Summary
Neopterin is primarily synthesized and released by activated macrophages/monocytes upon stimulation with interferon‐γ and is considered as a marker for macrophage activation. This study aimed to analyze the serum levels of neopterin in patients with dermatomyositis (DM) in association with clinical manifestations, laboratory data and patient prognosis. One hundred and eighty‐two consecutive DM patients and 30 healthy controls were retrospectively enrolled into the study. Serum levels of neopterin were significantly increased in DM patients compared to healthy controls (P < 0·001). High serum neopterin levels were associated with anti‐melanoma differentiation‐associated gene (MDA5) antibody, rapidly progressive interstitial lung disease (RP‐ILD) and characteristic DM cutaneous involvement. Longitudinal assessment of serum samples revealed that the serum neopterin levels were closely correlated with disease severity (β = 30·24, P < 0·001). In addition, a significant increase in serum neopterin concentration of non‐survivors was observed when compared to that of survivors (P < 0·001). Receiver operator characteristic curves showed that serum neopterin could distinguish non‐survivors and survivors at an optimal cut‐off level of 22·1 nmol/l with a sensitivity and specificity of 0·804 and 0·625, respectively (P < 0·001). Kaplan–Meier survival curves revealed that DM patients with serum neopterin > 22·1 nmol/l had a significantly higher mortality compared to the patient group with serum neopterin < 22·1 nmol/l (log‐rank P < 0·001). Multivariate regression analysis identified high serum neopterin concentration to be an independent risk factor for poor prognosis in DM (adjusted hazard ratio = 4·619, 95% confidence interval = 2·092–10·195, P < 0·001). In conclusion, increased serum levels of neopterin were significantly associated with RP‐ILD and reduced survival in DM patients, suggesting it as a promising biomarker in disease evaluation of DM.
Keywords: dermatomyositis, neopterin, prognostic factor, rapidly progressive interstitial lung disease
Serum neopterin was significantly increased in DM, especially in patients with anti‐MDA5 and RP‐ILD. Serum neopterin seems to parallel disease severity in DM patients. High baseline level of neopterin was associated with increased mortality and was identified as an independent prognostic factor in DM.

Introduction
Dermatomyositis (DM) is a common clinical subset of idiopathic inflammatory myopathies (IIMs) characterized by symmetrical proximal muscle weakness, decreased muscle endurance, characteristic skin lesions, the presence of myositis‐specific autoantibodies (MSAs) and inflammatory infiltrates in muscle and skin 1. Interstitial lung disease (ILD) is a significant extra‐muscular manifestation that affects approximately up to 80% DM patients 2, and is considered to be associated with poor prognosis 3. A subgroup of DM patients with ILD are acute and rapidly progressive, and these patients are especially found with anti‐melanoma differentiation‐associated gene (MDA5) 4, 5 and anti‐synthetase antibodies 6, 7.
Neopterin, a catabolic product of guanosine triphosphate (GTP), is primarily synthesized and released by activated macrophages/monocytes and dendritic cells upon stimulation with interferon (IFN)‐γ 8. It has been reported to increase in numerous clinical states with dysregulation of the immune system and chronic inflammation. Furthermore, neopterin is confirmed to be associated with disease severity and is considered to be a potential biomarker predicting adverse outcomes in patients with HIV infection 9, cardiovascular disease 10 and various types of cancer 11. Interestingly, several studies have revealed increased serum neopterin in rheumatic diseases such as systemic lupus erythematosus 12, rheumatoid arthritis 12, 13, Sjögren’s syndrome 14 and granulomatosis with polyangiitis 15. In addition, neopterin was found elevated in the sera and urine of juvenile and adult IIM patients 16, 17. Moreover, De Benedetti revealed that serum neopterin was significantly correlated with the severity of muscle strength impairment in juvenile DM 18. More recently, Nishioka found elevated serum neopterin may correlate with interstitial lung disease in anti‐MDA5 antibody‐positive clinically amyopathic dermatomyositis (CADM) 19. However, with increased understanding of the heterogeneous nature of DM, the clinical significance of neopterin in DM remains to be fully clarified and there is a paucity of longitudinal data concerning serum neopterin in relation to the outcome of DM patients.
Therefore, in the present study, we set out to investigate the expression and clinical relevance of serum neopterin in DM, especially its association with patient prognosis.
Methods
Study design and patients
We retrospectively identified DM patients from longitudinal cohorts at the China–Japan Friendship Hospital. Patients with probable or definite DM according to the diagnostic criteria of Bohan and Peter 20, 21 or with clinical amyopathic DM according to the Sontheimer criteria 22 were recruited for this study. Patients with the following conditions were excluded: (1) complicated with other connective tissue diseases; (2) age of disease onset less than 16 years; (3) complicated with cancer that occurred beyond 3 years since myositis onset. A total of 225 DM and 10 CADM patients were included. Among these patients, 36 were complicated with other connective tissue diseases, five were complicated with cancer beyond 3 years from myositis onset and 12 patients’ disease onset was less than 16 years. Finally, 182 DM patients referred to our hospital between September 2003 and September 2016 were enrolled into the study (as shown in Supporting information, Fig. S1). Some of the patients in this cohort were involved in our previous study 6, 23. The patients were followed‐up until December 2016, with a last follow‐up rate of 91%. In addition, 30 age‐ and sex‐matched healthy volunteers were included in the healthy control group.
All patients’ data were used anonymously, and written informed consents were obtained from all participating individuals. This study was performed with the approval of the Human Ethics Board of the China–Japan Friendship Hospital (approval number 2016‐117).
Data collection
A standard form was used to collect clinical and laboratory data from medical records. Data collection included age of onset, sex, duration, presence of ILD, rapidly progressive ILD (RP‐ILD), muscle weakness, myalgia, mechanic’s hands, Raynaud’s phenomenon, skin ulcer, Gottron papules, Heliotrope rash, calcinosis, arthritis/arthralgia, dysphagia and malignancy, as well as all the laboratory tests. The results of pulmonary function examination were also collected, including forced VC (FVC), forced expiratory volume in 1 s (FEV1) and diffusing capacity of carbon monoxide (DLco). The main outcome was death. Survival status was confirmed by telephone call follow‐up or hospital records. Twenty‐six patients were followed‐up as out‐ or in‐patients in December 2016; 140 were successfully reached by telephone; the remaining 16 patients could not be reached and were considered as lost to follow‐up.
Definition
Cancer‐associated myositis (CAM) was defined as cancer occurring within 3 years of myositis onset. The presence of ILD was evaluated by computed tomography (CT) and abnormal pulmonary function test 24. RP‐ILD was defined as a worsening of radiological interstitial changes with progressive dyspnea and hypoxemia within 3 months after the onset of respiratory symptoms 24, 25. Physician global activity (PGA) using a continuous 10‐cm visual analog scale was performed to evaluate the disease activity of DM patients. A higher PGA score represents more severe disease status. A change greater than 20% in the PGA compared to the last visit was considered as a clinical improvement or deterioration.
Biochemical analyses
Serum levels of neopterin were detected using a commercially available quantitative sandwich enzyme‐linked immunosorbent assay (ELISA) kit (IBL International, Hamburg, Germany), according to the manufacturer’s protocol. Briefly, 20 μl standard control serum was pipetted in duplicate to designated wells of a microplate coated with goat anti‐rabbit immunoglobulin (Ig)G. Following this step, 100 μl of enzyme conjugate and 50 μl of neopterin anti‐serum were added to the wells. After incubating at room temperature on an orbital shaker at 500 rpm in the dark for 90 min, the plates were washed and incubated with 150 μl 3,3′,5,5′‐tetramethylbenzidine (TMB) substrate solution for 10 min. Stop solution was then added and absorbance was measured using an ELISA reader at 450 nm, with 630 nm as the reference wavelength. A standard curve for each assay was generated, and serum neopterin concentration was calculated. Each sample was analyzed twice.
The myositis‐specific antibodies were examined using an immunoblot assay kit (EUROIMMUN, Luebeck, Germany), according to the manufacturer’s instructions, including anti‐Mi‐2, anti‐transcriptional intermediary factor (TIF1)‐γ, anti‐nuclear matrix protein 2 (NXP2), anti‐small ubiquitin‐like modifier‐activating enzyme A subunit (SAE1), anti‐MDA5, anti‐signal recognition particle (SRP), anti‐histidyl‐tRNA synthetase (Jo‐1), anti‐threonyl (PL)‐7, anti‐PL‐12, anti‐isoleucyl (OJ) and anti‐gylcyl (EJ).
Statistical analyses
Statistical analyses were performed using GraphPad Prism version 6.01 (GraphPad Software, San Diego, CA, USA) and spss version 23.0 (SPSS, Inc., Chicago, IL, USA). The normally distributed data were expressed as mean ± standard deviation (s.d.) and compared by independent Student’s t‐test; non‐parametric distribution data were expressed as median values and interquartile ranges (IQR), and the data of unpaired samples were analyzed by using the Mann–Whitney U‐test. Spearman’s correlation analysis was performed to test the correlations. Generalized estimating equation model (GEE) for repeated measures was performed to determine correlations between serum neopterin levels and disease activity scores. Wilcoxon’s signed‐rank and c2 tests were used when appropriate. The Kaplan–Meier survival curve and log‐rank tests were used to compare survival rates. Cox proportional hazards regression analysis was applied to identify the risk factors. A P‐value ≤ 0·05 was considered statistically significant.
Results
Baseline clinical characteristics and treatment of DM patients
In the present study, 182 DM patients were enrolled and their baseline clinical characteristics are presented in Table 1. Women outnumbered men in the cohort (127 women/55 men). The mean age of disease onset was 49·6 years, and the mean disease duration was 1·9 years. During follow‐up, 27 (14·8%) patients developed malignancy within 3 years of diagnosis of DM. In total, 134 (73·6%) patients were tested as MSAs‐positive.
Table 1.
Baseline characteristics of the study population
| Variables | DM patients |
|---|---|
| Female/male ratio | 127/55 |
| Age of onset, mean ± s.d. (range) years | 49·6 ± 13·3 (18–83) |
| Disease duration, mean ± s.d., years | 1·9 ± 3·3 |
| Clinical features, number of patients affected (%) | |
| ILD | 124 (68·1%) |
| RP‐ILD | 40 (22·0%) |
| Muscle weakness | 121 (66·5%) |
| Myalgia | 78 (42·9%) |
| Mechanic’s hands | 59 (32·4%) |
| Raynaud’s phenomenon | 16 (8·8%) |
| Skin ulcer | 32 (17·6%) |
| Gottron papules | 102 (56·0%) |
| Heliotrope rash | 95 (52·2%) |
| Calcinosis | 6 (3·3%) |
| Arthritis/arthralgia | 55 (30·2%) |
| Dysphagia | 47 (25·8%) |
| Malignancy | 27 (14·8%) |
| Pulmonary function testa | |
| FVC % | 88·9 ± 23·7 |
| FEV1 % | 86·7 ± 21·8 |
| DLco % | 63·8 ± 17·9 |
| Laboratory parameters | |
| ANA‐positive, number of patients (%) | 103 (56·6%) |
| Anti‐synthetase antibody‐positive, number of patients (%) | 37 (20·3%) |
| Anti‐MDA5‐positive, number of patients (%) | 48 (26·4%) |
| Anti‐TIF1γ‐positive, number of patients (%) | 26 (14·3%) |
| Anti‐NXP2‐positive, number of patients (%) | 10 (5·5%) |
| Anti‐SAE‐positive, number of patients (%) | 3 (1·6%) |
| Anti‐Mi2‐positive, number of patients (%) | 10 (5·5%) |
| CK, IU/l | 600·9 ± 1250·9 |
|
LDHb, IU/l |
347·1 ± 534·9 |
| ASTb, IU/l | 71·8 ± 180·2 |
| ALTb, IU/l | 63·3 ± 79·5 |
| CRPc, mg/dl | 1·75 ± 4·50 |
|
ESRd, mm/h |
26·6 ± 26·8 |
| Ferritine, ng/ml | 712·8 ± 1415·8 |
| sCD163, ng/ml | 845·0 ± 378·8 |
| CD3+ T cell numberf, cells/μl | 904 ± 537 |
| CD3+CD4+ T cell numberf, cells/μl | 555 ± 344 |
| CD3+CD8+T cell numberf, cells/μl | 336 ± 243 |
DM = dermatomyositis; ILD = interstitial lung disease; RP‐ILD = rapidly progressive ILD; ANA = anti‐nuclear antibodies; CAM = cancer‐associated myositis; FVC = forced VC; FEV1 = forced expiratory volume in 1 s; DLco = diffusing capacity of carbon monoxide; MDA5 = melanoma differentiation‐associated gene; TIF1 = transcriptional intermediary factor; NXP2 = nuclear matrix protein 2; SAE = small ubiquitin‐like modifier‐activating enzyme A subunit; CK = creatine kinase, normal limits: 26–200; LDH = lactate dehydrogenase, normal limits: 100–250; AST = aspartate aminotransferase, normal limits: 0–42; ALT = alanine aminotransferase, normal limits: 0–40; CRP = C‐reactive protein, normal limits: 0–0·8; ESR = erythrocyte sedimentation rate, normal limits: 0–20 for female, 0–15 for male; ferritin, normal limits: 11–306·8; CD3+ T cell number = normal limits: 808–2072. aData available for 101 patients; bdata available for 181 patients; cdata available for 171 patients; ddata available for 172 patients; edata available for 99 patients; fdata available for 164 patients.
All patients in our cohort received corticosteroids at doses between 0·5 mg/kg and 1 mg/kg as part of their initial therapy. In addition, approximately 84·6% patients (154 of 182) also received at least one or more immunosuppressants, including methotrexate, cyclophosphamide, cyclosporin A, azathioprine, intravenous immunoglobulin, hydroxychloroquine or mycophenolate mofetil.
Increased serum neopterin in DM patients
From the established biobank, the baseline serum samples taken from the patients at their first visit to our center were used (each sample was independently taken from one individual). ELISA was performed to examine the serum levels of neopterin, and the results showed that the median value of serum neopterin levels were significantly higher in DM patients (median = 21·2 nmol/l, IQR = 13·9–35·2 nmol/l) than healthy controls (median = 4·3 nmol/l, IQR = 2·9–5·6 nmol/l, P < 0·001), as shown in Fig. 1a. We further analyzed the associations of serum neopterin levels with laboratory parameters and clinical manifestations. Using analysis of variance (anova) comparison, we found that serum neopterin levels significantly differed between individual MSA groups (P < 0·001). Multiple comparison showed that serum neopterin levels in anti‐MDA5 group are significantly higher than those in the anti‐aminoacyl tRNA synthetase (ARS) group (adjusted P < 0·05) and MSA negative group (adjusted P < 0·01) (Fig. 1b). Spearman’s correlation analysis results revealed that the serum levels of neopterin positively correlated with serum ferritin concentrations (r = 0·617, P < 0·001), as shown in Fig. 1c. After excluding the highest outlier for neopterin, serum neopterin still significantly correlated with serum ferritin concentrations (r = 0·605, P < 0·001). The serum levels of neopterin were not correlated with serum creatine kinase (CK) levels (P > 0·05); however, serum levels of neopterin positively correlated with lactate dehydrogenase (LDH) (r = 0·364, P < 0·001), aspartate aminotransferase (AST) (r = 0·41, P < 0·001), alanine aminotransferase (ALT) (r = 0·308, P < 0·001), C‐reactive protein (CRP) (r = 0·42, P < 0·001) and erythrocyte sedimentation rate (ESR) (r = 0·451, P < 0·001). Moreover, the serum levels of neopterin were inversely correlated with circulated CD3+ T cell number (r = −0·311, P < 0·001). In addition, the serum neopterin concentration was markedly elevated in patients with RP‐ILD compared to those with non‐RP‐ILD or with no ILD (Fig. 1d). Consequently, we further analyzed the association between serum neopterin concentration and pulmonary function impairments, and the results showed that serum neopterin concentration inversely correlated with FVC% (r = −0·35, P < 0·001), FEV1% (r = −0·338, P < 0·01) and DLco/SB% (r = −0·286, P < 0·01), as shown in Fig. 1e–g. These correlations between serum neopterin concentration and pulmonary function impairments remain significant after excluding the highest outlier for neopterin. We also found an increase in serum neopterin concentration of patients with skin ulcer (median = 27·9 nmol/l, IQR = 19·9–46·6 nmol/l versus median = 20·8 nmol/l, IQR = 12·9–34·5 nmol/l, P < 0·05) and heliotrope rash (median = 25·6 nmol/l, IQR = 16·3–50·9 nmol/l versus median = 19·9 nmol/l, IQR = 12·9–27·7 nmol/l, P < 0·01) compared to those patients without these clinical features, while no statistical difference was seen in serum neopterin concentration of patients with or without malignancy (Fig. 1h). In addition, we found disease duration significantly differentiated serum neopterin levels in DM patients (Fig. 1i). Spearman’s correlation analysis revealed a reverse correlation between disease duration and serum neopterin level (r = −0·366, P < 0·001). Moreover, no significant differences in serum neopterin concentration were found when patients were subgrouped according to the presence of muscle weakness, myalgia, mechanic’s hands, Raynaud’s phenomenon, Gottron papules, arthritis/arthralgia, calcinosis or dysphagia (P‐values all greater than 0·05).
Figure 1.
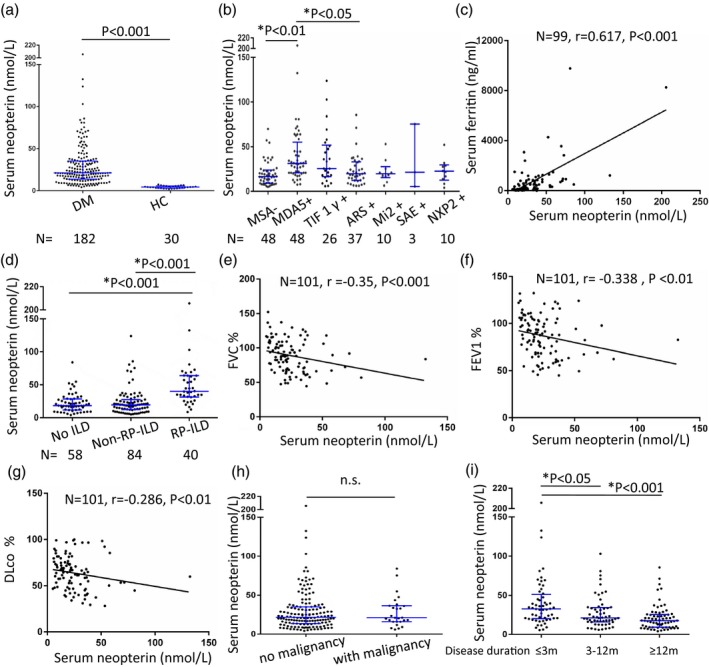
Serum concentrations of neopterin in dermatomyositis (DM) patients. Serum levels of neopterin were detected in 182 DM patients and 30 healthy controls using the enzyme‐linked immunosorbent assay (ELISA) method (each sample was independently from one individual). (a) Serum levels of neopterin in DM patients were significantly higher than that in healthy controls by using the Mann–Whitney U‐test. (b) Using analysis of variance (anova) comparison, we found that serum neopterin levels significantly differed between individual myositis‐specific autoantibody (MSA) groups (P < 0·001). Multiple comparison showed that serum neopterin levels in the anti‐melanoma differentiation‐associated gene (MDA5) group are significantly higher than those in the anti‐aminoacyl tRNA synthetase (ARS) group (adjusted P < 0·05) and MSA negative group (adjusted P < 0·01). (c) The serum levels of neopterin positively correlated with serum ferritin concentrations by Spearman’s correlation analysis. (d) The serum neopterin concentrations were markedly elevated in patients with rapidly progressive interstitial lung disease (RP‐ILD) compared to those with non‐RP‐ILD or with no ILD. (e–g) Serum neopterin concentration correlated inversely with pulmonary function impairment parameters by Spearman’s correlation analysis. (h) No difference was found in serum neopterin concentrations between cancer associated myositis (CAM) and non‐CAM patients using the Mann–Whitney U‐test. (i) Patients with disease duration of less than 3 months had significantly higher serum neopterin levels compared to patients whose disease durations were 3–12 months and those whose disease duration longer than 12 months. The displayed P‐values marked by asterisk * have been adjusted by Bonferroni correction and are from one‐way analysis of variance (anova) tests of logarithmically transformed data. HC = healthy control; FEV1 = forced expiratory volume in 1 s; FVC = forced VC; DLco = diffusing capacity of carbon monoxide; n.s. = not significant.
Serum neopterin and disease severity correlation
In order to investigate the variation in serum neopterin levels over time and in relation to disease severity, we analyzed the neopterin levels in longitudinal serum samples. Collectively, 72 serum samples and corresponding clinical data from repeated hospitalizations of 29 DM patients were collected. We obtained three longitudinal samples per patient in 14 patients. For the remaining 15 patients, we obtained two longitudinal samples per patient. By GEE, a significantly positive correlation was found between the serum neopterin levels and the PGA scores (β = 30·24, P < 0·001). The results showed a stepwise decrease in the median value of serum neopterin concentration in 17 patients who achieved clinical improvement with decreasing disease severity, as shown in Fig. 2a. In 12 DM patients who deteriorated clinically or did not improve, the median serum neopterin concentration matched the direction of PGA score, but this was not statistically significant (Fig. 2b). Fig. 2 also shows the trends of serum neopterin levels and the changes of PGA scores in all the patients with clinical improvement (Fig. 2c) and patients with no improvement or worsening (Fig. 2d). These results suggest that serum neopterin seems to parallel disease severity at least in some DM patients.
Figure 2.
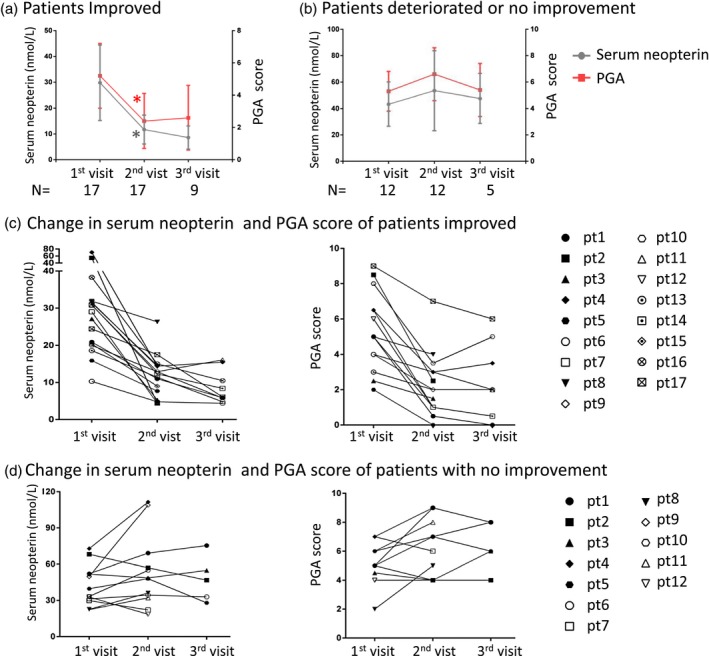
The change in serum neopterin levels over time in relation to disease status. (a) The median value of serum neopterin concentration stepwise decreased in 17 patients who achieved clinical improvement with decreasing disease severity. The intervals of each visit were 3–4 months. (b) In 12 dermatomyositis (DM) patients who deteriorated clinically or did not improve, median serum neopterin concentration matched the direction of physician global activity (PGA) score. The intervals of each visit were 1–2 months. The shorter follow‐up interval was due to uncontrolled disease development. The direction of serum neopterin levels and PGA scores are shown in all the patients with clinical improvement (c) and patients with no improvement or worsening (d). *P < 0·01 compared to the first visit by Wilcoxon’s paired signed‐rank test.
Survival analysis in relation to neopterin
In our cohort, the follow‐up duration ranged from 0·5 to 284 months, and the median follow‐up time was 27 months. During the follow‐up period, 46 subjects died due to all causes. The cumulative 10‐year overall survival rate of the cohort was 64·9%. The levels of baseline serum neopterin were compared between patients with survival and patients who died. As shown in Fig. 3a, for the entire DM group, there was a significant increase in the median baseline neopterin concentration of non‐survivors (median = 38·7 nmol/l, IQR = 23·5–65·3 nmol/l) compared to that of survivors (median = 19·0 nmol/l, IQR = 12·5–29·0 nmol/l, P < 0·001). As RP‐ILD and malignancy have been reported as significant factors associated with poor prognosis in DM, we subsequently compared the serum levels of neopterin within the patient group with RP‐ILD or CAM. When looking at the patients with RP‐ILD, we found the baseline serum levels of neopterin were statistically increased in non‐survivors (median = 60·7 nmol/l, IQR = 38·2–75·0 nmol/l) compared to survivors (median = 34·9 nmol/l, IQR = 23·5–53·8 nmol/l, P < 0·01) (Fig. 3b). In addition, for the patients with CAM, there was a significant difference in the baseline serum levels of neopterin between survivors and non‐survivors (P < 0·05) (Fig. 3c). As a higher neopterin level was found in DM patients with anti‐MDA5 antibodies, we further compared the baseline serum neopterin level of survivors and non‐survivors in patients with anti‐MDA5 antibodies. As shown in Fig. 3d, a significantly higher serum neopterin level was found in non‐survivors compared to survivors (P < 0·01).
Figure 3.
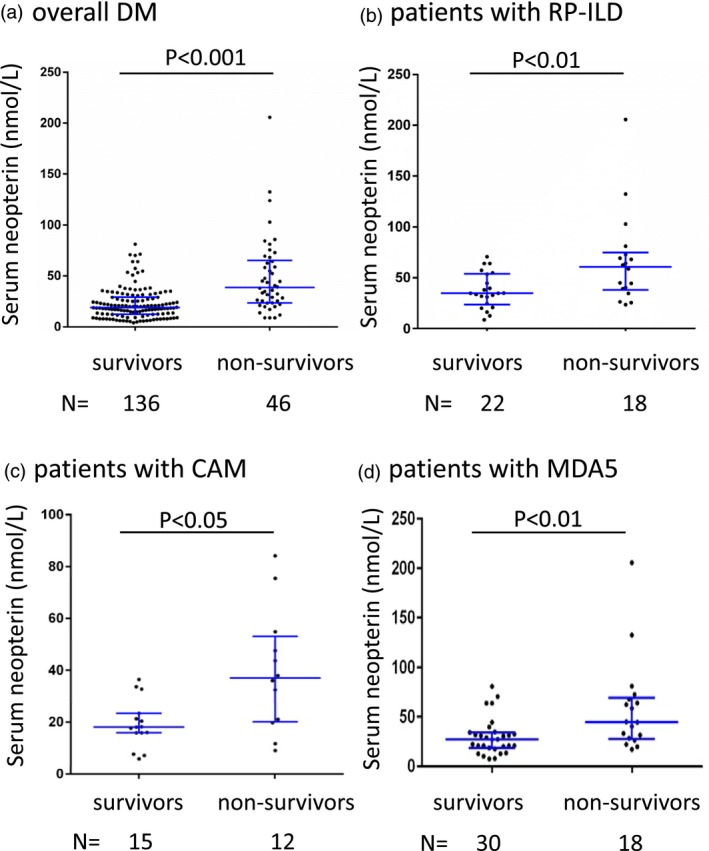
Serum levels of neopterin in dermatomyositis patient subgroups stratified by survival status. (a) All patients; (b) patients with rapidly progressive interstitial lung disease (RP‐ILD); (c) patients with cancer‐associated myositis (CAM); (d) patients with anti‐melanoma differentiation‐associated gene (MDA5) antibody‐positive.
Receiver operating characteristic (ROC) curves were generated to test the ability of serum neopterin in distinguishing between non‐survivors and survivors for sensitivity and specificity (Fig. 4a). At an optimal cut‐off level of 22·1 nmol/l, the sensitivity and specificity was 0·804 and 0·625, respectively, and the area under the curve (AUC) was 0·777 [P < 0·001, 95% confidence interval (CI) = 0·697–0·857]. Kaplan–Meier survival curves showed that DM patients with serum neopterin > 22·1 nmol/l had significantly higher mortality compared to the patient group with serum neopterin < 22·1 nmol/l (log‐rank P < 0·001), as shown in Fig. 4b. The 1‐ and 5‐year survival rates of the patient group with neopterin > 22·1 nmol/l were 0·689 and 0·47, respectively, while for the patient group with neopterin < 22·1 nmol/l the 1‐ and 5‐year survival rates were 0·968 and 0·854, respectively. Meanwhile, no significant differences were observed in the treatment regimens between the patient group with neopterin > 22·1 nmol/l (corticosteroid alone: 15 of 88, 17·0%, corticosteroid combined with immunosuppressants: 73 of 88, 83·0%) and the patient group with neopterin < 22·1 nmol/l (corticosteroid alone: 13 of 94, 13·8%, corticosteroid combined with immunosuppressants: 81 of 94, 86·2%, χ2 value = 0·361, P > 0·05). Furthermore, we tried to group the patients into three subgroups: those with baseline serum neopterin < 22·1, with baseline serum neopterin 22·1–44·2 (twice the cut‐off level) and with baseline serum neopterin > 44·2. Kaplan–Meier survival curves (Fig. 4c) revealed a significant difference in mortality between these groups (log‐rank P < 0·001). The 5‐year survival rates of these three groups were 0·854, 0·573 and 0·241, respectively. The 1‐year survival rates of these three groups were 0·968, 0·774 and 0·536, respectively. The deceased cases of these three groups until the end‐point of follow‐up comprised nine of 94 (9·6%), 17 of 56 (30·4%) and 20 of 32 (62·5%), respectively. Collectively, these data indicate that DM patients with higher baseline serum neopterin tend to have a poorer prognosis.
Figure 4.
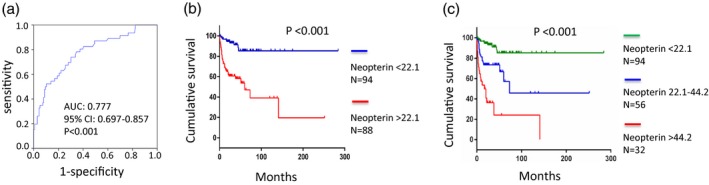
Survival analysis of dermatomyositis (DM) patients related to serum neopterin levels. (a) Receiver operating characteristics (ROC) for the prediction of fatal outcome in DM patients showed that a cut‐off value of 22·1 nmol/l could distinguish non‐survivors from survivors with an area under the curve (AUC) of 0·777 [95% confidence interval (CI) = 0·697–0·857, P < 0·001]. (b) Survival curves of DM patients subgrouped by serum neopterin levels at a cut‐off value of 22·1 nmol/l. (c) Survival curves of DM patients subgrouped by serum neopterin into three groups: those with baseline serum neopterin < 22·1, those with baseline serum neopterin 22·1–44·2 and those with baseline serum neopterin > 44·2.
Prognostic factors analysis
We further performed multivariate Cox regression analysis to identify factors associated with all‐cause mortality in our cohort of patients and to verify whether serum neopterin is a prognostic factor. The results of univariate analysis demonstrated several significant associations with poor outcomes in DM patients (Table 2), including age, RP‐ILD, muscle weakness, skin ulcer, dysphagia, CAM, anti‐MDA5 antibody, anti‐TIF1‐γ antibody, elevated CK, LDH, CRP, ESR, serum ferritin, decreased peripheral CD3+ T cell count and increased serum neopterin concentration. In contrast, the univariate analysis revealed that the presence of anti‐synthetase antibody was associated with decreased risk for all‐cause mortality with hazard ratios of 0·372 (95% CI = 0·147–0·942, P < 0·05). We further created a multivariable regression model to test whether serum neopterin was independently associated with poor outcome for DM patients. We chose the variables for inclusion of multivariable analysis carefully, given the number of events available 26, 27 and the clinical relevance. Finally, seven components, including age, sex, rp‐ILD, CAM, anti‐MDA5, anti‐TIF1‐γ antibody and serum neopterin, were included in the multivariate Cox analysis. As shown in Table 2, the resulting data revealed that age, RP‐ILD, CAM, anti‐MDA5 antibody and neopterin > 22·1 nmol/l were significant predictors of mortality. Of note, after adjustment for age, sex, RP‐ILD, CAM, anti‐MDA5 and anti‐TIF1‐γ antibody, the patients with serum neopterin > 22·1 nmol/l showed a fourfold increase in the relative risk of death compared to those with neopterin < 22·1 nmol/l (adjusted HR = 4·619, 95% CI = 2·092–10·195, P < 0·01), suggesting that high serum neopterin concentration was a significant clinical characteristic associated with increased mortality, independent of age, RP‐ILD, CAM or anti‐MDA5 antibody.
Table 2.
Multivariate Cox regression analysis of prognostic factors in patients with dermatomyositis
| Variables | HR (95% CI) | P‐value |
|---|---|---|
| Univariate analysis | ||
| Age of onset, years | 1·044 (1·020–1·070) | < 0·001 |
| Sex | 1·459 (0·800–2·663) | 0·218 |
| ILD | 0·982 (0·529–1·820) | 0·953 |
| RP‐ILD | 3·690 (2·019–6·743) | < 0·001 |
| Muscle weakness | 2·342 (1·127–4·867) | < 0·05 |
| Myalgia | 1·785 (0·997–3·195) | 0·051 |
| Mechanic’s hands | 1·050 (0·559–1·970) | 0·880 |
| Raynaud’s phenomenon | 1·386 (0·587–3·274) | 0·457 |
| Skin ulcer | 2·764 (1·483–5·151) | < 0·05 |
| Gottron papules | 1·154 (0·641–2·077) | 0·633 |
| Calcinosis | 0·045 (0·000–25·831) | 0·339 |
| Arthritis/arthralgia | 1·248 (0·680–2·290) | 0·475 |
| Dysphagia | 2·565 (1·430–4·601) | < 0·01 |
| CAM | 2·472 (1·273–4·803) | < 0·01 |
| Anti‐MDA5 antibody | 2·634 (1·433–4·841) | < 0·01 |
| Anti‐TIF1‐γ antibody | 2·592 (1·337–5·023) | < 0·01 |
| Anti‐synthetase antibodies | 0·372 (0·147–0·942) | < 0·05 |
| CK > 200 IU/l | 2·590 (1·451–4·624) | < 0·01 |
| LDHa > 250 IU/l | 2·313 (1·269–4·213) | < 0·01 |
| CRPb > 0·8 mg/dl | 3·124 (1·681–5·803) | < 0·001 |
| ESRc > 20 mm/h | 3·895 (1·995–7·605) | < 0·001 |
| Ferritind > 306·8 ng/ml | 6·124 (2·472–15·175) | < 0·001 |
| sCD163 | 1·000 (1·000–1·001) | 0·378 |
| CD3+ T cell numbere < 808 cells/μl | 2·079 (1·053–4·103) | < 0·05 |
| Neopterin > 22·1 nmol/l | 6·017 (2·896–12·501) | < 0·01 |
| Multivariate analysis | ||
| Age, years | 1·027 (1·000–1·055) | < 0·05 |
| Sex | 0·898 (0·475–1·698) | 0·742 |
| RP‐ILD | 2·451 (1·178–5·101) | < 0·05 |
| CAM | 3·327 (1·404–7·885) | < 0·01 |
| Anti‐MDA5 antibody | 2·994 (1·391–6·444) | < 0·01 |
| Anti‐TIF1‐γ antibody | 2·097 (0·846–5·197) | 0·11 |
| Neopterin > 22·1 nmol/l | 4·619 (2·092–10·195) | < 0·001 |
HR = hazard ratio; CI = confidence interval; ILD = interstitial lung disease; RP‐ILD = rapidly progressive ILD; CK = creatine kinase; LDH = lactate dehydrogenase; CRP = C‐reactive protein; ESR = erythrocyte sedimentation rate; CAM = cancer‐associated myositis; TIF1 = transcriptional intermediary factor; MDA5 = melanoma differentiation‐associated gene. aData available for 181 patients; bdata available for 171 patients; cdata available for 172 patients; ddata available for 99 patients; edata available for 164 patients.
In addition, as the data of serum ferritin were available from only approximately half the patients in our cohort, we carried out another set of multivariate Cox regression analyses including serum ferritin in 99 DM patients of our cohort. The resulting data showed that serum neopterin and ferritin were independently associated with the increased mortality of DM patients (Table 3).
Table 3.
Multivariate Cox regression analysis including serum ferritin*
| Variables | HR (95% CI) | P‐value |
|---|---|---|
| Age, years | 0·990 (0·953–1·028 | 0·597 |
| Sex | 0·648 (0·253–1·657) | 0·365 |
| RP‐ILD | 2·609 (1·060–6·425) | < 0·05 |
| CAM | 12·665 (3·134–51·186) | < 0·001 |
| Anti‐MDA5 antibody | 4·349 (1·447–13·069) | < 0·01 |
| Anti‐TIF1‐γ antibody | 3·888 (1·023–14·781) | < 0·05 |
| Ferritin > 306·8 ng/ml | 3·433 (1·128–10·449) | < 0·05 |
| Neopterin > 22·1 nmol/l | 4·84 (1·642–14·263) | < 0·01 |
Analysis performed in 99 patients. HR = hazard ratio; CI = confidence interval; RP‐ILD = rapidly progressive interstitial lung disease; CAM = cancer‐associated myositis; MDA5 = melanoma differentiation‐associated gene; TIF1 = transcriptional intermediary factor.
Discussion
In this study, we demonstrate that, in a large longitudinal cohort, the serum neopterin levels are significantly elevated in DM patients. Increased serum neopterin levels were significantly associated with anti‐MDA5 antibodies, RP‐ILD and characteristic DM cutaneous involvement such as skin ulcer and heliotrope rash. There seems to be a trend between serum neopterin level and DM disease activity, at least in some individuals. In addition, high levels of serum neopterin were strongly associated with increased mortality in DM patients. Furthermore, multivariate analysis identified high serum neopterin level as an independent risk factor for poor prognosis in DM patients.
Increased levels of neopterin have been reported in various clinical disorders, including several autoimmune diseases. The significant elevation of serum levels of neopterin in DM patients observed in our study are in agreement with previously published studies 16, 17, 19, 28. In addition, we found that serum levels of neopterin correlated with disease activity in DM patients, as previously reported in juvenile myositis patients 17, 18. With the increasing discovery of myositis‐specific autoantibodies and the consequently expanded understanding of the heterogeneity of DM, we analyzed the serum neopterin by subgrouping patients by MSAs and heterogeneous clinical manifestations to clarify the clinical relevance of serum neopterin in DM. Besides the findings of increased neopterin levels and the significant association of serum neopterin with disease severity we found that, interestingly, the DM patients with anti‐MDA5 antibodies had the highest serum levels of neopterin, and a significant increase was found in DM patients with RP‐ILD compared to those without RP‐ILD.
To our knowledge, this is the first study to demonstrate that serum neopterin is an independent prognostic factor for DM. An increasing number of studies have been performed to identify prognostic factors in myositis and several biomarkers or clinical complications have been suggested to be associated with poor outcome, such as anti‐MDA5 antibody 4, serum ferritin 25, malignancy 29 and ILD 3, 30. In line with these studies, we also confirmed anti‐MDA5 antibody, malignancy and RP‐ILD to be independent risk factors, based on our multivariate model. Intriguingly, our study revealed high serum neopterin as a prognostic factor independent of these currently known DM risk factors, indicating that DM patients with highly increased serum neopterin require more aggressive management in clinical practice.
In recent years a subset of DM with anti‐MDA5‐positive antibodies has been of great interest, and brings considerable challenges for rheumatologists. The high frequency of complications with RP‐ILD lead to poor survival in this subset of DM patients 31, 32. Nevertheless, patients with anti‐MDA5 antibodies or complicated with RP‐ILD have a distinct outcome. Similar to our clinical experience, Koga et al. showed that RP‐ILD did not worsen in any of the anti‐MDA5‐positive patients who survived the first 6 months 4. Although MSAs have shown great utility in identifying distinct clinical subsets with more homogeneity, increasing attention has been given to disease heterogeneity within a single MSA 33. Therefore, there is an extremely urgent need to find serum biomarkers which are able to categorize patients into more homogeneous groups and predict patient outcome. In the RP‐ILD group of our cohort, we found non‐survival patients had significantly higher serum neopterin concentrations compared to survival patients, indicating that serum neopterin may be a prognostic marker for predicting outcome in patients with RP‐ILD. Further validation of the clinical utility of serum neopterin may thus significantly aid physicians for disease assessment and treatment guidance.
Neopterin is produced by activated monocytes and macrophages after IFN‐γ stimulation 8. A growing body of evidence have suggested a pathogenic role of macrophage activation and polarization in lung fibrosis, including idiopathic pulmonary fibrosis 34, 35 and scleroderma‐related ILD 36, 37. With the identification of tissue‐resident alveolar macrophages in human lung and the clarification of their phenotype, location and gene expression 38, macrophages are thought to play a vital role in the physiological and pathological processes of the lung. As the most prevalent extra‐muscular complication, DM‐related ILD may be associated with macrophage over‐activation. In a previous study, we found significantly increased serum levels of soluble CD163 (sCD163) in polymyositis and DM patients, and patients with high serum sCD163 levels also showed a higher incidence of CD163+ macrophage infiltration in muscle tissue 39. The elevated serum sCD163 levels were further confirmed in two independent Japanese cohorts 40, 41. Enomoto et al. revealed that patients with higher serum sCD163 (a marker of macrophage activation) had significantly lower survival rates that those with lower sCD163. Alveolar infiltration of CD163‐positive macrophages was evident in lungs of patients with DM‐related ILD, and was particularly more severe in the non‐survivors’ lungs 40. Our findings indicate not only serum neopterin as a possible prognostic biomarker for DM, but also the potential role of activated macrophages in the immunopathogenesis of DM.
As we know, an IFN signature has previously been described to be elevated in dermatomyositis 42, 43. Because neopterin is produced in response to IFN‐γ, the high level of neopterin in DM patients may represent an IFN response. In particular, the higher neopterin noted in the anti‐MDA5 antibody‐positive DM may correlate with an elevated IFN response signature that has been reported within this antibody group 44. Of interest, IFN‐γ has also been noted to play a role in life‐threatening RP‐ILD 45, but this may also reflect how neopterin may correlate to both RP‐ILD and mortality.
However, we acknowledge that our study has some limitations. First, the retrospective design and inclusion of patients from a single center may lead to possible biases, including bias resulting from the missing data. Secondly, other than physician global disease activity by PGA, standard core disease activity measures in DM, such as manual muscle testing, and their relationship to neopterin levels were not evaluated. In addition, due to the limited sample size, the results from the multivariate analysis should be interpreted carefully. Because of the relatively small number of outcome events observed in our study, in the multivariate analysis model we could not include all the factors that were found to be associated with poor outcomes in univariate analysis. The good correlation of serum neopterin with ferritin, pulmonary function impairments and PGA score may suggest limited utilities in clinical practice, while these good correlations provide additional evidence that serum neopterin levels may reflect the disease severity of DM patients. Last, but not least, the source of neopterin was not examined. Further clarification of the source of neopterin may provide pathomechanical clues for DM pathogenesis.
In conclusion, our longitudinal cohort study confirmed that elevated serum levels of neopterin were associated with RP‐ILD and significantly higher mortality in DM patients. These findings provide new insights into the role of cellular immune activation, in addition to macrophage activation, in the pathogenesis of DM.
Disclosures
The authors declare no potential conflicts of interest.
Author contributions
Q. L. P. and G. C. W. designed the study. All authors were involved in data collection, statistical analysis, interpretation of data and drafting or revision of the manuscript.
Supporting information
Fig. S1. Flow map of patient inclusion. Patients with probable or definite DM (n = 225) according to the diagnostic criteria of Bohan and Peter or with clinical amyopathic DM (n = 10) according to the Sontheimer criteria were recruited for this study. Patients with the following conditions were excluded: (1) complicated with other connective tissue diseases (n = 36); (2) disease onset age less than 16 years (n = 12); (3) complicated with cancer that occurred beyond 3 years since myositis onset (n = 5). Finally, 182 DM patients were enrolled in the study. DM: dermatomyositis; CADM: clinical amyopathic DM; CTD: connective tissue disease.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81701615, 81571603, 81971531) and Beijing Municipal Science & Technology Commission (Z181100001718063, Z171100001017208).
References
- 1. Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet 2003; 362:971–82. [DOI] [PubMed] [Google Scholar]
- 2. Morisset J, Johnson C, Rich E et al Management of myositis‐related interstitial lung disease. Chest 2016; 150:1118–28. [DOI] [PubMed] [Google Scholar]
- 3. Johnson C, Pinal‐Fernandez I, Parikh R et al Assessment of mortality in autoimmune myositis with and without associated interstitial lung disease. Lung 2016; 194:733–7. [DOI] [PubMed] [Google Scholar]
- 4. Koga T, Fujikawa K, Horai Y et al The diagnostic utility of anti‐melanoma differentiation‐associated gene 5 antibody testing for predicting the prognosis of Japanese patients with DM. Rheumatology 2012; 51:1278–84. [DOI] [PubMed] [Google Scholar]
- 5. Moghadam‐Kia S, Oddis CV, Sato S et al Antimelanoma differentiation‐associated gene 5 antibody: expanding the clinical spectrum in North American patients with dermatomyositis. J Rheumatol 2017; 44:319–25. [DOI] [PubMed] [Google Scholar]
- 6. Shi J, Li S, Yang H et al Clinical profiles and prognosis of patients with distinct antisynthetase autoantibodies. J Rheumatol 2017; 44:1051–7. [DOI] [PubMed] [Google Scholar]
- 7. Chen F, Li S, Wang T et al Clinical heterogeneity of interstitial lung disease in polymyositis and dermatomyositis patients with or without specific autoantibodies. Am J Med Sci 2018; 355:48–53. [DOI] [PubMed] [Google Scholar]
- 8. Murr C, Widner B, Wirleitner B et al Neopterin as a marker for immune system activation. Curr Drug Metab 2002; 3:175–87. [DOI] [PubMed] [Google Scholar]
- 9. Mildvan D, Spritzler J, Grossberg SE et al Serum neopterin, an immune activation marker, independently predicts disease progression in advanced HIV‐1 infection. Clin Infect Dis 2005;40:853–8. [DOI] [PubMed] [Google Scholar]
- 10. Fuchs D, Avanzas P, Arroyo‐Espliguero R et al The role of neopterin in atherogenesis and cardiovascular risk assessment. Curr Med Chem 2009; 16:4644–53. [DOI] [PubMed] [Google Scholar]
- 11. Sucher R, Schroecksnadel K, Weiss G et al Neopterin, a prognostic marker in human malignancies. Cancer Lett 2010; 287:13–22. [DOI] [PubMed] [Google Scholar]
- 12. Rho YH, Solus J, Raggi P et al Macrophage activation and coronary atherosclerosis in systemic lupus erythematosus and rheumatoid arthritis. Arthritis Care Res 2011; 63:535–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. D’Agostino LE, Ventimiglia F, Verna JA et al Correlation between DAS‐28 and neopterin as a biochemical marker of immune system activation in early rheumatoid arthritis. Autoimmunity 2013; 46:44–9. [DOI] [PubMed] [Google Scholar]
- 14. Sfriso P, Ostuni P, Botsios C et al Serum and salivary neopterin and interferon‐gamma in primary Sjogren’s syndrome. Correlation with clinical, laboratory and histopathologic features. Scand J Rheumatol 2003; 32:74–8. [DOI] [PubMed] [Google Scholar]
- 15. Nassonov EL, Samsonov MY, Tilz GP et al Serum concentrations of neopterin, soluble interleukin 2 receptor, and soluble tumor necrosis factor receptor in Wegener’s granulomatosis. J Rheumatol 1997; 24:666–70. [PubMed] [Google Scholar]
- 16. Samsonov MY, Nassonov EL, Tilz GP et al Elevated serum levels of neopterin in adult patients with polymyositis/dermatomyositis. Br J Rheumatol 1997; 36:656–60. [DOI] [PubMed] [Google Scholar]
- 17. Rider LG, Schiffenbauer AS, Zito M et al Neopterin and quinolinic acid are surrogate measures of disease activity in the juvenile idiopathic inflammatory myopathies. Clin Chem 2002; 48:1681–8. [PubMed] [Google Scholar]
- 18. De Benedetti F, De Amici M, Aramini L et al Correlation of serum neopterin concentrations with disease activity in juvenile dermatomyositis. Arch Dis Child 1993; 69:232–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nishioka A, Tsunoda S, Abe T et al Serum neopterin as well as ferritin, soluble interleukin‐2 receptor, KL‐6 and anti‐MDA5 antibody titer provide markers of the response to therapy in patients with interstitial lung disease complicating anti‐MDA5 antibody‐positive dermatomyositis. Mod Rheumatol 2019; 29:814–20. [DOI] [PubMed] [Google Scholar]
- 20. Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med 1975; 292:344–7. [DOI] [PubMed] [Google Scholar]
- 21. Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts). N Engl J Med 1975; 292:403–7. [DOI] [PubMed] [Google Scholar]
- 22. Sontheimer RD. Would a new name hasten the acceptance of amyopathic dermatomyositis (dermatomyositis sine myositis) as a distinctive subset within the idiopathic inflammatory dermatomyopathies spectrum of clinical illness? J Am Acad Dermatol 2002; 46:626–36. [DOI] [PubMed] [Google Scholar]
- 23. Yang H, Peng Q, Yin L et al Identification of multiple cancer‐associated myositis‐specific autoantibodies in idiopathic inflammatory myopathies: a large longitudinal cohort study. Arthritis Res Therapy 2017; 19:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Travis WD, Costabel U, Hansell DM et al An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013; 188:733–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gono T, Kawaguchi Y, Satoh T et al Clinical manifestation and prognostic factor in anti‐melanoma differentiation‐associated gene 5 antibody‐associated interstitial lung disease as a complication of dermatomyositis. Rheumatology 2010; 49:1713–9. [DOI] [PubMed] [Google Scholar]
- 26. Peduzzi P, Concato J, Feinstein AR et al Importance of events per independent variable in proportional hazards regression analysis. II. Accuracy and precision of regression estimates. J Clin Epidemiol 1995; 48:1503–10. [DOI] [PubMed] [Google Scholar]
- 27. Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol 2007; 165:710–8. [DOI] [PubMed] [Google Scholar]
- 28. Prestridge A, Morgan G, Ferguson L et al Pulmonary function tests in idiopathic inflammatory myopathy: association with clinical parameters in children. Arthritis Care Res 2013; 65:1424–31. [DOI] [PubMed] [Google Scholar]
- 29. Trallero‐Araguas E, Grau‐Junyent JM, Labirua‐Iturburu A et al Clinical manifestations and long‐term outcome of anti‐Jo1 antisynthetase patients in a large cohort of Spanish patients from the GEAS‐IIM group. Semin Arthritis Rheum 2016; 46:225–31. [DOI] [PubMed] [Google Scholar]
- 30. Nuno‐Nuno L, Joven BE, Carreira PE et al Mortality and prognostic factors in idiopathic inflammatory myositis: a retrospective analysis of a large multicenter cohort of Spain. Rheumatol Int 2017; 37:1853–61. [DOI] [PubMed] [Google Scholar]
- 31. Chen F, Wang D, Shu X et al Anti‐MDA5 antibody is associated with A/SIP and decreased T cells in peripheral blood and predicts poor prognosis of ILD in Chinese patients with dermatomyositis. Rheumatol Int 2012; 32:3909–15. [DOI] [PubMed] [Google Scholar]
- 32. McHugh NJ, Tansley SL. Autoantibodies in myositis. Nat Rev Rheumatol 2018; 14:290–302. [DOI] [PubMed] [Google Scholar]
- 33. Yang H, Lu X, Peng Q et al Differential clinical associations of anti‐nuclear matrix protein 2 autoantibodies in patients with idiopathic inflammatory myopathies. Arthritis Rheumatol 2018; 70:1288–97. [DOI] [PubMed] [Google Scholar]
- 34. Zhang L, Wang Y, Wu G et al Macrophages: friend or foe in idiopathic pulmonary fibrosis? Respir Res 2018; 19:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wynn TA, Vannella KM. Macrophages in tissue repair, regeneration, and fibrosis. Immunity 2016; 44:450–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mathai SK, Gulati M, Peng X et al Circulating monocytes from systemic sclerosis patients with interstitial lung disease show an enhanced profibrotic phenotype. Lab Invest 2010; 90:812–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Christmann RB, Sampaio‐Barros P, Stifano G et al Association of Interferon‐ and transforming growth factor beta‐regulated genes and macrophage activation with systemic sclerosis‐related progressive lung fibrosis. Arthritis Rheumatol 2014; 66:714–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Desch AN, Gibbings SL, Goyal R et al Flow cytometric analysis of mononuclear phagocytes in nondiseased human lung and lung‐draining lymph nodes. Am J Respir Crit Care Med 2016; 193:614–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Peng QL, Zhang YL, Shu XM et al Elevated serum levels of soluble CD163 in polymyositis and dermatomyositis: associated with macrophage infiltration in muscle tissue. J Rheumatol 2015; 42:979–87. [DOI] [PubMed] [Google Scholar]
- 40. Enomoto Y, Suzuki Y, Hozumi H et al Clinical significance of soluble CD163 in polymyositis‐related or dermatomyositis‐related interstitial lung disease. Arthritis Res Ther 2017; 19:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kawasumi H, Katsumata Y, Nishino A et al Association of serum soluble CD163 with polymyositis and dermatomyositis, especially in anti‐MDA5 antibody‐positive cases. J Rheumatol 2018; 45:947–55. [DOI] [PubMed] [Google Scholar]
- 42. Giris M, Durmus H, Yetimler B et al Elevated IL‐4 and IFN‐gamma levels in muscle tissue of patients with dermatomyositis. In Vivo 2017; 31:657–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Moneta GM, Pires Marafon D, Marasco E. Muscle expression of Type I and Type II interferons is increased in juvenile dermatomyositis and related to clinical and histologic features. Arthritis Rheumatol 2019; 71:1011–21. [DOI] [PubMed] [Google Scholar]
- 44. Zhang SH, Zhao Y, Xie QB et al Aberrant activation of the type I interferon system may contribute to the pathogenesis of anti‐melanoma differentiation‐associated gene 5 dermatomyositis. Br J Dermatol 2019; 180:1090–8. [DOI] [PubMed] [Google Scholar]
- 45. Ishikawa Y, Iwata S, Hanami K et al Relevance of interferon‐gamma in pathogenesis of life‐threatening rapidly progressive interstitial lung disease in patients with dermatomyositis. Arthritis Res Ther 2018; 20:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Flow map of patient inclusion. Patients with probable or definite DM (n = 225) according to the diagnostic criteria of Bohan and Peter or with clinical amyopathic DM (n = 10) according to the Sontheimer criteria were recruited for this study. Patients with the following conditions were excluded: (1) complicated with other connective tissue diseases (n = 36); (2) disease onset age less than 16 years (n = 12); (3) complicated with cancer that occurred beyond 3 years since myositis onset (n = 5). Finally, 182 DM patients were enrolled in the study. DM: dermatomyositis; CADM: clinical amyopathic DM; CTD: connective tissue disease.


