Abstract
Introduction
Routine histopathological examination following appendicectomy and cholecystectomy has significant financial implications and comprises a substantial portion of the pathologists’ workload, while the incidence of unexpected pathology is low. The aim of the selective histopathological examination Following AppeNdicectomy and CholecystectomY (FANCY) study is to investigate the oncological safety and potential cost savings of selective histopathological examination based on macroscopic assessment performed by the surgeon.
Methods and analysis
This is a Dutch multicentre prospective observational study, in which removed appendices and gallbladders will be systematically assessed by the operating surgeon for macroscopic abnormalities suspicious for malignant neoplasms. After visual inspection and digital palpation of the removed specimen, the operating surgeon will report whether macroscopic abnormalities suspicious for a malignant neoplasm are present, and if he or she believes additional microscopic examination by the pathologist is indicated. Regardless of the surgeon’s assessment, all specimens will be sent for histopathological examination. In this way, routine histopathological examination can be compared with a hypothetical situation in which specimens are routinely examined by surgeons and only sent to the pathologist on indication. The two main outcomes are oncological safety and potential cost savings of a selective policy. Oncological safety of selective histopathological examination will be assessed by calculating the number of patients in whom a histopathological diagnosis of an appendiceal neoplasm or gallbladder cancer with clinical consequences benefitting the patient would have been missed. A cost analysis will be performed to quantify the potential cost savings.
Ethics and dissemination
The study protocol was reviewed by the Institutional Review Board of the Amsterdam UMC, location AMC, which decided that the Dutch Medical Research Involving Human Subjects Act is not applicable. In all participating centres, approval for execution of the FANCY study has been obtained from the local Institutional Review Board before the start of inclusion of patients. The study results will be disseminated through peer-reviewed publications and conference presentations. Guidelines will be revised according to the findings of the study.
Trial registration number
Keywords: surgery, histopathology, health economics
Strengths and limitations of this study.
This will be the first multicentre study that prospectively investigates both the oncological safety and potential cost savings of selective histopathological examination following appendicectomy and cholecystectomy in a large cohort of patients.
The robust and transparent instruction on how to perform a proper macroscopic assessment of the removed specimens results in a uniform performed examination.
As a result of the high participation rate of Dutch hospitals, a large number of surgeons and residents will become experienced in performing macroscopic examination of appendices and gallbladders, which will ease successful implementation of a selective policy, if oncological safety is proven.
Patients’ safety will not be compromised, since all specimens will be routinely sent for additional microscopic examination by the pathologist, regardless of the surgeon’s assessment.
Due to limited and inconclusive evidence on the prognostic impact of revisional surgery in patients with gallbladder cancer, all additional diagnostic or therapeutic procedures that performed ≥T1 b gallbladder were considered beneficial.
Introduction
In the Netherlands, approximately 16 000 appendicectomies and 22 500 cholecystectomies are performed annually. Traditionally, all removed appendices and gallbladders are microscopically examined by a pathologist to exclude the presence of a malignancy. However, most specimens show typical histopathological findings and unexpected neoplasms are only diagnosed in less than 1%.1 2 At the same time, histopathological examination (HPE) of the appendix and gallbladder constitutes a significant financial burden on healthcare, and comprises a considerable portion of the pathologists’ workload. Consequently, it is debatable whether routine HPE following appendicectomy and cholecystectomy is necessary.
In the past decade, several studies suggested that a selective HPE policy might be justified.3–8 Selective HPE entails that surgeons perform a macroscopic assessment of the removed specimen, and select specimens that require additional microscopic examination by the pathologist. Opponents fear that surgeons might not recognise neoplasms, resulting in missed diagnoses with potential disadvantageous consequences for the patient. However, it is hypothesised that tumours not detected during visual inspection or palpation are of early stage. These missed tumours are likely to be clinically inconsequential after the appendix or gallbladder have been completely resected. Most appendiceal neoplasms are neuro-endocrine tumours, which only require additional treatment if the diameter exceeds 2 cm or if the tumour exhibits unfavourable histopathological characteristics.9 In case of gallbladder cancer (GBC), an extended cholecystectomy is only indicated for stage T1b and above.10 Unfortunately, prospective studies regarding macroscopic assessment of appendiceal specimens by the surgeon are lacking, and the few prospective studies investigating the ability of surgeons to identify macroscopic abnormalities in gallbladder specimens are limited by the small numbers of patients.11–13
Currently, it is still standard practice in the Netherlands to send all appendices for HPE.14 The Dutch guideline for gallstone disease states that macroscopic normal appearing gallbladders can be refrained from HPE.15 However, implementation of this recommendation appeared to be suboptimal, and the need for more evidence was expressed.16 In order to draw definitive conclusions regarding safety and potential cost savings of a selective policy, a large prospective cohort of patients is required. The FANCY study will prospectively investigate the ability of surgeons to macroscopically recognise neoplasms with clinical consequences in a multicentre setting, resulting in definitive recommendations regarding the appropriate HPE policy of appendices and gallbladders.
Methods and analysis
Objectives
The primary aim of this study is to determine whether a selective HPE policy following appendicectomy and cholecystectomy for presumed benign diseases is oncologically safe. In addition, we will quantify the potential cost savings of selective HPE.
Study design
This is a Dutch multicentre prospective observational study, registered with ClinicalTrials.gov on 27 April 2018. The study was designed in accordance with the principles of collaborative resident-led snapshot research. This type of research is primarily led and conducted by surgical residents, supervised by consultants. Snapshot research is particularly suited to investigate a common condition or treatment. By generating a population-based overview, this design rapidly provides insight in current clinical practice. Previous snapshot studies performed by the Dutch Snapshot Research Group on appendicitis, rectal cancer, and acute left-sided obstructive colon cancer demonstrated that many data can be collected in a short period of time.17–19
In the FANCY study, appendices and gallbladders will be systematically assessed by the operating surgeon (or surgical resident) for macroscopic abnormalities suspicious for malignant neoplasms. After visual inspection and digital palpation of the removed specimen, the operating surgeon will report on a predefined scoring form whether macroscopic abnormalities suspicious for a malignant neoplasm are present, and if he or she believes additional microscopic examination by the pathologist is indicated (online supplementary material 1). In case of suspicious macroscopic abnormalities, the surgeon is asked to describe these on the same form. Similarly, the surgeon is requested to specify the indication for HPE, if present. Regardless of the surgeon’s assessment, all specimens will be sent for HPE. In this way, routine HPE can be compared with a hypothetical situation of selective HPE.
bmjopen-2019-035912supp001.pdf (46KB, pdf)
The FANCY study will be performed in 60 out of 74 Dutch hospitals, including academic (n=6), teaching (n=37) and non-teaching hospitals (n=17). Centres that have already implemented a selective HPE policy for gallbladder specimens may choose to either only participate in the appendix part of the FANCY study or to return to the routine policy for the duration of the study period. Hospitals will be allowed to start patient accrual after local approval has been obtained, and a site initiation visit has taken place, including a presentation of the study protocol and instructions for the macroscopic assessment of the specimens. Due to variation in completion of hospitals’ local approval procedures, it was decided to open study sites in phases. Every first day of a new month, a group of hospitals will start patient accrual. To avoid bias, all hospitals will include patients for a duration of 9 months, even if this means that the required sample size is exceeded.
Study population
Patients of all ages scheduled to undergo appendicectomy for appendicitis or cholecystectomy for cholecystitis or gallstone disease in the elective or non-elective setting will be included.
A potential subject who meets any of the following criteria will be excluded:
Appendix
Primary indication for surgery: strong clinical/radiological suspicion or histopathological proof of an appendiceal neoplasm.
Appendix removed as part of more extensive surgery, so-called incidental appendicectomies (eg, right colectomy).
Patients included in the The effect of Appendectomy on the Clinical Course of UlceRatiVe colitis (ACCURE) trial.20
Gallbladder
Primary indication for surgery: strong clinical/radiological suspicion or histopathological proof of GBC.
Gallbladder removed as part of more extensive surgery, so-called incidental cholecystectomies (eg, Whipple procedure).
The presence of a polyp of >10 mm on preoperative imaging.
Study observations
Macroscopic assessment
During the site initiation visits, all steps of a systematically performed macroscopic assessment will be discussed. Instruction videos showing how to perform the macroscopic assessment will be available on the study website during the entire study period.
Surgeon’s macroscopic assessment of the appendix
The examination starts with inspection of the appendix and mesoappendix, followed by digital palpation. In consultation with the pathologist, it was decided that opening the appendix is prohibited, since this might impede proper HPE.
Surgeon’s macroscopic assessment of the gallbladder
First, the outer surface of the gallbladder is inspected. Then, the gallbladder is incised along its longitudinal axis on the peritoneal side, leaving the cystic duct intact. After removal of stones and bile, the gallbladder mucosa is inspected and palpated. Examples of macroscopic abnormalities are masses, polyps, ulcers, cysts, hardening, irregularity and wall thickening.
Histopathological examination
All specimens will be sent to the department of pathology for macro/microscopic assessment. HPE will be conducted according to the local protocol of the pathology department where the specimen is assessed. In general, this includes macroscopic assessment of the complete specimen, followed by microscopic assessment of samples taken from macroscopic abnormalities and the top (in case of appendices) or cystic duct margin (in case of gallbladders). If no macroscopic abnormalities are present, random samples are taken for microscopic assessment. The latest histological tumour–node–metastasis (TNM) classification of the American Joint Committee on Cancer applicable at the time HPE was performed was used for staging malignancies.21
Additional treatment
In case of a histopathologically proven appendiceal neoplasm or GBC, the postoperative management will be discussed in a local multidisciplinary team meeting. If it is decided that an additional resection is required, the specimens of the re-resection will be evaluated for the presence of residual tumour and positive lymph nodes, according to the local protocol.
Outcomes
All outcomes will be analysed separately for appendiceal and gallbladder specimens.
Primary outcomes
Oncological safety
Oncological safety of selective HPE will be assessed by calculating the number of patients per 1000 examined appendices or gallbladders with the histopathological diagnosis of an appendiceal neoplasm or GBC with clinical consequences benefitting the patient that would have been missed. In case of an appendiceal neoplasm, the following consequences will be considered beneficial: (1) HPE of the re-resection specimen shows residual tumour and/or positive lymph nodes, (2) treatment with (adjuvant) systemic or local chemotherapy, radiotherapy, immunotherapy or stem cell transplantation and (3) palliative treatment for metastases detected during staging procedures. If an additional resection is performed following the diagnosis of an appendiceal neoplasm, and no residual tumour and/or positive lymph nodes are found during HPE, this is considered harmful due to the potential risks of surgery the patient is exposed to. For GBC, evidence on the prognostic impact of revisional surgery and adjuvant therapies is limited and inconclusive.22 For pragmatic reasons, it was decided that all cases of GBC requiring additional diagnostic or therapeutic procedures (ie, ≥T1 b GBC) will be considered beneficial.
To determine the number of missed diagnoses, only the specimens that would not have been sent for HPE in case of a selective policy (ie, specimens without an indication for HPE according to the surgeon) will be analysed. In general, it is difficult to determine what incidence of missed neoplasms is acceptable to omit routine HPE. The cut-off value for safety of selective HPE was chosen based on data from the Dutch national screening programme for colorectal cancer (CRC). The incidence of CRC in asymptomatic patients is 0.8%, and the sensitivity of the immunochemical faecal occult blood test (iFOBT) ranges from 65%-80%, depending on the number of screenings.23 As a result, the diagnosis of CRC is missed in 1.6–2.8 per 1000 patients. Since selective HPE implies cost savings, a reduced workload for pathologists, and less risk of overtreatment, a higher incidence of missed diagnoses is acceptable. Therefore, it was decided that selective HPE of appendices and gallbladders will be considered oncologically safe if the number of patients with a neoplasm with clinical consequences benefitting the patient that would have been missed is below 3 per 1000 examined specimens (approximately twice the incidence of missed CRC in the screening programme).
Cost analysis
The economic evaluation will be performed as a cost-minimisation analysis. In addition, a budget impact analysis of selective HPE will be performed from governmental, insurer and hospital provider perspectives.
Secondary outcomes
The incidence of different histopathological diagnoses following appendicectomy and cholecystectomy.
-
Value of the intraoperative assessment (ie, inspection and palpation) performed by the surgeon for detection of appendiceal neoplasms or GBC.
Incidence of specimens with a recognised appendiceal neoplasm or GBC.
Incidence of specimens with an unrecognised appendiceal neoplasm or GBC.
-
Indication for additional diagnostic or therapeutic procedures following histopathological diagnosis of appendiceal neoplasms or GBC and its clinical consequences, both in terms of benefit and harm.
Incidence of appendiceal neoplasms and GBC requiring additional diagnostic or therapeutic procedures.
Incidence of residual tumour and/or positive lymph nodes found in the re-resection specimen.
Incidence of postoperative complications within 90 days after additional resection.
-
Value of the intraoperative assessment (ie, inspection and palpation) performed by the surgeon for detection of aberrant findings other than appendiceal neoplasms and GBC.
Appendiceal specimens: incidence of parasite infection, endometriosis, granulomatosis and other aberrant findings that would and would not have been sent for HPE.
Gallbladder specimens: incidence of adenoma, biliary intra-epithelial neoplasm, cholesterol polyp, inflammatory/hyperplastic polyp, adenomyomatosis and other aberrant findings that would and would not have been sent for HPE.
Group size calculation
Group size calculation is based on the number of appendices and gallbladders with a tumour with clinical consequences benefitting the patient that would have been missed in case of a selective policy. According to systematic reviews, the incidences of appendiceal neoplasms and GBC are 7 per 1000 and 4 per 1000 patients, respectively.1 2 Data regarding the ability of surgeons to recognise these abnormalities and the consequences of these neoplasms are insufficient. It is however estimated that less than 1 out of 1000 examined specimens will contain an appendiceal neoplasm or GBC with clinical consequences benefitting the patient that is not recognised by the surgeon during the macroscopic assessment. Selective HPE will be considered safe if this number does not increase to 3 per 1000 patients. To demonstrate non-inferiority of selective compared with routine HPE, a sample size of 4462 per cohort achieves a 84% power to detect a difference of 0.002 using a one-sided binomial test at a target significance level of 0.025, assuming a baseline and actual proportion of 0.001, and a non-inferiority limit of 0.00299. The actual significance level achieved by the Fisher's exact test is 0.021. These two cohorts (one for appendices, one for gallbladders) only include the specimens that would not have been sent for HPE in case of a selective policy. If the rate of HPE can be reduced to 20%, 5578 patients per cohort (4462/0.8) should initially be included.
Study organisation
The FANCY study is coordinated by a PhD candidate (VPB) under supervision of the principle investigator (WAB). The steering committee consists of seven surgeons, of whom two working in academic hospitals (PRdR, PJT), and five in teaching hospitals (GJDvA, AAWvG, KHiH, CCvR, GDS), a pathologist working in an academic hospital (LK), and a pathologist working in a teaching hospital (VT), three surgical residents (ACK, HAS, JLPvV), three PhD candidates (BJGAC, EAJdSL, JdJ) and a clinical methodologist and health economist (MGWD), besides the coordinating PhD candidate and principle investigator. All local principal investigators and the residents, physician assistants and research nurses who are responsible for data collection, will be mentioned in alphabetical order as collaborators on all publications deriving from the FANCY study databases.
Data collection
The local study team of each participating hospital will be responsible for entering the prospectively collected data into an electronic case record form build with Castor EDC, which is ISO 27001 and NEN 7510 certified.24 Pre/intraoperative data will be processed after surgery, and complemented with the postoperative histopathological outcomes when the pathology report is available (±two weeks after surgery). Pre/postoperative data will be obtained from the electronic patient database and pathology reports. Intraoperative data will be obtained from the scoring form that will be filled in by the surgeon after examination of the specimen. In case a neoplasm is found during HPE, additional data about postoperative management, including details of additional diagnostic tests and/or treatment, postoperative morbidity and HPE of re-resection specimens, will be collected.
Monitoring of the primary endpoint
The reliability and quality of the primary endpoint will be assured in three ways: (1) revision of all pathology reports, (2) source data verification by remote monitoring of all cases with a histopathological diagnosis of appendiceal neoplasm or GBC and (3) estimation of the incidence of appendiceal neoplasms and GBC in the group of eligible patients that were unintentionally not included.
Revision of the pathology reports
Under supervision of the two pathologists of the steering committee (LK, VT), the coordinating investigator (VPB) will revise all pathology reports. All histopathological diagnoses will be assigned to one of the predefined categories, as shown in table 1.
Table 1.
Histopathological diagnoses after appendicectomy and cholecystectomy
| APPENDICES | GALLBLADDERS |
| Normal appendix | Normal gallbladder |
| Acute inflammation | Acute inflammation |
| Chronic inflammation and reactive changes | Chronic inflammation and reactive changes |
| Appendiceal neoplasms | Gallbladder neoplasms |
| Neuro-endocrine neoplasm | Adenoma |
| Non-invasive epithelial neoplasm | Biliary intraepithelial neoplasm |
| Invasive epithelial neoplasm | Carcinoma |
| Lymphoma | Other malignant neoplasms |
| Non-neoplastic aberrant findings | Non-neoplastic aberrant findings |
| Parasitic infection | Cholesterol polyp |
| Endometriosis | Inflammatory/hyperplastic polyp |
| Granulomatous disease | Adenomyomatosis |
| Other | Other |
Source data verification
Independent remote monitoring will be performed by a qualified monitor of the Clinical Research Unit of the Amsterdam UMC. Monitoring will be limited to all cases with a histopathological diagnosis of an appendiceal neoplasm or GBC. The quality assessment will focus on comparing entered data with source documents. Since no informed consent is obtained in this study, anonymised source documents of relevant patients will be supplied by the local study teams.
Estimation of the incidence of neoplasms in unintentionally not included patients
Since the macroscopic assessment of appendices and gallbladders is currently not routine practice, surgeons and residents might unintentionally forget to assess the specimen and fill in the scoring form. It is expected that the macroscopic assessment will not be performed in approximately 5%–10% of all eligible patients. In order to determine whether our patient cohort is representative for all patients undergoing an appendicectomy or cholecystectomy, the incidence of appendiceal neoplasms and GBC in the group of patients that were not included has to be determined. This will be done in collaboration with Pathologisch-Anatomisch Landelijk Geautomatiseerd Archief (PALGA), the Dutch nationwide network and registry of histopathology and cytopathology that contains pathology reports of all pathology laboratories in the Netherlands with complete coverage of reports since 1991.25 The PALGA database will be used to assess the number of patients in the participating centres that were unintentionally not included in the FANCY study. By means of comparing the total number of appendiceal neoplasms and GBC found in the PALGA database to the study database, we will be able to identify the number of patients that were not registered in the FANCY study. In collaboration with a staff member of PALGA, the individual pathology reports (without patient identifying information) of these patients will be checked for exclusion criteria. Consequently, the (estimated) incidence of appendiceal neoplasms and GBC in the group of unintentionally not included patients will be known.
Cleaning and locking of the database
The database will be locked and exported for statistical analysis as soon as all data are entered, and all missing items are checked with the local study team. After locking, the database will be archived in a licensed repository.
Predefined statistical analysis plan
General principles
The analyses will be performed after data entry is completed, monitoring and cleaning of the data have been performed and the statistical analysis plan is accepted for publication. For the primary analyses, all patients who underwent an appendicectomy for appendicitis or cholecystectomy for cholecystitis or gallstone disease will be included. All analyses described below will be performed using the latest version of SPSS statistics (IBM Corp) at the time of analysis.
Baseline characteristics
Baseline characteristics will be expressed as medians and IQR, or counts and percentages. Baseline characteristics will be presented as shown in table 2 (appendices) and table 3 (gallbladders).
Table 2.
Baseline characteristics (appendices)
| Total (n=) | |
| Age, years | Median (IQR) |
| Sex, n (%) | |
| Female | n (% of ‘Total’) |
| Male | n (% of ‘Total’) |
| Preoperative imaging, n (%) | |
| Ultrasound | n (% of ‘Total’) |
| Ultrasound+CT | n (% of ‘Total’) |
| Ultrasound+MRI | n (% of ‘Total’) |
| Ultrasound+CT+MRI | n (% of ‘Total’) |
| CT | n (% of ‘Total’) |
| MRI | n (% of ‘Total’) |
| CT+MRI | n (% of ‘Total’) |
| No preoperative imaging | n (% of ‘Total’) |
| Hospital | |
| Academic hospital | n (% of ‘Total’) |
| Teaching hospital | n (% of ‘Total’) |
| Non-teaching hospital | n (% of ‘Total’) |
| Macroscopic assessment performed by | |
| Surgeon | n (% of ‘Total’) |
| Resident | n (% of ‘Total’) |
| Both | n (% of ‘Total’) |
Table 3.
Baseline characteristics (gallbladders)
| Total (n=) | |
| Age, years | Median (IQR) |
| Sex, n (%) | |
| Female | n (% of ‘Total’) |
| Male | n (% of ‘Total’) |
| Preoperative diagnosis, n (%) | |
| Cholecystitis | n (% of ‘Total’) |
| Symptomatic cholelithiasis | n (% of ‘Total’) |
| Preoperative imaging, n (%) | |
| Ultrasound | n (% of ‘Total’) |
| Ultrasound+CT | n (% of ‘Total’) |
| Ultrasound+MRI | n (% of ‘Total’) |
| Ultrasound+CT+MRI | n (% of ‘Total’) |
| CT | n (% of ‘Total’) |
| MRI | n (% of ‘Total’) |
| CT+MRI | n (% of ‘Total’) |
| Other | n (% of ‘Total’) |
| No preoperative imaging | n (% of ‘Total’) |
| Surgical setting | |
| Acute | n (% of ‘Total’) |
| Elective | n (% of ‘Total’) |
| Hospital | |
| Academic hospital | n (% of ‘Total’) |
| Teaching hospital | n (% of ‘Total’) |
| Non-teaching hospital | n (% of ‘Total’) |
| Macroscopic assessment performed by | |
| Surgeon | n (% of ‘Total’) |
| Resident | n (% of ‘Total’) |
| Both | n (% of ‘Total’) |
Primary outcomes
Oncological safety
The number of patients with a histopathological diagnosis of an appendiceal neoplasm or GBC with clinical consequences benefitting the patient will be reported in absolute numbers and percentages, and as number per 1000 examined specimens for both strategies of HPE (routine and selective). The data will be presented as in figure 1 (appendices) and figure 2 (gallbladders). For analysis of the primary outcome, only the specimens that would not have been sent for HPE in case of a selective policy (ie, specimens without an indication for HPE according to the surgeon) will be analysed. A selective policy will be considered safe, if, following an exact test, the one-sided upper limit at a 97.5% CI of the proportion of missed malignancies falls below 3 per 1000 examined specimens. The influence of the assessor of the specimen (surgeon vs resident) and hospital (academic hospital vs teaching hospital vs non-teaching hospital) on the primary outcome will be assessed with Poisson regression.
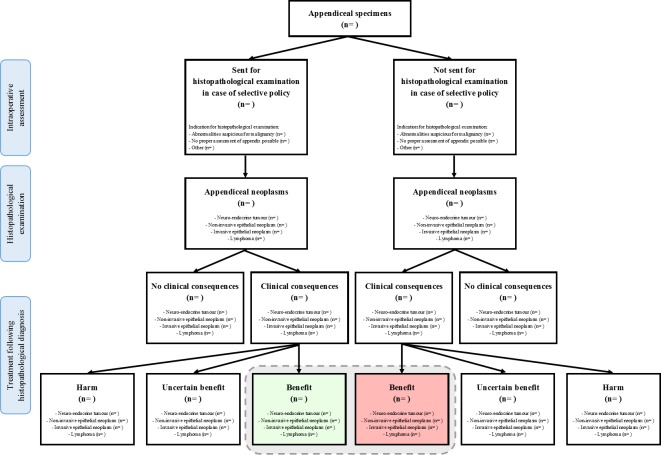
Figure 1.
Number of patients with an appendiceal neoplasm with clinical consequences benefitting the patient that would have been diagnosed (green box) and missed (red box) in case of a selective policy. The gray dotted line indicates the total number of patients benefitting from clinical consequences of an appendiceal neoplasm, that would have been diagnosed in case of a routine policy.
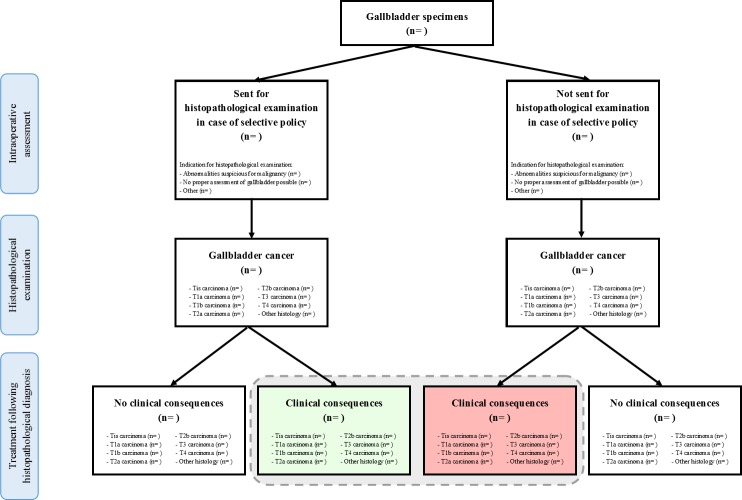
Figure 2.
Number of patients with gallbladder cancer with clinical consequences benefitting the patient that would have been diagnosed (green box) and missed (red box) in case of a selective policy. The gray dotted line indicates the total number of patients benefitting from clinical consequences of gallbladder cancer, that would have been diagnosed in case of a routine policy.
Costs
Economic evaluation
Considering that—under the non-inferiority hypothesis—1 or 2 per 1000 patients at maximum will experience health consequences from routine HPE, and 3 to 4 per 1000 patients may experience transient harm following unnecessary additional treatment, differences in effectiveness between the routine and selective strategy can best be addressed qualitatively (eg, by case reports). Quantitatively, a cost analysis from a healthcare provider will be the main focus of research.
During the study, all specimens will be sent for HPE, so the selective policy will not be observed. The comparison between both strategies will therefore be done through decision tree analysis. Observed distributions of histopathological findings under the routine policy will be used to define chance nodes in the reference tree of the model. Alternatively, both the distribution of histopathological findings in specimens that would have been sent for HPE as the distribution of histopathological findings in specimens that would not have been sent for HPE in case of a selective policy will be used to define chance nodes in the other main model tree.
In addition to the initial HPE, costs of other resources (eg, additional treatment, additional HPE, hospital stay) will be assigned to each end node in the model. If costs were observed under routine examination, but the specimen would not have been sent for HPE in case of a selective policy, they will be ignored if related to unnecessary use of healthcare resources. However, if these costs were justified, and the specimen would not have been sent for HPE in case of a selective policy, it will be assumed that these costs would nevertheless be generated at a later stage during the disease course, and thus included in the decision tree. In addition, a scenario analysis will be run with a 50% surplus penalty of these costs to compensate for yet unobserved extra costs of delayed healthcare at a later disease stage. Unit costing of hospital resources will be based on the Erasmus University Rotterdam/National Healthcare Institute guideline for costing in healthcare research.26 If specific unit costs are lacking in the guideline, local bottom-up or top-down costing initiatives in participating hospitals (eg, Amsterdam UMC) will be used.
All probabilities at the chance nodes will be assumed beta-distributed. Multiple theoretical distributions will be assessed for fitting the (observed) distributions of healthcare costs at the end nodes of the decision tree. If theoretical fits seem insufficient, a uniform distribution will be defined for the (observed) cost data. Monte Carlo simulation will be applied based on 25 000 draws from each distribution of input parameters. It is expected that a time horizon for the cost analysis of 6 months is sufficient to reliably estimate the cost difference between routine and selective HPE. Separate models will be built for the analyses of appendices and gallbladders.
Budget impact analysis
The budget impact analysis of selective HPE will be performed from governmental, insurance and hospital provider perspectives for a 4-year budget period, starting with the first full budget year after completion of the trial. The budget impact will be expressed in millions of euros. Primarily, the budget for care by medical specialists (code 0303), such as pathologists and surgeons, will be affected. For all perspectives, the reimbursement guidelines from the Dutch Healthcare Authority will be applied to the estimate actual expenses.
In case of negotiable reimbursement levels, the 10% trimmed mean purchase price per unit as provided by www.opendisdata.nl (eg, DBC code 119599010) will be used. Data on the incidences of performed appendicectomies and cholecystectomies will be gathered from public data sources (www.opendisdata.nl; www.statline.cbs.nl), and linearly extrapolated to forecast the numbers during the period for budget impact analysis.
Secondary outcomes
All incidences of histopathological diagnoses will be reported in absolute numbers and percentages. The incidence of different histopathological diagnoses will be presented as shown in table 4 (appendices) and table 5 (gallbladders). These tables will also provide information on which specimens would and would not have been sent for HPE in case of a selective policy. Table 6 will show whether or not specimens containing an appendiceal neoplasm or GBC were reported as suspicious by the surgeon and whether or not the surgeon believed HPE was indicated. Details on all patients with an appendiceal neoplasm or GBC will be presented as proposed in table 7 (appendices) and table 8 (gallbladders).
Table 4.
Histopathological diagnoses after appendicectomy for appendicitis
| Total (n=) | Indication for HPEa (n=) | No indication for HPEa (n=) | |||
| Histopathological diagnosis | Normal appendix | n (% of ‘Total’) | n | n | |
| Acute inflammation | n (% of ‘Total’) | nb | nc | ||
| Chronic inflammation and reactive changes | n (% of ‘Total’) | n | n | ||
| Appendiceal neoplasms | Neuro-endocrine neoplasm | n (% of ‘Total’) | n | n | |
| Non-invasive epithelial neoplasm | n (% of ‘Total’) | n | n | ||
| Invasive epithelial neoplasm | n (% of ‘Total’) | n | n | ||
| Lymphoma | n (% of ‘Total’) | n | n | ||
| Non-neoplastic aberrant findings | Parasitic infection | n (% of ‘Total’) | n | n | |
| Endometriosis | n (% of ‘Total’) | n | n | ||
| Granulomatous disease | n (% of ‘Total’) | n | n | ||
| Other | n (% of ‘Total’) | n | n | ||
a. According to the operating surgeon or surgical resident.
b. Uncomplicated acute appendicitis (n=), complicated acute appendicitis (n=); as reported in pathology report.
c. Uncomplicated acute appendicitis (n=), complicated acute appendicitis (n=); as reported in pathology report.
HPE, histopathological examination.
Table 5.
Histopathological diagnoses after cholecystectomy for presumed benign gallbladder diseases
| Total (n=) | Indication for HPEa (n=) | No indication for HPEa (n=) | |||
| Histopathological diagnosis | Normal gallbladder | n (% of ‘Total’) | n | n | |
| Acute inflammation | n (% of ‘Total’) | n | n | ||
| Chronic inflammation and reactive changes | n (% of ‘Total’) | n | n | ||
| Gallbladder neoplasms | Adenoma | n (% of ‘Total’) | n | n | |
| Biliary intraepithelial neoplasm | n (% of ‘Total’) | n | n | ||
| Carcinoma | n (% of ‘Total’) | n | n | ||
| Other malignant neoplasms | n (% of ‘Total’) | nb | nc | ||
| Non-neoplastic aberrant findings | Cholesterol polyp | n (% of ‘Total’) | n | n | |
| Inflammatory/hyperplastic polyp | n (% of ‘Total’) | n | n | ||
| Adenomyomatosis | n (% of ‘Total’) | n | n | ||
| Other | n (% of ‘Total’) | n | n | ||
a. According to the operating surgeon or surgical resident.
b. Details on histology.
c. Details on histology.
HPE, histopathological examination.
Table 6.
Value of the intraoperative assessment by the surgeon for detection of appendiceal neoplasms/GBC
| Appendiceal neoplasm/GBC (n=) | No appendiceal neoplasm/GBC (n=) | Total (n=) | |
| Presence of abnormalities suspicious for malignancy | n (% of ‘Appendiceal neoplasm/GBC’) | n (% of ‘No appendiceal neoplasm/GBC’) | n (% of ‘Total’) |
| Indication for HPE | n (% of ‘Appendiceal neoplasm/GBC’) | n (% of ‘No appendiceal neoplasm/GBC’) | n (% of ‘Total’) |
GBC, gallbladder cancer; HPE, histopathological examination.
Table 7.
Details of patients with a histopathological diagnosis of an appendiceal neoplasm
| Case | Sex, Age | Preoperative imaging | Assessor | Macroscopic abnormalities suspicious for neoplasm | Indication for HPE according to surgeon | Histopathological diagnosis | Additional diagnostic and/or therapeutic procedures | Remaining tumour tissue | Positive lymph nodes | 90-day complications |
| 1 | ||||||||||
| 2 | ||||||||||
| 3 | ||||||||||
| … |
HPE, histopathological examination.
Table 8.
Details of patients with a histopathological diagnosis of GBC
| Case | Sex, Age | Preoperative imaging | Preoperative diagnosis | Surgical setting | Assessor | Macroscopic abnormalities suspicious for neoplasm | Indication for HPE according to surgeon | Histopathological diagnosis | Additional diagnostic and/or therapeutic procedures | Residual disease | 90-day complications |
| 1 | |||||||||||
| 2 | |||||||||||
| 3 | |||||||||||
| … |
GBC, gallbladder cancer; HPE, histopathological examination.
Several exploratory subgroup analyses will be performed. For appendiceal specimens, the influence of age (adults vs children) on the incidence of different histopathological diagnoses will be evaluated and reported in a similar way as shown in table 4. For gallbladder specimens, a subgroup analysis on the influence of preoperative diagnosis (cholecystitis vs gallstone disease) on the incidence of different histopathological diagnoses will be performed. Furthermore, the influence of the assessor (surgeon vs resident) and hospital (academic hospital vs teaching hospital vs non-teaching hospital) on the rate of specimens that would have been sent for HPE will be reported.
Current status of the study
The study was registered with ClinicalTrials.gov on 27 April 2018 and in the Netherlands Trial Register on 16 April 2018 under number NTR7151 (www.trialregister.nl). Recruitment of patients started in May 2018. At time of submission, November 2019, 53 of 60 hospitals have finished the 13 months period of data collection (9 months accrual followed by 4 months for data entry) and 6902 and 8387 patients have been included in the appendices and gallbladders databases, respectively.
Manuscripts and authorship
The steering committee of the FANCY study will share the results irrespective of the outcomes. The outcomes as described in this protocol will be reported in two manuscripts, one for the appendices and one for the gallbladders. These manuscripts will be submitted with the steering committee as co-authors and all other investigators as collaborators. The coordinating investigator (VPB) and principal investigator (WAB) will be first and senior author on both manuscripts, respectively. If the results of the economic evaluation are reported separately, senior authorship for this manuscript will be shared by WAB and MGWD. For the appendices manuscript, the other PhD candidates will be second (JdJ), third (BJGAC) and fourth author (EAJdSL). If possible, JLPvV, EAJdSL and BJGAC will share second authorship on the gallbladder manuscript. The other members of the steering committee will be co-authors on both publications. All local principal investigators and the residents, physician assistants and research nurses who were responsible for data collection will be mentioned in alphabetical order as collaborators. All efforts will be made to link the collaborators to the final publications in indexed databases.
Patient and public involvement
Patients and public were not involved in designing the study.
Ethics and dissemination
Ethical aspects and informed consent
This study will be performed in accordance with the principles of Good Clinical Practice, the Dutch Agreement on Medical Treatment Act and the European General Data Protection Regulation.
In the FANCY study, a large number of patients will be included in a relatively short period of time. After consultation with the legal department of the Amsterdam UMC, it was decided that no written informed consent will be requested for the use of patients’ data. Obtaining written informed consent of all included patients during the usually short hospital admission would be futile and impede the execution of this study. Participation in the FANCY study does not have any treatment consequences for patients, as there is no change in current clinical practice. Patients will easily postpone their decision on participation. Moreover, it was suggested that certain patient groups (eg, young patients, patients with a complicated postoperative course, patients with histopathological findings requiring additional hospital visits) tend to provide informed consent more often, which would introduce selection bias. A deferred consent procedure including a phone call in the postoperative period was considered but deemed impractical due to the large number of healthcare providers involved. For these reasons, it was decided that the extensive effort to obtain informed consent does not compete with the relatively small amount of non-identifiable data that is collected in the FANCY study. Alternatively, patients will be offered the opportunity to refuse the use of their data by using an opt-out procedure. All patients that underwent an appendicectomy or cholecystectomy will receive a leaflet with brief information about the study. It will be explained that all data will be extracted from the patient’s charts followed by deidentification and no additional investigations are required. When a patient or its relatives object to participate, the patient will be excluded from the study and data will not be entered into the database.
Dissemination
During the study, all collaborators will be updated about the progress of the study by monthly newsletters. The results of the FANCY study will be presented at national and international conferences and submitted for publication in an international peer-reviewed scientific journal. The Dutch Surgical Society (NVVH), which is responsible for revision of the guidelines, recognises the relevance of this research and supports the implementation of the results. As secretary of the Board of Directors of the NVVH (GJDvA) and chairman of the guideline committee ‘Appendicitis’ (CCvR), two of our steering committee members are involved in the revision of the guidelines, which ensures that the guidelines will be adjusted according to the results of the FANCY study.
Supplementary Material
Footnotes
Contributors: VPB, MGWD and WAB have made substantial contributions to the conception and design of this study and have been involved in drafting the manuscript or revising it critically for important intellectual content. BJGAC has made substantial contributions to the design of this study and has been involved in drafting the manuscript. EAJdSL, JdJ, ACK, HAS, JLPvV, GJDvA, AAWvG, KHiH, LK, PRdR, CCvR, GDS, PJT and VT have made substantial contributions to the design of this study and the organisation of this trial and have revised the manuscript critically for important intellectual content. All authors have given final approval of the version to be published.
Funding: This investigator initiated study was supported by the Netherlands Organisation for Health Research and Development (ZonMw) under grant number 843002822, of which the Dutch Ministry of Health, Welfare and Sports and the Dutch Organisation for Scientific Research are the main commissioning organisations. ZonMw has not played a role in designing the study and data collection, nor will it influence the analysis and writing process of the manuscripts.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; peer reviewed for ethical and funding approval prior to submission.
References
- 1. Swank HA, Eshuis EJ, Ubbink DT, et al. . Is routine histopathological examination of appendectomy specimens useful? A systematic review of the literature. Colorectal Dis 2011;13:1214–21. 10.1111/j.1463-1318.2010.02457.x [DOI] [PubMed] [Google Scholar]
- 2. Swank HA, Mulder IM, Hop WC, et al. . Routine histopathology for carcinoma in cholecystectomy specimens not evidence based: a systematic review. Surg Endosc 2013;27:4439–48. 10.1007/s00464-013-3084-3 [DOI] [PubMed] [Google Scholar]
- 3. Khan RA, Ghani I, Chana RS. Routine histopathological examination of appendectomy specimens in children: is there any rationale? Pediatr Surg Int 2011;27:1313–5. 10.1007/s00383-011-2926-0 [DOI] [PubMed] [Google Scholar]
- 4. Jahan B, Najeeb S, Shaikh AW. Acute appendicitis correlating histopathological findings with clinical - is histopathology needed for all? PJMHS 2016;10:118–21. [Google Scholar]
- 5. van Vliet JLP, van Gulik TM, Verbeek PCM. Is it necessary to send gallbladder specimens for routine histopathological examination after cholecystectomy the use of macroscopic examination. Dig Surg 2013;30:472–5. 10.1159/000357259 [DOI] [PubMed] [Google Scholar]
- 6. Emmett CD, Barrett P, Gilliam AD, et al. . Routine versus selective histological examination after cholecystectomy to exclude incidental gallbladder carcinoma. Annals 2015;97:526–9. 10.1308/rcsann.2015.0013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Olthof PB, Metman MJH, de Krijger RR, et al. . Routine pathology and postoperative follow-up are not cost-effective in cholecystectomy for benign gallbladder disease. World J Surg 2018;42:3165–70. 10.1007/s00268-018-4619-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Deng Y-lei, Xiong X-ze, Zhou Y, et al. . Selective histology of cholecystectomy specimens--is it justified? J Surg Res 2015;193:196–201. 10.1016/j.jss.2014.07.039 [DOI] [PubMed] [Google Scholar]
- 9. Pape U-F, Niederle B, Costa F, et al. . ENETS consensus guidelines for neuroendocrine neoplasms of the appendix (excluding goblet cell carcinomas). Neuroendocrinology 2016;103:144–52. 10.1159/000443165 [DOI] [PubMed] [Google Scholar]
- 10. Aloia TA, Járufe N, Javle M, et al. . Gallbladder cancer: expert consensus statement. HPB 2015;17:681–90. 10.1111/hpb.12444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Romero-González RJ, Garza-Flores A, Martínez-PérezMaldonado L, et al. . Gallbladder selection for histopathological analysis based on a simple method: a prospective comparative study. Annals 2012;94:159–64. 10.1308/003588412X13171221589810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tayeb M, Rauf F, Ahmad K, et al. . Is it necessary to submit grossly normal looking gall bladder specimens for histopathological examination? Asian Pacific Journal of Cancer Prevention 2015;16:1535–8. 10.7314/APJCP.2015.16.4.1535 [DOI] [PubMed] [Google Scholar]
- 13. Corten BJGA, Alexander S, van Zwam PH, et al. . Outcome of surgical inspection of the gallbladder in relation to final pathology. J Gastrointest Surg 2019;23:1130–4. 10.1007/s11605-018-3921-8 [DOI] [PubMed] [Google Scholar]
- 14. NVVH. Evidence-based richtlijn Nederlandse Vereniging voor Heelkunde, acute appendicitis, 2019. [Google Scholar]
- 15. NVVH. Evidence-based richtlijn Nederlandse Vereniging voor Heelkunde, Galsteenlijden, 2016. [Google Scholar]
- 16. Corten BJGA, Leclercq WKG, Dejong CH, et al. . Selective histological examination after cholecystectomy: an analysis of current daily practice in the Netherlands. World J Surg 2019;43:2561–70. 10.1007/s00268-019-05077-w [DOI] [PubMed] [Google Scholar]
- 17. van Rossem CC, Bolmers MDM, Schreinemacher MHF, et al. . Prospective nationwide outcome audit of surgery for suspected acute appendicitis. Br J Surg 2016;103:144–51. 10.1002/bjs.9964 [DOI] [PubMed] [Google Scholar]
- 18. Borstlap WAA, Westerduin E, Aukema TS, et al. . Anastomotic leakage and chronic presacral sinus formation after low anterior resection: results from a large cross-sectional study. Ann Surg 2017;266:870–7. 10.1097/SLA.0000000000002429 [DOI] [PubMed] [Google Scholar]
- 19. Amelung FJ, Borstlap WAA, Consten ECJ, et al. . Propensity score‐matched analysis of oncological outcome between stent as bridge to surgery and emergency resection in patients with malignant left‐sided colonic obstruction. BJS 2019;106:1075–86. 10.1002/bjs.11172 [DOI] [PubMed] [Google Scholar]
- 20. Gardenbroek TJ, Pinkney TD, Sahami S, et al. . The ACCURE-trial: the effect of appendectomy on the clinical course of ulcerative colitis, a randomised international multicenter trial (NTR2883) and the ACCURE-UK trial: a randomised external pilot trial (ISRCTN56523019). BMC Surg 2015;15:30 10.1186/s12893-015-0017-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Amin MB, Edge S, Greene F, et al. . Ajcc cancer staging manual. 8th edition, 2017. [Google Scholar]
- 22. Søreide K, Guest RV, Harrison EM, et al. . Systematic review of management of incidental gallbladder cancer after cholecystectomy. Br J Surg 2019;106:32–45. 10.1002/bjs.11035 [DOI] [PubMed] [Google Scholar]
- 23. Wiersma T, de Wit N, Drenthen T. NHG-Standpunt Bevolkingsonderzoek darmkanker 2013.
- 24. Castor EDC. Castor Electronic Data Capture, 2019. Available: https://castoredc.com
- 25. Casparie M, Tiebosch AT, Burger G, et al. . Pathology databanking and biobanking in the Netherlands, a central role for PALGA, the nationwide histopathology and cytopathology data network and archive. Cell Oncol 2007;29:19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zorginstituut N. Richtlijn voor Het uitvoeren van economische evaluaties in de gezondheidszorg. Diemen 2015.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2019-035912supp001.pdf (46KB, pdf)