Abstract.
The Grand Magal of Touba (GMT) is an annual 1-day Muslim religious event that takes place in Touba in Senegal. The city of Touba swells from 800,000 to four million people during the GMT. All patients who attended one of the 154 dedicated medical care public healthcare structures of the medical region of Diourbel during the GMT were included in a cross-sectional survey from November 16 to November 21, 2016. Demographic, morbidity, and mortality data were collected on a daily basis using a standardized article form that allows data to be recorded in a free-text format. Data were obtained from a total of 20,850 healthcare encounters, and 30.9% patients were aged ≤ 15 years. The most frequent conditions were gastrointestinal and respiratory diseases. Most frequent gastrointestinal symptoms were abdominal and gastric pain, nausea and vomiting, and diarrhea, suggesting that most patients suffered gastroenteritis. The predominance of cough, rhinitis, influenza-like illness, and sore throat among patients with respiratory symptoms suggests that most patients suffered from upper respiratory tract infections. Other frequent symptoms were headaches and pain in various organs. Three percentage of patients were considered to have malaria, 29.8% of patients were prescribed antibiotics and 2.6% antimalarial drugs, and 1.5% of patients were hospitalized. Only one death was recorded. Preparedness of the medical infrastructure should target these syndromic features, in terms of diagnostic tools and specific treatments, including pediatric formulations. It is also essential to improve the quality and rapid availability of data to enable real-time analysis of medical events at the GMT and to implement a rapid response, if necessary.
INTRODUCTION
The Grand Magal of Touba (GMT) is an annual 1-day Muslim religious event that takes place in Touba, located 200 km to the northwest of Dakar, every year on the 18th of Safar month of the Islamic calendar. Touba was founded by Cheikh Amadou Bamba Mbacké (the founder of Mouridism, a Sufi Muslim order) in 1887, and its surface is about 120 km2. During the GMT, the population of Touba and its suburbs increases from approximately 1.5 million inhabitants to an estimated 4–5 million pilgrims coming from across Senegal and the surrounding countries, as well as from countries outside Africa (Figure 1). However, the accurate number of pilgrims is not available. People usually come earlier to the event and stay for several days in Touba. During this period, pilgrims are accommodated in private housing structures because there are no hotels in Touba. These accommodation facilities can be residences of the local inhabitants, where family members living in different parts of Senegal or who have emigrated abroad meet during the Magal period, or marabout houses with a capacity of up to hundreds of individuals. Many pilgrims sleep on carpets, on the floor, in houses, or on outdoor terraces. Food is prepared collectively by the family members or by marabout followers. Slaughtering of animals is mostly carried out in the streets, in front of housing structures. Various community associations of Mouride disciples (dahiras) are responsible for feeding pilgrims by providing them with free food in the streets and along the roads to Touba.
Figure 1.
Map of Senegal and surrounding countries. This figure appears in color at www.ajtmh.org.
The two central religious events of the GMT are visits of the mausoleum of Cheikh Ahmadou Bamba and of the Great Mosque of Touba, which involves a partial circumambulation of the mosque. In addition, pilgrims visit the mausoleums of several other important Mouride leaders who were descendants of the Cheikh. Within the city, the mosque and mausoleum area is a secluded place surrounded by walls. Access to the mosque area is allowed to everyone (including tourists), but flows are regulated by security staff. In addition, pilgrims visit places in Touba that are associated with the holy life of the Cheikh, including the “Well of Mercy,” a spring whose sacred water is known to heal all kinds of diseases and misfortunes. In addition, pilgrims often visit the central library of Touba, which contains the writings of the Cheikh and other influential Mouride theologians, and the Mouride University. Finally, pilgrims visit their personal Mouride spiritual guides, or marabouts, who receive their followers in their personal residence in the city. Recitation of the Koran and spiritual introspection complete these religious activities.
During the GMT period, in addition to religious celebrations, intense cultural activities take place, including conferences, seminaries, and debates between representatives of various Muslims communities from Senegal and from the diaspora. Recitation of poems by Ahmadou Bamba and collective hymn-singing sessions are organized at night, in the streets, under large open tents. Informally, pilgrims also visit Touba’s large temporary marketplace, one of the biggest in the country, where a wide range of products can be found with prices lower than those of any other market in Senegal.
Medical care is provided free of charge during the Grand Magal. Most of the available local public medical infrastructures are involved in the medical preparation, surveillance, and response during the event.1 As an example, three tertiary care and four secondary care hospitals (total bed capacity of 481 in seven hospitals) and about 300 primary healthcare centers and 50 private structures in Touba and surrounding cities were mobilized in 2015. The vast majority of the medical staff taking care of patients during the GMT is composed of nurses, midwives, and volunteer community health agents. Overall, 78 medical doctors were mobilized during the 2015 GMT, many of whom came from Dakar to temporarily reinforce local staff.
The WHO defines mass gatherings (MGs) as a “concentration of people at a specific location for a specific purpose over a set period of time which has the potential to strain the planning and response resources of the country or community.”2 The GMT can, therefore, be described as an MG event. A variety of health risks are associated with MGs, including the transmission of infectious diseases, noncommunicable diseases, trauma and injuries, environmental effects (such as heat-related illnesses, dehydration, and hypothermia), diseases related to drug and alcohol use, and deliberate acts, such as terrorist attacks.2 Infectious diseases are of particular concern at MGs, and the international component of some MGs may favor the globalization of local endemic diseases.3,4 Outbreaks of cholera and meningococcal disease have been described in the context of Hajj, one of the largest religious MGs at Mecca, Saudi Arabia. Today, respiratory tract infections are the leading cause of infectious diseases in Hajj pilgrims with a prevalence of 50–93%.5 In other MGs, such as Muslim, Christian, and Hindu religious events, sports events, and large-scale open-air festivals, outbreaks have been reported less frequently. The most common outbreaks at these events involved diseases preventable by vaccination, notably measles and influenza. Gastrointestinal infections caused by a variety of pathogens have also been recorded.5
The situation is particularly challenging in the context of the GMT, with regard to the relatively limited medical resources available during this largest religious event. The context of the GMT and the crowded conditions experienced during the event are likely to increase the prevalence of infectious diseases, including febrile illness, diarrheal diseases, and respiratory infections, as suggested by preliminary surveillance data obtained from local medical structures receiving ill pilgrims during the GMT in 2015.1 In addition, in a cohort survey conducted among 110 GMT pilgrims from south Senegal in 2017, 41.8% and 14.6% of participants suffered from respiratory and gastrointestinal symptoms, respectively.6
In connection with the public health authorities of the medical region of Diourbel, which is hosting the GMT, detailed clinical surveillance data from the 2016 season, targeting symptoms possibly attributed to infectious diseases are presented in this article and analyzed in relation with demographics.
MATERIALS AND METHODS
All patients who attended one of the 154 public healthcare structures of the medical region of Diourbel of the dedicated medical care system during the GMT, from November 16 to November 21, 2016, were included in a cross-sectional survey (the religious ceremony of the GMT took place on November 19th). Data were collected during the event, by local medical staff, including doctors, nurses, medical students, and other healthcare personal, on a daily basis using a standardized paper form provided by the Ministry of Health to record demographic, morbidity, and mortality data in free-text format. Body temperature was assessed using an electronic axillary thermometer. Patients with a body temperature > 37.5°C were considered febrile.7 Influenza-like illness was defined as the association of cough, sore throat, and fever.8 High blood pressure is defined as systolic blood pressure ≥ 130 mmHg and/or diastolic blood pressure ≥ 90 mmHg.9 Diarrhea was defined by at least three loose or liquid stools per day. The local public medical infrastructures involved in this cross-sectional study, including hospitals (n = 7), primary healthcare centers (n = 94), and dispensaries and advanced medical posts (n = 213), are presented in detail elsewhere.1 After the event, the paper forms were secondarily reviewed by a team of four data managers who entered anonymously the following items into a database: age, gender, clinical data, treatments related to infectious agents, hospitalization and referral to Dakar, and death. Data managers worked under the supervision of a medical doctor in Senegal, and inconsistencies were further corrected by a team of four medical doctors in Marseille. Based on the most frequent clinical conditions observed in 2015, a selection of 27 signs or symptoms was extracted from paper forms in 2016, notably targeting those possibly indicative of infectious diseases (Table 1). Signs and symptoms unrelated to infectious diseases were not analyzed. Apart from body temperature and blood pressure measurement, results of clinical examination were not reported consistently by healthcare providers, as many volunteer community health agents are not trained for clinical examination. In addition, results of Plasmodium falciparum rapid diagnostic test, when available, were documented (SD BIOLINE Malaria Ag P.f, Standard Diagnostics, Inc., Yongin, Republic of Korea).
Table 1.
Main symptoms presented by patients (N = 20,850)
| Symptoms | Number of patients | Proportion of all patients (%) |
|---|---|---|
| Headache | 5,913 | 28.4 |
| Fever | 3,593 | 17.2 |
| Fatigue | 3,277 | 15.7 |
| Vertigo | 1,476 | 7.1 |
| High blood pressure | 1,398 | 6.7 |
| Diffuse pain | 1,216 | 5.8 |
| Arthralgia | 567 | 2.7 |
| Myalgia | 566 | 2.7 |
| Gastrointestinal symptoms | 4,591 | 22.0 |
| Abdominal pain | 1,509 | 7.2 |
| Vomiting or nausea | 1,223 | 5.9 |
| Epigastric pain | 1,222 | 5.9 |
| Diarrhea | 803 | 3.9 |
| Constipation | 340 | 1.6 |
| Anorexia | 288 | 1.4 |
| Respiratory symptoms | 3,563 | 17.1 |
| Cough | 3,126 | 15.0 |
| Rhinitis | 1,791 | 8.6 |
| Influenza-like illness | 446 | 2.1 |
| Sore throat | 194 | 0.9 |
| Dyspnea | 153 | 0.7 |
| Skin infections | 600 | 2.9 |
| Wound | 1,039 | 5.0 |
| Dermatitis | 454 | 2.2 |
| Skin abscess | 146 | 0.7 |
| Dental pain | 750 | 3.6 |
| Trauma | 1,578 | 7.6 |
| Conjunctivitis | 300 | 1.5 |
| Heat stress | 282 | 1.4 |
| Urinary symptoms | 227 | 1.1 |
The protocol of this study was approved by the Ministry of Health of Senegal (SEN17/62).
Statistical analysis.
Pearson’s chi-squared test and Fisher’s exact test, as appropriate, were applied to analyze the categorical variables. Percentages and odds ratio with 95% CI estimations and comparisons were carried out using STATA 11.1 (Copyright 2009 StataCorp LP, College Station, TX, http://www.stata.com). P-values of 0.05 or less were considered significant.
RESULTS
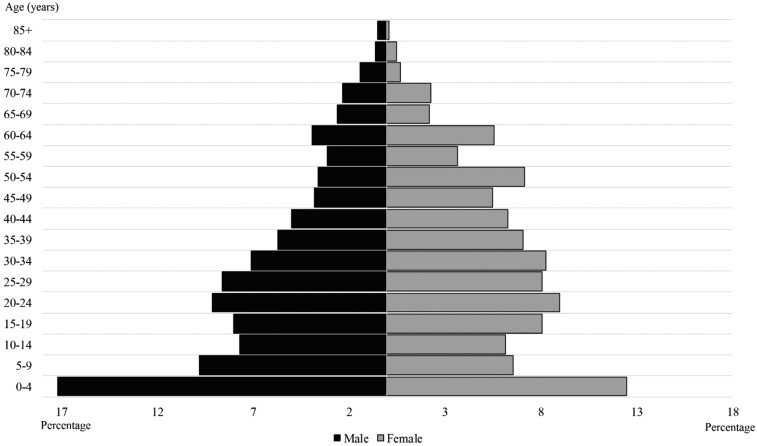
Data were obtained from a total of 20,850 healthcare encounters. The median age of patients was 26 years (interquartile range 11–45 years, range 0–96 years) with 30.9% of individuals aged ≤ 15 years and 11.4% ≥ 60 years. In total, 11,694 patients were female (56.1%), 8,960 were male (43.1%), and gender was undocumented in 170 cases. The age–gender pyramid is depicted in Figure 2. The most prevalent symptoms were headaches (5,913/20,850, 28.4%), gastrointestinal symptoms (4,591/20,850, 22.0%), fever (3,593/20,850, 17.2%), respiratory symptoms (3,563/20,850, 17.1%), and fatigue (3,277/20,850, 15.7%) (Table 1). Among patients with respiratory symptoms, cough was the most prevalent (3,126/3,563, 87.7%), followed by rhinitis (1,791/3,563, 50.3%). Among patients with gastrointestinal symptoms, abdominal or epigastric pain was the most common complaint (2,731/4,591, 59.5%), followed by nausea or vomiting (1,223/4,591, 26.6%).
Figure 2.
Distribution of patients by age and gender (N = 20,656 patients).
With regard to treatments related to infectious diseases (Table 2), 6,210/20,850 (29.8%) patients were prescribed antibiotics (including metronidazole), with penicillin ranking first (19.8% patients), quinolone second (5.3%), and co-trimoxazole third (5.1%), and 549 (2.6%) received antimalarial treatment, with artemisinin-based combination being the most frequent (2.4%) (Table 2). In addition, 1,085 (5.2%) patients received mebendazole, 529 (2.5%) albendazole, and three (0.01%) praziquantel.
Table 2.
Antibiotics and antimalarials prescribed according to symptoms (N = 20,850 patients)
| Treatment | Gastrointestinal symptoms (n = 4,591) | Respiratory tract symptoms (n = 3,563) | Urinary symptoms (n = 227) | Skin symptoms (n = 1,593) | Malaria (n = 624) | Total of population (n = 20,850) |
|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Antibiotics | 949 (20.7) | 2,210 (62.0) | 118 (52.0) | 890 (55.9) | – | 6,210 (29.8) |
| Penicillin | 495 (10.8) | 1,937 (54.4) | 50 (22.0) | 334 (21.0) | – | 4,122 (19.8) |
| Quinolone | 407 (8.9) | 108 (3.0) | 61 (26.9) | 38 (2.4) | – | 1,098 (5.3) |
| Co-trimoxazole | 61 (1.3) | 199 (5.6) | 3 (1.3) | 535 (33.6) | – | 1,072 (5.1) |
| Cycline | 2 (0.04) | 0 | 7 (3.1) | 1 (0.1) | – | 12 (0.06) |
| Cephalosporin | 1 (0.02) | 3 (0.1) | 1 (0.4) | 0 | – | 9 (0.04) |
| Macrolide | 1 (0.02) | 1 (0.03) | 0 | 1 (0.1) | – | 8 (0.04) |
| Metronidazole* | 535 (11.7) | 35 (1.0) | 28 (12.3) | 35 (2.2) | – | 866 (4.2) |
| Antimalarials | – | – | – | – | 549 (88.0) | 549 (2.6) |
| Artemisinin-based combination | – | – | – | – | 502 (80.4) | 502 (2.4) |
| Quinine | – | – | – | – | 69 (11.1) | 69 (0.3) |
* Metronidazole is both an antibacterial and an antiparasitic drug.
A total of 2,345/20,850 (11.2%) patients were tested for malaria, resulting in 508 positive P. falciparum cases (21.7% of the patients tested for malaria). An additional 116 patients were suspected of having malaria, based on the clinical criteria. Overall, 624/20,850 (3.0%) patients received a diagnosis of malaria (either confirmed or suspected), of whom 549 (88.0%) received a documented antimalarial treatment. Of note, information about antimalarial treatment was missing in 75 patients with a diagnosis of malaria.
At the time of data collection, 312 patients (1.5%) were hospitalized, of whom 201 were transferred to Dakar hospitals (1.0%). Among hospitalized patients, most frequent reasons for admission were gastrointestinal symptoms (26.6%), malaria (20.8%), trauma (12.8%), and respiratory symptoms (4.8%). During the collection period, only one death was recorded in a 4-month-old patient who arrived dead at the hospital with no other information available. No follow-up information was available following the data collection period.
Some symptoms have been more frequently reported by men, including fever, skin symptoms, and trauma. Malaria was 1.7 times more prevalent in males. Females were more likely to report headaches, vertigo, high blood pressure, diffuse pain, and arthralgia. Infants were more likely to be affected by fever and malaria, and gastrointestinal, respiratory, and skin symptoms. Older patients were more likely to report high blood pressure, diffuse pain, and arthralgia (Table 3).
Table 3.
Distribution of symptoms and treatment according to gender and age (N = 20,656 patients)
| Total | Gender | P-value | Age | P-value | ||||
|---|---|---|---|---|---|---|---|---|
| Male | Female | ≤ 15 | 16–59 | ≥ 60 | ||||
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |||
| Headache | 5,913 (28.4) | 2,053 (22.9) | 3,822 (32.7) | < 0.0001 | 1,211 (18.8) | 3,963 (33.0) | 735 (31.0) | < 0.0001 |
| Fever | 3,593 (28.4) | 1,718 (19.1) | 1,841 (15.7) | < 0.0001 | 2,085 (32.3) | 1,326 (11.1) | 177 (7.5) | < 0.0001 |
| Fatigue | 3,277 (15.7) | 1,463 (16.3) | 1,789 (15.3) | 0.054 | 224 (3.5) | 2,583 (21.5) | 470 (19.8) | < 0.0001 |
| Vertigo | 1,476 (7.1) | 352 (3.9) | 1,114 (9.5) | < 0.0001 | 110 (1.7) | 1,111 (9.3) | 255 (10.7) | < 0.0001 |
| High blood pressure | 1,398 (6.7) | 342 (3.8) | 1,050 (9.0) | < 0.0001 | 40 (0.6) | 738 (6.2) | 620 (26.1) | < 0.0001 |
| Diffuse pain | 1,216 (5.8) | 380 (4.2) | 828 (7.1) | < 0.0001 | 69 (1.1) | 885 (7.4) | 260 (11.0) | < 0.0001 |
| Arthralgia | 567 (2.7) | 188 (2.1) | 377 (3.2) | < 0.0001 | 38 (0.6) | 344 (2.9) | 185 (7.8) | < 0.0001 |
| Myalgia | 566 (2.7) | 277 (3.1) | 287 (2.5) | 0.006 | 48 (0.7) | 442 (3.7) | 76 (3.2) | < 0.0001 |
| Gastrointestinal symptoms | 4,591 (22.0) | 1,817 (20.2) | 2,518 (21.5) | 0.02 | 1,646 (25.5) | 2,377 (19.8) | 344 (14.5) | < 0.0001 |
| Respiratory symptoms | 3,563 (17.1) | 1,615 (18.0) | 1,921 (16.4) | 0.003 | 1,845 (28.6) | 1,479 (12.3) | 230 (9.7) | < 0.0001 |
| Skin symptoms | 1,593 (7.6) | 965 (10.7) | 616 (5.3) | < 0.0001 | 696 (10.8) | 813 (6.8) | 82 (3.5) | < 0.0001 |
| Dental pain | 750 (3.6) | 358 (4.0) | 385 (3.3) | 0.008 | 185 (2.9) | 532 (4.4) | 33 (1.4) | < 0.0001 |
| Trauma | 607 (2.9) | 412 (4.6) | 192 (1.6) | < 0.0001 | 199 (3.1) | 374 (3.1) | 34 (1.4) | < 0.0001 |
| Conjunctivitis | 300 (1.5) | 199 (2.2) | 221 (1.9) | 0.1 | 174 (2.7) | 198 (1.7) | 49 (2.1) | < 0.0001 |
| Heat stress | 282 (1.4) | 96 (1.1) | 185 (1.6) | 0.002 | 44 (0.7) | 209 (1.7) | 29 (1.2) | < 0.0001 |
| Urinary symptoms | 227 (1.1) | 77 (0.9) | 147 (1.3) | 0.006 | 58 (0.9) | 140 (1.2) | 29 (1.2) | 0.20 |
| Malaria | 624 (3.0) | 357 (4.0) | 264 (2.3) | < 0.0001 | 316 (4.9) | 281 (2.3) | 27 (1.1) | < 0.0001 |
DISCUSSION
Usually, whole families converge on Senegal’s holy city of Touba during the Grand Magal. The results of our study clearly show that people of all ages (children and adults), women and men, consult healthcare structures during the GMT in marked contrast with what is observed during the Hajj pilgrimage in Mecca, Saudi Arabia, where most pilgrims are adults.10 In a study conducted from 2012 to 2017, the median age of 783 French Hajj pilgrims was 62 years, with a minimum of 21 and maximum of 96 years and with a high proportion of chronic underlying diseases, including 28.0% diabetes mellitus and 9.7% chronic respiratory disease.11 The high proportion of young children in our results reflects the Senegalese population in general.12 The specific demographic characteristics of the GMT population suggest that pediatricians or healthcare workers with training in pediatrics should be part of the medical staff deployed during the GMT to attend patients. In total, the medical staff of the region of Diourbel has eight pediatricians who are reinforced by three pediatricians from the Ministry of Health during the GMT 2016.
The clinical profile of patients consulting during the GMT shows that the most frequent conditions were gastrointestinal and respiratory diseases. The most common gastrointestinal symptoms were abdominal and gastric pain, nausea, vomiting, and diarrhea, suggesting that most patients had gastroenteritis. The predominance of cough, rhinitis, influenza-like illness, and sore throat among patients with respiratory symptoms suggests that most patients suffered from upper respiratory tract infections (URTIs). According to the Institute for Health Metrics and Evaluation, diarrhea and respiratory tract infections were the most prevalent diseases in 2016, accounting for 8.42% and 7.48% of total disability-adjusted life years, respectively, in Senegal.13 Our results are in line with those documented by our team in 2017 in a small cohort of GMT pilgrims where respiratory tract infection (RTI) and Gastrointestinal infection (GI) infection symptoms were predominant.6 Interestingly, RTI symptoms and diarrhea also account for most of the reasons for consultation and hospitalization during Hajj and other religious MGs.14 The predominance of RTI symptoms in GMT pilgrims is probably due to interhuman transmission of respiratory pathogens during travel to Touba in overcrowded buses and other overcrowded areas, in and around the mosque during the rituals. GI infections are likely the result of poor sanitary conditions during the GMT, with food prepared collectively by nonprofessional persons.
Therefore, preparedness of the medical infrastructure dedicated to the GMT should target these syndromic features, in terms of diagnostic tools and specific treatments, including pediatric formulation, because these conditions were frequent among children. The high frequency of pain and fever among the general symptoms is also indicative of the need for analgesics and antipyretics. The high 30% prescription rate of antibiotics in patients during the GMT, which is mostly empiric, given that only 6% of patients benefit underwent laboratory analysis (out of the malaria rapid test) based on the 2015 GMT data,1 represents a significant expanse of likely unnecessary antibiotics as most URTIs are due to viruses with no formal need for antibiotic treatment.15 Identification of the main microbiological agents responsible for respiratory or digestive diseases and nonmalarial fever would help rationalizing the prescription of antibiotics and antiparasitic drugs at the GMT. Studies conducted on a representative sample of patients recruited in key healthcare settings could potentially provide useful insights into the etiology of the main clinical presentation to the GMT as a basis for more reliable treatment algorithms. Choice of first-line antibiotics was globally in accordance with WHO recommendations at the time the survey was carried out (available from https://www.who.int/medicines/publications/essentialmedicines/en/), with penicillin ranking first in patients suffering RTIs and GI symptoms. Nevertheless, quinolones ranked first in patients with urinary tract infection symptoms and second in those with RTI and GI symptoms, although the WHO recommends limiting their use because of the risk of development of resistance.
These data also provide indications on which to base individual preventive measures to limit the occurrence of respiratory tract and gastrointestinal infections, including reinforced hand hygiene, cough etiquette, and specific vaccination. In a cohort survey that our group conducted in 2017, 46.4% of pilgrims reported washing their hands more often than usual during the GMT and 63.6% used hand gel frequently; 32.3% and 2.8% of pilgrims reported using frequently disposable handkerchiefs and face mask, respectively, during the pilgrimage. Only one pilgrim was vaccinated against influenza.6 Control of the quality of drinking water and foodstuffs, inspection of main kitchens and catering areas, treatment of waste water and cleaning and disinfection of areas frequented by pilgrims, and disinfection of landfills are carried out by the Ministry of Health and municipal staff. However, strict microbiological control of quality of drinking water sources and food items, and microbiological testing of kitchen staff and kitchen environments are not conducted.6
The relatively low rate (3.0%) of malaria observed during the 2016 Magal is in line with the national surveillance data from the Senegalese National Malaria Control Program (2.7% malaria cases among 205,844 patients from November 16 to November 21, 2016).16 A substantial decrease in the burden of malaria has been observed over the past decade in many areas in Senegal, in relation to the massive scale-up of malaria interventions with artemisinin-based combination therapies (ACTs) and long-lasting insecticidal treated bed nets (LLINs).17–20 In Dielmo, a longitudinal malaria survey has been conducted since 1990.21 In 1990, the parasite and spleen rates in children were about 90%. Twenty-two years later, the parasite rate was only 0.3%, in both children and adults, and the incidence of malaria outbreaks in the community decreased 98-fold among children and 12-fold among adults between 2000 and 2012.20 The choice of ACTs used as first-line treatment and the universal deployment of LLINs were the most important factors for the great changes in malaria morbidity in Senegal.22 Drug selection and prompt access to treatment seem crucial. In addition, insecticide sprays are applied to the religious sites and houses of the main marabouts in Touba during the Grand Magal.
This successful situation is, however, fragile because LLINs are most of the time freely distributed to the population in Senegal through foreign funding (President’s Malaria Initiative funding, Global Fund), and malaria resurgences could happen if such resources are reduced.23
The low hospitalization rate observed in our survey should be interpreted with caution, given that only 481 beds are available in the medical region of Diourbel (391 for tertiary care hospitals and 90 for secondary care hospitals), based on the 2015 data. Nevertheless, the very low mortality rate is indicative of the relative mildness of diseases in the context of the GMT.
Some limitations were observed in this study. Notably, the only source of our data in this study is the general consultation registers from the medical region of Diourbel set up every year during the GMT. These registers are manually documented in free text, and, as a result, information was missing in some cases or difficult to classify. In addition, the clinical nature of the data does not allow the identification of the nature of pathogens potentially responsible for infectious diseases in the GMT. However, because comparison with data before and after the GMT has not been performed, it is, therefore, not possible to state if the clinical profile of diseases during the event is qualitatively influenced by the context beyond the numerical increase in consultations. Also, we have no information about the follow-up of the patients and were not able to confirm that the diagnosis was correct and the treatments were effective. Finally, we have no information about prevention behaviors. Slight adjustment of data collection forms would be useful to better document these aspects. Nevertheless, the large amount of data provides useful insights into the clinical profile of patients presenting in public health facilities during the GMT, on which to base the preparation of medical coverage for future GMTs.
In summary, maintaining a surveillance program is essential during the GMT because the epidemiology of infectious diseases is constantly evolving as exemplified by the recent dengue epidemic during the 2018 Magal.24,25 It is also necessary to improve the quality and rapid availability of data to enable real-time analysis of medical events at the GMT and to implement a rapid response if necessary. Our data, based essentially on syndromic surveillance, suggest that the most common causes of consultation are gastroenteritis, URTI, fever, and pain. In terms of preventive measures, hand hygiene and cough etiquette should be recommended. Pain killer and antipyretics (ibuprofen and paracetamol), oral rehydration salts, and antidiarrheal and antiemetic drugs, including pediatric formulation, should be widely available during the GMT.
Finally, microbiological documentation of infections should be carried out to identify the pathogens circulating at the GMT based on the implementation of point-of-care laboratories for rapid diagnosis and timely prescription of antibiotics, antimalarials, oseltamivir, antiamoebic and antigiardiasis medicines, or intestinal antihelminthics in patients with respiratory or digestive diseases and malarial or nonmalarial fever.26,27 Implementation of preventive measures and provision of adapted curative treatment would limit the spread of communicable diseases among Senegalese and surrounding populations with low level of financial resources and help reduce the financial burden of infections associated with the Grand Magal.
REFERENCES
- 1.Sokhna C, et al. 2017. Communicable and non-communicable disease risks at the Grand Magal of Touba: the largest mass gathering in Senegal. Travel Med Infect Dis 19: 56–60. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization , 2015. Public Health for Mass Gatherings: Key Considerations. Geneva, Switzerland: WHO. [Google Scholar]
- 3.Al-Tawfiq JA, Memish ZA, 2012. Mass gatherings and infectious diseases: prevention, detection, and control. Infect Dis Clin North Am 26: 725–737. [DOI] [PubMed] [Google Scholar]
- 4.Gautret P, Steffen R, 2016. Communicable diseases as health risks at mass gatherings other than Hajj: what is the evidence? Int J Infect Dis 47: 46–52. [DOI] [PubMed] [Google Scholar]
- 5.Hoang VT, Gautret P, 2018. Infectious diseases and mass gatherings. Curr Infect Dis Rep 20: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoang VT, et al. 2019. Respiratory and gastrointestinal infections at the 2017 Grand Magal de Touba, Senegal: a prospective cohort survey. Travel Med Infect Dis 32: 101410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization , 2015. Guidelines for the Treatment of Malaria – 3rd edition. Available at: https://apps.who.int/iris/bitstream/handle/10665/162441/9789241549127_eng.pdf?sequence=1. Accessed November 6, 2019. [PubMed] [Google Scholar]
- 8.Rashid H, Shaf S, El Bashir H, Haworth E, Memish ZA, Ali KA, Booy R, 2008. Influenza and the Hajj: defining influenza-like illness clinically. Int J Infect Dis 12: 102–103. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organisation, Regional Office of the Eastern Mediterranean , 2005. Technical Publications Series 29. Clinical Guidelines for the Management of Hypertension. Available at: http://applications.emro.who.int/dsaf/dsa234.pdf. Accessed November 6, 2019. [Google Scholar]
- 10.Memish ZA, et al. 2014. Hajj: infectious disease surveillance and control. Lancet 383: 2073–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoang VT, et al. 2019. Antibiotic use for respiratory infections among Hajj pilgrims: a cohortsurvey and review of the literature. Travel Med Infect Dis 30: 39–45. [DOI] [PubMed] [Google Scholar]
- 12.CIA World Factbook Senegal Age Structure – 2016. Available at: https://www.indexmundi.com/senegal/age_structure.html. [Google Scholar]
- 13.Institute for Health Metrics and Evaluation , 2016. GDB Compare. Available at: https://vizhub.healthdata.org/gbd-compare/. Accessed November 6, 2019. [Google Scholar]
- 14.Memish ZA, Steffen R, White P, Dar O, Azhar EI, Sharma A, Zumla A, 2019. Mass gatherings medicine: public health issues arising from mass gathering religious and sporting events. Lancet 393: 2073–2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Milani RV, Wilt JK, Entwisle J, Hand J, Cazabon P, Bohan JG, 2019. Reducing inappropriate outpatient antibiotic prescribing: normative comparison using unblinded provider reports. BMJ Open Qual 8: e000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.PNLP , 2016. Bulletin de surveillance sentinelle du paludisme du 21 au 27 novembre 2016, No BSEP-47/2016. Dakar, Sénégal: Ministère de la Santé et l’Action Sociale, Available at: www.pnlp.sn/wp-content/uploads/2016/12/Bulletin-Surveillance-Paludisme-au-SENEGAL-N°47_2016.pdf. Accessed November 6, 2019. [Google Scholar]
- 17.WHO , 2010. Roll Back Malaria: Focus on Senegal. Progress & Impact Series. vol. November. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 18.Brasseur P, Badiane M, Cisse M, Agnamey P, Vaillant MT, Olliaro PL, 2011. Changing patterns of malaria during 1996–2010 in an area of moderate transmission in southern Senegal. Malar J 10: 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wotodjo AN, et al. 2015. The implication of long-lasting insecticide-treated net use in the resurgence of malaria morbidity in a Senegal malaria endemic village in 2010–2011. Parasit Vectors 8: 267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trape JF, et al. 2014. The rise and fall of malaria in a west African rural community, Dielmo, Senegal, from 1990 to 2012: a 22 year longitudinal study. Lancet Infect Dis 14: 476–488. [DOI] [PubMed] [Google Scholar]
- 21.Trape JF, Rogier C, Konate L, Diagne N, Bouganali H, Canque B, Legros F, Badji A, Ndiaye G, Ndiaye P, 1994. The Dielmo project: a longitudinal study of natural malaria infection and the mechanisms of protective immunity in a community living in a holoendemic area of Senegal. Am J Trop Med Hyg 51: 123–137. [DOI] [PubMed] [Google Scholar]
- 22.WHO , 2012. World Malaria Report 2012. Geneva, Switzerland: WHO. [Google Scholar]
- 23.Cohen JM, Smith DL, Cotter C, Ward A, Yamey G, Sabot OJ, Moonen B, 2012. Malaria resurgence: a systematic review and assessment of its causes. Malar J 11: 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.WHO Regional Office for Africa , 2018. Weekly Bulletin on Outbreaks and Other Emergences. Week 43: 20–26 October 2018. Available at: http://apps.who.int/iris/bitstream/handle/10665/275620/OEW43-2026102018.pdf. Accessed November 6, 2019. [Google Scholar]
- 25.Sokhna C, Goumballa N, Gautret P, 2018. The Grand Magal of Touba in the time of a dengue outbreak in Senegal. Travel Med Infect Dis 28: 107–108. [DOI] [PubMed] [Google Scholar]
- 26.Sokhna C, Mediannikov O, Fenollar F, Bassene H, Diatta G, Tall A, Trape JF, Drancourt M, Raoult D, 2013. Point-of-care laboratory of pathogen diagnosis in rural Senegal. PLoS Negl Trop Dis 7: e1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abat C, Colson P, Chaudet H, Rolain J-M, Bassene H, Diallo A, Mediannikov O, Fenollar F, Raoult D, Sokhna C, 2016. Implementation of syndromic surveillance systems in two rural villages in Senegal. PLoS Negl Trop Dis 10: e0005212. [DOI] [PMC free article] [PubMed] [Google Scholar]