Abstract.
Children with malnutrition compared with those without are at higher risk of infection, with more severe outcomes. How clinicians assess nutritional risk factors in febrile children in primary care varies. We conducted a post hoc subgroup analysis of febrile children with severe malnutrition enrolled in a randomized, controlled trial in primary care centers in Tanzania. The clinical outcome of children with severe malnutrition defined by anthropometric measures and clinical signs was compared between two electronic clinical diagnostic algorithms: ePOCT, which uses weight-for-age and mid-upper arm circumference to identify and manage severe malnutrition, and ALMANACH, which uses the clinical signs of edema of both feet and visible severe wasting. Those identified as having severe malnutrition by the algorithms in each arm were prescribed antibiotics and referred to the hospital. From December 2014 to February 2016, 106 febrile children were enrolled and randomized in the parent study, and met the criteria to be included in the present analysis. ePOCT identified 56/57 children with severe malnutrition using anthropometric measures, whereas ALMANACH identified 2/49 children with severe malnutrition using clinical signs. The proportion of clinical failure, defined as the development of severe symptoms by day 7 or persisting symptoms at day 7 (per-protocol), was 1.8% (1/56) in the ePOCT arm versus 16.7% (8/48) in the Algorithm for the MANagement of Childhood illnesses arm (risk difference −14.9%, 95% CI −26.0%, −3.8%; risk ratio 0.11, 95% CI 0.01, 0.83). Using anthropometric measures to identify and manage febrile children with severe malnutrition may have resulted in better clinical outcomes than by using clinical signs alone.
INTRODUCTION
Malnutrition affects more than 150 million children aged less than 5 years around the world1 and has been suggested to reduce host defense and favor microbial translocation,2,3 leading to much higher incidence of infections in malnourished than well-nourished children.2,4–6 Furthermore, infections are often more severe among malnourished children, with higher mortality rates among malnourished children than children without malnutrition,7–11 contributing to 14% of child mortality worldwide.12 Complicating matters for clinicians is the fact that children with malnutrition present fewer clinical signs suggestive of serious infections than do children who are better nourished.2,6
Whereas substantial research has concentrated on preventing infections in children identified as having severe malnutrition, the point of entry into care is often the reverse: children present with an acute complaint to a primary care provider, who must then identify malnutrition as a risk factor for more severe outcomes. How primary care providers assess malnutrition in acute care settings varies widely.
The 2008 Integrated Management of Childhood Illness (IMCI) guideline by the WHO for primary care providers in low-resource settings first proposed the classification of severe malnutrition necessitating referral to a hospital for children with visible severe wasting or edema of both feet.13 In 2009, the WHO and United Nations Children’s Fund updated the recommendations for the diagnostic criteria for severe acute malnutrition, which was later included in the 2014 Integrated Management of Neonatal and Childhood Illness (IMNCI) guideline.14,15 They proposed the use of anthropometric measures (weight-for-height [WFH] or mid-upper arm circumference [MUAC]) and edema of both feet to identify children with severe acute malnutrition, and further stratify those who need to be referred: complicated or uncomplicated (Table 1). In reality however, primary care providers in low-resource settings rarely take anthropometric measures. Height measurements specifically are logistically challenging to accurately assess in the short consultation time available, and height measurement devices may not be available and are overall judged as not feasible by pediatric experts in low-resource settings.16–19 Mid-upper arm circumference and, to a lesser extent, weight-for-age (WFA) were found to be better than WFH for classifying malnutrition in terms of age independence, precision, accuracy, sensitivity, and specificity,20 and better or as well as WFH in predicting mortality.21,22 Meanwhile, the use of clinical signs alone for the detection of severe malnutrition (visible wasting and edema) by low-level health workers was found to be inaccurate, missing approximately half of children with severe malnutrition based on anthropometric measures or expert examination.23,24 Based on this evidence, WFA and MUAC were selected to assess nutritional status within a novel electronic clinical decision algorithm (eCDA) designed for the management of febrile children at the primary care level (ePOCT).25
Table 1.
Classification of severe malnutrition
| Source | Label | Definition | Need for hospital referral |
|---|---|---|---|
| Subgroup analysis study definition | Severe malnutrition | WFA < −3 z-scores* or | Yes |
| MUAC < 11.5 cm† or | |||
| Visible severe wasting or | |||
| Edema of both feet | |||
| ePOCT | Severe malnutrition | WFA < −3 z-scores * or | Yes |
| MUAC < 11.5 cm† | |||
| ALMANACH | Severe malnutrition | Visible severe wasting or | Yes |
| Edema of both feet | |||
| Integrated Management of Childhood Illness (IMCI) 2008‡ | Severe malnutrition | Visible severe wasting or | Yes |
| Edema of both feet | |||
| IMNCI 2014§ | Uncomplicated severe acute malnutrition | WFH/L < −3 z-scores§ or | No |
| MUAC < 11.5 cm† or | |||
| Able to finish RUTF | |||
| IMNCI 2014‡ | Complicated severe acute malnutrition | Edema of both feet or | Yes |
| WFH/L < −3 z-scores or MUAC < 11.5 cm, and any of the following:†‖ | |||
| Medical complication present | |||
| Unable to finish RUTF¶ | |||
| Breastfeeding problem |
* WFA is weight-for-age determined by using the WHO growth standards charts.
† MUAC is mid-upper arm circumference measured only in children aged 6 months or older.
‡ At the time of the study, Integrated Management of Childhood Illness 200 was in use in Tanzania.
§ IMNCI is Integrated Management of Childhood and Neonatal Ilness by the WHO.
‖ WFH/L is weight-for-height or weight-for-length determined using the WHO growth standards charts.
¶ RUTF is ready-to-use therapeutic food for conducting the appetite test and feeding children with severe acute malnutrition.
The aim of this post hoc subgroup analysis of patients with fever and severe malnutrition as defined by a combination of anthropometric measures and clinical signs was to compare the identification and clinical outcomes using two different clinical algorithms: ePOCT algorithm, which uses WFA and MUAC, and Algorithm for the MANagement of Childhood illnesses (ALMANACH), which uses clinical signs alone. We hypothesized that the ePOCT algorithm would identify more children with severe malnutrition than would ALMANACH and that identification would improve clinical management in this high-risk group, thus resulting in superior clinical outcomes among children managed with ePOCT than ALMANACH.
Key messages.
1. Children with malnutrition are at higher risk of infection, with more severe outcomes, yet assessment of malnutrition in acute care settings varies widely.
2. Anthropometric measures of WFA and MUAC identified more children with severe malnutrition than clinical signs (visible wasting and edema of both feet) alone.
3. Systematically identifying and managing severe malnutrition based on WFA and MUAC in children with acute febrile illness resulted in better clinical outcomes.
4. Systematic screening for malnutrition using anthropometric measurements should be implemented in the primary care management of children with acute infections.
METHODS
Study design.
This was a post hoc subgroup analysis of children with severe malnutrition from within an open-label randomized controlled non-inferior trial that compared a novel eCDA using point of care tests (ePOCT) with a validated eCDA derived from IMCI 2008 (ALMANACH) when managing febrile children aged less than 5 years.25 The overall aim of the parent study was to compare clinical outcome and antibiotic prescription between both eCDAs. The study was conducted at nine public outpatient clinics in the city of Dar es Salaam, Tanzania. The overall methods and results of this trial are reported elsewhere.25 The protocol, statistical analysis plan, and CONSORT checklist are available in the supporting files of the main publication.25
Ethics.
The study protocol and related documents were approved by the Institutional Review Board of the Ifakara Health Institute and by the National Institute for Medical Research Review Board in Tanzania, by the Ethikkommission beider Basel in Switzerland, and by the Boston Children’s Hospital Ethical Review Board.
Participants.
Consecutive patients presenting during normal business hours were screened for eligibility. For the main trial, the inclusion criteria were age 2–59 months, history of fever for more than 7 days or less, and axillary temperature ≥ 37·5°C. Exclusion criteria were weight < 2.5 kg, main complaint as injury or acute poisoning, and previous medical care for the present illness. There were, however, no children older than 2 months with weight less than 2.5 kg who were excluded. Children were enrolled if the guardian signed a detailed written informed consent. For this subgroup analysis, we included all children with severe malnutrition, defined as WFA less than −3 z-scores calculated using the WHO growth standards26; children aged >/ = 6 months with mid-upper arm circumference less than 11.5 cm; or children with clinical signs of severe malnutrition (visible severe wasting or edema of both feet) (Table 1). The use of the term severe malnutrition in this article, therefore, includes all the definitions outlined in Table 1, including severe acute malnutrition. The main study also included a routine care cohort; this cohort was not used for this subgroup analysis because routine clinicians did not systematically record anthropometric or clinical signs of malnutrition.
Randomization.
Patients were enrolled by the study clinicians and individually randomized to one of the management arms in blocks of four according to a computer-generated list provided by an independent, off-site researcher. Sealed, opaque forms were used for allocation concealment and opened only after the patient's enrollment.
Procedures.
The intervention consisted in having study clinicians use the ePOCT eCDA (intervention arm) or ALMANACH eCDA (control arm) during the consultation to manage the patient. The development and content of the ePOCT and ALMANACH management algorithms are described elsewhere.25,27 The overall goal of the algorithms was to better manage children with an acute illness. Identification of severe malnutrition was part of the many assessments carried out within the algorithms to improve clinical care. Weight and MUAC were measured in patients at enrollment and clinical signs for malnutrition recorded (visible severe wasting/edema). The ePOCT eCDA classified children with severe malnutrition using the combination of anthropometric measures of very low WFA < −3 z-scores and/or MUAC < 11.5 cm for patients aged >/ = 6 months. The ALMANACH classified severe malnutrition using the clinical signs of “visible severe wasting” or “edema of both feet’”as per WHO 2008 IMCI guidelines used at the time in Tanzania (Table 1).13 Although IMCI 2008 and ALMANACH used WFA to identify children in need of nutritional counseling, this did not alter the management of the acute febrile illness. Mid-upper arm circumference was, thus, recorded for research purposes only in the ALMANACH arm, whereas clinical signs were only recorded for research purposes in the ePOCT arm. Training and provision of standard operating procedures on the correct measurement of anthropometric measures were provided to all study clinicians. A standard MUAC measurement tape was provided, and weight scales were calibrated daily with standard calibration weights.
In the ePOCT arm, children identified as having severe malnutrition received per os (PO) amoxicillin (intramuscular [IM] ceftriaxone if unable to take PO medications), along with correction of low blood sugar and oral rehydration, and were referred to the hospital. In the ALMANACH arm, IM ceftriaxone, prevention of low blood sugar, and urgent referral to the hospital were part of the management of severe malnutrition. After identification, management, and referral for severe malnutrition by each respective algorithm, not all investigations for other severe diseases were performed. Patients referred to the hospital were admitted and managed at the discretion of the hospital personnel. For referred patients who were not admitted and those discharged after hospitalization, oral amoxicillin was continued for a total of 7 days, and enrollment in an outpatient therapeutic feeding program was initiated at the discretion of the hospital clinicians. The proportion of children enrolled in an outpatient therapeutic feeding program was not collected.
Laboratory point-of-care tests were performed on-site according to the recommendation given by the assigned eCDAs. Malaria rapid diagnostic test (mRDT) was carried out for all patients in both arms using either the SD BIOLINE Malaria Ag P.f/Pan™ (Standard Diagnostics Inc., Gyeonggi-do, Republic of Korea) or the CareStart Malaria HRP2™ (Access Bio, Inc., Sommerset, NJ) assay. Voluntary screening for HIV antibodies using the Determine™ HIV-1/2 (Alere Inc., Waltham, MA) assay was offered to all patients in both arms when HIV test kits were available at the health facilities.
All caregivers were asked to return with the child for a scheduled visit on day 3 (D3) and D7 post-randomization (D0), or at any time if the parent was concerned about the child’s condition. Patients cured at D3 (see Table 2 for definition of clinical failure/not cured) were followed up by a phone call on D7. Field workers traced patients missing on the D7 visit. Patients not cured at D3 were managed again according to the assigned eCDA and returned at D7, whereas patients not cured at D7 were treated according to the clinician’s judgment; however, children with persisting fever without focal clinical signs systematically received ciprofloxacin in both arms. Those not cured at D7 had another follow-up visit on D14 to assure that the child was cured. All patients were called by telephone on D30 to inquire about interim hospitalizations or deaths.
Table 2.
Definition of clinical failure by day 7 (primary outcome measure)
| At any time between initial assessment and day 7 | At day 3 | At day 7 |
|---|---|---|
| Severe disease | Clinical pneumonia | Fever or temperature ≥ 38°C |
| Coma | History of cough and tachypnea‡ | Clinical pneumonia |
| More than two convulsions within 24 hours | History of cough and lower chest indrawing | History of cough and tachypnea‡ |
| Inability to drink or breastfeed | Significant dehydration§ | History of cough and lower chest indrawing |
| Hypoxemia (SaO2 < 90%) | Diarrhea‖ | |
| Severe tachypnea* | Significant dehydration§ | |
| Severe tachycardia† | Serious skin infection¶ | |
| A new significant symptom or sign related to the acute episode but not present at day 0 |
* Respiratory rate (RR) ≥ 97th %ile for age and temperature.46
† Heart rate ≥ 90th %ile for age and temperature.47
‡ Respiratory rate ≥ 60/minutes and age < 12 months or RR ≥ 50/minutes and age ≥ 12 months.
§ Dehydration requiring facility-based treatment.
‖ ≥ 3 liquid stools per day.
¶ Skin infection requiring systemic antibiotic treatment and/or facility-based treatment.
Outcomes.
The primary outcome measure was the proportion of children with clinical failure (see definition in Table 2) by D7. At follow-up, clinicians recorded the variables that were used to calculate the criteria for clinical failure according to Table 2. (These variables were either already part of the eCDA assessment or otherwise recorded on paper forms.) However, the clinicians were unaware of the study criteria for clinical failure and how the variables recorded were used to calculate study outcomes. The study outcomes were not used to decide on patient management.
The secondary outcome measures were the proportion of primary referrals and admissions, the proportion of antibiotic prescription at D0 and between D1 and D6, and the proportion of severe adverse events (secondary hospitalizations and death) by D30.
Statistical analysis.
The sample size was computed for the primary analysis for the parent study.25 In the present subgroup analysis, for the observed proportion of clinical failure and total available sample size of 106 subjects, with an alpha level of 0.05, we had 76% power to detect the observed difference in clinical failures between the two groups.
The modified intention-to-treat (mITT) population comprised all randomized patients within the sub-study population for which the inclusion criteria for this subgroup analysis could be assessed (all patients for whom data were entered into the eCDA on D0). According to the definition, patients who were lost for follow-up were treated as clinical failures. The per-protocol (PP) population included all randomized patients who received the intervention and completed the D7 assessment (Figure 1). The primary outcome was calculated for both the intention-to-treat (ITT) and PP populations. Because the ITT and PP populations were almost the same, we used the PP analysis as our primary analysis. Risk difference (RD) and relative risk (RR) values with 95% CIs were calculated to estimate the intervention effects on the main study outcomes using the Stata cs procedure. Odds ratios were used as approximated RR because the primary outcome was rare. The trial was registered in ClinicalTrials.gov, identifier NCT02225769. We used Stata version 15.1 for the analysis.
Figure 1.
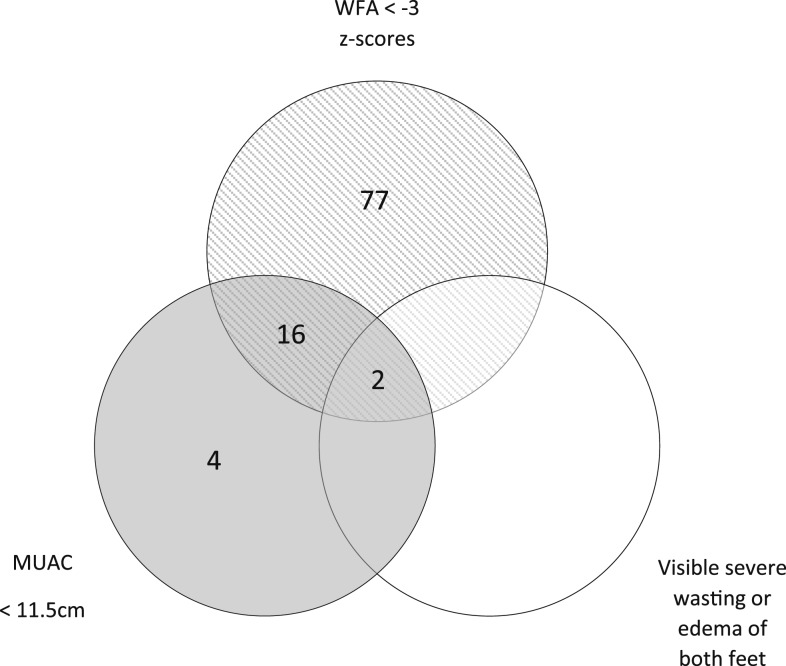
Venn diagram of severe malnutrition detection. Number of children aged 6–59 months identified as having severe malnutrition using mid-upper arm circumference < 11.5 cm, weight-for-age < − 3 z-scores, and visible severe wasting or edema of both feet.
RESULTS
Trial participants.
From December 2014 through February 2016, 4,729 age-eligible patients were screened and 3,192 randomized into the main parent trial (Figure 1). Five patients in each arm withdrew before data were entered into the eCDA. The mITT population of patients with severe malnutrition for this analysis included 106 patients: 57 (54%) in the ePOCT arm and 49 (46%) in the ALMANACH arm. Among patients older than 6 months, WFA identified severe malnutrition in 77/96 patients for whom the MUAC was above 11.5 cm, whereas the MUAC identified 4/96 patients in whom the WFA was above −3 z-scores (Figure 1). The two patients identified as having visible severe wasting also met the criteria for severe malnutrition using both WFA and MUAC. One patient in each arm was lost to follow-up for the D7 outcome assessment and D30 phone follow-up (Figure 2).
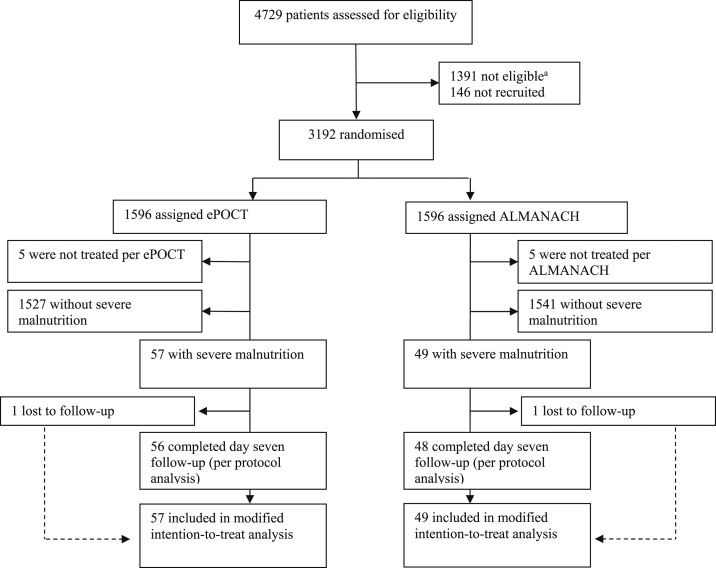
Figure 2.
Patient flowchart. Flow diagram of the progress of patients through the different phases of enrollment, allocation, and follow-up, and analysis of this randomized trial subgroup analysis.a “Not eligible” refers to patients who did not meet the inclusion or exclusion criteria.
Children in both arms were young and presented to care shortly after the onset of illness, with similar anthropometric values. Among the children with severe malnutrition classified based on a combination of anthropometric measures and clinical signs and included in this subgroup analysis, ePOCT identified 56/57 (98%) children with severe malnutrition based only on anthropometric values, whereas ALMANACH identified 2/49 (4%) children based only on clinical signs (Table 3).
Table 3.
Baseline characteristics
| Characteristic | ePOCT | ALMANACH | ||
|---|---|---|---|---|
| N | n (%) or median (IQR) or mean (SD) | N | n (%) or median (IQR) or mean (SD) | |
| Demographic | ||||
| Male gender | 57 | 31 (54%) | 49 | 27 (55%) |
| Age group (months) | 57 | 49 | ||
| 2–11 | 29 (51%) | 26 (53%) | ||
| 12–23 | 21 (37%) | 13 (27%) | ||
| ≥ 24 | 7 (12%) | 10 (20%) | ||
| Primary caregiver other than mother | 56 | 2 (4%) | 48 | 2 (4%) |
| Mother’s highest grade of education | 56 | 48 | ||
| None | 7 (13%) | 9 (19%) | ||
| Primary | 41 (75%) | 30 (63%) | ||
| Postprimary | 7 (13%) | 9 (19%) | ||
| Number of children in household, median (IQR) | 55 | 2 (1, 3) | 48 | 2 (1, 3) |
| Medical history | ||||
| Symptoms | ||||
| Diarrhea | 57 | 14 (25%) | 49 | 17 (35%) |
| Cough | 57 | 33 (58%) | 49 | 30 (61%) |
| Skin problem | 51 | 0 (0%) | 48 | 4 (8%) |
| Duration of fever (days) | 56 | 49 | ||
| 1 or less | 29 (52%) | 26 (51%) | ||
| 2–4 | 26 (46%) | 21 (43%) | ||
| 5 or more | 1 (2%) | 3 (6%) | ||
| Clinical characteristics | ||||
| Weight-for-age z-score <−3* | 57 | 54 (95%) | 49 | 47 (96%) |
| Weight-for-age z-score, median (IQR)* | 57 | −3.5 (−3.8, −3.2) | 49 | −3.6 (−4.0, −3.2) |
| Mid-upper arm circumference < 11.5 cm if age > 6 months | 53 | 10 (19%) | 43 | 10 (23%) |
| Mid-upper arm circumference for age z-score if > 6 months, mean (SD)* | 53 | −2.0 (1.1) | 43 | −1.9 (1.3) |
| Clinical signs of severe acute malnutrition | ||||
| Visible severe wasting | 57 | 0 | 49 | 2 (4%) |
| Edema of both feet | 57 | 0 | 49 | 0 |
| Complicated severe malnutrition† | 57 | 3 (5%) | 49 | 7 (14%) |
| Respiratory rate, median (IQR) | 57 | 40 (36, 48) | 49 | 47 (38, 58) |
| Heart rate, mean (SD) | 57 | 147 (16) | 49 | 146 (15) |
| Axillary temperature, median (IQR) | 57 | 38.0 (37.8, 38.4) | 49 | 38.3 (37.9, 38.7) |
| Laboratory values | ||||
| Malaria rapid diagnostic test positive | 57 | 3 (5%) | 49 | 1 (2%) |
| HIV-1/2 antibody positive | 50 | 2 (4%) | 43 | 3 (7%) |
| Hemoglobin (g/dL)‡ | 57 | 9.6 (8.8, 10.4) | 4 | 9.7 (9.3, 9.95) |
| Moderate anemia according to WHO | 57 | 34 (60%) | 4 | 3 (75%) |
| Severe anemia according to WHO | 57 | 5 (9%) | 4 | 0 |
| Positive blood cultures | 54 | 4 (7%)§ | 11 | 0 (0%) |
| Algorithm disease classification | ||||
| Severe malnutrition‖ | 57 | 56 (98%) | 49 | 2 (4%) |
| Severe disease other than malnutrition‖¶ | 57 | 2 (4%) | 49 | 5 (10%) |
| Gastrointestinal disease‖ | 57 | 13 (23%) | 49 | 15 (31%) |
| Pneumonia‖ | 57 | 0 (0%) | 49 | 21 (43%) |
| Fever without a clinical source‖ | 57 | 0 (0%) | 49 | 11 (22%) |
Data are n (%), median (IQR), mean (SD), or n/N (%).
* WHO 2006 growth curve.28
† Complicated severe malnutrition definition adapted from the 2014 Integrated Management of Neonatal and Childhood Ilnesses (IMNCI) guidelines, defined as for the purpose of this study: WFA < −3 z-score or MUAC < 11.5 cm if age >/ = 6 months and the presence of any danger sign, severe classification, or tachypnea with chest indrawing.
‡ Moderate anemia defined as Hb 7–9.9 g/dL, and severe anemia as Hb < 7g/dL. Only performed systematically with the ePOCT algorithm. Four patients in the ALMANACH arm had Hb measured at the referral hospital.
§ Excluding contaminants, the pathogens were Salmonella paratyphi B, Staphylococcus aureus, Acinetobacter baumannii/coacetius, and Vibrio vulnificus.
‖ As defined by each respective algorithm.
¶ The detection of other severe diseases was limited once one severe disease (such as severe malnutrition) was identified.
The ePOCT algorithm integrated POCT including hemoglobin (Hb), which identified 34 severely malnourished children (60%) with moderate anemia as defined by the WHO (Hb 7 to 9.9 g/dL) and five (9%) with severe anemia (Hb < 7 g/dL).29 Blood cultures were positive for a pathogen in four (8%) severely malnourished children in the ePOCT arm and negative in all severely malnourished children in the ALMANACH arm.
Outcomes.
In the PP analysis, 1.8% (1/56) of patients in the ePOCT arm experienced clinical failure by D7 versus 16.7% (8/48) in the ALMANACH arm (RD −14.9%, 95% CI −26.0% to −3.8%; Table 4). The RR of clinical failure among those with severe malnutrition was 89% lower in the ePOCT arm than the ALMANACH arm (RR 0.11, 95% CI 0.01 to 0.83). The proportion of clinical failures was the same across age groups. In the ePOCT arm, 98.2% (55/56) of children received antibiotics at day 0 and were referred to the hospital for management of severe malnutrition; 19.6% (11/56) were admitted based on the hospital staff’s judgment. In the ALMANACH arm, only 47.9% (23/48) of children received antibiotics at day 0, 14.6% (7/48) were referred to the hospital, and 10.4% (5/48) were admitted. Only two of those receiving antibiotics and referred to the hospital in the ALMANACH arm were referred because of severe malnutrition, and neither was admitted. There was no significant difference in the proportion of secondary admissions and death in the ePOCT arm compared with the ALMANACH arm: 1.8% (1/56) versus 4.2% (2/49, RR 0.43, 95% CI 0.04–4.58), respectively (Table 4). There was one death in each arm and one secondary admission in the ALMANACH arm. The results were similar in the intention-to-treat analysis (Supplemental Appendix Table 1).
Table 4.
Primary and secondary outcome measures (per-protocol analysis)
| ePOCT | ALMANACH | Risk difference | Risk ratio | |
|---|---|---|---|---|
| % (n/N) | % (n/N) | % (95% CI) | (95% CI) | |
| Primary outcome | ||||
| Clinical failure by day 7 | 1.8 (1/56) | 16.7 (8/48) | −14.9 (−26.0, −3.8) | 0.11 (0.01–0.83) |
| Secondary outcomes | ||||
| Primary referrals | 98.2 (55/56) | 14.6 (7/48) | 83.6 (73.1, 94.2) | 6.7 (3.4–13.4) |
| Primary admission | 19.6 (11/56) | 10.4 (5/48) | 9.2 (−4.3, 22.8) | 1.9 (0.7--5.1) |
| Severe malnutrition | 100 (11/11) | 0 (0/5) | ||
| Severe pneumonia | 0 | 100 (5/5) | ||
| Anemia (including severe anemia) | 36 (4/11) | 0 | ||
| Malaria | 9 (1/11) | 0 | ||
| Dehydration | 0 | 20 (1/5) | ||
| Antibiotic prescription at day 0 | 98.2 (55/56) | 47.9 (23/48) | 50.3 (35.7, 64.8) | 2.1 (1.5,-2.8) |
| Antibiotic prescription by day 7 | 98.2 (55/56) | 50.0 (24/48) | 48.2 (33.7, 62.8) | 2.0 (1.5-, 2.6) |
| Severe adverse events by day 30 | 1.8 (1/56) | 4.2 (2/48) | −2.4 (−9.0, 4.2) | 0.43 (0.04–4.6) |
| Secondary admissions | 0 (0/56) | 2.0 (1/48) | −2.1 (−6.1, 2.0) | NA |
| Deaths | 1.8 (1/56) | 2.1 (1/48) | −0.3 (−5.6, 5.0) | 0.86 (0.06, 13.3) |
Results for the modified intention-to-treat analysis are available in the Supplemental Appendix Table 1.
In the ePOCT arm, 1.8% (1/55) of children with severe malnutrition who received antibiotic treatment experienced clinical failure by D7, whereas the one patient who did not receive antibiotic treatment did not experience clinical failure. In the ALMANACH arm, 26% (6/23) of children who received antibiotic treatment versus 8% (2/25) of those who did not receive antibiotic treatment experienced clinical failure.
DISCUSSION
This post hoc subgroup analysis of malnourished children in a randomized trial of children with fever provides evidence that a diagnostic strategy using systematic anthropometric screening with measurements amenable to primary care (weight and MUAC) to identify nutritional risk factors may improve the clinical outcome of children with acute infections in the outpatient setting. The ePOCT tool identified more children with severe malnutrition than would be identified using other algorithms that focus on clinical signs alone, further informing more appropriate clinical management of children with both fever and severe malnutrition. Whereas many studies have looked at preventing infections in children hospitalized with severe malnutrition, our approach analyzes the effect of a different identification and classification method for malnutrition among children with acute febrile illness, to inform treatment and attempt to prevent adverse outcomes among this especially high-risk group.
Despite reports from other studies that 35% and 42% of children with severe acute malnutrition admitted to a tertiary referral hospital in Dar es Salaam, Tanzania, had edematous malnutrition,30,31 there were few patients identified with clinical signs of bipedal edema or visible severe wasting in our study. This was not surprising, given the probable low number of cases of edematous malnutrition which would be expected to present at the primary care setting compared with tertiary referral hospitals. Furthermore, numerous studies have demonstrated the difficulty of healthcare workers in identifying these signs and the lower yield in identifying severe malnutrition than anthropometric measures. Nurses in an outpatient clinic in The Gambia only identified half of the children with visible severe wasting and/or bipedal edema compared with an expert clinical evaluation from a physician, and this was after receiving training to identify malnutrition using IMNCI training materials.23 A study in Kenya found that clinical signs identified only half of the cases compared with anthropometric measures, a much higher proportion than that identified by clinical signs in our study.24 The two patients identified with clinical signs would have also been identified with very low WFA and MUAC, as clinical signs are often signs of more severe disease.
There are multiple potential mechanisms through which ePOCT may have resulted in improved outcomes among children with severe malnutrition and fever. Electronic clinical decision algorithms help encourage systematic performance of screening for nutritional risk factors, facilitate accurate z-score calculations, and support systematic and appropriate management of patients identified as at risk, including informing indications for appropriate antibiotic treatment and patient referral.32 The mere identification of severe malnutrition when using the eCDA, which indicates that the patient has a serious disease, may also partly explain the better clinical outcomes in the ePOCT arm as it may have led clinicians and their caregivers to provide more vigilant and comprehensive care to the child. This could include less quantifiable actions, such as better counseling to the caretakers and, thereafter, improved feedings by the caretakers.
Identifying more children with severe malnutrition may have resulted in a reduction in clinical failures. This study was underpowered to assess the effects on mortality and secondary hospitalizations, the beneficial effects of antibiotics and hospital referral, or the influence of clinician and caretaker awareness of severe malnutrition on the care provided. The prescription of antibiotics to all identified as having severe malnutrition (very low WFA or low MUAC) by ePOCT may, however, be the most plausible explanation for the better outcomes. The benefit of systematic antibiotic treatment, however, remains unclear for those with uncomplicated severe acute malnutrition or in those with more broad definitions of severe malnutrition, including very low WFA. Three randomized placebo-controlled trials comparing clinical outcome among children with uncomplicated severe acute malnutrition receiving prophylactic antibiotics to prevent infectious complications versus placebo showed contradictory results.33–35 Given the unclear benefit of systematic prophylactic antibiotics for children with uncomplicated severe acute malnutrition4,36 and the high rates of antimicrobial resistance among infected children with malnutrition,37,38 a more targeted approach should be explored among febrile children with uncomplicated severe malnutrition. Limiting antibiotic prescription to align with IMNCI 2014 recommendations in only treating those with severe acute malnutrition and not those with very low WFA must, however, be balanced with the observed potential benefit in this subgroup analysis and evidence of very low WFA being a better or equal predictor of mortality than WFH.39,40
A more targeted approach may also be necessary for identifying patients who require hospital referrals, as only 20% (11/55) of patients referred to the hospital using ePOCT were admitted, whereas 71% (5/7) in the ALMANACH arm were admitted, although none were admitted for severe malnutrition. This likely represents an over-referral as both eCDAs referred cases of uncomplicated severe malnutrition, who are unlikely to benefit from inpatient therapy.41,42 Such discrepancies in the criteria used could result in a source of tension, confusion, and dissatisfaction among caretakers, as well as reduced compliance of primary care clinicians to the eCDA recommendation to refer the patient, as was described in a community-based therapeutic care program.43 A more sensible approach would be to refer only cases of complicated severe malnutrition (including very low WFA) or only cases of complicated severe acute malnutrition, as proposed by IMNCI 2014, instead of the more conservative approach of ePOCT which referred all cases of severe malnutrition (based on WFA and MUAC) with fever. Cases of uncomplicated severe malnutrition, including those with very low WFA and normal MUAC, could be referred to nutrition programs, where WFH could be assessed.
The better overall identification and management of other problems identified by ePOCT, in addition to malnutrition identification, may have contributed to the favorable outcomes observed in this subpopulation.25 For example, the addition of systematic Hb testing identified that 68% of children had anemia in the ePOCT arm, whereas only 6% of those in the ALMANACH arm were determined to have anemia, and only a small proportion of the children in this arm were tested and managed accordingly. It is possible that among the vulnerable population of children with malnutrition, other components of the ePOCT algorithm, in addition to malnutrition classification, may account for some of the difference in clinical outcomes observed between the two arms.
The main limitation of this study is due to the intrinsic nature of a subgroup analysis, for which confounders could bias results. Another potential source of bias in our analysis is that most patients met the subgroup inclusion criteria based on the anthropometry measures used in the ePOCT arm and not because of the clinical signs as used in the ALMANACH arm. Because of practical reasons and difficulty with height measurements,20,44,45 height was not integrated in the ePOCT algorithm or as an independent anthropometric measure for this study. It was also not integrated into ALMANACH because at the time of development, in Tanzania the 2008 IMCI guidelines were being used, which only used clinical signs of malnutrition to diagnose severe malnutrition.13 ePOCT was thus compared with the 2008 IMCI guidelines and not the 2014 guidelines. However, given that height and other anthropometric measures are rarely measured,18 ALMANACH may actually better resemble routine care in Tanzania than what is recommended in the 2014 IMNCI guidelines. More practical and simpler tools to measure height in children (e.g., based on image analysis) would be beneficial if clinicians are to align with IMNCI. The generalizability of this study is limited by the fact that it took place in an urban area of a single country with low HIV prevalence and moderate malaria prevalence. Tanzania also has a low proportion of edematous malnutrition in the primary care setting, limiting the use of clinical signs for the identification of severe malnutrition. The sample size of this analysis was limited because of the relatively good nutritional status of children in Dar es Salaam, although we maintained adequate power to detect a difference, which we believe is of clinical significance.
In conclusion, this analysis has shown that anthropometric measurements assisted by an eCDA may be an important component of the systematic assessment to detect malnutrition among febrile children so that they can be managed appropriately.
Supplemental appendix
Acknowledgments:
We express our sincere thanks to the parents and children who agreed to participate in this study; the entire ePOCT team for their work in carrying out the trial; and the participating and collaborating hospitals, health centers, and dispensaries for their support.
Note: Supplemental appendix appears at www.ajtmh.org.
REFERENCES
- 1.United Nations Children’s Fund (UNICEF), WHO, World Bank Group , 2018. Levels and Trends in Child Malnutrition: Key Findings of the 2018 Edition of the Joint Child Malnutrition Estimates. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 2.Ibrahim MK, Zambruni M, Melby CL, Melby PC, 2017. Impact of childhood malnutrition on host defense and infection. Clin Microbiol Rev 30: 919–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muenchhoff M, et al. 2018. Malnutrition in HIV-infected children is an indicator of severe disease with an impaired response to antiretroviral therapy. AIDS Res Hum Retroviruses 34: 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lazzerini M, Tickell D, 2011. Antibiotics in severely malnourished children: systematic review of efficacy, safety and pharmacokinetics. Bull World Health Organ 89: 593–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uwaezuoke SN, 2016. The prevalence of urinary tract infection in children with severe acute malnutrition: a narrative review. Pediatric Health Med Ther 7: 121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Page AL, et al. 2013. Infections in children admitted with complicated severe acute malnutrition in Niger. PLoS One 8: e68699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lazzerini M, et al. 2016. Mortality and its risk factors in Malawian children admitted to hospital with clinical pneumonia, 2001–12: a retrospective observational study. Lancet Glob Health 4: e57–e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Reilly CE, et al. 2012. Risk factors for death among children less than 5 years old hospitalized with diarrhea in rural Western Kenya, 2005–2007: a cohort study. PLoS Med 9: e1001256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chisti MJ, Pietroni MA, Smith JH, Bardhan PK, Salam MA, 2011. Predictors of death in under-five children with diarrhoea admitted to a critical care ward in an urban hospital in Bangladesh. Acta Paediatr 100: e275–e279. [DOI] [PubMed] [Google Scholar]
- 10.Hicks RM, Padayatchi N, Shah NS, Wolf A, Werner L, Sunkari VB, O’Donnell MR, 2014. Malnutrition associated with unfavorable outcome and death among South African MDR-TB and HIV co-infected children. Int J Tuberc Lung Dis 18: 1074–1083. [DOI] [PubMed] [Google Scholar]
- 11.Preidis GA, McCollum ED, Mwansambo C, Kazembe PN, Schutze GE, Kline MW, 2011. Pneumonia and malnutrition are highly predictive of mortality among African children hospitalized with human immunodeficiency virus infection or exposure in the era of antiretroviral therapy. J Pediatr 159: 484–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Black RE, et al. 2013. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 382: 427–451. [DOI] [PubMed] [Google Scholar]
- 13.WHO , 2008. Integrated Management of Childhood Illness for High HIV Settings. Geneva, Switzerland: World Health Organization. [PubMed] [Google Scholar]
- 14.WHO , 2009. WHO Child Growth Standards and the Identification of Severe Acute Malnutrition in Infants and Children: A Joint Statement by the World Health Organization and the United Nations Children’s Fund. Geneva, Switzerland: World Health Organization. [PubMed] [Google Scholar]
- 15.WHO , 2014. Integrated Management of Childhood Illness: Chart Booklet. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 16.Zaman S, Ashraf RN, Martines J, 2008. Training in complementary feeding counselling of healthcare workers and its influence on maternal behaviours and child growth: a cluster-randomized controlled trial in Lahore, Pakistan. J Health Popul Nutr 26: 210–222. [PMC free article] [PubMed] [Google Scholar]
- 17.Horwood C, Vermaak K, Rollins N, Haskins L, Nkosi P, Qazi S, 2009. An evaluation of the quality of IMCI assessments among IMCI trained health workers in South Africa. PLoS One 4: e5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peck R, Mghamba J, Vanobberghen F, Kavishe B, Rugarabamu V, Smeeth L, Hayes R, Grosskurth H, Kapiga S, 2014. Preparedness of Tanzanian health facilities for outpatient primary care of hypertension and diabetes: a cross-sectional survey. Lancet Glob Health 2: e285–e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fung JST, Akech S, Kissoon N, Wiens MO, English M, Ansermino JM, 2019. Determining predictors of sepsis at triage among children under 5 years of age in resource-limited settings: a modified Delphi process. PLoS One 14: e0211274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Myatt M, Khara T, Collins S, 2006. A review of methods to detect cases of severely malnourished children in the community for their admission into community-based therapeutic care programs. Food Nutr Bull 27: S7–S23. [DOI] [PubMed] [Google Scholar]
- 21.Sachdeva S, Dewan P, Shah D, Malhotra RK, Gupta P, 2016. Mid-upper arm circumference v. weight-for-height Z-score for predicting mortality in hospitalized children under 5 years of age. Public Health Nutr 19: 2513–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berkley J, Mwangi I, Griffiths K, Ahmed I, Mithwani S, English M, Newton C, Maitland K, 2005. Assessment of severe malnutrition among hospitalized children in rural Kenya: comparison of weight for height and mid upper arm circumference. JAMA 294: 591–597. [DOI] [PubMed] [Google Scholar]
- 23.Hamer C, Kvatum K, Jeffries D, Allen S, 2004. Detection of severe protein-energy malnutrition by nurses in The Gambia. Arch Dis Child 89: 181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mogeni P, Twahir H, Bandika V, Mwalekwa L, Thitiri J, Ngari M, Toromo C, Maitland K, Berkley JA, 2011. Diagnostic performance of visible severe wasting for identifying severe acute malnutrition in children admitted to hospital in Kenya. Bull World Health Organ 89: 900–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keitel K, et al. 2017. A novel electronic algorithm using host biomarker point-of-care tests for the management of febrile illnesses in Tanzanian children (e-POCT): a randomized, controlled non-inferiority trial. PLoS Med 14: e1002411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.WHO , 2009. WHO Child Growth Standards: Growth Velocity Based on Weight, Length and Head Circumference: Methods and Development. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 27.Rambaud-Althaus C, Shao AF, Kahama-Maro J, Genton B, d’Acremont V, 2015. Managing the sick child in the era of declining malaria transmission: development of ALMANACH, an electronic algorithm for appropriate use of antimicrobials. PLoS One 10: e0127674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.WHO Multicentre Growth Reference Study Group , 2006. Assessment of differences in linear growth among populations in the WHO Multicentre Growth Reference Study. Acta Paediatr Suppl 450: 56–65. [DOI] [PubMed] [Google Scholar]
- 29.WHO , 2011. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 30.Walli NZ, Munubhi EK, Aboud S, Manji KP, 2017. Vitamin D levels in malnourished children under 5 years in a tertiary care center at Muhimbili National Hospital, Dar es Salaam, Tanzania-a cross-sectional study. J Trop Pediatr 63: 203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sunguya BF, Koola JI, Atkinson S, 2006. Infections associated with severe malnutrition among hospitalised children in east Africa. Tanzan Health Res Bull 8: 189–192. [DOI] [PubMed] [Google Scholar]
- 32.Rambaud-Althaus C, Shao A, Samaka J, Swai N, Perri S, Kahama-Maro J, Mitchell M, D’Acremont V, Genton B, 2017. Performance of health workers using an electronic algorithm for the management of childhood illness in Tanzania: a pilot implementation study. Am J Trop Med Hyg 96: 249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trehan I, Goldbach HS, LaGrone LN, Meuli GJ, Wang RJ, Maleta KM, Manary MJ, 2013. Antibiotics as part of the management of severe acute malnutrition. N Engl J Med 368: 425–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berkley JA, et al. 2016. Daily co-trimoxazole prophylaxis to prevent mortality in children with complicated severe acute malnutrition: a multicentre, double-blind, randomised placebo-controlled trial. Lancet Glob Health 4: e464–e473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Isanaka S, et al. 2016. Routine amoxicillin for uncomplicated severe acute malnutrition in children. N Engl J Med 374: 444–453. [DOI] [PubMed] [Google Scholar]
- 36.Alcoba G, Kerac M, Breysse S, Salpeteur C, Galetto-Lacour A, Briend A, Gervaix A, 2013. Do children with uncomplicated severe acute malnutrition need antibiotics? A systematic review and meta-analysis. PLoS One 8: e53184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woerther P-L, et al. 2011. Massive increase, spread, and exchange of extended spectrum β-lactamase–encoding genes among intestinal Enterobacteriaceae in hospitalized children with severe acute malnutrition in Niger. Clin Infect Dis 53: 677–685. [DOI] [PubMed] [Google Scholar]
- 38.Ndir A, Diop A, Faye PM, Cisse MF, Ndoye B, Astagneau P, 2016. Epidemiology and burden of bloodstream infections caused by extended-spectrum beta-lactamase producing Enterobacteriaceae in a pediatric hospital in Senegal. PLoS One 11: e0143729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McDonald CM, Olofin I, Flaxman S, Fawzi WW, Spiegelman D, Caulfield LE, Black RE, Ezzati M, Danaei G, 2013. The effect of multiple anthropometric deficits on child mortality: meta-analysis of individual data in 10 prospective studies from developing countries. Am J Clin Nutr 97: 896–901. [DOI] [PubMed] [Google Scholar]
- 40.Myatt M, Khara T, Dolan C, Garenne M, Briend A, 2019. Improving screening for malnourished children at high risk of death: a study of children aged 6–59 months in rural Senegal. Public Health Nutr 22: 862–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.WHO , 2013. Updates on the Management of Severe Acute Malnutrition in Infants and Children. Geneva, Switzerland: World Health Organization. [PubMed] [Google Scholar]
- 42.Picot J, Hartwell D, Harris P, Mendes D, Clegg AJ, Takeda A, 2012. The effectiveness of interventions to treat severe acute malnutrition in young children: a systematic review. Health Technol Assess 16: 1–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Collins S, Sadler K, Dent N, Khara T, Guerrero S, Myatt M, Saboya M, Walsh A, 2006. Key issues in the success of community-based management of severe malnutrition. Food Nutr Bull 27: S49–S82. [DOI] [PubMed] [Google Scholar]
- 44.Laar ME, Marquis GS, Lartey A, Gray-Donald K, 2018. Reliability of length measurements collected by community nurses and health volunteers in rural growth monitoring and promotion services. BMC Health Serv Res 18: 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mwangome MK, Fegan G, Mbunya R, Prentice AM, Berkley JA, 2012. Reliability and accuracy of anthropometry performed by community health workers among infants under 6 months in rural Kenya. Trop Med Int Health 17: 622–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nijman RG, Thompson M, van Veen M, Perera R, Moll HA, Oostenbrink R, 2012. Derivation and validation of age and temperature specific reference values and centile charts to predict lower respiratory tract infection in children with fever: prospective observational study. BMJ 345: e4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thompson M, Harnden A, Perera R, Mayon-White R, Smith L, McLeod D, Mant D, 2009. Deriving temperature and age appropriate heart rate centiles for children with acute infections. Archives of Disease in Childhood 94: 361–365. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.