Abstract
Background:
l-amino acids, such as monosodium glutamate (MSG), activate the umami receptor T1R1/T1R3. We previously showed increased peristalsis in response to activation of T1R1/T1R3 by MSG in mouse colon. However, the expression and function of these receptors in the different regions of the stomach are not clear.
Methods:
Mouse gastric smooth muscle cells (SMCs) were isolated and cultured in Dulbecco’s Modified Eagle Medium. Expression of T1R1 and T1R3 was measured by RT-PCR and Western blot. The effect of MSG with and without inosine monophosphate (IMP, an allosteric activator of T1R1/T1R3) on acetylcholine (ACh)-induced contraction was measured in muscle strips and isolated SMCs by scanning micrometry. The effect of MSG with or without IMP on activation of G proteins and ACh-induced Ca2+ release was measured in SMCs.
Key Results:
Monosodium glutamate inhibited ACh-induced contractions in muscle strips from both antrum and fundus and the effect of MSG was augmented by IMP; the effects were concentration-dependent and not affected by the nitric oxide synthase inhibitor, L-NNA, or tetrodotoxin suggesting a direct effect on SMCs. In isolated gastric SMCs, T1R1 and T1R3 transcripts and protein were identified. Addition of MSG with or without IMP inhibited ACh-induced Ca2+ release and muscle contraction; the effect on contraction was blocked by pertussis toxin suggesting activation of Gαi proteins. MSG in the presence of IMP selectively activated Gαi2.
Conclusions and Inferences:
Umami receptors (T1R1/T1R3) are present on SMCs of the stomach, and activation of these receptors induces muscle relaxation by decreasing [Ca2+]i via Gαi2.
Keywords: cellular signalling, gastric motility, smooth muscle, taste receptors, umami
1 |. INTRODUCTION
Ingestion and movement of food through the gastrointestinal (GI) tract are highly coordinated and require a synchronized interaction of regulatory systems including mucosal cells, smooth muscle cells (SMCs), interstitial cells of Cajal (ICC), enteric neurons, and glial cells. GI motility is controlled by neurohumoral agents released from the autonomic and enteric nervous system, glial cells, and enteroendocrine cells (EEC), which modulate the contractility of SMCs.1 Intraluminal nutrient effects have been characterized by their uptake and distribution in the body or secondary to release of neurohumoral agents.2 The effects of nutrients have recently been expanded by studies that showed the presence of extra-oral nutrient receptors, especially in the gut, and studies to understand their functional significance.3–8 These new roles for nutrients and their receptors have made a significant impact in the field of GI motility and secretion.
Of the five basic tastes, three are mediated by G protein-coupled receptors (GPCRs) for nutrients: sweet, umami, and bitter. Umami and sweet agonists activate specific heterodimers, T1R1/T1R3, and T1R2/T1R3, respectively, of the taste receptor type 1 (T1R) family. T1R1 provides selectivity for umami tastants, T1R2 provides selectivity for sweet tastants, and T1R3 is functionally co-expressed with both the sweet and umami receptor subunit.9 T1R1/T1R3 is sensitive to l-amino acids and especially monosodium glutamate (MSG), whereas T1R2/T1R3 is sensitive to sweet agonists. In contrast, bitter substances activate 25 subtypes of the taste receptor type 2 (T2Rs) family.10,11 Receptors for bitter tastants are present in EEC and SMCs of the gut, and a role for these receptors in chemosensation, control of gut hormone secretion, and satiety has been demonstrated.6,10
A functional role for T1R1/T1R3 activation has also been demonstrated in the gut. T1R1 and T1R3 subunits have been identified in EECs, and activation of these causes the release of cholecystokinin (CCK)7,12 and calcitonin gene-related peptide (CGRP).13 Our previous studies have reported that the classic T1R1 receptor agonist MSG increases peristalsis in whole colon and that this effect was enhanced by the addition of the T1R1/T1R3 specific positive allosteric modulator 5′ inosine monophosphate (IMP).13–15 The peristaltic effect was inhibited in T1R1 knockout mice, supporting a role for T1R1/T1R3 in gut motility.
While T1R1/T1R3 nutrient receptor expression and function have been detailed in the mucosa, there is less known about the expression, signaling, and function of these receptors in the smooth muscle. Recent studies by Vancleef et al demonstrated the presence of mRNA for multiple l-amino acid (l-AA) sensing receptors including the T1R1 receptor in human and mouse smooth muscle tissues.16 Interestingly, the T1R3 subunit was expressed in mouse but not human fundic smooth muscle tissue. In this study, l-AA caused contraction of fundic muscle of mouse through activation of the calcium sensing receptor (CaSR).
The present study identifies T1R1/T1R3 heterodimer expression in gastric SMCs and demonstrates that the T1R1 agonist MSG in the presence and absence of IMP attenuates acetylcholine (ACh)-induced contraction in smooth muscle tissue strips as well as in isolated SMCs. We also demonstrate that the muscle relaxation is due to activation of Gαi2 proteins and a decrease in intracellular calcium. These results suggest a role for the nutrient receptors in the regulation of GI motility at the level of smooth muscle in addition to their characterized role in mucosal cells of the gut and the gustatory system. The studies also suggest a potential role for T1R1/T1R3 agonist to treat disorders of gastric motility.
2 |. MATERIALS AND METHODS
2.1 |. Animals
Male and female C57BL/6J mice (approximately 6 weeks of age) were purchased from Jackson Laboratories. Mice were housed 3–4 per cage in an AAALAC-accredited facility at Virginia Commonwealth University with ad libitum access to food and water and subjected to 12 hour light and 12 hour dark cycles. All studies were approved by the Institutional Animal Care and Use Committee at Virginia Commonwealth University prior to the start of any experiments.
2.2 |. Chemicals
[35S]GTPγS was obtained from New England Nuclear Life Sciences; RNAqueous™ kit and TRIzol® Reagent were obtained from Ambion. High Capacity cDNA Reverse Transcription Kit was obtained from Life Technologies; Western blotting materials, 4× Laemmli Sample Buffer, and DC™ Protein Assay Reagents were obtained from Bio-Rad Laboratories; SuperSignal® West Pico Chemiluminescent Substrate, Dulbecco’s Modified Eagle Medium (DMEM), and Trypsin-EDTA (0.25%) were obtained from Thermo Fisher Scientific; Collagenase CLS type II and soybean trypsin inhibitor were obtained from Worthington; antibody to T1R1 (sc #50308) and T1R3 (sc #50352) was obtained from Santa Cruz Biologicals. DNA ladder 100 bp (#N3231) was purchased from New England Biolabs. Monosodium glutamate, IMP, acetylcholine (ACh), carbachol (CCh), L-NG-nitroarginine (L-NNA), NF449 (4,4′,4″,4‴-[Carbonylbis (imino-5,1,3-benzen-etriyl-bis(carbonylimino))] tetrakis-1,3-benzenedisulfonic acid), and pertussis toxin (PTx) were purchased from Millipore Sigma. All other chemicals were obtained from Sigma-Aldrich.
2.3 |. Methods
2.3.1 |. Measurement of relaxation in muscle strips
Muscle strips (approximately 5 × 15 mm) from the gastric fundus and antrum regions were isolated, the mucosa gently scraped, and each end secured with silk suture to a support rod at one end and an FT03c force displacement transducer (Grass Instrument) at other end. The muscle strip and support rod were then mounted at a resting tension of 1 g in organ baths containing Krebs solution with the following composition: 118 mmol/L NaCl, 4.8 mmol/L KCl, 1 mmol/L MgSO4, 1.15 mmol/L NaH2PO4, 15 mmol/L NaHCO3, 10.5 mmol/L glucose and 2.5 mmol/L CaCl2 (95% O2/5%CO2, pH 7.4) maintained at 37°C and allowed to equilibrate for 1 hour. Isometric responses were measured with the Grass FT03c force displacement transducers attached to muscle strips and recorded with Lab Chart software (AD Instruments). Muscle strips were precontracted with 100 μmol/L ACh to raise tone and/or induce phasic activity, and MSG (1–100 mmol/L) in the presence and absence of IMP (1–1000 μmol/L) was added to induce relaxation.13 Relaxation of tone was calculated as a decrease from ACh-induced tone which was sustained in fundic strips.17 Relaxation of antral phasic activity was calculated as a decrease from ACh-induced increase in the amplitude of phasic contractions and as decrease in ACh-induced increase in the frequency of phasic contractions. The amplitudes and frequencies of contractions were measured for the period before addition of ACh (basal) and the 2 minute plateau period in the presence of ACh prior to addition of MSG. The responses to MSG were measured as the maximal response obtained within 5 minutes of the addition of MSG. Measurements were done using the AD software.
The general neural involvement and the specific involvement of nitric oxide (NO) in the inhibitory response was evaluated in a separate set of muscle strips by the addition of TTX and L-NNA, respectively. Muscle strips were tested as described above, washed, and then incubated with TTX (5 μmol/L) or L-NNA (100 μmol/L) for 15 minutes before repeat administration of ACh and umami receptor agonists. Results are expressed as percent relaxation as compared to ACh-induced contraction.
2.3.2 |. Preparation of dispersed gastric muscle cells
Stomachs were removed and placed in buffer consisting of NaCl 120 mmol/L, KCl 4 mmol/L, KH2PO4 2.6 mmol/L, CaCl2 2.0 mmol/L, MgCl2 0.6 mmol/L, HEPES (N-2-hydroxyethylpiperazine-N′ 2-ethanesulfonic acid) 25 mmol/L, glucose 14 mmol/L, and basic Eagle Medium (essential amino mixture) 2.1% (pH 7.4). SMCs were isolated as described previously by bisecting the stomach, removing the mucosal layer via gentle scraping, cutting the stomach into strips and by incubation in HEPES medium containing 0.05% type II collagenase and 0.03% soybean trypsin inhibitor for 25 minutes.17–19 The partially digested tissue was then washed and re-suspended in collagenase-free medium for an additional 30 minutes to allow spontaneous dispersion of cells. Dispersed SMCs were filtered through 500 μm Nitex mesh and cultured in DMEM containing penicillin (200 U/mL), streptomycin (200 μg/mL), gentamycin (100 μg/mL), amphotericin B (2.5 μg/mL), and 10% fetal bovine serum (DMEM-10). The resulting suspension of gastric SMCs contained cells from both the fundic and antral regions; the SMC data represent responses of whole stomach rather than for a specific region. Freshly dispersed SMCs were used for functional studies. Cultured SMCs were used for biochemical measurements such as expression of receptors, G protein activation, and intracellular Ca2+. Previous studies have shown that SMCs in culture were devoid of ICC and enteric neurons.
2.3.3 |. Detection of T1R1 and T1R3 by RT-PCR
RNAqueous™ prep kits (Invitrogen) were used to collect total RNA from cultured gastric SMCs. RNA (2 μg) was reverse transcribed using high capacity cDNA reverse transcription kit (Applied Biosystems). GAPDH was used as an endogenous control reference gene. The RT-PCR primers for T1R1 were as follows: sense primer: 5′-acgtgattctctgccgtccaga-3′; antisense primer: 5′-gactacacgaggcgctgcgg-3′ and primers for T1R3 were as follows: sense primer: 5′-tgggtcagcctgggcttggt-3′; antisense primer: 5′-ggcctaccagccagctgtgc-3′. PCR conditions for GAPDH had initial denaturation step at 94°C for 2 minutes; 94°C for 30 seconds, 25 cycles of 55°C for 30 seconds, and 72°C for 1 minute; final extension step at 72°C for 10 minutes; hold at 4°C as needed. PCR conditions for T1R1, and T1R3, samples had an initial denaturation step at 94°C for 2 minutes; 94°C for 30 seconds, 39 cycles of 57°C for 30 seconds, and 72°C for 1 minute; final extension step at 72°C for 10 minutes; hold at 4°C as needed. The PCR products were separated by electrophoresis in a 1.5% agarose gel at 100 volts in the presence of ethidium bromide, and 100 bp DNA ladder was used as marker. Bands were visualized by ultraviolet fluorescence and recorded with a Bio-Rad Gel Doc EZ Imager.
2.3.4 |. Determination of T1R1 and T1R3 by Western blot
Lysates from isolated gastric SMCs were separated by SDS-PAGE and transferred to PVDF membranes. The membranes were blocked and incubated with T1R1 or T1R3 antibodies (1:1000), washed, and incubated with secondary antibody. Proteins on the membrane were detected by chemiluminescence detection system (Bio-Rad).
2.3.5 |. Immunofluorescence staining and imaging of cultured gastric cells
Isolated gastric SMCs were grown in eight-well chambered slides, fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) for 20 minutes, washed three times with PBS, and then incubated for 40 minutes with 5% normal goat serum as blocking agent (Jackson ImmunoResearch). Sections were then incubated overnight at 4°C with 1:100 dilution of primary antibody to T1R1 (sc #50308) or T1R3 (sc #50352) (Santa Cruz Biologicals) in 5% normal goat serum, or in the absence of primary antibody as a control. After incubation, slides were washed again four times with PBS, then incubated 90 minutes at room temperature with donkey anti-rabbit Alexa Fluor 488 (1:300), and finally washed four times with PBS before covering with Fluoroshield DAPI (Abcam). Slides were imaged with a Zeiss Imager Z1 controlled with ZEN software and using an EXFO X-Cite Series 120 fluorescence illumination source. Slides were imaged for Alexa488 fluorescence and DAPI, images combined, keeping contrast and brightness constant between images.
2.3.6 |. Assay for G protein activity
G protein activation was performed as described previously.19 Briefly, SMCs were homogenized in 20 mmol/L HEPES medium (pH 7.4) containing 2 mmol/L MgCl2, 1 mmol/L EDTA, and 2 mmol/L dithiothreitol. After centrifugation at 15 000 rpm for 30 mintues at 4°C, the membranes were incubated with 100 nmol/L [35S]GTPγS in a solution containing 10 mmol/L HEPES (pH 7.4), 0.1 mmol/L EDTA, and 10 mmol/L MgCl2 for 30 minutes at 37°C. The reaction was stopped with 10 volumes of 100 mmol/L Tris/HCl medium (pH 8.0) containing 10 mmol/L MgCl2, 100 mmol/L NaCl, and 20 μmol/L GTP, and the mixture was placed in wells precoated with specific G protein antibodies (1:1000 dilution). Coating with G protein antibodies was done after the wells were first coated with anti-rabbit or anti-mouse secondary antibody for 2 hours on ice. After incubation for 2 hours on ice, the wells were washed three times with phosphate buffer solution containing 0.05% Tween 20, and the radioactivity from each well was counted by liquid scintillation.
2.3.7 |. Measurement of relaxation in dispersed muscle cells
Contraction in freshly dispersed gastric SMCs from 4 to 5 mice per experiment was determined by scanning micrometry.17–19 An aliquot (0.5 mL) of cells containing approximately 104 cells/mL was treated with MSG (100 μmol/L) or MSG (100 μmol/L) with IMP (1 μmol/L) for 5 minutes followed by ACh (1 μmol/L) for 1 minutes, and the reaction terminated with 1% acrolein. The resting cell length was determined in control experiments in which muscle cells were incubated without MSG, IMP, or ACh. The lengths of 50 muscle cells in each treatment group were measured by scanning micrometry and the mean lengths compared with the mean of the lengths of 50 untreated cells. The contractile response to ACh was expressed as the percent decrease in mean cell length from mean control cell length. Relaxation was measured as a decrease in response to ACh in the presence of MSG with and without IMP. Separate sets of cells were incubated with the Gαi protein inhibitor pertussis toxin (PTx, 400 ng/mL) for 1 hour or Gαs protein inhibitor NF449 (10 μmol/L) for 15 minutes before treating with ACh and receptor agonists. Results are expressed as percent contraction compared to basal control cells.
2.3.8 |. Calcium imaging analysis
Smooth muscle cells were placed on coverslips 1–2 days prior to assay and loaded with fura-2 AM by incubation for 1 hour at 37°C in Ringers medium containing HEPES 10 mmol/L, NaCl 150 mmol/L, KCl 5 mmol/L, glucose 10 mmol/L, CaCl2 1 mmol/L, MgCl2 1 mmol/L (pH 7.4). Cells were washed and the coverslips mounted on an inverted fluorescence microscope (Olympus IX71) with a 40× oil-immersion objective. Excitation at 340 and 380 nm was attained using a 75 W Xenon arc lamp with a monochromator (Cairn Research) controlled by MetaFluor software (Universal Imaging; Cairn Research), and emission was recorded with a CCD camera (Orca ER, Hammamatsu; Cairn Research). Background-subtracted fluorescence was normalized to a baseline average measured before application of the first test reagent and expressed as a 340/380 nm ratio, and the response to receptor agonists was defined as increase in 340/380 nm ratio.
2.4 |. Statistical analysis
Results were analyzed by one-way analysis of variance (ANOVA), followed by Dunnett’s post hoc comparisons, or Student t test where appropriate. To evaluate potential sex differences, muscle strip data were analyzed by two-way ANOVA with sex and treatment as independent variables and relaxation as the dependent variable. Results were considered significant at P < .05.
3 |. RESULTS
3.1 |. Activation of T1R1/T1R3 causes inhibition of tonic contraction and phasic activity
Responses for MSG alone or MSG plus IMP in both fundus (P = .37) and antrum (P = .76) were not statistically different when compared between tissues from male and female mice; therefore, the data were combined (data not shown) and henceforth referred to as response of fundus or antrum, respectively.
In muscle strips from fundus, ACh (100 μmol/L) induced a rapid contraction that was sustained for more than 10 minutes. The response to different concentrations of MSG (1–100 mmol/L) alone or MSG (50 mmol/L) plus different concentrations of IMP (1 μmol/L −1 mmol/L) was examined when the contraction to ACh was stable. MSG caused a rapid, dose-dependent relaxation (ie, inhibition) of ACh-induced contraction which was significant at concentrations >10 mmol/L (F (3, 66) = 4.86; P < .01; Figure 1A,B). Additionally, IMP caused a concentration-dependent augmentation of relaxation induced by 50 mmol/L MSG which was significant at concentrations of IMP above 10 μmol/L (P < .01; Figure 1C). IMP alone did not affect ACh-induced muscle contraction (data not shown). These results with IMP strongly suggest that activation of T1R1/T1R3 receptors causes relaxation of fundus because IMP does not augment the response to MSG mediated by any other putative L-AA receptors.13,14
FIGURE 1.
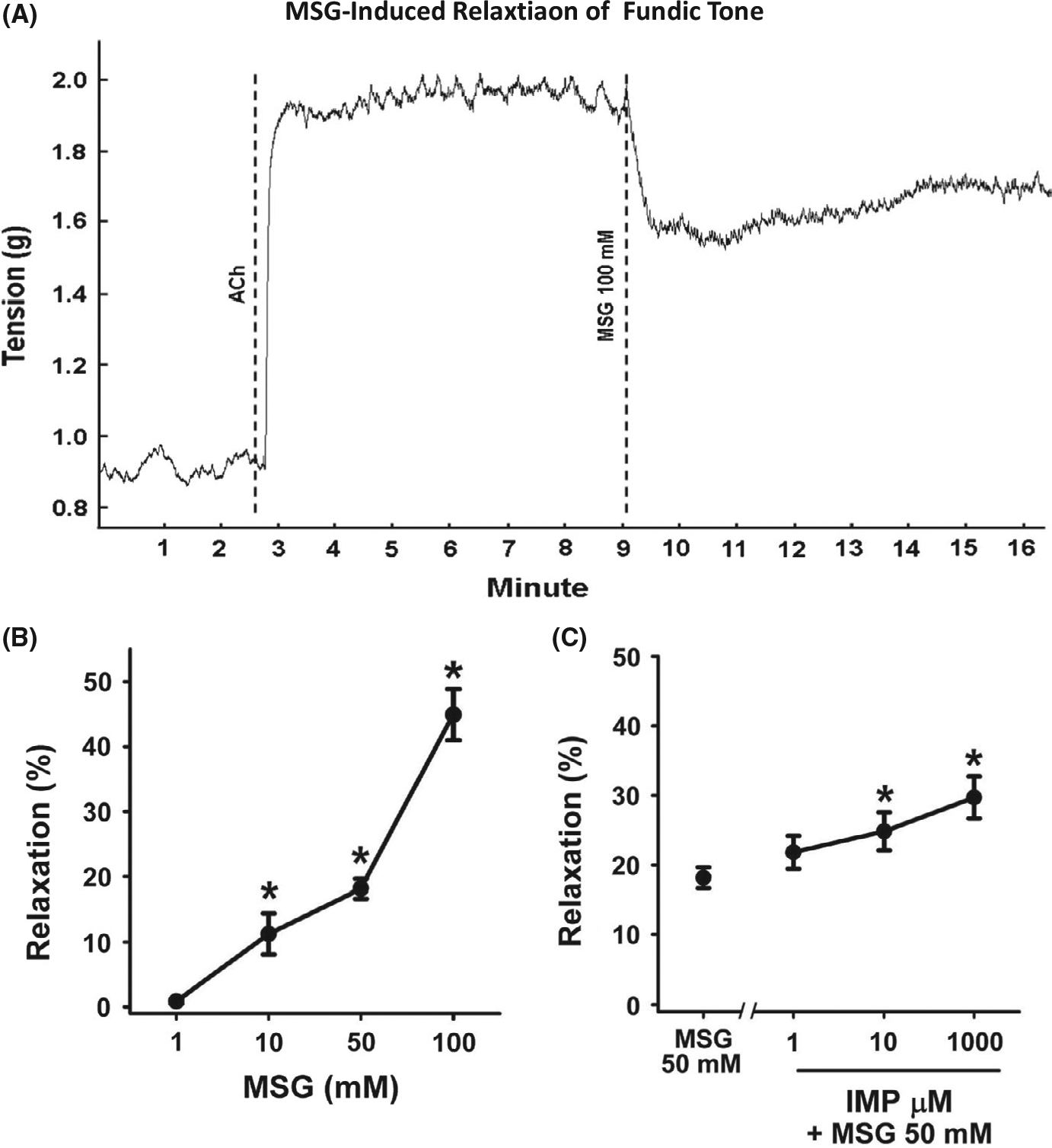
Inhibition of tonic muscle contraction by MSG and MSG plus IMP in muscle strips from fundus. A, Representative tracing illustrating the contraction of the muscle strip from fundus in response to acetylcholine (ACh, 100 μmol/L) and inhibition of contraction by MSG (100 mmol/L). The average increase in tension in response to ACh is 0.8 g. Dotted lines indicate the time at which the agents were added. B, Concentration-dependent relaxation by MSG. Relaxation was calculated as percent inhibition of ACh (100 μmol/L)-induced contraction. Data are mean ± SEM, *P < .05, (n = 12–27). C, Concentration-dependent augmentation of MSG (50 mmol/L)-induced relaxation by IMP. Data are mean ± SEM, *P < .05 vs MSG (50 mmol/L), (n = 12–27)
In the muscle strips from antrum, ACh also induced a rapid and sustained tonic contraction of lower amplitude than fundus. MSG caused a rapid, concentration-dependent relaxation (ie, inhibition) of ACh-induced contraction in antrum which was significant at concentrations greater than 10 mmol/L (F (3, 86) = 36.96; P < .01; Figure 2A,B). Although the effect of IMP at different concentrations showed a tendency to augment MSG-induced relaxation of ACh-induced tone, this augmentation did not achieve statistical significance (Figure 2C). This likely is due to the overall lower levels of tonic contraction, and therefore relaxation, in antral strips. Consistent with the notion that gastric antral tissue is a phasic rather than tonic muscle, ACh caused an increase in both amplitude and frequency of phasic contractile activity rather than increase in tone in many strips. In antral muscle strips demonstrating increased ACh-induced phasic activity, MSG inhibited both phasic contraction amplitude (F (3, 46) = 9.25; P < .01; Figure 3A,B) and phasic contraction frequency (F (4, 96) = 13.92; P < .01; Figure 3A,C). The decrease in phasic contraction amplitude achieved significance at concentrations >50 mmol/L whereas the inhibition of frequency was significant at 100 mmol/L. It is noteworthy that at this higher concentrations MSG abolished all phasic activity in some antral strips. These results suggest that activation of T1R1/T1R3 receptors also causes relaxation and inhibition of phasic contractile activity in gastric antrum.
FIGURE 2.
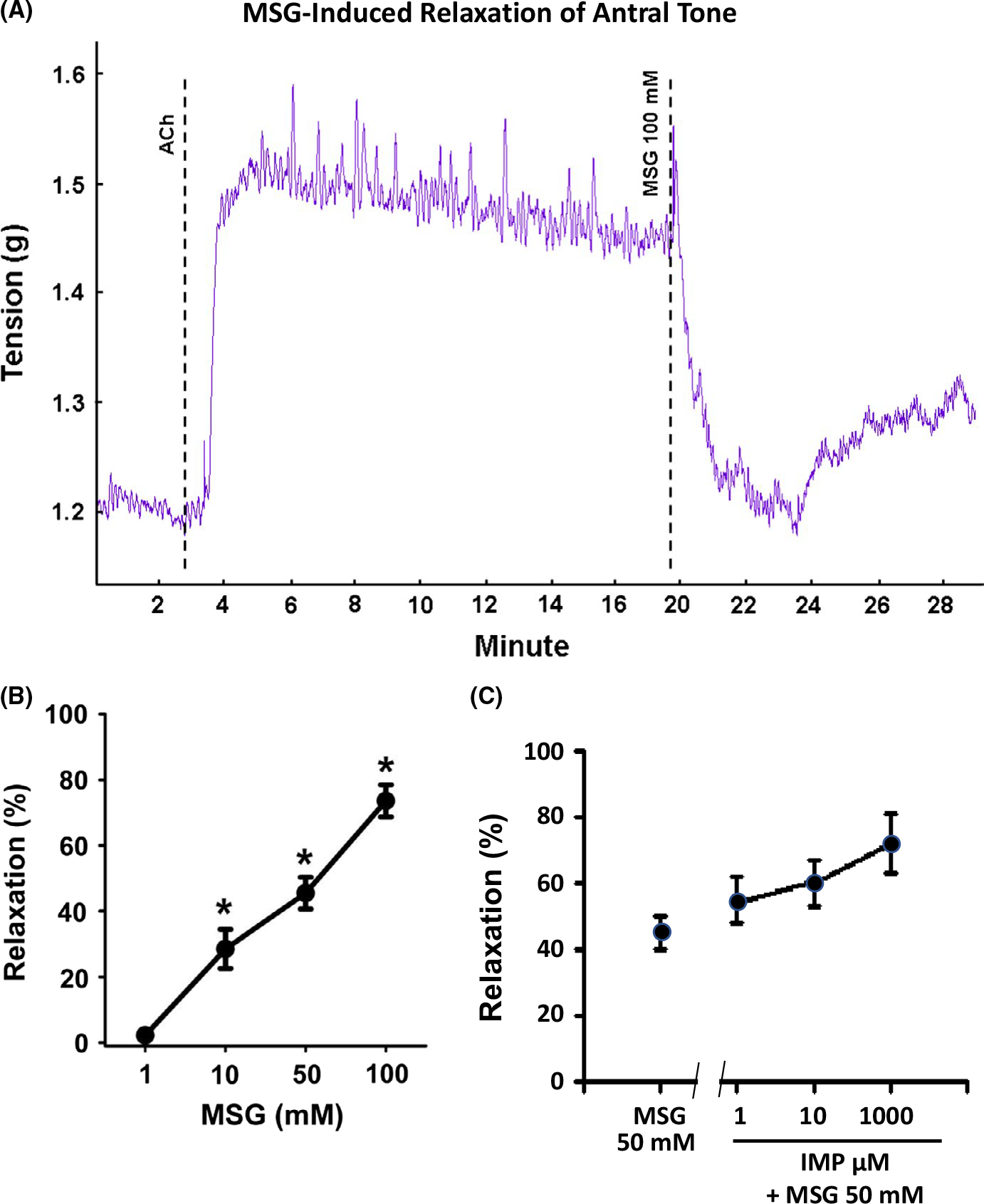
Inhibition of tonic muscle contraction by MSG and MSG plus IMP in muscle strips from antrum. A, Representative tracing illustrating the contraction of the muscle strip from antrum in response to acetylcholine (ACh, 100 μmol/L) and inhibition of contraction by MSG (100 mmol/L). The average increase in tension in response to ACh is 0.2 g. Dotted lines indicate the time at which the agents were added. B, Concentration-dependent relaxation by MSG. Relaxation was calculated as percent inhibition of ACh (100 μmol/L)-induced contraction. Data are mean ± SEM, *P < .05, (n = 7–18). C, Concentration-dependent augmentation of MSG (50 mmol/L)-induced relaxation by IMP. Relaxation was calculated as percent inhibition of ACh (100 μmol/L)-induced contraction. Data are mean ± SEM. Although MSG-induced relaxation appears to be increasing with IMP, the values are not statistically significant compared to relaxation by MSG (50 mmol/L), (n = 7–18)
FIGURE 3.
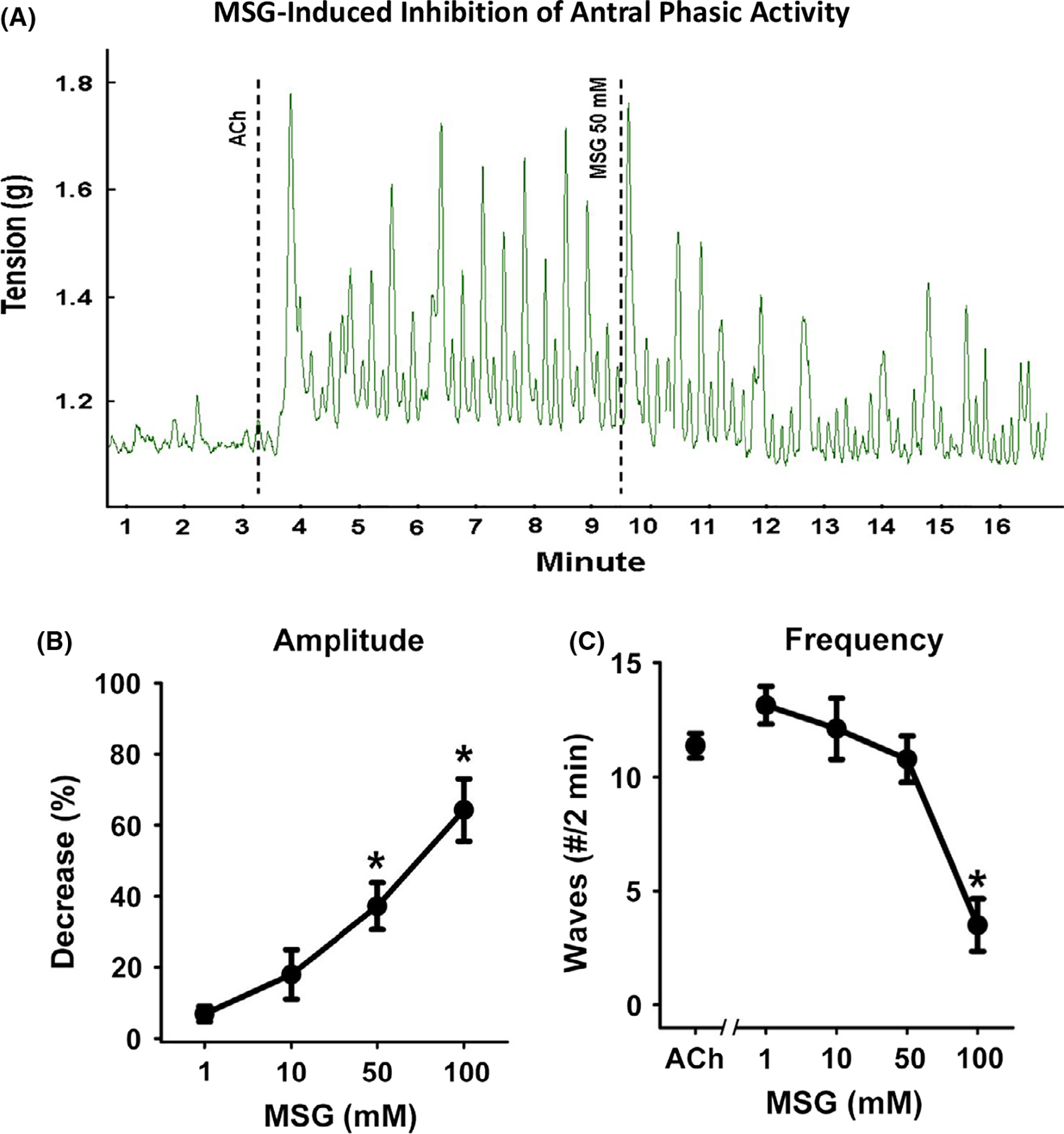
Inhibition of phasic muscle contraction by MSG and MSG plus IMP in muscle strips from antrum. A, Representative tracing illustrating the phasic contraction of the muscle strip from antrum in response to acetylcholine (ACh, 100 μmol/L) and inhibition of the amplitude and frequency of phasic contraction by MSG (100 mmol/L). Dotted lines indicate the time at which the agents were added. B, Concentration-dependent inhibition of amplitude by MSG. Inhibition by MSG was calculated as % decrease in ACh-induced increase in the mean amplitude of phasic contractions. Data are mean ± SEM, *P < .05, (n = 7–18). C, Concentration-dependent inhibition of frequency of both low and high amplitude phasic contraction by MSG. Inhibition by MSG was calculated as % decrease in the ACh-induced increase in the number of phasic contractions. Data are mean ± SEM, *P < .05, (n = 7–18)
In innervated muscle strips from fundus and antrum, relaxation in response to MSG could be due to activation of inhibitory neurons which have been shown to release NO, vasoactive intestinal peptide (VIP), and other putative agents. To assess involvement of enteric neurons in general and NO in particular, separate muscle strips were tested as described above with ACh (100 μmol/L) and MSG (50 mmol/L), then incubated with the neurotoxin tetrodotoxin (5 μmol/L) or the nitric oxide synthase inhibitor L-NNA (100 μmol/L) for 15 minutes and re-tested for the effect of MSG (50 mmol/L) on ACh-(100 μmol/L)-induced contraction. Neither TTX nor L-NNA affected MSG-induced relaxation in fundus or antrum (Figure 4). These results suggested that the effect of MSG is due to activation of T1R1/T1R3 receptor present on SMCs. This was tested first by determining the expression of T1R1 and T1R3 receptors in cultured SMCs devoid of neurons and ICC, and measurements of relaxation and intrinsic signaling pathways such as G protein activation and intracellular Ca2+ in SMCs isolated from the whole stomach.
FIGURE 4.
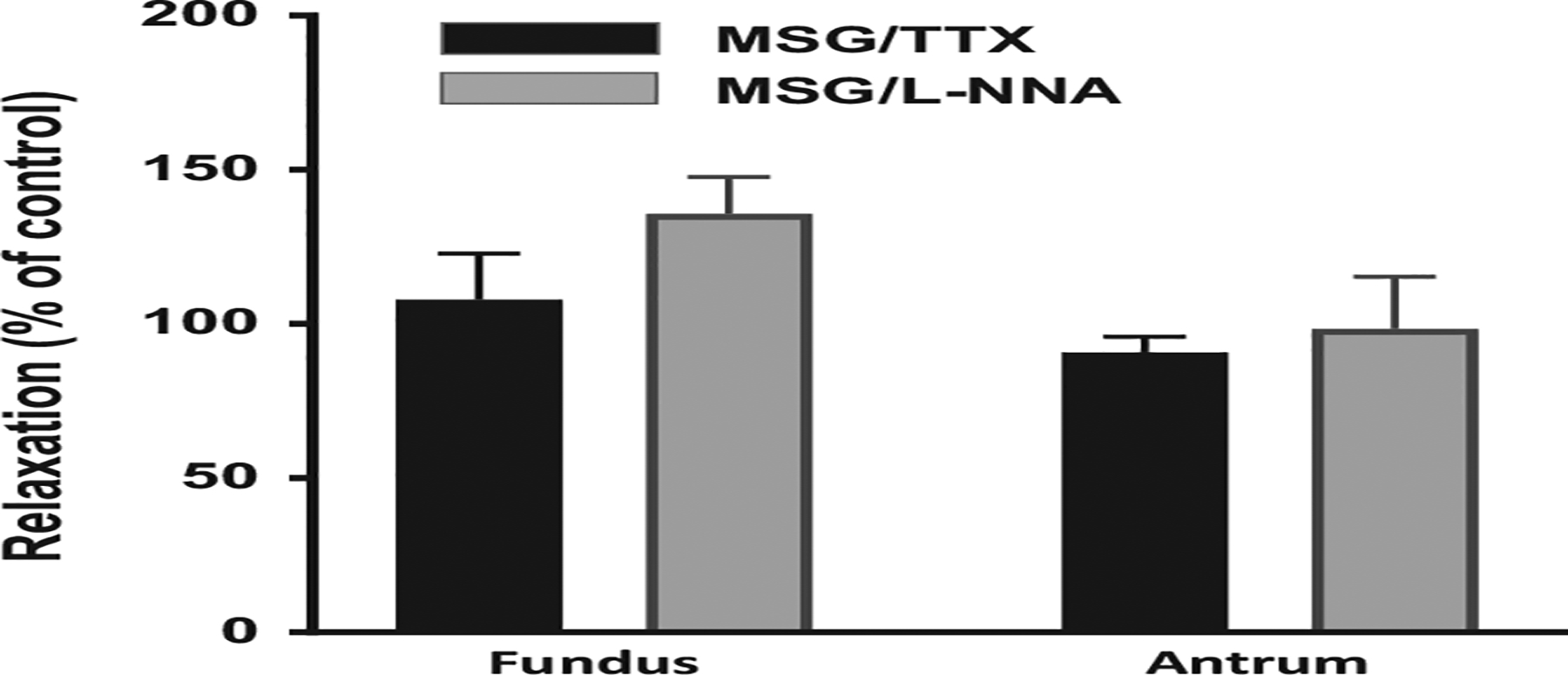
Lack of effect of tetrodotoxin (TTX) and nitric oxide synthase inhibitor (L-NNA) on MSG-induced relaxation. Muscle strips from fundus and antrum were treated with ACh (100 μmol/L) separately to induce contraction followed by treatment with MSG (50 mmol/L) to induce relaxation before and after the addition of either TTX (5 μmol/L) or L-NNA (100 μmol/L) for 15 minutes. Relaxation by MSG was calculated before (control) and after the addition of TTX or L-NNA and expressed as % of control response. L-NNA and TTX did not affect MSG-induced relaxation. Data are mean ± SEM, *P < .05, (n = 3–4 for L-NNA and 4–5 for TTX)
3.2 |. Expression of umami receptors (T1R1 and T1R3) on gastric smooth muscle cells
We first evaluated the presence of the T1R1 and T1R3 receptor subunits in isolated gastric SMCs. T1R1 and T1R3 were detected by RT-PCR using primers based on mouse cDNA (Figure 5A). Additionally, T1R1 (94 kDa) and T1R3 (93 kDa) proteins were detected via Western blot analysis using specific antibodies in lysates from isolated gastric SMCs (Figure 5B). The presence of T1R1 and T1R3 on gastric SMCs was confirmed by immunohistochemical detection using specific antibodies (Figure 5C). No staining was evident in the absence of primary antibody (Figure 5C).
FIGURE 5.
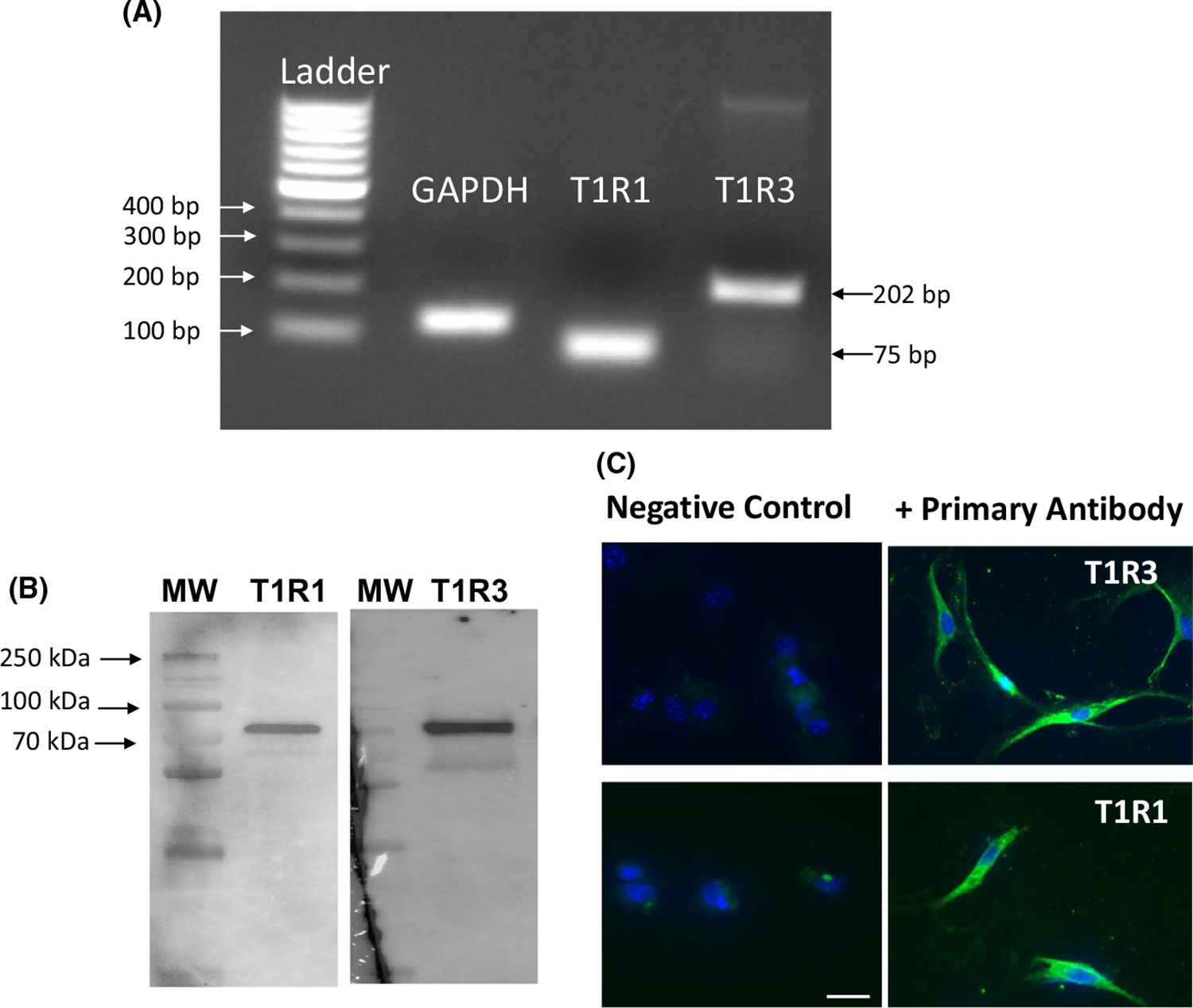
Expression of T1R1 and T1R3 in isolated gastric muscle cells. A, Total RNA isolated from cultured gastric SMCs was reverse transcribed, and cDNA amplified with specific primers for T1R1 and T1R3 as indicated in methods. Sizes are indicated for 100 bp DNA ladder. GAPDH was used as control. B, Lysates prepared from isolated gastric SMCs were homogenized and protein in the supernatant was separated by SDS-PAGE followed by Western blot analysis using antibodies specific for T1R1 and T1R3. Protein bands were visualized by chemiluminescence. Arrows indicate bands at T1R1 (94 kDa) and T1R3 (93 kDa), respectively. C, T1R1 and T1R3 were detected on cultured gastric SMCs using a specific antibody (sc-50308, 1:100 and sc-50352, 1:100) and Alexa Fluor 488 (1:300) (right panel; green). Cell nuclei were stained with DAPI (blue). In the absence of the primary antibody for T1R1 or T1R3 (left panels), there was no detectable staining for the secondary antibody. Scale bar equals 25 μm
3.3 |. Activation of T1R1 and T1R3 causes relaxation in smooth muscle cells
To evaluate the direct effect of MSG on gastric smooth muscle, isolated SMCs were treated with MSG (100 μmol/L) in the presence and absence of IMP (1 μmol/L) for 5 minutes before administration of ACh (1 μmol/L) for 1 minute. Change in cell length was measured via scanning micrometry. Treatment with ACh caused a 28 ± 3% decrease in cell length compared to control cells (basal cell length in controls: 80 ± 2 μm), indicating muscle cell contraction (Figure 6). Pretreatment with MSG in the absence and presence of IMP inhibited ACh-induced contraction (ie, relaxation). MSG administration decreased ACh-induced contraction to 14 ± 5% (or 49 ± 10% relaxation; P < .05 vs ACh) and MSG plus IMP further decreased ACh-induced contraction to 5 ± 2% (or 80 ± 6% relaxation; P < .05 vs MSG alone) (Figure 6).
FIGURE 6.
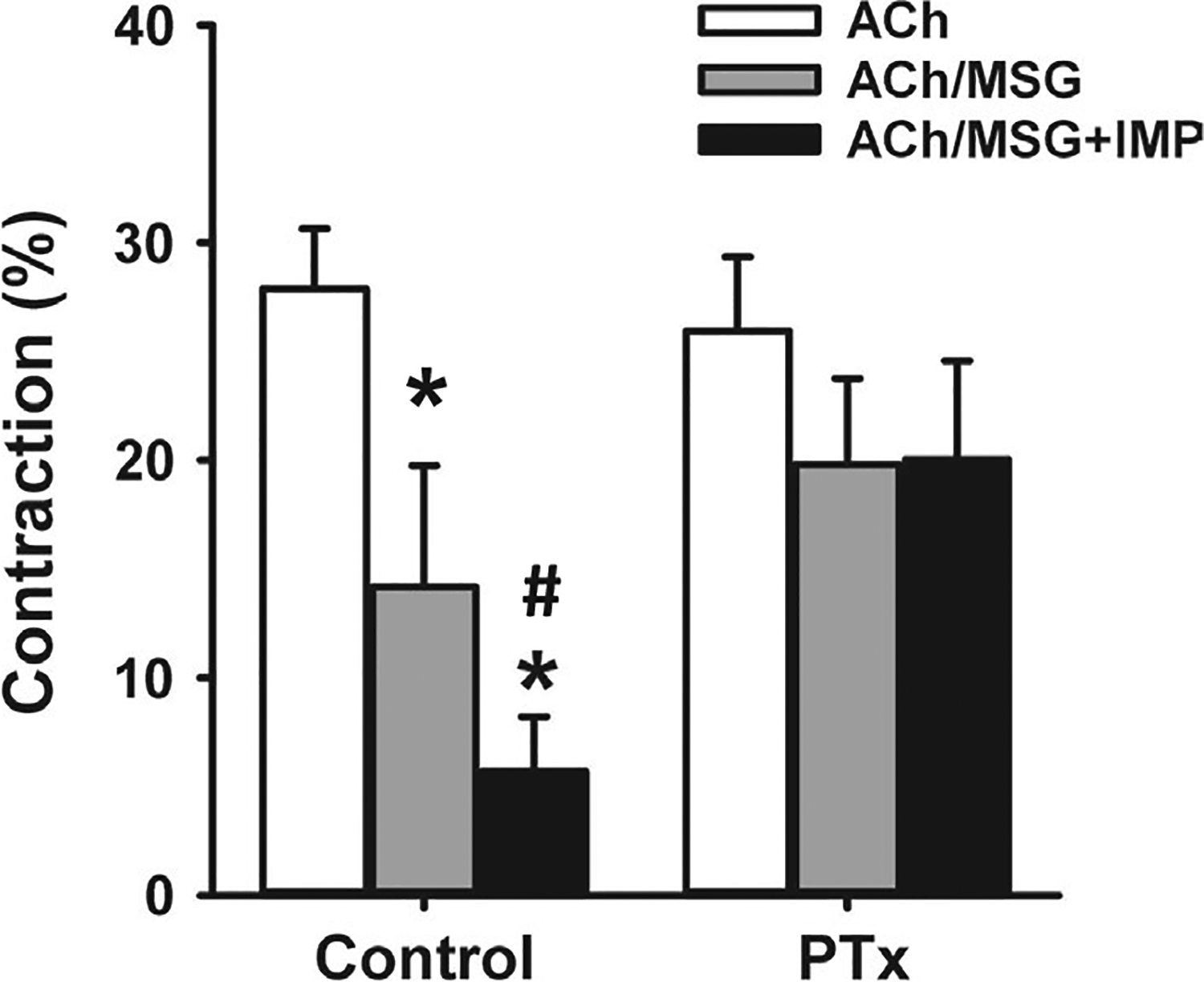
Inhibition of acetylcholine (ACh)-induced contraction by MSG and MSG plus IMP in isolated gastric muscle cells. Isolated gastric SMCs were treated with ACh (1 μmol/L) for 1 minute with or without pretreatment with MSG (100 μmol/L) alone or MSG plus IMP (1 μmol/L) for 5 minutes. In some experiments, SMCs were treated with Gαi inhibitor pertussis toxin (PTx, 400 ng/mL) for 1 h before treatment with ACh or ACh plus MSG and MSG plus IMP. Contraction was calculated as percent decrease from basal cell length (80 μm). MSG caused an inhibition of ACh-induced contraction (ie, relaxation) and IMP augmented MSG-induced relaxation. PTx had no effect on ACh-induced contraction but blocked relaxation by MSG and MSG plus IMP. Data are mean ± SEM, *P < .05 vs ACh response; # P < .05 between MSG and MSG plus IMP
Previous studies have shown that T1R1/T1R3 receptors activate canonical pathways via Gαgustducin (Gαgust; a member of the Gαi family) or non-canonical pathways via activation of Gαi or Gαs.20,21 To initially identify the G proteins activated by MSG in gastric SMCs, we used PTx which uncouples the receptors from Gαi proteins and NF449 which inhibits Gαs proteins.22 Dispersed SMCs were incubated with the PTx (400 ng/mL) for 1 hour before or the NF449 (10 μmol/L) for 15 minutes before treatment with MSG or MSG plus IMP. In cells treated with NF449, contraction in response to ACh was not affected (29 ± 3% decrease in cell length). Relaxation in response to MSG alone (70 ± 9% relaxation) or MSG plus IMP (85 ± 9% relaxation) was also not significantly different than in the absence of NF449. Previous studies showed that the relaxation in response to activation of Gαs-coupled receptors such as VPAC2 or GLP-1R was inhibited by NF449.22 These results suggest that T1R1/T1R3 receptors in smooth muscle are not coupled to Gαs proteins. In SMCs treated with PTx, contraction in response to ACh was not affected; however, relaxation in response to MSG alone or MSG plus IMP was strongly inhibited although only the inhibition by MSG plus IMP achieved statistical significance (Figure 6). In these cells, ACh caused a 26 ± 3% decrease in cell length and this contraction was not significantly affected by MSG with and without IMP. In the presence of MSG alone, ACh caused 20 ± 4% decrease in cell length (24 ± 6% relaxation by MSG), whereas in the presence of MSG plus IMP, ACh caused 20 ± 5% decrease in cell length (23 ± 8% relaxation by MSG plus IMP). These results suggest that T1R1/T1R3 receptors in gastric SMCs are coupled to PTx-sensitive Gαi proteins to mediate relaxation. It is noteworthy that although Gαgust belongs to the Gαi family, it is not sensitive to PTx.23
3.4 |. Activation of T1R1/T1R3 receptors stimulate Gαi2 protein activity
To corroborate the results with PTx and to directly demonstrate the stimulation of Gαi activity, G protein activation was measured by [35S]GTPγS loading in gastric SMCs membranes in response to MSG alone or MSG plus IMP using specific antibodies to G protein subunits. MSG (100 μmol/L) in the presence of IMP (1 μmol/L) increased the binding of [35S]GTPγS to Gαi2 in isolated SMC membranes (F (2,6) = 46.08; P < .01; Figure 7). Post hoc analysis revealed that MSG with IMP (P < .01) significantly increased binding compared to control. There was no significant increase of [35S]GTPγS binding over control when using antibodies to Gαgust and Gαs (Figure 7), Gαi1 and Gαi3, (data not shown). These results suggest that T1R1/T1R3 receptors in gastric SMCs are coupled selectively to Gαi2 in contrast to the canonical activation of Gαgust and confirm the results obtained with PTx.
FIGURE 7.
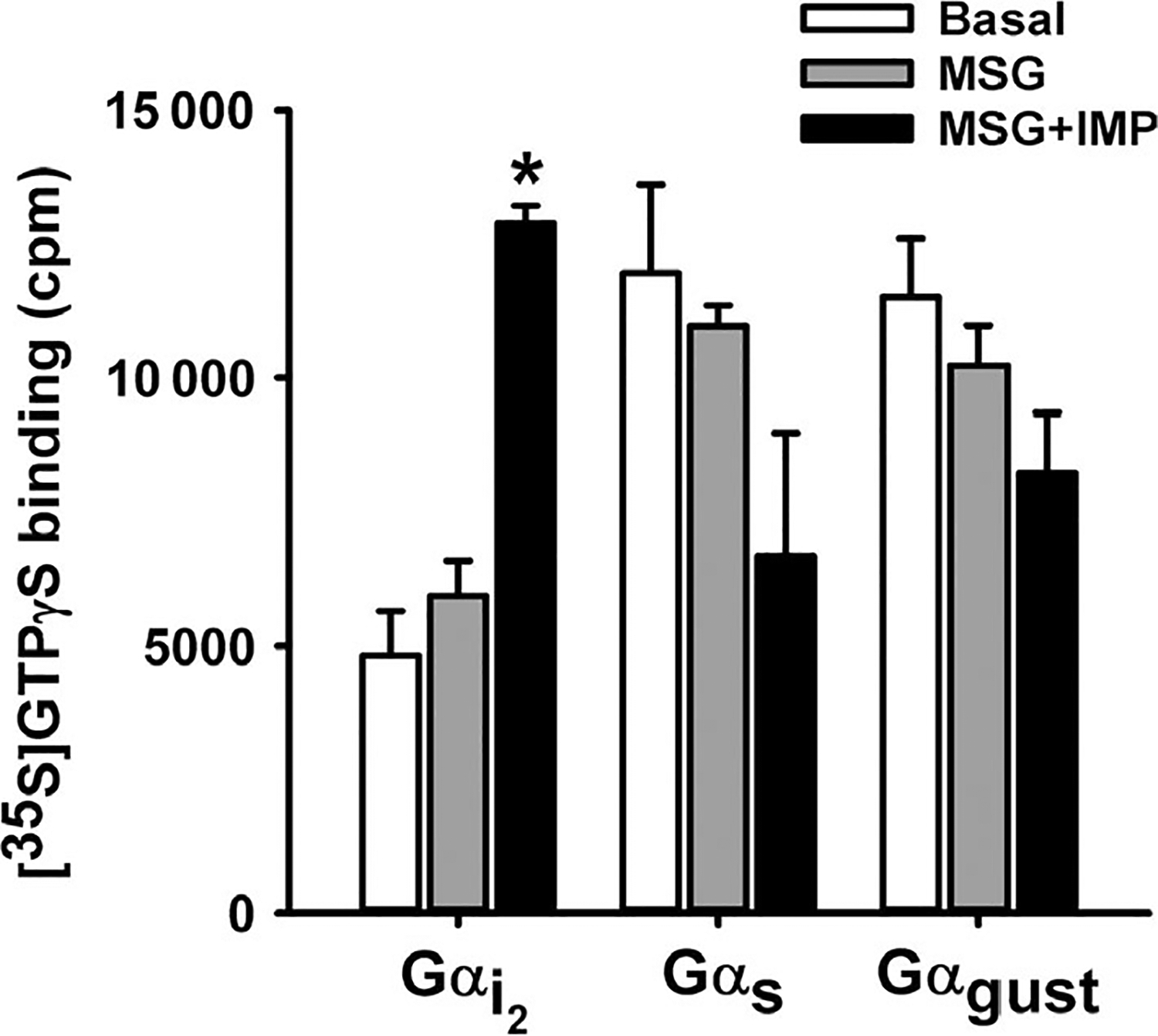
Activation of Gαi2 by MSG plus IMP in isolated muscle cells. Membranes derived from isolated gastric SMCs were incubated with [35S]GTPγS in the presence or absence of MSG (100 μmol/L) and MSG plus IMP (1 μmol/L) for 20 minutes. Aliquots were added to wells precoated with antibodies to Gαi2, Gαs, and Gαgustducin for 2 hours, and the bound radioactivity was measured. An increase in the binding of [35S]GTPγS.Gα reflects activation of G protein. A significant increase in the binding of [35S]GTPγS.Gα complexes was obtained to wells coated with Gαi2 antibody only. Data are mean ± SEM, *P < .05 vs basal (n = 3)
3.5 |. Activation of T1R1/T1R3 decreases [Ca2+]I in smooth muscle cells
Increase in intracellular Ca2+ mediates smooth muscle contraction whereas a decrease in intracellular Ca2+ mediates smooth muscle relaxation.18 To examine whether relaxation in response to activation of T1R1/T1R3 receptors is associated with a decrease in intracellular Ca2+, intracellular Ca2+ levels were measured in single SMCs by fura-2 fluorescence. Administration of MSG (100 μmol/L) in the presence and absence of IMP (1 μmol/L) decreased basal intracellular Ca2+ in fura-2 AM loaded gastric SMCs. Carbachol (CCh; 10 μmol/L) or ACh (1 μmol/L) caused a transient increase of intracellular Ca2+ (Figure 8), and this is consistent with activation of Gαq-coupled muscarinic m3 receptors. Cholinergic stimulation in the presence of MSG alone and in the presence of IMP attenuated muscarinic agonist induced increase in [Ca2+]i levels consistent with the inhibitory effect of MSG with or without IMP on ACh-induced SMC contraction. MSG (100 μmol/L) caused a 44 ± 28% decrease in calcium response and MSG with IMP caused a 89 ± 7% decrease in calcium response as measured by a decrease in 340/380 ratio in fura-2 loaded cells (Figure 8).
FIGURE 8.
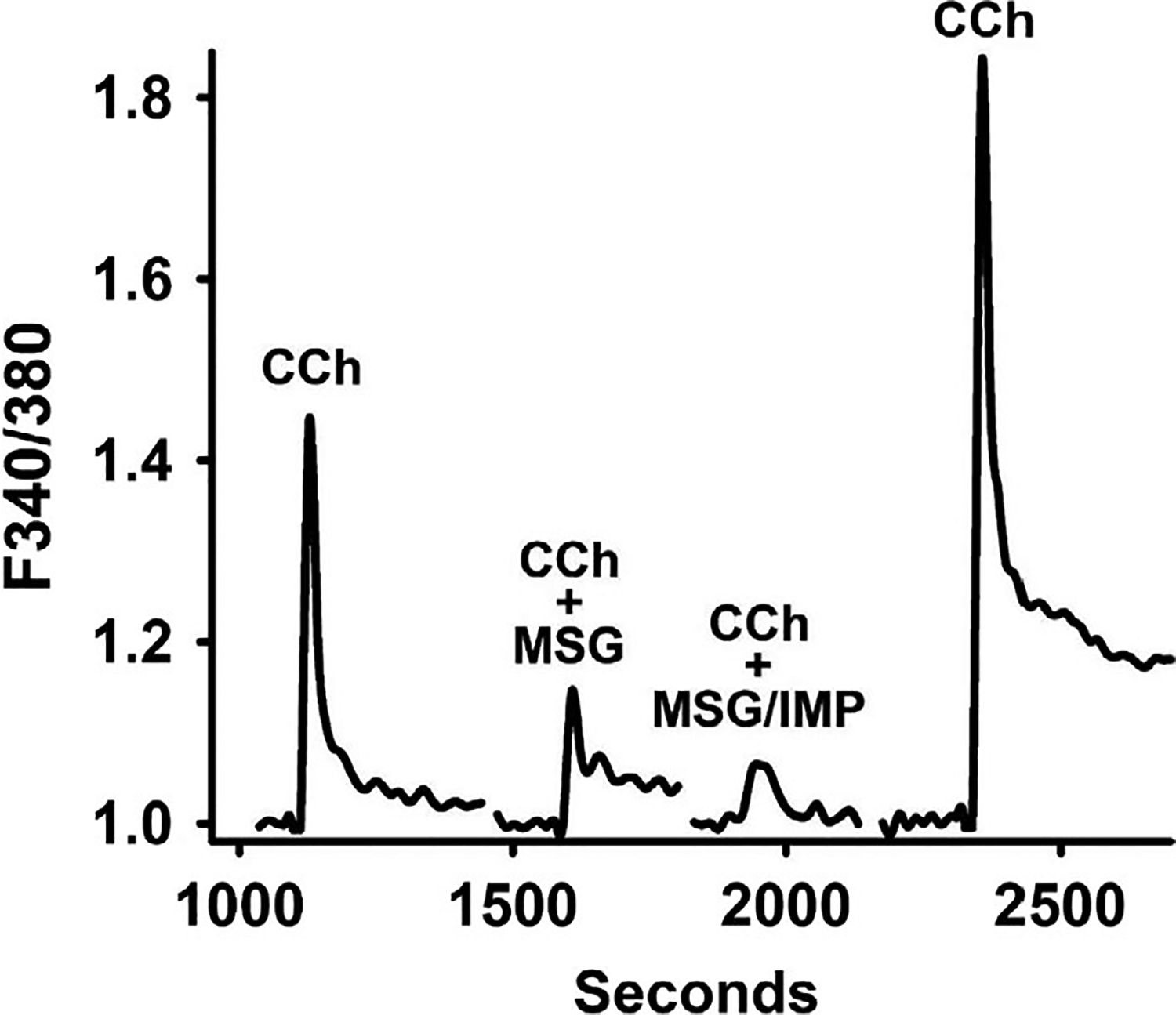
Inhibition of carbachol (CCh)-induced increase in intracellular Ca2+ by MSG and MSG plus IMP in isolated muscle cells. Isolated gastric SMCs were loaded with 5 μmol/L fura-2 and treated with 10 μmol/L CCh before and after pretreatment with MSG (100 μmol/L) alone or MSG plus IMP (1 μmol/L). The SMCs were alternately excited at 380 nm and 340 nm. The background and autofluorescence were corrected from images of a SMC without the fura-2. Results in the representative tracing are expressed as 340/380 ratio and an increase in ratio reflects an increase in cytosolic Ca2+
4 |. DISCUSSION
The physiological effects of nutrients in the gut are mediated by their uptake and distribution in the body or secondary release of neurohumoral agents. Recent studies that showed the presence of plasma membrane receptors for nutrients outside the gustatory system, especially in the gut, suggesting a much more complex role of nutrients in the control of gut function, appetite, and even emotional conditions.1–8 The majority of the research on the receptors for chemosensation has focused on EECs of gut mucosa. Plasma membrane GPCRs for umami and sweet agonists comprise heterodimers of T1R subtype; T1R1/T1R3 for umami and T1R2/T1R3 for sweet receptors. T1R2 activation in EECs of the intestine increased GLP-1, GLP-2, and GIP release,7,12 and T1R1 activation causes a release of CCK, CGRP, peptide YY, and neurotensin in intestine, colon and STC-1 cell line.12,13,24–26 Activation of T1R1/T1R3 receptors in mouse colon causes release of CGRP and elicits ascending contraction and descending relaxation components of the peristaltic reflex.13 While these studies provide evidence for expression of T1Rs on EECs mediating hormone release, the expression of T1Rs on SMCs and their role in the regulation of smooth muscle function itself has not been fully characterized. Therefore, the present study evaluated the expression and function of T1R1/T1R3 in gastric smooth muscle tissue as well as isolated SMCs and confirmed the presence of T1R1 and T1R3 on isolated gastric SMCs at the mRNA and protein levels.. In the present study, activation of these receptors by umami ligands MSG and MSG plus IMP causes relaxation of smooth muscle. The evidence for the notion that these ligands act directly on SMCs was based on the following results: (a) MSG with or without IMP inhibited ACh-induced contraction (ie, caused relaxation) in a concentration-dependent fashion in muscle strips from fundus and antrum; (b) the general neurotoxin TTX and the NO synthase inhibitor, L-NNA, had no effect on relaxation induced by MSG or MSG plus IMP; (c) T1R1 and T1R3 transcripts and protein are present in isolated SMCs; (d) MSG or MSG plus IMP decreased ACh-induced increase in intracellular Ca2+ in isolated gastric SMCs; (e) relaxation induced by MSG and MSG plus IMP was blocked in SMCs pretreated with Gαi blocker PTx, but not in cells pretreated with Gαs blocker NF449, suggesting that relaxation was mediated by activation of Gαi proteins; and (f) MSG plus IMP selectively stimulated Gαi2 activity in isolated SMCs.
Recent studies by Vancleef have identified the species-specific presence of mRNA transcripts for several types of L-AA receptors on human and mouse gastric tissues and the functional effect of their activation.16 While gastric tissue likely contains multiple cell types, the findings likely represent expression in smooth muscle and are consistent with the present study. This study differed from the present one in that most L-AAs and a casein hydrolysate caused a phasic and a tonic contraction rather than relaxation. Although several L-AA were tested, the paradigmatic T1R1/T1R3 receptor agonist MSG and MSG plus IMP were not tested in these studies. Several of those tested failed to cause contraction and L-Glu caused a nonsignificant decrease in tone. Since multiple L-AA receptors are present, it is possible relaxation and contraction reflects interaction with different receptors. This notion is supported by the finding that in human tissues, these authors found the T1R1/T1R3 antagonist, lactisole, increased phasic but not tonic contraction in response to casein suggesting an inhibitory action of T1R1/T1R3 activation. This study also found that the tonic but not phasic contraction was inhibited by 50% by a blocker of the CaSR receptor. The contractile effects of L-AAs were TTX-resistant and retained in α-gustducin knockout mice suggesting activation of non-canonical G proteins, a notion consistent with the activation of similar PTx-sensitive G proteins in our study. The similarity and difference of these studies with our study suggest that the response to L-AA in smooth muscle as in other tissues may be variable and highly depend on specific L-AA receptor(s), region of the gut, and species.
The ability of nutrient receptors to demonstrate differences from the established signaling pathways has been described in various tissues. Functional bitter taste receptors were demonstrated on human and mouse gut SMCs and activation of these receptors induced contraction and relaxation in an agonist- and region-specific manner. For example, lower concentrations of bitter agonists caused muscle contraction, while higher concentrations induced muscle relaxation, whereas other bitter agonists only affected gastric muscle and not colonic muscle.10 Contraction was suggested to be mediated by Gαgust and other Gαi proteins, whereas relaxation was suggested to be T2R-independent.10 In human airway smooth muscle, T2Rs are coupled to Gαi1–3, an effect supported by the finding that Gαgust knockdown had no effect on signaling.23 Consistent with studies on T2Rs, T1R1/T1R3 have also been shown to be coupled to Gαs in neuroblastoma cells.21
Smooth muscle relaxation is typically regulated by inhibitory transmitters such as NO that activate the soluble guanylyl cyclase (sGC)/cGMP/cGMP-dependent protein kinase (PKG) pathway and/or VIP that acts on Gαs-coupled VPAC2 receptors to activate adenylyl cyclase (AC)/cAMP/cAMP-dependent protein kinase (PKA) pathway and is characterized by a decrease in intracellular calcium.18,22 Our studies in gastric smooth muscle showed that relaxation in response to T1R1/T1R3 receptor activation was direct because it was evident in isolated SMCs and because relaxation in smooth muscle strips was not affected by the neurotoxin TTX. It was also not mediated by activation of sGC/cGMP/PKG or AC/cAMP/PKA pathway in SMCs because the nitric oxide synthase inhibitor L-NNA or the Gαs inhibitor NF449 had no effect on relaxation in response to MSG and MSG plus IMP. Relaxation in response to MSG and MSG plus IMP is blocked by PTx suggesting activation of T1R1/T1R3 receptors coupled to Gαi proteins. This was corroborated by direct measurements of G protein activation in SMCs, as shown to be the case in airway SMCs.23
Considering a decrease in Ca2+ is essential for smooth muscle relaxation, we evaluated intracellular Ca2+ release in isolated SMCs using single cell live imaging. The cholinergic agonist caused a transient peak in intracellular Ca2+ release which was significantly decreased by MSG and MSG plus IMP indicating T1R1/T1R3 activation decreases intracellular Ca2+. Our studies did not identify the mechanism involved in the decrease in the Ca2+, and however, one plausible mechanism could be activation of K+ channels and suppression of voltage-gated Ca2+ channels leading to hyperpolarization as shown with airway smooth muscle relaxation mediated by bitter taste receptors.27
In the present study, the classic umami T1R1/T1R3 agonist MSG significantly decreased ACh-induced contraction and phasic activity. However, MSG and other L-amino acids (L-AA) can activate a variety of other umami receptors including the CaSR, metabotropic glutamate receptors (mGlu 1 and 4), and G protein-coupled receptor family group 6 member A (GPR6A) receptor among others.6,28–30 While MSG has highest specificity for the T1R1/T1R3 heterodimer, the specific positive allosteric modulator IMP was used in conjunction with MSG to selectively identify T1R1/T1R3 mediated effects.10,31,32 In the present study, IMP augmented MSG-induced relaxation in fundic and antral strips in a concentration-dependent manner. Additionally, IMP augmented MSG-induced relaxation and MSG-induced inhibition of Ca2+ in isolated gastric muscle cells. In some cases, such as the antrum, the augmentation did not achieve statistical significance. This could be explained by the limited potential for augmentation of relaxation in antral muscle strips because of the small tonic contraction (about 0.2 g) induced by ACh in the antrum and the near maximal relaxation in response to MSG alone. It is also possible that the effect of MSG could be mediated, in part, by other receptors in conjunction with the T1R1/T1R3 heterodimer and the fact that this partial component is not augmented by IMP. Finally, it is possible that the expression of putative umami receptors by individual muscle cells differs within or between regions. Collectively, the present data strongly support the notion that MSG-induced gastric relaxation is mediated by specific T1R1/T1R3 receptors on the SMCs.
As nutrients in the lumen of the stomach do not directly interact with the smooth muscle, the effects of T1R1/T1R3 activation on smooth muscle may be due to more systemic effects following absorption. While we did not evaluate the systemic effect of MSG and MSG plus IMP, absorption of nutrients can have stimulatory effects via the vasculature that differs from direct interaction in the gut lumen.3,33 For example, a free fatty acid receptor 1 (FFAR1) agonist did not affect intestinal secretory functions when administered intraluminally, but induced GLP-1 release after intravascular administration.33 This indicates that nutrients can have complex effects that may include both direct intraluminal effects at the EECs and extraluminal effects following absorption and distribution by systemic vasculature to other tissues such as gut smooth muscle.33
In conclusion, the present study determined the presence, function, and signaling of T1R1/T1R3 activation in gastric smooth muscle. T1R1/T1R3 activation decreased intracellular Ca2+ via Gαi2 to induce gastric smooth muscle relaxation. As the GI tract acts as a gateway for the effect of nutrients, understanding the nutrient receptor function and potential multiple sites of action opens the possibility that gastric functions can be modulated by targeting these receptors on a variety of cell types.
Key Points.
The expression of umami (T1R1/T1R3) receptors is well characterized in enteroendocrine cells of gut mucosa but their presence and action in smooth muscle are less well known. The present study examined the expression and function of umami receptors in gastric smooth muscle.
Umami (T1R1/T1R3) receptors are present on gastric smooth muscle cells and activation of these receptors inhibited contraction (caused relaxation) in muscle strips and isolated smooth muscle cells. Relaxation was mediated by a decrease in intracellular Ca2+ via activation of Gαi2.
These studies suggest the role of nutrient receptors in the regulation of gut motility at the level of smooth muscle in addition to their characteristic role in mucosal enteroendocrine cells. The study also suggests a potential role for T1R1/T1R3 agonists to treat disorders of gastric motility.
ACKNOWLEDGMENTS
This work was supported by DK34153 (JR Grider), and DK28300 and DK15564 (KS Murthy) from the National Institute of Diabetes and Digestive and Kidney Diseases. MS Crowe was supported by NIH F32 Fellowship (DK118877). A preliminary report of this study was presented as an abstract at the 3rd Meeting of the Federation of Neurogastroenterology and Motility in Amsterdam, the Netherlands in 2018.
Footnotes
CONFLICT OF INTEREST
All the authors declare that they have no conflict of interest.
REFERENCES
- 1.Vella A, Camilleri M. The gastrointestinal tract as an integrator of mechanical and hormonal response to nutrient ingestion. Diabetes. 2017;66:2729–2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farré R, Tack J. Food and symptom generation in functional gastrointestinal disorders: physiological aspects. Am J Gastroenterol. 2013;108:698–706. [DOI] [PubMed] [Google Scholar]
- 3.Steensels S Depoortete I. Chemoreceptors in the gut. Annu Rev Physiol. 2018;80:117–141. [DOI] [PubMed] [Google Scholar]
- 4.Kokrashvili Z, Mosinger B, Margolskee R. T1R3 and α gustducin in gut regulate secretion of glucagon-like peptide. Ann N Y Acad Sci. 2009;1170:91–94. [DOI] [PubMed] [Google Scholar]
- 5.Rozengurt E, Sterninin C. Taste receptor signaling in the mammalin gut. Curr Opin Pharmacol. 2007;7:557–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raybould HE. Gut chemosensing: interactions between gut endocrine cells and visceral afferents. Auton Neurosci. 2010;153:41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shirazi-Beechey SP, Daly K, Al-Rammahi M, Moran AW, Bravo D. Role of nutrient-sensing taste 1 receptor (T1R) family members in gastrointestinal chemosensing. Br J Nutr. 2014;111(Suppl 1):S8–15. [DOI] [PubMed] [Google Scholar]
- 8.Kaji I, Kaunitz JD. Luminal chemosensing in the gastroduodenal mucosa. Curr Opin Gastroenterol. 2017;33:439–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu H, Staszewski L, Tang H, Adler E, Zoller M, Li X. Different functional roles of T1R subunits in the heteromeric taste receptors. Proc Natl Acad Sci USA. 2004;101:14258–14263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Avau B, Rotondo A, Thijs T, et al. Targeting extra-oral bitter taste receptors modulates gastrointestinal motility with effects on satiation. Sci Rep. 2015;5:15985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scott TR, Giza BK. Issues of gustatory neural coding: where they stand today. Physiol Behav. 2000;69:65–76. [DOI] [PubMed] [Google Scholar]
- 12.Daly K, Al-Rammahi M, Moran A, Marcello M, Ninomiya Y, Shirazi-Beechey SP. Sensing of amino acids by the gut-expressed taste receptor T1R1–T1R3 stimulates CCK secretion. Am J Physiol Gastrointest Liver Physiol. 2013;304:G271–G282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kendig DM, Hurst NR, Bradley ZL, et al. Activation of the umami taste receptor (T1R1/T1R3) initiates the peristaltic reflex and pellet propulsion in the distal colon. Am J Physiol Gastrointest Liver Physiol. 2014;307:G1100–G1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He W, Yasumatsu K, Varadarajan V, et al. Umami taste responses are mediated by alpha-transducin and alpha-gustducin. J Neurosci. 2004;24:7674–7680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nelson G, Chandrashekar J, Hoon MA, et al. An amino-acid taste receptor. Nature. 2002;416:199–202. [DOI] [PubMed] [Google Scholar]
- 16.Vancleef L, Thijs T, Baert F, et al. Depoortere I. Obesity impairs oligopeptide/amino acid-induced ghrelin release and smooth muscle contractions in human proximal stomach. Mol Nutr Food Res. 2018;62 10.1002/mnfr.201700804. [DOI] [PubMed] [Google Scholar]
- 17.Mahavadi S, Bhattacharya S, Kumar DP, et al. Increased PDE5 activity and decreased Rho kinase and PKC activities in colonic muscle from caveolin-1−/− mice impair the peristaltic reflex and propulsion. Am J Physiol Gastrointest Liver Physiol. 2013;305:G964–G974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murthy KS. cAMP inhibits IP3-dependent Ca2+ release by preferential activation of cGMP-primed PKG. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1238–G1245. [DOI] [PubMed] [Google Scholar]
- 19.Sriwai W, Mahavadi S, Al-Shboul O, Grider JR, Murthy KS. Distinctive G protein-dependent signaling by protease-activated receptor 2(PAR2) in smooth muscle: feedback inhibition of RhoA by cAMP-independent PKA. PLoS ONE. 2013;8:e66743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ozeck M, Busrt P, Xu H, Servant G. Receptors for bitter, sweet and umami taste couple to inhibitory G protein signaling pathways. Eur J Pharmacol. 2004;489:139–149. [DOI] [PubMed] [Google Scholar]
- 21.Muroi Y, Ishii T. Umami taste receptor functions as an amino acid sensor via Gαs subunit in N1E–115 neuroblastoma cells. J Cell Biochem. 2012;113:1654–1662. [DOI] [PubMed] [Google Scholar]
- 22.May T, Crowe MS, Blakeney BA, et al. Identification of expression and function of the glucagon-like peptide receptor in the colonic smooth muscle. Peptides. 2019;112:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim D, Woo JA, Geffken E, An SS, Liggett SB. Coupling of airway smooth muscle bitter taste receptors to intracellular signaling and relaxation Is via Gαi 1,2,3. Am J Respir Cell Mol Biol. 2017;56:762–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cordier-Bussat M, Bernard C, Levenez F, et al. Peptones stimulate both the secretion of the incretin hormone glucagon-like peptide 1 and the transcription of the proglucagon gene. Diabetes. 1998;477:1038–1045. [DOI] [PubMed] [Google Scholar]
- 25.Geraedts M, Troost FJ, Saris W. Peptide-YY is released by the intestinal cell line STC-1. J Food Sci. 2009;74:H79–82. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, Prpic V, Green GM, Reeve JR, Liddle RA. Luminal CCK-releasing factor stimulates CCK release from human intestinal endocrine and STC-1 cells. Am J Physiol Gastrointest Liver Physiol. 2002;282:G16–G22. [DOI] [PubMed] [Google Scholar]
- 27.Zhang CH, Lifshitz LM, Uy KF, Ikebe M, Fogarty KE, ZhuGe R. The cellular and molecualr basis of bitter tastant-induced bronchodilatin. PLoS Biol. 2013;11:e1001501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nguyen CA, Akiba Y, Kaunitz JD. Recent advances in gut nutrient chemosensing. Curr Med Chem. 2012;19:28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wellendorph P, Johansen LD, Bräuner-Osborne H. Molecular pharmacology of promiscuous seven transmembrane receptors sensing organic nutrients. Mol Pharmacol. 2009;76:453–465. [DOI] [PubMed] [Google Scholar]
- 30.Yasumatsu K, Manabe T, Yoshida R, et al. Involvement of multiple taste receptors in umami taste: analysis of gustatory nerve responses in metabotropic glutamate receptor 4 knockout mice. J Physiol. 2015;593:1021–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baryłko-Pikielna N, Kostyra E. Sensory interaction of umami substances with model food matrices and its hedonic effect. Food Qual Prefer. 2007;18:751–758. [Google Scholar]
- 32.Behrens M, Meyerhof W, Hellfritsch C, Hofmann T. Sweet and umami taste: natural products, their chemosensory targets, and beyond. Angew Chem Int Ed. 2011;50:2220–2242. [DOI] [PubMed] [Google Scholar]
- 33.Christensen LW, Kuhre RE, Janus C, Svendsen B, Holst JJ. Vascular, but not luminal, activation of FFAR1 (GPR40) stimulates GLP-1 secretion from isolated perfused rat small intestine. Physiol Rep. 2015;3:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]


