Abstract
It has been suggested that contextual fear conditioning can be supported by either an elemental system, where individual features of the environment are associated with shock, or a configural system, where environmental features are bound together and associated with shock. Although the retrosplenial cortex (RSC) is known to be involved in contextual fear conditioning, it is not clear whether it contributes to the elemental or configural system. In order to isolate the role of the RSC in contextual fear conditioning, the current experiments examined the influence of RSC lesions on the context pre-exposure facilitation effect, a procedure known to produce conditioning to a configural representation of context. In Experiment 1, rats that were pre-exposed to the conditioning context froze more compared to rats that were not, replicating the context pre-exposure facilitation effect. Although pre-training lesions of the RSC had no impact on the context pre-exposure facilitation effect (Experiment 2a), post-training lesions attenuated the effect (Experiment 2b), suggesting that the RSC normally contributes to a configural context representation. Retro-hippocampal contributions to contextual fear conditioning are discussed.
Keywords: retrosplenial, context, elemental, configural, fear conditioning
In a typical contextual fear conditioning experiment, rats are placed in a conditioning chamber and, after several minutes have elapsed, receive a series of mild foot-shocks. With this procedure, rats will freeze during the post-shock period, and will also freeze when they are re-exposed to the chamber at a later time (e.g., Fanselow, 1980). Freezing is considered to be a conditioned response (CR), as opposed to an unconditioned response (UR) to the shock, and is provoked by conditioning that occurs to the context as a result of pairings with the shock (Fanselow, 1986, 1990, 2000). Contexts are often operationally defined as the background stimuli that are provided by the experimental apparatus. They typically differ in their olfactory, tactile and visual characteristics (Bouton, 2010).
It has been suggested that contextual fear conditioning can be supported by either a configural or an elemental conditioning process (Anagnostaras, Gale, & Fanselow, 2001; Maren, 2001; Maren, Aharonov, & Fanselow, 1997; Rudy, 2009; Rudy, Barrientos, & O’Reilly, 2002; Rudy, Huff, & Matus-Amat, 2004; Sutherland & Rudy, 1989; but see Fanselow, 2010). According to a configural view, individual stimuli that comprise the environment are bound together into a cohesive and integrated representation, and it is this representation that is associated with shock (Fanselow, 2000; Rudy; 2009; Rudy et al., 2004). Alternatively, according to an elemental view, during contextual fear conditioning individual stimuli that make up the context become separately associated with shock. According to this theoretical framework, the hippocampus is assumed to support the configural system, while surrounding cortical regions are often assumed to support the elemental system (Rudy, Barrientos, & O’Reilly, 2002; Rudy & O’Reilly, 2001; Rudy, 2009).
One strategy for isolating configural context conditioning is to take advantage of the immediate shock deficit. It is well known that when rats are placed into a novel-conditioning chamber and receive an immediate shock, they show little or no fear conditioning (e.g, Fanselow, 1986). The lack of conditioning after immediate shock indicates that rats have not associated the context with shock, either elementally or configurally. However, if rats are pre-exposed to the conditioning context prior to receiving the immediate shock, they will freeze more to the context relative to control rats that receive either no pre-exposure or are pre-exposed to a different context (Fanselow, 1986, 1990; Landeira-Fernandez, Fanselow, DeCola, & Kim, 1995; Kiernan & Westbrook, 1993). This is known as the context pre-exposure facilitation effect. Furthermore, the elements of the context must be experienced together; pre-exposure to context elements separately does not alleviate the immediate shock deficit (Rudy & O’Reilly, 1999). When the elements are experienced together, it is assumed that they are associated or bound into an integrated representation. This configural representation can subsequently be activated when a subset of the context features are later experienced (Fanselow, 2000). It is for these reasons that the context pre-exposure methodology is thought to isolate contextual fear conditioning supported by configural processing and is thus considered a strong test of the configural account of contextual fear conditioning (Fanselow, 2010; Rudy et al., 2002).
The hippocampus is a site of convergence for poly-modal information (Suzuki & Amaral, 2004) and is therefore well positioned to process and associate multiple features of the environment to form a configural representation of the context (see Fanselow, 2010). Importantly, Rudy and colleagues have demonstrated that the hippocampus is necessary for the context pre-exposure facilitation effect (Matus-Amat, Higgins, Barrientos, & Rudy, 2004; Rudy et al., 2002; Rudy & Matus-Amat, 2005; see also Stote & Fanselow, 2004), consistent with the notion that the hippocampus has a critical role in representing the context as a unitary, cohesive whole (e.g., Fanselow, 2000, 2010). The purpose of the present experiments, however, was to determine if the retrosplenial cortex (RSC) contributes to configural representations of context. The RSC is positioned at the interface between sensory cortical regions and the parahippocampal – hippocampal system (Sugar, Witter, van Strein, & Cappaert, 2011; van Strein, Cappaert, & Witter, 2009), suggesting that it might have an important role in contextual information processing (Bucci & Robinson, 2014). Indeed, both pre- and post-training lesions of the RSC attenuate contextual fear conditioning (Keene & Bucci, 2008a, 2008b). Likewise, temporary blockade of NMDA receptors in the RSC impairs retrieval of contextual fear memories (Corcoran et al., 2011), and blocking protein synthesis in the RSC prior to conditioning impairs contextual fear conditioning (Kwapis, Jarome, Lee, & Helmstetter, 2015). Thus, a variety of evidence indicates the RSC plays some role in contextual fear conditioning. Indeed, one model of RSC function states that the RSC’s main contribution to context learning involves binding together or associating individual elements of the context, a seemingly configural process (e.g., Bucci & Robinson, 2014).
Nevertheless, the role of the RSC in contextual fear conditioning is unclear because most, if not all, studies examining RSC and contextual fear conditioning use a standard fear conditioning method where either an elemental or configural system might be involved. Therefore, in order to directly test if the RSC is involved in configural context processing, the present experiments utilized the context pre-exposure facilitation procedure. One possibility is that the RSC is part of the elemental system and does not contribute to configural processing (see Rudy, 2009). However, it is also possible that the RSC interacts with the hippocampus to produce configural representations. This would be consistent with recent studies suggesting that the RSC might in fact store a “unitary” representation of the context (Cowansage et al., 2014).
Experiment 1
Although the context pre-exposure facilitation effect has been reported with several different procedures (e.g., Fanselow, 1990; Kiernan & Westbrook, 1993; Rudy, Barrientos, & O’Reilly, 2002; Stote & Fanselow, 2004), in some cases similar procedures have failed to demonstrate a facilitative effect of context pre-exposure (e.g., Fanselow, 1990, Experiments 2 and 3). Our first objective was therefore to demonstrate a facilitative effect of context pre-exposure within our own laboratory. We elected to replicate the effect using the same procedure as Kiernan and Westbrook (1993, Experiment 3). The design of Experiment 1 is depicted in Figure 1. On four consecutive days, groups of rats were exposed to either Context A or Context B. On the fifth day, all rats were placed in Context A and shocked after 7 seconds. On the final day of the experiment, rats were returned to Context A for a shock-free retrieval test. It was expected that rats pre-exposed to Context A (Group Pre) would show higher levels of context fear than rats that were not (Group Non).
Figure 1.
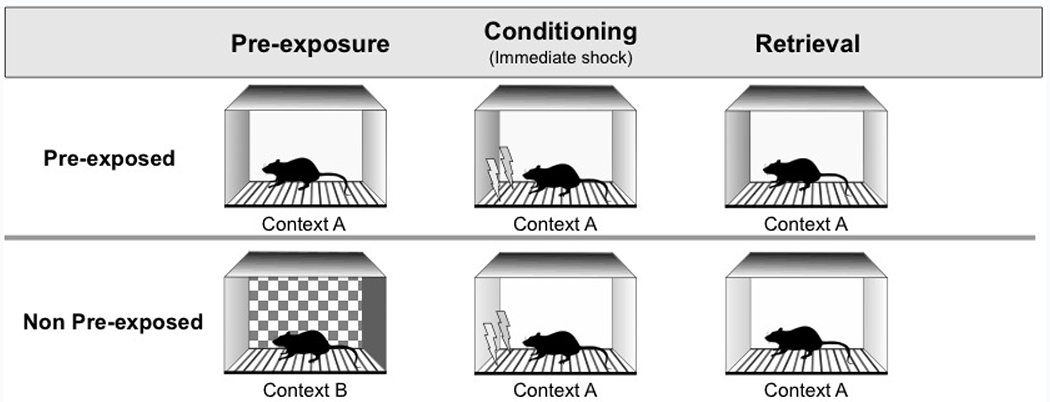
Behavioral procedures for Experiments 1, 2a, and 2b. Rats were exposed to either Context A (Pre-exposed) or Context B (Non pre-exposed) for 2 minutes on each of 4 consecutive days. All rats then received immediate shock in Context A (Conditioning), followed by a final shock-free retrieval test 24 hours later (Retrieval).
Method
Subjects
The subjects were 16 naïve adult male Long Evans rats, ~60 days old. They were obtained from Harlan Laboratories (Indianapolis, IN). Rats were housed individually and allowed at least 6 days to acclimate to the vivarium with food available ad libitum (Purina standard rat chow; Nestle Purina, St. Louis, MO). Throughout the study, rats were maintained on a 14:10 light-dark cycle and monitored and cared for in compliance with association for Assessment and Accreditation of laboratory Animal Care guidelines and the Dartmouth College Institutional Animal Care and Use Committee.
Behavioral apparatus
Two sets of four conditioning chambers housed in separate rooms of the laboratory served as two contexts. One set of chambers (Context A) measured 24 cm W × 30.5 cm L × 29 cm H. Each chamber (Med Associates, ENV-007) was housed in its own sound attenuation chamber (ENV-017M; 66 cm W × 56 cm H × 56 cm D) and outfitted with an exhaust fan to provide airflow and background noise (~68 dB). The sidewalls and ceiling were made of clear acrylic plastic and the front and rear walls were made of brushed aluminum. The grid floor was stainless steel rods (5-mm diameter) spaced 1.5 cm apart (center-to-center). Each chamber was outfitted with a food cup, recessed in the center of the front wall. Retractable levers (Med Associates, model ENV-112CM) were positioned to the left and right of the food cup (both levers remained retracted during the experiment). The chambers were illuminated with two 2.8-W bulbs (one with a red cover), mounted to the ceiling of the sound attenuating chamber. In addition, there were four panel lights (ENV-221M) in the chamber: one above each lever, one just above the food cup, and one ~16 cm above the grid floor centered over the food cup. None of the panel lights were illuminated throughout the experiment. Footshocks were generated by a Med associated unit (ENV-414) for each chamber. Approximately 0.5 grams of Vicks Vaporub (Proctor & Gamble, Cincinnati, Ohio) was smeared along the chamber tray below the grid floor to provide a distinct olfactory cue. The apparatus was controlled by computer equipment located in an adjacent room. Surveillance cameras located inside the sound-attenuating chambers were used to record the rats’ behavior.
The second set of chambers (Context B) measured 17.8 cm W × 21.6 cm L × 12.7 cm H. Each chamber (Med Associates, ENV-307W) was housed in its own sound attenuation chamber (ENV-022MD; 55.9 cm W × 38.1 cm H × 35.6 cm D). The front and back walls, as well as the ceiling were made of clear acrylic plastic and the sidewalls were made of brushed aluminum. The grid floor was stainless steel rods (0.32 cm diameter) spaced 0.79 cm apart (center-to-center). Each chamber was outfitted with a food cup, recessed in the center of the front wall. A house light provided illumination of the chamber (2.8 W). The front door and back wall had checkered panels (8 cm × 5 cm panels). The same checkered panel was taped to the roof of the sound-attenuating chamber. Approximately 3 ml of vinegar (Heinz, Pittsburgh, PA), was placed in the chamber tray to provide a distinct odor cue.
Behavioral procedures
Pre-exposure.
For the first four days of the experiments, rats received daily 2-min exposures to either Context A (Group Pre) or Context B (Group Non).
Conditioning (immediate shock).
On the fifth day, all rats were placed in Context A and received a 1 mA, 2-s footshock. Shock was delivered 7 s after placement into the chamber. Rats remained in the chamber for an additional 2 minutes after shock delivery.
Retrieval.
On Day 6, all rats were re-exposed to Context A for 2 minutes. No shock was delivered.
Data analysis.
The dependent measure was freezing, defined as the absence of all movements, except those related to respiration (Fanselow, 1980). Each rat was observed, and every 4th second behavior was scored as either freezing or not. This yielded 30 observations for the 2-min post-shock period of “Conditioning”, and the 2-min session for “Retrieval”, which were then converted to percentages with the following formula: (# of observations recorded as freezing / 30) × 100. For Conditioning, behavior was scored online by a single observer (TPT). For Retrieval, freezing was scored from DVD recordings by two observers (TPT and MYJ) blind to experimental condition (Pre vs. Non). The ratings for Retrieval were highly correlated across observers (r > .97). Due to the expectation of heterogeneity of variance (cf. Fanselow, 1990; Landeira-Fernandez, Fanselow, DeCola, & Kim, 1995), the data were analyzed with non-parametric tests (Mann-Whitney U).
Results and Discussion
The results of Conditioning and Retrieval are presented in Figure 2. Due to an equipment malfunction, data were lost for 2 rats in Group Pre for Conditioning only. Nevertheless, as seen in the figure, pre-exposure to the conditioning context (Context A) reliably increased freezing for both Conditioning and Retrieval. Group Non (Mdn = 6.66) froze less than Group Pre (Mdn = 40.00) during the postshock period (Conditioning), [U(8, 6) = 4.5, p = .01]. Group Non (Mdn = 1.66) also froze less than Group Pre (Mdn = 58.33) during the shock-free test 24 hours later (Retrieval), [U(8,8) = 2, p < .01]. Overall, Experiment 1 demonstrated a robust context pre-exposure facilitation effect, using the same procedure reported by Kiernan & Westbrook (1993).
Figure 2.
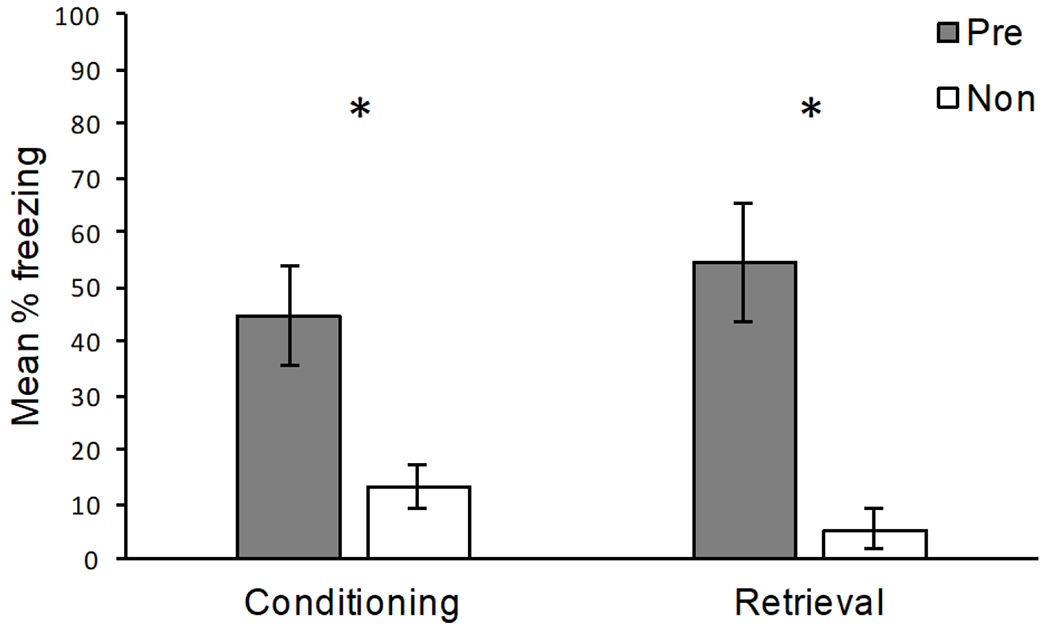
Results of Experiment 1. Mean percentage freezing (± SEM) for rats pre-exposed to the conditioning context (Pre) and rats that were exposed to a different context (Non). Conditioning: freezing during the 2 min post-shock period. Retrieval: freezing during the 2 min shock-free retrieval session 24 hours after conditioning. *Pre-exposed rats froze significantly more than non-exposed rats (p < .05).
Experiments 2a and 2b
The purpose of Experiments 2a and 2b was to determine if the RSC contributes to configural context representations. We therefore examined the effect of permanent RSC lesions on the context pre-exposure facilitation effect, using the same procedure as Experiment 1. If the RSC contributes to configural representations of context, either by providing input into the configural representation (see Rudy, 2009), or storing configural representations (e.g, Cowansage et al., 2014), then lesions of the RSC should reduce freezing in rats that are pre-exposed to the conditioning context. In Experiment 2a, lesions of the RSC were made prior to the start of the experiment. In Experiment 2b, they were made after the immediate shock conditioning session. The purpose of the post-training lesions (Experiment 2b) was to address the possibility that other brain regions are able to compensate for pre-training damage to the RSC (Experiment 2a).
Method
Subjects.
The subjects were 64 naïve adult male Long Evans rats, ~60 days old purchased from the same vendor as those in the previous experiment and maintained under the same conditions.
Surgery.
Subjects were anesthetized with isoflurane gas (1.5% - 3% in oxygen) and placed in a Kopf stereotaxis apparatus. The skin was retracted and holes were drilled through the skull above each of the intended lesion sites (see Table 1) based on the rat brain atlas of Paxinos and Watson (2009). Thirty-two rats received bilateral electrolytic lesions (2 mA, 15 s at each site) of the RSC prior to behavioral training. Control rats received sham lesions consisting of a craniotomy and shallow, non-puncturing burr holes to minimize damage to the underlying cortex. For Experiment 2a, rats were allowed to recover for at least 2 weeks prior to the pre-exposure phase of the experiment. In Experiment 2b, lesions occurred 24 hours after conditioning (i.e., immediate shock). Rats were allowed 14 days to recovery prior to the retrieval test.
Table 1.
Stereotaxic coordinates for restrosplenial cortex (RSC) lesions.
| AP | ML | DV |
|---|---|---|
| −2.0 | ± 0.3 | −2.0 and −2.7 |
| −3.5 | ± 0.4 | −2.0 and −2.7 |
| −5.0 | ± 0.4 and ± 1.0 | −2.0 and −2.7 (medial site) and −2.0 (lateral site) |
| −6.5 | ± 0.8 and ± 1.5 | −2.0 and −2.8 (medial site) and −3.4 (lateral site) |
| −8.0 | ± 1.6 and ± 2.4 | −2.5 (medial site) and −3.1 (lateral site) |
| −9.0 | ± 3.4 | −4.0 |
Note. All anterior-posterior (AP), medial-lateral (ML) and dorsal-ventral (DV) measurements are derived from bregma, midline, and skull surface, respectively (measurements are in mm).
Behavioral apparatus and procedures.
The apparatus and procedures were the same as those used in Experiment 1.
Behavioral observations and data analysis.
Freezing was assessed in the same manner as in Experiment 1, with the following exception. For Experiment 2a, both Conditioning and Retrieval were scored from DVD recordings by two observers (TPT and MYJ) unaware of experimental condition (Pre vs. Non) and lesion type (RSC vs. Sham). The ratings across observers were highly correlated (r > .92). In addition, we also report the percent freezing during the first and forth exposure to Context A for Group Pre, scored by TPT. For Experiment 2b, both Conditioning and Retrieval were scored from DVD recordings by two observers (TPT and NED) unaware of experimental condition (Pre vs. Non) and lesion type (RSC vs. Sham). The ratings across observers were highly correlated (r > .95).
Lesion verification and analysis.
After the behavioral procedures were completed, rats were deeply anesthetized with an overdose of sodium pentobarbital and transcardially perfused with 0.9% saline for 2 min, followed by 10% buffered formalin for 6 min. Coronal brain sections (60 μm) were collected using a freezing microtome and were Nissl-stained using thionin. Using a compound microscope (Axioskop I, Zeiss, Inc.), we identified gross tissue damage as necrosis, missing tissue, or marked thinning of the cortex. Outlines of the lesions were drawn onto digital images adapted from Paxinos and Watson (2009) using PowerPoint at 6 levels along the rostro-caudal extent of the RSC (−1.8, −3.0, −4.2, −5.4, −6.6, and −7.8 from bregma). At each level, area measurements where then made with ImageJ, including the total area of the target region and the area of the target region that exhibited gross tissue damage. From these measurements, we report the average percentage of RSC that was damaged. In addition, we report the average percentage of sections across the rostro-caudal plane that exhibited RSC damage (~22 sections collected for each rat), the average percentage of sections with damage outside the RSC, and the number of rats with damage to regions outside the RSC.
Results and Discussion
Histology.
Figure 3a shows a photomicrograph of a representative RSC lesion. In Figure 3b, lesion drawings from Experiment 2a are stacked onto a single atlas image for each of the 6 co-ordinates. Bilateral RSC damage was observed in all rats, and the average area of RSC damaged on each section analyzed was 61.38% (SEM = 3.76). The average RSC damage did not differ between pre-exposed and non pre-exposed rats (p = .96). Damage to the RSC was present on 99.5% (SEM = 0.32) of sections collected for each subject, indicating damage extended throughout the rostro-caudal extent of the RSC. There was minor damage outside the RSC in 15 rats (e.g., anterior cingulate, visual cortex, motor cortex, cingulum bundle, forceps major corpus callosum). In contrast to RSC damage, the minor damage outside RSC was present on only 36.15% (SEM = 6.57) of sections collected.
Figure 3.
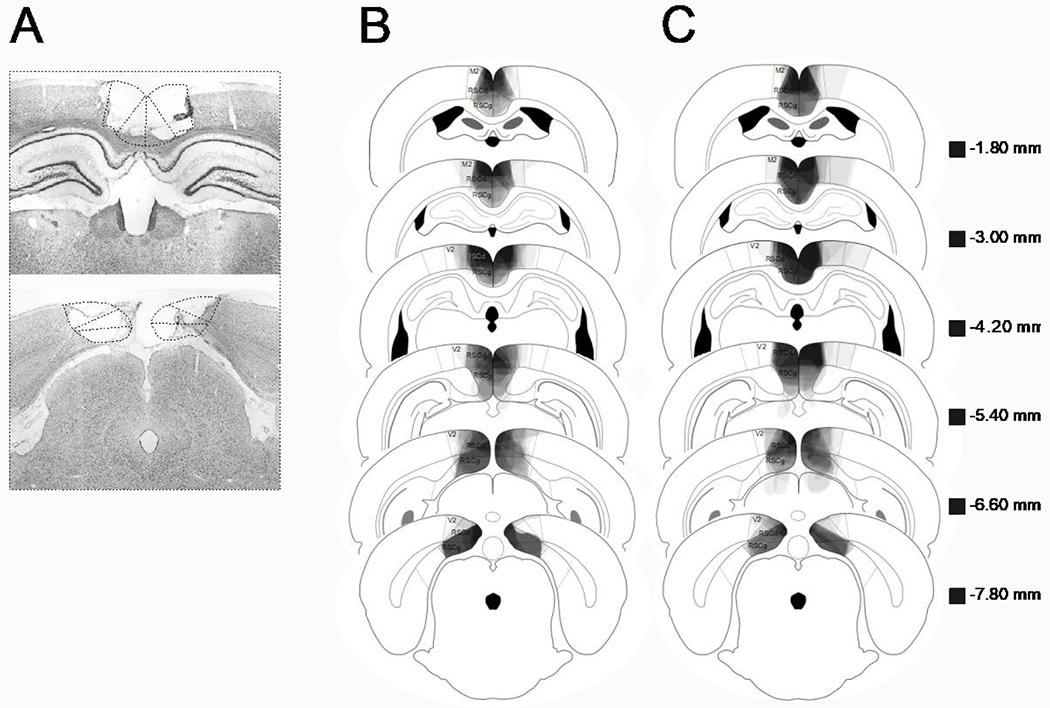
A: Photomicrograph of a representative RSC lesion. B (Experiment 2a) and C (Experiment 2b): drawings of lesions at six levels along the rostro-caudal extent of the RSC (−1.8, −3.0, −4.2, −5.4, −6.6, and −7.8 posterior to bregma). At each level, lesion drawings were stacked onto a single image. The darkness of an area indicates the number of lesions cases that include that area. Grey boxes (next to the bregma values) represents the expected darkness for overlap from all subjects. M2 = secondary motor cortex, RSCd = restrosplenial dysgranular, RSCg = retrosplenial granula, V2 = secondary visual cortex.
Lesions drawings for Experiment 2b are presented in Figure 3c. One RSC lesioned rat was removed due to extensive damage outside the RSC. The average area of RSC damaged on each section analyzed was 71.2% (SEM = 2.95), and once again pre-exposed and non pre-exposed rats did not differ (p = .40). There was minor damage outside the RSC in all rats (e.g., anterior cingulate, visual cortex, motor cortex, cingulum bundle, forceps major corpus callosum, superficial gray superior colliculus). Although RSC damage was present on 100% of sections collected, the extra-RSC damage was only present on 49.02% (SEM = 6.26) of sections collected. The majority of the extra-RSC damage was to areas of neocortex (visual, motor) that are not necessary for contextual fear conditioning (e.g., Kim & Fanselow, 1992).
Comparing across Experiments 2a and 2b, the average RSC damage did not differ between pre-exposed lesioned rats (p = .10), nor did the average percent of sections with RSC damage (p = .18) or extra-RSC damage (p = .21).
Behavior.
In Experiment 2a, neither Sham nor RSC lesioned rats froze during the first pre-exposure session to Context A, and there was virtually no freezing for either Sham (M = 2.92, Mdn = 0) or RSC (M = 2.08, Mdn = 0) rats during the 4th pre-exposure to Context A. Thus, pre-training lesions of the RSC had no measurable impact on unconditioned exploratory behavior.
Mean percentage freezing for Conditioning and Retrieval of Experiment 2a are presented in Figure 4. For Conditioning, Sham lesioned rats froze more when they were pre-exposed to Context A (Pre) compared to rats exposed to B (Non), [U(8,8) = 3.5, p < .01]. This replicates the context pre-exposure facilitation effect reported in Experiment 1. Likewise, during Conditioning, rats with lesions of the RSC also showed a context pre-exposure facilitation effect, [U(8,8) = 2, p < .01]. More so, Sham and RSC rats that were pre-exposed to Context A (Pre) did not reliably differ, [U(8,8) = 25.5, p = .49], nor did Sham and RSC rats that were exposed to Context B (Non) [U(8,8) = 19.5, p = .17]. For Conditioning, the median percent freezing for each group was: 33.33 (Sham – Pre), 30.00 (RSC – Pre), 5.00 (Sham – Non), and 0.00 (RSC – Non).
Figure 4.
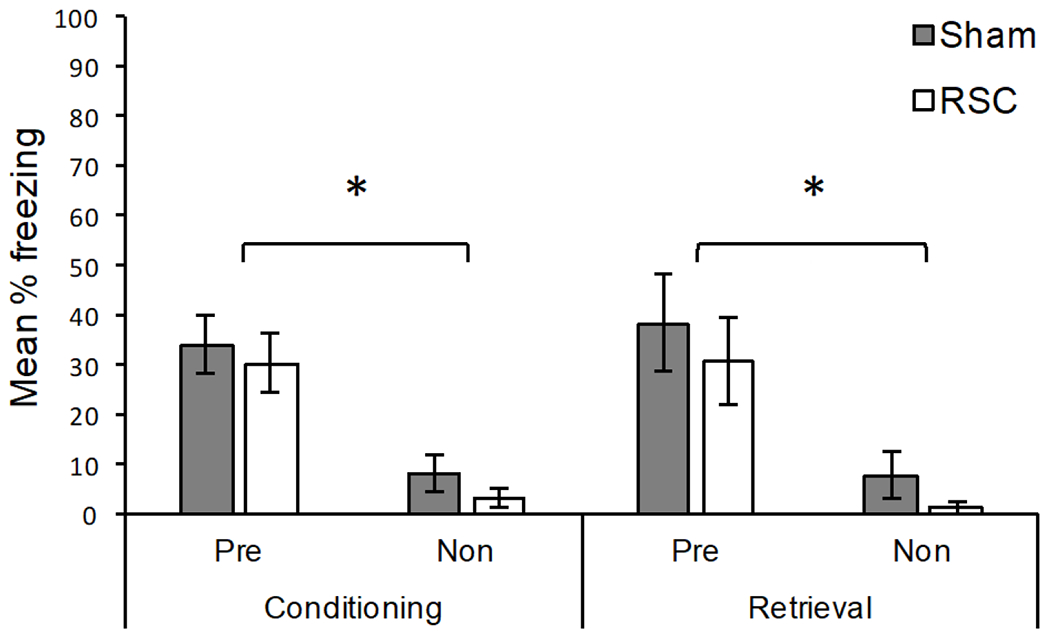
Results of Experiment 2a. Mean percentage freezing (± SEM) for rats pre-exposed to the conditioning context (Pre) and rats that were pre-exposed to a different context (Non). Sham = sham lesioned rats; RSC = retrosplenial cortex lesioned rats. Conditioning: freezing during the 2 min post-shock period. Retrieval: freezing during the 2 min shock-free retrieval session 24 hours after conditioning. *Pre-exposed rats froze significantly more than non-exposed rats (p < .05). Sham and RSC lesioned rats did not differ in either the pre-exposed non pre-exposed conditions.
An identical pattern of results was observed during the shock-free test that occurred 24 hours later (Retrieval). Both Sham, [U(8,8) = 8, p = .01], and RSC lesioned rats, [U(8,8) = 5, p < .01], froze more when they were pre-exposed to Context A (Pre) as opposed to Context B (Non). Sham and RSC lesioned rats did not differ in either the pre-exposed condition (Pre), [U(8,8) = 27, p = .60], nor the non pre-exposed condition (Non), [U(8,8) = 23, p = .28]. For Retrieval, the median percent freezing for each group was: 36.67 (Sham – Pre), 26.66 (RSC – Pre), 1.66 (Sham – Non), and 0.00 (RSC – Non).
In sum, pre-training lesions of the RSC had no impact on the context pre-exposure facilitation effect. During both Conditioning and Retrieval, a context pre-exposure facilitation effect was observed in both Sham and RSC lesioned rats. Further, RSC lesioned rats did not differ from Sham controls in either the pre-exposed or non pre-exposed conditions. Although the RSC lesions in the current study had no impact on behavior, the average size and extent of damage throughout the rostro-caudal plane of the RSC was comparable to previous studies from our laboratory in which lesions of the RSC produced deficits in contextual fear conditioning deficits (e.g., Keene & Bucci, 2008a; Todd et al., 2016). Thus, the failure to observe any behavioral deficit is unlikely due to insufficient lesions.
The results of Experiment 2b are presented in Figure 5. During Conditioning (left portion of Figure 5), both groups of rats that were pre-exposed to Context A (Pre) froze more than rats exposed to Context B (Non), ps < .05. The pre-exposed groups did not differ from each other, [U(8,7) = 20.00, p = .40], which was expected given that lesions did not occur until after Conditioning. Likewise, the groups exposed to Context B (Non) did not differ, [U(8,8) = 27.00, p = .65]. For Conditioning, the median percent freezing for each group was: 55.00 (Sham – Pre), 56.66 (RSC – Pre), 5.00 (Sham – Non), and 1.66 (RSC – Non).
Figure 5.
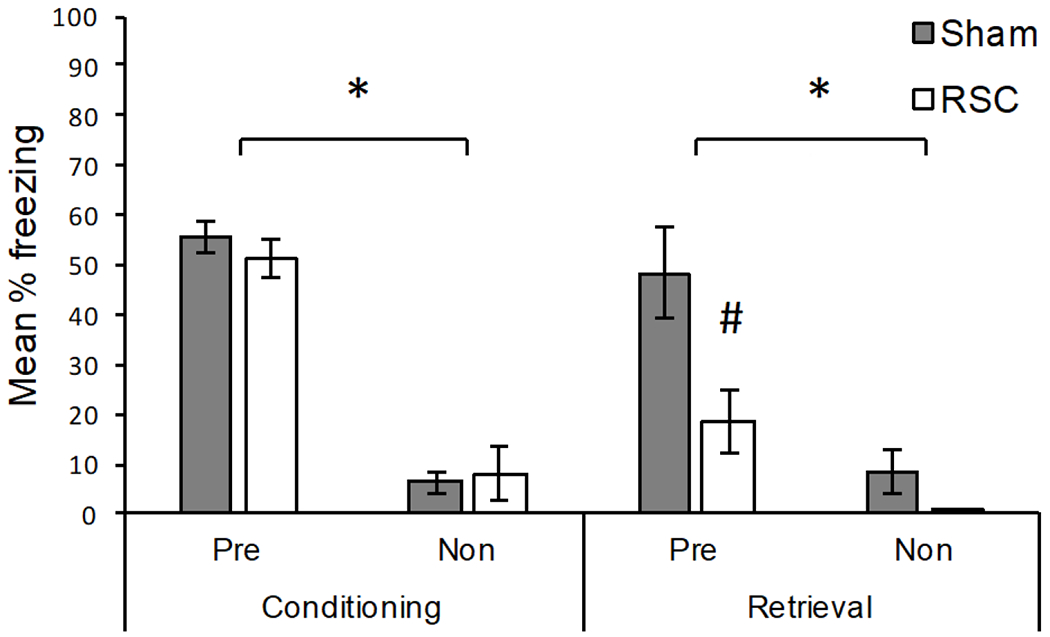
Results of Experiment 2b. Mean percentage freezing (± SEM) for rats pre-exposed to the conditioning context (Pre) and rats that were pre-exposed to a different context (Non). Sham = sham lesioned rats; RSC = retrosplenial cortex lesioned rats. Conditioning: freezing during the 2 min post-shock period. Retrieval: freezing during the 2 min shock-free retrieval test. Lesions occurred following conditioning. *Pre-exposed rats froze significantly more than non-exposed rats (p < .05). #Pre-exposed Sham rats froze significantly more than pre-exposed RSC lesioned rats.
Retrieval occurred following recovery from either Sham or RSC lesions. As shown in the right portion of Figure 5, RSC lesioned rats pre-exposed to Context A froze significantly less than pre-exposed Sham lesioned rats, [U(8,7) = 8.0, p < .05], indicating that the context pre-exposure facilitation effect was attenuated via damage to the RSC. Although freezing was reduced for RSC lesioned rats in Context A, RSC rats still showed significantly more freezing when they were pre-exposed to Context A (Pre) relative to when they were not (Non), [U(8,7) = 4.50, p < .01]. As expected, Sham rats pre-exposed to Context A (Pre) also froze more during Retrieval than rats exposed to Context B (Non), [U(8,8) = 4.0, p < .01]. Sham and RSC lesioned rats exposed to Context B (Non) did not differ, [U(8,8) = 18.50, p = .16]. For Retrieval, the median percent freezing for each group was: 46.66 (Sham – Pre), 13.33 (RSC – Pre), 1.66 (Sham – Non), and 0.00 (RSC – Non). Overall, the results of Experiment 3 demonstrate that post-training lesions of the RSC attenuate the context pre-exposure facilitation effect.
General Discussion
Although previous studies have demonstrated a role for the RSC in contextual fear conditioning, the exact nature of the RSC’s contribution has been unclear, in part because with most standard contextual fear conditioning methods conditioning can be supported, at least theoretically, by either an elemental or configural conditioning process (e.g., Rudy, 2009). The purpose of the present experiments was to determine if the RSC contributes to configural representations of context. We therefore examined the role of the RSC in contextual fear conditioning using the context pre-exposure facilitation method, a procedure known to produce conditioning to a configural context representation (Rudy & O’Reilly, 1999; Rudy et al., 2002). In Experiment 1, rats that were pre-exposed to the conditioning context froze more after receiving an immediate shock relative to rats that were exposed to a different context, demonstrating a context pre-exposure facilitation effect (see also Kiernan & Westbrook, 1993). Pre-training lesions of the RSC had no impact on the context pre-exposure facilitation effect (Experiment 2a). However, when lesions of the RSC occurred after training in Experiment 2b, the context pre-exposure facilitation effect was weakened. This finding encourages the perspective that the RSC contributes to configural context representations.
Similar to the findings reported here, the hippocampus is also necessary for the context pre-exposure facilitation effect. For example, temporary inactivation of the dorsal hippocampus during pre-exposure, immediate shock, or at the time of retrieval attenuates the context pre-exposure facilitation effect (Matus et al., 2004). The fact that both the hippocampus and the RSC contribute to the context pre-exposure facilitation effect suggests the possibility that coordinated hippocampal-cortical activity is required to produce and/or retrieve a configural context representation. One possibility is that cortical activity in the RSC converges on a hippocampal “index” (Eichenbaum, 2000; Teyler & DiScenna, 1986) and successful retrieval requires reinstatement of this cortical activity (Santoro & Frankland, 2014). Consistent with this notion, Tanaka et al. (2014) have recently demonstrated that when CA1 cells are active and then tagged during contextual fear conditioning, subsequent silencing of these cells not only impairs retrieval of contextual fear conditioning, but also attenuates neural activity in the RSC, as well as the entorhinal and perirhinal cortices. This suggests that successful retrieval of contextual fear conditioning requires reactivation of cortical cells, including those in the RSC.
The idea that RSC activity can coherently represent context is also supported by a recent series of studies by Cowansage et al. (2014). In these studies, neurons in the mouse RSC that were active during exploration of Context A were tagged to selectively express a channelrhodopsin variant (ChEF), prior to fear conditioning in Context A. In a final test, mice were exposed to Context C (a novel context) and the previously tagged RSC neurons were optogenetically activated. Activation of RSC neurons produced a freezing response in mice, and also resulted in increased cFos levels in the basal and central amygdala, areas critically involved in fear learning (Maren, 2001; Maren & Fanselow, 1997). In a separate experiment, activation of neurons in the RSC produced context-elicited freezing in mice that simultaneously had their hippocampus inactivated. Based on their results, Cowansage et al. (2014) suggested the RSC might store “unitary cellular representations of context” (pg. 5), although the training procedures did not isolate configural processing. Nevertheless, the current results support the conclusion of Cowansage et al. (2014) by demonstrating the RSC is necessary for contextual fear conditioning in a procedure that requires the formation of an integrated and cohesive context representation.
The current experiments focused on the context pre-exposure facilitation effect, considered a strong test of configural context conditioning (see Fanselow, 2010), because it has previously been suggested that contextual fear conditioning can be supported by either a configural (hippocampus) or an elemental (neocortex) process (Anagnostaras, Gale, & Fanselow, 2001; Maren, 2001; Maren, Aharonov, & Fanselow, 1997; Rudy, 2009; Rudy, Barrientos, & O’Reilly, 2002; Rudy, Huff, & Matus-Amat, 2004; Sutherland & Rudy, 1989). However, Fanselow (2010) has alternatively suggested that all contextual fear conditioning involves configural context processing, whether it occurs with the context pre-exposure method or more standard procedures. Part of this argument rests on the finding that pre-training lesions of the hippocampus (i.e., the configural system) results in quantitative as opposed to qualitative changes in contextual fear conditioning (Fanselow, 2010; see also Wiltgen et al., 2006). Instead of configural and elemental processes, Fanselow (2010) has instead suggested there are multiple (primary vs. alternative) pathways for context learning. The primary pathway is more efficient and typically dominates, thus post-training lesions are expected produce strong retrograde amnesia. Our current finding that post-training lesions of the RSC attenuate the context pre-exposure facilitation effect (Experiment 2b) thus suggests that the RSC is part of the primary contextual fear conditioning pathway.
According to Fanselow’s (2010) perspective, pre-training lesions of the primary system do not always impair conditioning because under some circumstances the alternative pathway is able to compensate. In Experiments 2a, pre-training lesions of the RSC had no impact on the context pre-exposure facilitation effect. Thus, it is possible that in the absence of the RSC, other systems are able to compensate and produce a strong representation of the context. However, it is also possible that low levels of fear observed in Experiment 2a made it difficult to detect a lesion effect. In Experiment 2a the level of freezing for Sham rats during Test 2 was about 10% lower than Experiment 2b, where a lesion effect was observed (compare Figures 4 and 5). Thus, although the findings from Experiment 2b suggest the RSC is part of the primary system, it is not clear if pre-training lesions of the RSC can in fact be overcome. Additional experiments are required to resolve this issue.
The present data suggest that the RSC contributes to contextual fear learning as part of a configural system, consistent with previous research demonstrating an important role of the RSC in processing multiple stimuli and forming associations between multiple stimuli in the environment (e.g., Keene & Bucci, 2008b; Robinson et al., 2012; Robinson et al., 2014; see also Bucci & Robinson, 2014). Nevertheless, more recent data suggests the RSC contributes to some forms of fear conditioning with individual cues (Kwapis et al., 2014, 2015; Robinson et al., 2012; Todd et al., 2016), a seemingly elemental process. However, it is important to note that procedures involving individual stimuli can be solved by either elemental or configural systems, and likewise procedures that involve multiple stimuli do not always require configural processing (e.g., Rescorla & Wagner, 1972; see also see Honey, Iordanova, & Good, 2014). Thus, it remains to be fully determined the degree by which the RSC contributes to elemental and/or configural processing across learning and memory paradigms, especially those involving discrete cues.
In sum, although previous studies have demonstrated that the RSC contributes to contextual fear conditioning, the current findings indicate that the RSC contributes to contextual fear conditioning through configural processing. This is because the current experiments have utilized the context pre-exposure facilitation method, which constitutes a strong test of configural processing during contextual fear conditioning. Overall, these data are generally consistent with the notion that one critical RSC function is to associate sensory stimuli in the environment (Bucci & Robinson, 2014) and the view that context memories are encoded and stored within a distributed hippocampal-cortical network (Santoro & Frankland, 2014).
Acknowledgments
This work was supported by a National Science Foundation Grant IOS1353137 (D.J.B.) and a National Institutes of Health Grant MH105125 (T.P.T.). We thank Dr. Robert Leaton and Dr. Meghan Eddy for valuable comments on a previous version of this manuscript. Experiment 2a was presented at the annual meeting of the Society for Neuroscience (2015).
References
- Albasser MM, Poirier GL, Warburton EC, & Aggleton JP (2007). Hippocampal lesions halve immediate-early gene protein counts in retrosplenial cortex: distal dysfunctions in a spatial memory system. European Journal of Neuroscience, 26, 1254–1266. [DOI] [PubMed] [Google Scholar]
- Anagnostaras SG, Gale GD, & Fanselow MS (2001). Hippocampus and contextual fear conditioning: recent controversies and advances. Hippocampus, 11, 8–17. [DOI] [PubMed] [Google Scholar]
- Anagnostaras SG, Maren S, & Fanselow MS (1999). Temporally graded retrograde amnesia of contextual fear after hippocampal damage in rats: within-subject examination. The Journal of Neuroscience, 19, 1106–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME (2010). The multiple forms of “context” in associative learning theory In Mesquita B, Feldman Barrett L, & Smith ER (Eds.), The mind in context (pp. 233–258). New York: The Guilford Press. [Google Scholar]
- Bucci DJ, Phillips RG, & Burwell RD (2000). Contributions of the postrhinal and perirhinal cortex to contextual information processing. Behavioral Neuroscience, 114, 882–894. [DOI] [PubMed] [Google Scholar]
- Bucci DJ, & Robinson S (2014). Toward a conceptualization of retrohippocampal contributions to learning and memory. Neurobiology of Learning and Memory, 116, 197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Donnan MD, Tronson NC, Guzmán YF, Gao C, Jovasevic V, & Radulovic J (2011). NMDA receptors in retrosplenial cortex are necessary for retrieval of recent and remote context fear memory. The Journal of Neuroscience, 31, 11655–11659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowansage KK, Shuman T, Dillingham BC, Chang A, Golshani P, & Mayford M (2014). Direct reactivation of a coherent neocortical memory of context. Neuron, 84, 432–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS (1980). Conditional and unconditional components of post-shock freezing in rats. Pavlovian Journal of Biological Sciences, 15, 177–182. [DOI] [PubMed] [Google Scholar]
- Fanselow MS (1986). Associative vs. topographical accounts of the immediate shock deficit in rats: Implications for the response selection rules governing species specific defensive reactions. Learning and Motivation, 17, 16–39. [Google Scholar]
- Fanselow MS (1990). Factors governing one-trial contextual conditioning. Animal Learning and Behavior, 18, 264–270. [Google Scholar]
- Fanselow MS (2000). Contextual fear, gestalt memories, and the hippocampus. Behavioural Brain Research, 110, 73–81. [DOI] [PubMed] [Google Scholar]
- Furtak SC, Wei SM, Agster KL, & Burwell RD (2007). Functional neuroanatomy of the parahippocampal region in the rat: The perirhinal and postrhinal cortices. Hippocampus, 17, 709–722. [DOI] [PubMed] [Google Scholar]
- Honey RC, Iordanova MD, & Good M (2014). Associative structures in animal learning: dissociating elemental and configural processes. Neurobiology of Learning & Memory, 108, 96–103. [DOI] [PubMed] [Google Scholar]
- Jacobs NS, Cushman JD, & Fanselow MS (2010). The accurate measure of fear memory in Pavlovian conditioning: Resolving the baseline issue. Journal of Neuroscience Methods, 190, 235–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene CS, & Bucci DJ (2008a). Contributions of the retrosplenial and posterior parietal cortices to cue-specific and contextual fear conditioning. Behavioral Neuroscience, 122, 89–97. [DOI] [PubMed] [Google Scholar]
- Keene CS, & Bucci DJ (2008b). Neurotoxic lesions of retrosplenial cortex disrupt signaled and unsignaled contextual fear conditioning. Behavioral Neuroscience, 122, 1070–1077. [DOI] [PubMed] [Google Scholar]
- Kiernan MJ, & Westbrook RF (1993). Effects of exposure to a to-be-shocked environment upon the rat’s freezing response: evidence for facilitation, latent inhibition, and perceptual learning. The Quarterly Journal of Experimental Psychology, 46B, 271–288. [DOI] [PubMed] [Google Scholar]
- Kim JJ, & Fanselow M (1992). Modality specific retrograde amnesia of fear following hippocampal lesions. Science, 256, 675–677. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y & Amaral DG (2003). Macaque monkey retrosplenial cortex: II. Cortical afferents. J. Comp. Neurol, 466, 48–79. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y & Amaral DG (2007). Macaque monkey retrosplenial cortex: III. Cortical efferents. J. Comp. Neurol, 502, 810–833. [DOI] [PubMed] [Google Scholar]
- Kwapis JL, Jarome TJ, Lee JL, Gilmartin MR, & Helmstetter FJ (2014). Extinguishing trace fear engages the retrosplenial cortex rather than the amygdala. Neurobiology of Learning and Memory, 113, 41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwapis JL, Jarome TJ, Lee JL, & Helmstetter FJ (2015). The retrosplenial cortex is involved in the formation of memory for context and trace fear conditioning. Neurobiology of Learning and Memory, 123, 110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landeira-Fernandez J, Fanselow MS, DeCola JP, & Kim JJ (1995). Effects of handling and context preexposure on the immediate shock deficit. Animal Learning & Behavior, 23, 335–339. [Google Scholar]
- Maren S (2001). Neurobiology of Pavlovian fear conditioning. Annu. Rev. Neurosci, 24, 897–931. [DOI] [PubMed] [Google Scholar]
- Maren S, Aharonov G, & Fanselow MS (1997). Neurotoxic lesions of the dorsal hippocampus and Pavlovian fear conditioning in rats. Behavioral Brain Research, 88, 261–274. [DOI] [PubMed] [Google Scholar]
- Maren S, & Fanselow MS (1997). The amygdala and fear conditioning: has the nut been cracked? Neuron, 16, 237–240. [DOI] [PubMed] [Google Scholar]
- Matus-Amat P, Higgins EA, Barrientos RM, & Rudy JW (2004). The role of the dorsal hippocampus in the acquisition and retrieval of context memory representations. The Journal of Neuroscience, 24, 2431–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, & Watson C (2009). The rat brain in stereotaxic coordinates. (Compact 6th ed.). San Diego, CA: Academic Press. [Google Scholar]
- Rescorla RA, & Wagner AR (1972). A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement In Black AH & Prokasy WF (Eds.), Classical conditioning II: current research and theory (pp.64–99). New York: Appleton-Century-Crofts. [Google Scholar]
- Robinson S, Keene CS, Iaccarino HF, Duan D, & Bucci DJ (2012). Involvement of retrosplenial cortex in forming associations between multiple sensory stimuli. Behavioral Neuroscience, 125, 578–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S, Poorman CE, Marder TJ, & Bucci DJ (2012). Identifaction of functional circuitry between retrosplenial and postrhinal cortices during fear conditioning. The Journal of Neuroscience, 32, 12076–12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S, Todd TP, Pasternak AR, Luikart BW, Skelton PD, Urban DJ, & Bucci DJ (2014). Chemogenetic silencing of neurons in the retrosplenial cortex disrupts sensory preconditioning. The Journal of Neuroscience, 34, 10982–10988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy JW (2009). Context representations, context functions, and the parahippocampal – hippocampal system. Learning & Memory, 16, 573–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy JW, Barrientos RM, & O’Reilly RC (2002). Hippocampal formation supports conditioning to memory of a context. Behavioral Neuroscience, 116, 530–538. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Huff NC, & Matus-Amat P (2004). Understanding contextual fear conditioning: insights from a two-process model. Neuroscience and Biobehavioral Reviews, 28, 675–685. [DOI] [PubMed] [Google Scholar]
- Rudy JW, & O’Reilly RC (2001). Conjunctive representations, the hippocampus, and contextual fear conditioning. Cognitive, affective, & Behavioral Neuroscience, 1, 66–82. [DOI] [PubMed] [Google Scholar]
- Rudy JW, & O’Reilly RC (1999). Contextual fear conditioning, conjunctive representations, pattern completion, and the hippocampus. Behavioral Neuroscience, 113, 867–880. [DOI] [PubMed] [Google Scholar]
- Rudy JW, & Matus-Amat P (2005). The ventral hippocampus supports a memory representation of context and contextual fear conditioning: implications for a unitary function of the hippocampus. Behavioral Neuroscience, 119, 154–163.\ [DOI] [PubMed] [Google Scholar]
- Sugar J Witter MP, van Strein NM, & Cappaert NL (2011). The retrosplenial cortex: Intrinsic connectivity and connections with the (para)hippocampal region in the rat. An interactive connectome. Frontiers in Neuroinformatics, 5, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland RS, & Rudy JW (1989). Configrual association theory: The role on the hippocampus in learning, memory and amnesia. Psychobiology, 17, 129–144. [Google Scholar]
- Stote DL, & Fanselow MS (2004). NMDA receptor modulation of incidental learning in Pavlovian context conditioning. Behavioral Neuroscience, 118, 253–257. [DOI] [PubMed] [Google Scholar]
- Tanaka KZ, Pevzner A, Hamidi AB, Nakazawa Y, Graham J, & Wiltgen BJ (2014). Cortical representations are reinstated by the hippocampus during memory retrieval. Neuron, 84, 347–354. [DOI] [PubMed] [Google Scholar]
- van Strein NM, Cappaert NL, Witter MP (2009). The anatomy of memory: An interactive overview of the parahippocampal – hippocampal network. Nature Reviews Neuroscience, 10, 272–282. [DOI] [PubMed] [Google Scholar]


