Summary
Secretory IgA (SIgA) is a well‐known mucosal‐surface molecule in first‐line defense against extrinsic pathogens and antigens. Its immunomodulatory and pathological roles have also been emphasized, but it is unclear whether it plays a pathological role in lung diseases. In the present study, we aimed to determine the distribution of IgA in idiopathic pulmonary fibrosis (IPF) lungs and whether IgA affects the functions of airway epithelial cells. We performed immunohistochemical analysis of lung sections from patients with IPF and found that mucus accumulated in the airspaces adjacent to the hyperplastic epithelia contained abundant SIgA. This was not true in the lungs of non‐IPF subjects. An in‐vitro assay revealed that SIgA bound to the surface of A549 cells and significantly promoted production of vascular endothelial growth factor (VEGF), transforming growth factor (TGF)‐β and interleukin (IL)‐8, important cytokines in the pathogenesis of IPF. Among the known receptors for IgA, A549 cells expressed high levels of transferrin receptor (TfR)/CD71. Transfection experiments with siRNA targeted against TfR/CD71 followed by stimulation with SIgA suggested that TfR/CD71 may be at least partially involved in the SIgA‐induced cytokine production by A549 cells. These phenomena were specific for SIgA, distinct from IgG. SIgA may modulate the progression of IPF by enhancing synthesis of VEGF, TGF‐β and IL‐8.
Keywords: fibrosis, human, lung, secretory IgA
Secretory IgA is a well‐known mucosal‐surface molecule in first‐line defense against extrinsic pathogens and antigens. It is unclear whether it plays a pathological role in lung diseases. In the present study, we showed that accumulated mucus in the airspaces of idiopathic pulmonary fibrosis lungs contained abundant SIgA in direct contact with the epithelia of remodeled tissue, and that SIgA bound to the surface of A549 cells, leading to production of profibrotic and inflammatory cytokines, VEGF, TGF‐β and IL‐8.

Introduction
Immunoglobulin A (IgA) is composed of two heavy chains (α chain) and two light chains (κ/λ chain), and is the most abundant immunoglobulin on mucosal surfaces 1. In secretions, IgA is present mainly in dimeric form, i.e. two monomers are linked by a chain, the ‘J chain’. The dimer binds to polymeric immunoglobulin receptors (pIgR) expressed on the basal surface of epithelial cells, is transported to the apical side and is released with the cleaved pIgR extracellular domain, a secretory component (SC) 2, called secretory IgA (SIgA). SIgA’s basic function is as a first‐line defense by neutralizing extrinsic pathogens and antigens on mucosal surfaces, thereby protecting mucosal tissues from infections and allergic sensitization 2, 3, 4. In more recent years, attention has been focused on other immunomodulatory and pathological roles of SIgA.
SIgA on the intestinal mucosa contributes to intestinal homeostasis by controlling commensal bacteria and inducing immunological tolerance via cross‐talk between the host and its intestinal contents 5. SIgA binds to gastrointestinal M cells of Peyer’s patches, is transported to the subepithelial dome region and may influence immunological tolerance 5. Interestingly, SIgA exerts its effects on tissue cells not only under physiological conditions, but also in pathological states. In celiac disease, gliadin‐specific SIgA binds to transferrin receptor (TfR)/CD71 that is over‐expressed on intestinal epithelial cells and is transported to subepithelial areas, leading to accumulation of gliadin 6. IgA is associated with the pathogenesis of IgA nephropathy. It is thought that mainly high‐molecular‐weight serum IgA is deposited in the kidney, but SIgA can also play a pathogenic role by promoting proinflammatory cytokine production by mesangial cells via an unknown receptor 7. Regarding hematocytes, cell surface binding and cross‐linking of SIgA induces degranulation of eosinophils 8 and basophils 9, which can exacerbate allergic reactions. Despite these findings showing binding of SIgA to myriad cells, evoking multiple physiological and pathological effects, there has been no investigation of whether or how SIgA influences the cells comprising respiratory mucosal tissue.
Idiopathic pulmonary fibrosis (IPF) is the most common disease among idiopathic interstitial pneumonia, and has a poor prognosis 10. It is thought that repeated injury to alveolar epithelial cells and aberrant repair due to excess fibrotic signaling lead to progressive and irreversible fibrosis 11, 12. The pathological findings include accumulation of fibroblasts and myofibroblasts, excess extracellular matrix deposition, formation of so‐called fibroblastic foci 13 and abnormal epithelial cells, some of which have an alveolar type 2 (AT2) cell‐like identity 14. Moreover, it is notable that mucus, having an altered composition 15, 16, accumulates in IPF lungs 16, 17. The amount of mucus accumulation in IPF lungs is associated with the degree of lung inflammation and FVC decline 17. These novel findings led to a hypothesis that drainage impairment caused by aberrant mucus production affects the disease progression in IPF lungs, but the precise mechanism has not been elucidated.
In this study, we show that epithelia of remodeled tissue in IPF lungs was exposed to SIgA‐rich mucus that had accumulated in the airspaces. An in‐vitro assay revealed that SIgA bound to the surface of A549 cells, a cell line with AT2 cell phenotype, and promoted production of vascular endothelial growth factor (VEGF), transforming growth factor (TGF)‐β and interleukin (IL)‐8, cytokines reported to be involved in the pathogenesis of IPF 12, 18, 19, 20, 21, 22. We also found that TfR/CD71 was involved in the cytokine production evoked by SIgA stimulation.
Materials and methods
Reagents
The following reagents were purchased as indicated: purified human secretory IgA (MP Biomedicals, Santa Ana, CA, USA); phosphate‐buffered saline (PBS) and fetal bovine serum (FBS) (Thermo Fisher Scientific, Waltham, MA, USA); citrate buffer (Muto Pure Chemicals Co., Tokyo, Japan); histofine antigen retrieval solution pH 9 (Nichirei, Tokyo, Japan); Dulbecco’s modified Eagle’s medium (DMEM)/Ham’s F‐12 medium, hydrogen peroxide, 0·5 M ethylenediamine tetraacetic acid (EDTA) and methanol (Wako Pure Chemical Industries, Osaka, Japan); 0·25% trypsin (Life Technologies Corporation, Grand Island, NY, USA); carbonate–bicarbonate buffer (Sigma Aldrich, St Louis, MO, USA); and BioFX TMB One Component HRP Microwell Substrate (Surmodics IVD, Inc., Eden Prairie, MN, USA).
The following antibodies were purchased as indicated: fluorescein isothiocyanate (FITC)‐conjugated mouse anti‐human CD71 monoclonal antibody (mAb) (IgG2aκ, clone CY1G4), phycoerythrin (PE)‐conjugated mouse anti‐human CD89 mAb (IgG1κ, clone A59), allophycocyanin (APC)‐conjugated mouse anti‐human CD206 mAb (IgG1, clone 15‐2), APC‐conjugated mouse anti‐human CD209 mAb (IgG2a, clone 9E9A8), PE‐conjugated mouse anti‐human CD351 mAb (IgG1κ, clone TX61), FITC‐conjugated mouse IgG2aκ isotype control (clone MOPC‐173), PE‐conjugated mouse IgG1κ isotype control (clone MOPC‐21) and mouse IgG1κ isotype control (clone MOPC‐21) (BioLegend, San Diego, CA, USA); PE‐conjugated mouse anti‐asialoglycoprotein receptor (ASGP‐R) mAb (IgG1κ, clone 8D7) and PE‐conjugated mouse IgG1κ isotype control (clone MOPC‐21) (BD Biosciences, San Jose, CA, USA); PE‐conjugated goat F(ab′)2 anti‐mouse IgG pAb, APC‐conjugated mouse IgG1 isotype control (clone P3.6.2.8.1) and APC‐conjugated mouse IgG2a isotype control (clone eBM2a) (eBioscience, San Diego, CA, USA); APC‐conjugated mouse anti‐human IgA mAb (IgG1, clone IS11‐8E10) (Miltenyi Biotec, Bergisch Gladbach, Germany); goat horseradish peroxidase (HRP)‐conjugated anti‐IgA antibody (polyclonal, whole IgG) (Bethyl Laboratories, Inc., Montgomery, TX, USA); mouse anti‐human transferrin receptor/CD71 mAb (IgG1, clone H68.4) and mouse anti‐human secretory component mAb [IgG1, clone COMPO2 (SC‐05)] (Thermo Fisher Scientific); and mouse anti‐β 1, 4‐galactosyltransferase 1 mAb (IgG1) (Santa Cruz Biotechnology, Inc., Dallas, TX, USA).
Immunohistochemistry
Formalin‐fixed, paraffin‐embedded lung tissue samples from three non‐IPF patients and samples from three IPF patients who underwent lobectomy for lung cancer were used. Non‐diseased parts of the resected lungs from the non‐IPF patients were used as controls. The diagnosis of IPF was based on the official American Thoracic Society/European Respiratory Society/Japanese Respiratory Society/Latin American Thoracic Society (ATS/ERS/JRS/ALAT) statement, and diagnosis of usual interstitial pneumonia was confirmed by an independent pathologist. The study was approved by the Ethics Committee of Tokyo Hospital. Written informed consent was obtained from each of the patients before surgery.
After deparaffinization and blocking of endogenous peroxide with 0·9% hydrogen peroxide in methanol, heat‐induced antigen retrieval was performed in citrate buffer pH 6 at 98ºC for 40 min for secretory component staining and in histofine antigen retrieval solution pH 9 at 98ºC for 40 min for IgA and TfR/CD71 staining. Immunohistochemistry was performed using a Vectastain ABC kit (Funakoshi Co., Ltd., Tokyo, Japan) in combination with an avidin/biotin blocking kit (Vector Laboratories, Inc., Burlingame, CA, USA). Mouse anti‐human secretory component mAb, goat anti‐human IgA mAb and mouse anti‐human transferrin receptor/CD71 mAb were used at 40 µg/ml, 0·2 µg/ml and 0·5 µg/ml, respectively, and comparison was made to sections stained with isotype‐matched control antibodies at the same concentrations. The color was developed by the diaminobenzidine (DAB) reaction using a peroxidase substrate kit DAB (Vector Laboratories, Inc.). Photographs were taken with a Keyence fluorescence microscope BZ‐X710 (Keyence Japan, Osaka, Japan).
Cells and culture
An adenocarcinomic human alveolar basal epithelial cell line, A549, was maintained in DMEM/Ham’s F‐12 medium containing 5% FBS, 100 U/ml penicillin and 100 µg/ml streptomycin at 37°C, 5% CO2 until the experiments.
Flow cytometric analysis
SIgA binding to the A549 cell surface was analyzed by flow cytometry. After detaching from the plates with 5 mM EDTA in PBS, 2 × 105 cells were washed with fluorescence activated cell sorter (FACS) buffer (PBS containing 3% FBS), blocked with human polyclonal IgG at 20 μg/ml, and incubated with or without SIgA at 300 µg/ml for 30 min at 4°C. For binding inhibition assay with IgG, human polyclonal IgG was added as well as SIgA at a final concentration of 300 µg/ml. After washing with FACS buffer, the cells were incubated with APC‐conjugated mouse anti‐human IgA mAb for 30 min at 4°C. Then the cells were washed again with FACS buffer, resuspended in FACS buffer and analyzed by FACSVerse (BD Biosciences). For each sample, at least 10 000 events were collected, and dot‐plots and histograms were generated using FlowJo (Tree Star Inc., Ashland, OR, USA).
For binding inhibition assay with EDTA, the cells were preincubated with 10 mM EDTA in FACS buffer for 15 min at 4°C. Then human polyclonal IgG at 20 μg/ml was added for blocking, and finally SIgA was added. For binding inhibition assay with trypsin treatment, the cells were preincubated with 0·25% trypsin for 5 min at room temperature, washed with FACS buffer, blocked with human polyclonal IgG at 20 μg/ml, and finally SIgA was added. After incubation for 30 min at 4°C, the cells were washed with FACS buffer and incubated with APC‐conjugated mouse anti‐human IgA mAb for 30 min at 4°C. The cells were washed with FACS buffer and analyzed by flow cytometry in the same manner as described above. To investigate the cell surface expression of each of TfR/CD71, FcαRI/CD89, mannose receptor/CD206, dendritic cell‐specific intercellular adhesion molecule‐3‐grabbing non‐integrin (DC‐SIGN)/CD209, Fcα/μR/CD351, asialoglycoprotein receptor (ASGP‐R) and β 1, 4‐galactosyltransferase 1, after blocking with human polyclonal IgG at 20 μg/ml, 2 × 105 cells were stained with specific antibody, or with isotype control antibody for 30 min on ice. Cells stained with non‐conjugated antibody were incubated with PE‐conjugated anti‐mouse IgG antibody for 30 min on ice. Finally, the cells were analyzed by flow cytometry as described above.
Quantitation of cell‐derived cytokines
A549 cells were seeded at 1 × 105/well in 24‐well plates. After 24 h, the cells were incubated with SIgA or human polyclonal IgG at 300 µg/ml in FBS‐free DMEM/Ham’s F‐12 medium for 24 or 48 h. For concentration analysis, the cells were incubated with SIgA at 10, 30, 100 and 300 µg/ml or human polyclonal IgG at 300 µg/ml for 24 h. The levels of VEGF‐A and IL‐8 in the culture supernatants were measured by cytometric bead array (CBA; BD Biosciences), according to the manufacturer’s instructions. The levels of TGF‐β1 in the culture supernatants were measured by human/mouse TGF‐β1 ELISA Ready‐SET‐Go (Thermo Fisher Scientific), according to the manufacturer’s instructions. The supernatants were stored at −80°C until assay.
Real‐time quantitative polymerase chain reaction (RT–PCR) analysis
A549 cells were seeded at 1 × 105/well in 24‐well plates. After 24 h, the cells were incubated with or without SIgA at 300 µg/ml in FBS‐free DMEM/Ham’s F‐12 medium, and the total RNA was then extracted from the cultured cells using an RNeasy Mini Kit (Qiagen, Hilden, Germany), according to the manufacturer’s instructions. The extracted mRNA was reverse‐transcribed to cDNA using an iScript cDNA Synthesis Kit (Bio‐Rad Laboratories, Hercules, CA, USA). RT–PCR was performed using primers and Taqman probes designed by Thermo Fisher Scientific, Inc. Data were calculated by the ΔΔCt method using the cDNA and, as a reference, β‐actin cDNA. The relative quantitation (RQ) values were calculated using the following equation: RQ = 2–ΔΔCt.
Down‐regulation of TfR/CD71 by siRNA transfection
A549 cells were transfected with small interference RNA (siRNA) against TfR/CD71 (Sigma Genosys siRNA Service, Hs_TFRC_9216_s: #1590001; 5′rGrGUUrArCUrGrGrGrCrArAUUUrCUrATT3′, Hs_TFRC_9216_as: #1590002; 5′UrArGrArArAUUrGrCrCrCrArGUrArArCrCTT3′) (Sigma Aldrich) or control RNA (Sigma Genosys siRNA Service, Mission_SIC‐001_s, Mission_SIC‐001_as) at 1000 nM by the electroporation method using 4D‐Nucleofector and a SF cell line 4D‐Nucleofector X kit L (Lonza, Basel, Switzerland), according to the manufacturer’s instructions. The transfected cells were cultured for 24 h and then used for the experiments.
Statistics
Statistical analyses were performed using an unpaired, two‐tailed t‐test for pairwise comparisons, a one‐sample t‐test for comparisons of relative values and analysis of variance (anova) with Tukey’s test for multiple comparisons. Statistical significance was defined as a P‐value of less than 0·05. All data are expressed as the mean ± standard error of the mean (s.e.m.) of three to 12 independent experiments.
Results
Accumulated mucus containing SIgA was in direct contact with epithelia in IPF lungs
We first performed immunohistochemical analysis of lung sections from the control subjects and IPF patients to investigate the morphological relationships between SIgA and epithelial cells. The details of the control subjects and IPF patients are summarized in Table 1. We found that, in IPF lungs, accumulated mucus was highly positive for both IgA and SC and was in direct contact with the epithelia of remodeled tissue (Fig. 1e–g,i–k). In contrast, mucus stained with anti‐IgA antibody was not observed in the alveoli of control lungs, whereas their bronchial glands, cells infiltrated around the glands 23 and sera in vessels were clearly stained for IgA, as expected in their normal state (Fig. 1a–d).
Table 1.
Characteristics of the IPF patients and control subjects
| IPF patients | Control subjects | |||||
|---|---|---|---|---|---|---|
| Age (years) | 73 | 78 | 69 | 72 | 63 | 80 |
| Gender | Male | Male | Female | Female | Female | Female |
| Smoking history | Yes | Yes | Yes | Never | Never | Never |
| Brinkman index | 1000 | 1600 | 800 | – | – | – |
| Pulmonary function tests | ||||||
| FVC% predicted | 80·1 | 79 | 83·4 | 98·2 | 133·9 | 127·5 |
| DLco% predicted | 29·9 | Not performed | 40·8 | Not performed | Not performed | Not performed |
| Medication for IPF | No | No | Pirfenidone | – | – | – |
DLco = diffusing capacity for carbon monoxide; FVC = forced vital capacity; IPF = idiopathic pulmonary fibrosis.
Figure 1.
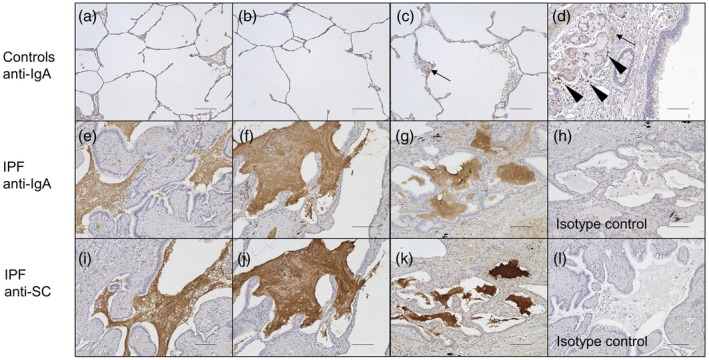
Immunohistochemistry of lung sections of control subjects (n = 3) and idiopathic pulmonary fibrosis (IPF) patients (n = 3). Lung sections were stained with anti‐immunoglobulin (Ig)A antibody (controls, a–d; IPF, e–g) or anti‐SC antibody (IPF, i–k). Representative images from all patients’ lung sections are shown. Accumulated mucus was highly positive for IgA and was adjacent to the epithelia of remodeled tissue in IPF lungs (e–g). These findings were not observed in control lungs (a–c), while bronchial glands, mononuclear cells around bronchial glands (d, arrowhead) and serum in vessels (c,d, arrow) were stained by anti‐IgA antibody. The mucus in IPF lungs was also positive for secretory component (SC) (i–k). Non‐specific staining was not observed with the isotype control antibodies (isotype control for anti‐IgA antibody, h; isotype control for anti‐SC antibody, l). The bars represent 100 µm.
These results indicate that (1) aberrantly accumulated mucus in IPF lungs were rich in SIgA, the production and distribution of which are restricted to bronchi in normal states 23 and (2) the epithelia of remodeled tissue in IPF lungs were exposed to that mucus.
SIgA bound to the surface of A549 cells
Next, using flow cytometry, we found that SIgA clearly bound to the surface of A549 cells (Fig. 2a). The binding was not inhibited by polyclonal human IgG (Fig. 2b), indicating that SIgA does not share its receptor with IgG. Because some IgA receptors bind to IgA cation‐dependently 24, 25, we also examined whether the SIgA binding to A549 cells is cation‐dependent or ‐independent. As shown in Fig. 2c, the SIgA binding was not affected by chelation with EDTA. Conversely, trypsin treatment significantly reduced the SIgA binding to A549 cells (Fig. 2d), showing that a trypsin‐sensitive protein expressed on the surface of A549 cells is involved in SIgA binding.
Figure 2.
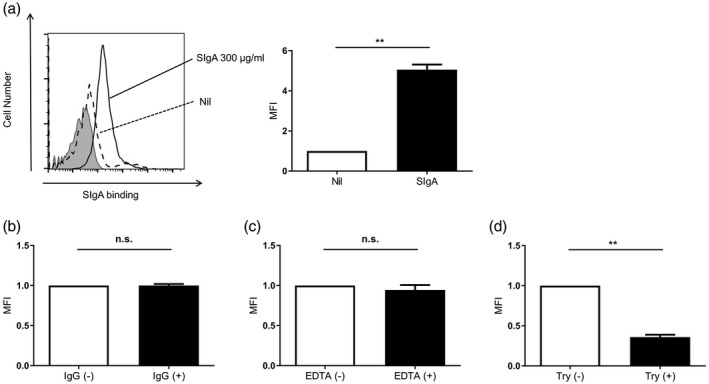
Secretory immunoglobulin A (SIg)A bound to the surface of A549 cells. A549 cells were incubated with SIgA at 300 µg/ml (solid line) or without SIgA dashed line), followed by staining with allophycocyanin (APC)‐conjugated anti‐IgA antibody. The shaded area shows cells without staining. Representative histograms from three independent experiments are shown. Mean fluorescence intensity (MFI) of the histograms from three independent experiments are shown as relative values to those of cells incubated without SIgA (□). Bars represent the standard error of the mean (s.e.m.). **P < 0·01. (b) A549 cells were incubated with SIgA at 300 µg/ml and with (■) or without (□) IgG at 300 µg/ml. The cells were stained and analyzed as described above. MFI of the histograms from three independent experiments are shown as relative values to those of cells incubated without (□) IgG. Bars represent the s.e.m.; n.s. = not significant. (c) A549 cells were incubated with SIgA at 300 µg/ml and with (■) or without (□) ethylenediamine tetraacetic acid (EDTA). The cells were stained and analyzed as described above. MFI of the histograms from three independent experiments are shown as relative values to those of cells incubated without (□) EDTA. Bars represent the s.e.m.; n.s. = not significant. (d) After the treatment with (■) or without (□) trypsin, A549 cells were incubated with SIgA at 300 µg/ml. The cells were stained and analyzed as described above. MFI of the histograms from three independent experiments are shown as relative values to those of cells without trypsin treatment (□). Bars represent the standard error of the mean (s.e.m.). **P < 0·01.
These results indicate that SIgA binds to A549 cells in a cation‐independent and trypsin‐sensitive manner.
SIgA‐induced VEGF‐A, TGF‐β1 and IL‐8 production by A549 cells
Following the binding assay, we investigated for VEGF, TGF‐β and IL‐8 production by A549 cells stimulated with SIgA. We measured VEGF‐A and TGF‐β1 because they are well‐studied subtypes known to be involved in the pathogenesis of IPF 21, 22. The levels of VEGF‐A and TGF‐β1 in the culture supernatant were significantly increased by high‐dose SIgA stimulation for both 24 and 48 h (Fig. 3a). IL‐8 was also up‐regulated in a dose‐dependent manner and increased significantly in response to high‐dose SIgA (Fig. 3a). Conversely, human polyclonal IgG was unable to similarly induce cytokine production (Fig. 3a). Moreover, SIgA up‐regulated mRNA expression for both VEGF‐A and IL‐8 at 6 h and for TGF‐β1 at 24 h (Fig. 3b).
Figure 3.
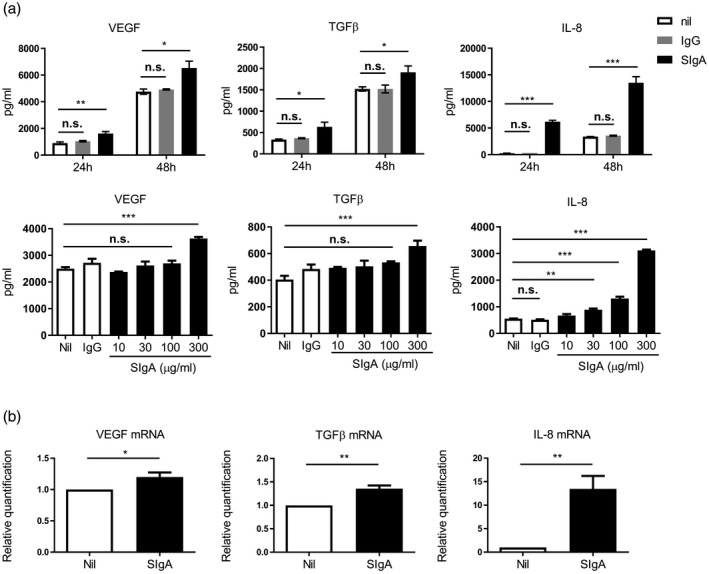
Production of each of vascular endothelial growth factor (VEGF), transforming growth factor (TGF)‐β and interleukin (IL)‐8 was enhanced by secretory immunoglobulin A (SIg)A stimulation. (a) Upper panels show the levels of VEGF, TGF‐β and IL‐8 in the culture supernatant after stimulation with SIgA or IgG at 300 µg/ml for 24 and 48 h. Lower panels show the levels of VEGF, TGF‐β and IL‐8 in the culture supernatant after stimulation with SIgA at the indicated concentrations or IgG at 300 µg/ml for 24 h. After stimulation with SIgA under each condition, the levels of VEGF, TGF‐β and IL‐8 in the culture supernatant were measured. Bars represent the standard error of the mean (s.e.m.) (n = 3–5); n.s. = not significant. *P < 0·05, **P < 0·01, *** P < 0·001. (b) mRNA expressions of VEGF and IL‐8 at 6 h after stimulation with (■) or without (□) SIgA at 300 µg/ml and those of TGF‐β 24 h after stimulation. Bars represent the s.e.m. (n = 5–6). *P < 0·05, **P < 0·01.
Collectively, SIgA up‐regulated A549 cells production of VEGF‐A, TGF‐β1 and IL‐8 at both the protein and mRNA levels. These phenomena were specific for SIgA, and not seen with human polyclonal IgG.
A549 cells and hyperplastic epithelial cells in IPF lungs expressed TfR/CD71
We next investigated the cell‐surface expression of seven well‐established IgA receptors: TfR/CD71 26, FcαRI/CD89 27, mannose receptor/CD206 28, DC‐SIGN/CD209 24, Fcα/μR/CD351 29, ASGP‐R 30 and β 1, 4‐galactosyltransferase 1 31. Flow cytometry found that only TfR/CD71 was expressed on the surface of A549 cells (Fig. 4a). Because TfR/CD71 is trypsin‐sensitive 32, these findings are compatible with our finding that the SIgA binding to A549 cells was trypsin‐sensitive (Fig. 2d).
Figure 4.
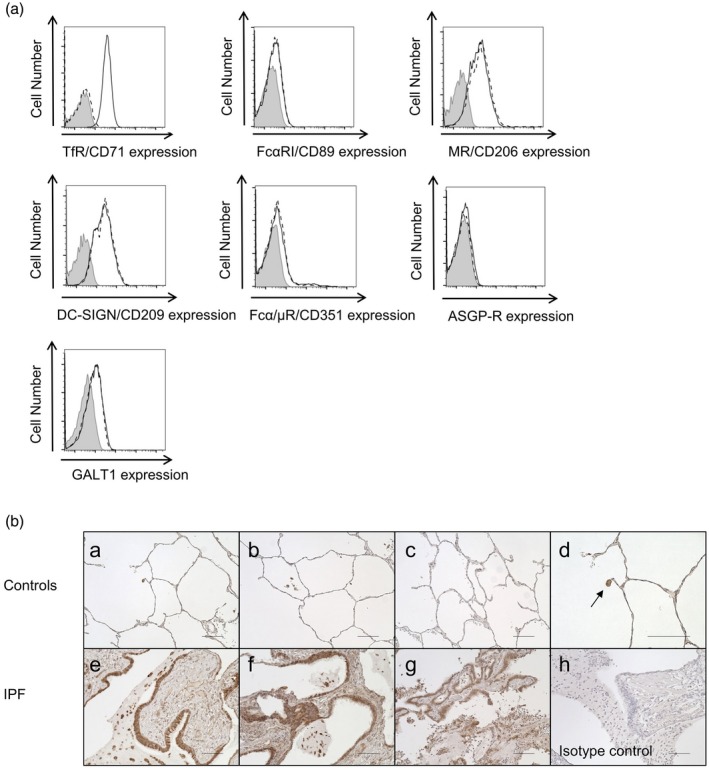
A549 cells and epithelial cells in idiopathic pulmonary fibrosis (IPF) lungs expressed transferrin receptor (TfR)/CD71. (a) Expressions of TfR/CD71, FcαRI/CD89, mannose receptor (MR)/CD206, dendritic cell‐specific intercellular adhesion molecule‐3‐grabbing non‐integrin (DC‐SIGN)/CD209, Fcα/μR/CD351, asialoglycoprotein receptor (ASGP‐R) and β 1, 4‐galactosyltransferase 1 (GALT1) on the surface of A549 cells were examined. A549 cells were stained with specific antibodies for the indicated receptors (solid line) or with isotype control antibody (dashed line). Expression of each receptor was analyzed by flow cytometry. Representative histograms from three independent experiments are shown. The shaded area shows cells without staining. (b) Immunohistochemistry of lung sections from control subjects (n = 3) and IPF patients (n = 3). Lung sections were stained with anti‐TfR/CD71 antibody (controls, a–d; IPF, e–g). Representative images from all patients’ lung sections are shown. The epithelial cells of remodeled tissue in IPF lungs were clearly positive for TfR/CD71 (e–g). These findings were not observed in normal control lungs (a–c), while alveolar macrophages were positive for TfR/CD71 (d, magnified figure of image; a, arrow). Non‐specific staining was not observed with the isotype control antibodies (h). The bars represent 100 µm.
We also investigated expression of TfR/CD71 by epithelial cells of IPF lungs. Immunohistochemical analysis revealed that hyperplastic epithelial cells in IPF lungs highly expressed TfR/CD71 compared to alveolar epithelial cells in the control lungs (Fig. 4b).
TfR/CD71 was involved in the VEGF, TGF‐β and IL‐8 production evoked by SIgA
Finally, we examined the effect of siRNA targeting TfR/CD71 on cytokine production in response to SIgA. A549 cells were transfected with TfR/CD71 siRNA to knock down its expression. Flow cytometric analysis revealed significant suppression of expression of cell‐surface TfR/CD71 at 24 h after transfection (Fig. 5a), and that knock‐down persisted for up to 48 h after transfection. After transfection with siRNA or control RNA, A549 cells were stimulated with SIgA at 300 μg/ml. As shown in Fig. 5b, VEGF‐A, TGF‐β1 and IL‐8 production by TfR/CD71 siRNA‐treated cells was significantly decreased compared to the control RNA‐treated cells.
Figure 5.
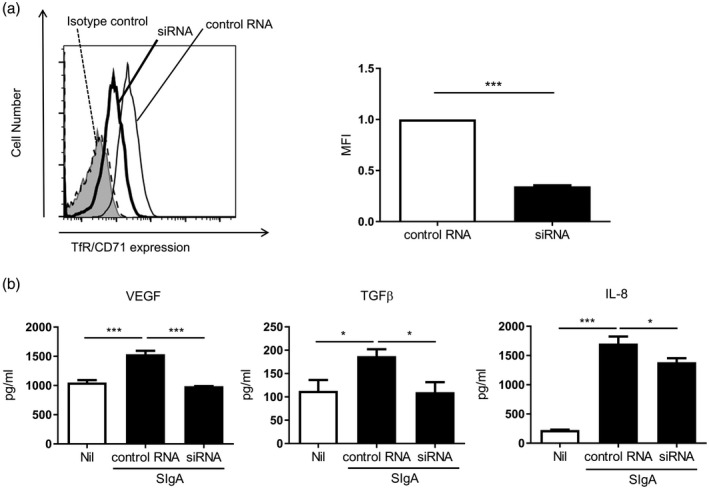
Knock‐down experiments on transferrin receptor (TfR)/CD71. (a) A549 cells were transfected with TfR/CD71 siRNA (bold line) or negative control RNA (solid line). The cells were stained with specific antibody for TfR/CD71 or with isotype control antibody (dashed line) and analyzed by flow cytometry. Representative histograms from three independent experiments are shown. The shaded area shows cells without staining. Mean fluorescence intensity (MFI) of the histograms from three independent experiments are shown as relative values. Bars represent the standard error of the mean (s.e.m.). ***P < 0·001. (b) After transfection with TfR/CD71 siRNA or control RNA, A549 cells were stimulated with secretory immunoglobulin A (SIg)A at 300 μg/ml for 24 h. The levels of vascular endothelial growth factor (VEGF), transforming growth factor (TGF)‐β and interleukin (IL)‐8 in the culture supernatant were measured. Bars represent the s.e.m. (n = 4–12); n.s. = not significant. *P < 0·05, **P < 0·01, ***P < 0·001.
These results suggest that TfR/CD71 is at least partially involved in the cytokine production induced by SIgA stimulation.
Discussion
In this study, we show that accumulated mucus in the airspaces of IPF lungs contained abundant SIgA and was in direct contact with the epithelia of remodeled tissue (Fig. 1). We also found that SIgA bound to the surface of A549 cells (Fig. 2a), leading to production of profibrotic and inflammatory cytokines; namely, VEGF, TGF‐β and IL‐8 (Fig. 3). Among multiple cytokines involved in the pathogenesis of IPF, VEGF and TGF‐β play pivotal roles 12, 18, 19, 20, 21, 22 and are the targets of anti‐fibrotic agents used currently in clinical practice 33, 34, 35. Importantly, one of the main sources of these cytokines is from hyperplastic AT2 cells 21, 36. IL‐8 is an inflammatory cytokine known to be a potent chemotactic factor for neutrophils 37, which are often observed to be recruited to IPF lungs 38, 39. The level of serum IL‐8 and the bronchoalveolar lavage fluid neutrophil count are associated with the prognosis of IPF 20, 39. Taken together, SIgA may be involved in the progression of IPF by promoting production of these mediators by epithelial cells in remodeled tissue in IPF lungs.
The serum IgA concentration is reported to reflect the severity of liver fibrosis in non‐alcoholic fatty liver disease 40 and also the prognosis of IPF 41. While IgA can be a biomarker of fibrosis based on these findings, it is not clear whether IgA itself up‐regulates profibrotic cytokine synthesis. TGF‐β can be a confounding factor, because it induces IgA class‐switch 42 and promotes fibrosis in the liver and lungs 12, 22, 43. Interestingly, serum IgA1 from patients with IgA nephropathy was reported to induce TGF‐β synthesis via the renin–angiotensin system in human mesangial cells 44. In line with this, our previous study showed that SIgA induces inflammatory cytokine production and collagen synthesis by human lung fibroblasts 45. In our present study, using alveolar epithelial cells, we show for the first time that high‐dose SIgA can induce production of two profibrotic cytokines, VEGF and TGF‐β.
TfR/CD71 was first reported as a serum IgA receptor expressed on mesangial cells in IgA nephropathy 26. It was also shown to function as an SIgA receptor 6, although it is still controversial whether TfR/CD71 binds SIgA 6 or not 46. Our preliminary experiments, using recombinant TfR/CD71 and an ELISA technique, confirmed that SIgA bound to TfR/CD71 to the same extent as serum IgA (Supporting information, Fig. S1). Moreover, among well‐established IgA receptors – namely, TfR/CD71, FcαRI/CD89, mannose receptor/CD206, DC‐SIGN/CD209, Fcα/μR/CD351, ASGP‐R and β 1, 4‐galactosyltransferase 1 – only TfR/CD71 was expressed on A549 cells (Fig. 4a). In general, compared to slowly proliferating cells, highly proliferating cells express high levels of TfR/CD71 to meet their iron demands, and this was also the case with A549 cells 47, 48, 49. Hyperplastic epithelial cells in IPF lungs also expressed a high level of TfR/CD71 (Fig. 4b), which is compatible with the finding that serial gene expressions related to cell proliferation are up‐regulated in abnormal epithelial cells with an AT2 cell‐like identity in IPF lungs 14. In addition, our knock‐down experiment using siRNA suggests that TfR/CD71 may be at least partially involved in the cytokine production induced by SIgA stimulation (Fig. 5b). To further confirm the importance of TfR/CD71 on airway epithelial cells, the expression levels of TfR/CD71 and cytokine synthesis evoked by SIgA were also investigated using BEAS‐2B, a bronchial epithelial cell line that expresses TfR/CD71 on its surface. BEAS‐2B cells expressed TfR/CD71, but no other established SIgA receptors were detectable on its surface. SIgA induced cytokine production, including IL‐8, by BEAS‐2B (data not shown).
Despite these findings, we must note that our study has some important limitations. First, our knock‐down study using siRNA targeted TfR/CD71, but we cannot conclude that it is the only functional receptor for SIgA, because IL‐8 production evoked by SIgA stimulation was not suppressed to the control level (Fig. 5b). There may be other unknown IgA receptor(s) 7, or TfR/CD71 might work only as a molecule that uptakes SIgA intracellularly, while there are other intracellular receptors or signal transducers. Secondly, our in‐vitro assay did not use epithelial cells obtained from IPF lungs. Although there is an emerging novel method for maintaining iPS cell‐derived AT2 cells in vitro 50, it is still impossible to culture disease‐specific epithelial cells from IPF lungs without their phenotype being changed. A549 is one of the ideal alternative materials available, because it shares some important traits with abnormal epithelial cells in IPF lungs. That is, A549 cells have a AT2 phenotype, and cell proliferation signals are up‐regulated with high TfR/CD71 expression, as noted previously 49. Finally, structural differences among individual SIgA samples obtained from different sources should be taken into account. Although the basic structure of IgA is preserved, IgA is variously modified by glycosylation 1 and, importantly, the bioactivity of IgA is likely to be altered by glycosylation. Aberrant glycosylation is an important factor in the pathogenesis of IgA nephropathy 46, so we should note that the response of A549 cells to SIgA could vary depending on the differences in glycosylation among the SIgA samples.
In conclusion, we show that SIgA bound to A549 cells and evoked production of profibrotic and inflammatory cytokines involved in the pathogenesis of IPF. Further studies are needed to investigate whether SIgA contributes to and modifies the progression of IPF in vivo, and to confirm the involvement of TfR/CD71. Our study sheds new light on the role of SIgA in respiratory diseases from a novel viewpoint.
Disclosure
The authors have no conflicts of interest to declare.
Supporting information
Fig. S1. TfR/CD71 ‐ SIgA ELISA.
Acknowledgements
The authors thank Dr Hiroyuki Tashimo, Osamu Narumoto and Takeshi Fukami for participating in meaningful discussions throughout the research. The authors also thank Ms Miyuki Wagatsuma and Mr Kenji Urata for their skilled technical assistance and Ms Mariko Yoshizawa for her efficient secretarial work.
Contributor Information
M. Suzukawa, Email: fueta-tky@umin.ac.jp.
K. Ohta, Email: ohtaken3dr@yahoo.co.jp.
References
- 1. Kerr MA. The structure and function of human IgA. Biochem J 1990; 271:285–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brandtzaeg P. Secretory IgA: designed for anti‐microbial defense. Front Immunol 2013; 4:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gloudemans AK, Lambrecht BN, Smits HH. Potential of immunoglobulin A to prevent allergic asthma. Clin Dev Immunol 2013; 2013:542091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Papadopoulou A, Mermiri D, Taousani S, Triga M, Nicolaidou P, Priftis KN. Bronchial hyper‐responsiveness in selective IgA deficiency. Pediatr Allergy Immunol 2005; 16:495–500. [DOI] [PubMed] [Google Scholar]
- 5. Corthésy B. Role of secretory IgA in infection and maintenance of homeostasis. Autoimmun Rev 2013; 12:661–5. [DOI] [PubMed] [Google Scholar]
- 6. Matysiak‐Budnik T, Moura IC, Arcos‐Fajardo M et al Secretory IgA mediates retrotranscytosis of intact gliadin peptides via the transferrin receptor in celiac disease. J Exp Med 2008; 205:143–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Oortwijn BD, van der Boog PJM, Roos A et al A pathogenic role for secretory IgA in IgA nephropathy. Kidney Int 2006; 69:1131–8. [DOI] [PubMed] [Google Scholar]
- 8. Bartemes KR, Cooper KM, Drain KL, Kita H. Secretory IgA induces antigen‐independent eosinophil survival and cytokine production without inducing effector functions. J Allergy Clin Immunol 2005; 116:827–35. [DOI] [PubMed] [Google Scholar]
- 9. Iikura M, Yamaguchi M, Fujisawa T et al Secretory IgA induces degranulation of IL‐3‐primed basophils. J Immunol 1998; 161:1510–5. [PubMed] [Google Scholar]
- 10. Bjoraker JA, Ryu JH, Edwin MK et al Prognostic significance of histopathologic subsets in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 1998; 157:199–203. [DOI] [PubMed] [Google Scholar]
- 11. Wynn TA. Integrating mechanisms of pulmonary fibrosis. J Exp Med 2011; 208:1339–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aschner Yael, Downey GP. Transforming growth factor‐β: master regulator of the respiratory system in health and disease. Am J Respir Cell Mol Biol 2016; 54:647–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. King TE Jr, Schwarz MI, Brown K et al Idiopathic pulmonary fibrosis: relationship between histopathologic features and mortality. Am J Respir Crit Care Med 2001; 164:1025–32. [DOI] [PubMed] [Google Scholar]
- 14. Yan X, Mizuno T, Sridharan A et al Single‐cell RNA sequencing identifies diverse roles of epithelial cells in idiopathic pulmonary fibrosis. JCI Insight 2016; 1:e90558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yang IV, Fingerlin TE, Evans CM, Schwarz MI, Schwartz DA. MUC5B and idiopathic pulmonary fibrosis. Ann Am Thorac Soc 2015; 12:S193–S199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Seibold MA, Wise AL, Speer MC et al A common MUC5B promoter polymorphism and pulmonary fibrosis. N Engl J Med 2011; 364:1503–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Turato G, Floriani AF, Baraldo S et al Mucus production, lung inflammation and functional decay in IPF. Eur Respir J 2014; 44:P3503. [Google Scholar]
- 18. Hamada N, Kuwano K, Yamada M et al Anti‐vascular endothelial growth factor gene therapy attenuates lung injury and fibrosis in mice. J Immunol 2005; 175:1224–31. [DOI] [PubMed] [Google Scholar]
- 19. Simler N, Brenchley P, Horrocks A, Greaves S, Hasleton P, Egan J. Angiogenic cytokines in patients with idiopathic interstitial pneumonia. Thorax 2004; 59:581–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tomos I, Manali E, Karakatsani A et al IL‐6 and IL‐8 in stable and exacerbated IPF patients and their association to outcome. Eur Respir J 2016; 48:PA3890. [Google Scholar]
- 21. Barratt SL, Blythe T, Jarrett C et al Differential expression of VEGF‐Axxx isoforms is critical for development of pulmonary fibrosis. Am J Respir Crit Care Med 2017; 196:479–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Khalil N, Parekh TV, O'Connor R et al Regulation of the effects of TGF‐β1 by activation of latent TGF‐β1 and differential expression of TGF‐β receptors (TβR‐I and TβR‐II) in idiopathic pulmonary fibrosis. Thorax 2001; 56:907–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Soutar CA. Distribution of plasma cells and other cells containing immunoglobulin in the respiratory tract of normal man and class of immunoglobulin contained therein. Thorax 1976; 31:158–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Baumann J, Park CG, Mantis NJ. Recognition of secretory IgA by DC‐SIGN: implication for immune surveillance in the intestine. Immunol Lett 2010; 131:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tomana M, Zikan J, Moldoveanu Z, Kulhavy R, Bennett JC, Mestecky J. Interactions of cell‐surface galactosyltransferase with immunoglobulins. Mol Immunol 1993; 30:265–75. [DOI] [PubMed] [Google Scholar]
- 26. Moura IC, Centelles MN, Arcos‐Fajardo M et al Identification of the transferrin receptor as a novel immunoglobulin (Ig)A1 receptor and its enhanced expression on mesangial cells in IgA nephropathy. J Exp Med 2001; 194:417–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Morton HC, Brandtzaeg P. CD89: the human myeloid IgA Fc receptor. Arch Immunol Ther Exp 2001; 49:217–29. [PubMed] [Google Scholar]
- 28. Heystek HC, Moulon C, Woltman AM, Garonne P, van Kooten C. Human immature dendritic cells efficiently bind and take up secretory IgA without the induction of maturation. J Immunol 2002; 168:102–7. [DOI] [PubMed] [Google Scholar]
- 29. Sakamoto N, Shibuya K, Shimizu Y et al A novel Fc receptor for IgA and IgM is expressed on both hematopoietic and non‐hematopoietic tissues. Eur J Immunol 2001; 31:1310–6. [DOI] [PubMed] [Google Scholar]
- 30. Stockert RJ, Kressner MS, Collins JC, Sternlieb I, Morell AG. IgA interaction with the asialoglycoprotein receptor. Proc Natl Acad Sci USA 1982; 79:6229–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Molyneux K, Wimbury D, Pawluczyk I et al β 1, 4‐galactosyltransferase 1 is a novel receptor for IgA in human mesangial cells. Kidney Int 2017; 92:1458–68. [DOI] [PubMed] [Google Scholar]
- 32. Laube F, Glanz D. Modulation of melanotransferrin and transferrin receptor 1 (TFRC)‐ and CD44‐based signaling for TFRC up‐regulation in human melanoma cells. Anticancer Res 2017; 37:3001–7. [DOI] [PubMed] [Google Scholar]
- 33. Conte E, Gili E, Fagone E, Fruciano M, Iemmolo M, Vancheri C. Effect of pirfenidone on proliferation, TGF‐β‐induced myofibroblast differentiation and fibrogenic activity of primary human lung fibroblasts. Eur J Pharm Sci 2014; 58:13–9. [DOI] [PubMed] [Google Scholar]
- 34. King TE Jr, Bradford WZ, Castro‐Bernardini S et al A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med 2014; 370:2083–92. [DOI] [PubMed] [Google Scholar]
- 35. Richeldi L, Du Bois RM, Raghu G et al Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med 2014;370:2071–82. [DOI] [PubMed] [Google Scholar]
- 36. Khalil N, O’Connor RN, Unruh HW et al Increased production and immunohistochemical localization of transforming growth factor‐beta in idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol 1991; 5:155–62. [DOI] [PubMed] [Google Scholar]
- 37. Ribeiro RA, Flores CA, Cunha FQ, Ferreira SH. IL‐8 causes in vivo neutrophil migration by a cell‐dependent mechanism. Immunology 1991; 73:472–7. [PMC free article] [PubMed] [Google Scholar]
- 38. Hunninghake GW, Gadek JE, Lawley TJ, Crystal RG. Mechanisms of neutrophil accumulation in the lungs of patients with idiopathic pulmonary fibrosis. J Clin Invest 1981; 68:259–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kinder BW, Brown KK, Schwarz MI et al Baseline BAL neutrophilia predicts early mortality in idiopathic pulmonary fibrosis. Chest 2008; 133:226–32. [DOI] [PubMed] [Google Scholar]
- 40. Maleki I, Aminafshari MR, Taghvaei T. Serum immunoglobulin A concentration is a reliable biomarker for liver fibrosis in non‐alcoholic fatty liver disease. World J Gastroenterol 2014; 20:12566–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ten Klooster L, van Moorsel CH, Kwakkel‐van Erp JM, van Velzen‐Blad H, Grutters JC. Immunoglobulin A in serum: an old acquaintance as a new prognostic biomarker in idiopathic pulmonary fibrosis. Clin Exp Immunol 2015; 181:357–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stavnezer J, Kang J. The surprising discovery that TGFβ specifically induces the IgA class switch. J Immunol 2009; 1:5–7. [DOI] [PubMed] [Google Scholar]
- 43. Yang L, Roh YS, Song J, Zhang B, Liu C, Loomba R, Seki E. TGF‐β signaling in hepatocytes participates in steatohepatitis through regulation of cell death and lipid metabolism in mice. Hepatology 2014; 59:483–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lai KN, Tang SCW, Guh J‐Y et al Polymeric IgA1 from patients with IgA nephropathy upregulates transforming growth factor‐beta synthesis and signal transduction in human mesangial cells via the renin‐angiotensin system. J Am Soc Nephrol 2003; 14:3127–37. [DOI] [PubMed] [Google Scholar]
- 45. Arakawa S, Suzukawa M, Watanabe K et al Secretory immunoglobulin A induces human lung fibroblasts to produce inflammatory cytokines and undergo activation. Clin Exp Immunol 2019; 195:287–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Moura IC, Arcos‐Fajardo M, Sadaka C et al Glycosylation and size of IgA1 are essential for interaction with mesangial transferrin receptor in IgA nephropathy. J Am Soc Nephrol 2004; 15:622–34. [DOI] [PubMed] [Google Scholar]
- 47. Yihe W, Jinming X, Chen J, Zou M, Rusidanmu A, Yang R. Blocking transferrin receptor inhibits the growth of lung adenocarcinoma cells in vitro . Thorac Cancer 2018; 9:253–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nagai K, Nakahata S, Shimosaki S et al Development of a complete human anti‐human transferrin receptor C antibody as a novel marker of oral dysplasia and oral cancer. Cancer Med 2014; 3:1085–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Neckers LM, Trepel JB. Transferrin receptor expression and the control of cell growth. Cancer Invest 1986; 4:461–70. [DOI] [PubMed] [Google Scholar]
- 50. Yamamoto Y, Gotoh S, Korogi Y et al Long‐term expansion of alveolar stem cells derived from human iPS cells in organoids. Nat Methods 2017; 14:1097–106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. TfR/CD71 ‐ SIgA ELISA.


