Abstract
Background and Study
Cumin seed oil (extracted from Cuminum cyminum) has many applications but conclusive evidence of its therapeutic uses has not been presented. This study has explored the anticancer and antibacterial properties of the seed oil.
Methods
The cumin nanoemulsion was prepared with Tween 80 non-ionic surfactant employing ultra-sonication technology. The anticancer activity of the nanoscale-based emulsion was evaluated through cell viability (MTT), antiproliferation evaluation through clonogenic assay, and apoptosis through Annexin V-FITC assay. Agar well diffusion was used to study the antimicrobial activity, and this was supported by membrane integrity analysis.
Results
A thorough study of process parameters, aimed at obtaining the optimal surface concentration and emulsification time, was completed. GC-MS data indicated cumaldehyde as a major component. The resultant droplet diameter after a sonication time of 5 min was 10.4 ± 0.5 nm. MTT assay revealed the IC50 value at 1.5 µL/mL and the early induction of apoptosis was evident. Tongue carcinoma cell line treated with cumin nanoemulsion presented a diminished colony formation. The nanoemulsion exhibited significant antibacterial activity against S. aureus. A significant cytoplasmic leakage was observed on treatment with cumin nanoemulsion. The consequences of the analysis projected cumin as a potential component for cancer therapy.
Conclusion
This study provides definitive evidence for cumin essential oil nanoemulsion as a legitimate plant-based medicine that can bypass the drawbacks of the present aggressive treatment of cancer, can overcome the antimicrobial resistance, and can also meet all prerequisites.
Keywords: cumin oil, nanoemulsion, tongue carcinoma cell line, apoptosis, antibacterial activity, anticancer activity
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
Phytochemicals (bioactive compounds) are well known to prevent cell proliferation, tumor formation, angiogenesis and metastases through their various mechanisms of action and diverse structure. Bioactive compounds are exhaustively studied to estimate their consequences on health. Cancer inhibition is controlled by the molecular pathways of these natural compounds through cell cycle arrest, apoptosis, necrosis and autophagy.1,2 In spite of these advantages, factors associated with gastric residence time and instability can affect the efficacy of these bioactives.3 Besides, drug resistance due to suppression of apoptosis and expressive levels of anti-apoptotic proteins has intensified in cancer treatment.4 Henceforth, these bioactive compounds must be equipped to overcome the resistance mechanism occurring in these cells.5 The pharmaceutical industry today faces a great challenge to develop an effective treatment for cancer in view of side-effects observed in conventional therapies and the sharp hike in mortality rates to 8.8 million in the year 2015.6
Antimicrobial resistance has turned in to a global health threat that needs to be tackled from its very root.7 Unbefitting usage of antibiotics and incremental intake of medicinal drugs has made antimicrobial resistance a substantial cause of health deterioration in developing countries.8 Rise in antimicrobial resistance (AMR) has resulted in ineffective control of various diseases in the community. Moreover, the spectrum of AMR varies by region.9 The consequences of AMR are, however, complicated by the toxic side effects of the expensive drugs which often result in mortality.10
Emergence of nanotechnology in the medicinal field has led to rapid progress in the recent years. Among nanocarriers to achieve tumor selective drug delivery, nanoemulsions are highly regarded owing to their remarkable properties.11 Nanoemulsion applications populate diverse fields by virtue of their kinetic stability, high surface area per unit volume, tuneable rheology and many more positive attributes.12 As nanocarriers, they improve therapeutic efficacy, reduce adverse toxic effects and are biocompatible.13 Based on their potential, drug-containing nanoemulsions such as Liple, Cleviprex, Limethasone, Diazemuls, Lipfen, Ropion, Diprivan, and Vitalipid have been made commercially available in markets, and many others are at their clinical and preclinical stages of development.14 Devising oil-in-water nanoemulsions is the preferred route in commercialization owing to their enhanced bioavailability and improved absorption capability of lipophilic compounds.15
Black cumin (Nigella sativa) has been predominantly used in traditional medicine for centuries due to significant biological properties of its active ingredients such as essential oils and proteins.16 Studies support N. sativa essential oil formulated into nanoemulsion for its use in cancer therapy.17 Cumin (Cuminum cyminum) seed is used as a condiment and also in perfumes in very small amount largely for its flavour and aroma. At present, there exists scant literature about the anticancer activity of cumin seed (Cuminum cyminum) oil nanoemulsion. In this study, the nanoemulsion was formulated to assess its cytotoxicity using MTT assay, antiproliferative activity by clonogenic assay and apoptosis indicated by Annexin V. Membrane integrity analysis and agar well diffusion assay were performed to assess the antibacterial activity of the seed oil nanoemulsion. GC-MS analysis of Cumin seed essential oil was also conducted.
Methods
Materials
Cumin seed oil (Cuminum cyminum) of Indian origin was purchased from Cyrus Enterprises, Chennai, India. The non-ionic surfactant, polyethylene glycol sorbitan monooleate also commonly known as Tween 80 was purchased from Merck, India. MTT was acquired from Hi Media Laboratories, India. Annexin V-FITC Early Apoptosis Detection Kit was purchased from Cell Signaling Technology, Inc. The water utilized for all the operations was double distilled with the help of a milli-Q system.
Gas Chromatography-Mass Spectrometry (GC-MS)
GC analyses of cumin essential oil were carried out on a Hewlett Packard 6890 N gas chromatograph, equipped with HP-5 MS capillary fused silica column (30 m × 0.25 μm). The oven temperature was held at 45°C for 1 min, then programmed at 5°C/min to 250°C, and held for 20 min. Other operating conditions were as follows: carrier gas, He (99.999%); inlet pressure, 85.4 kPa; linear velocity, 44.2 cm/sec; injector temperature, 280°C; detector temperature, 250 °C; and split ratio, 1:50.
Formulation Technique
Cumin nanoemulsion was formulated using the three components— Cumin seed oil, water and Tween 80. The non-ionic surfactant was preferred due to its favourable oil-in-water (O/W) characteristic. Tween 80 displays efficient solubility with essential oils, and also effectively minimises droplet diameter by adhering to the droplet’s surface, improving the overall stability of O/W emulsion system. Nanoemulsion formulation process is a two step-process, initiating with preparations of O/W macroemulsions by blending of oil (cumin seed oil), surfactant (Tween 80) and water at specific concentrations using a magnetic stirrer at a speed of 500 rpm for 10 min (Table 1). The various oil: surfactant ratio (v/v%) used in this formulation was 1:1 (Cum-A), 1:2 (Cum-B), and 1:3 (Cum-C). The oil concentration was kept to a constant of 6%. Subsequently, these emulsions were prepared by forming their respective nanoemulsions using an ultrasonicator with 750 W input power processor [PCI (Probe sonicator-Advanced model) 20 kHz]. Each concentration was subjected to different sonication time periods of 5, 10 and 15 min, respectively. Turbulence produced by the high-energy shockwaves is capable of disrupting the droplets. The mild heat generated was regulated by positioning the sample in a container filled with ice.
Table 1.
Different Formulations Used in Optimizing Cumin Oil Nanoemulsion
| Formulation Code | Oil: Surfactant Ratio (v/v) | Cumin Oil: Tween 80: Water (v/v) | Sonication Time | Mean Droplet Size Diameter (nm) | Polydispersity Index |
|---|---|---|---|---|---|
| Cum-A1 | 1:1 | 6:6:88 | 5 min | 149.33 ± 1.15 | 0.257 ± 0.082 |
| Cum-B1 | 1:2 | 6:12:82 | 5 min | 11.26 ± 1.20 | 0.257 ± 0.009 |
| Cum-C1 | 1:3 | 6:18:76 | 5 min | 10.4 ± 0.51 | 0.212 ± 0.105 |
| Cum-A2 | 1:1 | 6:6:88 | 10 min | 132.93 ± 1.18 | 0.148 ± 0.086 |
| Cum-B2 | 1:2 | 6:12:82 | 10 min | 11.96 ± 0.70 | 0.262 ± 0.039 |
| Cum-C2 | 1:3 | 6:18:76 | 10 min | 10.76 ± 0.56 | 0.215 ± 0.117 |
| Cum-A3 | 1:1 | 6:6:88 | 15 min | 128.5 ± 2.00 | 0.211 ± 0.025 |
| Cum-B3 | 1:2 | 6:12:82 | 15 min | 12.83 ± 2.17 | 0.273 ± 0.258 |
| Cum-C3 | 1:3 | 6:18:76 | 15 min | 12.23 ± 0.89 | 0.255 ± 0.040 |
Characterization of Nanoemulsion
Absorbances of the emulsions were measured at 600 nm with the help of UV–visible spectrophotometer (V-630 Series, Jasco Analytical Instruments, Asia).
The droplet size and polydispersity index of emulsions of varying ratios were measured using dynamic light scattering technique (Horiba, Nanopartica SZ-100 series). Prior to the experiment, all the samples were diluted to 10% with deionised water in order to reduce the effects caused by multiple scattering effects.
The viscosity of the undiluted samples was measured using Brookfield Viscometer with Brookfield Rheocalc Software [DV-II+ Pro EXTRA, LV-II, UL Adapter] at 25 ± 1°C, 60 rpm. The pH was examined quantitatively with a Digital pH meter [EUTECH Instruments, Oakton, Singapore], at 25 ± 1°C.
Stability studies such as centrifugation and subjecting the formulation to various extreme temperature conditions were carried out.
Subsequently, the best formulation was identified on the basis of reduced surfactant concentration utilization, stable physicochemical properties and lower droplet sizes. All trials were carried out in triplicate as three independent experiments, and results expressed as the mean ± standard deviation.
Anticancer Activity
Cell Culture
Tongue carcinoma cell line (SAS) and Hek-293 (non-tumorigenic) cell line were obtained as a kind gift from Professor Devarajan Karunagaran, Department of Biotechnology, Indian Institute of Technology Madras, Chennai, India. SAS cell line was procured from an accredited commercial source Japanese Collection of Research Bioresources Cell Bank, Japan by our Institute IIT-Madras, Department of Biotechnology. Professor Devarajan Karunnagaran group procured this cell line and used this cell line for their research work and published recently in Mol Cell Biol 2019 Mar 15 (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6399668/). SAS cells were maintained in Roswell Park Memorial Institute (RPMI) 1640 Medium, and Hek 293 cells were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% Fetal Bovine Serum (FBS) at 37° C in 5% CO2 and penicillin (100 units/mL)/streptomycin (100 μg/mL) (Gibco, Grand Island, NY, USA).
Cell Viability Measurement
SAS cells were seeded in 96-well plates (10, 000 cells/well), and cultured in Roswell Park Memorial Institute (RPMI) 1640 Medium, 37°C, 5% CO2 for a day. On the next day, they were treated with an increasing concentration of optimized cumin nanoemulsion formulation. A surfactant-water mixture was the negative control. After 48 h of treatment, MTT assay was performed by adding MTT solution (0.5 mg/mL) to each well and incubating for about 3 hrs. Dark blue formazan crystals formed, implying the presence of viable cells on solubilization with DMSO. Nonviable cells do not form those crystals. The absorbance was measured by a microplate reader at 570 nm. The cell viability was expressed as the percentage of formazan absorbance.18
Clonogenic Assay
The colony formation assay in this study was carried out by culturing cells in 6-well culture plates (1000 cells/well) and after 24 h, treated with cumin nanoemulsion at IC50 concentration and incubated at 37°C and 5% CO2 for 7–10 days till optimal clones were acquired. Later, methanol was employed to fix and then stained with crystal violet to observe the colonies.19
Annexin V-FITC Assay
SAS cell line (1 x 105) was cultured overnight after it was plated onto 25-cm2 flasks. Subsequently, it was incubated with cumin nanoemulsion at IC50 for 48 h. All the cells were collected and then washed off with PBS or ice-cold medium through centrifugation. In due time, these cells were resuspended with Annexin V binding buffer. One microliter of Annexin V-FITC conjugate and 12.5 µL of Propidium Iodide (PI) solution were added up together to each aliquot of 96 µL cell suspension and then incubated for 10 min on ice in dark. Finally, it was diluted to a final volume containing 250 µL/assay with ice-cold 1X Annexin V binding buffer and immediately analyzed using a BD FACSVERSE flow cytometer with an in-built BD FACSuiteTM Software.
Antibacterial Activity
Bacterial Culture
Staphylococcus aureus (ATCC 29213), a bacterial pathogen, was sustained at 4° C in agar slants as stock cultures. A few selected colonies were segregated from these cultures and were inoculated into Mueller–Hinton Broth. A separate subculture was made to ensure both the purity and viability and was further diluted with fresh medium to achieve an optical density of 1.5 x 107–108 CFU/mL.
Agar Well Diffusion Assay
A 100 µL volume of the microbial inoculum was inoculated by spreading it over the entire agar surface. A hole of diameter 6 to 8 mm is punched aseptically with a cork borer and a volume of 20–100 µL of the optimized concentration of cumin nanoemulsion is introduced. All the resulting effects of cumin oil, surfactant, and DMSO were scrutinized independently. The agar plates were then incubated in accordance with appropriate and suitable conditions. The phytochemical compound was diffused in the agar medium and inhibited the growth of the microbial strain tested. At the end of incubation for 24 h, the diameter of the zone of inhibition was measured.20
Membrane Integrity Analysis
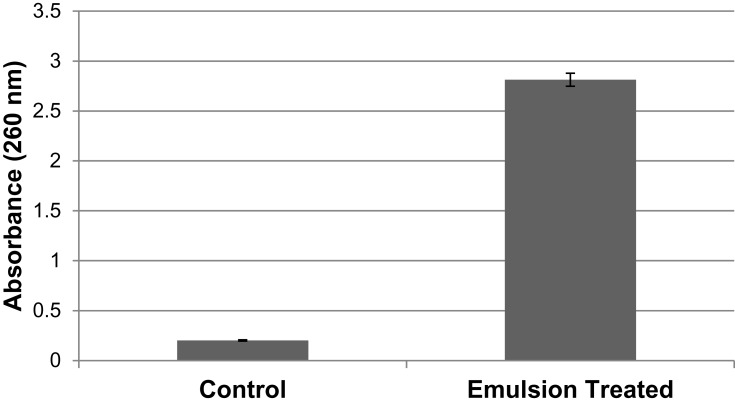
With a slight alteration as indicated by the convention of Hou et al,21 an overnight-developed culture was gathered, washed, resuspended, and changed in accordance with 1 × 108 CFU/mL utilizing PBS. Test samples were made by weakening a 0.5 mL of the microbial suspension bringing about a volume of 9.5 mL of cumin oil and its nanoemulsion. The microbial suspension diluted to 9.5 mL of PBS was functioned as a negative control. All the test samples were then incubated for 1 h in the laboratory environment. To force out the cytoplasmic substance from the microbial cells, the previously incubated samples were further centrifuged at 6000 G for 10 min. The supernatant was then estimated for absorbance at 260 nm utilizing UV–Vis Spectrophotometer. This test was conducted in triplicate.
Statistical Analysis
Graphpad Prism 4.0 (GraphPad Software, Inc., San Diego, CA) was used to produce concentration-dependent curves along with statistical analysis. Every investigation was repeated for a minimum of three times. These analyses enable the estimation of mean ± STD, one- or two-way ANOVA, and Bonferroni post-test in every relevant (*P<0.05; **P<0.01; ***P<0.001).
Results
Identification of Cumin Essential Oil Constituents by GC-MS
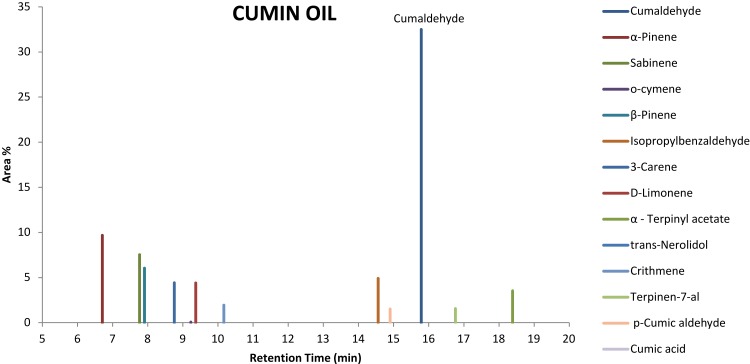
Gas Chromatography-Mass Spectroscopy was performed for the cumin oil. The peak area% and the retention time of active components are shown in Figure 1. The tests revealed cumaldehyde with the highest peak area % of 32.50, followed by α-Pinene (9.68), sabinene (7.54), o-cymene (6.55), β-Pinene (6.0), isopropyl benzaldehyde (4.91), 3-carene (4.42), D-limonene (4.41), α - Terpinyl acetate (3.53), trans-nerolidol (3.02), crithmene (1.95), terpinen-7-al (1.56), p-Cumic aldehyde (1.51), Cumic acid (1.41) in the chromatogram.
Figure 1.
Gas Chromatography-Mass Spectroscopy of cumin essential oil extract.
Nanoemulsion Characterization
Visual appearances of the formulations of varying oil-to-surfactant ratio after a sonication time of 5, 10 and 15 min are shown in Figure 2. The increase in oil-to-surfactant ratio ranging from 1:1 to 1:2 and 1:3 led to the change in colour of emulsions from milky white to transparent.
Figure 2.
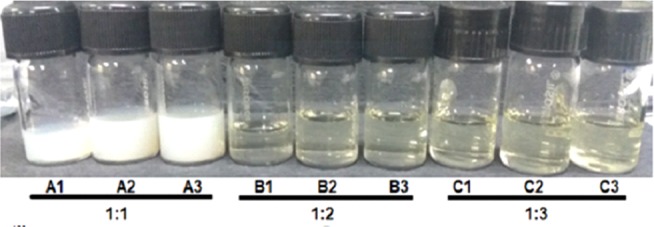
Visual appearances of all the formulations with varying sonication time depict colour gradient from milky white to clear emulsion.
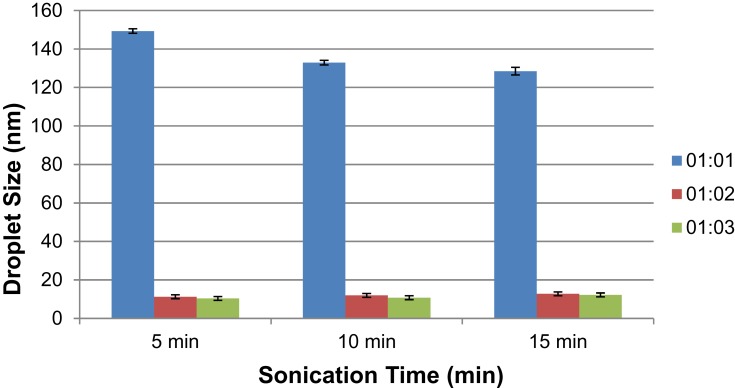
During the preparation, a rapid decrease in the droplet diameter from Cum-A to Cum-C formulations with a rise in the oil-to-surfactant ratio was observed (Figure 3). Their polydispersity indices affirm uniformity of the droplets formed.
Figure 3.
Mean droplet size of cumin oil-based formulations with respect to emulsification time and sonication time of the varying formulations.
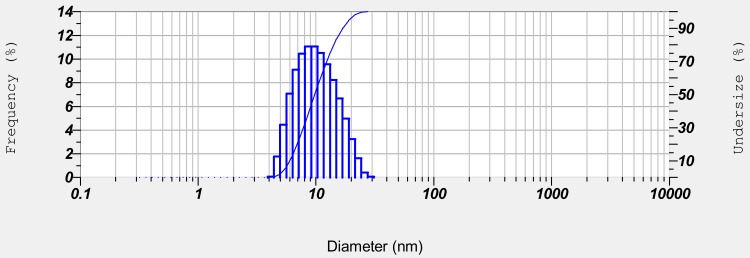
Cum-C1 was further optimized for application studies owing to stable uniform droplets with relatively low surfactant concentration and minimized droplet size diameter formed within a sonication period of 5 min (Figure 4).
Figure 4.
Droplet size distribution of optimized cumin nanoemulsion with respect to parameters of frequency and undersize percentage.
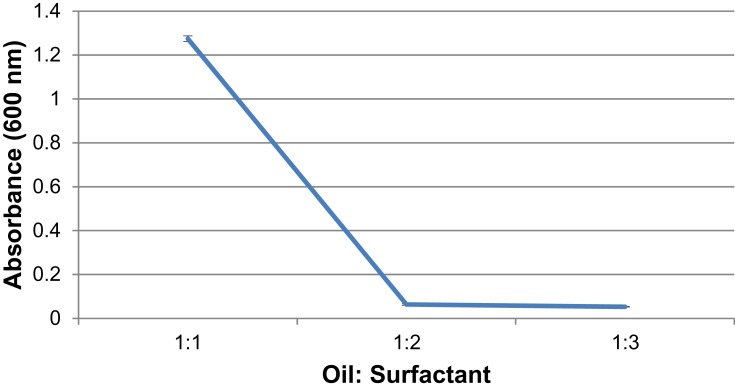
Physico-chemical characterization was done for all ratios with an optimized sonication time of 5 min. A decrease in absorbance is observed with increasing surfactant concentration (Figure 5).
Figure 5.
UV-Spectroscopic measurements of formulations with oil: surfactant ratios of 1:1, 1:2, 1:3 at 600 nm displays a decrease in absorbance with increased surfactant concentration.
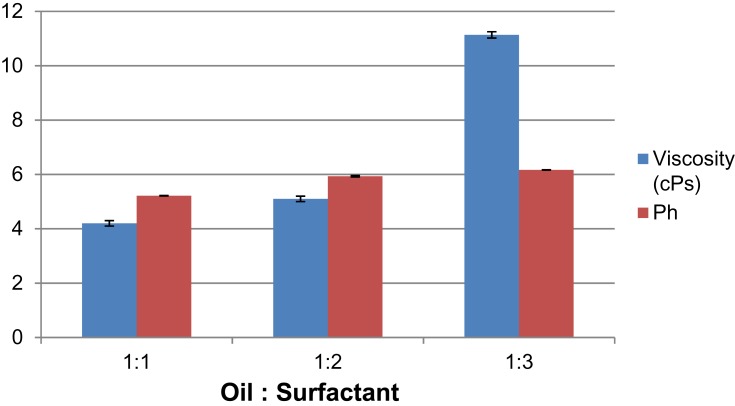
The surfactant concentration manifests a clear direct relation with viscosity. With an increase in surfactant concentration, the viscosity rises (Figure 6).
Figure 6.
The effect of oil-surfactant ratio (1:1 to 1:3) on viscosity and pH.
pH values increase with increasing surfactant concentration (Figure 6). The pH values of Cum-A1, Cum-B1 and Cum-C1 at room temperature were found to be in the range of 5.21 ± 0.005, 5.93 ± 0.025 and 6.16 ± 0.005, respectively.
Cum-A formulations did not exhibit stability, as they phase-separated within 72 h of preparation. Cum-B and Cum-C formulations passed through all stress conditions displaying good stability.
Anticancer Activity
Cytotoxicity of Nanoemulsion
Cell viability was studied through quantification of the formazan crystal formation associated with the reduced MTT tetrazolium by living cells. MTT technique (related to cell metabolism and activity), is a colorimetric quantitative evaluation of cytotoxicity, cell viability and also cell proliferation.22,23
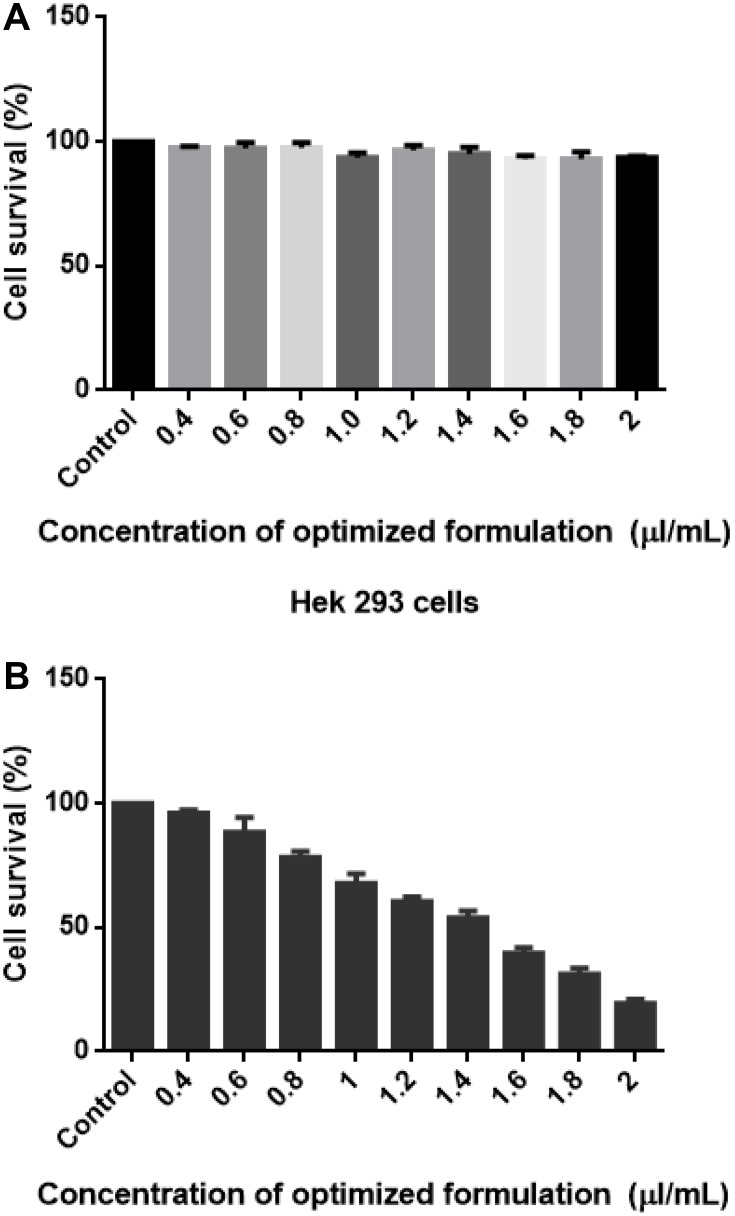
The MTT assay expresses cytotoxicity against SAS cell line on interaction with nanoemulsion at an IC50 concentration (50% reduction in cell viability) of 1.5 µL/mL (Figure 7A). However, the nanoemulsion did not show any cytotoxicity on the non-tumorigenic cell line Hek 293 cell line (Figure 7B), implying that the nanoemulsion targets and inhibits cell proliferation of only the cancerous cells. With an increase in the exposure concentration, there was a decrease in cell viability. This effect was observed at 48 hrs. However, there was no significant change for 24 h and 48 h of exposure duration in relation to the control. The vehicle control did not contribute any cytotoxicity.
Figure 7.
(A and B) Cell viability measurement of Hek 293 (A) left and HTh-7 (B). Cells were treated with 1.5 µL/mL and after 48 hr of treatment MTT assay was performed by assessing formazan crystal formation (purple colour formation), which was then measured by a microplate reader at 570 nm.
Clonogenic Assay
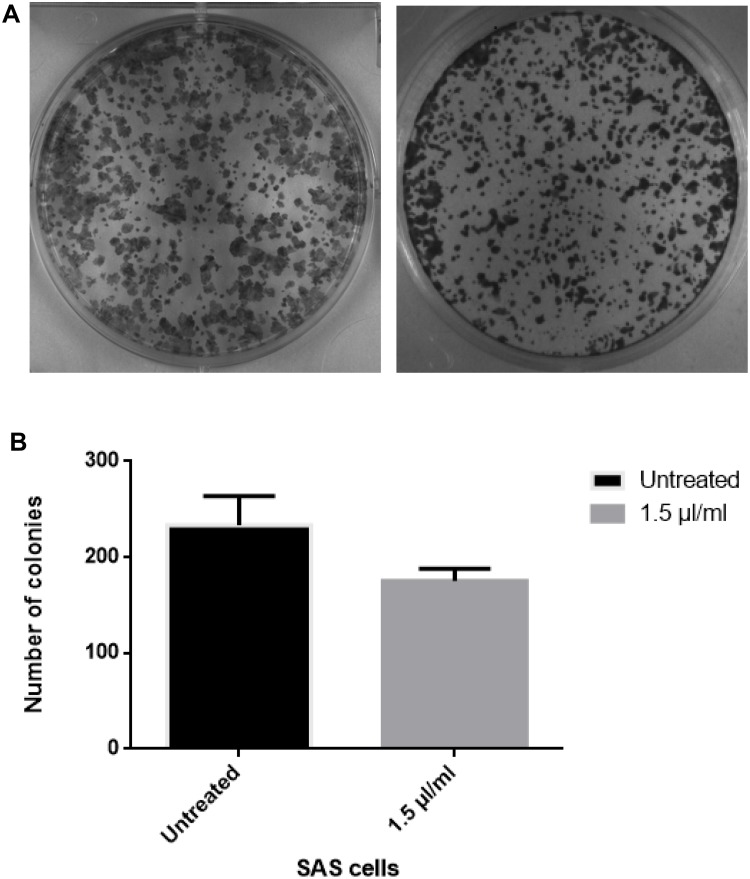
Clonogenic assays are frequently used to investigate the survival of irradiated cancer cells.24 Clonogenic assay is a method based on ionizing radiation to determine cell reproductive death after treatment.25 Clonogenic assay was conducted to verify the antiproliferative activity of cumin nanoemulsion. SAS cell line was treated with 1.5 µL/mL of concentrated cumin nanoemulsion for a duration of 7–10 days. The results depicted a diminished colony formation, as shown in Figure 8A and B. This result confirms the antiproliferative effect of cumin nanoemulsion against SAS cells.
Figure 8.
(A and B) Colony formation assay reveals a significant decline on the treatment of tongue carcinoma cell line with nanoemulsion (right) as compared to untreated control (left). Cells were treated with nanoemulsion formulation and incubated at 37°C and 5% CO2 for 7–10 days until the formation of optimal clones. After incubation, the cells were fixed with methanol and stained with Crystal violet.
Flow Cytometry
Organisms evolve the cell suicide mechanism termed apoptosis to eliminate the redundant, damaged, or infected cells.26 The Annexin V-FITC assay perceives such early apoptotic cells through flow cytometry assay in a cell population.
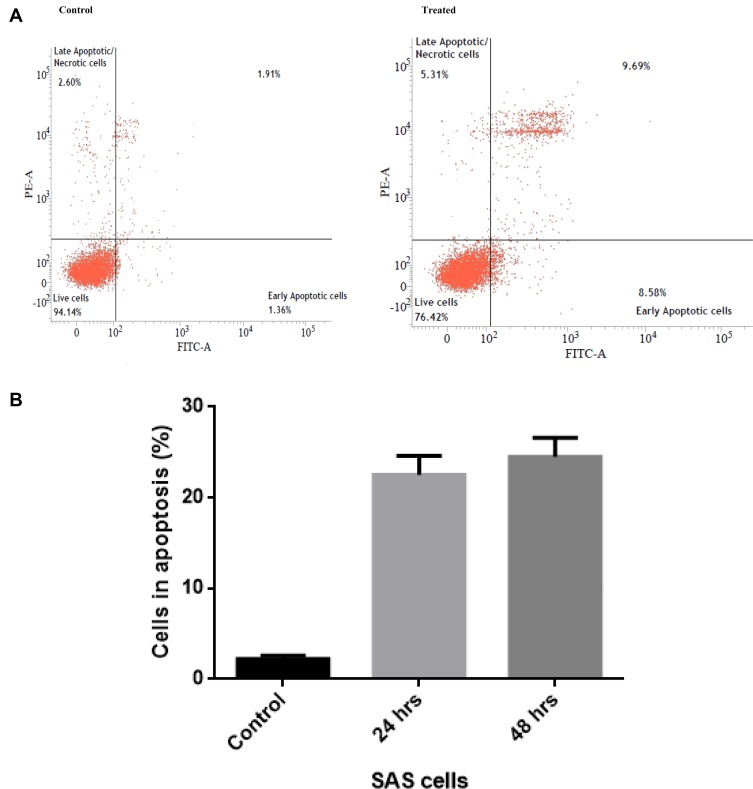
In order to determine whether the cumin nanoemulsion induces cell death by apoptosis, Annexin V-FITC Assay was performed using cumin nanoemulsion at a concentration of 1.5 µL/mL. The assay demonstrated early apoptosis (Annexin V+/PI−) in the SAS cell line (Figure 9A and B). Slight necrosis (Annexin V−/PI+) was observed, as shown in Figure 9A.
Figure 9.
(A and B) Annexin V-FITC assay to detect early apoptosis in the SAS cell line that is treated with the formulated cumin nanoemulsion on contrary with control.
Antibacterial Activity
Agar Well Diffusion
Agar well diffusion is extensively employed for assessing the antimicrobial activity of plants and microbial extracts.27 Agar well diffusion assay was performed and both mean and standard deviation were calculated. At the optimized concentration of cumin nanoemulsion, a clear and sensitive zone of inhibition for three replicates was observed with a mean diameter of 20.3 ± 0.11 mm (Figure 10). However, cumin oil displayed a zone size of 18.6 ± 0.11 mm. The oil and emulsion showed no significant difference, though there was a slight increase in the zone size with respect to cumin nanoemulsion.
Figure 10.
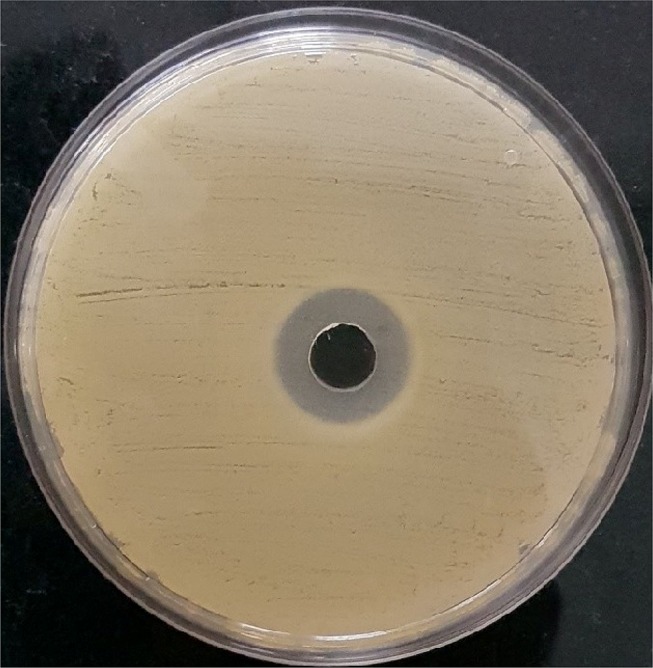
Antibacterial activity of the formulated nanoemulsion against Staphylococcus aureus by agar well diffusion assay.
Membrane Integrity
Membrane integrity was tested to support the results. UV-Vis Spectroscopy technique was used to measure the absorption of light across the ultra-violet and visible-light wavelengths through the liquid sample. The relation between absorbance estimated and measure of cytoplasmic leakage was directly in proportion.28 A significant release of cytoplasmic contents was observed on nanoemulsion interaction in comparison with control cells (Figure 11).
Figure 11.
Membrane integrity analysis displays potential cytoplasmic leakage from Staphylococcus aureus on treatment with the optimized Cum-C1 formulation in comparison with control.
Discussion
Accelerating progress in the field of cancer treatment has brought down the rate of deaths.19 Optimal therapeutic methods for cancer involve targeting multiple molecules found in both the tumor and supportive tissues. This paradigm for anticancer therapy involving targeting multiple signalling pathways is indeed the need of the hour.29
Many natural compounds from various plant extracts have been investigated for their chemopreventive effects.30 Extracts from the essential oils contain a wide range of saturated and unsaturated hydrocarbons and gas chromatography has turned out to be the best-suited separation process for multicomponent analysis.31 The peak area % obtained indicated cuminaldehyde to be a major contributor to the biological properties of cumin seed oil extract. Characterisation studies were conducted to establish a preferred cumin nanoemulsion formulation in order to substantiate the idea of antiproliferative and anti-bacterial effects of the chemo-preventive agents affecting multiple pathways. The cumin nanoemulsion was prepared using cumin seed oil, water and Tween 80 owing to its low polarity, high solubilization capacity, and lesser molecular weight.32 The colour change observed on visual inspection is attributable to Rayleigh scattering effect from nanoemulsions with minimized droplet size diameter.33 The contribution of the surfactant in stabilizing and controlling the droplet size diameter is pivotal for the formulation of nanoemulsions.34 The formulation process is termed to be non-spontaneous in nature as the Gibbs free energy for the formation of cumin nanoemulsion is positive. The surfactant significantly reduces the free energy utilized in nanoemulsion formulation.35 The ultrasonication technique generated small droplets. A lower polydispersity index of these droplets was indicative of the homogeneity and stability of the formulations.36
The small droplets produced through this technique result in greater surface area, facilitating easier permeation of the active compounds through the blood vessels.37 A slight increase in emulsification time and surfactant concentration from 1:1 to 1:2 v/v led to an effective reduction in the droplet diameter. However, further increase in surfactant concentration to 1:3 v/v and sonication time (10 min, 15 min) was not very effective in reducing droplet size, considering that the droplet size was controlled and stabilized at 1:3 concentration after a sonication time of 5 min.
Dynamic light scattering is a technique used extensively for particle size measurement, and also for size distribution studies. DLS is a technique based on the Brownian motion of spherical particles which incurs a doppler shift of incident laser light. The polydispersity index characterizes the stability of the system.38 The literature asserts that the relatively weak scattering from optically transparent emulsions is likely the cause for the decrease in absorbance.39,40 Viscosity serves as a key parameter influencing the effective delivery of bioactive compounds. The effects of oil concentration, surfactant concentration and water are related to the viscosity of nanoemulsions.41 The entrapment of water molecules in the cross-linking portions of surfactant molecules causes an increase in viscosity.42,43
After refrigeration, the pH values demonstrated a variation of <0.2 and these minute variations are in parallel with the reports of Bhushani et al.44 Surfactant concentration and emulsification time play a crucial role in imparting stability to the nanoemulsions. It is observed that an increment in surfactant concentration and emulsification time caused a direct effect on the stability of the nanoemulsions. Although both Cum-B1 and Cum-C1 showed a nanometric particle size after 5 min of sonication, Cum-C1 was chosen for application in this study based on smaller droplet size with minimum polydispersity index in comparison to other formulations.
The MTT assay was effective in evaluating the cell viability of cancerous tongue carcinoma (SAS) cell line and the non-cancerous (Hek 293) cell line on treatment with cumin nanoemulsion. The nanoemulsion displayed a concentration-dependent cytotoxicity profile. The presence of bioactive compounds in the nanoemulsion might have altered its route of internalization to the target cell.45 It is understood that the amount of MTT formazan has a direct relation to the number of viable cells.46 This calorimetric assay measures the MTT reduced by mitochondrial dehydrogenases in metabolically active cells.47 These mitochondrial succinate dehydrogenases of viable cells result in converting MTT to their corresponding formazan.48 MTT is also presumed to be reduced in the mitochondria and to behave as an indicator of mitochondrial function.49–51 The diminished colony formation observed in the clonogenic assay indicates the anticancer potential of cumin nanoemulsion against SAS cells. The results are in agreement with the reports of Khan et al as they observed a decrease in colony formation of A549 cells on treatment with carvacrol nanoemulsion in a dose-dependent manner.52
Apoptosis and necrosis are the basic types of cell death. Apoptosis is defined by a number of distinct morphological features and biochemical processes which distinguish it from necrosis, a premature cell death triggered by external exogenous damage.53 Cancer cells have been suppressing apoptosis as exemplified by a few proteins and between cell death and cell proliferation.54 In general, cell death attributable to necrosis or apoptosis could be recognized by morphology and cellular pathways.55–58 The treatments devised are better owing to apoptotic cell death which gives a clear insight into the pathogenesis of a disease.59,60 Flow cytometry technique was conducted with distinct dyes to simplify the task of differentiating apoptosis from necrosis. Annexin V assay is a marker of apoptosis, and propidium iodide acts as a fluorescent dye necrotic indicator.61 Furthermore, Annexin V-FITC binds to cell surfaces and reveals phosphatidylserine, an early apoptosis indicator. Apoptosis, necrosis, autophagy and cell cycle arrest are diverse pathways through which cells enter the death phase.62 In order to perceive the possible mechanism of cytotoxicity in SAS by cumin nanoemulsion, MTT, clonogenic assay and Annexin V-FITC assay results were analysed thoroughly. The achieved results confirm potential anticancer activity against tongue carcinoma cell lines.
The lipid membranes of S. aureus readily fuse with these oil-based nanoemulsions.27 The antimicrobial activity of cumin nanoemulsion against S. aureus was evident from the zone of inhibition. Studies suggest diffusion techniques such as agar well diffusion are more sensitive to antibacterial activity.63 Contrary to other substance union methods, the assessment of the antimicrobial impacts of nanoemulsions utilizing common sources delivers specific advantages for pharmaceutical and biomedical applications.64 The impact of cumin nanoemulsion on cytoplasmic leakage from bacterial pathogen was expressed as absorbance measured at 260 nm in membrane integrity assay. Loss of cell contents can be the reason for the significant release.63 Moreover, there was only a slight increase in the cytoplasmic leakage of nanoemulsion-treated in comparison with oil-treated samples. The cumin nanoemulsion possessed high antibacterial activity owing to the presence of bioactive compounds. Since these nanoemulsions fuse with the lipid membrane of the pathogen, this paves the way for the destabilization of membrane integrity and cell death as cited in the literature.65 The alterations in membrane permeability due to changes in structure could, therefore, be a possible reason for increased cytoplasmic leakage on treatment with cumin nanoemulsion.
Conclusion
Cumin nanoemulsion system was formulated through cumin seed oil through nanoemulsion by ultrasonication technology which produced uniform and spherical droplets in the nanometer size range. The essential oil was subjected to membrane integrity analysis and agar well diffusion assay. The nanoemulsion exhibited compelling potential cytotoxicity in resistance to tongue carcinoma cell lines. Its antibacterial activity against S. aureus was also considerable. The prevalence of tongue cancer has prioritized the need to develop non-aggressive methods of cancer treatment. Cumin used as an edible spice may be biocompatible, and will pose relatively low or no toxicity-a potentially significant competitive advantage. Facilitation of more in-vivo studies will help to achieve possible use in translational medicine.
Acknowledgments
We acknowledge CLRI, Adyar for availing FACS facility. We sincerely thank Dr. D. Karunagaran, Head, Department of Biotechnology, Bhupat & Jyoti Mehta School of Biosciences, IIT Madras for allowing access to the cell culture laboratory.
Disclosure
The authors declare no conflicts of interest in this work.
References
- 1.Mondal S, Bandyopadhyay S, K Ghosh M, et al. Natural products: promising resources for cancer drug discovery. Cancer Agents Med Chem. 2012;12:49–75. doi: 10.2174/187152012798764697 [DOI] [PubMed] [Google Scholar]
- 2.Safarzadeh E, Sandoghchian Shotorbani S, Baradaran B, et al. Herbal medicine as inducers of apoptosis in cancer treatment. Adv Pharm Bull. 2014;4:421–427. doi: 10.5681/apb.2014.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rein MJ, Renouf M, Cruz-Hernandez C, Actis-Goretta L, Thakkar SK, da Silva Pinto M. Bioavailability of bioactive food compounds; a challenging journey to bioefficacy. Br J Clin Pharmacol. 2013;3:588–602. doi: 10.1111/j.1365-2125.2012.04425.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hassan M, Watari H, AbuAlmatty A, Ohba Y, Sakuragi N. Apoptosis and molecular targeting therapy in cancer. Biomed Res Int. 2014;2014:150845. doi: 10.1155/2014/150845 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Renukuntla J, Vadlapudi AD, Patel A, Boddu SK, Mitra AK. Approaches for enhancing oral bioavailability of peptides and proteins. Int J Pharm. 2013;447:75–93. doi: 10.1016/j.ijpharm.2013.02.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meghani N, Patel P, Kansara K, et al. Formulation of vitamin D encapsulated cinnamon oil nanoemulsion: its potential anti-cancerous activity in human alveolar carcinoma cells. Colloids Surf B Biointerfaces. 2018;166:349–357. doi: 10.1016/j.colsurfb.2018.03.041 [DOI] [PubMed] [Google Scholar]
- 7.Roca I, Akova M, Baquero F, et al. The global threat of antimicrobial resistance: science for intervention. New Microbes New Infect. 2015;6:22–29. doi: 10.1016/j.nmni.2015.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar SG, Adithan C, Harish BN, Sujatha S, Roy G, Malini A. Antimicrobial resistance in India: a review. J Nat Sci Biol Med. 2013;4:286–291. doi: 10.4103/0976-9668.116970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raghunath D. Emerging antibiotic resistance in bacteria with special reference to India. J Biosci. 2008;33:593–603. doi: 10.1007/s12038-008-0077-9 [DOI] [PubMed] [Google Scholar]
- 10.Manoharan A, Chatterjee S, Mathai D, Study Group S; Mathai D SARI Study group. Detection and characterization of metallo beta lactamases producing P. aeruginosa. Indian J Med Microbiol. 2010;28:241–244. doi: 10.4103/0255-0857.66486 [DOI] [PubMed] [Google Scholar]
- 11.Souto EB, Nayak AP, Murthy RSR. Lipid nanoemulsions for anti-cancer drug therapy. Pharmazie. 2011;66:473–478. [PubMed] [Google Scholar]
- 12.Gupta A, Eral HB, Hatton TA, et al. Nanoemulsions: formation, properties and applications. Soft Matter. 2016;12:2826–2841. doi: 10.1039/C5SM02958A [DOI] [PubMed] [Google Scholar]
- 13.Jaiswal M, Dudhe R, Sharma PK. Nanoemulsion: an advanced mode of drug delivery system. 3 Biotech. 2015;5:123–127. doi: 10.1007/s13205-014-0214-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ganta S, Talekar M, Singh A, Coleman TP, Amiji MM. Nanoemulsions in translational research-opportunities and challenges in targeted cancer therapy. AAPS PharmSciTech. 2014;15:694–708. doi: 10.1208/s12249-014-0088-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walia N, Dasgupta N, Ranjan S, Chen L. Fish oil based vitamin D nanoencapsulation by ultrasonication and bioaccessibility analysis in simulated gastro-intestinal tract. UltrasonSonochem. 2017;39:623–635. doi: 10.1016/j.ultsonch.2017.05.021 [DOI] [PubMed] [Google Scholar]
- 16.Khan A, Chen H-C, Tania M, et al. Anticancer activities of Nigella sativa (black cumin). Afr J Tradit Complement Altern Med. 2011;8(5S). doi: 10.4314/ajtcam.v8i5S.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Periasamy VS, Athinarayanan J, Alshatwi AA. Anticancer activity of an ultrasonic nanoemulsion formulation of Nigella sativa L. essential oil on human breast cancer cells. Ultrason Sonochem. 2016;31:449–455. doi: 10.1016/j.ultsonch.2016.01.035 [DOI] [PubMed] [Google Scholar]
- 18.Yoon HJ, Zhang X, Kang MG, et al. Cytotoxicity evaluation of turmeric extract incorporated oil-in-water nanoemulsion. Int J Mol Sci. 2018;19:280. doi: 10.3390/ijms19010280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franken NA, Rodermond HM, Stap J, Haveman J, van Bree C. Clonogenic assay of cells in vitro. Nat Protoc. 2006;1:2315–2319. doi: 10.1038/nprot.2006.339 [DOI] [PubMed] [Google Scholar]
- 20.Balouiri M, Sadiki M, Ibnsouda SK. Methods for in vitro evaluating antimicrobial activity: a review. J Pharm Anal. 2016;6:71–79. doi: 10.1016/j.jpha.2015.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hou L, Shi Y, Zhai P, Le G. Inhibition of foodborne pathogens by Hf1, a novel antibacterial peptide from the larvae of the housefly (Musca domestica) in medium and orange juice. Food Control. 2007;18:1350–1357. doi: 10.1016/j.foodcont.2006.03.007 [DOI] [Google Scholar]
- 22.Berridge MV, Herst PM, Tan AS. Tetrazolium dyes as tools in cell biology: new insights into their cellular reduction. Biotechnol Annu Rev. 2005;11:127–152. doi: 10.1016/S1387-2656(05)11004-7 [DOI] [PubMed] [Google Scholar]
- 23.Horobin RW, Kiernan JA. Conn’s Biological Stains. A Handbook of Dyes, Stains and Fluorochromes for Use in Biology and Medicine. 10th ed. Oxford: Bios Scientific Publishers; 2002. [Google Scholar]
- 24.Nuryadi E, Permata TBM, Komatsu S, et al. Inter-assay precision of clonogenic assays for radiosensitivity in cancer cell line A549. Oncotarget. 2018;9:13706–13712. doi: 10.18632/oncotarget.24448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buch K, Peters T, Nawroth T, et al. Determination of cell survival after irradiation via clonogenic assay versus multiple MTT assay - a comparative study. Radiat Oncol. 2012;7:1. doi: 10.1186/1748-717X-7-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koopman G, Reutelingsperger CP, Kuijten GA, et al. Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood. 1994;84:1415–1420. doi: 10.1182/blood.V84.5.1415.bloodjournal8451415 [DOI] [PubMed] [Google Scholar]
- 27.Valgas C, De Souza SM, Smânia EFA, Smania A. Screening methods to determine antibacterial activity of natural products. Braz J Microbiol. 2007;38:369–380. doi: 10.1590/S1517-83822007000200034 [DOI] [Google Scholar]
- 28.Sugumar S, Nirmala J, Ghosh V, Anjali H, Mukherjee A, Chandrasekaran N. Bio-based nanoemulsion formulation, characterization and antibacterial activity against food-borne pathogens. J Basic Microbiol. 2013;53:677–685. doi: 10.1002/jobm.v53.8 [DOI] [PubMed] [Google Scholar]
- 29.Faivre S, Djelloul S, Raymond E. New paradigms in anticancer therapy: targeting multiple signaling pathways with kinase inhibitors. Semin Oncol. 2006;33:407–420. doi: 10.1053/j.seminoncol.2006.04.005 [DOI] [PubMed] [Google Scholar]
- 30.Chanda S, Nagani K. In vitro and in vivo methods for anticancer activity evaluation and some Indian medicinal plants possessing anticancer properties: an overview. J Pharmacogn Phytochem. 2013;2:140–152. [Google Scholar]
- 31.Marriott PJ, Shellie R, Cornwell C, et al. Gas chromatographic technologies for the analysis of essential oils. J Chromatogr A. 2001;936:1–22. doi: 10.1016/s0021-9673(01)01314-0 [DOI] [PubMed] [Google Scholar]
- 32.Cheng M, Zeng G, Huang D, et al. Advantages and challenges of tween 80 surfactant-enhanced technologies for the remediation of soils contaminated with hydrophobic organic compounds. Chem Eng J. 2017;314:98–113. doi: 10.1016/j.cej.2016.12.135 [DOI] [Google Scholar]
- 33.Mason TG, Wilking JN, Mcleson K, Chang CB, Graves SM. Nanoemulsions: formation, structure, and physical properties. J Phys Condens Matter. 2006;18:R635–R666. doi: 10.1088/0953-8984/18/41/R01 [DOI] [Google Scholar]
- 34.Hasai S, Pezashki A, Hamishehkar H. Effect of surfactant and oil type on size droplets of betacarotene-bearing nanoemulsions. Int J Curr Microbiol Appl Sci. 2015;4:146. [Google Scholar]
- 35.Tadros T, Izquierdo P, Esquena J, Solans C. Formation and stability of nano-emulsions. Adv Colloid Interface Sci. 2004;108–109:303–318. doi: 10.1016/j.cis.2003.10.023 [DOI] [PubMed] [Google Scholar]
- 36.Constantinides PP, Yiv SH. Particle size determination of phase-inverted water-in-oil microemulsions under different dilution and storage conditions. Int J Pharm. 1995;115:225–234. doi: 10.1016/0378-5173(94)00272-7 [DOI] [Google Scholar]
- 37.Nirmala MJ, Mukherjee A, Chandrasekaran N. Improved efficacy of fluconazole against candidiasis using bio-based microemulsion technique. BiotechnolApplBiochem. 2013;60:417–429. doi: 10.1002/bab.1116 [DOI] [PubMed] [Google Scholar]
- 38.Liu X, Dai Q, Austin L, et al. A one-step homogeneous immunoassay for cancer biomarker detection using gold nanoparticle probes coupled with dynamic light scattering. J Am Chem Soc. 2008;130:2780–2782. doi: 10.1021/ja711298b [DOI] [PubMed] [Google Scholar]
- 39.McClements DJ. Colloidal basis of emulsion color. Curr Opin Colloid Interface Sci. 2002;7:451–455. doi: 10.1016/S1359-0294(02)00075-4 [DOI] [Google Scholar]
- 40.McClements DJ. Theoretical prediction of emulsion color. Adv Colloid Interface Sci. 2002;97:63–89. doi: 10.1016/S0001-8686(01)00047-1 [DOI] [PubMed] [Google Scholar]
- 41.Lovelyn C, Attama AA. Current state of nanoemulsions in drug delivery. J Biomater Nanobiotechnol. 2011;2:626–639. [Google Scholar]
- 42.El Eini DID, Barry BW, Rhodes CT. Micellar size, shape, and hydration of long-chain polyoxyethylene non-ionic surfactants. J Colloid Interface Sci. 1976;54:348–351. doi: 10.1016/0021-9797(76)90314-3 [DOI] [Google Scholar]
- 43.Barry BW, El Eini DID. Solubilization of hydrocortisone, dexamethasone, testosterone and progesterone by long-chain polyoxyethylene surfactants. J Pharm Pharmacol. 1976;28:210–218. doi: 10.1111/j.2042-7158.1976.tb04133.x [DOI] [PubMed] [Google Scholar]
- 44.Bhushani JA, Karthik P, Anandharama krishnan C. Nanoemulsion based delivery system for improved bioaccessibility and Caco-2 cell monolayer permeability of green tea catechins. Food Hydrocoll. 2016;56:372–382. doi: 10.1016/j.foodhyd.2015.12.035 [DOI] [Google Scholar]
- 45.Milhomem-Paixão SS, Fascineli ML, Muehlmann LA, et al. Andiroba oil (carapa guianensis aublet) nanoemulsions: development and assessment of cytotoxicity, genotoxicity, and hematotoxicity. J Nanomater. 2017;2017:1–11. doi: 10.1155/2017/4362046 [DOI] [Google Scholar]
- 46.Van Meerloo J, Kaspers GJ, Cloos J. Cell sensitivity assays: the MTT assay. Methods Mol Biol. 2011;731:237–245. [DOI] [PubMed] [Google Scholar]
- 47.Bergantini APF, Castro FA, Souza AM, Fett-Conte AC. Chronic myeloid leukemia and the Fas-FasL system. Rev Bras Hematol Hemoter. 2005;27:120–125. doi: 10.1590/S1516-84842005000200012 [DOI] [Google Scholar]
- 48.Saravanan BC, Sreekumar C, Bansal GC, Ray D, Rao JR, Mishra AK. A rapid MTT colorimetric assay to assess the proliferation index of two Indian strains of Theileriaannulata. Vet Parasitol. 2003;113:211–216. doi: 10.1016/s0304-4017(03)00062-1 [DOI] [PubMed] [Google Scholar]
- 49.Huet O, Petit JM, Ratinaud MH, Julien R. NADH-dependent dehydrogenase activity estimation by flow cytometric analysis of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction. Cytometry. 1992;13:532–539. doi: 10.1002/cyto.990130513 [DOI] [PubMed] [Google Scholar]
- 50.Feuerstein T, Schauder A, Malik Z. Silencing of ALA dehydratase affects ALA-photodynamic therapy efficacy in K562 erythroleukemic cells. Photochem Photobiol Sci. 2009;8:1461–1466. doi: 10.1039/b9pp00007k [DOI] [PubMed] [Google Scholar]
- 51.Du XM, Yan Y, Bai ZL, et al. Evaluation of the cytotoxicity of a two photon absorbing fluorescence compound on human hepatic cancer cells in mice. Biotech Histochem. 2010;85:107–113. doi: 10.1080/10520290903149588 [DOI] [PubMed] [Google Scholar]
- 52.Khan I, Bahuguna A, Bhardwaj M, et al. Carvacrol nanoemulsion evokes cell cycle arrest, apoptosis induction and autophagy inhibition in doxorubicin resistant-a549 cell line. Artif Cells Nanomed Biotechnol. 2018;46:664–675. doi: 10.1080/21691401.2018.1434187 [DOI] [PubMed] [Google Scholar]
- 53.Nikoletopoulou V, Markaki M, Palikaras K, et al. Crosstalk between apoptosis, necrosis and autophagy. Biochim Biophys Acta Mol Cell Res. 2013;1833:3448–3459. doi: 10.1016/j.bbamcr.2013.06.001 [DOI] [PubMed] [Google Scholar]
- 54.Massano J, Regateiro FS, Januário G, et al. Oral squamous cell carcinoma: review of prognostic and predictive factors. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:67–76. doi: 10.1016/j.tripleo.2005.07.038 [DOI] [PubMed] [Google Scholar]
- 55.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4 [DOI] [PubMed] [Google Scholar]
- 56.Wong RSY. Apoptosis in cancer: from pathogenesis to treatment. J Exp Clin Cancer. 2011;30:87. doi: 10.1186/1756-9966-30-87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Foster I. Cancer: a cell cycle defect. Radiography. 2008;14:144–149. doi: 10.1016/j.radi.2006.12.001 [DOI] [Google Scholar]
- 58.Kuwana T, Newmeyer DD. Bcl-2-family proteins and the role of mitochondria in apoptosis. Curr Opin Cell Biol. 2003;15:691–699. [DOI] [PubMed] [Google Scholar]
- 59.Kerr JFR, Gobe GC, Winterford CM, Harmon BV. Anatomical methods in cell death. Method Cell Biol. 1995;46:1–27. [DOI] [PubMed] [Google Scholar]
- 60.Boujrad H, Gubkina O, Robert N, Krantic S, Susin AS. AIF-mediated programmed necrosis: a highly regulated way to die. Cell Cycle. 2007;6:2612–2619. doi: 10.4161/cc.6.21.4842 [DOI] [PubMed] [Google Scholar]
- 61.Vermelho AB, Cardoso VS, Junior ER, dos Santos EP, Supuran CT. Nanoemulsions of sulfonamide carbonic anhydrase inhibitors strongly inhibit the growth of Trypanosoma cruzi. J Enzyme Inhib Med Chem. 2018;33:139–146. doi: 10.1080/14756366.2017.1405264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang H, Shen FY, Weng P, Zhao G, Feng F, Zheng X. Antimicrobial activity of a food-grade fully dilutable microemulsion against Escherichia coli and Staphylococcus aureus. Int J Food Microbiol. 2009;135:211–215. doi: 10.1016/j.ijfoodmicro.2009.08.015 [DOI] [PubMed] [Google Scholar]
- 64.Maiti S, Krishnan D, Barman G, Ghosh SK, Laha SK. Antimicrobial activities of silver nanoparticles synthesized from Lycopersicon esculentum extract. J Anal Sci Technol. 2014;5:40. doi: 10.1186/s40543-014-0040-3 [DOI] [Google Scholar]
- 65.Baker JR, Hamouda T, Shih A, Andrzej M. Non-toxic antimicrobial compositions and methods of use. US Patent. 2003;6:559,189. [Google Scholar]