Abstract.
Infection with Strongyloides stercoralis can cause life-threatening disease in immunocompromised patients. Strongyloidiasis is thought to be hyper-endemic in tropical Australia, but there are limited contemporary seroprevalence data to inform local elimination strategies. To define the temporospatial epidemiology of strongyloidiasis in Far North Queensland, tropical Australia, the serology results of 2,429 individuals tested for the infection between 2000 and 2018 were examined. The proportion of positive tests fell from 36/69 (52.2%) in 2000 to 18/222 (8.1%) in 2018 (P < 0.001). Indigenous patients were more likely to have a positive result (Odds Ratio [OR]: 3.9, 95% CI: 3.0–5.0); however, by the end of the study period, residence in a rural or remote location (OR 3.9 (95% CI: 1.2–13.0), P = 0.03) was a more important risk factor for seropositivity than Indigenous status (OR 1.1 (95% CI: 0.4–3.1) P = 0.91). Ivermectin prescription data were available for the period 2004–2018, with annual prescriptions increasing from 100 to 185 boxes (P = 0.01). The volume of ivermectin dispensed correlated negatively with seropositivity (Spearman’s rho = −0.62, P = 0.02). An expanded environmental health program was implemented during the study period and likely contributed to the declining seroprevalence; however, the relative contributions of the individual components of this program are difficult to quantify. The seroprevalence of strongyloidiasis has declined markedly in this region of tropical Australia despite there being no targeted campaign to address the disease. Expanded prescription of ivermectin and public health interventions targeting the few remaining high-prevalence communities would be expected to expedite disease elimination.
INTRODUCTION
Strongyloidiasis, a neglected tropical disease caused by the soil-transmitted nematode, Strongyloides stercoralis, is estimated to infect 370 million individuals worldwide.1,2 The auto-infective life cycle of S. stercoralis means that the parasite can persist in individuals for life. Not only does this mean that individuals remain infective but also in the event of immunosuppression, the parasite can develop an accelerated life cycle, proliferating rapidly and disseminating from the gut to the rest of the body (hyperinfection syndrome). The case fatality rate of untreated hyperinfection syndrome approaches 100%, and even with treatment, it can exceed 25%.3
Strongyloides stercoralis has been hyper-endemic in tropical Australia,4 but studies examining the burden of strongyloidiasis have used heterogeneous methodologies and were often conducted in a single town.5–10 Accordingly, the data are not generalizable and, crucially, they do not reflect recent improvements in sanitation, socioeconomic gains, or regional variation in the prescription of antiparasitic therapy. Accurate seroprevalence data are important because the increasing use of immunosuppressive medications in Australia increases the risk of hyperinfection syndrome11 and it is essential for clinicians who work in high-burden settings to identify patients at risk of this potentially fatal complication.1,12,13
Ivermectin has been available for use in humans in Australia since 1996 and is used to treat strongyloidiasis and scabies.14 Australian guidelines recommend ivermectin as the first-line therapy for strongyloidiasis.15 Immunocompetent individuals receive 200 mg/kg with fatty food, followed by a second dose 7–14 days later. To reduce the risk of relapse, immunosuppressed patients receive 200 mg/kg with fatty food, on days 1, 2, 15, and 16. Repeat serology, stool examination, and blood examination for eosinophilia are recommended three months after therapy, and if any of the tests remain positive, re-treatment is recommended.16
There have been no coordinated public health strategies to eliminate strongyloidiasis in Australia despite multiple calls to action.17,18 Diseases such as malaria and filariasis have been eliminated, while enormous progress has been made in reducing the burden of leprosy and hookworm, suggesting that the elimination of strongyloidiasis in Australia is a realistic aim.19 Elimination would require sustained, well-funded, community-led programs with multiple components, including health education, improved sanitation, and, possibly, population-based chemotherapy.18 However, to this point, efforts to address the infection have been passive, relying on improvements in general sanitation and the standard of living, supplemented by opportunistic treatment from interested clinicians.
The Indigenous Aboriginal and Torres Strait Islander peoples have borne the greatest burden of strongyloidiasis in Australia.4 Indigenous Australians have poorer health outcomes than non-Indigenous Australians on almost every health metric, a source of continuing national shame.20,21 The “gap” between the life expectancy at birth for Indigenous and non-Indigenous Australian males is 8.6 years; for women, this difference is 7.8 years. The “Closing the Gap” campaign was launched in 2006 in an effort to address Indigenous Australians’ disadvantage, but the program has had only mixed success.20 Given the significant burden of strongyloidiasis in Indigenous Australians and its strong relationship with socioeconomic disadvantage, the infection’s seroprevalence may represent one proxy measure of the country’s progress in addressing factors responsible for the disparities between Indigenous and non-Indigenous health outcomes.
This study was performed to determine the temporospatial epidemiology of strongyloidiasis in the Far North Queensland (FNQ) region of tropical Australia. It also aimed to evaluate the impact of improved access to ivermectin and environmental health interventions, with the goal of providing data to inform a program to eliminate strongyloidiasis in the region.
MATERIALS AND METHODS
This retrospective study was performed in FNQ, tropical Australia, between January 1, 2000 and October 31, 2018. FNQ covers an area of 380,000 km2 and has a population of almost 280,000 people, 12% of whom identify as Aboriginal or Torres Strait Islanders.22 Cairns Hospital, a 531-bed tertiary referral hospital, has the only intensive care unit (ICU) in the region.
All patients who had serological testing for S. stercoralis in the public health system during the study period were eligible for inclusion. Tests were identified using the electronic Queensland Health pathology database AUSLAB. If patients were tested on multiple occasions, only the first test result was included in the analysis. The patients’ characteristics, including their age, gender, Indigenous status, and their residential address were recorded. Patients who did not reside in FNQ were excluded from the analysis.
Testing was performed using different serology test kits over the duration of the study period. From 2000 until 2008, testing was referred to a private Australian laboratory using their in-house ELISA kit (QML Pathology, Brisbane, Queensland), and after July 31, 2008, two commercial Strongyloides ratti ELISA kits were used: IVD Research (Carlsbad, CA) until August 2010 (reported sensitivity of 91.9% and specificity of 97.4%)23 and then Bordier Affinity Products (Crissier, Switzerland) until the end of the study period (reported sensitivity of 88% and specificity of 94%).23
Cairns is the largest city in FNQ and its administrative hub. For the purposes of this study, Cairns and its surrounding suburbs were defined as metropolitan, while the rest of FNQ was defined as rural or remote. Socioeconomic disadvantage was defined using the publicly available Socio-Economic Indexes for Areas (SEIFA) scores calculated for each postcode by the Australian Bureau of Statistics.24 A SEIFA score of less than 900 was used to define a significantly socioeconomically disadvantaged community. Indigenous status was self-reported by patients at the time of blood collection.
Local health service pharmacy records were examined to identify the volume of ivermectin that had been dispensed, although complete data were only available from 2004. Meanwhile, hospital and ICU admission data and the hospital’s infectious diseases consultation database were also analyzed to identify cases of hyperinfection syndrome.
Statistical analysis.
Data were entered into an electronic database (Microsoft Excel, Redmond, WA) and analyzed with statistical software (Stata version 14.2, College Station, TX). Groups were analyzed using the Kruskal–Wallis test, the chi-squared tests, and Fisher’s exact test, where appropriate. Odds ratios (ORs) were determined using logistic regression. Regions were divided by postcode, based on key clinical hubs, with prevalence maps constructed using mapping software (MapInfo version 17, Stamford, CT). Population data were collected from the Australian Bureau of Statistics.22
Ethics review.
The FNQ Human Research Ethics Committee approved the study (HREC/17/QCH/25 – 1125, AM/QCH/34791). The requirement for informed consent was waived given the retrospective nature of the data and their aggregated presentation. However, to prevent the stigmatization of high-burden communities, the names of individual communities were de-identified and presented in an aggregated manner.
RESULTS
Serological testing for S. stercoralis was performed on a total of 3,062 occasions during the study period, although 633 (20.7%) represented repeated testing of the same individual or of patients who did not live in FNQ. This left 2,429 tests for analysis (Supplemental Table 1).
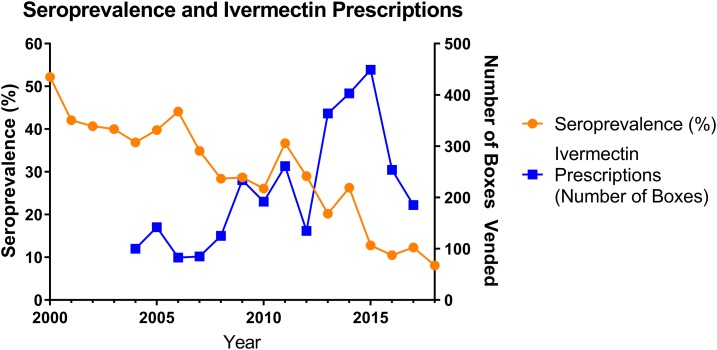
The patients’ median (interquartile range [IQR]) age was 48 (33–62) years, 185 (7.6%) were children (age < 18 years). Gender was available in 2,425 patients, 1,229 (50.7%) of whom were female; Indigenous status was available in 2,370, 1,649 (69.6%) of whom identified as Indigenous Australians. Of the 2,429 tests, 645 (26.6%) were positive. The proportion of positive tests declined from 36/69 (52.2%, 95% CI: 40.3–63.5) in 2000 to 18/222 (8.1% (95% CI: 5.2–12.5) in 2018 (P for trend < 0.001) (Figure 1).
Figure 1.
Changing annual seroprevalence compared with rate of ivermectin prescription. This figure appears in color at www.ajtmh.org.
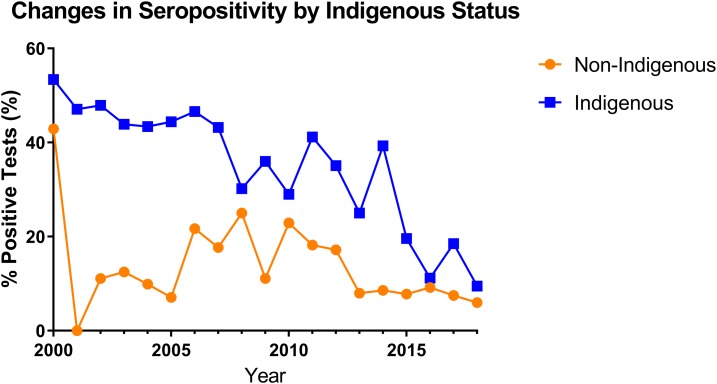
Positive serology was more common in Indigenous patients than in non-Indigenous patients over the entire study period (OR 3.9, 95% CI: 3.0–5.0). However, the proportion of positive tests in Indigenous patients fell from 31/58 (53.5%, 95% CI: 40.8–65.7) in 2000 to 10/105 (9.5%, 95% CI: 5.3–16.6) in 2018 (P for trend < 0.001). In 2018, the final year of the study period, there was no difference in the seropositivity rate between Indigenous patients and non-Indigenous patients (10/105 [9.5%, 95% CI: 5.3–16.6] versus 7/116 [6.0%, 95% CI: 3.0–11.9]), P = 0.45) (Figure 2).
Figure 2.
Proportion of serological tests positive for Strongyloides stercoralis over the course of the study period; comparison between patients identifying as Indigenous and non-Indigenous Australians. This figure appears in color at www.ajtmh.org.
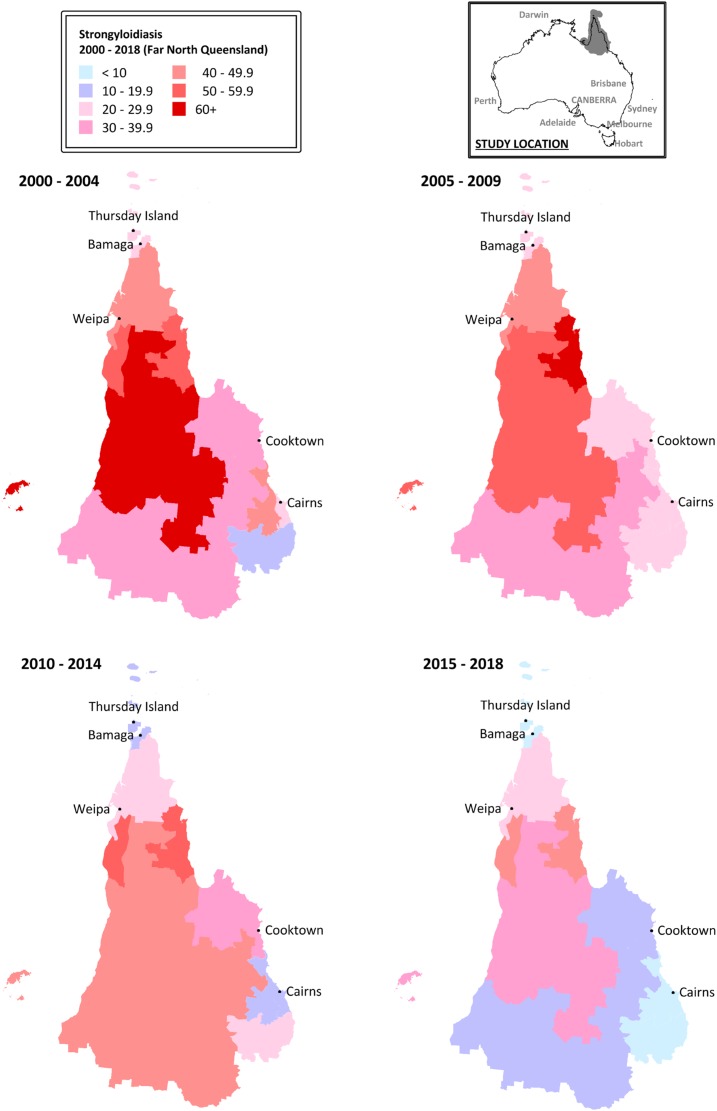
There was significant heterogeneity in seropositivity rates across the region. Positive results were more common in patients from a rural or remote location than a metropolitan location (OR 3.0 [95% CI: 2.3–3.8]). This remained the case at the end of the study period (OR 4.3 [95% CI: 1.4–13.4]). Indeed, at the end of the study period, rural or remote location was a more important risk factor for seropositivity than Indigenous status (residence in a rural or remote location: OR 3.9 [95% CI: 1.2–13.0], P = 0.03; Indigenous status: OR 1.1 [95% CI: 0.4–3.1] P = 0.91). However, even when examining rural or remote locations, there was significant variation between regions (Table 1, Figure 3).
Table 1.
Strongyloides stercoralis seropositivity rates by region of Far North Queensland between 2000 and 2018
| 2000–2004 | 2005–2009 | 2010–2014 | 2015–2018 | |||||
|---|---|---|---|---|---|---|---|---|
| Remote North FNQ | 12/41 | 29.3% | 9/37 | 24.3% | 16/93 | 17.2% | 18/263 | 6.8% |
| North West Cape | 32/79 | 40.5% | 9/20 | 45.0% | 6/21 | 28.6% | 6/30 | 20.0% |
| North East Cape | 25/48 | 52.1% | 24/38 | 63.2% | 12/22 | 54.5% | 7/16 | 43.8% |
| South West Cape | 33/48 | 68.8% | 16/32 | 50.0% | 10/22 | 45.5% | 4/13 | 30.8% |
| West Cape | 30/56 | 53.6% | 34/60 | 56.7% | 25/44 | 56.8% | 17/41 | 41.5% |
| Southern Cape | 25/64 | 39.1% | 15/58 | 25.9% | 11/32 | 34.4% | 7/37 | 18.9% |
| Metropolitan outskirts | 6/15 | 40.0% | 14/53 | 26.4% | 9/48 | 18.8% | 7/82 | 8.5% |
| Metropolitan | 13/49 | 26.5% | 28/118 | 23.7% | 27/178 | 15.2% | 25/351 | 7.1% |
| Southern FNQ | 1/9 | 11.1% | 3/14 | 21.4% | 6/30 | 20.0% | 3/35 | 8.6% |
| South West FNQ | 35/97 | 36.1% | 40/128 | 31.3% | 57/140 | 40.7% | 5/38 | 13.2% |
FNQ = Far North Queensland.
Figure 3.
Changing proportion of positive Strongyloides serology tests by region over the study period. Map data based on Australian Bureau of Statistics Postal Areas.25 This figure appears in color at www.ajtmh.org.
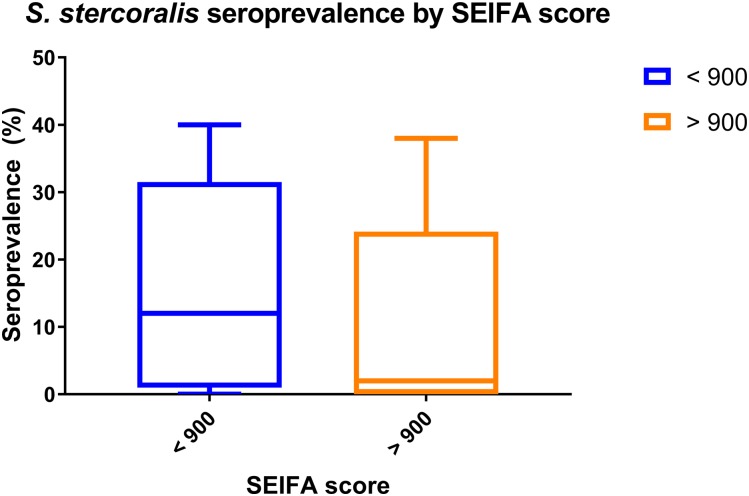
An association between the communities’ seroprevalence and the degree of socioeconomic disadvantage did not reach statistical significance: at the end of the study period, the median (IQR) proportion of tests that were positive in disadvantaged communities was 12% (1.5–23) compared with 2% (0–10.3) in communities which were not (P = 0.07) (Figure 4).
Figure 4.
Association between Socio-Economic Indexes for Areas (SEIFA) score and Strongyloides stercoralis seroprevalence in Far North Queensland. This figure appears in color at www.ajtmh.org.
During the study period, there was an increase in the number of ivermectin prescriptions in the region, from 100 boxes dispensed in 2004 to 185.4 boxes in 2018 (P for trend = 0.01). The fall in the proportion of positive tests correlated with the amount of ivermectin dispensed (Spearman’s rho = −0.61, P = 0.02) (Figure 1).
Hyperinfection in FNQ.
There was only one case of Strongyloides hyperinfection diagnosed in the region’s tertiary referral hospital during the entire study period. An 80-year-old man had a history of S. stercoralis infection that had been diagnosed and treated 6 years previously while living in the Northern Territory, a separate region of Australia. He presented with 12 months of progressive anorexia, nausea, diarrhea, and weight loss. A colonoscopy demonstrated multiple colonic ulcers with histology suggestive of Crohn’s disease. He was prescribed corticosteroids and mesalazine for 2 weeks without improvement. During a subsequent gastroscopy, he became hypoxic and hypotensive and required ICU support. Gastric biopsies and subsequent bronchoalveolar lavage identified S. stercoralis larvae and blood cultures isolated Bacteroides thetaiotaomicron. Despite broad-spectrum antibiotics and intravenous doramectin, he died 6 days after his admission to the ICU.
DISCUSSION
There has been a remarkable recent fall in the proportion of positive serological tests for S. stercoralis in this region of tropical Australia. There has been no dedicated strongyloidiasis-elimination program, but the proportion of tests returning a positive result has declined by more than 85% in less than 20 years, suggesting a significant decrease in the burden of the disease. The study period coincides with the approval of ivermectin for use in humans in Australia and the decline in seropositivity correlates with the significant increase in its local prescription.
Concomitant improvements in sanitation infrastructure26 and socioeconomic conditions in the region are also likely to have contributed to the decline in seropositivity.24 In 2002, the Queensland Government implemented the Aboriginal and Torres Strait Islander Public Health Program, a suite of environmental health interventions that aimed to improve the health and wellbeing of remote communities. Initiatives have included delivering personal hygiene education programs at public meetings and schools, addressing domestic and community waste management, upgrading sewerage systems and improving water quality. There has been a focus on animal management, with mass canine ivermectin therapy a major component.
Historically, strongyloidiasis in Australia has occurred more commonly in its Indigenous population. Strongyloidiasis is linked consistently to lower socioeconomic status and reduced access to health care, issues for many Indigenous Australians, particularly those in remote communities.27–30 Indigenous Australians were again almost four times as likely as non-Indigenous patients in this cohort to have positive serology across the study period. However, by the end of the study, there was no significant difference between the two populations. While the “gap” in overall health outcomes between Indigenous and non-Indigenous Australians remains stubbornly persistent,21 the closing of the gap in S. stercoralis seropositivity rates demonstrates that—at least for some health conditions—this can be remedied over a relatively short period of time.
The persistence of parasitic infections has been attributed to a variety of factors, including poverty, poor health literacy, inadequate sanitation, and poorly coordinated public health campaigns.18 Efforts to eliminate strongyloidiasis would benefit from a community-specific targeting of these factors. Up to date, reliable prevalence data such as that presented in this study will assist in identifying those communities that are most in need.17 Increased serological testing and treatment with ivermectin, a safe, inexpensive, and effective therapy, would be expected to reduce the communities’ burden.31 This could be complemented by education campaigns that stress the importance of hand hygiene, wearing appropriate footwear, and caring for pets that may carry the parasite.32 Finally, public health bodies could work with governments and the private sector to improve housing and sanitation, while general measures to increase education, boost employment, and improve the overall socioeconomic status of these communities continue.
Far North Queensland is not the only region of Australia that is likely to have seen progress toward the elimination of strongyloidiasis. The decreasing trend reported in this study was also seen in a studies conducted in the Northern Territory of Australia33 and the Kimberley region of Western Australia.31,33 However, the lack of any national prevalence studies makes drawing conclusions regarding the true prevalence of strongyloidiasis across Australia challenging. Despite this, the availability of ivermectin, environmental health interventions, and general improvements in sanitation are likely to be reducing the infection’s prevalence, even in what have been considered to be high-burden communities.
These findings may be instructive for countries where strongyloidiasis remains endemic. Ivermectin is also an inexpensive, safe, and effective therapy for scabies and lice, which, like strongyloidiasis, affect predominantly disadvantaged communities.7,9,31 Treatment of scabies would be expected to reduce the burden of streptococcal skin infections and the complications of rheumatic heart disease and post-streptococcal glomerulonephritis, which also affect these communities disproportionately.18 Beyond eliminating strongyloidiasis, environmental health interventions will reduce the burden of infection with other parasites, the incidence of diarrheal disease, and would also have salutary effect on social and economic development.34
It is interesting to note that despite the high rates of S. stercoralis seropositivity in many parts of the region, hyperinfection was rare, with the condition confirmed only once in almost two decades. This may represent under-diagnosis, but the increasing prescription of ivermectin might suggest that clinicians are aware of the complication and following national guidelines for preventative therapy.35 Human T lymphotropic virus-1 infection, an infection which significantly increases the risk of hyperinfection and which is common in some other parts of Australia, is rare in the region and this may also have contributed to the apparent rarity of the syndrome.36 However, these findings are no grounds for complacency and local clinicians need to remain aware of the complication given its significant case-fatality rate and relatively simple prevention.
This retrospective study has many limitations. The study only reports the data of patients who were tested for strongyloidiasis, which almost certainly overestimates the prevalence of the infection in the general community. The indication for testing was not available—it is not clear whether the testing was performed for general screening, before immunosuppression or to investigate symptoms or eosinophilia. Increases in population screening of asymptomatic patients may have artificially reduced the proportion of positive tests. However, although there was an increase in testing from 2016, the trend in declining seropositivity was present before this date (Supplemental Table 1). Ivermectin—a relatively specialized medication—was only dispensed from the Cairns hospital pharmacy during the study period. By contrast, an evolving range of immunosuppresive therapies were dispensed from a number of private pharmacies. Unfortunately, it was not possible to access these data to quantify the clinical impression of their increased prescription and the ensuing risk of hyperinfection syndrome. All laboratory tests were also performed in the public health sector, which is also likely to overestimate the infection’s prevalence as public sector patients are more likely to have a lower socioeconomic status. Although acknowledging this to be the case, most of the region’s remote communities have no private laboratories so this is less likely to be an issue in these locations. The diagnostic test used varied over the study period and none of the tests have perfect sensitivity or specificity; however, they were the best available tests at the time and were similar to other tests from the Strongyloides literature, facilitating comparison with other studies. Finally, the study lacked the statistical power to confirm the association between strongyloidiasis and socioeconomic disadvantage.
CONCLUSION
There has been a marked reduction in the seropositivity of patients tested for strongyloidiasis in FNQ since 2000. The reduction is likely to be at least partly due to improved access to ivermectin, a medication which is safe, inexpensive, and simple to take, and which also has activity against other infections that are prevalent in areas where strongyloidiasis is endemic. Australia’s Indigenous people have borne the disproportionate burden of strongyloidiasis in the past, another example of the stubborn gap in health outcomes that exist in the country; however, by the end of the study, a difference in seropositivity was no longer apparent. The greater seropositivity in rural and remote locations provides data to inform targeted local elimination programs. The impressive decline in seropositivity in the region over a short period of time—despite the absence of a coordinated program—augurs well for more directed programs in similar high-prevalence settings.
Supplemental tables
Acknowledgment:
We thank Annemarie Black, Cairns Hospital Pharmacy, who provided valuable advice during the manuscript’s revision.
Note: Supplemental tables appear at www.ajtmh.org.
REFERENCES
- 1.Centers for Disease Control , 2016. Resources for Health Professionals. Available at: https://www.cdc.gov/parasites/strongyloides/health_professionals/index.html. Accessed August 30, 2018. [Google Scholar]
- 2.Schär F, Trostdorf U, Giardina F, Khieu V, Muth S, Marti H, Vounatsou P, Odermatt P, 2013. Strongyloides stercoralis: global distribution and risk factors. PLoS Negl Trop Dis 7: e2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vadlamudi RS, Chi DS, Krishnaswamy G, 2006. Intestinal strongyloidiasis and hyperinfection syndrome. Clin Mol Allergy 4: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paltridge M, Traves A, 2018. The health effects of strongyloidiasis on pregnant women and children: a systematic literature review. Trop Med Infect Dis 3: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fisher D, McCarry F, Currie B, 1993. Strongyloidiasis in the Northern Territory. Under-recognised and under-treated? Med J Aust 159: 88–90. [PubMed] [Google Scholar]
- 6.Flannery G, White N, Flannery G, White N, 1993. Immunological Parameters in Northeast Arnhem Land Aborigines: Consequences of Changing Settlement Patterns and Lifestyles. Urban Ecology and Health in the Third World. Cambridge, UK: Cambridge University Press, 202–220. [Google Scholar]
- 7.Kearns TM, et al. 2017. Strongyloides seroprevalence before and after an ivermectin mass drug administration in a remote Australian aboriginal community. PLoS Negl Trop Dis 11: e0005607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meloni BP, Thompson RC, Hopkins RM, Reynoldson JA, Gracey M, 1993. The prevalence of giardia and other intestinal parasites in children, dogs and cats from aboriginal communities in the Kimberley. Med J Aust 158: 157–159. [DOI] [PubMed] [Google Scholar]
- 9.Prociv P, Luke R, 1993. Observations on strongyloidiasis in Queensland aboriginal communities. Med J Aust 158: 160–163. [DOI] [PubMed] [Google Scholar]
- 10.Shield J, Aland K, Kearns T, Gongdjalk G, Holt D, Currie B, Prociv P, 2015. Intestinal parasites of children and adults in a remote aboriginal community of the Northern Territory, Australia, 1994–1996. Western Pac Surveill Response J 6: 44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bossingham D, 2003. Systemic lupus erythematosus in the far north of Queensland. Lupus 12: 327–331. [DOI] [PubMed] [Google Scholar]
- 12.Furuya-Kanamori L, Yakob L, Riley TV, Paterson DL, Baker P, McKenzie SJ, Robson J, Clements AC, 2016. Community-acquired Clostridium difficile infection, Queensland, Australia. Emerg Infect Dis 22: 1659–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hollingworth SA, Chan R, Pham J, Shi S, Ford PJ, 2017. Prescribing patterns of analgesics and other medicines by dental practitioners in Australia from 2001 to 2012. Community Dent Oral Epidemiol 45: 303–309. [DOI] [PubMed] [Google Scholar]
- 14.Therapeutic Goods Administration , 2013. Product Information for AusPAR Stromectol Ivermectin Merck Sharp Dohme (Australia). Canberra, Australia: Department of Health, Therapeutic Goods Administration. [Google Scholar]
- 15.Therapeutic Guidelines , 2014. Strongyloidiasis. Available at: https://tgldcdp-tg-org-au./viewTopic?topicfile=gastrointestinal-helminths&guidelineName=Antibiotic#toc_d1e204. Accessed December 1, 2018. [Google Scholar]
- 16.Davis JS, et al. 2003. Prevention of opportunistic infections in immunosuppressed patients in the tropical Top End of the Northern Territory of Australia. Commun Dis Intell 27: 7. [DOI] [PubMed] [Google Scholar]
- 17.Bisoffi Z, et al. 2013. Strongyloides stercoralis: a plea for action. PLoS Negl Trop Dis 7: e2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCarthy JS, Garrow SC, 2002. Parasite elimination programs: at home and away. Med J Aust 176: 456–457. [DOI] [PubMed] [Google Scholar]
- 19.Hempenstall A, Smith S, Hanson J, 2019. Leprosy in Far North Queensland: almost gone, but not to be forgotten. Med J Aust 211: 182–183. [DOI] [PubMed] [Google Scholar]
- 20.Holland C, 2018. A Ten-Year Review the Closing the Gap Strategy and Recommendations for Reset: The Close the Gap Campaign Steering Committee. Sydney, NSW, Australia: Australian Human Rights Committee. [Google Scholar]
- 21.Commonwealth of Australia , 2019. Closing the Gap Report 2019. Canberra, Australia: Department of the Prime Minister and Cabinet. [Google Scholar]
- 22.Australian Bureau of Statistics , 2012. Regional Population Growth, Australia. Canberra, Australia: Australian Bureau of Statistics, Australian Government. [Google Scholar]
- 23.Bisoffi Z, et al. 2014. Diagnostic accuracy of five serologic tests for Strongyloides stercoralis infection. PLoS Negl Trop Dis 8: e2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Commonwealth of Australia , 2018. Socio-Economic Indexes for Areas. Canberra, Australia: Australian Bureau of Statistics. [Google Scholar]
- 25.Commonwealth of Australia , 2006. ABS Postal Area Concordances, Aug 2006. Canberra, Australia: Australian Bureau of Statistics. [Google Scholar]
- 26. Hall N, Barbosa M, Currie D, Dean A, Head S, Hill P, Naylor S, Reid S, Selvey L, Willis J, 2017. Water, Sanitation and Hygiene in Remote Indigenous Australian Communities: A Scan of Priorities. Queensland TUo, ed. Global Change Institute discussion paper: Water for equity and wellbeing series. Brisbane: University of Queensland.
- 27.Australian Institute of Health and Welfare , 2015. The Health and Welfare of Australia’s Aboriginal and Torres Strait Islander Peoples. Canberra, Australia: Australian Institute of Health and Welfare. [Google Scholar]
- 28.Beknazarova M, Whiley H, Ross K, 2016. Strongyloidiasis: a disease of socioeconomic disadvantage. Int J Environ Res Public Health 13: E517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olsen A, van Lieshout L, Marti H, Polderman T, Polman K, Steinmann P, Stothard R, Thybo S, Verweij JJ, Magnussen P, 2009. Strongyloidiasis – the most neglected of the neglected tropical diseases? Trans R Soc Trop Med Hyg 103: 967–972. [DOI] [PubMed] [Google Scholar]
- 30.Senephansiri P, Laummaunwai P, Laymanivong S, Boonmar T, 2017. Status and risk factors of Strongyloides stercoralis infection in rural communities of Xayaburi province, Lao PDR. Korean J Parasitol 55: 569–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hays R, Esterman A, McDermott R, 2017. Control of chronic Strongyloides stercoralis infection in an endemic community may be possible by pharmacological means alone: results of a three-year cohort study. PLoS Negl Trop Dis 11: e0005825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paradies P, Iarussi F, Sasanelli M, Capogna A, Lia RP, Zucca D, Greco B, Cantacessi C, Otranto D, 2017. Occurrence of strongyloidiasis in privately owned and sheltered dogs: clinical presentation and treatment outcome. Parasit Vectors 10: 345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mayer-Coverdale KJ, Crowe A, Smith P, Baird WR, 2017. Trends in Strongyloides stercoralis faecal larvae detections in the Northern Territory, Australia: 2002 to 2012. Trop Med Infect Dis 2: E18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mara D, Lane J, Scott B, Trouba D, 2010. Sanitation and health. PLoS Med 7: e1000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Therapeutic Guidelines , 2014. Immunosuppressed patients: prevention of other infections. Prevention of infection: immunosuppressed patients. West Melbourne, Victoria, Australia: Therapeutic Guidelines Limited. [Google Scholar]
- 36.Smith S, Russell D, Horne P, Hanson J, 2019. HTLV-1 is rare in Far North Queensland despite a significant burden of classically associated diseases. Pathology 51: 91–94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.