Abstract
Objective
The purpose of this study was to assess the effects of glucose fluctuation targeted intervention on neurologic function, independent living skills, and quality of life in type 2 diabetes patients following the first episode of cerebral infarction (CI).
Methods
This was a randomized control trial. Following confirmed cerebral infarction, 75 patients with type 2 diabetes were randomized into 2 groups: control group (n = 37) with usual care, focused on hemoglobin A1c (HbA1c) control, targeting A1c < 7%, and intervention group (n = 37) with usual care, focused on hemoglobin A1c (HbA1c) control, targeting A1c < 7%, and intervention group (
Results
After 6 months, data from 63 patients were analyzed (30 in the control group, 33 in the intervention group). There was no difference (P > 0.05) in the reduction of A1c between the 2 groups, but the reductions of LAGE (P > 0.05) in the reduction of A1c between the 2 groups, but the reductions of LAGE (P > 0.05) in the reduction of A1c between the 2 groups, but the reductions of LAGE (P > 0.05) in the reduction of A1c between the 2 groups, but the reductions of LAGE (P > 0.05) in the reduction of A1c between the 2 groups, but the reductions of LAGE (P > 0.05) in the reduction of A1c between the 2 groups, but the reductions of LAGE (P > 0.05) in the reduction of A1c between the 2 groups, but the reductions of LAGE (P > 0.05) in the reduction of A1c between the 2 groups, but the reductions of LAGE (P > 0.05) in the reduction of A1c between the 2 groups, but the reductions of LAGE (
Conclusion
Glucose fluctuation targeted intervention can improve nerve function for patients with T2DM following the first CI episode. This trial is registered with NCT03932084.
1. Introduction
Diabetes mellitus is an independent risk factor for cerebral infarction. People with diabetes have more than double the risk for cerebrovascular diseases, after controlling for confounding, relative to individuals without the disease [1]. Interestingly, even in patients without known diabetes, hyperglycemia occurs in 20-40% following acute ischemic stroke [2].
The relationship between HbA1c and risk of microvascular disease in patients with diabetes is well-established; the impact of tighter glycemic control on macrovascular complications, however, is not as robust [3–5]. Since HbA1c neither captures glycemic variation nor does it provide any information on glucose dynamics [6], we propose that the use of HbA1c alone is not sufficient to assess the risk for macrovascular complications and the metabolic status of patients with type 2 diabetes mellitus (T2DM).
Glucose fluctuation, also known as glucose variability (GV), refers to variations in blood glucose levels and, more broadly, to blood glucose oscillations that occur throughout the day. These may include periods of hypoglycemia and postprandial hyperglycemia, as well as day-to-day glycemic variations [7]. Recent studies have demonstrated that compared with sustained hyperglycemia, increased glycemic variation has been considered a major risk factor in the development of diabetes macrovascular complications [8–10]. Utilizing a continuous glucose monitoring system (CGMS), an Italian observational study with a cohort of 1,409 subjects with T2DM aged 56–74 years followed up to 10 years found that fasting plasma glucose variation coefficient (CV-FPG) was an independent predictor of cardiovascular mortality [11], even in patients with similar mean glucose levels [12]. A recent study [13] revealed that patients with elevated glycemic variability had significantly higher SYNTAX scores, an indicator of coronary artery lesion severity. Indeed, SYNTAX scores remained significantly associated with high GV, independent of HbA1c. There is a significant association between glycemic fluctuation and the incidence of diabetes macrovascular complications. Therefore, understanding the impact of glycemic fluctuations on diabetic macroangiopathy could be inform strategy for prevention and treatment of macroangiopathy in patients with diabetes.
Previous studies have been observational. To our knowledge, however, there has been no prospective intervention study confirming the effects of glucose fluctuation targeted management on the prognosis of T2DM patients following CI. The present randomized control trial sought to establish glucose fluctuation as an important target for management on metabolic index, neurologic function, and quality of life in T2DM patients following CI.
2. Methods
2.1. Participants
Participants were recruited between February 2017 and July 2017 from the Departments of Neurology and Endocrinology at the Hospital on Integration of Chinese and Western Medicine, an affiliate of the Nanjing University of Chinese Medicine. One hundred and four participants with a history of T2DM following CI were recruited. All participants underwent a medical examination, and a total of eighty-four participants fulfilled the inclusion criteria. Finally, 75 participants were willing to participate in this project.
2.2. Inclusion and Exclusion Criteria
The inclusion criteria were subjects (1) aged ≥18 years; (2) with CI within one month, diagnosed by magnetic resonance imaging (MRI) or computed tomography (CT) according to 1995 acute cerebral infarction diagnosis standards promulgated by the Fourth National Cerebrovascular Disease Conference [14]; and (3) having T2DM (as defined by the WHO diagnostic criteria in 1999) [15]. Exclusion criteria were (1) coexisting acute complications of diabetes including diabetic ketoacidosis (DKA), hyperglycemic hyperosmolar syndrome (HHS), and metabolic acidosis; (2) severe comorbid chronic complications of diabetes; (3) active malignancy; (4) subjects with mental illness and communication disorders; and (5) those actively participating in other research studies.
2.3. Sample Size
Sample size was calculated considering the expected change of the NIHSS score of the participants. NIHSS score was the primary study endpoint; our previous preliminary experiment has reported a reduction of the NIHSS score by 2.18. Sample size was calculated to have 90% power to detect a moderate 2.0-SD difference in the NIHSS score, with an alpha/α value of 0.05. Additional participants were recruited until the required number was achieved (n = 23) per group, with an anticipated dropout rate of 20%. Thus, we planned to recruit 66 subjects. Among 84 eligible participants, 75 subjects finally participated in this study.
2.4. Trial Design and Randomization
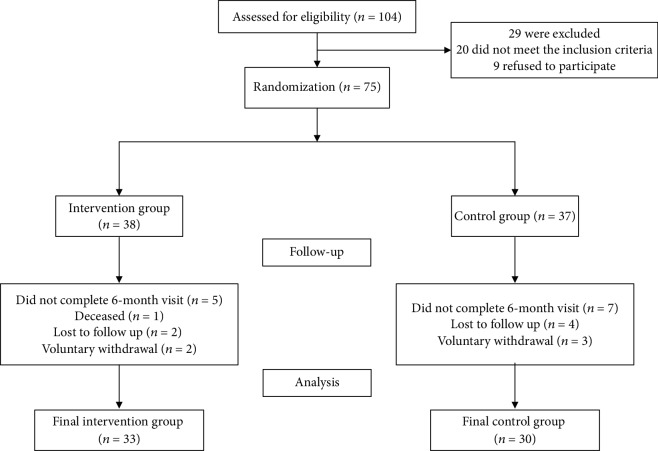
A total of 75 participants met inclusion criteria and were willing to participate in the study. Participants were randomized into two groups: (1) control group (n = 37) and (2) intervention group (n = 38) (Figure 1). The study was approved by the ethics committee of the Affiliated Hospital of Integrated Traditional Chinese and Western Medicine. Participants provided written informed consent prior to enrollment. All methods were carried out in accordance with approved guidelines and regulations.
Figure 1.
Flow diagram of study enrollment.
2.5. Scales
2.5.1. National Institutes of Health Stroke Scale (NIHSS)
The NIHSS is an impairment scale used to measure stroke severity. It was originally developed in 1989 [16] and is now widely used in clinics and is recommended as a valid tool to assess stroke severity in emergency departments.
The NIHSS includes the following domains: level of consciousness, sensory, neglect, visual field, gaze, facial palsy, motor arm, motor leg, limb ataxia, language, and dysarthria. Each domain is scored on an ordinal scale ranging from 0 to 2, 0 to 3, or 0 to 4. Item scores are summed to a total score ranging from 0 to 42 (the higher the score, the more severe the stroke).
2.5.2. Modified Rankin Scale (MRS)
Stroke outcome is most commonly rated by the modified Rankin scale [17] because of its well-established validity and rapid application; it can discriminate clinically relevant levels of disability and recovery in clinical trials [18–20]. We used a modified Rankin scale [21] to create a more comprehensive assessment of CI patients' independence and living ability to also include physical function and activities of daily living (ADLs). The 0-6 Likert scale is as follows: 0, no symptoms; 1, no significant disability; 2, slight disability; 3, moderate disability; 4, moderately severe disability; 5, severe disability; and 6, dead. The higher the score, the worse the patient's prognosis. When evaluating prognosis, a score≦2 was classified as a “good”.
2.5.3. Stroke Impact Scale (SIS)
The Stroke Impact Scale is a specific scale that evaluates disability and health-related quality of life after stroke [22]. The scale has been translated into Chinese, with well-validated reliability and sensitivity [23]. The overall Cronbach coefficient is 0.923, and the test-retest reliability is 0.712-0.912, accurately reflecting quality of life in patients with CI as well as and potential change over time. The SIS includes the following eight domains: strength, memory, thinking, emotion, communication and ADLs, mobility, hand function, and participation. A total of 8 domains with 59 items are scored on a 5-point scale, of which the sixth, eighth, and ninth questions of the emotional dimension are reversed, and the other items are positive. Each domain scores range from 0 to 100 and are calculated using the following equation: calculated score = [(actual score − the lowest possible score in this domain)/(the difference between the highest possible score and the lowest score in this domain)] × 100. The total score of the SIS scale is the sum of all domains. A higher score indicates better quality of life and less functional damage.
2.6. Study Procedure
2.6.1. Control Group
The control group received usual care as follows:
(1) During Hospitalization.
Monitor subjects' blood glucose by point-of-care capillary blood glucose testing (Yueyou II, Yuwell) during hospitalization (fasting, two-hour postprandial, and bedtime) and calculate as the difference between the highest and the lowest blood glucose values during one day (LAGE), the ratio of standard deviation and mean (CV-FPG)
Individual diabetes education is the one-on-one education for patients and their families by diabetes specialist nurses. Educational content takes 60 minutes to deliver and includes skills related to diabetes self-management, basic knowledge of diabetes, diet, exercise, medication, blood glucose monitoring, risks of glucose fluctuations, and hypoglycemia. Insulin self-administration skills were taught and reinforced to those patients requiring insulin as part of their management plan
Teaching patients and their families to use blood glucose meters and correctly record results. The diabetes specialist nurses demonstrate correct methods for self-monitoring blood glucose, explain the operation precautions, and ask the patient or family to perform repeated operation training under supervision until skills are mastered
(2) During Discharge. Patients were given standard hospital discharge instructions, home blood glucose monitors, test strips, and log books and were asked to monitor their blood glucose 5 times (fasting, 2 hours postprandially, and before sleep) daily following hospital discharge.
(3) Follow-Up. Within-range blood sugars did not prompt intervention (fasting plasma glucose (FPG) < 7 mmol/L(126 mg/dL), 2hPG < 10 mmol/L (180 mg/dL), and A1c < 7%, no hypoglycemia). If hyper- or hypoglycemia did occur, researchers (diabetes educators) explored potential causes with each patient. Causes related to lifestyle (such as dietary indiscretion and unanticipated exertion) prompted exploration of self-care behavioral solutions between the patient and the educator. For an absent obvious cause, physician endocrinology referral was made for potential medication adjustment and further evaluation. Participants received telephone follow-up one week following discharge; thereafter, follow-up was conducted monthly for six months.
2.6.2. Intervention Group
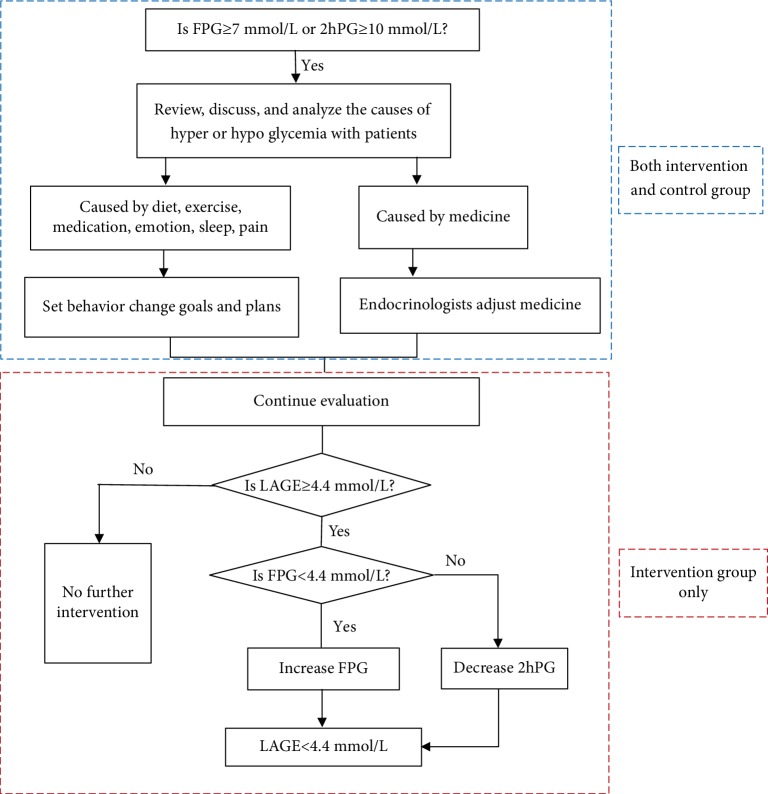
In addition to control group intervention parameters, we also added LAGE < 80 mg/dL as one management goal. Even if FBG, 2hPG, and A1c were all within the target range, LAGE ≥ 80 mg/dL prompted initial careful assessment of dietary intake, physical activity, and activities of daily living. If out-of-range LAGE was attributed to lifestyle, researchers would work with patients to find a self-care behavioral solution for the glycemic fluctuation and set behavioral goals. For an absent apparent cause for abnormal LAGE, patients were referred to a physician endocrinologist for further guidance. If a patient's FPG was <80 mg/dL, the physician would adjust the medications for the patient to increase his or her FPG to minify the LAGE; otherwise, the doctor would increase the patient's dosage or medications to decrease the 2hPG. At subsequent follow-up visit, we evaluated glucose fluctuation and target completion. Figure 2 details follow-up visit procedures. As an example, if a patient's FPG was 5 mmol/L (90 mg/dL), and his 2hPG was 9.5 mmol/L (171 mg/dL), he would receive no intervention if he was in the control group, but if he was in the intervention group, the team would aim to reduce his 2hPG, thus targeting a LAGE < 80 mg/dL.
Figure 2.
Flow diagram for two groups.
2.7. Data Analysis
All analyses were performed using IBM SPSS version 22 (IBM Corp, Armonk, NY, USA). Data was presented as mean ± SD; the significance of differences in outcomes between the two groups was assessed using t-tests when the data was normally distributed and using nonparametric tests of two independent samples when the data was not normally distributed. Within-group differences were tested by the Wilcoxon signed-rank or paired t-test. Categorical variables were tested by chi-square test. Statistical significance was established at the P < 0.05 level.
3. Results
A total of 75 participants were enrolled in the study and were randomly assigned to the intervention group (n = 38) or the control group (n = 37). By study's end, a total of 63 participants completed the 6-month intervention (16% dropout rate). The reasons for dropping out were the voluntary withdrawal and the loss to follow-up (n = 5, n = 6), and one participant was deceased (Figure 1).
As shown in Table 1, the two groups did not differ significantly at baseline. Following intervention, there was no difference in HbA1c between the intervention group and the control group (P > 0.05) (Table 2); the difference values of LDL-c (P = 0.046), 2hPG (P = 0.041), and LAGE (P = 0.030) were significantly decreased, whereas the 1,5-AG (P = 0.023) level was significantly increased in the intervention group compared to the control group (Figures 3–5).
Table 1.
Demographic characteristics and clinical variables at baseline (n = 63).
| Characteristics | Control n = 30 |
Intervention n = 33 |
χ 2 | P value |
|---|---|---|---|---|
| Gender, n (%) | 0.551 | 0.458 | ||
| Male | 20 (66.7%) | 19 (57.6%) | ||
| Female | 10 (33.3%) | 14 (42.4%) | ||
| Family history of CI, n (%) | 0.002 | 0.961 | ||
| No | 18 (60.0%) | 20 (60.6%) | ||
| Yes | 12 (40.0%) | 13 (39.4%) | ||
| Family history of diabetes, n (%) | 0.148 | 0.701 | ||
| No | 16 (53.3%) | 16 (48.5%) | ||
| Yes | 14 (46.7%) | 17 (51.5%) | ||
| Marital status, n (%) | 0.223 | 0.223 | ||
| Not married | 0 (0.0%) | 0 (0.0%) | ||
| Married | 28 (93.3%) | 33 (100%) | ||
| Divorced/widowed | 2 (6.7%) | 0 (0.0%) | ||
| Educational level, n (%) | 5.418 | 0.228 | ||
| Illiterate | 0 (0.0%) | 0 (0.0%) | ||
| Primary school | 4 (13.3%) | 3 (9.1%) | ||
| Middle school | 16 (53.4%) | 18 (54.6%) | ||
| High school/secondary school | 4 (13.3%) | 10 (30.3%) | ||
| Associate | 5 (16.7%) | 1 (3.0%) | ||
| Bachelor and above | 1 (3.3%) | 1 (3.0%) | ||
| Medical fee payment method, n (%) | 0.007 | 0.933 | ||
| Free medical care | 0 (0.0%) | 0 (0.0%) | ||
| Self-payment | 2 (6.7%) | 1 (3.0%) | ||
| National health insurance | 28 (93.3%) | 32 (97.0%) | ||
| Career, n (%) | 1.371 | 0.242 | ||
| In-service | 7 (23.3%) | 4 (12.1%) | ||
| Nonservice | 23 (76.7%) | 29 (87.9%) | ||
| Comorbidities, n (%) | 2.618 | 0.106 | ||
| No | 4 (13.3%) | 10 (30.3%) | ||
| Yes | 26 (86.7%) | 23 (69.7%) | ||
| Diabetes complications, n (%) | 1.801 | 0.18 | ||
| No | 15 (50.0%) | 11 (33.3%) | ||
| Yes | 15 (50.0%) | 22 (66.7%) | ||
| Injecting insulin, n (%) | 0.716 | 0.397 | ||
| No | 15 (50.0%) | 13 (39.4%) | ||
| Yes | 15 (50.0%) | 20 (60.6%) | ||
| Smoking, n (%) | 0.682 | 0.409 | ||
| No | 23 (76.7%) | 28 (84.8%) | ||
| Yes | 7 (23.3%) | 5 (15.2%) | ||
| Drinking, n (%) | 3.067 | 0.08 | ||
| No | 21 (70.0%) | 29 (87.9%) | ||
| Yes | 9 (30%) | 4 (12.1%) | ||
| Exercise, n (%) | 0.05 | 0.824 | ||
| No | 11 (36.7%) | 13 (39.4%) | ||
| Yes | 19 (63.3%) | 20 (60.6%) | ||
| Age (years) | 65.37 ± 9.01 | 64.03 ± 6.93 | 0.663 | 0.51 |
| Weight (kg) | 67.81 ± 11.94 | 66.28 ± 10.29 | 0.546 | 0.587 |
| BMI (kg/m2) | 25.05 ± 3.37 | 25.03 ± 3.26 | 0.015 | 0.988 |
| WC (cm) | 89.00 ± 10.21 | 87.67 ± 10.37 | 0.513 | 0.61 |
| HC (cm) | 94.85 ± 7.03 | 96.14 ± 5.94 | -0.787 | 0.435 |
| SBP (mmHg) | 148.10 ± 20.41 | 144.27 ± 20.53 | 0.741 | 0.461 |
| DBP (mmHg) | 85.10 ± 10.20 | 82.42 ± 20.19 | 0.654 | 0.516 |
| TC (mmol/L) | 4.32 ± 1.02 | 4.20 ± 1.39 | 0.38 | 0.705 |
| TG (mmol/L) | 1.83 ± 1.16 | 1.36 ± 0.90 | 1.874 | 0.061 |
| HDL-c (mmol/L) | 1.21 ± 0.39 | 1.35 ± 0.54 | 1.572 | 0.116 |
| LDL-c (mmol/L) | 2.62 ± 0.97 | 2.63 ± 1.11 | -0.026 | 0.98 |
| FPG (mmol/L) | 8.92 ± 3.27 | 7.54 ± 2.72 | 1.809 | 0.076 |
| 2hPG (mmol/L) | 12.40 ± 3.58 | 10.66 ± 3.66 | 1.894 | 0.063 |
| HbA1c (%) | 8.15 ± 2.46 | 7.91 ± 2.16 | 0.404 | 0.688 |
| 1,5-AG (μg/mL) | 90.14 ± 36.69 | 97.81 ± 34.85 | -0.841 | 0.402 |
| CV-FPG (%) | 0.20 ± 0.12 | 0.19 ± 0.11 | 0.277 | 0.783 |
| LAGE (mmol/L) | 5.96 ± 2.16 | 5.84 ± 2.21 | 0.202 | 0.841 |
The data are shown as n (%) and mean ± SD. CI: cerebral infarction; BMI: body mass index; WC: waist circumference; HC: hip circumference; SBP: systolic blood pressure; DBP: diastolic blood pressure; TC: total cholesterol; TG: triglyceride; HDL-c: high-density lipoprotein cholesterol; LDL-c: low-density lipoprotein cholesterol; FPG: fasting plasma glucose; 2hPG: 2-hour postprandial blood glucose; HbA1c: hemoglobin A1c; 1,5-AG: 1,5-anhydroglucitol; CV-FPG: fasting plasma glucose variation coefficient; LAGE: largest amplitude of glycemic excursions.
Table 2.
Before and after glucose fluctuation target management between-group comparison of changes on glycemic control and lipid profile variables (postintervention baseline).
| Variables | Control n = 30 |
Intervention n = 33 |
T/Z | P value |
|---|---|---|---|---|
| Weight (kg) | 0.97 ± 2.47 | 0.94 ± 4.00 | -0.031 | 0.975 |
| BMI (kg/m2) | 0.38 ± 0.91 | 0.40 ± 1.52 | 0.079 | 0.938 |
| WC (cm) | 1.80 ± 6.59 | 0.70 ± 6.33 | -0.639 | 0.525 |
| HC (cm) | 3.76 ± 6.55 | 2.41 ± 6.69 | -0.765 | 0.447 |
| SBP (mmHg) | 4.52 ± 17.40 | 1.58 ± 18.13 | -0.6 | 0.551 |
| DBP (mmHg) | 1.83 ± 12.67 | 1.71 ± 17.16 | -0.027 | 0.978 |
| TC (mmol/L) | 0.26 ± 0.64 | −0.06 ± 1.06 | -1.331 | 0.189 |
| TG (mmol/L) | −0.31 ± 1.11 | 0.17 ± 0.99 | 1.686 | 0.098 |
| HDL-c (mmol/L) | 0.17 ± 0.23 | −0.12 ± 0.46 | -1.805 | 0.077 |
| LDL-c (mmol/L) | 0.25 ± 0.57 | −0.12 ± 0.77 | -2.043 | 0.046∗ |
| FPG (mmol/L) | −0.89 ± 2.60 | −0.34 ± 3.03 | 0.734 | 0.466 |
| 2hPG (mmol/L) | 0.79 ± 3.70 | −1.35 ± 3.65 | -2.092 | 0.041∗ |
| HbA1c (%) | −0.61 ± 1.75 | −0.65 ± 1.33 | 0.143 | 0.887 |
| 1,5-AG (μg/mL) | 21.64 ± 47.81 | 49.65 ± 42.10 | -2.342 | 0.023∗ |
| CV-FPG (%) | −0.05 ± 0.31 | −0.01 ± 0.15 | 0.916 | 0.378 |
| LAGE (mmol/L) | 0.38 ± 2.91 | −2.01 ± 2.13 | 2.324 | 0.030∗ |
Data are shown as mean ± SD. Statistically significant figures are indicated by symbols, ∗P < 0.05.
Figure 3.
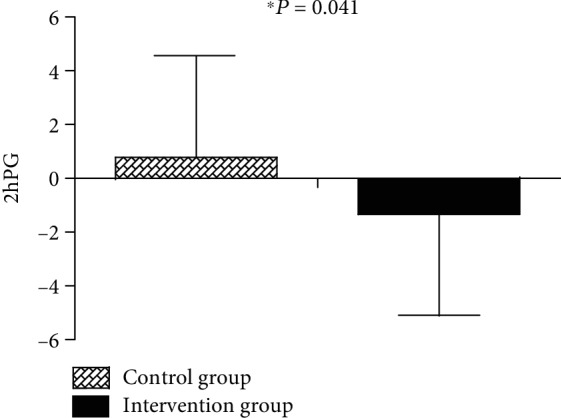
Changes in 2hPG from baseline to 6 months between the control and glucose fluctuation target management groups.
Figure 4.
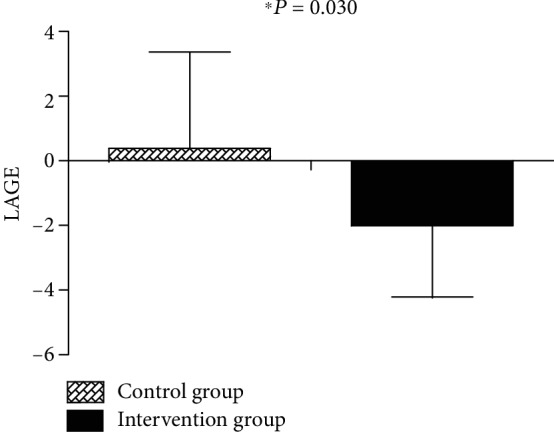
Changes in LAGE from baseline to 6 months between the control and glucose fluctuation target management groups.
Figure 5.
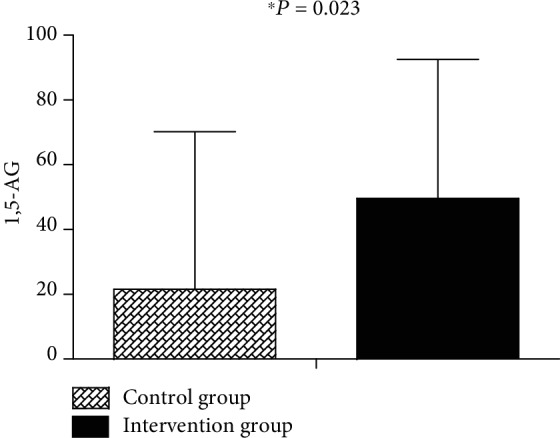
Changes in 1,5-AG from baseline to 6 months between the control and glucose fluctuation target management groups.
Within the intervention group, 2hPG (P = 0.041), HbA1c (P = 0.011), and LAGE (P = 0.015) were significantly reduced, whereas the 1,5-AG was significantly improved (P < 0.001) after 6 months (Table 3).
Table 3.
Comparisons of glycemic control and lipid profile variables within the glucose fluctuation target management group.
| Variables | Preintervention | Postintervention | T/Z | P value |
|---|---|---|---|---|
| Weight (kg) | 66.28 ± 10.29 | 67.22 ± 9.91 | -1.348 | 0.187 |
| BMI (kg/m2) | 25.03 ± 3.26 | 25.44 ± 3.43 | -1.523 | 0.138 |
| WC (cm) | 87.44 ± 10.45 | 88.14 ± 11.36 | -0.625 | 0.536 |
| HC (cm) | 95.86 ± 5.81 | 98.27 ± 9.43 | -2.035 | 0.050 |
| SBP (mmHg) | 144.00 ± 21.08 | 145.58 ± 17.08 | -0.486 | 0.631 |
| DBP (mmHg) | 82.06 ± 20.79 | 83.77 ± 12.99 | -0.555 | 0.583 |
| TC (mmol/L) | 4.20 ± 1.39 | 4.14 ± 1.13 | 0.308 | 0.760 |
| TG (mmol/L) | 1.36 ± 0.90 | 1.51 ± 1.40 | -0.805 | 0.427 |
| HDL-c (mmol/L) | 1.35 ± 0.54 | 1.34 ± 0.27 | 0.148 | 0.884 |
| LDL-c (mmol/L) | 2.63 ± 1.11 | 2.50 ± 0.99 | 0.875 | 0.389 |
| FPG (mmol/L) | 7.54 ± 2.72 | 7.20 ± 2.45 | 0.630 | 0.533 |
| 2hPG (mmol/L) | 10.66 ± 3.66 | 9.30 ± 2.40 | 2.132 | 0.041∗ |
| HbA1c (%) | 7.91 ± 2.16 | 7.26 ± 1.81 | 2.722 | 0.011∗ |
| 1,5-AG (μg/mL) | 96.76 ± 34.87 | 148.78 ± 31.12 | -7.270 | <0.001∗ |
| CV-FPG (%) | 0.19 ± 0.11 | 0.15 ± 0.12 | 0.951 | 0.348 |
| LAGE (mmol/L) | 5.84 ± 2.21 | 3.98 ± 2.32 | 2.545 | 0.015∗ |
Data are shown as mean ± SD, ∗P < 0.05 for baseline vs. after 6-month intervention within-group.
Within the control group, hip circumference (HC), high-density lipoprotein cholesterol (HDL-c), LDL-c, and 1,5-AG were improved compared to baseline (P < 0.001) (Table 4).
Table 4.
Comparisons of glycemic control and lipid profile variables within the control group.
| Variables | Preintervention | Postintervention | T/Z | P value |
|---|---|---|---|---|
| Weight (kg) | 66.05 ± 11.92 | 67.02 ± 11.86 | -1.962 | 0.061 |
| BMI (kg/m2) | 24.45 ± 3.16 | 24.82 ± 3.21 | -2.060 | 0.050 |
| WC (cm) | 87.20 ± 9.56 | 89.00 ± 10.59 | -1.366 | 0.185 |
| HC (cm) | 93.74 ± 6.53 | 97.50 ± 7.38 | -2.871 | 0.008∗ |
| SBP (mmHg) | 145.35 ± 18.71 | 149.87 ± 23.81 | -1.247 | 0.226 |
| DBP (mmHg) | 83.74 ± 9.08 | 85.57 ± 15.76 | -0.691 | 0.497 |
| TC (mmol/L) | 4.41 ± 1.03 | 4.67 ± 0.97 | -2.053 | 0.051 |
| TG (mmol/L) | 1.94 ± 1.22 | 1.63 ± 0.83 | 1.387 | 0.178 |
| HDL-c (mmol/L) | 1.22 ± 0.41 | 1.39 ± 0.37 | -3.638 | 0.001∗ |
| LDL-c (mmol/L) | 2.68 ± 0.99 | 2.94 ± 0.84 | -2.239 | 0.035∗ |
| FPG (mmol/L) | 8.48 ± 3.28 | 7.59 ± 2.82 | 1.721 | 0.098 |
| 2hPG (mmol/L) | 12.25 ± 3.72 | 12.77 ± 4.21 | -0.688 | 0.499 |
| HbA1c (%) | 7.92 ± 2.54 | 7.31 ± 1.58 | 1.985 | 0.059 |
| 1,5-AG (μg/mL) | 89.33 ± 40.20 | 110.97 ± 34.41 | -2.217 | 0.037∗ |
| CV-FPG (%) | 0.20 ± 0.12 | 0.16 ± 0.88 | 1.024 | 0.313 |
| LAGE (mmol/L) | 5.96 ± 2.16 | 6.40 ± 3.08 | -0.445 | 0.662 |
Data are shown as mean ± SD, ∗P < 0.05 for baseline vs. after 6-month within-group.
There was no difference in the NIHSS score between the groups (P > 0.05) at baseline; after 6 months, the NIHSS score was significantly lower in the intervention group compared to the control group (P = 0.012) (Table 5).
Table 5.
Between-group differences in the score of the NIHSS and MRS after 6 months.
| Control (n = 30) | Intervention (n = 33) | P value | ||
|---|---|---|---|---|
| NIHSS | After | 3.50 ± 2.24 | 2.35 ± 0.81 | |
| Change | −2.04 ± 1.10 | −2.90 ± 1.33 | 0.012∗ | |
|
| ||||
| MRS | After | 1.53 ± 0.90 | 1.48 ± 0.76 | |
| Change | −0.80 ± 0.65 | −0.91 ± 0.63 | 0.509 | |
Data are shown as mean ± SD, ∗P < 0.05. NIHSS: National Institutes of Health Stroke Scale; MRS: modified Rankin scale.
There was no significant difference in the scores of each dimension and total scores of SIS between the two groups before intervention (P > 0.05). After 6 months, the control group had a total score of 559.43 ± 112.53, and the intervention group had a total score of 615.47 ± 87.94. There was no statistically significant difference between the two groups. However, in the two dimensions of strength and hand function, the intervention group had a statistically significant better performance (P < 0.05) (Table 6).
Table 6.
Comparisons of various dimensions of SIS between groups after 6 months.
| Variables | Control (n = 30) | Intervention (n = 33) | P value | |
|---|---|---|---|---|
| Total score | After | 559.43 ± 112.53 | 615.47 ± 87.94 | |
| Change | 59.11 ± 44.73 | 89.53 ± 70.43 | 0.141 | |
|
| ||||
| Strength | After | 57.08 ± 19.03 | 70.83 ± 19.09 | |
| Change | 8.75 ± 15.27 | 16.67 ± 20.67 | 0.014∗ | |
|
| ||||
| Memory and thinking | After | 78.57 ± 16.86 | 78.23 ± 18.49 | |
| Change | −0.71 ± 2.77 | 0.01 ± 6.59 | 0.534 | |
|
| ||||
| Emotions | After | 62.04 ± 14.50 | 62.57 ± 16.35 | |
| Change | 3.89 ± 11.78 | 8.33 ± 16.32 | 0.078 | |
|
| ||||
| Communication | After | 90.48 ± 14.58 | 92.35 ± 13.52 | |
| Change | 7.86 ± 14.98 | 4.25 ± 9.08 | 0.068 | |
|
| ||||
| Activities of daily living | After | 79.67 ± 21.79 | 86.31 ± 15.16 | |
| Change | 12.50 ± 19.18 | 15.60 ± 20.78 | 0.716 | |
|
| ||||
| Mobility | After | 81.93 ± 20.91 | 88.09 ± 15.96 | |
| Change | 11.67 ± 17.58 | 14.18 ± 20.84 | 0.387 | |
|
| ||||
| Hand function | After | 53.00 ± 23.13 | 67.14 ± 18.48 | |
| Change | 2.67 ± 10.33 | 8.33 ± 14.94 | 0.016∗ | |
|
| ||||
| Participation | After | 56.67 ± 23.38 | 69.94 ± 15.58 | |
| Change | 12.50 ± 21.68 | 22.17 ± 19.56 | 0.171 | |
Data are shown as mean ± SD, ∗P < 0.05. SIS: Stroke Impact Scale.
The changes in the blood glucose measurement value at the 5 points (blood glucose self-monitoring by finger stick) and hypoglycemia were reported in Table 7. There was no difference between two groups in the changes of 5 points of blood glucose measurement and hypoglycemia occurrence.
Table 7.
Comparisons of the amount of blood glucose change between groups after 6 months.
| Variables | Control (n = 30) | Intervention (n = 33) | P value | |
|---|---|---|---|---|
| FBG (mmol/L) | After | 7.54 ± 2.03 | 6.46 ± 1.00 | |
| Change | 0.90 ± 2.12 | −0.38 ± 1.95 | 0.241 | |
| 2hBG (mmol/L) | ||||
| After breakfast | After | 10.10 ± 2.26 | 9.64 ± 2.72 | |
| Change | −0.18 ± 0.76 | −0.68 ± 2.64 | 0.692 | |
| After lunch | After | 10.04 ± 2.82 | 10.04 ± 3.49 | |
| Change | −1.02 ± 4.85 | −0.64 ± 4.53 | 0.880 | |
| After dinner | After | 10.08 ± 2.47 | 9.69 ± 3.64 | |
| Change | −1.82 ± 3.92 | −0.37 ± 6.64 | 0.658 | |
| Before sleep | After | 8.22 ± 1.12 | 7.68 ± 2.85 | |
| Change | −2.38 ± 2.94 | −0.85 ± 4.13 | 0.464 | |
| Hypoglycemia, n (%) | Before | 8 (26.7%) | 9 (27.3%) | 0.958 |
| After | 4 (13.3%) | 3 (9.1%) | 0.600 | |
Data are shown as n (%) and mean ± SD, ∗P < 0.05. FBG: fasting blood glucose tested by finger stick.
4. Discussion
This is the first prospective study examining the impact of glucose fluctuation targeted intervention on outcomes in patients suffering a neurological insult. We found that after 6-month individualized glucose fluctuation target management, nonsignificant differences were observed in HbA1c between groups. Interestingly, our data noted a statistical improvement in LAGE, 1,5-AG, nerve function, and quality of life in the intervention group compared with the control group.
In the current study, due to the synergistic effects of behavioral intervention and drug regimen adjustment, LAGE, an index reflecting intraday blood glucose fluctuations, decreased significantly in the intervention group. Because of nonsignificant differences in HbA1c at baseline, we attributed the benefits in LAGE to glucose fluctuation target management. Our study suggested that it is worth paying additional attention to the LAGE (≥4.4 mmol/L) and making corrections by means of lifestyle intervention or medication adjustment.
After 6-month individualized glucose fluctuation target management, 1,5-AG increased significantly in the intervention group when compared with controls. 1,5-AG is a very sensitive and reliable clinical index. It has proven useful in predicting short-term blood glucose fluctuation (within 1-2 weeks), HbA1c is normal or only slightly elevated and can adequately reflect postprandial hyperglycemia and blood glucose fluctuation [24]. 1,5-AG can predict CD8+T and CD4+T cell modifications related to atherosclerosis [25]. A Japanese study verified that decreased levels of 1,5-AG were a risk factor for macrovascular diseases, especially in males [26]. In addition, patients with low levels of 1,5-AG were 4.07 times more likely to have CI than those with high levels of 1,5-AG [27]. In our study, we included patients with CI. Therefore, whether increased 1,5-AG is beneficial to prevent recurrence of CI remains to be studied.
In our study, the intervention group demonstrated decreased neurologic impairment when compared to controls. It is worth noting that there was no statistically significant difference between the two groups in HbA1c following intervention, but LAGE and 1,5-AG were significantly improved in the intervention group. This indicates that improvement in neurologic function in the intervention group may have been due to reduction in glucose fluctuation. Long-term data from UKPDS, DCCT, VADT, and other large studies have confirmed that controlling HbA1c to the ideal level can significantly reduce the occurrence of microvascular complications but cannot necessarily effectively reduce the incidence of cardiovascular and cerebrovascular events in patients with diabetes [28].
Our findings conclude that following 6-month glucose fluctuation target management, the MRS score, used as the evaluation tool for predicting CI prognosis [18], was lower in the intervention group (1.48 ± 0.76) than the control group (1.53 ± 0.90). Furthermore, 90.91% of patients in the intervention group had a good prognosis (MRS ≤ 2) with only 86.67% in the control group. Nevertheless, we did not observe statistically significant between-group differences in the MRS scores. Two reasons might account for this difference: first, the present study had a relatively small sample size and short intervention duration; and, second, subjects included in this study were motivated patients with mild disease. Previous data showed that amplitude of glucose fluctuation may directly affect or even determine the prognosis of T2DM patients with CI [29], indicating that glucose fluctuation target management has an important effect on the prognosis of vascular complications of diabetes. Therefore, in a future study, we could extend the intervention duration and include patients with more severe disease to verify the effects of glucose fluctuation target management on patient prognosis.
Our finding revealed that after 6-month glucose fluctuation target management, there was no statistically significant difference in total scores of SIS in patients with glucose fluctuation target management when compared to usual care. However, in the intervention group, strength, hand function, and participation dimensions of SIS increased significantly relative to the control group. In this study, we found that patients with postprandial hyperglycemia benefitted from focusing on dietary guidance, postprandial exercise guidance by way of increased strength, and hand function. Furthermore, “one-to-one” follow-up encouraged patients to communicate with their clinical team, to investigate causes of dysglycemia and fluctuations in overall glycemic control and set behavioral change goals, greatly improving the patient's ability to participate in diabetes self-care. In contrast, Ma et al. [30] reported that blood glucose management in patients with T2DM combined with CI can improve ADLs. This discrepancy may be related to the fact that patients in their study received systematic rehabilitation early in their hospitalization. But in our study, we mainly focused on glucose fluctuation target management and provided some exercise guidance according to the patient's glycemic fluctuation value, for an absent global approach to systematic rehabilitation training. Thus, it should be noted that both the glucose fluctuation target management and the rehabilitation of nerve function are indispensable for patients with T2DM combined with CI. A future study to standardize the rehabilitation training of patients after discharge is needed.
At present, most studies on the glycemic fluctuation in T2DM patients with CI were cross-sectional studies. To our knowledge, ours is the first prospective study to target glucose fluctuation management. Glucose fluctuation target management can be generalized to T2DM patients with cerebrovascular disease in the further research.
Limitations of this study warrant mention. First, the overall sample size was relatively small and the subjects included in this study were patients with mild disease who were willing to cooperate, which may be biased and limit the power of some conclusions. Second, the 6-month intervention in this study was relatively short. Future studies involving the intervention time can be extended to 1-2 years. Third, due to budget limitations, instead of continuing glucose monitoring (CGM), self-monitoring of blood glucose (SMBG) was used to evaluate glycemic fluctuation, and blood glucose values before lunch and dinner were not routinely checked, which could increase the risk of hypoglycemia. In future studies, CGM will be used to assess glucose fluctuation for more comprehensive and accurate data.
In conclusion, the results of this study suggested that after 6 months, patients receiving glucose fluctuation management achieved better 2hPG, 1,5-AG, LAGE, and LDL-c, reduced neurologic defect, and improved the quality of life when compared to controls. However, both the control and intervention groups had similar effects in increasing functional status. This study provides valuable information to guide diabetes clinicians in targeting glucose fluctuation in order to promote better glycemic control, lipid profile, and overall quality of life in patients with DM-related macrovascular disease.
Acknowledgments
The authors sincerely thank all participants for their time and effort in this study. This study was supported by the Health and Family Planning Research Project in 2017 (grant number N2017003).
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Ethical Approval
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Consent
Informed consent was obtained from all patients for being included in the study.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Authors' Contributions
We certify that all authors have participated sufficiently in the work to take public responsibility for the appropriateness of the study design and method; the collection, analysis, interpretation of the data; and the manuscript revision. H. L conceived and designed the study, QQ. L and XD. Y contributed to drafting and revising the manuscript critically for important intellectual content. SJ. H supervised data collection and management. J. Y and PP. Z assisted with patient recruitment and follow-up. JN. L and LH. Y developed the statistical analysis plan for outcomes. H. L and JD. M oversaw the data quality and revised the manuscript as needed. All authors reviewed the manuscript. Qingqing Lou and Xiaodan Yuan contributed equally to this work.
References
- 1.Goldstein L. B., Bushnell C. D., Adams R. J., et al. Guidelines for the primary prevention of stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(2):517–584. doi: 10.1161/STR.0b013e3181fcb238. [DOI] [PubMed] [Google Scholar]
- 2.Kiers L., Davis S. M., Larkins R., et al. Stroke topography and outcome in relation to hyperglycaemia and diabetes. Journal of Neurology, Neurosurgery, and Psychiatry. 1992;55(4):263–270. doi: 10.1136/jnnp.55.4.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The Diabetes Control and Complications Trial Research Group. The relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the diabetes control and complications trial. Diabetes. 1995;44(8):968–983. doi: 10.2337/diab.44.8.968. [DOI] [PubMed] [Google Scholar]
- 4.Nathan D. M., Buse J. B., Davidson M. B., et al. Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2006;29(8):1963–1972. doi: 10.2337/dc06-9912. [DOI] [PubMed] [Google Scholar]
- 5.Temelkova-Kurktschiev T. S., Koehler C., Henkel E., Leonhardt W., Fuecker K., Hanefeld M. Postchallenge plasma glucose and glycemic spikes are more strongly associated with atherosclerosis than fasting glucose or HbA1c level. Diabetes Care. 2000;23(12):1830–1834. doi: 10.2337/diacare.23.12.1830. [DOI] [PubMed] [Google Scholar]
- 6.Kohnert K. D., Heinke P., Vogt L., Salzsieder E. Utility of different glycemic control metrics for optimizing management of diabetes. World Journal of Diabetes. 2015;6(1):17–29. doi: 10.4239/wjd.v6.i1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suh S., Kim J. H. Glycemic variability: how do we measure it and why is it important? Diabetes and Metabolism Journal. 2015;39(4):273–282. doi: 10.4093/dmj.2015.39.4.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuroda M., Shinke T., Sakaguchi K., et al. Association between daily glucose fluctuation and coronary plaque properties in patients receiving adequate lipid-lowering therapy assessed by continuous glucose monitoring and optical coherence tomography. Cardiovascular Diabetology. 2015;14:p. 78. doi: 10.1186/s12933-015-0236-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiao X. M., Zhang X. G., Xu X. U., et al. Blood glucose fluctuation aggravates lower extremity vascular disease in type 2 diabetes. European Review for Medical and Pharmacological Sciences. 2014;18(14):2025–2030. doi: 10.4314/tjpr.v13i7.24. [DOI] [PubMed] [Google Scholar]
- 10.Ito T., Ichihashi T., Fujita H., et al. The impact of intraday glucose variability on coronary artery spasm in patients with dysglycemia. Heart and Vessels. 2019;34(8):1250–1257. doi: 10.1007/s00380-019-01353-w. [DOI] [PubMed] [Google Scholar]
- 11.Muggeo M., Zoppini G., Bonora E., et al. Fasting plasma glucose variability predicts 10-year survival of type 2 diabetic patients: the Verona diabetes study. Diabetes Care. 2000;23(1):45–50. doi: 10.2337/diacare.23.1.45. [DOI] [PubMed] [Google Scholar]
- 12.Hsu C. W. Glycemic control in critically ill patients. World Journal of Critical Care Medicine. 2012;1(1):31–39. doi: 10.5492/wjccm.v1.i1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benalia M., Zeller M., Mouhat B., et al. Glycaemic variability is associated with severity of coronary artery disease in patients with poorly controlled type 2 diabetes and acute myocardial infarction. Diabetes & Metabolism. 2019;45(5):446–452. doi: 10.1016/j.diabet.2019.01.012. [DOI] [PubMed] [Google Scholar]
- 14.Wu C. H., Liu B. The Fourth National Cerebrovascular Disease Conference. Vol. 6. China Medical News; 1996. [Google Scholar]
- 15.Colman P. G., Thomas D. W., Zimmet P. Z., Welborn T. A., Garcia-Webb P., Moore M. P. New classification and criteria for diagnosis of diabetes mellitus. The Australasian Working Party on Diagnostic Criteria for Diabetes Mellitus. The New Zealand Medical Journal. 1999;112(1086):139–141. [PubMed] [Google Scholar]
- 16.Brott T., Adams HP Jr, Olinger C. P., et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20(7):864–870. doi: 10.1161/01.STR.20.7.864. [DOI] [PubMed] [Google Scholar]
- 17.Rankin J. Cerebral vascular accidents in patients over the age of 60: II. Prognosis. Scottish Medical Journal. 1957;2(5):200–215. doi: 10.1177/003693305700200504. [DOI] [PubMed] [Google Scholar]
- 18.Sulter G., Steen C., De Keyser J. Use of the Barthel index and modified Rankin scale in acute stroke trials. Stroke. 1999;30(8):1538–1541. doi: 10.1161/01.STR.30.8.1538. [DOI] [PubMed] [Google Scholar]
- 19.Banks J. L., Marotta C. A. Outcomes validity and reliability of the modified Rankin scale: implications for stroke clinical trials: a literature review and synthesis. Stroke. 2007;38(3):1091–1096. doi: 10.1161/01.STR.0000258355.23810.c6. [DOI] [PubMed] [Google Scholar]
- 20.de Haan R., Limburg M., Bossuyt P., van der Meulen J., Aaronson N. The clinical meaning of Rankin ‘handicap’ grades after stroke. Stroke. 1995;26(11):2027–2030. doi: 10.1161/01.STR.26.11.2027. [DOI] [PubMed] [Google Scholar]
- 21.Kwon S., Hartzema A. G., Duncan P. W., Min-Lai S. Disability measures in stroke relationship among the Barthel index, the functional independence measure, and the modified Rankin scale. Stroke. 2004;35(4):918–923. doi: 10.1161/01.STR.0000119385.56094.32. [DOI] [PubMed] [Google Scholar]
- 22.Duncan P. W., Wallace D., Lai S. M., Johnson D., Embretson S., Laster L. J. The stroke impact scale version 2.0: evaluation of reliability, validity, and sensitivity to change. Stroke. 1999;30(10):2131–2140. doi: 10.1161/01.str.30.10.2131. [DOI] [PubMed] [Google Scholar]
- 23.Lan Y., Huang D. F., Hu X. Q., Xu G. Q., Li K. Reliability of the Chinese Version of stroke Impact Scale. Chinese Journal of Clinical Rehabilitation. 2004;8(28):6009–6011. [Google Scholar]
- 24.Koga M., Murai J., Saito H., et al. Serum 1,5-anhydroglucitol levels in patients with fulminant type 1 diabetes are lower than those in patients with type 2 diabetes. Clinical Biochemistry. 2010;43(15):1265–1267. doi: 10.1016/j.clinbiochem.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 25.Dworacka M., Winiarska H., Borowska M., Abramczyk M., Bobkiewicz-Kozlowska T., Dworacki G. Pro-atherogenic alterations in T-lymphocyte subpopulations related to acute hyperglycaemia in type 2 diabetic patients. Circulation Journal. 2007;71(6):962–967. doi: 10.1253/circj.71.962. [DOI] [PubMed] [Google Scholar]
- 26.Watanabe M., Kokubo Y., Higashiyama A., Ono Y., Miyamoto Y., Okamura T. Serum 1,5-anhydro-D-glucitol levels predict first-ever cardiovascular disease: an 11-year population-based cohort study in Japan, the Suita study. Atherosclerosis. 2011;216(2):477–483. doi: 10.1016/j.atherosclerosis.2011.02.033. [DOI] [PubMed] [Google Scholar]
- 27.Ikeda N., Hara H., Hiroi Y., Nakamura M. Impact of serum 1,5-anhydro-d-glucitol level on prediction of major adverse cardiac and cerebrovascular events in non-diabetic patients without coronary artery disease. Atherosclerosis. 2016;253:1–6. doi: 10.1016/j.atherosclerosis.2016.08.016. [DOI] [PubMed] [Google Scholar]
- 28.Lachin J. M., Genuth S., Nathan D. M., Zinman B., Rutledge B. N., for the DCCT/EDIC Research Group Effect of glycemic exposure on the risk of microvascular complications in the diabetes control and complications trial--revisited. Diabetes. 2008;57(4):995–1001. doi: 10.2337/db07-1618. [DOI] [PubMed] [Google Scholar]
- 29.Yoon J. E., Sunwoo J. S., Kim J. S., et al. Poststroke glycemic variability increased recurrent cardiovascular events in diabetic patients. Journal of Diabetes and its Complications. 2017;31(2):390–394. doi: 10.1016/j.jdiacomp.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 30.Ma G., Xu J. H. Effects of blood glucose management on rehabilitation after ischemic stroke in patients with type 2 diabetes mellitus. Modern Diagnosis and Treatment. 2013;24(16):3752–3753. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.