Abstract
Background: Several epidemiological studies have been performed to evaluate the association of dietary intake of vitamin C-oriented foods (DIVCF) with risk of fracture and bone mineral density (BMD) loss, but the results remain controversial. Therefore, we conducted a systematic meta-analysis to assess this correlation.
Methods: We searched EmBase, PubMed, Web of Science, and the Chinese database CNKI for relevant articles published up to August 2019. Pooled relative risks (RRs) with 95% confidence intervals (CIs) were calculated using the random- or fixed-effects model. Discrepancies were resolved by consultation with a third expert.
Results: A total of 13 eligible articles (including 17 studies) with 19,484 subjects were identified for the present meta-analysis. The pooled RR of hip fracture for the highest vs. lowest category was 0.66 (95% CI, 0.47–0.94) for DIVCF, i.e., people with a greater frequency of Vitamin C uptake had a 34% (95% CI, 6%−53%) lower prevalence of hip fracture. In subgroup analyses stratified by study design, gender, and age, the negative associations were statistically significant. Furthermore, the statistical analysis of the association between DIVCF and risk of osteoporosis (RR, 0.66; 95% CI, 0.48–0.92), BMD at the lumbar spine (pooled r, 0.15; 95% CI, 0.09–0.23), and BMD at the femoral neck (pooled r, 0.20; 95% CI, 0.11–0.34) showed beneficial effects of DIVCF.
Conclusion: Our meta-analysis indicates that DIVCF is negatively associated with the risk of hip fracture, osteoporosis, and BMD loss, suggesting that DIVCF decreases the risk of hip fracture, osteoporosis, and BMD loss.
Keywords: BMD loss, dietary intake, osteoporosis or fracture, risk reduction, vitamin C-oriented foods, meta-analysis
Background
Osteoporosis is a systemic skeletal disease, which is common in postmenopausal women and is characterized by an increased risk of bone fragility and a decrease in bone mass (1). Bone homeostasis requires a balance between bone-forming osteoblasts and bone-resorbing osteoclasts (2). When this balance is impaired, normal bone remodeling cannot keep bone mass stable, leading to osteopenia and osteoporosis (3). Traditionally, dual X-ray absorptiometry (DEXA), which measures bone mineral density (BMD), is routinely used to assess the risk of fracture in osteoporosis patients. According to the World Health Organization, osteoporosis is one of the most common disorders, and 30–50% of all women in the world suffer from fractures due to osteoporosis throughout their lives (4).
Increasing evidence demonstrates that osteoporosis is affected by genetic factors, low body mass (weight), and lifestyle factors, such as coffee intake, alcohol consumption, soft drink intake, and dietary intake of vitamin C-oriented foods (DIVCF) (5). Diet and physical activity, the two key determinants of body weight, might influence osteoporosis directly. Vitamin C-oriented foods comprise one of the most important components of the diet and have been reported to exert many potential health benefits, because they are rich in vitamins, fiber, phytochemicals, and minerals (6). Several studies have found that DIVCF significantly lowers the risk of breast cancer, hypertension, metabolic syndrome, type 2 diabetes mellitus, depression, inflammatory bowel disease, and all-cause mortality (7, 8). Antioxidants and anti-inflammatory components from such diets are hypothesized to play a vital role in the protective effects against osteoporosis (9).
Although a series of epidemiological studies have been performed to evaluate the association between DIVCF and the risk of osteoporosis (10–22), the results remain controversial. A negative association between DIVCF and the risk of hip fractures was observed in two studies (11, 16), whereas no association was shown in other studies (10, 12–15). The purpose of the present systematic meta-analysis was to assess the correlation between DIVCF and the risk of osteoporosis, hip fracture, and BMD loss.
Methods
The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines and others were followed in the current analysis (23, 24).
Literature Search Strategy
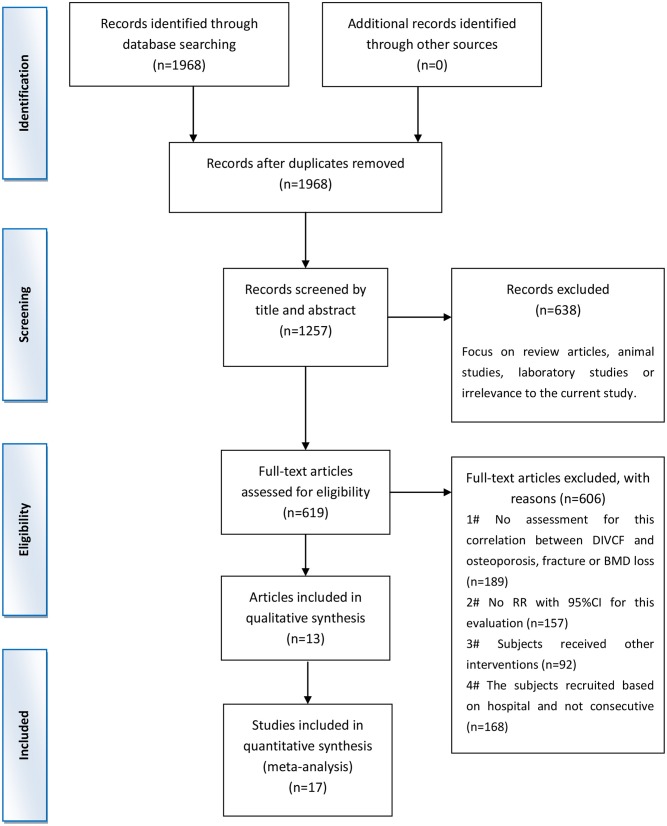
A systemic search was performed for potential articles in four databases, i.e., EmBase, PubMed, Web of Science, and the Chinese database CNKI, published in or before August 2019. We used the search terms “Vitamin C,” “ascorbic acid,” “ascorbate,” and “acid ascorbic” in combination with “osteoporosis” or “fracture.” The reference lists from the articles included were manually screened for undetected relevant studies. The final results of the literature search were updated on August 31, 2019. The detailed steps of the literature search are shown in Figure 1.
Figure 1.
Flow diagram of the trial selection process. DIVCF, dietary intake of vitamin C-oriented foods; BMD, bone mineral density.
Inclusion and Exclusion Criteria
Articles were included in this review if they met the following criteria: (1) observational study published as an original study; (2) exposure of interest was defined as DIVCF, i.e., dietary intake of foods that are rich in Vitamin C; (3) outcome of interest was defined as osteoporosis, fracture, and/or BMD loss in the subjects; and (4) study included estimates of relative risks (RRs), comparing the highest DIVCF category with the lowest, with corresponding 95% confidence intervals (CIs) for the associations or other information that could be used for inference. Furthermore, the following exclusion criteria applied: (1) studies describing animal experiments, review papers, and mechanistic studies; (2) studies lacking specified data of osteoporosis in relation to low bone mass; and (3) articles that only contained an abstract. All identified articles were reviewed independently by two researchers, and discrepancies were discussed with and resolved by a third expert.
Data Extraction and Quality Assessment
Two researchers extracted data independently, following the standardized norms for literature collection. Any discrepancies were resolved by consulting with a third expert. The following information was extracted from the articles: name of the first author, publication year, country where the study was conducted, baseline age, gender, study design, sample size and number of cases, assessment approach of DIVCF, assessment approach of osteoporosis, the measurement location of osteoporosis, and adjusted RR with 95% CI of osteoporosis for DIVCF consumption. The Newcastle–Ottawa Scale (NOS) was used to assess the quality of case–control and cohort studies (25). The Agency for Healthcare Research and Quality guidelines were applied to assess the quality of cross-sectional studies (26).
Statistical Analysis
The risk of osteoporosis, fracture, and BMD loss was estimated, comparing the highest DIVCF category to the lowest. Pooled RRs with 95% CIs were calculated using the random-effects model (REM) if moderate or high heterogeneity (I2 ≥ 50%) was observed; otherwise, the fixed-effects model (FEM) was used. The I-squared (I2) statistic and chi-square test were adopted to explore the possible heterogeneity of the studies. Potential publication bias was inspected by funnel plots. Subgroup analyses were performed by study type, gender, and age to assess potential influencing factors. All statistical analyses were performed using the statistical software package STATA SE, version 14.1 (Stata Corp, College Station, TX, USA).
Patient and Public Involvement Statement
The study is a systemic literature review that did not involve experimental participants or human subjects, and we did not collect any private data or sensitive information during the review process.
Results
Results of the Literature Search
As shown in Figure 1, a total of 1,968 articles were identified in the database search; 1,349 articles were excluded because of the following reasons: 711 articles were duplications, and 638 articles were excluded due to their nature (e.g., reviews, animal studies, or laboratory studies). Of the remaining 619 articles, 189 did not assess the association between DIVCF and the risk of osteoporosis, fracture, or BMD loss; 157 studies did not report the RR (with 95% CI) for the relationship between DIVCF and osteoporosis risk; 92 articles were excluded for other reasons; and 168 studies were based on a hospital-based sample (not on a population-based sample). As a result, 13 published articles (with 17 studies, i.e., 4 cohort, 11 case–control, and 2 cross-sectional studies) were identified as eligible for the present meta-analysis (10–22) (Figure 1).
Study Characteristics and Quality Assessment
The basic characteristics for DIVCF with risk of osteoporosis are shown in Table 1. In our study quality assessment, the studies received a quality score of ≥5, indicating that the methodological quality of the studies was generally good.
Table 1.
Characteristics of the studies included in the Meta-analysis.
| References (sources) | Study design | Gender/Age (y) | No. of cases (study size) | DIVCF assessment | Duration time (y) | Outcome | Study quality | Adjusted factors |
|---|---|---|---|---|---|---|---|---|
| Finck et al. (10) (Male subjects), UK | Cohort | M, 39–79 | 112/1,842 124/2,334 78/1,808 | 7-d Food record | 12.6 | Hip fracture Spine fracture | 7 | Age, family history of osteoporosis, BMI, smoking, physical activity, steroid medication, energy, Ca intake, Ca and vitamin D supplemental status, HRT |
| Finck et al. (10) (Female subjects), UK | Cohort | F, 39–79 | 339/2,525 | 7-d Food record | 12.6 | Hip fracture Spine fracture | 7 | Age, family history of osteoporosis, BMI, smoking, physical activity, steroid medication, energy, Ca intake, Ca and vitamin D supplemental status, HRT |
| Sun et al. (11) China | Case control | F/M, 42–79 | 726/1452 | Food Frequency Questionnaire | NA | Hip fracture | 5 | Age, sex, drugs, BMI, education, occupation, income, family history of fracture, smoking, alcohol, Ca and multi-vitamin supplement, physical activity, energy intake |
| Sahni et al. (12) USA | Cohort | F/M, 70–80 | 100/958 | Food Frequency Questionnaire | 15 | Hip fracture | 6 | Age, sex, energy intake, estrogen use, BMI, multi-vitamin use, height |
| Zhang et al. (13) USA | Case control | F/M, ≥50 | 1215/2,564 | Food Frequency Questionnaire | NA | Hip fracture | 8 | Age, sex, BMI, physical activity, energy, Ca, vitamin D, protein, caffeine, alcohol intake |
| Michaelsson et al. (14) Sweden | Case control | F, 40–75 | 247/1,140 | Food Frequency Questionnaire | NA | Hip fracture | 7 | Diabetes, fracture history, HRT, smoking, physical activity, BMI, energy intake |
| Nieves et al. (15) USA | Case control | F, 50–103 | 161/328 | Food Frequency Questionnaire | NA | Hip fracture | 6 | BMI, estrogen use, chronic disease/age and hospital matching |
| Sun et al. (16) China | Case control | F/M, 40–70 | 725/1,450 | Food Frequency Questionnaire | 5 | Hip fracture | 5 | Age, sex, drugs, BMI, educational level, occupation, household income, family history of fracture, smoking, alcohol drinking, calcium supplement use, multivitamin supplement use, physical activity, daily energy intake, and selected dietary nutrients intakes (protein, calcium, and phosphorous:energy-adjusted) |
| Kim and Lee (17) South Korea | Cross sectional | F/M, ≥50 | 1212/3,047 | 24-h recall | NA | Osteoporosis (<-2·5 T-score/LS-FN-TH), BMD | 8 | Age, sex, income, education, smoking, HRT, survey year, energy intake, Ca intake, blood vitamin D level |
| Sugiura et al. (18) Japan | Cohort | F, 30–70 | 17/187 | Food Frequency Questionnaire | 4 | Osteoporosis (T-sore exceeded 70 %/FA) | 6 | Age, weight, height, years since menopause, current tobacco use, alcohol intake, exercise habit, supplement use, energy intake, intake of Ca, Mg, K, vitamin D |
| Zhang et al. (19) China | Case control | F, 58.1 ± 6.7 | 60/159 | Food Frequency Questionnaire | NA | Osteoporosis (<-2·5 T-score/LS-FN-TH), BMD | 6 | Age, sex, BMI, physical activity, energy, Ca, vitamin D, protein, caffeine, alcohol intake |
| Yang and Kim (20) South Korea | Cross-sectional | M, 50–79 | 189/2,305 | 24-h recall | 3 | BMD | 7 | Age, weight, education, alcohol intake, exercise, vitamin D, parathyroid hormone |
| Park et al. (21) South Korea | Case control | F, 50–70 | 72/144 | Food Frequency Questionnaire | NA | Osteoporosis (<-2.5 T-score/LS-FN-FT), BMD | 5 | Energy intake, age, BMI, HRT/ age-matching |
| Macdonald et al. (22) UK | Cohort | F, 45–55 | 5/891 | Food Frequency Questionnaire | 5 | BMD | 7 | Age, weight, weight change, height, smoking, physical activity, socioeconomic status |
DIVCF, dietary intake of vitamin C-oriented foods; F, female; M, male; NA, Not application.
Quantitative Synthesis
Meta-Analysis of DIVCF and the Risk of Hip Fracture
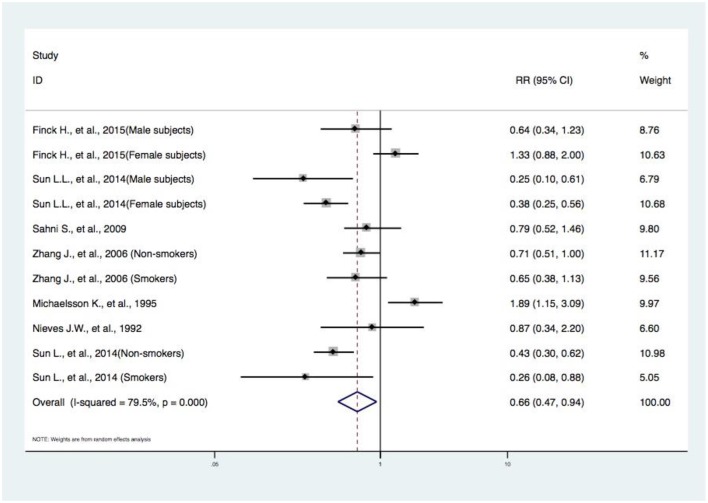
Eleven studies from seven articles (10–16) reported the events of hip fracture, and our random-effects meta-analysis showed that there is a significant difference between subjects in the highest category of DIVCF and the lowest. The RR for hip fracture events between the two groups was 0.66 (95% CI, 0.47–0.94) (Figure 2). These results indicate that the people with a higher consumption of Vitamin C-oriented foods had a 34% (95% CI, 6%−53%) lower risk of hip fracture. Because of the high heterogeneity among the abovementioned studies (I2 = 79.5%; P = 0.000), we conducted subgroup analyses based on study type (i.e., cohort and case–control studies), gender, and age (middle-aged subjects, older subjects) (Figures 3–5).
Figure 2.
Forest plot of meta-analysis of DIVCF and the risk of hip fracture. The size of the boxes is proportional to the weight assigned to each study, and horizontal lines represent the 95% confidence interval. DIVCF, dietary intake of vitamin C-oriented foods.
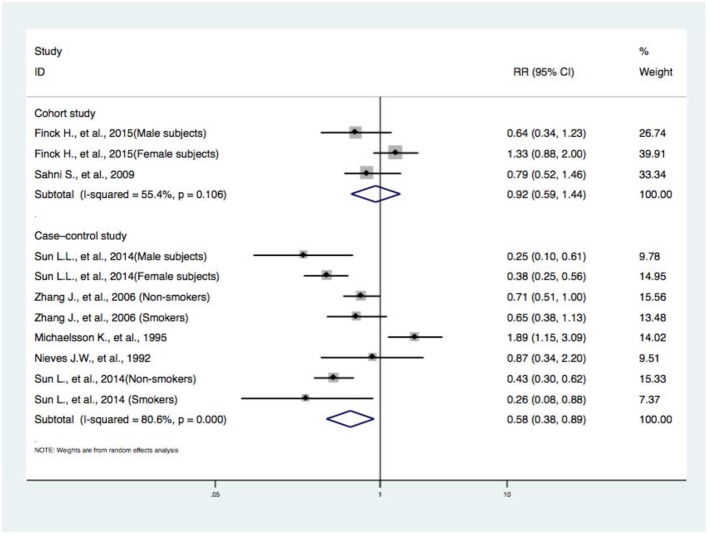
Figure 3.
Subgroup analysis of study design concerning DIVCF and the risk of hip fracture. The size of the boxes is proportional to the weight assigned to each study, and horizontal lines represent the 95% confidence interval. DIVCF, dietary intake of vitamin C-oriented foods.
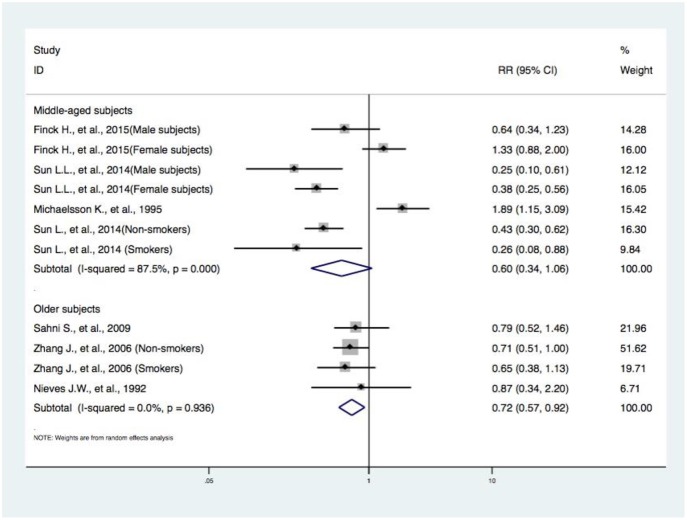
Figure 5.
Subgroup analysis of age concerning DIVCF and the risk of hip fracture. The size of the boxes is proportional to the weight assigned to each study, and horizontal lines represent the 95% confidence interval. DIVCF, dietary intake of vitamin C-oriented foods.
Subgroup Analyses by Study Design Concerning DIVCF and the Risk of Hip Fracture
Regarding the studies assessing the correlation between DIVCF and risk of hip fracture, two articles (including four case–control studies) (11, 16) reported that higher DIVCF could lower the risk of hip fracture, while other case–control studies (13–15) failed to reflect such a relationship. Also, two articles (including three cohort studies) (10, 12) failed to identify such a positive association. The RR for DIVCF and risk of hip fracture was much lower in the case–control studies (RR, 0.58; 95% CI, 0.38–0.89), and no significant results were found in the cohort studies (RR, 0.92; 95% CI, 0.59–1.44) (Figure 3).
Subgroup Analyses by Gender Concerning DIVCF and the Risk of Hip Fracture
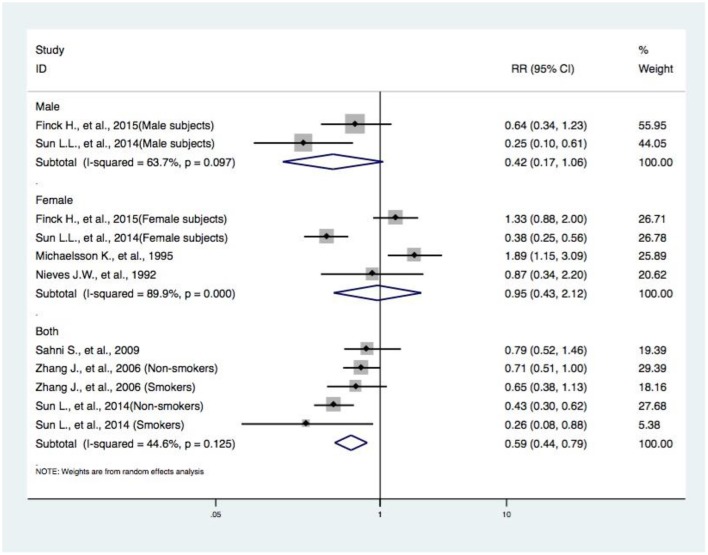
Seven articles (with 11 studies) (10–16) reported the effects of gender in the association between DIVCF and risk of hip fracture. A reduced risk of hip fracture was found for the pooled results of three articles (with five studies) (12, 13, 16) on both male and female subjects (RR, 0.59; 95% CI, 0.4–0.79). However, no positive effects were observed in the synthesized data of two articles (with two studies) (10, 11) on male subjects (RR, 0.42; 95% CI, 0.17–1.06) and four articles (with four studies) (10, 11, 14, 15) on female subjects (RR, 0.95; 95% CI, 0.43–2.12) (Figure 4).
Figure 4.
Subgroup analysis of gender concerning DIVCF and the risk of hip fracture. The size of the boxes is proportional to the weight assigned to each study, and horizontal lines represent the 95% confidence interval. DIVCF, dietary intake of vitamin C-oriented foods.
Subgroup Analyses by Age Concerning DIVCF and the Risk of Hip Fracture
The RR of hip fracture, from three articles (with four studies) (12, 13, 15) on older subjects (RR, 0.72; 95% CI, 0.57–0.92), is lower among subjects in the highest category of DIVCF. No statistically significant correlations were observed in the analysis of four articles (with seven studies) (10, 11, 14, 16) on middle-aged subjects (RR, 0.60; 95% CI, 0.34–1.06) (Figure 5).
Publication Bias Analysis
Funnel plots were used to evaluate the potential publication bias, with RRs generated from the articles concerning DIVCF and the risk of hip fracture. In the absence of publication bias, most of the formulated points are symmetrically placed around the vertical line given by the pooled RRs. No evidence of publication bias was found in the evaluation of DIVCF and the risk of hip fracture (Supplementary Figure 1).
Meta-Analysis of DIVCF and the Risk of Osteoporosis
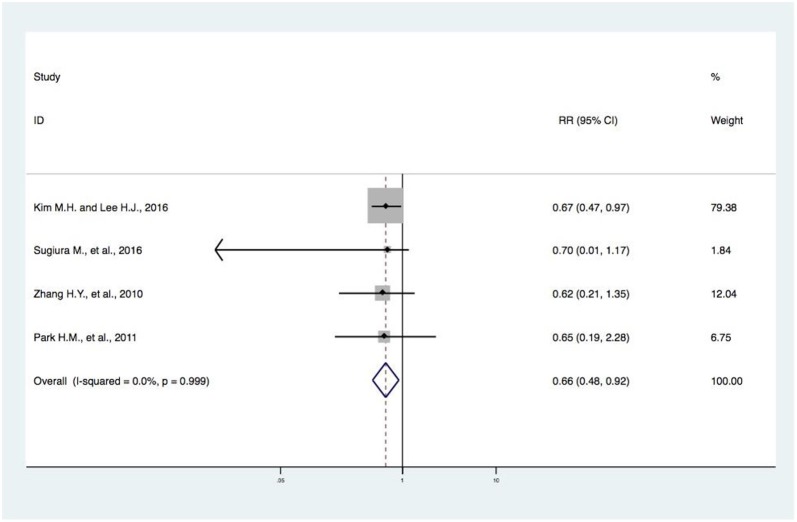
Four studies (17–19, 21) reported on DIVCF and the risk of osteoporosis. One study (17) found a negative association between DIVCF and risk of osteoporosis (RR, 0.67; 95% CI, 0.47–0.97), while no significant association was found in the other three studies (18–20). The evidence synthesis for DIVCF and risk of osteoporosis showed obviously beneficial effects (RR, 0.66; 95% CI, 0.48–0.92) (Figure 6).
Figure 6.
Forest plot of meta-analysis of DIVCF and the risk of osteoporosis. The size of the boxes is proportional to the weight assigned to each study, and horizontal lines represent the 95% confidence interval. DIVCF, dietary intake of vitamin C-oriented foods.
Meta-Analysis of DIVCF and BMD at the Lumbar Spine and Femoral Neck
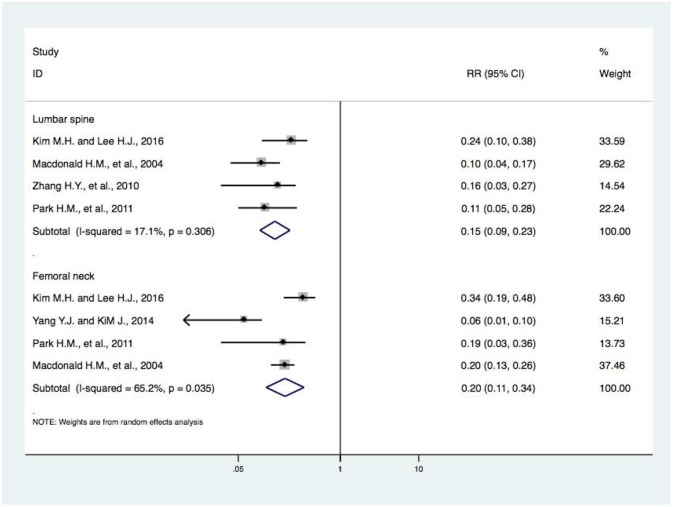
The risk of BMD loss at the lumbar spine was considered in four studies (17, 18, 21, 22). In our meta-analysis, we found that higher DIVCF was negatively associated with the risk of BMD loss at the lumbar spine (pooled r, 0.15; 95% CI, 0.09–0.23). Furthermore, four studies (17, 20–22) reported on DIVCF and the risk of BMD loss at the femoral neck. We found a significant negative association between them (pooled r, 0.20; 95% CI, 0.11–0.34) (Figure 7).
Figure 7.
Forest plot of meta-analysis of DIVCF and BMD at the lumbar spine and at the femoral neck. The size of the boxes is proportional to the weight assigned to each study, and horizontal lines represent the 95% confidence interval. DIVCF, dietary intake of vitamin C-oriented foods; BMD, bone mineral density.
Discussion
Summary of Evidence
Our meta-analysis included 13 eligible articles (including 17 studies) with 19,484 subjects. The pooled RR of hip fracture for the highest DIVCF category vs. the lowest category was 0.66 (95% CI, 0.47–0.94), i.e., people with a high frequency of Vitamin C-oriented food intake had a 34% (95% CI, 6%−53%) lower prevalence of hip fracture. In our subgroup analyses stratified by study design, gender, and age, the negative associations were found to be statistically significant. Furthermore, the analysis of DIVCF and risk of osteoporosis (RR, 0.66; 95% CI, 0.48–0.92), BMD at the lumbar spine (pooled r, 0.15; 95% CI, 0.09–0.23), and BMD at the femoral neck (pooled r, 0.20; 95% CI, 0.11–0.34) showed high DIVCF has beneficial effects. Our meta-analysis indicates that DIVCF is negatively associated with the risk of hip fracture, osteoporosis, and BMD loss, suggesting that people should consume more Vitamin C to decrease the risk of hip fracture, osteoporosis, and BMD loss.
Comparison of the Findings With Other Results in the Literature
In this paper, we carried out a meta-analysis of the possible protective effect of DIVCF against osteoporosis, fracture, and BMD loss. A similar article (27) was published in April 2018. The authors found no significant association between Vitamin C intake and the risk of hip fracture (RR, 0.74; 95% CI, 0.51–1.08). In our analysis, three more articles (with four studies) (16, 19, 20) were included, providing the chance to access documents that were published in Chinese. In this paper, we also construct a sound basis for further clinical research in this field (28). In addition to assessing the potential correlation between DIVCF and the risk of osteoporosis, we included meta-analyses on the effect of DIVCF on the risk of hip fracture and BMD loss. Finally, our pooled-effects analysis revealed a negative correlation between DIVCF and risk of hip fracture (RR, 0.66; 95% CI, 0.47–0.94); i.e., subjects with a higher intake of Vitamin C-oriented foods had a 34% (95% CI, 6%−53%) lower risk of hip fracture (Figure 2). Our results differ from those of Dr. Malmir, published in April 2018 (27). Due to the major heterogeneity among the studies, we performed subgroup analyses based on study type (i.e., cohort and case–control studies), gender, and age (middle-aged subjects, older subjects). Between DIVCF and risk of osteoporosis, a negative correlation was found (RR, 0.66; 95% CI, 0.48–0.92) (Figure 6). Also, we found that DIVCF was negatively associated with the risk of BMD loss at the lumbar spine (pooled r, 0.15; 95% CI, 0.09–0.23) and at the femoral neck (pooled r, 0.20; 95% CI, 0.11–0.34) (Figure 7).
Although a series of studies on Vitamin C and the risk of osteoporosis have been published, including controlled trials, case series, and case reports, to the best of our knowledge, no systematic meta-analyses have been published focusing on the correlation between DIVCF and osteoporosis, hip fracture, and BMD loss.
Several biological mechanisms may underlie the impact of DIVCF on the risk of osteoporosis. First, DIVCF includes not only Vitamin C but also Vitamin A, Vitamin B1, Vitamin B2, Vitamin B3, iron, and potassium intake. The positive effects of these vitamins and minerals on bone health have been well-established (29). Osteoporosis is a systemic skeletal disease, which is common in postmenopausal women and is characterized by an increased risk of bone fragility and a decrease in bone mass. Osteoporosis is often associated with metabolic syndrome (MBS), which involves oxidative stress and insulin resistance (30). Vitamin C-oriented foods are rich in several antioxidants, including Vitamin C, Vitamin K, and other phytochemicals. A high antioxidant consumption could contribute to lowering reactive oxygen species levels and enhancing the antioxidant status in animal models and human subjects (31, 32); this may slow down the progression of systemic oxidative damage resulting from overproduction of reactive oxygen species (33–35). Moreover, many phytochemicals can increase insulin production (36), and therefore potentially aid in the prevention of insulin resistance and hence MBS. Furthermore, DIVCF could protect against MBS by regulating the levels/activity of C-reactive protein and other inflammatory markers (37, 38). Higher DIVCF is correlated with reduced plasma concentrations of C-reactive protein (39, 40), and the latter might protect against MBS. Finally, people who eat many Vitamin C-oriented foods may consume much dietary fiber and little fat, decreasing the risk of MDS (41, 42). All the above mentioned factors are positively associated with BMD and negatively correlated with bone loss, thus reducing the risk of osteoporosis and fractures.
Meaning of the Study for Possible Clinical Use
A positive link was found between DIVCF and a reduced risk of osteoporosis, fracture, and BMD loss. Several observations support the use of this information in clinical settings. First, our meta-analysis included a large number of subjects, reducing the sampling error to a large extent. Second, large efforts have been made to control the interference of possible confounding factors (such as age, BMI, physical activity level, and smoking status), resulting from the adjusted RR being fully extracted. Third, similar correlations were observed after performing subgroup analyses stratified by study type, gender, and age, confirming that the results are credible and robust.
Limitation of the Review
Nevertheless, our study has several limitations. One of the key points is that only DIVCF was taken into account (and no dietary intake of other foods, such as fruit and vegetable), while many people with high DIVCF may in general eat a healthy diet, so an analysis of other healthy diets may give similar results. Therefore, it is necessary to verify that DIVCF is the active component of the diet. Also, it would be helpful to include intake of other dietary components (such as fruit and vegetable) for further study. Furthermore, Vitamin D plays a large role in the maintenance of BMD; its influence on the development of secondary hyperparathyroidism and osteomalacia, states where BMD is reduced reversibly, has been reported. Only a few studies in our meta-analysis adjusted for Vitamin D intake, though it is of great importance in the majority of nutritional studies (10, 12, 18–20). All the above mentioned factors could result in bias or inaccurate interpretations.
Another important point is that most of the articles we identified were cross-sectional or case–control studies; larger prospective cohort studies are warranted to verify the results. Although we have adjusted for most of the confounders the articles included, other confounders may still have existed. Moreover, the confounders that were adjusted for were different in each research; this may certainly have affected the results. Moreover, the approach of osteoporosis assessment differed between articles, which could influence the observed correlation. Because of the limited number of included articles, the effects of an inconsistent assessment approach on the strength of the correlation could not be further explored. Finally, due to the limited available data, we could not assess the dose–response association between DIVCF and the risk of osteoporosis.
Between-study heterogeneity is identified in most meta-analyses, and it is of importance to explore the sources of heterogeneity between studies. In our meta-analysis, there was high heterogeneity in the correlation between DIVCF and risk of osteoporosis. The factors contributing to such heterogeneity are complex. First, differences in the definition, classification, and assessment of DIVCF were found, which could result in heterogeneity. Second, the variables adjusted for (including age, BMI, energy intake, parathyroid hormone levels, 25-hydroxyvitamin D levels, smoking, alcohol intake, physical activity, supplement use, and oral contraceptive use) were not consistent between studies. In addition, because of the limited sample size, the evaluation of publication bias calls for further research.
Conclusion
Our meta-analysis indicates that DIVCF is negatively associated with the risk of osteoporosis, hip fracture, and BMD loss and suggests that people should consume more DIVCF to reduce these risks.
Author Contributions
JL and Z-PL conceived and designed the study. The literature searches, study selection, critical appraisal, data extraction, and contacting authors of included studies for additional information were carried out by L-FZ, M-HL, XX, Z-TG, DZ, J-LZ, J-TL, and J-KP. G-HL, JY, XW, H-YC, H-TH, QL, Y-HH, J-HL, S-RH, MW, and L-FZ conducted the interpretation and analysis of the relevant data. L-FZ, W-YY, DG, W-XL, QW, and JL drafted the paper. JL, A-HO, and L-FZ further revised the text. The final version of this article was rechecked and approved by L-FZ, M-HL, G-HL, M-HL, W-YY, XX, XW, JY, DG, H-YC, J-KP, H-TH, QL, Z-TG, Y-HH, DZ, J-LZ, S-RH, MW, J-TL, J-HL, W-XL, A-HO, QW, Z-PL, and JL.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Prof. Fabio Monzani, Prof. Giorgia and other editors/reviewers for the helpful comments and suggestions. We thank LetPub (www.letpub.com) for its linguistic assistance during the proofreading of this manuscript.
Footnotes
Funding. This work was funded by the China Postdoctoral Science Foundation (No. 2018M633036), the Medical Science Research Foundation of Guangdong Province (No. B2019091), the National Natural Science Foundation of China (No. 81873314), the Project of Guangdong Provincial Department of Finance (Nos. [2014]157, [2018]8), Key scientific research platforms and research projects of universities in Guangdong Province (No. 2018KQNCX041), and the Science and Technology Research Project of Guangdong Provincial Hospital of Chinese Medicine (Nos. YN2019ML08, YK2013B2N19, and YN2015MS15).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2019.00844/full#supplementary-material
References
- 1.National Institutes of Health (NIH) Osteoporosis and Related Bone Diseases National Resource Center Osteoporosis overview. Bethesda, MD: NIH (2015). Available online at: https://www.bones.nih.gov/health-info/bone/osteoporosis/overview (accessed February 2019). [Google Scholar]
- 2.NIH Consensus Development Panel on Osteoporosis Prevention Diagnosis and Therapy Osteoporosis prevention, diagnosis, and therapy. JAMA. (2001) 285:785–95. 10.1001/jama.285.6.785 [DOI] [PubMed] [Google Scholar]
- 3.Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet. (2011) 377:1276–87. 10.1016/S0140-6736(10)62349-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization Assessment of Fracture Risk and its Application to Screening for Postmenopausal Osteoporosis. Report of a WHO Study Group. WHO Technical Report Series, no. 843. Geneva: WHO; (1994). [PubMed] [Google Scholar]
- 5.Kanis JA, Melton LJ, III, Christiansen C, Johnston CC, Khaltaev N. The diagnosis of osteoporosis. J Bone Miner Res. (1994) 9:1137–41. 10.1002/jbmr.5650090802 [DOI] [PubMed] [Google Scholar]
- 6.Boonen S, Dejaeger E, Vanderschueren D, Venken K, Bogaerts A, Verschueren S, et al. Osteoporosis and osteoporotic fracture occurrence and prevention in the elderly: a geriatric perspective. Best Pract Res Clin Endocrinol Metab. (2008) 22:765–785. 10.1016/j.beem.2008.07.002 [DOI] [PubMed] [Google Scholar]
- 7.US Preventive Services Task Force. Curry SJ, Krist AH, Owens DK, Barry MJ, Caughey AB, et al. Screening for osteoporosis to prevent fractures: US preventive services task force recommendation statement. JAMA. (2018) 319:2521–31. 10.1001/jama.2018.7498 [DOI] [PubMed] [Google Scholar]
- 8.Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. (2006) 17:1726–33. 10.1007/s00198-006-0172-4 [DOI] [PubMed] [Google Scholar]
- 9.Becker DJ, Kilgore ML, Morrisey MA. The societal burden of osteoporosis. Curr Rheumatol Rep. (2010) 12:186–91. 10.1007/s11926-010-0097-y [DOI] [PubMed] [Google Scholar]
- 10.Finck H, Hart AR, Lentjes MA, Jennings A, Luben RN, Khaw KT, et al. Cross-sectional and prospective associations between dietary and plasma vitamin C, heel bone ultrasound, and fracture risk in men and women in the European Prospective Investigation into Cancer in Norfolk cohort. Am J Clin Nutr. (2015) 102:1416–24. 10.3945/ajcn.115.111971 [DOI] [PubMed] [Google Scholar]
- 11.Sun LL, Li BL, Xie HL, Fan F, Yu WZ, Wu BH, et al. Associations between the dietary intake of antioxidant nutrients and the risk of hip fracture in elderly Chinese: a case-control study. Br J Nutr. (2014) 112:1706–14. 10.1017/S0007114514002773 [DOI] [PubMed] [Google Scholar]
- 12.Sahni S, Hannan MT, Gagnon D, Blumberg J, Cupples LA, Kiel DP, et al. Protective effect of total and supplemental vitamin C intake on the risk of hip fracture – a 17-year follow-up from the Framingham Osteoporosis Study. Osteoporos Int. (2009) 20:1853–61. 10.1007/s00198-009-0897-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang J, Munger RG, West NA, Cutler DR, Wengreen HJ, Corcoran CD. Antioxidant intake and risk of osteoporotic hip fracture in Utah: an effect modified by smoking status. Am J Epidemiol. (2006) 163:9–17. 10.1093/aje/kwj005 [DOI] [PubMed] [Google Scholar]
- 14.Michaëlsson K, Holmberg L, Mallmin H, Sörensen S, Wolk A, Bergström R, et al. Diet and hip fracture risk: a case-control study. Study Group of the Multiple Risk Survey on Swedish Women for Eating Assessment. Int J Epidemiol. (1995) 24:771–82. 10.1093/ije/24.4.771 [DOI] [PubMed] [Google Scholar]
- 15.Nieves JW, Grisso JA, Kelsey JL. A case-control study of hip fracture: evaluation of selected dietary variables and teenage physical activity. Osteoporos Int. (1992) 2:122–7. 10.1007/BF01623818 [DOI] [PubMed] [Google Scholar]
- 16.Sun L, Xue WQ, Cao WT, Fan F, Xie HL, Chen YM, et al. Dietary intake of Vitamin C and risk of hip fracture in elderly Chinese in Guangdong: a case-control study. Acta Nutrimenta Sin. (2014) 36:430–4. 10.13325/j.cnki.acta.nutr.sin.2014.05.004 [DOI] [Google Scholar]
- 17.Kim MH, Lee HJ. Osteoporosis, vitamin C intake, and physical activity in Korean adults aged 50 years and over. J Phys Ther Sci. (2016) 28:725–30. 10.1589/jpts.28.725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sugiura M, Nakamura M, Ogawa K, Ikoma Y, Yano M. High Vitamin C intake with high serum β-cryptoxanthin associated with lower risk for osteoporosis in post-menopausal Japanese female subjects: mikkabi cohort study. J Nutr Sci Vitaminol. (2016) 62:185–91. 10.3177/jnsv.62.185 [DOI] [PubMed] [Google Scholar]
- 19.Zhang HY, Ma LX, Feng XJ. Nutritional status and osteoporosis in postmenopausal women. Mater Child Health Care China. (2010) 25:810–2. [Google Scholar]
- 20.Yang YJ, Kim J. Factors in relation to bone mineral density in Korean middle-aged and older men: 2008-2010 Korea National Health and Nutrition Examination Survey. Ann Nutr Metab. (2014) 64:50–9. 10.1159/000362425 [DOI] [PubMed] [Google Scholar]
- 21.Park HM, Heo J, Park Y. Calcium from plant sources is beneficial to lowering the risk of osteoporosis in postmenopausal Korean women. Nutr Res. (2011) 31:27–32. 10.1016/j.nutres.2010.12.005 [DOI] [PubMed] [Google Scholar]
- 22.Macdonald HM, New SA, Golden MH, Campbell MK, Reid DM. Nutritional associations with bone loss during the menopausal transition: evidence of a beneficial effect of calcium, alcohol, and fruit and vegetable nutrients and of a detrimental effect of fatty acids. Am J Clin Nutr. (2004) 79:155–65. 10.1093/ajcn/79.1.155 [DOI] [PubMed] [Google Scholar]
- 23.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. (2009) 339:b2535. 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA. (2000) 283:2008–12. 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 25.Stang A. Critical Evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 26.Zeng X, Zhang Y, Kwong JS, Zhang C, Li S, Sun F, et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med. (2015) 8:2–10. 10.1111/jebm.12141 [DOI] [PubMed] [Google Scholar]
- 27.Malmir H, Shab-Bidar S, Djafarian K. Vitamin C intake in relation to bone mineral density and risk of hip fracture and osteoporosis: a systematic review and meta-analysis of observational studies. Br J Nutr. (2018) 119:847–58. 10.1017/S0007114518000430 [DOI] [PubMed] [Google Scholar]
- 28.Zeng LF, Wang NS, Wang Q, Zou YP, Liang ZH, Kong LS, et al. Oral Chinese herbal medicine for kidney nourishment in Alzheimer's disease: a systematic review of the effect on MMSE index measures and safety. Complement Ther Med. (2015) 23:283–97. 10.1016/j.ctim.2015.01.014 [DOI] [PubMed] [Google Scholar]
- 29.Mazidi M, Kengne AP, Vatanparast H. Association of dietary patterns of American adults with bone mineral density and fracture. Public Health Nutr. (2018) 21:2417–23. 10.1017/S1368980018000939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen XW, Garner SC, Anderson JJ. Isoflavones regulate interleukin-6 and osteoprotegerin synthesis during osteoblast cell differentiation via an estrogen-receptor-dependent pathway. Biochem Biophys Res Commun. (2002) 295:417–22. 10.1016/S0006-291X(02)00667-8 [DOI] [PubMed] [Google Scholar]
- 31.Cashman KD. Diet, nutrition, and bone health. J Nutr. (2007) 137:2507–12. 10.1093/jn/137.11.2507S [DOI] [PubMed] [Google Scholar]
- 32.Tucker KL. Osteoporosis prevention and nutrition. Curr Osteoporos Rep. (2009) 7:111–7. 10.1007/s11914-009-0020-5 [DOI] [PubMed] [Google Scholar]
- 33.Yang TC, Aucott LS, Duthie GG, Macdonald HM. An application of partial least squares for identifying dietary patterns in bone health. Arch Osteoporos. (2017) 12:63. 10.1007/s11657-017-0355-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buclin T, Cosma M, Appenzeller M, Jacquet AF, Décosterd LA, Biollaz J, et al. Diet acids and alkalis influence calcium retention in bone. Osteoporos Int. (2001) 12:493–9. 10.1007/s001980170095 [DOI] [PubMed] [Google Scholar]
- 35.Arnett TR. Extracellular pH regulates bone cell function. J Nutr. (2008) 138:415–8. 10.1093/jn/138.2.415S [DOI] [PubMed] [Google Scholar]
- 36.Brandao-Burch A, Utting JC, Orriss IR, Arnett TR. Acidosis inhibits bone formation by osteoblasts in vitro by preventing mineralization. Calcif Tissue Int. (2005) 77:1671–4. 10.1007/s00223-004-0285-8 [DOI] [PubMed] [Google Scholar]
- 37.Aghajanian P, Hall S, Wongworawat MD, Mohan S. The roles and mechanisms of actions of Vitamin C in bone: new developments. J Bone Miner Res. (2015) 30:1945–55. 10.1002/jbmr.2709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Macdonald HM. Influence of organic salts of potassium on bone health: possible mechanisms of action for the role of fruit and vegetables. Int Congr. (2007) 1297:268-81. 10.1016/j.ics.2006.08.019 [DOI] [Google Scholar]
- 39.Expert Panel on Musculoskeletal Imaging. Ward RJ, Roberts CC, Bencardino JT, Arnold E, Baccei SJ. ACR appropriateness criteria® osteoporosis and bone mineral density. J Am Coll Radiol. (2017) 14:189–202. 10.1016/j.jacr.2017.02.018 [DOI] [PubMed] [Google Scholar]
- 40.Microsurgery Department of the Orthopedics Branch of the Chinese Medical Doctor Association Group from the Osteonecrosis and Bone Defect Branch of the Chinese Association of Reparative and Reconstructive Surgery Microsurgery and Reconstructive Surgery Group of the Orthopedics Branch of the Chinese Medical Association Chinese guideline for the diagnosis and treatment of osteonecrosis of the femoral head in adults. Orthop Surg. (2017) 9:3–12. 10.1111/os.12302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cotts KG, Cifu AS. Treatment of Osteoporosis. JAMA. (2018) 319:1040–1. 10.1001/jama.2017.21995. [DOI] [PubMed] [Google Scholar]
- 42.Tarantino U, Iolascon G, Cianferotti L, Masi L, Marcucci G, Giusti F, et al. Clinical guidelines for the prevention and treatment of osteoporosis: summary statements and recommendations from the italian society for orthopaedics and traumatology. J Orthop Traumatol. (2017) 18:3–36. 10.1007/s10195-017-0474-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.