Abstract
Bhutanese–Nepali refugees are one of the largest refugee groups to be resettled in the U.S. in the past decade. Cervical cancer is a leading cause of cancer disparity in this population, yet screening rates are suboptimal. Nepali-speaking interviewers administered a community health needs questionnaire to a convenience sample of Bhutanese–Nepali refugees in a Midwestern city between July to October of 2015. Descriptive statistics were used to describe socio-demographic characteristics, Pap smear beliefs, post-migration living difficulties, and screening status. Differences in Pap test uptake between groups were tested using t test and Chi square statistics. Of the 97 female participants, 44.3% reported ever having had a Pap smear. Screening rates were lowest among women who did not know English at all. Most women had positive perceptions of Pap smears (80%) and 44.4% had received a Pap test recommendation from their healthcare provider, family, or friends. Pap testing was significantly higher among those who had positive perceptions (58.3 vs. 11.1% for women of negative perception, p = 0.01) and those who had received a recommendation (87.5 vs. 18.6% for women who had no recommendations, p < 0.001). Significant predictors of having a Pap smear were having a healthcare provider/family/friends recommendation (OR 65.3, 95% CI 11.4–373.3) and greater number of post-migration living difficulties (OR 1.18, 95% CI 1.02–1.37). The results of this study have important implications for the development of cervical cancer prevention programs targeting Bhutanese–Nepali refugees. Providing cancer prevention interventions early in the resettlement process could impact Pap test uptake in this population.
Keywords: Pap test, Cervical cancer, Bhutanese–Nepali refugee, Women, Post-migration living difficulties
Introduction
Foreign-born individuals make up 42 million, or 13.2%, of the United States (U.S.) population [1], and is expected to rapidly increase by 85% from 42 to 78 million by 2060 for reasons such as, but not limited to, displacement, war, or violence [2]. Cervical cancer, a major cause of premature death and disability worldwide, is high for certain foreign-born groups compared to U.S.-born [3–5]. Globally, more than 527,000 new cases of cervical cancer and 265,000 deaths were reported in 2012 [4]. Despite the low incidence of cervical cancer in the U.S. (6.7 per 100,000) [6], the rate of cervical cancer remains exceedingly high in many countries around the world, especially in low- and middle-income countries (LMICs). In fact, 85% of women diagnosed and 87% of women who die from cervical cancer live in LMICs [4].
Among Bhutanese–Nepali women, cervical cancer is the most commonly diagnosed cancer and the second most common cause of cancer mortality [7]. In 2011, cervical cancer accounted for 32% of all cancers among women in Bhutan aged 15–64 years, with the large majority diagnosed at late stage, where the disease is more advanced, resulting in high mortality [8]. There are limited studies on cervical cancer and Bhutanese–Nepali women. Among the few published literature we found, studies have shown a paucity of knowledge regarding cervical cancer and screening practices among this population. Only 22.2% of Bhutanese–Nepali women reported having ever heard of a Pap test and 13.9% ever having had one [7]. In addition, Bhutanese–Nepali refugee women generally have low knowledge of the Human Papilloma Virus (HPV), a primary cause of cervical cancer [7]. In 2009, the government of Bhutan, in collaboration with the World Health Organization, implemented a program to provide the HPV vaccine to 12-year old girls [8]. While this program has been very effective in its implementation of girls living in Bhutan, it has no effect on the refugees who have recently been resettled in the U.S. from refugee camps in Nepal.
Despite solid evidence that regular screening through Pap testing reduces cervical cancer mortality [9–11], refugee and immigrant women are least likely to be screened [12–14]. Foreign-born women are more than three times as likely to have never had a Pap smear, [13] and Asian immigrant women continue to have strikingly low screening rates (75.4% for Pap smears) [15] compared with the general U.S. screening rate of 84.5%. Another study found that cervical cancer screening rates among refugee women before resettlement is low with a mean age of 36 at the time of abnormal Pap smear [16]. Continued limited knowledge of cancer detection and misconceptions about cervical cancer screening further exacerbates low screening rates. Furthermore, foreign-born individuals are more likely to have lower educational attainment, no health insurance coverage, and higher poverty rates compared to U.S.-born individuals (31.7, 65.7, and 18.8% vs. 11, 87.3, and 14.8%, respectively) [17].
In 2016, an estimated 85,000 refugees arrived in the U.S., with Bhutanese–Nepali refugees being one of the largest groups to have been resettled [18]. Bhutanese–Nepali refugees are of Nepali origin. In the 1980s, changes in the government of Bhutan led to ethnic and religious cleansing, forcing thousands of Bhutanese to escape their home country and flee to refugee camps in Nepal [19]. Of the 41 states where Bhutanese–Nepali refugees have been resettled, Ohio ranks fifth in the top ten states for resettlement following Pennsylvania, Texas, New York, and Georgia [18]. From 1983 to 2014, nearly 17,000 refugees were resettled in the Columbus metropolitan area, many of them Bhutanese–Nepali [20]. Through secondary migration, the number of Bhutanese–Nepali refugees living in Columbus, Ohio has continued to grow [21].
To our knowledge, few studies have examined cervical cancer screening behavior, perceptions of Pap testing, and post-migration living difficulties with Bhutanese–Nepali refugees in the U.S. In this article, we present findings on following research questions: (1) What is the cervical cancer screening status among Bhutanese–Nepali women? (2) What are barriers and facilitators to cervical cancer screening among Bhutanese–Nepali women? (3) What are Bhutanese–Nepali women’s beliefs about Pap smears? and (4) What are post-migration living difficulties for Bhutanese–Nepali women?
Materials and Methods
Overview
We used a community-engaged approach to develop and implement a community health needs assessment with Bhutanese–Nepali refugee women and men living in Columbus, OH. Community-engaged research is a collaborative approach between communities and researchers and is important for establishing trust and mutual respect [22]. The study methods are described in detail elsewhere [23]. Bilingual and bicultural Nepali-speaking Bhutanese interviewers recruited participants and administered the community health needs assessment questionnaire to a convenience sample of 201 Bhutanese–Nepali refugees. Data were collected between July to October of 2015. The questions relevant to the present study include items related to women’s cancer knowledge, Pap smear screening behavior, beliefs about Pap smears, and post-migration living difficulties. The study was approved by the Institutional Review Board at the Ohio State University.
Cultural Community Advisory Board
We worked closely with two local, non-profit partner agencies serving a large number of Bhutanese–Nepali refugees. Members of the Bhutanese Nepali Community of Columbus (BNCC) and Refugee Women in Action (RWIA) served on the project’s Cultural Community Advisory Board (CCAB). Founded by Bhutanese–Nepali refugees, the BNCC provides programs and services for families in the community. Throughout the study, the nine-member CCAB helped identify potential bilingual and bicultural interviewers and advised the investigator to ensure that the questionnaire and recruitment methods were culturally appropriate. Given the leadership on the CCAB and the interviewers recruited from the community, the support we received gave our project credibility and greater acceptability in the community, an important key facet of community-engaged research [24].
Recruitment and Data Collection Procedures
Participants were eligible to participate in the study if they self-identified as Bhutanese–Nepali, were 18 years or older, and lived in Franklin County, OH (where the city of Columbus is located). Using a recruitment script, recruitment was conducted in English, Nepali, or both, depending on the language preference of the participant, by trained bilingual and bicultural interviewers at community locations where Bhutanese–Nepali community members frequent. Participants were recruited primarily by word-of-mouth. Interviewers also conducted recruitment through various community organizations such as the BNCC and other local non-profit community-based organizations. Questionnaires were administered after obtaining participants’ consent. We obtained a waiver of documentation of informed consent from the university’s Institutional Review Board [23]. We expected that many participants would not read English, nor would they read Nepali; therefore, translating the informed consent form into Nepali may not be a good use of our limited resources. In addition, we were confident that the interviewers would be able to seamlessly go between English and Nepali without adding any stigma to situations in which participants do not speak English and need anywhere from a few words, or the whole interview, provided in Nepali.
Measures
Sociodemographic variables included as covariates were age, marital status, number of children, religion, employment, family income, health insurance, home ownership, country of birth, and years lived in refugee camp. We measured acculturation with a 3-point Likert Scale reading, speaking, and writing English and Nepalese (1 = not at all to 3 = well). Health status was measured by asking participants to rate their health with 1 = excellent health to 5 = poor health. Smoking status was assessed by asking participants if they smoke cigarettes 1 = every day, 2 = some days, 3 = not at all, never a smoker, or 4 = not at all, used to be a smoker.
Cervical Cancer Screening
Cervical cancer screening was obtained by asking participants if they had ever had a Pap smear. Response was measured by yes or no.
Pap Smear Beliefs
Pap smear beliefs were measured with questions addressing perceptions, barriers, and recommendations [25]. Questions about perceptions include, “Do you think a woman needs a Pap smear if she is not having sexual intercourse?” “Do you think a woman needs a Pap smear after menopause (when her periods have stopped)?” Questions related to barriers to screening included, “Does lack of a time prevent you from getting Pap smears?” “Are you afraid of having a Pap smear?” Questions pertaining to recommendations for Pap smears were, “Has a healthcare provider (e.g., doctor or nurse practitioner) ever recommended that you have a Pap smear?” and “Have any of your friends ever suggested that you have a Pap smear?” Responses to these sets of questions were yes, no, or don’t know.
Post-Migration Living Difficulties
The post-migration living difficulties (PMLD) checklist was used to measure the level of distress due to stressors related to post-migration [26, 27]. The 25-item checklist was shortened to 19 questions for the purposes of the present study and covered difficulties accessing medical treatment, healthcare and counseling services, government benefits, employment, communication, and acculturation difficulties. The response categories were originally on a 5-point Likert scale from 0 = no problem to 5 = serious problem; however, after iterative discussions with our CCAB and bilingual/bicultural research staff, we condensed the response to 3 categories (no problem, somewhat of a problem, and serious problem). A participant’s total number of PMLD was calculated by summing the responses of the 19 questions of PMLD (1 = somewhat of a problem or serious problem; 0 = no problem).
Data Analysis
Descriptive statistics were used to describe sample socio-demographic characteristics, Pap smear beliefs, PMLD, and cervical cancer screening status. Differences in the use of Pap test between groups were tested using t test for continuous variables and Chi square statistics for categorical variables. The Wilcoxon rank sum test and the exact Pearson Chi square test were used instead if the assumption of normal distribution was violated for continuous variables or the expected cell count were less than 5 for categorical variables [28]. Principal component analysis was used to identify the underlying factors of Pap smear beliefs (perceptions, barriers, and recommendations of having a Pap test) based on screen plots of eigenvalues and factor loadings. Multiple logistic regression analysis was conducted to identify predictors of Pap test use. Predictors entered into the logistic regression model include (i) socio-demographic characteristics that were significantly associated with Pap test use in the bivariate tests, (ii) three underlying factors (perceptions, barriers, and recommendations of having a Pap test) of Pap smear beliefs measures, and (iii) total number of post-migration living difficulties. All the significant tests were two-sided with significance level of 0.05. SAS 9.4 (SAS Institute®, Cary, NC) was used for the data analyses.
Results
The sociodemographic characteristics of the 97 female Bhutanese-Nepali refugees in the study are summarized in Table 1. A majority of the participants (83.5%) were born in Bhutan. Slightly more than half (55.7%) of the participants were between the ages of 25–44 years, 21.6% between 18 and 24 years, and 22.7% of 45 years or older. Most of the women were Hindu (76.3%), married (80.4%), had 0–2 children (52.6%), were employed (61.9%), had family income of ≤$30,000 (43.3% less than $15,000 and 27.8% between $15,000 and $30,000), and lived in a rented home (78.4%). A majority of the women reported good to excellent health (90.8%), more than half were non-smokers (64.9%), and nearly three-quarters had medical insurance from Medicare or Medicaid (74.2%). Half of the women (50.5%) had been in refugee camp for more than 20 years and can read/speak/write English well (54.6%).
Table 1.
Cervical cancer screening by sample characteristics
| N (%) | % Ever had a Pap smear | |
|---|---|---|
| All | 97 (100.0) | 44.3 |
| Country of birth | ||
| Bhutan | 81 (83.5) | 44.4 |
| Nepal | 12 (12.4) | 33.3 |
| India | 4 (4.1) | 75.0 |
| Age** | ||
| 18–24 | 21 (21.6) | 38.1 |
| 25–44 | 54 (55.7) | 55.6 |
| 45+ | 22 (22.7) | 22.7 |
| Number of children | ||
| 0–2 | 51 (52.6) | 41.2 |
| 3+ | 46 (47.4) | 47.8 |
| General health | ||
| Excellent/very good | 28 (28.9) | 35.7 |
| Good/fair | 60 (61.9) | 48.3 |
| Poor | 9 (9.3) | 44.4 |
| Smoking status | ||
| Current smoker | 5 (5.2) | 40.0 |
| Former smoker | 20 (20.6) | 45.0 |
| Non-smoker | 63 (64.9) | 49.2 |
| Religion | ||
| Hindu | 74 (76.3) | 47.3 |
| Other | 20 (20.6) | 35.0 |
| English* | ||
| Not at all | 13 (13.4) | 23.1 |
| Not too well | 30 (30.9) | 56.7 |
| Read or Speak or Write well | 53 (54.6) | 43.4 |
| Marital status | ||
| Married | 78 (80.4) | 44.9 |
| Divorced/separated/widowed | 6 (6.2) | 50.0 |
| Never married | 12 (12.4) | 41.7 |
| Insurance | ||
| Employer | 14 (14.4) | 35.7 |
| Medicare/medicaid | 72 (74.2) | 44.4 |
| No | 8 (8.2) | 62.5 |
| Home ownership | ||
| Rent | 76 (78.4) | 47.4 |
| Own | 17 (17.5) | 29.4 |
| Employment | ||
| Yes | 60 (61.9) | 46.7 |
| No | 35 (36.1) | 40.0 |
| Family income | ||
| <$15,000 | 42 (43.3) | 35.7 |
| $15,000–$30,000 | 27 (27.8) | 55.6 |
| $30,001–$50,000 | 20 (20.6) | 45.0 |
| Years in refugee camp | ||
| <10 years | 2 (2.1) | 50.0 |
| 10–19 years | 45 (46.4) | 51.1 |
| 20+ years | 49 (50.5) | 38.8 |
p < 0.05
p < 0.01
Table 1 also shows the proportion of women who reported having ever had a Pap smear, by sociodemographic characteristics. Overall, only 44.3% of the women reported having had a Pap smear before. Women aged 25–44 had the highest screening rate (55.6%), followed by those aged 18–24 (38.1%) and 45 years or older (22.7%) (p = 0.005). Women who did not know English at all had the lowest screening rate (23.1%) compared to 43.4% for those who could read/speak/write English well and 56.7% for those who know some English, but not too well (p = 0.01). The disparities in Pap testing by other sociodemographic characteristics did not reach statistical significance.
Table 2 describes the women’s Pap smear beliefs (12items) and the associated rates of Pap testing. We present the results by items of the underlying factors (perceptions, barriers, and recommendations).
Table 2.
Pap smear beliefs
| Beliefs | N (%) | % Ever had |
|---|---|---|
| Perceptions | ||
| Do you think a woman needs a Pap smear if she is not having sexual intercourse with a man?*** | ||
| No | 18 (21.4) | 27.8 |
| Yes | 31 (36.9) | 80.6 |
| Don’t know | 35 (41.7) | 20.0 |
| Do you think a woman needs a Pap smear after menopause (when her periods have stopped)?*** | ||
| No | 15 (17.9) | 20.0 |
| Yes | 33 (39.3) | 78.8 |
| Don’t know | 36 (42.9) | 22.2 |
| Do you think having a Pap smear could help you live longer?*** | ||
| No | 13 (15.5) | 30.8 |
| Yes | 34 (40.5) | 79.4 |
| Don’t know | 37 (44.0) | 16.2 |
| Do you think having a Pap smear could help you prevent cancer?** | ||
| No | 3 (3.6) | 0.0 |
| Yes | 57 (67.9) | 59.6 |
| Don’t know | 24 (28.6) | 12.5 |
| Do you think having a Pap smear could help you find cancer early?* | ||
| No | 4 (4.8) | 0.0 |
| Yes | 56 (66.7) | 53.6 |
| Don’t know | 24 (28.6) | 29.2 |
| Barriers | ||
| Does lack of time prevent you from getting Pap smears? | ||
| No | 45 (53.6) | 46.7 |
| Yes | 17 (20.2) | 64.7 |
| Don’t know | 22 (26.2) | 22.7 |
| Does shyness prevent you from getting Pap smears?* | ||
| No | 24 (28.6) | 54.2 |
| Yes | 48 (57.1) | 47.9 |
| Don’t know | 12 (14.3) | 8.3 |
| Do you think Pap smears are painful or uncomfortable?*** | ||
| No | 12 (14.3) | 41.7 |
| Yes | 36 (42.9) | 83.3 |
| Don’t know | 36 (42.9) | 5.6 |
| Are you afraid of having Pap smears?** | ||
| No | 25 (29.8) | 68.0 |
| Yes | 43 (51.2) | 44.2 |
| Don’t know | 16 (19.0) | 6.3 |
| Recommendations | ||
| Has a healthcare provider (e.g., doctor or nurse practitioner) ever recommended that you have a Pap smear?*** | ||
| No | 44 (52.4) | 22.7 |
| Yes | 30 (35.7) | 86.7 |
| Don’t know | 10 (11.9) | 10.0 |
| Have any of your family members ever suggested that you have a Pap smear?** | ||
| No | 64 (76.2) | 35.9 |
| Yes | 12 (14.3) | 100.0 |
| Don’t know | 8 (9.5) | 25.0 |
| Have any of your friends ever suggested that you have a Pap smear?* | ||
| No | 70 (83.3) | 41.4 |
| Yes | 7 (8.3) | 100.0 |
| Don’t know | 7 (8.3) | 14.3 |
p < 0.05
p < 0.01
p<0.001
Perceptions
About 40% believed that a woman needs a Pap smear even if she is not having sexual intercourse with a man or after menopause (39.3%). Over 60% of the women believed that having a Pap smear could help in preventing cancer (67.9%) or finding cancer early (66.7%). Forty percent believed that a Pap smear could help in prolonging life. The Pap smear receipt were significantly higher among those who had greater beliefs on the needs/benefits of Pap smear than those who had lower beliefs.
Barriers
The reported barriers that prevent a woman from getting Pap smears include lack of time (20.2%), shyness (57.1%), pain/discomfort from the procedure (42.9%), and feeling afraid of having the procedure (51.2%). The screening rates were significantly higher among those who thought Pap smears as painful or uncomfortable (83.3 vs. 41.7% for those who thought otherwise, p = 0.001) and were significantly lower among those who were afraid of having Pap smears (44.2 vs. 68.0% for those who were not afraid, p = 0.01).
Recommendations
A small proportion of women reported a healthcare provider (35.7%), family member (14.3%), or friend (8.3%) had recommended or suggested them to have Pap smear. The screening rates in these women (over 85%) were significantly higher than those who did not have such recommendations.
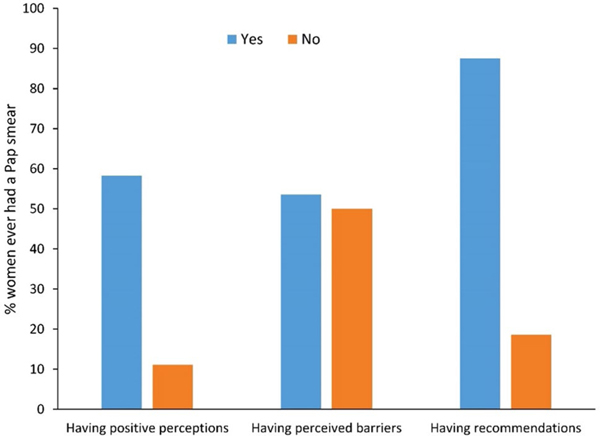
For almost all the items of Pap smear beliefs measure, study participants who reported ‘don’t know’ had the lowest screening rates. Each of the three underlying factors of Pap smear beliefs was summarized as a binary variable such as ‘yes’ if any of the item had an answer of ‘yes’ versus ‘no’ if all the items had an answer of ‘no’. For example, a woman was categorized as having recommendation(s) of having a Pap smear if her healthcare provider or family member or friends had ever recommended her to have a Pap smear. Overall, the majority of the women (80.0%) had positive perceptions of Pap smear; 76.7% had perceived barriers to have a Pap smear; and 44.4% had recommendations from their healthcare provider, family, or friends to have a Pap smear. The rate of Pap testing was significantly higher among those who had positive perceptions (58.3 vs. 11.1% for women of negative perception, p = 0.01) and those who had recommendations from provider/family/friends (87.5 vs. 18.6% for women who had no such recommendations, p < 0.001). Screening rates were similar among both those who had perceived barriers and those who had no perceived barriers (53.6 vs. 50.0%, p = 0.99) (Fig. 1). We included the three factors in the subsequent multiple logistic regression model to reduce collinearity and increase the model efficiency.
Fig. 1.
Proportion of women who ever had a Pap smear, by perceptions, barriers, and recommendations of having a Pap smear. (Color figure online)
Bhutanese–Nepali women’s post-migration living difficulties (PMLD, 19-items) and the associated rates of Pap testing are presented in Table 3. Communication/language difficulties appeared to be the greatest challenge for the women with 51.3% reporting having such difficulties. Fears of being deported was of the least concern, with only 15.8% reporting such concern. Surprisingly, the screening rates were higher among those who had PMLD for all the items. Overall, the women reported an average of 4.9 PMLD (SD = 5.1). The average number of PMLD were 6.1 (SD = 5.0) for women who reported ever having had a Pap smear versus 3.8 (SD = 5.0) for women who had not had a Pap smear (p = 0.008).
Table 3.
Post-migration living difficulties (PMLD)
| PMLD | N (%) | % Ever had a Pap smear |
|---|---|---|
| Getting treatment for health problems | ||
| No problem | 53 (69.7) | 37.7 |
| Somewhat of a problem | 16 (21.1) | 68.8 |
| Serious problem | 7 (9.2) | 71.4 |
| Access to emergency medical care | ||
| No problem | 55 (72.4) | 41.8 |
| Somewhat of a problem | 16 (21.1) | 62.5 |
| Serious problem | 5 (6.6) | 60.0 |
| Access to long term medical care (family doctor, Primary Care Physician)* | ||
| No problem | 51 (67.1) | 37.3 |
| Somewhat of a problem | 19 (25.0) | 68.4 |
| Serious problem | 6 (7.9) | 66.7 |
| Access to dental care* | ||
| No problem | 50 (65.8) | 38.0 |
| Somewhat of a problem | 15 (19.7) | 60.0 |
| Serious problem | 11 (14.5) | 72.7 |
| Access to counseling services* | ||
| No problem | 51 (67.1) | 39.2 |
| Somewhat of a problem | 19 (25.0) | 63.2 |
| Serious problem | 6 (7.9) | 66.7 |
| Not enough government help with welfare (unemployment benefits, financial help) | ||
| No problem | 47 (61.8) | 38.3 |
| Somewhat of a problem | 20 (26.3) | 60.0 |
| Serious problem | 9 (11.8) | 66.7 |
| Not enough help with welfare from charities (social services, Red Cross, Salvation Army) | ||
| No problem | 49 (64.5) | 44.9 |
| Somewhat of a problem | 21 (27.6) | 52.4 |
| Serious problem | 6 (7.9) | 50.0 |
| Communication difficulties/language difficulties | ||
| No problem | 37 (48.7) | 35.1 |
| Somewhat of a problem | 19 (25.0) | 52.6 |
| Serious problem | 20 (26.3) | 65.0 |
| Discrimination (because of language, color, religion, etc.)* | ||
| No problem | 60 (78.9) | 40.0 |
| Somewhat of a problem | 11 (14.5) | 81.8 |
| Serious problem | 5 (6.6) | 60.0 |
| Being unable to find work | ||
| No problem | 45 (59.2) | 40.0 |
| Somewhat of a problem | 13 (17.1) | 61.5 |
| Serious problem | 18 (23.7) | 55.6 |
| Bad working conditions** | ||
| No problem | 57 (75.0) | 38.6 |
| Somewhat of a problem | 8 (10.5) | 87.5 |
| Serious problem | 11 (14.5) | 63.6 |
| Separation from family | ||
| No problem | 51 (67.1) | 41.2 |
| Somewhat of a problem | 9 (11.8) | 55.6 |
| Serious problem | 16 (21.1) | 62.5 |
| Worries about family back home | ||
| No problem | 50 (65.8) | 42.0 |
| Somewhat of a problem | 10 (13.2) | 60.0 |
| Serious problem | 16 (21.1) | 56.3 |
| Unable to return home to family in an emergency | ||
| No problem | 54 (71.1) | 40.7 |
| Somewhat of a problem | 10 (13.2) | 50.0 |
| Serious problem | 12 (15.8) | 75.0 |
| Loneliness and boredom* | ||
| No problem | 51 (67.1) | 37.3 |
| Somewhat of a problem | 17 (22.4) | 70.6 |
| Serious problem | 8 (10.5) | 62.5 |
| Poor access to traditional foods* | ||
| No problem | 63 (82.9) | 42.9 |
| Somewhat of a problem | 5 (6.6) | 80.0 |
| Serious problem | 8 (10.5) | 62.5 |
| Fears of being sent home | ||
| No problem | 64 (84.2) | 46.9 |
| Somewhat of a problem | 8 (10.5) | 25.0 |
| Serious problem | 4 (5.3) | 100.0 |
| Being unable to practice your religion | ||
| No problem | 62 (81.6) | 41.9 |
| Somewhat of a problem | 8 (10.5) | 75.0 |
| Serious problem | 6 (7.9) | 66.7 |
| Difficulty adjusting to the weather/climate | ||
| No problem | 47 (61.8) | 38.3 |
| Somewhat of a problem | 21 (27.6) | 61.9 |
| Serious problem | 8 (10.5) | 62.5 |
Mean (SD) of total number of PMLD – Overall: 4.9 (5.1); subjects had a Pap smear subjects versus those who had not had a Pap smear: 6.1 (5.0) versus 3.8 (5.0) (p = 0.008 using Wilcoxon rank sum test)
p < 0.05
p < 0.01
We present the odds ratio (OR) and its 95% confidence interval (CI) for each predictor in the multiple logistic regression model in Table 4. After adjusting for other factors in the model, the significant predictors of higher odds of having a Pap smear were having a healthcare provider/family member/friends recommendation (OR 65.3, 95% CI 11.4–373.3) and greater number of post-migration living difficulties (OR 1.18, 95% CI 1.02–1.37). Other variables in the model did not reach statistical significance. However, the directions of the effect were consistent to that found in the bivariate tables. Specifically, younger age, better English, higher beliefs of benefits, and higher perceived barriers were associated with higher likelihood of having a Pap smear.
Table 4.
Odds ratio (95% CI) estimates from multiple logistic regression model
| Model | OR (95% CI) |
|---|---|
| Age | |
| 18–24 | Reference |
| 25–44 | 0.65 (0.10, 4.38) |
| 45+ | 0.30 (0.02, 6.05) |
| English | |
| Not at all | Reference |
| Not too well | 3.42 (0.15, 76.39) |
| Read or speak or write well | 2.46 (0.10, 55.56) |
| Positive perceptions of having a Pap smear | |
| Yes | 15.28 (0.49, 480.41) |
| No | Reference |
| Perceived barriers on having a Pap smear | |
| Yes | 2.90 (0.35, 23.84) |
| No | Reference |
| Provider/family/friends recommended a Pap smear | |
| Yes | 65.26 (11.41, 373.28) |
| No | Reference |
| Number of post-migration living difficulties | 1.18 (1.02, 1.37) |
Discussion
This study provides new insight into cervical cancer screening behavior and perceptions about screening among recently arrived Bhutanese-Nepali refugees in the U.S. Knowledge about cervical cancer and Pap smears is significantly low among women in this study. Similar findings have been published among Bhutanese–Nepali resettled elsewhere in the U.S [7]. Future intervention studies are warranted and should include culturally appropriate measures and methods to address cervical cancer knowledge deficits and promote screening uptake.
The findings from this study also suggest that Bhutanese–Nepali women have low rates of cervical cancer screening. Low screening rates among our study population are similar to that of Haworth et al. (2014) and other studies conducted with refugee and immigrant women [12, 14, 29]. Perceived barriers to screening among study participants were also similar to those of Asian immigrant women and included barriers such as shyness, fear of having a Pap smear, and experiencing pain or discomfort from having the Pap smear [30, 31]. Having a screening recommendation by a healthcare provider, friend, or family was the strongest predictor for a Pap test. Several studies with immigrant women have found the same to be true regarding cervical [32–34] and breast cancer screening [35, 36]. Healthcare providers, especially doctors and nurses, play an important role in motivating refugee women to obtain preventive screenings (e.g., Pap smear and mammography) as they are often seen as experts or authority figures. Furthermore, in some cultures, healthcare providers are highly revered and can be effective in motivating positive screening behavior. Future interventions should integrate healthcare providers to improve cervical cancer screening uptake among newly arrived refugees.
The results of higher Pap smear receipt among some of the more disadvantaged groups were contradictory to what we expected. For example, 62.5% uninsured women reported having had a Pap smear before, compared to 44.4% for those insured by Medicare/Medicaid, and 35.7% for those who had employer-sponsored insurance. Future studies should examine how health insurance impact preventive screenings for refugees as their Refugee Medical Assistance health benefits are discontinued 8-months after resettlement.
Refugees in the U.S. face significant health challenges, including low utilization of preventive screening services (e.g., Pap smears) [37, 38]. The idea of preventive screenings, such as those procedures in the U.S., is a foreign concept to many refugees. Preventive screenings in the refugee camp is not commonplace. Also, perceptions of illness, such that if one is not sick, then they do not need to go see a doctor, or the idea that fate played a role in one getting a disease, further exacerbates barriers to screening [38, 39]. In addition, the list of competing priorities, such as finding employment, education, childcare, etc., often take precedence over preventive care. Lastly, culture also influences perceptions of cancer risk, trust in the Western healthcare system and healthcare providers, and health seeking behaviors [40]. Future cervical cancer screening interventions should consider culturally tailoring messages to overcome screening barriers and promote screening uptake.
Limitations
This study has some limitations. The small sample size of the study limited the number of predictors to be included in the multiple logistic regression model and also the power to identify significant associations. Therefore, the significance tests may fail to identify important factors. On the other hand, the study had high specificity in identifying statistical significant factors; thus, the factors identified by significance tests were true findings. We emphasize more on the direction of the associations rather than on the definite degree of the associations due to the low precision of the estimates (or wide confidence intervals). We also relied on women’s self-report of Pap test receipt, which may raise issues of recall error and response bias [41]. In addition, approximately half of the participants in this study were recruited from a refugee resettlement agency limiting diversity in post-migration needs. Participants in this study may not be representative of the larger Bhutanese–Nepali population in Columbus, OH.
Despite these limitations, this study also has considerable strengths. This study contributes to the literature by examining cervical cancer screening perceptions and screening behavior among this new refugee group. This study is the first to our knowledge to assess Bhutanese–Nepali refugee women’s beliefs about Pap smears, screening behavior, and post-migration living difficulties. We used a community-engaged approach by working with community leaders and members from the conceptualization to study implementation. Early engagement of community members established trust and respect, and by having a cultural community advisory board to help guide the research process reduced barriers into the community. Hiring bilingual and bicultural interviewers from the Bhutanese–Nepali community also ensured successful participant recruitment.
Conclusions
Cervical cancer is highly preventable through Pap testing and HPV vaccination, yet awareness and screening uptake is low among refugee populations. The results of this study have important implications for the development and implementation of cervical cancer prevention programs for Bhutanese–Nepali refugees. It is important to understand the cultural beliefs and experiences of this refugee group to better inform Bhutanese-Nepali women of preventive measures to detect cervical cancer early and prevent unnecessary morbidity and mortality. More importantly, promoting preventive measures early in the resettlement process could impact Pap test uptake in this population.
Acknowledgements
We thank the Bhutanese–Nepali Community of Columbus for allowing us into their community and for their guidance throughout this project. We also thank Refugee Women in Action for their support. We want to acknowledge Jhuma Acharya, Dil Pyakurel, Sudarshan Pyakurel, and Kelly Yotebieng for their engagement in the project.
Funding This project was supported by the Ohio State University Center for Clinical and Translational Science and Award # UL1TR001070 from the National Center for Advancing Translational Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Footnotes
Compliance with Ethical Standards
Conflict of interest The authors have no conflict of interest to declare.
References
- 1.Brown A, Stepler R (2016). Statistical portrait of the foreign-born population in the United States. http://www.pewhispanic.org/2016/04/19/statistical-portrait-of-the-foreign-born-population-in-the-united-states/. Retrieved Oct 2016.
- 2.Office of Refugee Resettlement. (2014). http://www.acf.hhs.gov/programs/orr/resource/refugee-arrival-data. Retrieved Oct 2016.
- 3.Ginsburg O, Bray F, Coleman MP, Vanderpuye V, Eniu A, Kotha SR, & Conteh L (2016). The global burden of women’s cancers: A grand challenge in global health. Lancet, 389(10071), 847–860. doi: 10.1016/S0140-6736(16)31392-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Torre LA, Siegel RL, Ward EM, & Jemal A (2016). Global cancer incidence and mortality rates and trends—an update. Cancer Epidemiology, Biomarkers & Prevention, 25(1), 16–27. doi: 10.1596/978-1-4648-0349-9_ch2. [DOI] [PubMed] [Google Scholar]
- 5.Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Naghavi M (2015). Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990–2015: A systematic analysis for the global burden of disease study. JAMA Oncology. doi: 10.1001/jamaoncol.2016.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Cancer Society (2016). Cancer facts & Figs. 2016. Atlanta, GA: American Cancer Society. [Google Scholar]
- 7.Haworth RJ, Margalit R, Ross C, Nepal T, & Soliman AS (2014). Knowledge, attitudes, and practices for cervical cancer screening among the Bhutanese refugee community in Omaha, Nebraska. Journal of Community Health, 39(5), 872–878. doi: 10.1007/s10900-014-9906-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dorji T, Tshomo U, Phuntsho S, Tamang TD, Tshokey T, Baussano I, & Clifford G (2015). Introduction of a national HPV vaccination program into Bhutan. Vaccine, 33(31), 3726–3730. doi: 10.1016/j.vaccine.2015.05.078. [DOI] [PubMed] [Google Scholar]
- 9.American Cancer Society. (2015). Guidelines for the early detection of cancer. http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-046343.pdf. Retrieved Sept 2016.
- 10.Committee on Practice Bulletins-Gynecology. (2012). ACOG practice bulletin number 131: Screening for cervical cancer. Obstetrics & Gynecology, 120(5), 1222–1238. [DOI] [PubMed] [Google Scholar]
- 11.CDC (2016). Breast cancer http://www.cdc.gov/cancer/breast/basic_info/screening.htm. Retrieved Sept 2016.
- 12.Kandula NR, Wen M, Jacobs EA, & Lauderdale DS (2006). Low rates of colorectal, cervical, and breast cancer screening in Asian Americans compared with non-Hispanic whites: Cultural influences or access to care? Cancer, 107(1), 184–192. doi: 10.1002/cncr.21968. [DOI] [PubMed] [Google Scholar]
- 13.Tsui J, Saraiya M, Thompson T, Dey A, & Richardson,(2007). Cervical cancer screening among foreign-born women by birthplace and duration in the United States. Journal of Women’s Health, 16(10), 1447–1457. doi: 10.1089/jwh.2006.0279. [DOI] [PubMed] [Google Scholar]
- 14.Reyes AM, & Miranda PY (2015). Trends in cancer screening by citizenship and health insurance, 2000–2010. Journal of Immigrant and Minority Health, 17(3), 644–651. doi: 10.1007/s10903-014-0091-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.CDC. (2012). Cancer screening—United States, 2010. (No. 61(03)). Atlanta, GA: Morbidity and Mortality Weekly Report. [PubMed] [Google Scholar]
- 16.Pickle S, Altshuler M, & Scott K (2014). Cervical cancer screening outcomes in a refugee population. Journal of Immigrant & Refugee Studies, 12(1), 1–8. [Google Scholar]
- 17.Grieco EM, Acosta YD, de la Cruz GP, Gryn T, Larsen LJ, Trevelyan EN, & Walters NP (2012). The foreign-born population in the United States: 2010. Washington, D.C.: U.S. Census Bureau. [Google Scholar]
- 18.Office of Refugee Resettlement. (2015). Refugee arrival data. http://www.acf.hhs.gov/orr/resource/refugee-arrival-data. Retrieved Oct 2016.
- 19.Quigley J (2004). Bhutanese refugees in Nepal: What role now for the European Union and the United Nations high commission for refugees? Contemporary South Asia, 13(2), 187–200. [Google Scholar]
- 20.Community Research Partners. (2015). Impact of refugees in central Ohio: 2015 report. http://www.communityresearch-partners.org/portfolios/impact-of-refugees-in-central-ohio/. Retrieved Aug 2016.
- 21.Ferenchik M, & Pyle E (2015). Ohio sees growing Bhutanese refugee population. Washington, D.C.: The Washington Times [Google Scholar]
- 22.Pasick RJ, Oliva G, Goldstein E, Nguyen T (2010). Community-engaged research with community-based organizations: A resource manual for UCSF researchers. San Francisco: Clinical Translational Science Institute. [Google Scholar]
- 23.Kue J, Pyakurel S, & Yotebieng K (2016). Building community-engaged research partnerships with Bhutanese-Nepali refugees: Lessons learned from a community health needs assessment project. Practicing Anthropology, 38(4), 37–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Minkler M (2005). Community-based research partnerships: Challenges and opportunities. Journal of Urban Health, 82, ii3–ii12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taylor VM, Schwartz SM, Jackson JC, Kuniyuki A, Fischer M, Yasui Y, & Thompson B (1999). Cervical cancer screening among Cambodian-American women. Cancer Epidemiology, Biomarkers & Prevention, 8(6), 541–546. [PubMed] [Google Scholar]
- 26.Aragona M, Pucci D, Mazzetti M, & Geraci S (2012). Post-migration living difficulties as a significant risk factor for PTSD in immigrants: A primary care study. Italian Journal of Public Health, 9(3), e7525–1–e7525–8. [Google Scholar]
- 27.Schweitzer RD, Brough M, Vromans L, & Asci-Kobe M (2011). Mental health of newly arrived Burmese refugees in Australia: Contributions of pre-migration and post-migration experience. Australian and New Zealand Journal of Psychiatry, 45, 299–307. [DOI] [PubMed] [Google Scholar]
- 28.Agresti A (1992). A survey of exact inference for contingency tables. Statistical Science, 7(1), 131–177. [Google Scholar]
- 29.Goel MS, Wee CC, McCarthy EP, Davis RB, Ngo-Metzger Q, & Phillips RS (2003). Racial and ethnic disparities in cancer screening: The importance of foreign birth as a barrier to care. Journal of General Internal Medicine, 18(12), 1028–1035. doi: 10.1111/j.1525-1497.2003.20807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor VM, Yasui Y, Burke N, Nguyen T, Acorda E, Thai H, & Jackson JC (2004). Pap testing adherence among Vietnamese American women. Cancer Epidemiology, Biomarkers & Prevention, 13(4), 613–619. [PubMed] [Google Scholar]
- 31.Jackson JC, Taylor VM, Chitnarong K, Mahloch J, Fischer M, Sam R, & Seng P (2000). Development of a cervical cancer control intervention program for Cambodian American women. Journal of Community Health, 25(5), 359–375. [DOI] [PubMed] [Google Scholar]
- 32.Nguyen TT, McPhee SJ, Nguyen T, Lam T, & Mock J (2002). Predictors of cervical pap smear screening awareness, intention, and receipt among Vietnamese–American women. American Journal of Preventive Medicine, 23(3), 207–214. doi: 10.1016/S0749-3797(02)00499-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pham CT, & McPhee SJ (1992). Knowledge, attitudes, and practices of breast and cervical cancer screening among Vietnamese women. Journal of Cancer Education, 7(4), 305–310. doi: 10.1080/08858199209528187. [DOI] [PubMed] [Google Scholar]
- 34.Taylor VM, Jackson JC, Yasui Y, Kuniyuki A, Acorda E, Marchand A, & Thompson B (2002). Evaluation of an outreach intervention to promote cervical cancer screening among Cambodian American women. Cancer Detection and Prevention, 26(4), 320–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fox SA, Heritage J, Stockdale SE, Asch SM, Duan N, Reise SP (2009). Cancer screening adherence: Does physician-patient communication matter? Patient Education and Counseling, 75(2), 178–184. doi: 10.1016/j.pec.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 36.O’Malley AS, Forrest CB, & Mandelblatt J (2002). Adherence of low-income women to cancer screening recommendations. Journal of General Internal Medicine, 17(2), 144–154. doi: 10.1046/j.1525-1497.2002.10431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morrison TB, Wieland ML, Cha SS, Rahman AS,Chaudhry R (2012). Disparities in preventive health services among Somali immigrants and refugees. Journal of Immigrant and Minority Health, 14(6), 968–974. doi: 10.1007/s10903-012-9632-4. [DOI] [PubMed] [Google Scholar]
- 38.Saadi A, Bond BE, & Percac-Lima S (2015). Bosnian, Iraqi, and Somali refugee women speak: A comparative qualitative study of refugee health beliefs on preventive health and breast cancer screening. Women’s Health Issues, 25(5), 501–508. doi: 10.1016/j.whi.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 39.Kue J, Zukoski A, Keon KL, & Thorburn S (2014). Breast and cervical cancer screening: Exploring perceptions and barriers with Hmong women and men in Oregon. Ethnicity & Health, 19(3), 311–327. doi: 10.1080/13557858.2013.776013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kagawa-Singer M, Valdez Dadia A, Yu M, & Surbone A (2010). Cancer, culture, and health disparities: Time to chart a new course? CA, 60, 12–39. doi: 10.3322/caac.20051. [DOI] [PubMed] [Google Scholar]
- 41.Donaldson SL, & Grant-Vallone EJ (2002). Understanding self-report bias in organizational behavior research. Journal of Business and Psychology, 17(2), 245–260. [Google Scholar]