Abstract
Background and purpose — BoneMaster (BM) is an electrochemically deposited hydroxyapatite (HA) implant-coating, which is evenly distributed, thin, and quickly resorbed. It is designed to stimulate osseointegration and early implant stability and alleviate longer-term HA-induced third-body polyethylene wear. This study evaluates early cup migration and functional outcomes of cementless porous-coated hemispherical cups with or without BM.
Patients and methods — In a patient-blinded, randomized, controlled trial 53 patients at mean 64 years (55–75) with coxarthritis were operated with an Exceed cup (Zimmer Biomet) and Bi-Metric stem (Zimmer Biomet) with porous and BM coating (PBM) or with porous coating alone (P). Follow-ups were performed postoperatively and at 3, 6, 12, and 24 months. Effect measures were cup migration measured with RSA and PROMs.
Results — At 6-month follow-up, proximal cup migration in the PBM group (0.09 mm, 95% CI 0.02–0.20) was higher than in the P group (0.25 mm, CI 0.15–0.35). At 1- and 2-year follow-up, cup migration in all 6 degrees of freedom was similar between groups (p > 0.2). From before surgery to 2-year follow-up, Oxford Hip Score (OHS) increased by 17 points (CI 14–20). Hip disability and Osteoarthritis Outcome Score (HOOS) increased in all sub-scores, but was more pronounced for PBM cups compared with P cups in the Symptoms sub-score (p = 0.04).
Interpretation — Contrary to expectations, PBM cups had higher early migration than P cups. At 2-year follow-up, migration was similar between groups. There seems to be no early benefit of BM coating on acetabular cups.
Aseptic loosening remains one of the most common reasons for cup revision. In Denmark up to 86% of cups are cementless and 35% of these are coated with hydroxyapatite (HA) (DHR 2016).
Plasma-sprayed HA coating results in better bony ingrowth and early implant fixation in experimental studies (Soballe et al. 1992, Daugaard et al. 2010). However, clinical results have not truly been able to show superior cementless cup fixation with HA over porous coating at midterm (Rohrl et al. 2004, Valancius et al. 2013) or long-term (Otten et al. 2016, Lazarinis et al. 2017).
Plasma-sprayed HA applies coating in a line-of-sight, which may reduce the microstructure in the porous coating. Electrochemical application of HA is a new technique resulting in a thinner coating layer with better topographic structure and distribution as compared with plasma-sprayed HA. Electrochemically applied HA may stimulate early implant osseointegration and is resorbed within a few months (Daugaard et al. 2010). Therefore, it may not contribute to polyethylene wear.
The osteoconductive properties of electrochemical HA coating have been validated in experimental studies (Wang et al. 2006, Daugaard et al. 2010) and clinically on a femoral stem using RSA (Boe et al. 2011, Flatoy et al. 2016) in accordance with the guidelines for phased introduction (Nelissen et al. 2011). The suggested thresholds for proximal cup migration are 0.2 mm (at risk) and 1.0 mm (unacceptable), and for every 1 mm of proximal migration the risk of later revision increases by 10% (Pijls et al. 2012).
We evaluated the effect of electrochemically applied HA coating on early cup migration and functional outcomes using porous coated Exceed cups (Zimmer Biomet, Warsaw, IN, USA). We hypothesized that cementless Exceed ABT cups with electrochemically applied HA coating would have superior or equal early fixation compared to identical cups without hydroxyapatite coating.
Patients and methods
Study design
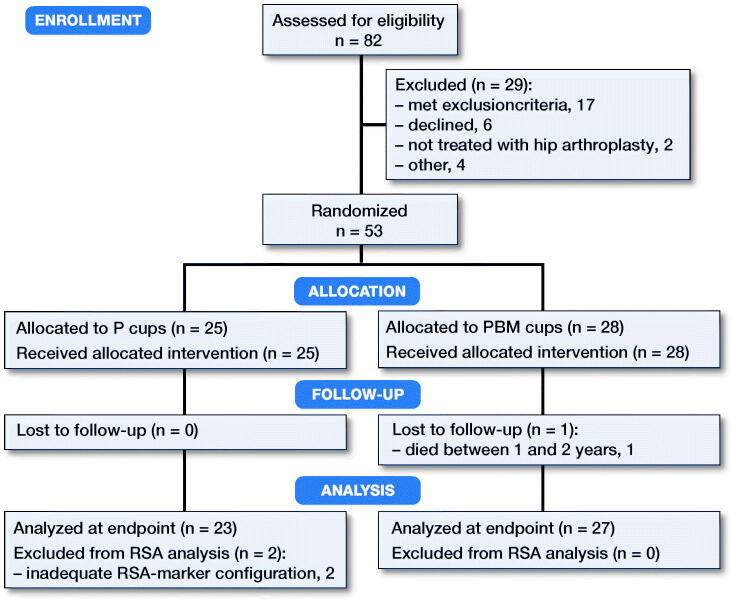
In this patient-blinded, randomized controlled trial, 82 patients were assessed for eligibility between January 2013 and March 2015. Randomization was done in the theater within blocks of 10 patients (5 porous [P], and 5 porous with BoneMaster [PBM]) by drawing concealed labels from sequentially numbered closed envelopes. We obtained written consent of 56 patients who met the inclusion criteria; non-osteoporotic by a pre-op dual energy X-ray absorptiometry (DEXA) scan, age 55–75 years, and severe coxarthrosis (Figure 1, Table 1).
Figure 1.
Consort flow chart
Table 1.
Baseline characteristics, mean (95% CI)
| Baseline demographics | P cups | PBM cups |
|---|---|---|
| N | 25 | 28 |
| Age | 65 (63–67) | 64 (62–66) |
| Men/women | 11/14 | 14/14 |
| T-score | –0.3 (–0.8 to 0.2) | –0.8 (–1.2 to –0.4) |
| OHS-score | 27 (23–30) | 22 (19–25) |
| HOOS | ||
| Pain | 48 (43–57) | 36 (31–45) |
| Symptoms | 45 (40–55) | 28 (28–42) |
| ADL | 57 (46–63) | 39 (37–50) |
| Sport | 38 (26–43) | 13 (13–26) |
| QoL | 38 (30–42) | 22 (17–29) |
Criteria of exclusion were: other diseases of affected hip than primary coxarthrosis at time of inclusion, secondary osteoarthritis, neuromuscular or vascular condition in lower extremity, arthroplasties of other lower-extremity joints, BMI at time of inclusion ≥ 35 or < 18.5, rheumatoid and similar arthritis, metabolic bone disease, reduced kidney function, previous treatment of skeleton with radiation therapy, pharmaceuticals that effect calcium–phosphorus metabolism and bone density, alcohol abuse, medication abuse, and psychological instability.
Sample size
A sample size calculation indicated 23 patients per group based on a clinically relevant difference in migration of 0.6 mm (SD 0.6) with a power of 90% and alpha set to 0.05 (Charnley 1982). To balance postoperative dropout, we aimed for 25 patients per group. To balance for exclusions during the inclusion period, we continued inclusion per block randomization until there were minimum 25 patients in each group, and in total 53 patients in the study.
Implants
25 patients received a cementless Exceed cup (Exceed ABT RingLoc-x shell) size 50-62 mm, and a cementless Bi-Metric stem (Zimmer Biomet). Cup and stem were treated with plasma-spray porous titanium coating with a porosity of 45% and an average pore size of 250 µm (range, 100–1,000 µm), providing a scratch fit (Lindgren et al. 2018). Another 28 patients received similar porous coated Exceed cups (Exceed ABT RingLoc-x shell) size 50–62 mm and Bi-Metric stem (Zimmer Biomet), both with an additional electrochemically applied HA coating (BoneMaster, Zimmer Biomet). The BoneMaster coating consisted of 70% crystalline HA with a thickness of 5 µm and with a 2.0 Ca/P ratio. The amorphous phase in the coating was primarily amorphous calcium phosphate (ACP) but also β-tricalcium phosphate (TCP). All patients received cobalt-chromium-molybdenum modular femoral heads, size 36 mm (1 patient had a size 32), and an E1 highly cross-linked UHMWPE liner (Zimmer Biomet).
Surgery
All patients were operated at Aarhus University Hospital, Denmark. Preoperative planning was done with the AGFA OT3000 digital templating software (Agfa-Gevaert NV, Mortsel, Belgium) and calibrated digital radiographs. All procedures used a posterolateral approach and the acetabulum was under-reamed by 1 mm in all patients. During surgery 6–8 1-mm tantalum beads were inserted into the peri-prosthetic pelvic bone. Preoperatively, patients received 1.5 g cefuroxime intravenously as antimicrobial prophylaxis, and 1 dose of tranexamic acid 10 mg/kg IV. Postoperatively patients received 1 dose of tranexamic acid IV and 3 doses of 1.5 g cefuroxime IV within the first 24 hours. Postoperatively, the patients were mobilized with full weight-bearing and walking aids as needed, using a “fast track” protocol.
Radiostereometric analysis
RSA recordings were performed on a standard RSA system with 2 synchronized ceiling-fixed roentgen tubes (Arco-Ceil/Medira; Santax Medico, Aarhus, Denmark) angled toward each other at 40°. The radiographs were digital (Fuji CR, image size 35 x 43) and stored in DICOM file-format without compression. During the study the RSA equipment was replaced with a newer direct digital dedicated stereo X-ray system, AdoraRSA suite (Nordic X-ray Technique, Aarhus, Denmark) with CXDI-70C wireless detectors (Canon, Tokyo, Japan). X-ray tubes remained at a 40-degree angle and a uniplanar carbon calibration box (Box 24, Medis Specials, Leiden, Netherlands) was used for all recordings.
RSA recordings were obtained by experienced radiographers and analyses were performed by the same blinded investigator (PBJ) using CAD surface implant models (Zimmer Biomet) with Model-Based RSA 4.1 (RSAcore, Leiden, The Netherlands). The maximum rigid body error was set to 0.35 mm and the condition number (CN) at 200. The mean CN was 99.8 (95% CI 89.9–109.6). 4 patients (2 in each group) with CN between 150 and 200 had a sufficient and non-linear bone model as judged by visual evaluation, and were kept in the analyses to maintain power. 1 patient with CN above 200 and 1 patient with only 2 markers were excluded from the RSA data.
2 patients had inadequate marker-configuration; 1 had inferior marker position and the other had only 2 visible markers. These patients were removed from the RSA analysis but contributed with patient-reported outcomes. 2 patients received new liners and femoral heads due to instability (PBM 3 days after primary operation) and recurrent dislocation of the hip (P 16 months after primary operation). These patients continued in the study.
RSA precision
When accepting condition numbers higher than 150 validation of precision becomes essential (Valstar et al. 2005). Precision calculations were based on double RSA examinations recorded at 6-month follow-up (on both RSA systems) (Table 2).
Radiographs and DEXA scans
Preoperative DEXA scans of the lumbar spine and dual hip region were performed using the 2 fan-beam GE Lunar iDXA with Encore software version 13 (Minneapolis, MN, USA). Patients with a T-score below –2.5 on hip or lumbar spine were defined as osteoporotic according to WHO criteria and excluded.
Standard anterior-posterior and medio-lateral hip radiographs were recorded postoperatively and at 2-year follow-up. Cup position was measured by 1 experienced hip surgeon (KS) using the known diameter of the femoral head to calibrate and avoid magnification errors. Radiolucent lines of 1 mm or more were described according to DeLee and Charnley (1976).
Patient-reported and clinical outcomes
Patient-reported outcomes were hip disability and osteoarthritis outcome score (HOOS) and Oxford hip score (OHS). HOOS were recorded in 5 subscales (pain, symptoms, ADL, sport, and quality of life) and have been validated for use in patients receiving total hip arthroplasty (Paulsen et al. 2012). Outcome was evaluated in each subscale giving from 0 (worst) to 100 (best) points. OHS was evaluated on a scale from 0 (worst) to 48 (best) points.
Postoperative complications were recorded at the 2-year follow-up.
Statistics
The RSA dataset consisted of signed cup migrations along and rotations about the 3 orthogonal axes (x, y, z), total translation (TT = sqrt(Tx2 + Ty2 + Tz2)), total rotation (TR = sqrt(Rx2 + Ry2 + Rz2)) and maximum total point motion (MTPM).
All migration measures were normally distributed as evaluated on q–q plots and hence presented as mean and 95% confidence intervals (CI) and were tested using Student’s t-test. Due to the non-normal nature of summed RSA data (TT, TR, and MTPM) the Mann–Whitney U-test was used for statistical testing of those.
Changes in HOOS and OHS were normally distributed as evaluated on q–q plots and tested using Student’s t-test, 1- and 2-year group comparisons were done with the Mann–Whitney U-test. Sub-analysis of proximal migration was performed comparing patients dichotomized on normal bone quality (T score > –1) and osteopenia (T score < –1).
Statistics were calculated using Stata/IC version 13.0 (StataCorp, College Station, TX, USA) and the significance level was set at 0.05.
Ethics, registration, funding, and potential conflicts of interest
The study was approved by the Data Protection Agency (1-16-02-175-11) and the Central Denmark Regions Committee on Biomedical Research Ethics (M-20110224) and was performed in agreement with the Helsinki II declaration. The study was registered at ClinicalTrials.gov (NCT02311179). Zimmer Biomet supported the study financially but had no influence on the manuscript or publication. The authors have no conflicts of interest.
Results
RSA results
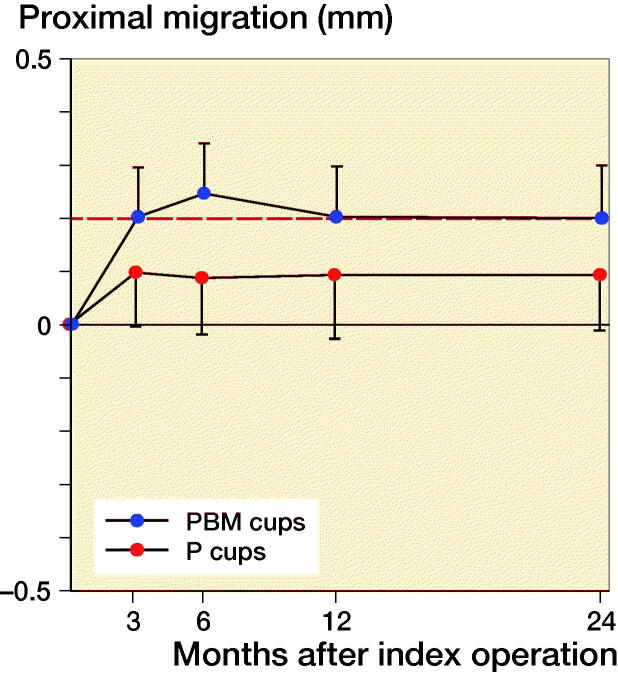
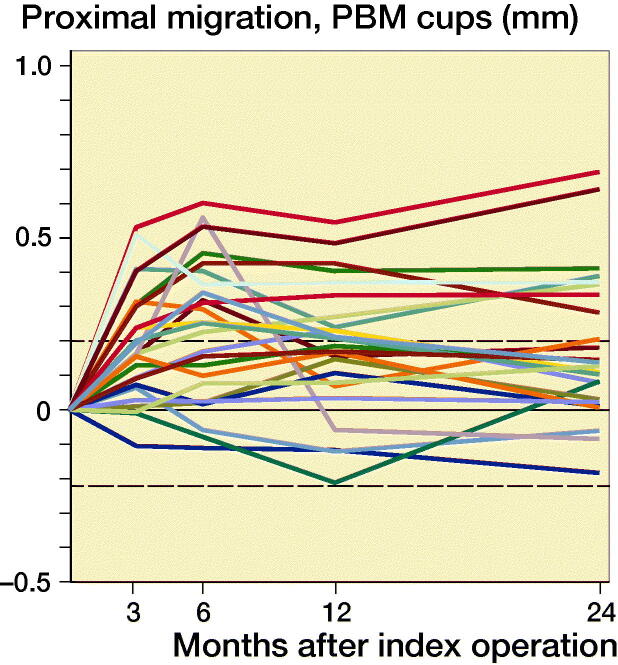
Proximal migration was 0.16 mm (CI 0.02–0.30) higher for PBM coated cups (0.25 mm CI 0.15–0.35) compared with P coated cups (0.09 CI –0.02–0.20) at 6-month follow-up. From 1-year follow-up to 2-year follow-up, the proximal migration was 0.0 mm (CI –0.06 to 0.06) for P-coated cups and 0.0 mm (CI –0.05 to 0.04) for PBM-coated cups. At 2-year follow-up, 8 P-coated cups and 10 PBM-coated cups had a proximal migration exceeding the precision limit of 0.2 mm, and the mean 2-year proximal cup migration was 0.09 mm (CI –0.02 to 0.20) for P-coated cups and 0.2 mm (CI 0.10–0.30) for PBM-coated cups. We found no statistically significant difference in proximal migration between the groups at 1-year follow-up (p = 0.2) and at 2-year follow-up (p = 0.2). At 3-month follow-up, we found a statistically significant difference in y-rotation (p = 0.03).
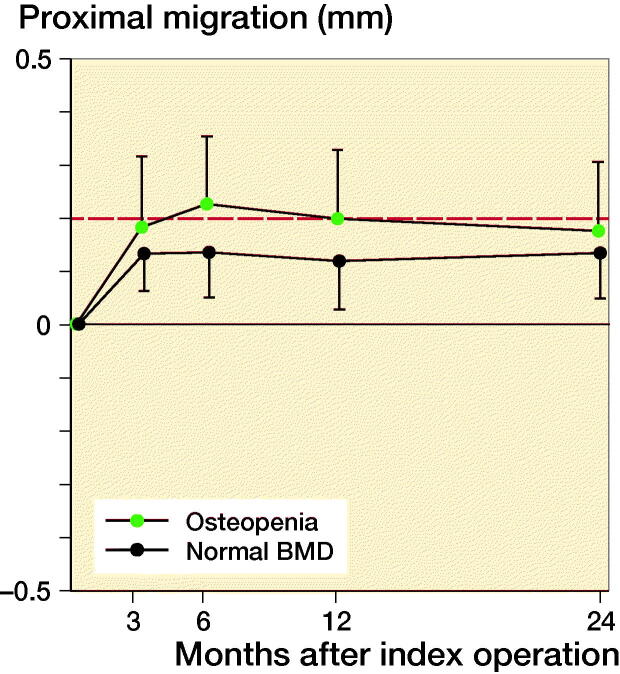
Based on preoperative DEXA scan, 21 patients had osteopenia (T-score < –1) and 29 patients had normal bone quality (T-score > –1). The ratio of normal BMD/osteopenia was 15/8 in the P-coated group and 13/14 in the PBM- coated group. Proximal cup migration was 0.09 mm (CI –0.06 to 0.24) higher for osteopenic patients compared with patients with normal BMD at 6-month follow-up, although this difference was not statistically significant (p = 0.2) (Figure 4). Further RSA results are presented in Figures 2, 3 and 4, and in Tables 2 and 3.
Figure 4.
Mean proximal migration (CI) dependent on bone mineral density with limit for safe 2-year migration (red line).
Figure 2.
Mean proximal migration (CI) with limit for safe 2-year migration (red line).
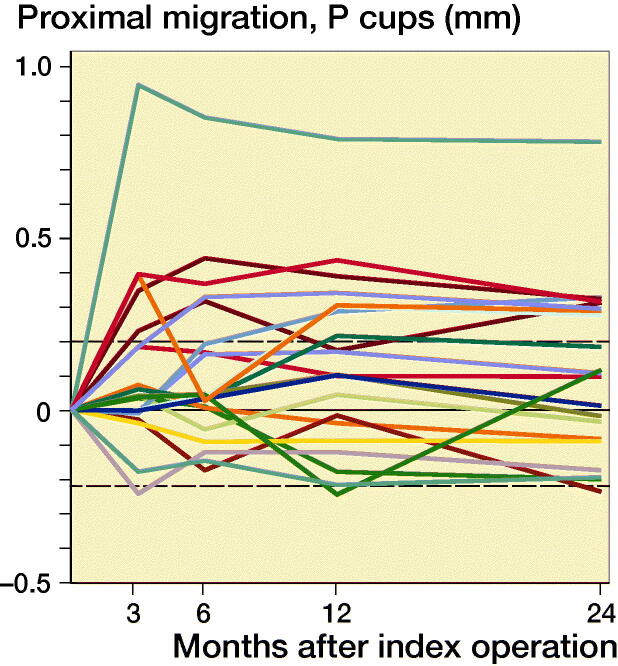
Figure 3.
Individual y-migration. Dashed lines are limits of agreement.
Table 3.
Migration, mean (95% CI)
| P cups, n = 23 | PBM cups, n = 27 | |
|---|---|---|
| Translations, mm | ||
| x-axis (+medial/–lateral) | ||
| 3 months | –0.05 (–0.16 to 0.06) | 0.01 (–0.14 to 0.16) |
| 6 months | –0.01 (–0.17 to 0.15) | –0.03 (–0.17 to 0.11) |
| 1 year | –0.06 (–0.22 to 0.11) | 0.02 (–0.15 to 0.18) |
| 2 years | –0.00 (–0.12 to 0.12) | –0.02 (–0.19 to 0.16) |
| y-axis (+proximal/–distal) | ||
| 3 months | 0.10 (–0.01 to 0.21) | 0.20 (0.11 to 0.30) |
| 6 months | 0.09 (–0.02 to 0.20) | 0.25 (0.15 to 0.35) a |
| 1 year | 0.09 (–0.03 to 0.22) | 0.20 (0.10 to 0.30) |
| 2 years | 0.09 (–0.02 to 0.20) | 0.20 (0.10 to 0.30) |
| z-axis (+anterior/–posterior) | ||
| 3 months | 0.09 (–0.02 to 0.21) | –0.02 (–0.17 to 0.13) |
| 6 months | 0.12 (–0.00 to 0.24) | –0.01 (–0.20 to 0.17) |
| 1 year | 0.19 (0.05 to 0.33) | 0.05 (–0.13 to 0.23) |
| 2 years | 0.12 (–0.03 to 0.26) | 0.08 (–0.11 to 0.28) |
| Rotations, degrees | ||
| x-axis (+anterior/–posterior tilt) | ||
| 3 months | –0.04 (–0.52 to 0.44) | 0.03 (–0.30 to 0.36) |
| 6 months | –0.09 (–0.63 to 0.45) | 0.04 (–0.32 to 0.41) |
| 1 year | 0.24 (–0.26 to 0.75) | –0.15 (–0.55 to 0.24) |
| 2 years | 0.15 (–0.40 to 0.71) | 0.02 (–0.35 to 0.40) |
| y-axis (+internal/–external rotation) | ||
| 3 months | –0.32 (–0.82 to 0.18) | 0.33 (–0.04 to 0.71) a |
| 6 months | –0.30 (–0.87 to 0.27) | 0.23 (–0.24 to 0.70) |
| 1 year | 0.14 (–0.55 to 0.82) | –0.12 (–0.63 to 0.39) |
| 2 years | –0.02 (–0.58 to 0.53) | 0.27 (–0.28 to 0.82) |
| z-axis (+increased/–decreased inclination) | ||
| 3 months | 0.05 (–0.30 to 0.39) | –0.17 (–0.54 to 0.19) |
| 6 months | –0.11 (–0.48 to 0.26) | –0.19 (–0.59 to 0.20) |
| 1 year | –0.32 (–0.78 to 0.14) | –0.13 (–0.57 to 0.32) |
| 2 years | –0.15 (–0.54 to 0.25) | –0.20 (–0.65 to 0.25) |
| Maximum total point movement b | ||
| 3 months | 0.94 (0.74 to 1.20) | 1.01 (0.85 to 1.20) |
| 6 months | 1.05 (0.81 to 1.38) | 1.19 (0.97 to 1.47) |
| 1 year | 1.16 (0.91 to 1.47) | 1.28 (1.05 to 1.57) |
| 2 years | 1.16 (0.94 to 1.43) | 1.38 (1.14 to 1.67) |
| Total translation b | ||
| 3 months | 0.36 (0.29 to 0.46) | 0.42 (0.33 to 0.54) |
| 6 months | 0.40 (0.30 to 0.53) | 0.52 (0.41 to 0.66) |
| 1 year | 0.47 (0.38 to 0.58) | 0.52 (0.41 to 0.66) |
| 2 years | 0.41 (0.32 to 0.51) | 0.51 (0.38 to 0.68) |
| Total rotationb | ||
| 3 months | 1.31 (1.01 to 1.70) | 1.23 (0.99 to 1.52) |
| 6 months | 1.37 (0.97 to 1.95) | 1.40 (1.11 to 1.77) |
| 1 year | 1.50 (1.10 to 2.05) | 1.55 (1.24 to 1.94) |
| 2 years | 1.60 (1.23 to 2.09) | 1.69 (1.42 to 2.01) |
aStatistically significant difference between P and PBM.
b2-sample Mann–Whitney U-test.
Radiographic results
Radiographic evaluation revealed a mean inclination of 42°(CI 40–43) and mean anteversion of 20° (CI 18–21) with no statistically significant difference between groups (p > 0.3). Only 1 patient (PBM) had a radiolucent line of more than 1 mm (1.03 mm) in zone 3 evaluated on 2-year radiographs. This patient had an early migration of the cup of 0.3 mm medially and 0.3 mm proximally at 3-month follow-up, and stabilized hereafter.
Patient-reported and clinical outcomes
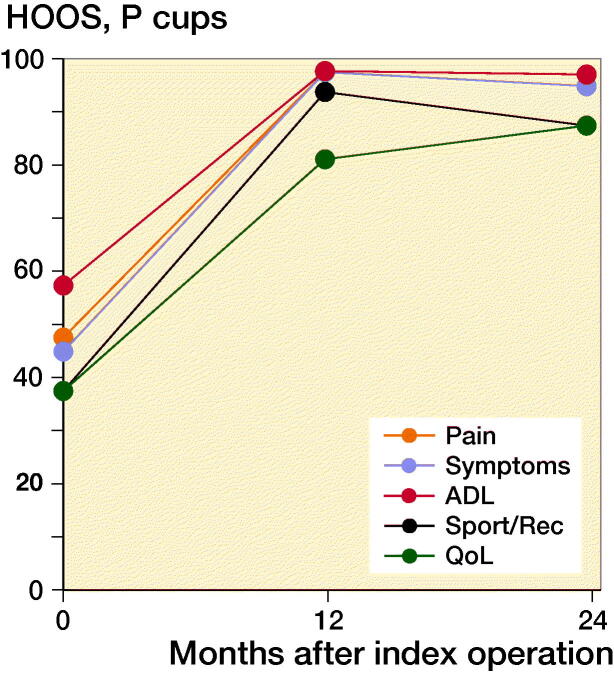
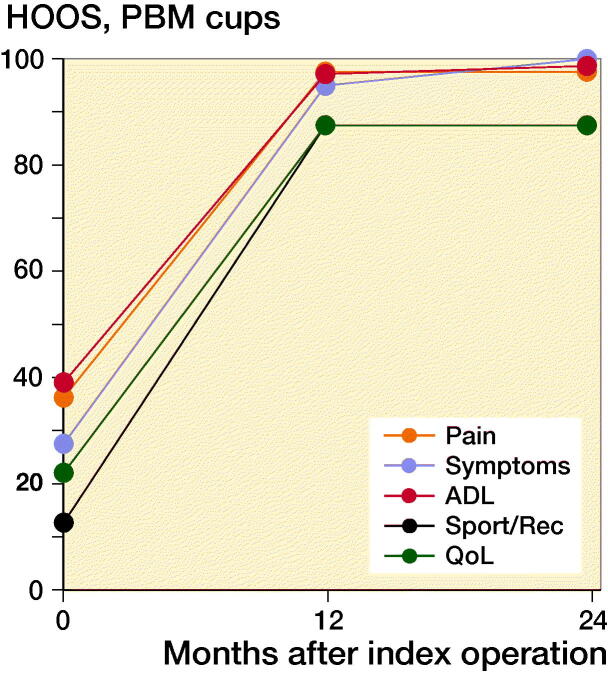
At 2-year follow-up OHS reached a median value of 46 points (7–48). OHS showed clinically relevant improvement in self-perceived hip function for both groups from baseline to 2-year follow-up of 17 points (CI 14–20) with no statistically significant difference between groups (p = 0.1). Similarly, HOOS increased significantly in all groups from baseline to 2-year follow-up (p < 0.001). PBM-coated cups had a greater increase in HOOS symptoms score than P-coated cups (p = 0.04). However, the difference of 14 points (CI 27–50) was not considered clinically relevant (Paulsen et al. 2014). At 1-year and 2-year follow-up, there were no statistically significant differences between the groups in any HOOS category or OHS (p > 0.5) (Figures 5 and 6).
Figure 5.
Median HOOS (0–100).
Figure 6.
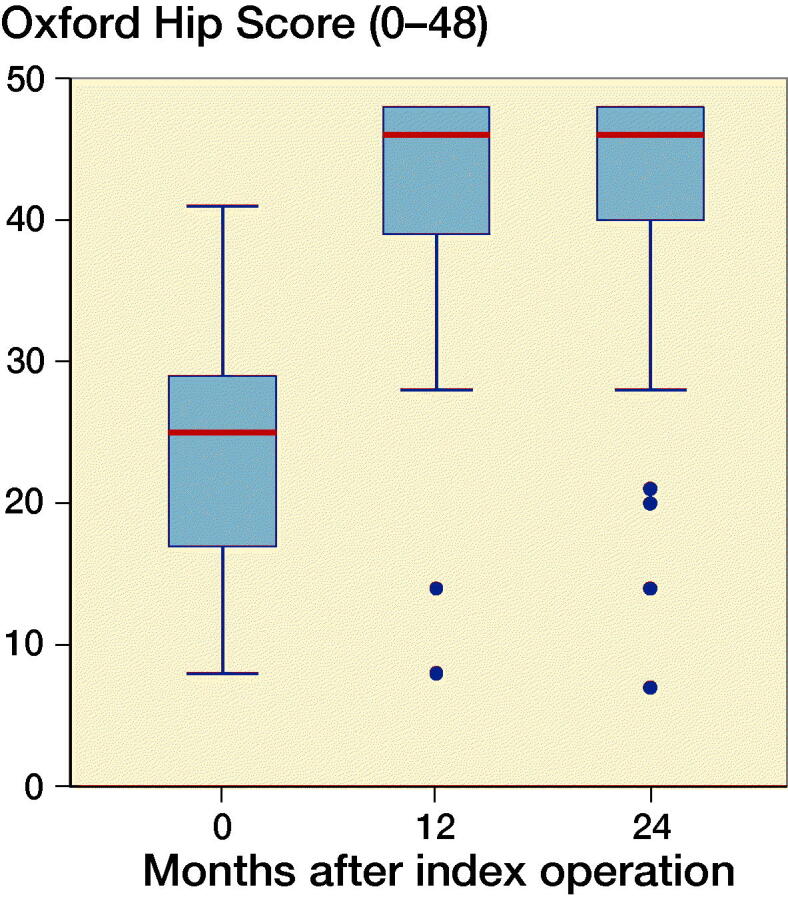
Oxford Hip Score in a box plot. The line tags the median, the box tags the interquartile range (IQR). Whiskers indicate the most extreme value within upper/lower quartile ±1.5ЧIQR.
A clinical evaluation at 2-year follow-up comprised 2 head/liner replacements related to dislocation and instability (1 P and 1 PBM).
Discussion
This study evaluates the migration of porous Exceed cups with or without BM coating, and we found that the PBM-coated cups had higher proximal migration at 6-month follow-up, but there was no statistically significant difference between groups at 2-year follow-up.
RSA
Building on the experimental results of BM we expected better early bone ingrowth and consequently better fixation in the bone/implant interface in the PBM group (Schmidmaier et al. 2002, Daugaard et al. 2010). Clinically we found that PBM-coated cups had a higher proximal migration at 6-month follow-up, but no statistically significant difference in comparison with P-coated cups was found at 2-year follow-up.
An earlier study on dogs showed that electrochemically applied HA coating was not visible after 4 weeks (Daugaard et al. 2010). This suggests that the coating has been resorbed leaving a space between the implant and bone. Such a gap may explain the increased migration of the PBM implant during the earliest months in the present study.
To our knowledge, this is the first randomized clinical study of electrochemically applied HA coating on cups. Lazarinis et al. (2014) studied a cohort of hemispherical trabeculae-oriented-pattern cups with electrochemically applied HA. Similar to our results, they found an initial proximal migration that seemed to stabilize after 3 months.
We also found statistically significant difference in the y-rotation (anteversion/retroversion) at 3-month follow-up. However, y-rotation varies over time and this finding is likely to be caused by variation.
In accordance with studies on plasma-sprayed HA coatings we found little or no positive effect of electrochemically applied HA coating on migration of hemispherical cups after 2 years (Rohrl et al. 2004, Valancius et al. 2013). Our findings also indicate no improved long-term survival effect of electrochemically applied HA coating. This is in agreement with recent larger registry-based analysis and meta-analysis of HA coatings on cementless cups (Chen et al. 2015, Lazarinis et al. 2017).
Both the P-coated cups and the PBM-coated cups stabilized after 6 months, and early RSA-measured cup stabilization is in agreement with a number of other studies of cementless hemispheric cups (Lazarinis et al. 2014, Salemyr et al. 2015, Hjorth et al. 2017, Nilsson et al. 2017).
Only 1 other clinical study on BoneMaster coating exists in the literature, and it concerns findings of increased early retroversion of femoral stems coated with BM compared with plasma-sprayed HA (Flatoy et al. 2016). In agreement with our results, their femoral stems all stabilized after 3-month follow-up.
BMD
Low systemic bone quality (T-score < –1.0) has been shown to increase proximal cup migration at 3- and 6-month follow-up (Finnila et al. 2016). In order to avoid migration bias from patients with poor bone quality we excluded all patients with osteoporosis (T-score < –2.5) based on a preoperative DEXA scan of systemic BMD. In spite of randomization, there was a difference in distribution of BMD between the 2 groups. This may explain some of the early difference in proximal cup migration.
Precision
We included 4 patients with a CN between 150 and 200 on RSA analysis, and upgraded the RSA system during the study. However, the precision of proximal migration was comparable to similar studies on hemispheric cups (Hjorth et al. 2017, Shareghi et al. 2017).
Patient-reported outcome
In general, patients receiving P cups had a better self-perceived hip function at baseline compared with the PBM group. Although preoperative score does not affect the Patient Acceptable Symptom State (PASS) postoperatively it may explain why PBM cups had superior improvement from baseline to 2-year follow-up in HOOS Symptoms sub-score (Paulsen et al. 2014). Postoperatively, patients reached OHS exceeding 40 points, which is the threshold corresponding to PASS after THR, and also corresponding to the Danish background population-based value of OHS (Paulsen et al. 2012, Keurentjes et al. 2014).
Strengths and weaknesses
This study sample size calculation was originally based on group mean comparisons of cup migration in general after 5 years. After initiation of this study, Pijls et al. (2012) published a paper validating 2-year follow-up of proximal migration (Y-axis) as proxy measure for later revision. Therefore, we focused the primary endpoint of this study on proximal cup migration after 2 years, and reduced multiple testing of outcomes. The decision to change endpoint was made without knowledge of actual data as described by Evans (2007).
The randomized blinded design and high precision of RSA are major strengths, along with a high degree of patient compliance with almost full follow-up. The generalizability is limited to patients within the study criteria, which were many, because we wanted to make groups as comparable as possible in order to limit variation and be able to find even small differences between coating groups. Longer-term effects of BoneMaster on polyethylene wear cannot be evaluated before 5 years’ follow-up because of the low wear in highly crosslinked UHMWPE liners.
Summary
We found a higher early proximal cup migration with porous and BoneMaster coating as compared with porous coating alone, which indicates no relevant positive effect of electrochemically applied HA (BoneMaster) on cup osseointegration. Based on this study and the current literature, we see no advantage in additional BoneMaster coating on porous-coated acetabular cups.
Supplementary data
Table 2 is available as supplementary data in the online version of this article, http://dx.doi.org/10.1080/17453674.2019.1687860
Supplementary Material
Acknowledgements
PBJ and MS wrote the manuscript and performed the statistical analyses. PBJ and KS performed image analyses. HD, SSJ, ML, KS, and MS contributed to planning, data interpretation, and manuscript revision.
Acta thanks Stergios Lazarinis for help with peer review of this study.
References
- Boe B, Heier T, Nordsletten L. Measurement of early bone loss around an uncemented femoral stem. Acta Orthop 2011; 82(3): 321–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnley J. Long-term results of low-friction arthroplasty. Hip 1982:42–9. [PubMed] [Google Scholar]
- Chen Y L, Lin T, Liu A, Shi M M, Hu B, Shi Z L, Yan S G. Does hydroxyapatite coating have no advantage over porous coating in primary total hip arthroplasty? A meta-analysis. J Orthop Surg Res 2015; 10: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugaard H, Elmengaard B, Bechtold J E, Jensen T, Soballe K. The effect on bone growth enhancement of implant coatings with hydroxyapatite and collagen deposited electrochemically and by plasma spray. J Biomed Mater Res A 2010; 92(3): 913–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLee J G, Charnley J. Radiological demarcation of cemented sockets in total hip replacement. Clin Orthop Relat Res 1976; (121): 20–32. [PubMed] [Google Scholar]
- DHR . National Report 2016. The Danish Hip Arthroplasty Registry; 2016.
- Evans S. When and how can endpoints be changed after initiation of a randomized clinical trial? PLoS Clin Trials 2007; 2(4): e18–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnila S, Moritz N, Svedstro M E, Alm J J, Aro H T. Increased migration of uncemented acetabular cups in female total hip arthroplasty patients with low systemic bone mineral density: a 2-year RSA and 8-year radiographic follow-up study of 34 patients. Acta Orthop 2016; 87(1): 48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatoy B, Rohrl S M, Boe B, Nordsletten L. No medium-term advantage of electrochemical deposition of hydroxyapatite in cementless femoral stems: 5-year RSA and DXA results from a randomized controlled trial. Acta Orthop 2016; 87(1): 42–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjorth M H, Lorenzen N D, Soballe K, Jakobsen S S, Stilling M. Equal primary fixation of resurfacing stem, but inferior cup fixation with anterolateral vs posterior surgical approach: a 2-year blinded randomized radiostereometric and dual-energy X-ray absorptiometry study of metal-on-metal hip resurfacing arthroplasty. J Arthroplasty 2017; 32(11): 3412–20. [DOI] [PubMed] [Google Scholar]
- Keurentjes J C, Van Tol F R, Fiocco M, So-Osman C, Onstenk R, Koopman-Van Gemert A W, Poll R G, Nelissen R G. Patient acceptable symptom states after total hip or knee replacement at mid-term follow-up: thresholds of the Oxford hip and knee scores. Bone Joint Res 2014; 3(1): 7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarinis S, Milbrink J, Mattsson P, Mallmin H, Hailer N P. Bone loss around a stable, partly threaded hydroxyapatite-coated cup: a prospective cohort study using RSA and DXA. Hip Int 2014; 24(2): 155–66. [DOI] [PubMed] [Google Scholar]
- Lazarinis S, Makela K T, Eskelinen A, Havelin L, Hallan G, Overgaard S, Pedersen A B, Karrholm J, Hailer N P. Does hydroxyapatite coating of uncemented cups improve long-term survival? An analysis of 28,605 primary total hip arthroplasty procedures from the Nordic Arthroplasty Register Association (NARA). Osteoarthritis Cartilage 2017; 25(12): 1980–7. [DOI] [PubMed] [Google Scholar]
- Lindgren V, Galea V P, Nebergall A, Greene M E, Rolfson O, Malchau H. Radiographic and clinical outcomes of porous titanium-coated and plasma-sprayed acetabular shells: a five-year prospective multicenter study. J Bone Joint Surg Am 2018; 100(19): 1673–81. [DOI] [PubMed] [Google Scholar]
- Nelissen R G, Pijls B G, Karrholm J, Malchau H, Nieuwenhuijse M J, Valstar E R. RSA and registries: the quest for phased introduction of new implants. J Bone Joint Surg Am 2011; 93 (Suppl. 3): 62–5. [DOI] [PubMed] [Google Scholar]
- Nilsson K G, Theodoulou A, Mercer G, Quinn S J, Krishnan J. Mid-term migration of a cementless, porous acetabular cup: a 5 year radiostereometric analysis. J Orthop 2017; 14(4): 454–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otten V T, Crnalic S, Rohrl S M, Nivbrant B, Nilsson K G. Stability of uncemented cups: long-term effect of screws, pegs and HA coating: a 14-Year RSA follow-up of total hip arthroplasty. J Arthroplasty 2016; 31(1): 156–61. [DOI] [PubMed] [Google Scholar]
- Paulsen A, Odgaard A, Overgaard S. Translation, cross-cultural adaptation and validation of the Danish version of the Oxford hip score: assessed against generic and disease-specific questionnaires. Bone Joint Rese 2012; 1(9): 225–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen A, Roos E M, Pedersen A B, Overgaard S. Minimal clinically important improvement (MCII) and patient-acceptable symptom state (PASS) in total hip arthroplasty (THA) patients 1 year postoperatively. Acta Orthop 2014; 85(1): 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pijls B G, Nieuwenhuijse M J, Fiocco M, Plevier J W M, Middeldorp S, Nelissen R G H H, Valstar E R. Early proximal migration of cups is associated with late revision in THA: a systematic review and meta-analysis of 26 RSA studies and 49 survival studies. Acta Orthop 2012; 83(6): 583–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrl S M, Nivbrant B, Strom H, Nilsson K G. Effect of augmented cup fixation on stability, wear, and osteolysis: a 5-year follow-up of total hip arthroplasty with RSA. J Arthroplasty 2004; 19(8): 962–71. [DOI] [PubMed] [Google Scholar]
- Salemyr M, Muren O, Eisler T, Boden H, Chammout G, Stark A, Skoldenberg O. Porous titanium construct cup compared to porous coated titanium cup in total hip arthroplasty: a randomised controlled trial. Int Orthop 2015; 39(5): 823–32. [DOI] [PubMed] [Google Scholar]
- Schmidmaier G, Wildemann B, Schwabe P, Stange R, Hoffmann J, Sudkamp N P, Haas N P, Raschke M. A new electrochemically graded hydroxyapatite coating for osteosynthetic implants promotes implant osteointegration in a rat model. J Biomed Mater Res 2002; 63(2): 168–72. [DOI] [PubMed] [Google Scholar]
- Shareghi B, Johanson P E, Karrholm J. Clinical evaluation of model-based radiostereometric analysis to measure femoral head penetration and cup migration in four different cup designs. J Orthop Res 2017; 35(4): 760–7. [DOI] [PubMed] [Google Scholar]
- Soballe K, Hansen E S, H B R, Jorgensen P H, Bunger C. Tissue ingrowth into titanium and hydroxyapatite-coated implants during stable and unstable mechanical conditions. J Orthop Res 1992; 10(2): 285–99. [DOI] [PubMed] [Google Scholar]
- Valancius K, Soballe K, Nielsen P T, Laursen M B. No superior performance of hydroxyapatite-coated acetabular cups over porous-coated cups. Acta Orthop 2013; 84(6): 544–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valstar E R, Gill R, Ryd L, Flivik G, Borlin N, Karrholm J. Guidelines for standardization of radiostereometry (RSA) of implants. Acta Orthop 2005; 76(4): 563–72. [DOI] [PubMed] [Google Scholar]
- Wang H, Eliaz N, Xiang Z, Hsu H P, Spector M, Hobbs L W. Early bone apposition in vivo on plasma-sprayed and electrochemically deposited hydroxyapatite coatings on titanium alloy. Biomaterials 2006; 27(23): 4192–203. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.