with and without BBD.
Methods
One hundred and eleven (111) subjects with BBD and 104 healthy women attending the Iranian Center for Breast Cancer affiliated with the Academic Center for Education, Culture and Research were enrolled in a case–control study. Dietary data collected using a 168‑item validated food frequency questionnaire (FFQ). Energy-adjusted DII was calculated based on FFQ. Socio demographic data were collected by interview. In addition, physical activity was measured by the International Physical Activity Questionnaire (IPAQ). Weight, height and waist circumference were also measured.
Results
After adjustment for multiple confounding variables, participants at the highest tertile of DII had increased OR for BBD (OR=1.7, 95% CI=0.75–3.95) (P-trend =0.04).
Conclusion
The increased chance of BBD was suggested with a higher consumption of diets with inflammatory potential. However, this result should be interpreted with caution as OR was not statistically significant. Interventional studies are warranted to elucidate the role of inflammatory diets in the development of BBD.
Keywords: benign breast disease, breast cancer, diet, dietary inflammatory index, inflammation
Introduction
Benign Breast Diseases (BBD) are noncancerous mammary gland diseases classified into three groups based on the risk of breast cancer: (1) non-proliferative, (2) proliferative lesions without atypia and (3) proliferative diseases with atypia. For example, diseases such as idiopathic granulomatous mastitis (IGM), benign proliferative breast disease (BPBD) and lobular hyperplasia are placed in these three groups, respectively. The second and third groups are associated with a higher risk of breast cancer.1
Previous studies showed the role of chronic inflammation in some diseases2–4 including cancer.5 IGM is known as an unusual kind of chronic inflammatory diseases.6 The levels of inflammation biomarkers such as interleukin-33 (IL-33) and soluble ST2 receptor of IL-33 (sST2) were significantly higher in women with IGM compared with controls.7 In addition, based on the result of a nested case–control study on 667 women with BPBD and 1321 controls, serum levels of C-reactive protein (CRP) were positively and adiponectin were inversely associated with the early stages of BPBD.8
In addition to the role of inflammation, other various causes such as endogenous hormones, oral contraceptives, estrogen replacement therapy and dietary intake during adolescence are important factors in the development of BBD.9–11 There are some foods and nutrients with pro- or anti-inflammatory properties. For instance, dietary intake of magnesium, fiber, ω-3 polyunsaturated fatty acids (PUFAs), monounsaturated fatty acids (MUFAs), flavonoids, and carotenoids was associated with lower serum inflammatory biomarkers, whereas intake of saturated fatty acids (SFAs), trans fatty acids (TFAs), high-glycemic-index (GI) carbohydrates, and a high ω-6 to ω-3 PUFAs ratio were associated with higher levels of these biomarkers.12 In addition, adherence to a healthy dietary pattern, especially rich in vegetables and fruits, was associated with lower concentrations of inflammatory biomarkers.13,14 Similar results were shown for adherence to Mediterranean dietary pattern, which is high in MUFAs, ω-3, fruits, vegetables, legumes, and grains.12
Some studies investigated the relationship between dietary factors and BBD. In a prospective study in adolescents, higher β-carotene intake was associated with a lower risk of BBD.15 Intake of alcohol during adolescence was positively associated with the risk of BBD, whereas intake of vegetable fat, vitamin E, fiber and nuts was inversely associated with the risk of BBD.16 In addition, consuming fried dishes more often than boiled dishes were positively associated with the risk of BBD.17
In Iran, some preparation methods such as frying are very common which may have a substantial effect on inflammation.18 In addition, the oils used for frying in Iran have high amounts of TFAs19 which was related to inflammation.12
Dietary inflammatory index (DII) was introduced in 2009 as an applicable tool that could categorize individuals’ dietary intake from anti-inflammatory to pro-inflammatory to assess the overall inflammatory potential of a diet.20 This index is associated with serum inflammatory markers, including CRP, interleukin 6 (IL-6) and tumor necrosis factor alpha (TNF-α).21–23 In the present study, we aimed to investigate the association between DII and chance of BBD, which has not been investigated in any other study. The results of this investigation can be used to design prospective and interventional studies.
Subjects and Methods
Design and Participants
The present study is part of a case–control study, which designed to compare dietary intakes between subjects with BBD or breast cancer with healthy women from February 2014 to April 2015. This study was performed in accordance with the Declaration of Helsinki. All participants completed written informed consent. The study was approved by the Ethics Committee of Tehran University of Medical Sciences. Participants were attending the Iranian Center for Breast Cancer affiliated with the Academic Center for Education, Culture and Research (ACECR) in Tehran. The present study included 115 patients with BBD (cases) and 116 controls. The cases were diagnosed by a pathologist within the previous month of study. The sample size was determined according to Haung et al study,24 with α = 0.05 and β = 0.2, the risk of breast cancer in Quartile 1 DII equal to 0.43 and the odds ratio (OR) 2.08. The two groups were matched by age (20–30, 31–40, 41–50 and 51–65 years) and menopause status using the frequency matching method. Women diagnosed within the past 1 month with one type of BBD such as, fibrocystic diseases, ductal ectasia, fat necrosis, papillomatosis, adenosis with and without sclerosing, fibroadenoma, ductal hyperplasia, atypical lobular hyperplasia and atypical ductal hyperplasia by the physicians of ACECR were considered as cases. The controls were women accompanying patients or women who had no symptoms or complaints of BBD after referring to the center for check-up. They were healthy at the clinical examination. Exclusion criteria were, BMI≥40 kg/m2, subjects with history of heart disease, diabetes, hypertension, dyslipidemia, kidney or liver diseases, stroke, food allergies, multiple sclerosis, Parkinson’s disease, alcohol or drug addiction and the use of any tobacco at the moment or quit within the past 3 months based on their reports. In addition, participants with current pregnancy or lactation in the past year, hormone therapy in the last 3 months, taking any special, edible or injectable dietary supplement in the past month and have any particular diet in the last 2 months were excluded from the study. The mentioned diseases and severe obesity are related to inflammation.25 In addition, smoking causes a strong inflammatory reaction.26 Therefore, we excluded women with these conditions.
Socio-Demographic, Anthropometric and Physical Activity Measurements
Socio-demographic and medical data such as age, place of residence, level of education, occupation, marital status, age of menarche, age of the first pregnancy, lactation, menopause, contraceptive methods and estrogen therapy, family history of breast diseases, smoking, tobacco and dietary supplement intake were collected using face-to-face interview.
Height and weight were measured when participants wearing minimal clothing and no shoes by a manufacturer tape to the nearest 0.5 cm and digital weighing scale (Seca: 813; Seca United Kingdom) to the nearest 0.1 kg, respectively. BMI was calculated through weight (kg) divided by square of the height (m2). Measurement of waist circumference was done by using a tape at the standing position at the level midway between the lower rib margin and the iliac crest at the end of a gentle expiration to the nearest 1 mm. The short form of the International Physical Activity Questionnaire (IPAQ)27 was used in order to measure the physical activity of the participants. In this regard, participants reported frequency (d) and duration (min) of severe, moderate, jogging and sedentary physical activity during the past 7 days, expressed as metabolic equivalent hours per week (METs h/wk).
Dietary Assessment and Dietary Inflammatory Index Calculation
Dietary intake of participants was calculated using the validated semi-quantitative food frequency questionnaire, with 168 food items, utilized in the Tehran Lipid and Glucose study.28 Participants were asked about the frequency of dietary intake during the past year on a daily, weekly, or monthly basis. Then, portion sizes of consumed foods in household measures converted to grams. Energy and nutrient content of foods and beverages analyzed by Nutritionist 4 software (First Data bank Inc., Hearst Corp., San Bruno, CA) modified for Iranian foods.
DII was calculated through 7 steps, which has been explained by Shivappa.20 In this regard, 31 parameters were included in the calculation of DII which was energy, carbohydrate, protein, total fat, cholesterol, SFAs, MUFAs, PUFAs, n-3 and n-6 PUFAs, vitamins such as, B12, B9, B6, B3, B2, B1, A, C, D and E, micronutrients such as, iron, zinc, selenium, magnesium, caffeine, tea, garlic, onion, pepper and β-carotene. First, energy-adjusted DII was computed using the residual method.29 Then, a variable called Z-score is obtained by the ratio of the difference between the global average intake of dietary factor from the reported intake of each participant to the global standard deviation. Then, we converted Z-score to the percentile and used the following formula;
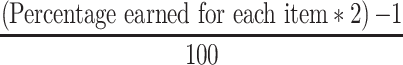 |
The resulting value for each dietary factor was multiplied by the inflammatory score of each item to achieve the dietary factor DII score. Then, all dietary factor DII scores were summed to calculate the DII score for each participant.
Statistical Analysis
The data analyzed using SPSS 16 for Windows (SPSS Inc., Chicago, IL). Differences in the continuous and categorical variables were compared using independent-sample t-test or analysis of variance (ANOVA) and Chi-square test, respectively. Participants with energy intake out of 500–3500 kcal per day were excluded from the study.30,31 In this regard, four cases and twelve controls were excluded from the data analysis. Using simple logistic regression, we estimated the odds ratio (OR) and 95% confidence intervals (CIs) of BBD for DII. In model 1 we adjusted for age and menopause status. In model 2 we adjusted further for estrogen therapy, family history of breast disease, use of dietary supplement, matrimony, BMI, physical activity and energy intake to assess the relation between DII and BBD. Variables used for matching of the two groups and baseline covariates that showed signs of having too large a difference between groups were selected for adjustment which included age (year), menopause status (menopause/nonmenopause), estrogen therapy (yes/no), family history of breast cancer (yes/no), intake of dietary supplement (yes/no), and matrimony (single/married). In addition, we adjusted for physical activity (METs/h/wk),32,33 BMI (kg/m2),34,35 and energy intake (kcal)36 that known to be related to BBD. P-values <0.05 were considered significant.
Results
Characteristics of the participants are shown in Table 1. Most of baseline characteristics of participants including age, educational levels, age of menarche, age of first pregnancy, lactation history, physical activity, energy intake, BMI, waist circumference, menopause status, occupation and marital status were similar between the two groups (Table 1). However, cases reported a higher family history of breast diseases (68% versus 31%) and higher dietary supplement intake (49% versus 35%) and lower estrogen therapy (5% versus 23%) (data not shown).
Table 1.
Characteristics of Participants with Benign Breast Diseases (Cases) and the Controls
| Variables | Cases | N(%) | Controls | N(%) |
|---|---|---|---|---|
| Age (years) | 111 | 104 | ||
| 20–40(y) | 40(36) | 43(41.3) | ||
| 41–65(y) | 71(64) | 61(58.7) | ||
| Education (years) | 111 | 104 | ||
| ≤10(y) | 29(26.1) | 22(21.2) | ||
| >10(y) | 82(73.9) | 82(78.8) | ||
| Menarche (years) | 102 | 101 | ||
| <15(y) | 84(82.4) | 82(81.2) | ||
| ≥15(y) | 18(17.6) | 19(18.8) | ||
| First pregnancy (years) | 93 | 73 | ||
| <23(y) | 56(60.2) | 39(53.4) | ||
| ≥23(y) | 37(39.8) | 34(46.6) | ||
| Lactation history (months) | 82 | 69 | ||
| 2–35(m) | 43(52.4) | 29(42) | ||
| 36–70(m) | 35(42.7) | 30(43.5) | ||
| 71–98(m) | 4(4.9) | 10(14.5) | ||
| Physical activity (MET-min/week) | 110 | 104 | ||
| Low(<600MET-min/week) | 83(75.5) | 73(70.2) | ||
| Moderate(600–1500 MET-min/week) | 19(17.3) | 21(20.2) | ||
| High(>1500MET-min/week | 8(7.3) | 10(9.6) | ||
| BMI (kg/m2) | 111 | 103 | ||
| <25 | 32(28.8) | 38(36.9) | ||
| 25–30 | 54(48.6) | 40(38.8) | ||
| >30 | 25(22.5) | 25(24.3) | ||
| Matrimony | 110 | 104 | ||
| Single | 14(12.7) | 23(22.1) | ||
| Married | 96(87.3) | 81(77.9) | ||
| Menopause | 111 | 104 | ||
| Non-menopause | 92(82.9) | 77(74) | ||
| Menopause | 19(17.1) | 27(26) | ||
| Energy-adjusted Dietary inflammatory index(EDII) | 111 | 0.07(1.72)* | 104 | −0.13(1.67)* |
Note: *Mean (SD).
The characteristics of the control group across the tertiles of the DII score based on the distribution of the control group are presented in Table 2. There were no significant differences in general characteristics across the tertiles of the DII score.
Table 2.
Characteristics of 104 Control Group Across the Dietary Inflammatory Index (DII) Tertiles (T) in a Case-Control Study
| T1 (Low) (−3.80-(−0.91)) N(%) |
T2 (−0.82–0.39) N(%) |
T3 (High) (0.45–4.53) N(%) |
P-Trend | |
|---|---|---|---|---|
| Job Housewife Working |
19(55.9) 15(44.1) |
21(60) 14(40) |
19(54.3) 16(45.7) |
0.8a |
| Matrimony Single Married |
8(23.5) 26(76.5) |
6(17.1) 29(82.9) |
9(25.7) 26(74.3) |
0.6a |
| Menopause Status Non-menopause Menopause |
26(76.5) 8(23.5) |
24(68.6) 11(31.4) |
27(77.1) 8(22.9) |
0.6a |
| Oral Contraceptive Pill Intake No Yes |
21(61.8) 13(38.2) |
19(54.3) 16(45.7) |
19(54.3) 16(45.7) |
0.7a |
| History of Estrogen Therapy No Yes |
27(79.4) 7(20.6) |
28(80) 7(20) |
25(71.4) 10(28.6) |
0.6a |
| Family History of Breast Disease No Yes |
22(64.7) 12(35.3) |
27(77.1) 8(22.9) |
22(62.9) 13(37.1) |
0.3a |
| Number of Pregnancy ≤2 >2 |
11(47.8) 12(52.2) |
13(50) 13(50) |
14(58.3) 10(41.7) |
0.7a |
| Intake of Dietary Supplement No Yes |
21(61.8) 13(38.2) |
20(57.1) 15(42.9) |
26(74.3) 9(25.7) |
0.3a |
| Mean(SD) | ||||
| Age (years) | n=34 44.7(10.6) |
n=35 41.4(10.1) |
n=35 39.06(9.8) |
0.07b |
| Education (years) | n=34 13.4(3.8) |
n=35 11.7(4.4) |
n=35 12.2(4.5) |
0.2b |
| Menarche (years) | n=34 13.3(1.3) |
n=32 13.2(1.3) |
n=35 13.06(1.5) |
0.7b |
| Age at first pregnancy (years) | n=23 24.1(4.5) |
n=26 22.1(5.5) |
n=24 22.04(4.4) |
0.2b |
| Lactation history (months) | n=23 38.5(21.05) |
n=24 37.1(20.9) |
n=22 41.5(4.3) |
0.7b |
| BMI (kg/m2) | n=34 27.5(4.4) |
n=34 27.2(3.9) |
n=35 25.7(4.5) |
0.1b |
| Energy intake (kcal) | n=34 2207.5(465.1) |
n=35 2081.3(609.02) |
n=35 2195.5(579.02) |
0.5b |
| Physical activity (MET-min/week) | n=34 695.2(666.1) |
n=35 579.1(635.6) |
n=35 406.1(500.8) |
0.1b |
Notes: aChi-square test. bANOVA test.
Table 3 presents the distribution of inflammatory and anti-inflammatory food parameters across the DII tertiles. Compared with women in the first tertile, women in the third tertile had much higher consumption of total fat (p<0.001), cholesterol (p=0.02), SFAs (p<0.001), MUFAs (p<0.001) but lower intake of fiber (p<0.01), vitamin A (p<0.001), vitamin C (p<0.001), vitamin B9 (p=0.003), vitamin B6 (p<0.001), magnesium (p<0.001), garlic (p=0.007), onion (p=0.04) and beta carotene (p<0.001).
Table 3.
Distribution of Inflammatory and Anti-Inflammatory Parameters Across Tertiles (T) of Energy-Adjusted Dietary Inflammatory Index (DII)
| T1 (−3.8-(−0.84)) |
T2 (−0.82–0.63) Mean (SD) |
T3 (0.64–4.53) |
P-Trenda | |
|---|---|---|---|---|
| Inflammatory Parameters | ||||
| Carbohydrate (g) | 319.4(79.1) | 300.3(85.6) | 314.2(88.4) | 0.3 |
| Protein (g) | 74.07(18.6) | 68.3(21.5) | 68.6(20.3) | 0.1 |
| Total fat (g) | 65.9(19.6) | 66.1(22.2) | 79.8(24.6) | <0.001 |
| Cholesterol (mg) | 199.4(73.8) | 226.8(129.3) | 253.8(147.1) | 0.02 |
| Saturated fatty acids (g) | 20.9(7.2) | 21.5(8.4) | 26.3(8.2) | <0.001 |
| Vitamin B12 (µg) | 3.4(1.5) | 3.4(1.6) | 3.5(1.5) | 0.9 |
| Iron (mg) | 15.2(4.1) | 14.1(4.4) | 13.6(4.1) | 0.08 |
| Anti-Inflammatory Parameters | ||||
| Mono unsaturated fatty acids (g) | 21.4(6.4) | 21.5(7.9) | 26.1(8.8) | <0.001 |
| Poly unsaturated fatty acids (g) | 13.9(5.2) | 13.6(5.3) | 15.5(7.3) | 0.1 |
| Omega-3 fatty acids (g) | 1.2(0.6) | 1.07(0.5) | 1.2(0.6) | 0.1 |
| Omega-6 fatty acids (g) | 11.5(4.6) | 11.4(4.7) | 13.2(6.7) | 0.09 |
| Fiber (g) | 39.6(14.01) | 36.6(17.3) | 32.0(15.7) | 0.01 |
| Vitamin B9 (µg) | 542.7(137.2) | 477.7(123.7) | 476.2(123.7) | 0.003 |
| Vitamin B3 (mg) | 20.7(5.07) | 19.5(6.4) | 19.4(5.9) | 0.3 |
| Vitamin B2 (mg) | 1.9(0.6) | 1.7(0.6) | 1.7(0.5) | 0.2 |
| Vitamin B1 (mg) | 1.7(0.4) | 1.6(0.5) | 1.7(0.4) | 0.7 |
| Vitamin A (RE) | 926.6(425.2) | 614.7(275.2) | 554.5(289.8) | <0.001 |
| Vitamin C (mg) | 233.6(97.1) | 167.5(88.4) | 146.2(85.9) | <0.001 |
| Vitamin D (µg) | 1.9(1.4) | 1.6(1.5) | 1.4(1.2) | 0.057 |
| Vitamin E (mg) | 12.4(4.1) | 11.1(4.5) | 12.3(5.2) | 0.1 |
| Vitamin B6 (mg) | 1.9(0.4) | 1.6(0.4) | 1.6(0.4) | <0.001 |
| Magnesium (mg) | 411.3(109.3) | 351.8(120.3) | 331.6(116.7) | <0.001 |
| Zinc (mg) | 10.5(2.6) | 9.7(3.1) | 9.6(2.9) | 0.1 |
| Selenium (µg) | 93.2(30.4) | 89.6(34.5) | 90.2(31.6) | 0.7 |
| Caffeine (mg) | 159.2(101.8) | 137.1(81.2) | 138.4(79.7) | 0.2 |
| Garlic (g) | 1.3(2.4) | 0.9(2.4) | 0.2(0.5) | 0.007 |
| Onion (g) | 24.4(15.2) | 20.1(14.6) | 18.5(13.2) | 0.04 |
| Pepper (g) | 0.1(0.3) | 0.1(0.2) | 0.1(0.2) | 0.8 |
| Tea (g) | 688.1(374.3) | 643.4(390.5) | 647.2(386.8) | 0.7 |
| Beta carotene (µg) | 7244.9(4064.2) | 4189.5(2138.9) | 3420.06(2451.4) | <0.001 |
Note: aANOVA test.
Abbreviation: RE, retinol equivalents.
Multivariable-adjusted OR for the association of DII score and odds of BBD in the total study population is illustrated in Table 4. There was no significant trend across DII tertiles in the first model (p for trend=0.2, Model 1). However, after adjustment for age, estrogen therapy, family history of breast disease, use of dietary supplement, physical activity, energy, BMI, matrimony and menopause status, an increased OR for BBD was found across the tertiles of DII (OR T3=1.7, 95% CI=0.75–3.95) (P-trend =0.04, Model 2).
Table 4.
Odds Ratio (OR) and 95% Confidence Intervals (CIs) for Benign Breast Diseases Across Tertiles (T) of Dietary Inflammatory Index (DII) *, n=215
| T1 (-3.8-(−0.91)) |
T2 (−0.82–0.39) |
T3 (0.45–4.53) |
P-Trend | Continuous Estimate | ||
|---|---|---|---|---|---|---|
| Model 1 | OR 95% CI P-value |
Ref. | 0.9 (0.46–1.79) 0.7 |
1.2 (0.63–2.63) 0.4 |
0.2 | 1.11 (0.94–1.32) 0.2 |
| Model 2 | OR 95% CI P-value |
Ref. | 0.8 (0.39–1.83) 0.6 |
1.7 (0.75–3.95) 0.1 |
0.04 | 1.22 (1.003–1.48) 0.04 |
Notes: *DII was categorized into tertiles according to the distribution of the control group. DII for overall participants (T1: −3.80-(−0.84), T2: −0.82–0.63, T3: 0.64–4.53). To test for a trend across tertiles, the median for each tertile category was used as a continuous variable. Model 1: adjusted for age and menopause status. Model 2: adjusted for age, estrogen therapy, family history of breast disease, use of dietary supplement, matrimony, menopause status, BMI, physical activity and energy intake.
Discussion
To the best of our knowledge, this is the first study investigating the association between DII and BBD. After adjusting for multiple confounding variables, we found a positive association between DII and BBD. This study provides some evidence to suggest the association between DII and BBD. However, this result should be interpreted with caution as OR was not statistically significant.
The relationship between dietary intake and BBD has been examined in several studies. Previous data reported that alcohol is associated with a greater risk for proliferative BBD,16 though intake of vegetable fat and nuts is associated with a lower risk.9,37 Walnuts contain bioactive molecules such as alpha-linolenic acid (ALA) and phytosterols that affect mammary epithelial cells.38 Unsaturated fatty acids and other bioactive compounds in nuts and peanuts could produce metabolic benefits.39,40 Carotenoids may also reduce the risk of developing BBD.15 In adolescents, intake of vegetable fat, carotenoids, and vitamin E was associated with a reduced risk of BBD and breast cancer.9,15,41,42 These items of food parameters are considered anti-inflammatory due to their low overall inflammatory effect documented in the Shivappa’s study.20
Several studies have indicated that diet and chronic inflammation are closely related to each other.21,43,44 The inflammatory potential of a diet could be a risk factor for most chronic diseases such as breast cancer24 and IGM.6 Actually, a higher and lower DII score indicates a more pro-inflammatory and anti-inflammatory diet, respectively.45 In this regard, several studies have assessed the relationship between DII score and breast cancer.46,47 BBD is a marker of subsequent breast cancer and may even be in the pathway for a subset of breast cancers.48 Results of a case–control study showed that more pro-inflammatory diets may increase the OR of breast cancer.49 Findings from the large prospective study conducted in Sweden showed a pro-inflammatory diet may increase the incidence of breast cancer in women.50 The results of other studies47,51 in this context are in line with the results of the studies mentioned above.
The exact mechanism linking DII scores to a reduced risk of BBD remains to be elucidated. Pro-inflammatory diets have been reported to be associated with increasing cytokine concentration including CRP, insulin-like growth factor 1R, IL-1β and TNF-α.20,52 DII scores can play an important role through these cytokines in breast cancer, such as induced methylation of the estrogen receptor via IL-1β53 and TNF-α with proliferation effects on breast cancer tumors.54
There are some limitations in this study that should be noted. Our study is a case–control research that does not allow cause and effect conclusion. Second, DII was computed based on the food frequency questionnaire which carried recall bias. Third, for DII calculation using FFQ, about 13 parameters were not available which include; anthocyanidin, flavon, isoflavone, flavontriol, flavonol, flavanone, ginger, thyme or pineapple, saffron, turmeric, rosemary and eugenol. However, this is the first study to examine the relationship between DII and BBD in a case–control design that is one of the main strengths of this study. The appropriate sample size, use of valid tools and trained dietitians to measure and collect data are other strengths of the present study.
In conclusion, our findings suggest that a lower chance of BBD may be associated with a lower DII score. Since the obtained OR was not statistically significant, the result should be interpreted with caution. It is possible women who have shown to be more prone to BBD may reap the benefits of anti-inflammatory diets to decrease BBD and the burden of cancer. However, interventional studies are needed to clarify the effect of DII on BBD prevention.
Acknowledgments
This research has been supported by Quality Of Life Department, Breast Cancer Research Center, Motamed Cancer Institute, ACECR (Grant No.2365_20).
Informed Consent
Informed consent was obtained from all individual participants included in the study. The study was approved by the ethics committee of Tehran University of Medical Sciences (IR.TUMS VCR.REC.1396.4029).
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflict of interests.
References
- 1.Hartmann LC, Sellers TA, Frost MH, et al. Benign breast disease and the risk of breast cancer. N Engl J Med. 2005;353:229–237. doi: 10.1056/NEJMoa044383 [DOI] [PubMed] [Google Scholar]
- 2.Galassetti P. Inflammation and oxidative stress in obesity, metabolic syndrome, and diabetes. Exp Diabetes Res. 2012;2012:943706. doi: 10.1155/2012/943706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Killeen K, Skora E. Pathophysiology, diagnosis, and clinical assessment of asthma in the adult. Nurs Clin North Am. 2013;48:11–23. doi: 10.1016/j.cnur.2012.11.001 [DOI] [PubMed] [Google Scholar]
- 4.Wium-Andersen MK, Orsted DD, Nielsen SF, Nordestgaard BG. Elevated C-reactive protein levels, psychological distress, and depression in 73, 131 individuals. JAMA Psychiatry. 2013;70:176–184. doi: 10.1001/2013.jamapsychiatry.102 [DOI] [PubMed] [Google Scholar]
- 5.Keibel A, Singh V, Sharma MC. Inflammation, microenvironment, and the immune system in cancer progression. Curr Pharm Des. 2009;15:1949–1955. doi: 10.2174/138161209788453167 [DOI] [PubMed] [Google Scholar]
- 6.Kessler E, Wolloch Y. Granulomatous mastitis: a lesion clinically simulating carcinoma. Am J Clin Pathol. 1972;58:642–646. doi: 10.1093/ajcp/58.6.642 [DOI] [PubMed] [Google Scholar]
- 7.Yigitbasi MR, Guntas G, Atak T, et al. The role of interleukin-33 as an inflammatory marker in differential diagnosis of idiopathic granulomatous mastitis and breast cancer. J Invest Surg. 2017;30:272–276. doi: 10.1080/08941939.2016.1240270 [DOI] [PubMed] [Google Scholar]
- 8.Catsburg C, Gunter MJ, Chen C, et al. Insulin, estrogen, inflammatory markers, and risk of benign proliferative breast disease. Cancer Res. 2014;74:3248–3258. doi: 10.1158/0008-5472.CAN-13-3514 [DOI] [PubMed] [Google Scholar]
- 9.Baer HJ, Schnitt SJ, Connolly JL, et al. Adolescent diet and incidence of proliferative benign breast disease. Cancer Epidemiol Biomarkers Prev. 2003;12:1159–1167. [PubMed] [Google Scholar]
- 10.Ruder EH, Dorgan JF, Kranz S, et al. Examining breast cancer growth and lifestyle risk factors: early life, childhood, and adolescence. Clin Breast Cancer. 2008;8:334–342. doi: 10.3816/CBC.2008.n.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goehring C, Morabia A. Epidemiology of benign breast disease, with special attention to histologic types. Epidemiol Rev. 1997;19:310–327. doi: 10.1093/oxfordjournals.epirev.a017960 [DOI] [PubMed] [Google Scholar]
- 12.Galland L. Diet and inflammation. Nutr Clin Pract. 2010;25:634–640. doi: 10.1177/0884533610385703 [DOI] [PubMed] [Google Scholar]
- 13.Fung TT, McCullough ML, Newby P, et al. Diet-quality scores and plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr. 2005;82:163–173. doi: 10.1093/ajcn/82.1.163 [DOI] [PubMed] [Google Scholar]
- 14.Wood AD, Strachan AA, Thies F, et al. Patterns of dietary intake and serum carotenoid and tocopherol status are associated with biomarkers of chronic low-grade systemic inflammation and cardiovascular risk. Br J Nutr. 2014;112:1341–1352. doi: 10.1017/S0007114514001962 [DOI] [PubMed] [Google Scholar]
- 15.Boeke CE, Tamimi RM, Berkey CS, et al. Adolescent carotenoid intake and benign breast disease. Pediatrics. 2014;133:e1292–e1298. doi: 10.1542/peds.2013-3844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Y, Tamimi RM, Berkey CS, et al. Intakes of alcohol and folate during adolescence and risk of proliferative benign breast disease. Pediatrics. 2012;129:e1192–e1198. doi: 10.1542/peds.2011-2601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sochacka-Tatara E, Pac A, Florek M, et al. Preferring fried dishes increases risk of benign breast disease, but not breast cancer. Folia Med Cracov. 2018;58:43–52. [PubMed] [Google Scholar]
- 18.Bhaskar N, Narasimhulu CA, Keewan E, Rohr M, Parthasarathy S. Proinflammatory properties of peroxidized fat may contribute to the etiology of crohn’s disease. J Med Food. 2019;22:162–169. doi: 10.1089/jmf.2018.0132 [DOI] [PubMed] [Google Scholar]
- 19.Hajimahmoodi M, Arami S, Nosrati M, et al. Trans fatty acid content of Iranian edible oils. Food Nutr Sci. 2013;04(11):8. doi: 10.4236/fns.2013.411150 [DOI] [Google Scholar]
- 20.Shivappa N, Steck SE, Hurley TG, et al. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014;17:1689–1696. doi: 10.1017/S1368980013002115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shivappa N, Steck SE, Hurley TG, et al. A population-based dietary inflammatory index predicts levels of C-reactive protein in the seasonal variation of blood cholesterol study (SEASONS). Public Health Nutr. 2014;17:1825–1833. doi: 10.1017/S1368980013002565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tabung FK, Steck SE, Zhang J, et al. Construct validation of the dietary inflammatory index among postmenopausal women. Ann Epidemiol. 2015;25:398–405. doi: 10.1016/j.annepidem.2015.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shivappa N, Hebert JR, Marcos A, et al. Association between dietary inflammatory index and inflammatory markers in the HELENA study. Mol Nutr Food Res. 2017;61(6):1600707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang WQ, Mo XF, Ye YB, et al. A higher dietary inflammatory index score is associated with a higher risk of breast cancer among Chinese women: a case-control study. Br J Nutr. 2017;117:1358–1367. doi: 10.1017/S0007114517001192 [DOI] [PubMed] [Google Scholar]
- 25.He Y, Yue Y, Zheng X, et al. Curcumin, inflammation, and chronic diseases: how are they linked? Molecules. 2015;20:9183–9213. doi: 10.3390/molecules20059183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johannsen A, Susin C, Gustafsson A. Smoking and inflammation: evidence for a synergistic role in chronic disease. Periodontol 2000. 2014;64:111–126. doi: 10.1111/prd.2013.64.issue-1 [DOI] [PubMed] [Google Scholar]
- 27.Moghaddam MB, Aghdam FB, Jafarabadi MA, et al. The Iranian Version of International Physical Activity Questionnaire (IPAQ) in Iran: content and construct validity, factor structure, internal consistency and stability. World Appl Sci. 2012;18:1073–1080. [Google Scholar]
- 28.Esfahani FH, Asghari G, Mirmiran P, Azizi F. Reproducibility and relative validity of food group intake in a food frequency questionnaire developed for the Tehran Lipid and Glucose Study. J Epidemiol. 2010;20:150–158. doi: 10.2188/jea.JE20090083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65:1220S–1228S. doi: 10.1093/ajcn/65.4.1220S [DOI] [PubMed] [Google Scholar]
- 30.Glintborg D, Hermann AP, Hagen C, et al. A randomized placebo-controlled study on the effects of pioglitazone on cortisol metabolism in polycystic ovary syndrome. Fertil Steril. 2009;91:842–850. doi: 10.1016/j.fertnstert.2007.12.082 [DOI] [PubMed] [Google Scholar]
- 31.Banna JC, McCrory MA, Fialkowski MK, Boushey C. Examining plausibility of self-reported energy intake data: considerations for method selection. Front Nutr. 2017;4:45. doi: 10.3389/fnut.2017.00045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berkey CS, Tamimi RM, Willett WC, et al. Adolescent physical activity and inactivity: a prospective study of risk of benign breast disease in young women. Breast Cancer Res Treat. 2014;146:611–618. doi: 10.1007/s10549-014-3055-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jung MM, Colditz GA, Collins LC, et al. Lifetime physical activity and the incidence of proliferative benign breast disease. Cancer Causes Control. 2011;22:1297–1305. doi: 10.1007/s10552-011-9803-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berkey CS, Tamimi RM, Rosner B, et al. Young women with family history of breast cancer and their risk factors for benign breast disease. Cancer. 2012;118:2796–2803. doi: 10.1002/cncr.26519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frazier AL, Rosenberg SM. Preadolescent and adolescent risk factors for benign breast disease. J Adolesc Health. 2013;52:S36–S40. doi: 10.1016/j.jadohealth.2013.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lubin F, Wax Y, Ron E, et al. Nutritional factors associated with benign breast disease etiology: a case-control study. Am J Clin Nutr. 1989;50:551–556. doi: 10.1093/ajcn/50.3.551 [DOI] [PubMed] [Google Scholar]
- 37.Su X, Tamimi RM, Collins LC, et al. Intake of fiber and nuts during adolescence and incidence of proliferative benign breast disease. Cancer Causes Control. 2010;21:1033–1046. doi: 10.1007/s10552-010-9532-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vanden Heuvel JP, Belda BJ, Hannon DB, et al. Mechanistic examination of walnuts in prevention of breast cancer. Nutr Cancer. 2012;64:1078–1086. doi: 10.1080/01635581.2012.717679 [DOI] [PubMed] [Google Scholar]
- 39.Ros E. Health benefits of nut consumption. Nutrients. 2010;2:652–682. doi: 10.3390/nu2070652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kris-Etherton PM, Hu FB, Ros E, Sabate J. The role of tree nuts and peanuts in the prevention of coronary heart disease: multiple potential mechanisms. J Nutr. 2008;138:1746s–1751s. [DOI] [PubMed] [Google Scholar]
- 41.Frazier AL, Ryan CT, Rockett H, et al. Adolescent diet and risk of breast cancer. Breast Cancer Res. 2003;5:R59–R64. doi: 10.1186/bcr583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berkey CS, Willett WC, Tamimi RM, et al. Vegetable protein and vegetable fat intakes in pre-adolescent and adolescent girls, and risk for benign breast disease in young women. Breast Cancer Res Treat. 2013;141:299–306. doi: 10.1007/s10549-013-2686-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cavicchia PP, Steck SE, Hurley TG, et al. A new dietary inflammatory index predicts interval changes in serum high-sensitivity C-reactive protein. J Nutr. 2009;139:2365–2372. doi: 10.3945/jn.109.114025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Giugliano D, Ceriello A, Esposito K. The effects of diet on inflammation: emphasis on the metabolic syndrome. J Am Coll Cardiol. 2006;48:677–685. doi: 10.1016/j.jacc.2006.03.052 [DOI] [PubMed] [Google Scholar]
- 45.Fan Y, Jin X, Man C, et al. Meta-analysis of the association between the inflammatory potential of diet and colorectal cancer risk. Oncotarget. 2017;8:59592. doi: 10.18632/oncotarget.v8i35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shivappa N, Blair CK, Prizment AE, et al. Prospective study of the dietary inflammatory index and risk of breast cancer in postmenopausal women. Mol Nutr Food Res. 2017;61:1600592. doi: 10.1002/mnfr.201600592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shivappa N, Hebert JR, Rosato V, et al. Association between the dietary inflammatory index and breast cancer in a large Italian case-control study. Mol Nutr Food Res. 2017;61(3):1600500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Solin L, Harris E, Orel S, Glick J. Local-regional recurrence after breast conservation treatment or mastectomy In: JR H, ME L, Morrow M, CK O, editors. Diseases of the Breast. 3e éd ed. Philadelphie: Lippincott Williams & Wilkins; 2004:1067–1087. [Google Scholar]
- 49.Jalali S, Shivappa N, Hebert JR, et al. Dietary inflammatory index and odds of breast cancer in a case-control study from Iran. Nutr Cancer. 2018;70(7):1–9. [DOI] [PubMed] [Google Scholar]
- 50.Shivappa N, Sandin S, Lof M, et al. Prospective study of dietary inflammatory index and risk of breast cancer in Swedish women. Br J Cancer. 2015;113:1099–1103. doi: 10.1038/bjc.2015.304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vahid F, Shivappa N, Hatami M, et al. Association between dietary inflammatory index (DII) and risk of breast cancer: a case-control study. Asian Pac J Cancer Prev. 2018;19:1215–1221. doi: 10.22034/APJCP.2018.19.5.1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tabung FK, Smith-Warner SA, Chavarro JE, et al. An empirical dietary inflammatory pattern score enhances prediction of circulating inflammatory biomarkers in adults. J Nutr. 2017;147:1567–1577. doi: 10.3945/jn.117.248377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jimenez-Garduno AM, Mendoza-Rodriguez MG, Urrutia-Cabrera D, et al. IL-1beta induced methylation of the estrogen receptor ERalpha gene correlates with EMT and chemoresistance in breast cancer cells. Biochem Biophys Res Commun. 2017;490:780–785. doi: 10.1016/j.bbrc.2017.06.117 [DOI] [PubMed] [Google Scholar]
- 54.Rota LM, Wood TL. Crosstalk of the insulin-like growth factor receptor with the Wnt signaling pathway in breast cancer. Front Endocrinol (Lausanne). 2015;6:92. doi: 10.3389/fendo.2015.00092 [DOI] [PMC free article] [PubMed] [Google Scholar]


