Abstract
Apicomplexan parasites such as Toxoplasma gondii possess an unusual heme biosynthesis pathway whose enzymes localize to the mitochondrion, cytosol, or apicoplast, a nonphotosynthetic plastid present in most apicomplexans. To characterize the involvement of the apicoplast in the T. gondii heme biosynthesis pathway, we investigated the role of the apicoplast-localized enzyme uroporphyrinogen III decarboxylase (TgUroD). We found that TgUroD knockdown impaired parasite proliferation, decreased free heme levels in the parasite, and decreased the abundance of heme-containing c-type cytochrome proteins in the parasite mitochondrion. We validated the effects of heme loss on mitochondrial cytochromes by knocking down cytochrome c/c1 heme lyase 1 (TgCCHL1), a mitochondrial enzyme that catalyzes the covalent attachment of heme to c-type cytochromes. TgCCHL1 depletion reduced parasite proliferation and decreased the abundance of c-type cytochromes. We further sought to characterize the overall importance of TgUroD and TgCCHL1 for both mitochondrial and general parasite metabolism. TgUroD depletion decreased cellular ATP levels, mitochondrial oxygen consumption, and extracellular acidification rates. By contrast, depletion of TgCCHL1 neither diminished ATP levels in the parasite nor impaired extracellular acidification rate, but resulted in specific defects in mitochondrial oxygen consumption. Together, our results indicate that the apicoplast has a key role in heme biology in T. gondii and is important for both mitochondrial and general parasite metabolism. Our study highlights the importance of heme and its synthesis in these parasites.
Keywords: Toxoplasma gondii, cytochrome, mitochondria, heme, electron transport, apicomplexa, apicoplast, cytochrome c/c1 heme lyase, heme biosynthesis, uroporphyrinogen III decarboxylase
Introduction
Apicomplexans are a diverse phylum of intracellular parasites, containing species of medical, veterinary, and agricultural importance. Of particular note are Plasmodium spp., the causative agents of malaria, and Toxoplasma gondii, the causative agent of toxoplasmosis. Most apicomplexan parasites possess a reduced plastid organelle, the apicoplast (1–3), that was derived from an endosymbiotic event whereby the heterotrophic ancestor of the apicomplexans engulfed a chloroplast-containing red alga (4, 5). During the shift to parasitism, apicomplexans lost the need and ability for photosynthesis, resulting in a reduction in plastid functions (6). Apicoplasts are no longer photosynthetic but are predicted to play a variety of functionally important roles, including in heme biosynthesis (2, 5, 7, 8).
Heme is a ubiquitous molecule that functions in a variety of essential life processes (7–9). Heme is a porphyrin molecule that consists of a cyclic tetrapyrrole structure with a central iron atom. The dual oxidation states of iron (which can exist as either Fe(II) or Fe(III)) allow heme to exist in either a reduced or an oxidized state (7, 9), enabling heme (and proteins containing heme as a prosthetic group) to participate in various electron transport and redox reactions. For example, heme is an important component of the mitochondrial electron transport chain (ETC)2 (8–10). Complex III (coenzyme Q:cytochrome c oxidoreductase) and Complex IV (cytochrome c oxidase) of the ETC contain heme molecules or heme-containing proteins (called cytochromes) that facilitate the transfer of electrons through these complexes, and the hemoprotein cytochrome c acts as an electron carrier between Complexes III and IV (8–10).
To obtain sufficient quantities of heme for their requirements, most eukaryotes possess a heme biosynthesis pathway (7). Although most of the heme biosynthesis enzymes are conserved among eukaryotes, the localization of these enzymes differs between different phyla (8). In animals, heme biosynthesis enzymes localize variously to the cytosol or mitochondrion, with final enzymes of the pathway occurring in the mitochondrion, the organelle that requires most heme in these organisms (8). By contrast, the heme biosynthesis pathway of plants, which shares numerous enzymes with the chlorophyll biosynthesis pathway, localizes predominantly to the plastid (8). The apicomplexan heme biosynthesis pathway is an unusual hybrid between the animal and plant pathways, with the eight enzymes in this pathway dispersed between the mitochondrion, cytosol, and apicoplast (8, 11). This unusual distribution likely evolved from reduction of the two heme biosynthesis pathways that existed in the ancestral apicomplexan host and its red algal symbiont early in apicomplexan evolution, leading to the hybrid pathway that exists today (12).
As in animals, the first enzyme in the apicomplexan heme biosynthesis pathway localizes to the mitochondrion, synthesizing δ-aminolevulinic acid from glycine and succinyl-CoA (13). δ-Aminolevulinic acid is then thought to be transported out of the mitochondrion and into the apicoplast (8). The next four enzymes are predicted to localize to the apicoplast, mirroring the heme synthesis pathway that occurs in plant plastids. Uroporphyrinogen III decarboxylase (UroD) is predicted to catalyze the final apicoplast-localized step of heme synthesis in apicomplexans, mediating the decarboxylation of uroporphyrinogen III to form coproporphyrinogen III (8). Coproporphyrinogen III is thought to be transported from the apicoplast into the cytosol, where it is oxidized. The final two enzymes, as in the animal pathway, localize back in the mitochondrion where heme is synthesized as the final product (14–16).
Numerous studies have examined the contribution of various enzymes in this “hybrid” heme biosynthesis pathway to the viability of Plasmodium parasites across the parasite life cycle. These studies have demonstrated that the pathway is dispensable in blood stages, where parasites likely scavenge heme from host erythrocytes, but important in insect and liver stages of the parasite life cycle (14, 16–18). Although it is clear that the heme biosynthesis pathway is important for Plasmodium parasites to complete their life cycles, the role of synthesized heme in these parasites is not well-understood. One study demonstrated that parasite-synthesized heme is incorporated into parasite proteins (16), and another predicts the presence of numerous hemoproteins in these parasites (8). The role of these putative hemoproteins in parasite biology, however, remain to be elucidated.
In contrast to the limited host cell range of Plasmodium parasites, T. gondii is capable of infecting virtually all nucleated cells in warm-blooded animals. Porphopbilinogen synthase, the second enzyme of the heme biosynthesis pathway, localizes to the apicoplast of T. gondii, and inhibitors of this enzyme impair parasite proliferation (19). Beyond this, the contribution of the parasite heme biosynthesis pathway to heme levels in T. gondii, and the importance of these enzymes for parasite proliferation and specific biological processes, have not been characterized.
In this study, we utilized genetic, biochemical, and physiological approaches to show that the apicoplast-localized heme biosynthesis enzyme TgUroD is important for parasite proliferation and various biological processes within the parasite. Specifically, we demonstrate the importance of TgUroD for the stability of heme-containing mitochondrial cytochromes, ETC function, and general parasite metabolism. Taken together, our data elucidate the importance of the apicoplast in a range of metabolic processes in these parasites.
Results
TgUroD is expressed in tachyzoites and localizes to the apicoplast
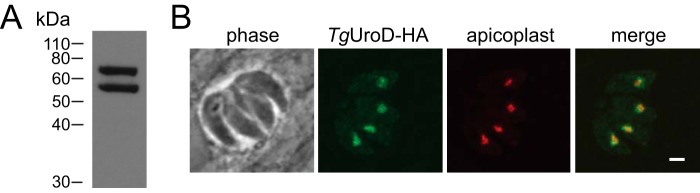
To determine whether TgUroD (ToxoDB number: TGME49_289940) is expressed in the disease-causing tachyzoite stage of T. gondii, we integrated a 3 × hemagglutinin (HA) tag into the 3′ region of the ORF in the native TgUroD locus. Western blotting revealed the presence of two protein isoforms, one of ∼70 kDa and a second of ∼57 kDa (Fig. 1A). Immunofluorescence assays revealed that TgUroD localized to a single, punctate structure in the parasite, overlapping with the apicoplast marker TgCpn60 (Fig. 1B). We conclude that TgUroD is an apicoplast-localized enzyme, with the two molecular mass species likely the precursor and mature forms of the protein that are commonly observed in apicoplast-targeted proteins (20).
Figure 1.
Expression and localization of TgUroD in T. gondii. A, Western blotting of proteins extracted from TgUroD-HA parasites in T. gondii tachyzoites and probed with anti-HA antibodies. B, immunofluorescence assay of four intracellular T. gondii parasites located within the same parasitophorous vacuole. TgUroD-HA (green) co-localizes with an apicoplast marker TgCpn60 (red). Scale bar is 2 μm.
TgUroD is important for tachyzoite proliferation
To facilitate a functional characterization of TgUroD in T. gondii, we generated a regulatable knockdown strain (which we term rTgUroD) wherein the native promoter of TgUroD was replaced with an anhydrotetracycline (ATc)-regulatable promoter. Successful integration of the ATc-regulatable promoter was verified through PCR analysis (Fig. S1).
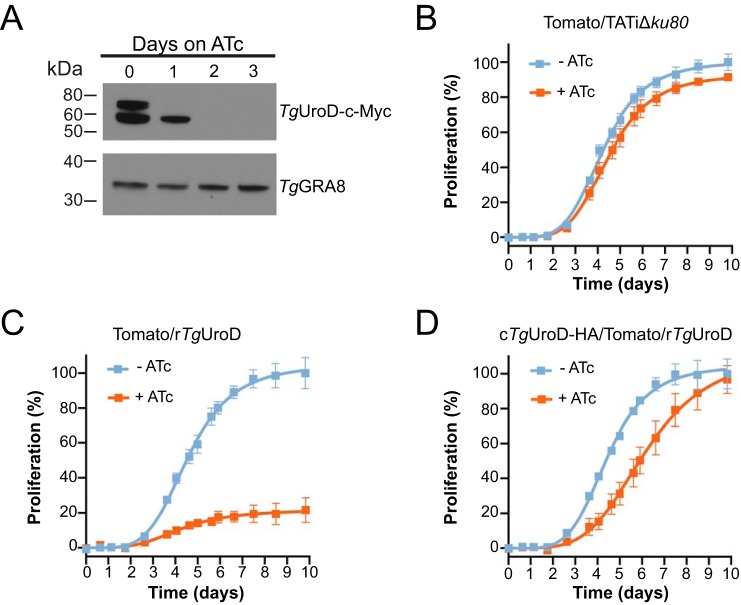
To determine whether TgUroD expression could be down-regulated upon the addition of ATc, we integrated a c-Myc tag into the 3′ end of the TgUroD ORF, generating a strain we termed rTgUroD-c-Myc. We grew parasites in the presence of ATc for 0 to 3 days and performed Western blotting on protein extracts. This revealed that the high molecular mass precursor species of TgUroD-c-Myc was undetectable after 1 day on ATc, and both molecular mass species were undetectable after 2 days (Fig. 2A).
Figure 2.
Knockdown of TgUroD causes a defect in parasite proliferation. A, Western blotting of the rTgUroD-c-Myc line grown in the presence of ATc for 0–3 days and probed with antibodies against c-Myc (to detect rTgUroD-c-Myc) and TgGRA8 (as a loading control). B–D, fluorescence growth assays depicting parasite proliferation in (B) parental Tomato/TATiΔku80, (C) tomato/rTgUroD, and (D) cTgUroD-HA/Tomato/rTgUroD strain parasites grown in the absence (blue) or presence (orange) of ATc. Proliferation is expressed as a percentage of that measured in parasites grown in the absence of ATc on the final day of each experiment. Data depict the mean ± S.D. from three technical replicates and are representative of three independent experiments.
To determine the importance of TgUroD for parasite proliferation, we introduced a tandem dimeric Tomato red fluorescent protein into the rTgUroD line, generating a Tomato/rTgUroD cell line. We inoculated wells of a 96-well plate with Tomato/rTgUroD or parental (Tomato/TATiΔku80) strain parasites, grew parasites in the absence or presence of ATc, and measured well fluorescence daily as a proxy for parasite proliferation, as described previously (21). Addition of ATc caused no difference in growth of the parental strain but severely impaired parasite proliferation in the Tomato/rTgUroD strain (Fig. 2, B and C). Complementation of the Tomato/rTgUroD cell line with a constitutively expressed, HA-tagged copy of TgUroD (a strain we termed cTgUroD-HA/Tomato/rTgUroD) largely restored parasite proliferation in the presence of ATc (Fig. 2D). We conclude that TgUroD is important for tachyzoite proliferation.
Depletion of TgUroD leads to reduced heme levels and reduced abundance of mitochondrial c-type cytochromes
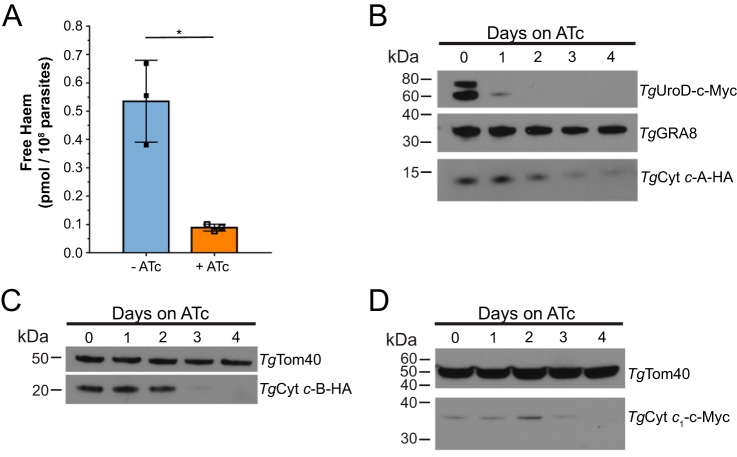
Next, we asked whether TgUroD was important for maintaining free heme levels in the parasite. To test this, we established a chemiluminescence assay based on the activity of the heme-requiring protein HRP (22). We grew rTgUroD parasites in the absence or presence of ATc for 3 days, extracted whole cell lysates containing free heme, mixed these with apo-HRP, which is inactive in the absence of an exogenous source of heme, and measured chemiluminescence. Following calibration of HRP activity with known amounts of heme, this allowed us to estimate free heme levels in parasite. We found that parasites grown in the presence of ATc showed a significant, 6-fold reduction in the levels of free heme compared with parasites grown in the absence of ATc (Fig. 3A) (p = 0.032, two-tailed unpaired Student's t test, n = 3). This is consistent with the hypothesis that parasites lacking TgUroD are unable to synthesize heme de novo.
Figure 3.
Knockdown of TgUroD reduces free heme and mitochondrial c-type cytochrome abundance in parasites. A, free heme levels were measured in parasites grown in the absence (blue) or presence (orange) of ATc for 3 days. Data show the mean ± S.D. from three independent experiments (*, p < 0.05; two-tailed unpaired Student's t test). B, Western blots of rTgUroD-c-myc/TgCyt c-A-HA parasites grown in the presence of ATc for 0–4 days and probed with antibodies against c-Myc, HA and TgGRA8 (loading control). C, Western blots of rTgUroD/TgCyt c-B-HA parasites grown in the presence of ATc for 0–4 days and probed with antibodies against HA and anti-TgTom40 (loading control). D, Western blots of rTgUroD/TgCyt c1-c-Myc parasites grown in the presence of ATc for 0–4 days and probed with antibodies against c-Myc and anti-TgTom40 (loading control). All Western blots are representative of three independent experiments.
To investigate the downstream effects of heme depletion in TgUroD knockdown parasites, we examined the abundance of the heme-containing c-type cytochromes in the parasite. C-type cytochromes include cytochrome c (Cyt c) and cytochrome c1 (Cyt c1), proteins with central roles in electron transfer reactions in the ETC of the mitochondrion (10). Two homologues of Cyt c are encoded in the T. gondii genome, which we term Cyt c-A and Cyt c-B (10). We incorporated an HA-epitope tag into the 3′ end of both the Cyt c-A and Cyt c-B open reading frames, and a 3 × c-Myc tag into the 3′ end of the Cyt c1 ORF, in the rTgUroD-c-Myc or rTgUroD parasite strains. We verified expression and mitochondrial localization of the tagged proteins by immunofluorescence assays (Fig. S2).
We grew the resultant strains in ATc for 0–4 days and performed Western blotting to measure changes in hemoprotein abundance. Cyt c-A abundance progressively decreased after 2 days on ATc, becoming virtually undetectable after 4 days (Fig. 3B). Cyt c-B protein was barely detectable after 3 days on ATc (Fig. 3C). c-Myc-tagged Cyt c1 was more difficult to detect, but also appeared to decrease in abundance upon prolonged ATc exposure (Fig. 3D). These data indicate that the abundance of c-type cytochrome hemoproteins decreased upon TgUroD knockdown.
Depletion of cytochrome c/c1 heme lyase results in a decrease in cytochrome c abundance
The observation that mitochondrial hemoprotein levels decrease upon knockdown of TgUroD led us to hypothesize that loss of heme synthesis leads to the inability of parasites to add heme prosthetic groups to these protein. In turn, the lack of a heme moiety may cause instability, and subsequent degradation, of these proteins. Heme is covalently attached to c-type cytochromes in a reaction catalyzed by cytochrome c/c1 heme lyases (CCHLs) (8, 23). The T. gondii genome encodes two candidate CCHL enzymes, which we termed TgCCHL1 (TGME49_293390) and TgCCHL2 (TGME49_314042). With a view to understanding the importance of heme prosthetic groups for the stability of c-type cytochromes, we set out to characterize the roles of these CCHL proteins in the maturation of c-type cytochromes in T. gondii.
We first investigated the role of TgCCHL1 protein in T. gondii biology. We generated a regulatable knockdown strain of TgCCHL1 wherein the native promoter was replaced with an ATc-regulatable promoter, simultaneously integrating a 3×HA-tag into the 5′ end of the TgCCHL1 ORF (Fig. S3A). Successful integration of the ATc-regulatable promoter was verified through PCR analysis (Fig. S3, B and C). We termed the resultant parasite strain rHA-TgCCHL1.
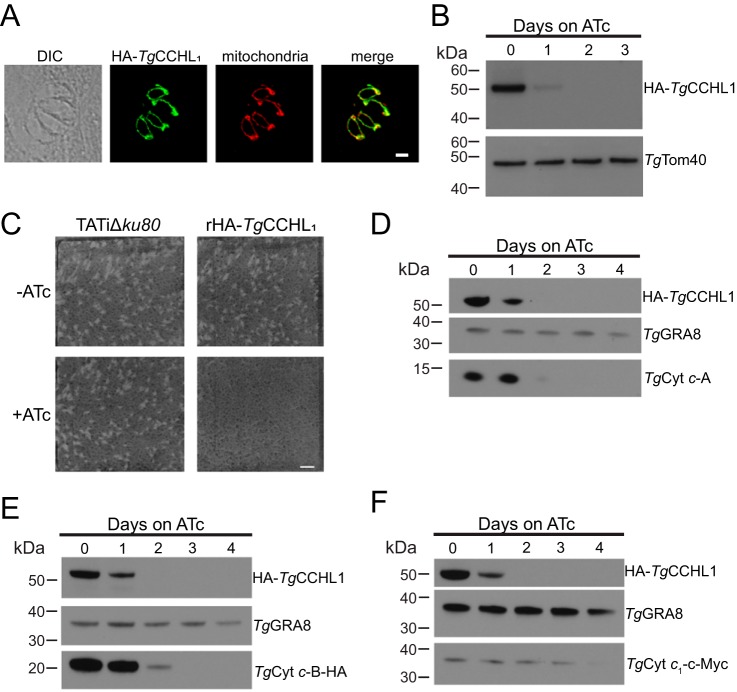
We performed an immunofluorescence assay and found that HA-TgCCHL1 localized to the parasite mitochondrion (Fig. 4A). We then performed Western blotting on proteins extracted from rHA-TgCCHL1 parasites grown in the presence of ATc for 0–3 days and found that HA-TgCCHL1 was undetectable after 2 days on ATc (Fig. 4B).
Figure 4.
Knockdown of TgCCHL1 results in defects in parasite proliferation and a depletion in the abundance of c-type cytochromes. A, immunofluorescence assay of a four-cell parasite vacuole with HA-TgCCHL1 (green) co-localizing with the mitochondrial marker Tom40 (red). Scale bar is 2 μm. B, Western blots of iHA3-TgCCHL1 lines grown in the presence of ATc for 0–3 days and probed with antibodies against HA and TgTom40 (as a loading control). C, plaque assay of parental TATiΔku80 and rHA-TgCCH1L parasites grown in the absence or presence of ATc over 8 days. Data are from a single experiment and representative of three independent experiments. Scale bar is 10 mm. D–F, Western blots of rHA3-TgCCHL1 parasite strains grown in the presence of ATc for 0–4 days and probed with antibodies against (D) TgCyt c-A, (E) HA-tagged TgCyt c-B, and (F) c-Myc-tagged TgCyt c1. GRA8 is included as a loading control, and data are representative of three independent experiments.
To determine the importance of TgCCHL1 for parasite proliferation we performed plaque assays. We grew rHA-TgCCHL1 parasites in the absence or presence of ATc for 8 days and observed the formation of zones of clearance (“plaques”) in the host cell monolayer, the sizes of which correlate with parasite proliferation. Plaque formation in rHA-TgCCHL1 parasites grown in the presence of ATc was severely impaired, whereas parental TATiΔku80 strain parasites grew normally (Fig. 4C). This suggests that TgCCHL1 is important for parasite proliferation.
Next, we investigated the importance of TgCCHL1 for the abundance of c-type cytochromes. We incorporated an HA tag into the 3′ region of the Cyt c-B ORF, or a c-Myc tag into the 3′ region of the Cyt c1 locus, in rHA-TgCCHL1 strain parasites. We then monitored the abundance of c-type cytochromes upon HA-TgCCHL1 knockdown. We grew parasites for 0 to 4 days in ATc and measured protein abundance by Western blotting, utilizing an anti-Cyt c-A antibody (24) to detect Cyt c-A. Cyt c-A was virtually undetectable after 2 days in ATc (Fig. 4D). Cyt c-B-HA abundance decreased considerably after 2 days and was undetectable after 3 days (Fig. 4E). Cyt c1-c-Myc was again difficult to detect, but its abundance appeared to decrease after 4 days (Fig. 4F). We conclude that knockdown of TgCCHL1 results in a depletion of mitochondrial c-type cytochromes, which mirrors the effect we observe upon TgUroD knockdown. This is consistent with the hypothesis that integration of the heme prosthetic group is important for stabilizing c-type cytochromes, and that loss of heme synthesis upon TgUroD knockdown leads to defects in the abundance of these proteins.
We also examined the importance of TgCCHL2 for T. gondii proliferation. We replaced the native promoter of TgCCHL2 with an ATc-regulatable promoter to generate a strain we termed rTgCCHL2 (Fig. S4A), verifying successful integration by PCR analysis (Fig. S4, B–E). We measured proliferation of rTgCCHL2 parasites in the presence or absence of ATc by plaque assay. We observed no obvious defects in proliferation in the rTgCCHL2 strain cultured in the presence of ATc compared with the parental control (Fig. S4F). As we did not incorporate an epitope tag into TgCCHL2 locus, we could not verify successful knockdown of the TgCCHL2 protein upon the addition of ATc. However, our observations are in line with a genome-wide phenotypic screen of T. gondii parasites, which predicted that TgCCHL2 is dispensable for tachyzoite proliferation (25). Given the lack of a growth phenotype in our mutant, we did not pursue the role of TgCCHL2 in parasites any further.
Investigating the importance of heme biosynthesis in parasite metabolism
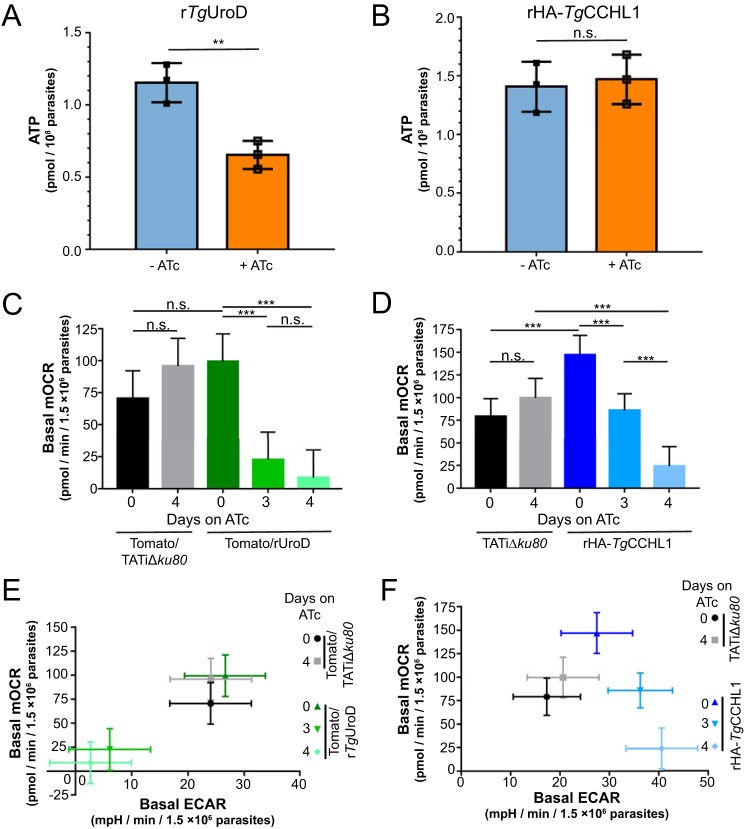
We next sought to establish the role of heme biosynthesis in parasite metabolism. Given the importance of c-type cytochromes in the mitochondrial ETC, and the role of the ETC in mitochondrial ATP synthesis (10, 26), we reasoned that knockdown of TgUroD would lead to defects in ATP levels in the parasite. We measured ATP levels in rTgUroD parasites grown in the presence or absence of ATc and found significantly lower amounts of cellular ATP upon TgUroD knockdown (Fig. 5A) (p = 0.008, two-tailed unpaired Student's t test, n = 3). To determine whether this decrease in ATP levels resulted from the effects of TgUroD knockdown on mitochondrial cytochromes, we measured ATP levels in rHA-TgCCHL1 parasites grown in the absence or presence of ATc. Surprisingly, we found no significant change in ATP levels upon HA-TgCCHL1 knockdown (Fig. 5B) (p = 0.738, two-tailed unpaired Student's t test, n = 3). This indicates that parasites are able to maintain cellular ATP levels despite the decrease in mitochondrial hemoprotein abundance that occurs upon TgCCHL1 knockdown, and suggests that the effects of TgUroD knockdown on parasite ATP levels are independent of their effect on c-type cytochrome abundance.
Figure 5.
Knockdown of TgUroD leads to general metabolic defects in parasites, whereas knockdown of TgCCHL1 results in selective defects in the mitochondrial ETC. A and B, whole cell ATP levels were measured in (A) rTgUrOD and (B) rHA-TgCCHL1 parasites grown in the absence of ATc (blue) or the presence of ATc for 3 days (orange). Data show the mean ± S.D. from three independent experiments (**, p < 0.01; n.s. = not significant; p > 0.05; two-tailed unpaired Student's t test). C and D, basal mOCR in (C) Tomato/TATiΔku80 parental (black/gray) and rTgUroD (green) parasites or (D) TATiΔku80 parental (black/gray) and rHA-TgCCHL1 parasites (blue) grown in the absence of ATc or the presence of ATc for 3 or 4 days. Data depict the least square means from a linear mixed model ± 95% confidence limits from three independent experiments (***, p < 0.001; n.s. = not significant; p > 0.05; ANOVA with Tukey's post hoc test). E and F, basal mOCR plotted against basal ECAR in (E) Tomato/TATiΔku80 parental (black/gray) and rTgUroD (green) parasites, or (F) TATiΔku80 parental (black/gray) and rHA-TgCCHL1 parasites (blue). Parasites were grown in the absence of ATc, or in the presence of ATc for 3 or 4 days. Data depict the least square means from a linear mixed model ± 95% confidence limits from three independent experiments.
To further investigate mitochondrial function upon knockdown of TgUroD, we measured mitochondrial membrane potential (ΔΨm) using the dye JC-1, as described previously (27). The mitochondrial ETC mediates ATP generation by establishment of a proton gradient across the inner mitochondrial membrane (10), and we reasoned that the defects in c-type cytochrome abundance upon TgUroD knockdown would impair the ETC, which would lead to a depletion of ΔΨm. As predicted, knockdown of TgUroD resulted in a dissipation of ΔΨm (Fig. S5). To determine whether this change in ΔΨm resulted selectively from impairment of mitochondrial cytochromes, we measured ΔΨm upon TgCCHL1 knockdown. Surprisingly, we found that knockdown of TgCCHL1 did not impair ΔΨm (Fig. S5). This indicates that mitochondrial membrane potential can be maintained in the absence of detectable amounts of c-type cytochromes, and that the defects in ΔΨm that we observe upon TgUroD knockdown are probably independent of the down-regulation of mitochondrial cytochromes that result from impairing parasite heme synthesis.
Loss of ΔΨm can be indicative of a general loss of cell viability (28), and we therefore sought an alternative measure of ETC function upon TgUroD knockdown. We grew TgUroD parasites in the absence of ATc, or in the presence of ATc for 3 or 4 days and measured mitochondrial oxygen consumption rate (mOCR) using a Seahorse XFe96 flux analyzer, as described previously (29). We observed a significant decrease in mOCR upon TgUroD knockdown (Fig. 5C) (p < 0.001, linear mixed model ANOVA, n = 3). This indicates that depletion of TgUroD, and concomitant loss of heme synthesis, leads to defects in ETC function. We observed a similar, significant decrease in mOCR upon TgCCHL1 knockdown (Fig. 5D) (p < 0.001, linear mixed model ANOVA, n = 3), indicating that depletion of c-type cytochromes also results in defects in ETC function.
The Seahorse XFe96 flux analyzer also measures the rate at which parasites acidify their extracellular environment (29). In mammalian cells, extracellular acidification rate (ECAR) corresponds primarily to glycolytic activity (30). Whether ECAR in T. gondii is a specific measure for glycolysis, or whether other metabolic processes in the parasite also contribute to ECAR, has not been experimentally demonstrated. Nevertheless, we have used ECAR as a general measure for parasite metabolic activity (29). We found that knockdown of TgUroD resulted in a significant ∼80% decrease in ECAR (Fig. 5E and Fig. S6A) (p < 0.001, linear mixed model ANOVA, n = 3). By contrast, knockdown of TgCCHL1 resulted in a significant ∼30% increase in ECAR (Fig. 5F and Fig. S6B) (p = 0.045 and p = 0.001 after 3 days and 4 days on ATc, respectively; linear mixed model ANOVA; n = 3). These data are consistent with knockdown of TgUroD resulting in a general impairment of parasite metabolism, whereas TgCCHL1 knockdown appears to result in a selective defect in the mitochondrial ETC and a concomitant increase in other aspects of parasite metabolism.
Discussion
In this study, we have demonstrated that TgUroD, an apicoplast-localized enzyme, is important for survival and proliferation of the disease-causing tachyzoite stage of the T. gondii life cycle. Knockdown of TgUroD led to a decrease in free heme in the parasites (Fig. 3A), implying a key role for the apicoplast in parasite heme biosynthesis. Our data are consistent with a recent study by Bergmann and colleagues (31), who demonstrated that loss of mitochondrial and cytosolic heme biosynthesis enzymes led to defects in T. gondii proliferation, virulence and heme levels.
Knockdown of TgUroD resulted in a decrease in the abundance of heme-containing c-type cytochrome proteins in the parasite mitochondrion (Fig. 3, B–D). We postulate that the decrease in free heme levels upon TgUroD knockdown diminishes heme incorporation into c-type cytochromes, resulting in instability and subsequent degradation of these proteins. In support of this, we found that knockdown TgCCHL1, an enzyme that catalyzes the covalent attachment of heme to c-type cytochromes (23), also resulted in a depletion in the abundance of c-type cytochromes (Fig. 4, D–F). Our data also demonstrate that TgUroD knockdown results in impairment of mitochondrial O2 consumption in the parasite, a phenotype mirrored upon TgCCHL1 knockdown (Fig. 5, C and D). These data indicate a key role of heme in the ETC of the mitochondrion, both as part of c-type cytochromes, and likely also the b- and a-type cytochromes that occur in the coenzyme Q:cytochrome c oxidoreductase and cytochrome c oxidase complexes of the ETC (8, 10). Our study, therefore, links apicoplast metabolism to a central function of the mitochondrion. Other products of apicoplast metabolism are also thought to be utilized in the mitochondrion, including isoprenoids (precursors of coenzyme Q) and fatty acids (precursors of lipids that are synthesized by the mitochondrion) (32). Together, these observations suggest that the apicoplast organelle plays a key “support” role for mitochondrial processes (32).
Our data demonstrate that knockdown of TgUroD leads to a depletion in parasite ATP levels (Fig. 3A), an impairment in the ability of parasites to acidify their extracellular environment (Fig. 5E), and a loss of mitochondrial ΔΨm (Fig. S5). These processes all require active parasite metabolism, pointing to a role for TgUroD in maintaining metabolic processes in the parasite. The ECAR defects we observe upon TgUroD knockdown resemble similar defects we have observed previously upon treating parasites with cycloheximide (29), a translation inhibitor that rapidly depletes protein synthesis leading to generalized cell death (33). Loss of mitochondrial ΔΨm is also frequently interpreted as an indicator of cell death (28). In concert, these data suggest that knockdown of TgUroD may lead to a general cell death response in the parasite. This response is probably independent of the impact of heme synthesis on the mitochondrial ETC and oxidative phosphorylation, because we did not observe defects in parasite ATP levels, ECAR, or ΔΨm upon TgCCHL1 knockdown (Fig. 4, B and F, and Fig. S5), and have previously reported that knockdown of key proteins in the parasite cytochrome c oxidase and ATP synthase complexes do not impair ECAR (26, 29).
One possibility is that heme is required for metabolic processes beyond the mitochondrial ETC. In addition to mitochondrial cytochrome proteins, the T. gondii genome encodes seven cytochrome b5-containing proteins and a homologue of cytochrome P450 (8). The functions of these proteins are currently still unstudied, but one of the cytochrome b5 proteins (TGME49_276110) is predicted to be important for parasite growth (25). Loss of heme synthesis is likely to impair the function of this and other nonmitochondrial ETC cytochromes, which may explain the resultant effects of TgUroD knockdown on parasite metabolism and survival.
An alternative explanation for the postulated cell death phenotype is that knockdown of TgUroD leads to a buildup of toxic porphyrin intermediates in the cell, similar to the effects observed in human porphyrias. Human porphyrias result from partial deficiencies in heme synthesis enzymes (34). A decrease in UroD activity in hepatic cells in humans is the cause for the most common porphyria in humans, termed porphyria cutanea tarda (35, 36). Porphyria cutanea tarda results from the accumulation of uroporphyrinogen III (the substrate of UroD) and other by-products of heme synthesis, leading to oxidative stress and cell death. This manifests as skin photosensitivity and the formation of skin lesions or blisters in affected patients. In a similar vein, the buildup of heme precursors in Plasmodium-infected erythrocytes results in parasite photosensitivity and a concomitant inhibition of parasite proliferation (37). It is conceivable then that a buildup of uroporphyrinogen III or other heme precursors upon TgUroD knockdown may be toxic to parasite cells, leading to the cell death phenotypes we observed in our study.
Determining whether the cell death effect we observe upon TgUroD knockdown results from a loss of heme synthesis and/or from the accumulation of toxic porphyrins will require further study. It will be of particular interest to examine whether similar cell death phenotypes are observed in heme biosynthesis pathway mutants that do not lead to the accumulation of potentially toxic porphyrins (e.g. mutants in δ-aminolevulinic acid synthase, the first enzyme of the pathway) (31).
In addition to our examination of the heme biosynthesis pathway, our study examines the maturation and role of c-type cytochromes in the mitochondrial ETC of T. gondii. We demonstrate that knockdown of TgCCHL1 leads to depletion of both cytochrome c isoforms in the parasite, as well as cytochrome c1, a component of the coenzyme Q:cytochrome c oxidoreductase complex (Fig. 4, D–F). In turn, this leads to a selective defect in mitochondrial O2 consumption (Fig. 5, D and F), as we have seen previously when inhibiting other components of the ETC (29). Notably, loss of c-type cytochromes upon TgCCHL1 knockdown did not result in decreased ATP levels in the parasite (Fig. 5B) or in mitochondrial ΔΨm (Fig. S5). This suggests that parasites can maintain ΔΨm and generate ATP in the absence of a functional ETC. It is possible that parasites are able to up-regulate ATP generation through glycolysis to compensate for defects in oxidative phosphorylation. Consistent with this, we observed a significant increase in ECAR upon TgCCHL1 knockdown (Fig. 5F and Fig. S6B). ECAR is, in part, probably dependent on the extrusion of lactate from the parasite. Lactate is an end-product of anaerobic glycolysis, and the increase in ECAR we observe suggests that parasites respond to impairment of oxidative phosphorylation by increasing anaerobic glycolysis. This hypothesis is consistent with other studies that highlight considerable metabolic flexibility in central carbon metabolism in T. gondii parasites (38–43). These studies have revealed that parasites are able to utilize numerous carbon sources for energy generation (including glucose, glutamine, and storage products such as amylopectin and γ-aminobutyric acid) and are able to metabolize these substrates differently (e.g. anaerobic glycolysis versus aerobic glycolysis versus glutaminolysis) in different life stages, or when parasites lack enzymes involved particular aspects of central carbon metabolism. Examining the effects of TgCCHL1 knockdown (and the concomitant loss of cytochrome maturation) on central carbon metabolism in the parasite may reveal how these parasites compensate for the predicted loss of mitochondrial ATP production.
Some eukaryotes, such as yeast, contain separate heme lyase enzymes for catalyzing heme attachment in cytochrome c and cytochrome c1 (44). We observe a depletion of both cytochrome c isoforms and cytochrome c1 upon TgCCHL1 knockdown (Fig. 4, D–F), consistent with TgCCHL1 being capable of catalyzing the insertion of heme into all three c-type cytochromes in the parasite. We observe a more rapid depletion of the two cytochrome c isoforms than of cytochrome c1 upon TgCCHL1 knockdown (Fig. 4, D–F). It is conceivable that the second parasite heme lyase, TgCCHL2 (which is annotated as a cytochrome c1 heme lyase on ToxoDB), may also be capable of inserting heme into cytochrome c1, although it appears that TgCCHL2 is not crucial for growth of T. gondii (Fig. S4) (25), suggesting that its role is, at most, redundant with that of TgCCHL1. Studies in yeast have demonstrated that CCHL enzymes, and the heme incorporation that they catalyze, is critical for the transport of cytochrome c into the mitochondrial inner membrane space (23, 45). The more rapid depletion of the two cytochrome c isoforms than of cytochrome c1 that we observe (Fig. 4, D–F) may, therefore, result from degradation of cytochrome c associated with its inability to import into the mitochondrion of the parasite.
Overall, our study indicates that the apicoplast of T. gondii has an important role in de novo heme synthesis in the parasite, contributing to the mitochondrial ETC as well as to other metabolic processes in the parasite. Recent studies have highlighted the potential of heme biosynthesis as a drug target (31) and revealed the role of parasite heme synthesis in modulating the susceptibility of apicomplexan parasites to existing drugs such as artemisinin (46). Our study, therefore, contributes to a growing body of literature that highlights the importance of understanding heme and its synthesis in these parasites.
Experimental procedures
Host cell and parasite culture and growth assays
T. gondii was cultured in human foreskin fibroblasts (HFF), as described previously (47). HFF cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% (v/v) bovine calf serum, 50 units/ml penicillin, 50 μg/ml streptomycin, 10 μg/ml gentamicin, 0.25 μg/ml amphotericin B and 0.2 mm l-glutamine. HFF cells were grown in tissue culture flasks in a humidified 5% CO2 incubator set to 37 °C. Parasites were grown in confluent HFF monolayers, and were incubated in supplemented DMEM containing 1% (v/v) fetal calf serum, 50 units/ml penicillin, 50 μg/ml streptomycin, 10 μg/ml gentamicin, 0.25 μg/ml amphotericin B, and 0.2 mm l-glutamine (Ed1). Where applicable, ATc was added to culture medium at a final concentration of 0.5 μg/ml. Parasites in paired ATc experiments grown without ATc had ethanol added as a vehicle control. Fluorescence growth assays were performed as described previously (48, 49). Parasites were cultured in phenol red-free Roswell Park Memorial Institute 1640 medium supplemented with 1% (v/v) fetal calf serum, 50 units/ml penicillin, 50 μg/ml streptomycin, 10 μg/ml gentamicin, 0.25 μg/ml amphotericin B and 0.2 mm l-glutamine (Ed1), and fluorescence was read daily using a FLUOstar OPTIMA Microplate Reader (BMG LABTECH). Plaque assays were performed as described previously (47), with 500 parasites added per flask, and flasks incubated for 8–10 days before crystal violet staining.
Genetic modification of T. gondii
TATiΔku80 strain parasites (50) were used as the parental cell line for generating the genetically modified parasites described in this study. To generate fluorescent parasites used in the fluorescence growth assays, we introduced tandem dimeric Tomato red fluorescent protein into the relevant cell lines, as described previously (49). All genetically modified parasite strains were cloned by limiting dilution or flow cytometry before being characterized.
To incorporate a 3′ HA tag into the TgUroD locus, we PCR-amplified the 3′ region of the TgUroD gene with primers 1 and 2 (all primers listed in Table S1), and cloned the resulted product into the pLIC-3xHA/DHFR vector by ligation-independent cloning, as described previously (51). The resulting vector was linearized with NsiI before transfection into parasites and selection on pyrimethamine, as described previously (47).
To generate an ATc-regulatable TgUroD parasite strain, we amplified the region including and immediately downstream of the TgUroD start codon with primers 3 and 4. We digested this with BglII and NotI and ligated into the equivalent sites of the vector pPR2-HA3 (52). We termed the resulting vector pPR2(TgUroD 3′flank). We next amplified a region upstream of the TgUroD start codon with primers 5 and 6. We digested the resulting product with PacI and NsiI, and ligated into the equivalent sites of the pPR2(TgUroD 3′flank) vector. We linearized the resulting vector with NotI, transfected into parasites, and selected on pyrimethamine. Clonal parasites were screened for the presence of the native TgUroD locus with primers 7 and 8, and for presence of the modified locus with primers 8 and 9. A 3′ c-Myc tag was integrated into the ATc-regulatable TgUroD locus by amplifying the 3′ region of the TgUroD gene using primers 10 and 11. The resulting product was digested with BglII and AvrII and ligated into the equivalent sites of the pgCM3 vector (52). The resulting vector was linearized with PstI, transfected into rTgUroD strain parasites, and selected on chloramphenicol, as described (47).
To complement the rTgUroD line with a constitutively expressed copy of TgUroD, we amplified the entire TgUroD ORF using primers 11 and 12 and cDNA as template. We digested the resultant product with BglII and AvrII, and ligated this into the BglII and AvrII sites of the vector pUgCTH3 (49). We linearized the resultant vector with MfeI, transfected it into the rTgUroD strain, and selected on chloramphenicol.
To incorporate a 3′ HA tag in the TgCyt c-A locus, we amplified the 3′ region of the TgCyt c-A ORF using the primers 13 and 14. We digested the resultant product with BglII and AvrII, and ligated this into the BglII and AvrII sites of the vector pgCH (49). The resultant vector was linearized with MfeI, transfected into the relevant parasite strains, and selected on chloramphenicol.
To incorporate a 3′ HA tag in the TgCyt c-B locus, we amplified the 3′ region of the TgCyt c-B ORF using the primers 15 and 16. We digested the resultant product with BglII and AvrII, and ligated this into the BglII and AvrII sites of the vector pgCH. The resultant vector was linearized with AfeI, transfected into the relevant parasite strains, and selected on chloramphenicol.
To incorporate a 3′ c-Myc tag in the TgCyt-c1 locus, we amplified the 3′ region of the TgCyt-c1 ORF using the primers 17 and 18. We digested the resultant product with BglII and AvrII, and ligated this into the BglII and AvrII sites of the vector pgCM3. The resultant vector was linearized with PstI, transfected into the relevant parasite strains, and selected on chloramphenicol.
To generate the TgCCHL1 knockdown strain, we amplified the region including and immediately downstream of the TgCCHL1 start codon with primers 19 and 20. The resultant PCR product was digested with XmaI and NotI and ligated into the equivalent sites of the pPR2-HA3 vector, generating a vector we termed pPR2-HA3(TgCCHL1 3′ flank). We next amplified the region upstream of the TgCCHL1 start codon with primers 21 and 22. We digested the resultant product with PspOMI and NdeI, and ligated this into the equivalent sites of the pPR2-HA3(TgCCHL1 3′ flank) vector. We linearized the resulting vector with NotI, transfected into parasites, and selected on pyrimethamine. Clonal parasites were screened for the presence of the native TgCCHL1 locus using primers 23 and 24, and presence of the modified locus using primers 9 and 24.
To generate the TgCCHL2 knockdown cell line, we first amplified the 3′ flank region of the TgCCHL2 locus with primers 25 and 26. We digested the resultant product with AvrII and NotI, and ligated this into the equivalent sites of the vector pPR2-HA3, generating a vector we termed pPR2(TgCCHL2 3′flank). We then amplified the 5′ flank region of the TgCCHL2 locus with primers 27 and 28. We digested the resultant product with ApaI and NdeI, and ligated this into the equivalent sites of the pPR2(TgCCHL2 3′flank) vector. The resultant vector was linearized with NotI, transfected into parasites, and selected on pyrimethamine. To verify successful integration of the construct, we used primer combinations 29/30 and 31/32 to test for the presence of the native TgCCHL2 locus, and primer combinations 9/30 and 31/33 to test for the presence of the modified locus.
Immunofluorescence assays, SDS-PAGE, and Western blotting
Immunofluorescence assays and SDS-PAGE/Western blotting were performed as described previously (21). The primary antibodies used were rat anti-HA (1:100 to 1:250; Roche clone 3F10), mouse anti-c-Myc (1:100 to 1:500; Santa Cruz Biotechnology clone 9E10), mouse anti-GRA8 (1:50,000 to 1:200,000; a kind gift from Gary Ward, University of Vermont (53)), anti-Tom40 (1:2000 to 3000 (24)), anti-Cpn60 (1:2000 (54)), and anti-Cyt c-A (1:250 to 1:500 (29)). Secondary antibodies used were HRP-conjugated goat anti-rat IgG (1:5000; Santa Cruz Biotechnology cat. no. sc-2006), HRP-conjugated goat anti-mouse IgG (1:5000 to 10,000; Santa Cruz Biotechnology cat. no. sc-2005), HRP-conjugated goat anti-rabbit IgG (1:5000; Santa Cruz Biotechnology cat. no. sc-2004), Alexa Fluor 488–conjugated goat highly cross-adsorbed anti-rat IgG (1:250, Thermo Scientific cat. no. A11006), Alexa Fluor 488–conjugated goat highly cross-adsorbed anti-mouse IgG (1:200 to 250, Thermo Scientific cat. no. A11029), Alexa Fluor 546–conjugated goat highly cross-adsorbed anti-mouse IgG (1:250, Thermo Scientific cat. no. A11030), Alexa Fluor 546–conjugated goat anti-rabbit IgG (1:250, Thermo Scientific cat. no. A11035), Alexa Fluor 647–conjugated goat anti-rabbit IgG (1:250, Thermo Scientific cat. no. A21244), and CF647-conjugated goat anti-rabbit (1:500, Sigma cat. no. SAB4600177). Immunofluorescence images were acquired on a DeltaVision Elite system (GE Healthcare) using an inverted Olympus IX71 microscope with a 100× UPlanSApo oil immersion lens (Olympus) paired with a Photometrics CoolSNAP HQ2 camera, or on Leica TCS SP2 inverted laser scanning confocal microscope. Images taken on the DeltaVision setup were deconvolved using SoftWoRx Suite 2.0 software. Images were adjusted linearly for contrast and brightness. Western blots were exposed onto X-ray films and scanned.
Free heme measurements
Parasites were harvested from 175 cm2 tissue culture flasks, passed through a 3 μm filter to remove host cell debris, then centrifuged at 1500 × g for 10 min to pellet them. Free heme was extracted from parasite pellets by the addition of acidified acetone (100% v/v acetone with 0.05% v/v concentrated HCl). This was followed by 10 s of vigorous vortexing, 10 min of incubation on ice, and 10 min of incubation at ambient temperature. Cell debris was pelleted by centrifugation at 20,000 × g for 10 min at 4 °C. Free heme in the supernatant was transferred to a separate tube, and the extraction was repeated with an acidified acetone/water solution mix (acidified acetone solution with 20% v/v distilled water) Supernatants were pooled and were subsequently concentrated until dry using a Savant SpeedVac SC100 (Thermo Scientific) with no heat and protected from light.
An HRP-based heme detection assay was adapted from Ref. 22. Apo-HRP was derived from HRP enzyme (Sigma) with Teale's butanone extraction method (55, 56). Briefly, an equivolume of ice-cold 2-butanone was added to HRP (25 μm, pH 2.5 with HCl), mixed thoroughly, and incubated at 4 °C until clear layers formed. The top and middle layer were aspirated, leaving the bottom aqueous layer containing apo-HRP. An Illustra NAP-5 size exclusion column packed with Sephadex G-25 DNA Grade resin (GE Healthcare Life Sciences) was used to filter and remove excess 2-butanone from the aqueous layer. The initial flow-through of the column was collected and the concentration of apo-HRP was calculated with a millimolar extinction coefficient of 20 at 278 nm.
Heme reconstitution reactions were set up with 10 nm of apo-HRP enzyme, 100 mm Tris-HCl, pH 8.4, and 10 mm KOH. Heme solutions (known standards or cell extracts) were added at 10% of the total reaction volume and incubated in white, nontreated, low auto-luminescence flat bottom 96-well plate (Nunc, Thermo Scientific) for 30 min at room temperature. An equivolume of reaction solution containing 10 mm luminol, 100 mm Tris-HCl, pH 8.4, and 200 μm of H2O2 was added to start the reaction and luminescence was read immediately using a FLUOstar OPTIMA Microplate Reader (BMG LABTECH). Heme concentration in the cell samples was calculated from a standard curve generated from known heme concentrations.
ATP measurements
Parasites were harvested from 175 cm2 tissue culture flasks, passed through a 3 μm filter to remove host cell debris, then centrifuged at 1500 × g for 10 min to pellet them. Parasites were washed in Hanks' Balanced Salt Solution (Sigma), then resuspended in buffer A with glucose (116 mm NaCl, 5.4 mm KCl, 0.8 mm MgSO4, 5.5 mm d-glucose, 5 mm HEPES, pH 7.2) to a concentration of 1 × 108 parasites/ml. Parasites were incubated in a 5% CO2 incubator for 1 h at 37 °C, pelleted by centrifugation, and resuspended in a 1:1 mixture of buffer A with glucose and 0.5 m HClO4. Parasite suspensions were incubated on ice for 30 min, then pelleted by centrifugation at 20,000 × g for 5 min at 4 °C. The reaction was neutralized with 33.3% (v/v) neutralizing solution (0.72 m KOH, 0.16 m KHCO3) and stored at −20 °C or used immediately.
The Invitrogen ATP Determination Kit (Thermo Scientific cat. no. A22066) was used to calculate ATP levels according to the manufacturer's instructions. Parasite extracts or ATP standards were added at 10% of the total volume of each reaction, and luminescence was read immediately after commencing the reaction on a FLUOstar OPTIMA microplate reader (BMG LABTECH). ATP concentrations in the samples were calculated from a standard curve generated from known ATP concentrations.
Mitochondrial membrane potential measurements
Mitochondrial membrane potential was measured using the ΔΨm-sensitive dye JC-1, as described previously (27). Briefly, parasites were harvested, washed once in phenol red–free Ed1, and resuspended to 2.5 × 107 parasites/ml in Ed1. The protonophore carbonyl cyanide 3-chlorophenylhydrazone was added to the appropriate control tubes and incubated for 30 min. JC-1 was added to a final concentration of 1.5 μm and parasites were incubated for 15 min before being analyzed by flow cytometry using a BD FACSCalibur (BD Biosciences) flow cytometer.
Seahorse XFe96 flux analysis
Seahorse XFe96 flux analysis experiments were performed with slight modifications of a protocol described previously (29). Briefly, parasites were grown in the absence or presence of ATc for 3–4 days, mechanically egressed from host cells through a 26-gauge needle, filtered through a 3 μm polycarbonate filter to remove host cell debris, washed once in base medium (Agilent Technologies) supplemented with 1 mm l-glutamine and 5 mm d-glucose, then resuspended in base medium to 1.5 × 107 cells/ml. 1.5 × 106 parasites were seeded into wells of a Seahorse XFe96 cell culture plate coated with 3.5 μg/cm2 CellTak cell adhesive (Corning), and attached to the bottom of wells by centrifugation at 800 × g for 3 min. Final volumes in wells were made up to 175 μl with supplemented base medium. ETC inhibitors were loaded into the sensor cartridge ports, and injected into wells at designated points during the experiment. OCR and ECAR measurements were obtained every 3 min for five repeats before and after injection of compounds. Injections 1 and 2 contained 1 μm carbonyl cyanide-p-trifluoromethoxyphenylhydrazone (Sigma) and 1 μm atovaquone (Sigma), respectively. The basal mOCR was calculated by subtracting the nonmitochondrial OCR (the value following atovaquone addition) from the basal OCR value obtained. A minimum of 4 background wells were used in each plate, and 3 technical replicates (i.e. wells subjected to identical treatments) were used for each condition.
Statistical and data analyses
Statistical differences between conditions differing in the presence or absence of ATc (free heme and cellular ATP levels) were tested through application of unpaired two-tailed Student's t-tests as described in the relevant sections.
Data from the Seahorse flux analysis were compiled and exported from the Seahorse Wave Desktop software (Agilent Technologies). A linear mixed effects model was applied to the data (29), setting the error between plates (between experiments) and wells (within experiments) as random effects, and the mOCR or ECAR values between cell lines and days on drug (ATc) as fixed effects. Analysis of the least square means of the values was performed on the R software environment. Statistical differences in these values were tested through ANOVA (linear mixed effects), with a post hoc Tukey test.
Author contributions
E. T. T., G. I. M., and G. G. v. D. conceptualization; E. T. T., J. A. H., and G. G. v. D. formal analysis; E. T. T., J. A. H., and G. G. v. D. validation; E. T. T., J. A. H., and G. G. v. D. investigation; E. T. T., J. A. H., G. I. M., and G. G. v. D. visualization; E. T. T., J. A. H., and G. G. v. D. methodology; E. T. T. and G. G. v. D. writing-original draft; E. T. T., J. A. H., G. I. M., and G. G. v. D. writing-review and editing; G. I. M. and G. G. v. D. supervision; G. I. M. and G. G. v. D. project administration; G. G. v. D. resources; G. G. v. D. funding acquisition.
Supplementary Material
Acknowledgments
We thank Gary Ward for providing the anti-GRA8 antibody, and Harpreet Vohra and Michael Devoy for performing FACS.
This work was supported by Australian Research Council (ARC) Discovery Project Grant DP110103144 (to G. G. v. D.) and the Research School of Biology Innovation Fund Grant (to G. G. v. D.). The authors declare that they have no conflicts of interest with the contents of this article.
This article contains Figs. S1–S6 and Table S1.
- ETC
- electron transport chain
- ΔΨm
- mitochondrial membrane potential
- ANOVA
- analysis of variance
- ATc
- anhydrotetracycline
- CCHL
- cytochrome c/c1 heme lyase
- Cyt c
- cytochrome c
- Cyt c1
- cytochrome c1
- ECAR
- extracellular acidification rate
- HFF
- human foreskin fibroblasts
- JC-1
- 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolyl-carbocyanine iodide
- mOCR
- mitochondrial O2 consumption rate
- UroD
- uroporphyrinogen III decarboxylase.
References
- 1. McFadden G. I., and Roos D. S. (1999) Apicomplexan plastids as drug targets. Trends Microbiol. 7, 328–333 10.1016/S0966-842X(99)01547-4 [DOI] [PubMed] [Google Scholar]
- 2. Ralph S. A., van Dooren G. G., Waller R. F., Crawford M. J., Fraunholz M. J., Foth B. J., Tonkin C. J., Roos D. S., and McFadden G. I. (2004) Tropical infectious diseases: Metabolic maps and functions of the Plasmodium falciparum apicoplast. Nat. Rev. Microbiol. 2, 203–216 10.1038/nrmicro843 [DOI] [PubMed] [Google Scholar]
- 3. Sato S. (2011) The apicomplexan plastid and its evolution. Cell Mol. Life Sci. 68, 1285–1296 10.1007/s00018-011-0646-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Janouskovec J., Horák A., Oborník M., Lukes J., and Keeling P. J. (2010) A common red algal origin of the apicomplexan, dinoflagellate, and heterokont plastids. Proc. Natl. Acad. Sci. U.S.A. 107, 10949–10954 10.1073/pnas.1003335107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van Dooren G. G., and Striepen B. (2013) The algal past and parasite present of the apicoplast. Annu. Rev. Microbiol. 67, 271–289 10.1146/annurev-micro-092412-155741 [DOI] [PubMed] [Google Scholar]
- 6. Janouškovec J., Paskerova G. G., Miroliubova T. S., Mikhailov K. V., Birley T., Aleoshin V. V., and Simdyanov T. G. (2019) Apicomplexan-like parasites are polyphyletic and widely but selectively dependent on cryptic plastid organelles. Elife 8, e49662 10.7554/eLife.49662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sigala P. A., and Goldberg D. E. (2014) The peculiarities and paradoxes of Plasmodium heme metabolism. Annu. Rev. Microbiol. 68, 259–278 10.1146/annurev-micro-091313-103537 [DOI] [PubMed] [Google Scholar]
- 8. van Dooren G. G., Kennedy A. T., and McFadden G. I. (2012) The use and abuse of heme in apicomplexan parasites. Antioxid. Redox Signal. 17, 634–656 10.1089/ars.2012.4539 [DOI] [PubMed] [Google Scholar]
- 9. Koený L., Oborník M., and Lukeš J. (2013) Make it, take it, or leave it: Heme metabolism of parasites. PLoS Pathog. 9, e1003088 10.1371/journal.ppat.1003088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hayward J. A., and van Dooren G. G. (2019) Same same, but different: Uncovering unique features of the mitochondrial respiratory chain of apicomplexans. Mol. Biochem. Parasitol. 232, 111204 10.1016/j.molbiopara.2019.111204 [DOI] [PubMed] [Google Scholar]
- 11. Seeber F., Limenitakis J., and Soldati-Favre D. (2008) Apicomplexan mitochondrial metabolism: A story of gains, losses and retentions. Trends Parasitol. 24, 468–478 10.1016/j.pt.2008.07.004 [DOI] [PubMed] [Google Scholar]
- 12. Koreny L., Sobotka R., Janouskovec J., Keeling P. J., and Lukeš M. (2011) Tetrapyrrole synthesis of photosynthetic chromerids is likely homologous to the unusual pathway of apicomplexan parasites. Plant Cell 23, 3454–3462 10.1105/tpc.111.089102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Varadharajan S., Dhanasekaran S., Bonday Z. Q., Rangarajan P. N., and Padmanaban G. (2002) Involvement of δ-aminolaevulinate synthase encoded by the parasite gene in de novo haem synthesis by Plasmodium falciparum. Biochem. J. 367, 321–327 10.1042/BJ20020834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ke H., Sigala P. A., Miura K., Morrisey J. M., Mather M. W., Crowley J. R., Henderson J. P., Goldberg D. E., Long C. A., and Vaidya A. B. (2014) The heme biosynthesis pathway is essential for Plasmodium falciparum development in mosquito stage but not in blood stages. J. Biol. Chem. 289, 34827–34837 10.1074/jbc.M114.615831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nagaraj V. A., Prasad D., Rangarajan P. N., and Padmanaban G. (2009) Mitochondrial localization of functional ferrochelatase from Plasmodium falciparum. Mol. Biochem. Parasitol. 168, 109–112 10.1016/j.molbiopara.2009.05.008 [DOI] [PubMed] [Google Scholar]
- 16. Nagaraj V. A., Sundaram B., Varadarajan N. M., Subramani P. A., Kalappa D. M., Ghosh S. K., and Padmanaban G. (2013) Malaria parasite-synthesized heme is essential in the mosquito and liver stages and complements host heme in the blood stages of infection. PLoS Pathog. 9, e1003522 10.1371/journal.ppat.1003522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rathnapala U. L., Goodman C. D., and McFadden G. I. (2017) A novel genetic technique in Plasmodium berghei allows liver stage analysis of genes required for mosquito stage development and demonstrates that de novo heme synthesis is essential for liver stage development in the malaria parasite. PLoS Pathog. 13, e1006396 10.1371/journal.ppat.1006396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rizopoulos Z., Matuschewski K., and Haussig J. M. (2016) Distinct prominent roles for enzymes of Plasmodium berghei heme biosynthesis in sporozoite and liver stage maturation. Infect. Immun. 84, 3252–3262 10.1128/IAI.00148-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shanmugam D., Wu B., Ramirez U., Jaffe E. K., and Roos D. S. (2010) Plastid-associated porphobilinogen synthase from Toxoplasma gondii: Kinetic and structural properties validate therapeutic potential. J. Biol. Chem. 285, 22122–22131 10.1074/jbc.M110.107243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. van Dooren G. G., Su V., D'Ombrain M. C., and McFadden G. I. (2002) Processing of an apicoplast leader sequence in Plasmodium falciparum and the identification of a putative leader cleavage enzyme. J. Biol. Chem. 277, 23612–23619 10.1074/jbc.M201748200 [DOI] [PubMed] [Google Scholar]
- 21. van Dooren G. G., Tomova C., Agrawal S., Humbel B. M., and Striepen B. (2008) Toxoplasma gondii Tic20 is essential for apicoplast protein import. Proc. Natl. Acad. Sci. U.S.A. 105, 13574–13579 10.1073/pnas.0803862105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Masuda T., and Takahashi S. (2006) Chemiluminescent-based method for heme determination by reconstitution with horseradish peroxidase apo-enzyme. Anal. Biochem. 355, 307–309 10.1016/j.ab.2006.04.008 [DOI] [PubMed] [Google Scholar]
- 23. Dumont M. E., Cardillo T. S., Hayes M. K., and Sherman F. (1991) Role of cytochrome c heme lyase in mitochondrial import and accumulation of cytochrome c in Saccharomyces cerevisiae. Mol. Cell. Biol. 11, 5487–5496 10.1128/mcb.11.11.5487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. van Dooren G. G., Yeoh L. M., Striepen B., and McFadden G. I. (2016) The import of proteins into the mitochondrion of Toxoplasma gondii. J. Biol. Chem. 291, 19335–19350 10.1074/jbc.M116.725069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sidik S. M., Huet D., Ganesan S. M., Huynh M. H., Wang T., Nasamu A. S., Thiru P., Saeij J. P. J., Carruthers V. B., Niles J. C., and Lourido S. (2016) A genome-wide CRISPR screen in Toxoplasma identifies essential apicomplexan genes. Cell 166, 1423–1435.e12 10.1016/j.cell.2016.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huet D., Rajendran E., van Dooren G. G., and Lourido S. (2018) Identification of cryptic subunits from an apicomplexan ATP synthase. eLife 7, e38097 10.7554/eLife.38097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brooks C. F., Johnsen H., van Dooren G. G., Muthalagi M., Lin S. S., Bohne W., Fischer K., and Striepen B. (2010) The Toxoplasma apicoplast phosphate translocator links cytosolic and apicoplast metabolism and is essential for parasite survival. Cell Host Microbe 7, 62–73 10.1016/j.chom.2009.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pasini E. M., van den Ierssel D., Vial H. J., and Kocken C. H. (2013) A novel live-dead staining methodology to study malaria parasite viability. Malar. J. 12, 190 10.1186/1475-2875-12-190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Seidi A., Muellner-Wong L. S., Rajendran E., Tjhin E. T., Dagley L. F., Aw V. Y., Faou P., Webb A. I., Tonkin C. J., and van Dooren G. G. (2018) Elucidating the mitochondrial proteome of Toxoplasma gondii reveals the presence of a divergent cytochrome c oxidase. eLife 7, e38131 10.7554/eLife.38131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang J., Nuebel E., Wisidagama D. R., Setoguchi K., Hong J. S., Van Horn C. M., Imam S. S., Vergnes L., Malone C. S., Koehler C. M., and Teitell M. A. (2012) Measuring energy metabolism in cultured cells, including human pluripotent stem cells and differentiated cells. Nat. Protoc. 7, 1068–1085 10.1038/nprot.2012.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bergmann A., Floyd K., Key M., Dameron C., Rees K. C., Whitehead D. C., Hamza I., and Dou Z. (2019) Toxoplasma gondii requires its plant-like heme biosynthesis pathway for infection. bioRxiv 10.1101/753863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. van Dooren G. G., and Hapuarachchi S. V. (2017) The dark side of the chloroplast: Biogenesis, metabolism and membrane biology of the apicoplast. in Advances in Botanical Research (Hirakawa Y., ed), pp 145–185, Academic Press, Oxford, UK [Google Scholar]
- 33. Beckers C. J., Roos D. S., Donald R. G., Luft B. J., Schwab J. C., Cao Y., and Joiner K. A. (1995) Inhibition of cytoplasmic and organellar protein synthesis in Toxoplasma gondii. Implications for the target of macrolide antibiotics. J. Clin. Invest. 95, 367–376 10.1172/JCI117665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Badminton M. N., and Elder G. H. (2009) Inherited disorders of haem synthesis: The human porphyrias. in Tetrapyrroles: Birth, Life and Death (Warren M. J., and Smith A. G., eds), pp. 89–100, Landes Bioscience, Austin, TX [Google Scholar]
- 35. Kauppinen R. (2005) Porphyrias. Lancet 365, 241–252 10.1016/S0140-6736(05)70154-9 [DOI] [PubMed] [Google Scholar]
- 36. Singal A. K. (2019) Porphyria cutanea tarda: Recent update. Mol. Genet. Metab. 128, 271–281 10.1016/j.ymgme.2019.01.004 [DOI] [PubMed] [Google Scholar]
- 37. Sigala P. A., Crowley J. R., Henderson J. P., and Goldberg D. E. (2015) Deconvoluting heme biosynthesis to target blood-stage malaria parasites. Elife 4, e09143 10.7554/eLife.09143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jacot D., Waller R. F., Soldati-Favre D., MacPherson D. A., and MacRae J. I. (2016) Apicomplexan energy metabolism: Carbon source promiscuity and the quiescence hyperbole. Trends Parasitol. 32, 56–70 10.1016/j.pt.2015.09.001 [DOI] [PubMed] [Google Scholar]
- 39. Lin S. S., Blume M., von Ahsen N., Gross U., and Bohne W. (2011) Extracellular Toxoplasma gondii tachyzoites do not require carbon source uptake for ATP maintenance, gliding motility and invasion in the first hour of their extracellular life. Int. J. Parasitol. 41, 835–841 10.1016/j.ijpara.2011.03.005 [DOI] [PubMed] [Google Scholar]
- 40. MacRae J. I., Sheiner L., Nahid A., Tonkin C., Striepen B., and McConville M. J. (2012) Mitochondrial metabolism of glucose and glutamine is required for intracellular growth of Toxoplasma gondii. Cell Host Microbe 12, 682–692 10.1016/j.chom.2012.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nitzsche R., Zagoriy V., Lucius R., and Gupta N. (2016) Metabolic cooperation of glucose and glutamine is essential for the lytic cycle of obligate intracellular parasite Toxoplasma gondii. J. Biol. Chem. 291, 126–141 10.1074/jbc.M114.624619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shukla A., Olszewski K. L., Llinas M., Rommereim L. M., Fox B. A., Bzik D. J., Xia D., Wastling J., Beiting D., Roos D. S., and Shanmugam D. (2018) Glycolysis is important for optimal asexual growth and formation of mature tissue cysts by Toxoplasma gondii. Int. J. Parasitol. 48, 955–968 10.1016/j.ijpara.2018.05.013 [DOI] [PubMed] [Google Scholar]
- 43. Uboldi A. D., McCoy J. M., Blume M., Gerlic M., Ferguson D. J., Dagley L. F., Beahan C. T., Stapleton D. I., Gooley P. R., Bacic A., Masters S. L., Webb A. I., McConville M. J., and Tonkin C. J. (2015) Regulation of starch stores by a Ca2+-dependent protein kinase is essential for viable cyst development in Toxoplasma gondii. Cell Host Microbe 18, 670–681 10.1016/j.chom.2015.11.004 [DOI] [PubMed] [Google Scholar]
- 44. Steiner H., Kispal G., Zollner A., Haid A., Neupert W., and Lill R. (1996) Heme binding to a conserved Cys-Pro-Val motif is crucial for the catalytic function of mitochondrial heme lyases. J. Biol. Chem. 271, 32605–32611 10.1074/jbc.271.51.32605 [DOI] [PubMed] [Google Scholar]
- 45. Kranz R., Lill R., Goldman B., Bonnard G., and Merchant S. (1998) Molecular mechanisms of cytochrome c biogenesis: Three distinct systems. Mol. Microbiol. 29, 383–396 10.1046/j.1365-2958.1998.00869.x [DOI] [PubMed] [Google Scholar]
- 46. Harding C. R., Sidik S. M., Petrova B., Gnadig N. F., Okombo J., Ward K. E., Markus B. M., Fidock D. A., and Lourido S. (2019) Genetic screens reveal a central role for heme biosynthesis in artemisinin susceptibility. bioRxiv, 10.1101/746974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jacot D., Meissner M., Sheiner L., Soldati-Favre D., and Striepen B. (2014) Genetic manipulation of Toxoplasma gondii. in Toxoplasma gondii: The Model Apicomplexan (Weiss L. M., and Kim K. eds), pp. 577–611, Elsevier, Amsterdam [Google Scholar]
- 48. Gubbels M. J., Li C., and Striepen B. (2003) High-throughput growth assay for Toxoplasma gondii using yellow fluorescent protein. Antimicrob. Agents Chemother. 47, 309–316 10.1128/AAC.47.1.309-316.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rajendran E., Hapuarachchi S. V., Miller C. M., Fairweather S. J., Cai Y., Smith N. C., Cockburn I. A., Bröer S., Kirk K., and van Dooren G. G. (2017) Cationic amino acid transporters play key roles in the survival and transmission of apicomplexan parasites. Nat. Commun. 8, 14455 10.1038/ncomms14455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sheiner L., Demerly J. L., Poulsen N., Beatty W. L., Lucas O., Behnke M. S., White M. W., and Striepen B. (2011) A systematic screen to discover and analyze apicoplast proteins identifies a conserved and essential protein import factor. PLoS Pathog. 7, e1002392 10.1371/journal.ppat.1002392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Huynh M. H., and Carruthers V. B. (2009) Tagging of endogenous genes in a Toxoplasma gondii strain lacking Ku80. Eukaryot. Cell 8, 530–539 10.1128/EC.00358-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Katris N. J., van Dooren G. G., McMillan P. J., Hanssen E., Tilley L., and Waller R. F. (2014) The apical complex provides a regulated gateway for secretion of invasion factors in Toxoplasma. PLoS Pathog. 10, e1004074 10.1371/journal.ppat.1004074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Carey K. L., Donahue C. G., and Ward G. E. (2000) Identification and molecular characterization of GRA8, a novel, proline-rich, dense granule protein of Toxoplasma gondii. Mol. Biochem. Parasitol. 105, 25–37 10.1016/S0166-6851(99)00160-7 [DOI] [PubMed] [Google Scholar]
- 54. Agrawal S., van Dooren G. G., Beatty W. L., and Striepen B. (2009) Genetic evidence that an endosymbiont-derived endoplasmic reticulum-associated protein degradation (ERAD) system functions in import of apicoplast proteins. J. Biol. Chem. 284, 33683–33691 10.1074/jbc.M109.044024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fruk L., Kuhlmann J., and Niemeyer C. M. (2009) Analysis of heme-reconstitution of apoenzymes by means of surface plasmon resonance. Chem. Commun. (Camb.), 230–232 10.1039/b817206d [DOI] [PubMed] [Google Scholar]
- 56. Teale F. W. (1959) Cleavage of the haem-protein link by acid methylethylketone. Biochim. Biophys. Acta 35, 543 10.1016/0006-3002(59)90407-X [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.