Abstract
Acting in the interfaces between environment and membrane compartments, membrane ion channels and receptors transduce various physical and chemical cues into downstream signaling events. Not surprisingly, these membrane proteins play essential roles in a wide range of cellular processes such as sensory perception, synaptic transmission, cellular growth and development, fate determination, and apoptosis. However, except insulin and insulin-like growth factor receptors, the functions of membrane receptors in animal lifespan modulation have not been well appreciated. On the other hand, although ion channels are popular therapeutic targets for many age-related diseases, their potential roles in aging itself are largely neglected. In this review, we will discuss our current understanding of the conserved functions and mechanisms of membrane ion channels and receptors in the modulation of lifespan across multiple species including Caenorhabditis elegans, Drosophila, mouse, and human.
Keywords: GPCRs, growth factor receptors, ion channels, lifespan, sensory
1 ∣. INTRODUCTION
Many cellular structures are enclosed by distinct biomembranes. In order to communicate with the environment, multiple types of ion channels, transporters, and membrane receptors function as signal detectors, relayers, and amplifiers. It has been estimated that ion channels, transporters, and membrane receptors account for 20–30% of human proteome (Fagerberg, Jonasson, von Heijne, Uhlen, & Berglund, 2010). These membrane proteins have been well established to be essential players in many cellular processes including sensory perception, synaptic transmission, cellular growth and development, fate determination, and apoptosis. However, relatively little is known about their functions in aging, a fundamental biological process present in nearly all animal species.
The traditional view attributes aging to the passive accumulation of DNA mutations, damaged proteins, and reactive oxygen species (ROS). However, pioneering genetic studies in Caenorhabditis elegans have clearly shown that single gene mutations, such as age-1 (a phosphoinositide 3-kinase) and daf-2 (an insulin receptor), can dramatically affect lifespan, supporting that aging is actively modulated by intrinsic genetic programs (Friedman & Johnson, 1988; Kenyon, Chang, Gensch, Rudner, & Tabtiang, 1993). In addition to genetic factors, various environmental cues also have profound impacts on aging, as human twin studies have suggested that environmental factors may determine 70–80% of human lifespans (Barzilai et al., 2012). Since many membrane ion channels and receptors act as cellular sensors for various environmental and intrinsic cues, one would expect that they must play important roles in aging. So far, insulin and insulin-like growth factor receptors remain the best studied membrane proteins that modulate aging across species (Finch & Ruvkun, 2001; Kenyon, 2010; Riera, Merkwirth, De Magalhaes Filho, & Dillin, 2016). A reduced function in insulin and insulin-like growth factor receptors has been linked to extended lifespan in C. elegans, Drosophila, rodent, and probably human (Finch & Ruvkun, 2001; Kenyon, 2010; Riera et al., 2016).
Ion channels are pore-forming membrane proteins that conduct ion exchanges and convert chemical and physical inputs into electrical signals. As popular therapeutic targets, ion channels have been targeted to treat many age-related diseases, including Alzheimer’s disease, cardiovascular diseases, and pain (Bagal et al., 2013; Mathie, 2010; Skaper, 2011). However, the functions of ion channels in aging itself have not been well studied. As many ion channels play key roles in sensory transduction, some ion channels have recently emerged as important players in the sensory modulation of longevity (Linford, Kuo, Chan, & Pletcher, 2011). Below, we discuss the important findings on the functions and mechanisms of membrane ion channels and receptors in lifespan modulation.
2 ∣. ION CHANNELS IN LIFESPAN MODULATION
Ion channels are widely expressed in both excitable cells such as neurons and muscle cells as well as non-excitable cells such as epithelial cells and adipocytes. As a prominent component of the nervous system, distinct types of ion channels play essential roles in the neuronal modulation of aging.
2.1 ∣. TRP channel
The first transient receptor potential (TRP) channel was cloned from a mutant Drosophila that exhibits a transient instead of plateau elevation of potential upon light stimulation (Cosens & Manning, 1969; Montell & Rubin, 1989). Next to the potassium channels, TRP channels form the second largest ion channel superfamily in the animal kingdom, which includes TRPC (TRP-Canonical), TRPV (TRP-Vanilloid), TRPM (TRP-Melastatin), TRPN (TRP-NompC), TRPA (TRP-Ankyrin), TRPP (TRP-Polycystin), andTRPML(TRP-MucoLipin)subfamilies (Venkatachalam & Montell, 2007). As important cellular sensors, TRP channels function as the transduction channels in many sensory modalities including thermosensation, mechanosensation, vision, smell, and taste (Clapham, 2003; Venkatachalam & Montell, 2007).
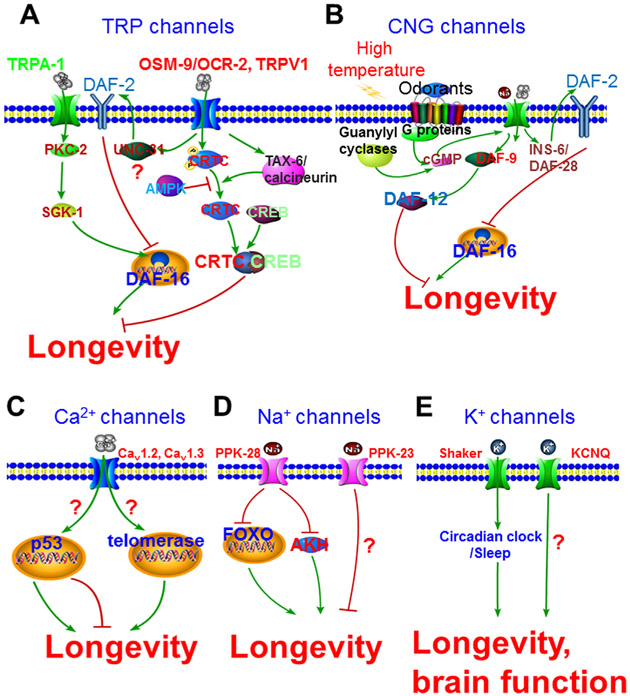
It has been reported nearly one century ago that temperature modulates animal aging with an apparent inverse correlation between temperature and lifespan (Loeb & Northrop, 1916). However, its underlying mechanisms are little understood. TRP channels are best-studied thermosensitive ion channels (Patapoutian, Peier, Story, & Viswanath, 2003; Xiao & Xu, 2009; Xiao, Liu, & Xu, 2015). Among all thermosensitive TRP channels, TRPA1 is unique in that its temperature sensitivity is species-dependent. Namely, it is a cold-activated channel in C. elegans and mouse but functions as a heat-activated channel in Drosophila and snake (Chatzigeorgiou et al., 2010; Gracheva et al., 2010; Story et al., 2003; Viswanath et al., 2003; Xiao et al., 2013). TRPA-1 plays an important role in the cold-promoted longevity in C. elegans as trpa-1(ok999)-null mutant exhibits shortened lifespan only at low, but not high, temperatures (Xiao et al., 2013). Upon activation by the low temperatures, TRPA-1 initiates a genetic program to actively promote longevity. This program includes Ca2+ influx, calcium-sensitive kinase PKC-2, FOXO kinase SGK-1, and FOXO family transcription factor DAF-16 (Fig. 1A) (Xiao et al., 2013). Interestingly, mammalian TRPA1 can functionally substitute for its worm homolog in lifespan extension, suggesting that TRPA1 might have a conserved role in mammalian aging. Notably, although adulthood low temperature extends lifespan, larval low temperature actually shortens it. Again, TRPA-1 is required for this developmental stage-dependent differential effects of low temperature on lifespan (Zhang et al., 2015). It is possible that TRPA-1 might activate distinct sets of transcriptional target genes during larval stage and adulthood (Zhang et al., 2015).
FIGURE 1.
Ion channels in lifespan modulation. A: C. elegans TRPA-1 channel mediates the cold-promoted longevity through FOXO transcription factor DAF-16, while mouse TRPV1 shortens lifespan through transcription co-factor CRTC1. B: CNG channels TAX-2 and TAX-4 are involved in the odorant- and heat-modulated lifespan through FOXO transcription factor DAF-16 and nuclear hormone receptor DAF-12, respectively. C: Multiple voltage-gated calcium channels modulate brain aging. D: ENaC channels PPK-28 and PPK-23 suppress longevity in Drosophila. E: Potassium channel Shaker may influence aging through circadian clock
TRPV1 is another well-characterized thermosensitive TRP channel that is activated by heat and the pungent ingredient capsaicin from hot chili pepper (Caterina et al., 1997). A recent study in mice showed that animals lacking TRPV1 are long-lived and exhibit improved metabolic health at the advanced ages (Riera et al., 2014). In-depth characterization of TRPV1-deficient mice revealed that the loss of TRPV1 alters downstream Ca2+ signaling and blocks the nuclear shuttling of CRTC1 (CREB-regulated transcriptional coactivator 1) that will otherwise lead to the reduced longevity (Fig. 1A) (Riera et al., 2014). Notably, the functions of TRPV1 in shortening lifespan appears to be evolutionarily conserved. In C. elegans, osm-9 and ocr-2 encode two TRPV channels (Colbert, Smith, & Bargmann, 1997; Tobin et al., 2002). Consistent with results from TRPV1 knockout mice, loss of osm-9 and ocr-2 in worms results in an increased lifespan (Lee & Ashrafi, 2008; Riera et al., 2014). Mechanistically, the opening of OSM-9 and OCR-2 facilitates Ca2+ influx and activates UNC-31, a calcium-activated regulator of neural dense-core vesicle release. This leads to the release of insulin, neuropeptides, and biogenic amines and activates insulin signaling pathway, which ultimately inhibits the FOXO transcription factor DAF-16 and suppresses longevity (Fig. 1A) (Lee & Ashrafi, 2008). However, it should be noted that, unlike TRPV1, neither OSM-9 nor OCR-2 has been established to be heat-activated. Additionally, it remains elusive whether TRPV1, OSM-9, and OCR-2 are involved in the high temperature-suppressed lifespan observed in many species.
2.2 ∣. CNG channel
Another type of ion channel involved in animal lifespan modulation is cyclic nucleotide-gated (CNG) channels that are activated upon binding of cyclic nucleotides. In C. elegans, CNG channels TAX-2 and TAX-4 play essential roles in sensory transduction (Bargmann, 2006). Mutations in tax-2 and tax-4 extend lifespan at low (15 and 20°C), but not high (25°C), temperatures (Apfeld & Kenyon, 1999; Lee & Kenyon, 2009). The extended lifespan of tax-2 and tax-4 mutants at low temperatures is dependent on DAF-16 since the loss-of-function of daf-16 almost completely abolishes the longevity phenotype of tax-2 and tax-4 mutants (Fig. 1B) (Apfeld & Kenyon, 1999). Furthermore, studies showed that TAX-2 and TAX-4 might modulate DAF-16 activity by regulating the expression of various insulin-like peptides (ILPs) including INS-6 and DAF-28 (Fig. 1B) (Artan et al., 2016). Interestingly, the effect of tax-2 and tax-4 mutations on lifespan is opposite at high temperatures—they shorten lifespan at 25°C (Lee & Kenyon, 2009). This lifespan shortening effect of tax-2 and tax-4 mutations at high temperatures requires a steroid signaling pathway, but not DAF-16 (Fig. 1B) (Lee & Kenyon, 2009). Specifically, the activation of the heat-sensitive neuron AFD (a pair of amphid sensory neuron involved in C. elegans thermosensation and CO2 sensing) by high temperatures promotes cGMP production and activates TAX-2 and TAX-4, which lead to an increase in DAF-9/cytochrome P450 level and alter the activity of nuclear hormone receptor DAF-12 (Fig. 1B). Eventually, it causes accelerated aging at high temperatures. Taken together, CNG channels might utilize distinct mechanisms to modulate the rate of aging under different temperatures.
2.3 ∣. Calcium channel
Calcium channels play critical roles in normal neuronal and muscular functions. Dysregulation of intracellular Ca2+ homeostasis may contribute to age-related decline in brain functions. For example, aging is accompanied by increased surface/total protein ratio of two L-type Ca2+ channels (Cav1.2 and Cav1.3) in the hippocampus region (Nunez-Santana et al., 2014). The loss of Cav1.2 can prevent animals from age-related memory loss in a sex-dependent manner (Zanos et al., 2015). By contrast, transgenic mice with overexpressed Cav1.3 has been used as a model to study brain aging (Krueger et al., 2016). The dysfunctions of calcium channels may also contribute to the age-related vascular disorders since calcium channel blockers are commonly used to treat hypertension in the elderly (Caballero-Gonzalez, 2015). For instance, the calcium channel blocker nifedipine can delay senescence and prevent telomerase activity decline in human endothelial cells (Hayashi et al., 2014). These anti-senescence effects are likely achieved by reducing the production of ROS and enhancing the activity of nitric oxide synthase (eNOS) (Hayashi et al., 2014).
The mechanisms through which calcium channels modulate aging and age-related conditions remain largely unknown. Transcription factor-mediated transcriptional reprogramming might be a key mechanism. For example, many calcium channels can modulate the expression and function of the tumor suppressor p53 which plays an important role in integrating diverse physiological signals and balancing tumor suppression and longevity (Fig. 1C) (Rodier, Campisi, & Bhaumik, 2007). Alternatively, calcium channels could influence aging through modulating telomerase activity. As an important modulator for replicative lifespan (Riera et al., 2016), telomere length is regulated by Ca2+ homeostasis and the activities of telomerase are enhanced by the increased extracellular Ca2+ level (Fig. 1C) (Alfonso-De Matte, Moses-Soto, & Kruk, 2002).
2.4 ∣. Sodium channel
The Degenerin/Epithelial sodium channels (DEG/ENaC) Pickpocket (PPK) represent a large family of ion channels in Drosophila (Zelle, Lu, Pyfrom, & Ben-Shahar, 2013). PPK28 is an osmo-sensitive ENaC channel that mediates the behavioral responses to water (Cameron, Hiroi, Ngai, & Scott, 2010). The loss-of-function of ppk28 increases lifespan and promotes health performance in fly. The lifespan extension of ppk28 mutant requires both FOXO transcription factor and AKH signaling which regulates lipid metabolism (Fig. 1D) (Waterson et al., 2014). The activation of another ENaC channel PPK23, which is a receptor for female pheromones, decreases male lifespan through neuropeptide signaling (Gendron et al., 2014).
2.5 ∣. Potassium channel
The voltage-gated potassium channel Shaker was originally cloned from a mutant Drosophila with atypical leg-shaking phenotype (Tempel, Papazian, Schwarz, Jan, & Jan, 1987). The loss-of-function mutations in Shaker disrupt sleep patterns and reduce lifespan in Drosophila (Cirelli et al., 2005). As the disturbance of circadian rhythm and sleep contributes to aging, Shaker might modulate lifespan through regulating circadian rhythm (Fig. 1E). Other types of potassium channels are also associated with aging. For example, mutations in KCNQ channels cause cardiac arrhythmias in young Drosophila, a phenotype typically observed in aged animals (Ocorr et al., 2007). In addition, mutations in KCNQ channels accelerate age-dependent memory impairment and this memory defect can be rescued by overexpressing KCNQ in a specific subset of neurons in Drosophila (Cavaliere, Malik, & Hodge, 2013). Importantly, a similar effect of KCNQ channels in brain aging has been reported in primates (Wang et al., 2011), indicating the conserved roles of KCNQ channels in aging (Fig. 1E). On the other hand, aging also has a profound influence on the functions of potassium channels. Studies in C. elegans revealed the age-dependent oxidation of potassium channel KVS-1 due to the accumulated ROS (Cai & Sesti, 2009). The oxidized KVS-1 contributes to the age-associated decline in sensory functions (Cai & Sesti, 2009).
2.6 ∣. Other ion channels
In addition to the channels discussed above, other types of ion channels are also involved in lifespan modulation. In C. elegans, cup-4 encodes an ion channel with high homology to mammalian nicotinic acetylcholine receptors (nAChRs). Interestingly, CUP-4 is required for the dietary restriction-mediated lifespan extension in C. elegans through NLP-7 signaling and transcription factors SKN-1 and PHA-4 (Park, Link, & Johnson, 2010). In Drosophila, the broadly expressed odorant receptor Or83b encodes a non-selective cation channel for odorant perception. Mutations in Or83b extend lifespan (Libert et al., 2007). However, since most insulin signaling components are unchanged in Or83b mutant, insulin-independent signaling might underlie the Or83b-modulated longevity (Libert et al., 2007).
3 ∣. GPCRs IN LIFESPAN MODULATION
G protein-coupled receptors (GPCRs) constitute a large family of membrane receptors that sense various environmental and intrinsic signals. As molecular receptors for neurotransmitters, hormones, chemokines, and neuromodulators, GPCRs make up approximately 5% of eukaryotic genome (Bargmann, 1998; Marchese, George, Lynch, & O'Dowd, 1999). Ligand-binding causes conformational changes in GPCRs which promote the exchange of GDP for GTP and activate heterotrimeric G proteins. Activated G proteins modulate the activities of downstream effector proteins such as adenylyl cyclase (AC), phospholipase C (PLC), and some ion channels, which in turn regulates the activities of second and third messengers, and eventually generates both acute and prolonged responses to distinct stimuli.
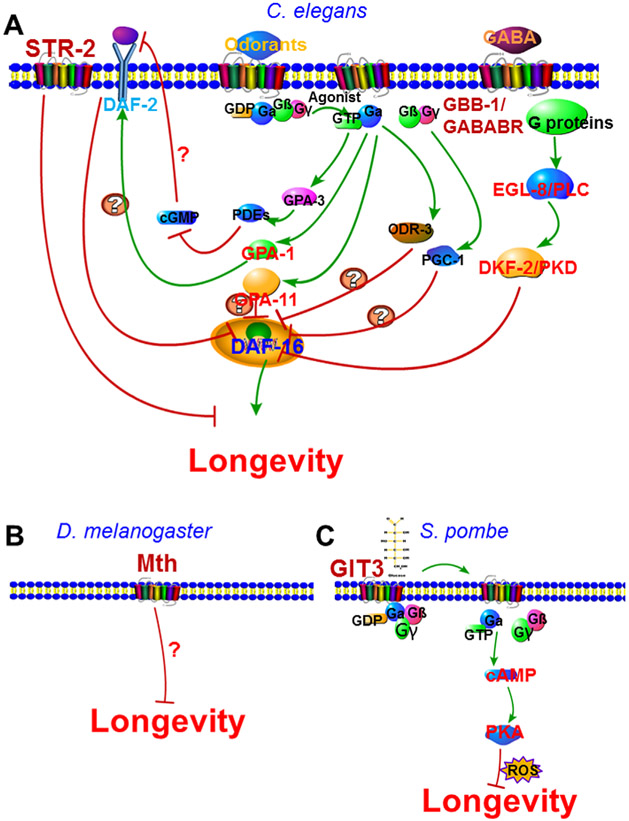
Although GPCRs are widely involved in nearly every aspect of cellular physiology, their roles in lifespan modulation have not been well recognized. C. elegans GBB-1 is a metabotropic receptor (GABAB) for the inhibitory neurotransmitter GABA. Deficiency in GABA signaling or loss-of-function of gbb-1 extends lifespan through PLCβ which transduces the longevity signal to DAF-16 via protein kinase D (PKD) (Fig. 2A) (Chun et al., 2015). str-2 encodes a putative chemosensory GPCR (Troemel, Sagasti, & Bargmann, 1999) and str-2 RNA interference (RNAi) extends lifespan (Fig. 2A) (Alcedo & Kenyon, 2004). However, the agonist for STR-2 is yet to be determined and the mechanism of how str-2 influences aging also remains elusive (Alcedo & Kenyon, 2004).
FIGURE 2.
G protein-coupled receptors in lifespan modulation. A: Metabotropic GABAB receptor GBB-1 and odorant receptor STR-2 modulate C. elegans longevity through G protein signaling and DAF-16. B: Methuselah (Mth) suppresses lifespan in Drosophila. C: Glucose receptor GIT3 suppresses Schizosaccharomyces pombe longevity through G protein signaling and protein kinase A
GPCRs also modulate lifespan in other species. In Drosophila, GPCR methuselah (mth) mutants display a substantial increase in mean lifespan and enhanced resistance to diverse stressors (Fig. 2B) (Lin, Seroude, & Benzer, 1998; McGarrigle & Huang, 2007). In yeast S. pombe, the inhibition of Git3, a GPCR for glucose, extends lifespan while constitutive activation of Git3 signaling accelerates aging and abolishes the lifespan extension due to dietary restriction (Fig. 2C) (Roux et al., 2009).
GPCRs trigger downstream signaling through heterotrimeric G proteins. Therefore, distinct G proteins might mediate the effects of GPCRs in lifespan modulation. A genetic screen was conducted in C. elegans to investigate the role of sensory G proteins in longevity regulation (Lans & Jansen, 2007). The loss-of-function mutations in Gα subunits odr-3 and gpa-1, Gγ subunit gpc-1, and the overexpression of Gα subunit gpa-11 all extend lifespan (Lans & Jansen, 2007). It appears that sensory G proteins regulate lifespan through FOXO signaling because the loss-of-function of DAF-16/FOXO completely abolishes the longevity phenotype of these G protein mutants (Fig. 2A) (Lans & Jansen, 2007). Among the G protein subunits that affect lifespan, gpa-1 regulates longevity by influencing DAF-2 activity while odr-3, gpc-1, and gpa-11 may signal to DAF-16 in a DAF-2-independent fashion (Fig. 2A). In a separate study, the activation of Gα subunit GPA-3 extended lifespan by inhibiting neuronal cGMP level through cGMP-specific phosphodiesterases (PDEs) (Fig. 2A) (Hahm, Kim, & Paik, 2009). Again, DAF-16 acts downstream of GPA-3 in the cGMP signaling-dependent lifespan extension (Hahm et al., 2009).
4 ∣. RECEPTOR TYROSINE KINASES IN LIFESPAN MODULATION
As membrane receptors for various polypeptide growth factors, cytokines, and hormones, receptor tyrosine kinases (RTKs) are essential for many aspects of cellular physiology including cell growth, proliferation, differentiation, and tissue repair. After activation, RTKs either transiently exert their kinase activity or recruit intracellular tyrosine or serine/threonine kinases to form a multiprotein complex. Among all RTKs, insulin and insulin-like growth factor receptors are probably the best studied modulators of longevity. Additionally, epidermal growth factor receptor (EGFR) also plays a role in aging. Below, we will focus our discussion on these two types of growth factor receptors in lifespan modulation.
4.1 ∣. Insulin and insulin-like growth factor receptor
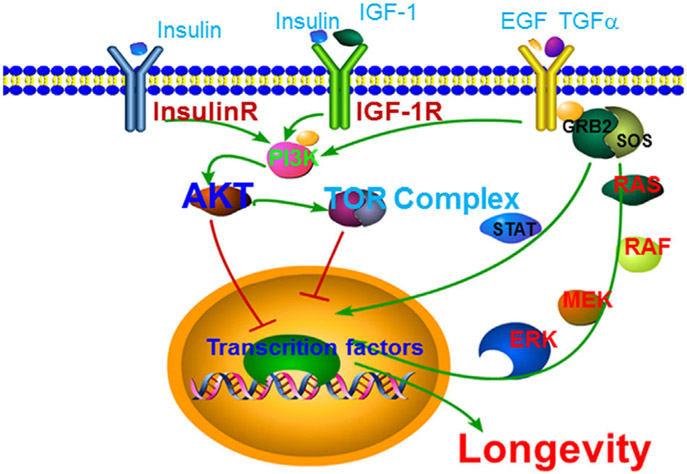
Insulin/insulin-like growth factor signaling (IIS) modulates lifespan in an evolutionarily conserved manner (Kenyon, 2010). Upon agonist binding, insulin/insulin-like growth factor receptors dimerize and activate their intracellular tyrosine kinase domain, which will then bind and activate phosphoinositide 3-kinases (PI3Ks) through their SH2 domain. The activated PI3Ks can convert the membrane phosphatidylinositol 4,5-bisphosphate (PIP2) to phosphatidylinositol 3,4,5-triphosphate (PIP3), which leads to the activation of protein kinase B (PKB, also called AKT) through phosphoinositide-dependent kinase (PDK). By phosphorylating multiple downstream aging-related effectors such as FOXOs and mechanistic target of rapamycin (mTOR), AKT functions as a key regulator of animal longevity (Fig. 3).
FIGURE 3.
Receptor tyrosine kinases in lifespan modulation. Insulin and insulin-like growth factor receptors and EGFR modulate lifespan in an evolutionarily conserved manner. AKT plays a central role downstream of growth factor receptors in lifespan modulation
The core components of IIS pathway, including DAF-2/insulin receptor, AGE-1/PI3K, PDK-1/PDK, AKT-1 and AKT-2/AKT, all negatively modulate C. elegans lifespan by inhibiting the FOXO transcription factor DAF-16 (Fig. 3) (Finch & Ruvkun, 2001; Kenyon, 2010). Very interestingly, human insulin can activate DAF-2 and suppress C. elegans lifespan through DAF-16 (Kimura, Tissenbaum, Liu, & Ruvkun, 1997; Pierce et al., 2001), strongly supporting that IIS modulates aging in an evolutionarily conserved fashion. In line with this notion, a mutant Drosophila insulin receptor extends the adult lifespan up to 85% in female flies and reduces the late age-specific mortality in male flies (Tatar et al., 2001). Furthermore, the loss of CHICO, a Drosophila insulin receptor substrate protein, also significantly extends lifespan in flies (Clancy et al., 2001). In mice, a reduced level in plasma growth factor or IGF-1 can cause up to 50% of lifespan extension (Brown-Borg, Borg, Meliska, & Bartke, 1996; Coschigano, Clemmons, Bellush, & Kopchick, 2000; Holzenberger et al., 2003), indicating that IGF-1 receptor might suppress longevity in mammals in a similar fashion as their C. elegans and Drosophila counterparts. Although homozygous deletion of IGF-1 receptor in mice leads to embryonic lethality, heterozygous IGF-1 knockout mice (Igf1r+/−) are long-lived, particularly in females (Holzenberger et al., 2003). Moreover, the disruption of insulin receptor substrate 1 (IRS-1), a major intracellular effector of the IGF receptor, also extends lifespan in female mice (Selman et al., 2008; Selman, Partridge, & Withers, 2011). Lastly, genetic variations in FOXO3, a homolog of C. elegans DAF-16 and Drosophila FOXO, are strongly associated with human longevity in multiple ethnic groups (Flachsbart et al., 2009; Li et al., 2009; Morris, Wilicoxa, Donlon, & Willcox, 2015; Wilicox et al., 2008). Taken together, the genetic evidence from multiple species strongly suggest that insulin and insulin-like growth factor receptors play very important roles in regulating animal longevity.
4.2 ∣. EGFR
EGFR (also called ErbB-1 or HER1) is another important RTK involved in lifespan modulation. After binding to its agonists EGF or transforming growth factor α (TGFα), EGFR forms homodimers and auto-phosphorylates several key intracellular tyrosine residues. These activated tyrosine residues bind the SH2 domain of PI3K and activate AKT-FOXO signaling pathway. Additionally, Grb2 and Shc also bind to the activated EGFR via their phosphorylated tyrosine residues, which then serve as an adaptor for the downstream Ras signaling and STAT signaling, respectively (Fig. 3). The last major signaling output of EGFR is through PLC gamma (PLCγ), which triggers calcium release from endoplasmic reticulum (ER) calcium store and PKC activation.
Studies in C. elegans suggest that EGFR can promote longevity and healthspan independent of IIS pathway (Iwasa, Yu, Xue, & Driscoll, 2010; Liu, Rogers, Murphy, & Rongo, 2011). The LET-23/EGFR gain-of-function mutant let-23(sa62) is long-lived (Iwasa et al., 2010) and EGFR may promote lifespan through ubiquitin proteasome system (UPS)-mediated protein homeostasis (Liu et al., 2011). UPS is known to regulate both FOXO transcription factor DAF-16 and FOXA transcription factor PHA-4 (Carrano, Liu, Dillin, & Hunter, 2009; Ghazi, Henis-Korenblit, & Kenyon, 2007; Li, Gao, Lee, Bennett, & Fang, 2007). Thus, the signaling outputs of EGFR in lifespan modulation might converge on DAF-16 and PHA-4, two well-established master regulators of C. elegans lifespan (Kenyon, 2010; Riera et al., 2016). In Drosophila, EGFR signaling plays a crucial role in intestinal stem cell (ISC) proliferation and promotes longevity and maintains tissue health during organismal aging in homeostatic and stress conditions (Biteau & Jasper, 2011; Park, Kim, & Yoo, 2009). In rodents, impaired EGFR signaling has also been linked to the age-related decline in hepatocytes (Chen et al., 2000; Hutter et al., 2000). In human, reduced EGFR signaling is associated with age-related decline in stress response (Enwere et al., 2004). Nevertheless, the precise roles and mechanisms of EGFR in animal lifespan modulation are less understood compared to that of insulin/insulin-like growth factor receptors.
5 ∣. OTHER MEMBRANE ION CHANNELS AND RECEPTORS IN LIFESPAN MODULATION
Many plasma membrane accessory proteins and intracellular organelle ion channels and receptors also participate in lifespan regulation. Here, we will discuss the functions of these aging-related membrane proteins based on their subcellular localization.
5.1 ∣. Klotho
klotho was originally discovered as a suppressor of several aging-related phenotypes and klotho-defective mice display short lifespan, infertility, arteriosclerosis, skin atrophy, osteoporosis, and emphysema (Kuro-o et al., 1997). There are two forms of Klotho proteins: membrane Klotho and secreted Klotho. The membrane Klotho functions as an obligate co-receptor for fibroblast growth factor-23 (FGF23) (Consortium, 2000; Kurosu et al., 2006). As FGF23 does not have a heparan sulfate-binding domain, it needs the membrane-bound Klotho for high affinity binding to the ubiquitously expressed FGFRs in target tissues (Kurosu et al., 2006; Urakawa et al., 2006).
The interaction between Klotho and FGF23 plays a key role in systemic phosphate homeostasis (Razzaque, 2009). Upon activation, Klotho and FGFR trigger a signaling cascade involving ERK1/2 and SGK1 kinases. SGK1 will phosphorylate Na+/H+ exchange regulatory cofactor (NHERF-1) which leads to the internalization and degradation of NaPi-2a, an important sodium/phosphate co-transporter required for phosphate homeostasis (Erben & Andrukhova, 2016). Excessive phosphate accelerates aging and phosphatopathy is commonly observed in patients with chronic kidney disease (CKD) (Ohnishi, Nakatani, Lanske, & Razzaque, 2009; Stubbs et al., 2007). The clinical outcomes of CDK include increased mortality, vascular calcification, cardiac hypertrophy, osteopenia, and sarcopenia (Tonelli et al., 2006). Remarkably, all these outcomes are linked to Klotho decrease (Shimamura et al., 2012) and the exogenous Klotho administration can exert protective effects in animal models of CDK (Razzaque, 2009). Therefore, the anti-aging protein Klotho may provide an attractive drug target for certain age-associated diseases.
5.2 ∣. ER membrane proteins
The endoplasmic reticulum (ER) has many essential cellular functions including protein folding, lipid biosynthesis, and calcium storage. Disrupted ER functions are linked to many age-related diseases including Alzheimer's disease (AD), Parkinson's disease (PD), amyotrophic lateral sclerosis (ALS), Huntington's disease, and type 2 diabetes mellitus.
Presenilins are multi-transmembrane domain proteins involved in AD (Annaert et al., 1999). It has been reported that more than 40% of familial AD are caused by mutations in the presenilin 1 and presenilin 2 (Tandon & Fraser, 2002). Presenilins act as catalytic subunits of γ-secretase that is a key enzyme for amyloid precursor protein (APP) processing and amyloid β-peptide Aβ) release (De Strooper et al., 1998; Wolfe et al., 1999). Mutations of presenilins in familiar AD also cause deranged calcium signaling (Smith, Green, & LaFerla, 2005) and presenilins may function as passive ER Ca2+-leak channels (Tu et al., 2006). Another ER membrane protein stromal interaction molecule (STIM) acts as an ER calcium sensor and gates the Ca2+ release-activated Ca2+ (CRAC) channel. In mouse cortical neurons, the knockdown of STIM1 promotes neuronal survival after traumatic neuronal injury (Hou, Liu, Li, Cheng, & Guo, 2015), indicating that STIM1 may play a role in maintaining neuronal functions during aging. Nevertheless, the precise roles of both presenilins and STIM in lifespan modulation have yet to be determined.
5.3 ∣. Mitochondrial membrane proteins
The functional decline in mitochondria is considered as a hallmark of aging. As the cellular power plant, mitochondria generate ROS, metabolites, iron-sulfur clusters (ISC), and protein and DNA fragments, all of which are involved in animal aging. The aged, dysfunctional, damaged, or excessive mitochondria present a big challenge for cells and can accelerate cellular senescence. To defend themselves, cells mainly rely on mitochondrial fusion and fission as well as mitophagy (Leonov & Titorenko, 2013; Richard et al., 2013). These mitochondria-related cellular processes all actively modulate longevity.
Several mitochondrial membrane ion channels and transporters have been reported to be involved in lifespan modulation. The mitochondrial permeability transition pore (mPTP) is composed of adenine nucleotide translocator (ANT) at the inner mitochondrial membrane and voltage-dependent anion channel (VDAC) at the outer mitochondrial membrane. During aging, the production of ROS and calcium overload open mPTP and promote cellular senescence via apoptosis (Kroemer, Galluzzi, & Brenner, 2007). Another mitochondrial membrane transporter involved in lifespan modulation is uncoupling protein 2 (UCP2) (Rousset et al., 2004). UCP2 plays an important role in glucose and lipid metabolism that is critical for aging. Mice carrying a hypocretin promoter-driven UCP2 transgene have a longer lifespan while UCP2 knockout mice exhibit a significantly shortened lifespan (Andrews & Horvath, 2009; Conti et al., 2006), strongly supporting that UCP2 is a prolongevity factor. Consistent with the findings in mice, polymorphisms in UCP2 are also associated with human longevity (Barbieri et al., 2012; Rose, Crocco, De Rango, Montesanto, & Passarino, 2011). Mechanistically, UCP2 knockout increases the circulating IGF-1 level, indicating a crosstalk between UCP2 and IIS pathway (Hirose et al., 2016).
5.4 ∣. Lysosomal membrane proteins
Lysosome is the major intracellular organelle to break down various biomolecules including proteins, nucleic acids, carbohydrates, and lipids. In addition, lysosome is required to recycle other damaged organelles and large cellular structures during autophagy. The lysosomal membrane receptor LAMP2 regulates the chaperone-mediated autophagy through substrate binding and selective uptake of cytosolic proteins (Majeski & Dice, 2004). LAMP2-deficient mice have a very short lifespan and exhibit massive accumulation of autophagic structures in many tissues (Tanaka et al., 2000). Similarly, LAMP2 deficiency in human causes the Danon's disease which is associated with the accumulation of autophagic materials in striated myocytes (Nishino et al., 2000). Although the detailed mechanism remains elusive, LAMP2 seems to modulate lifespan through autophagy pathway.
6 ∣. CONCLUSION
Human twin studies suggest that environmental factors are equally, if not more, important for lifespan modulation compared to genetic factors (Christensen, Johnson, & Vaupel, 2006; vB Hjelmborg et al., 2006). However, considering the extensively studied genetic programs involved in lifespan modulation, relatively little is known about how distinct environmental factors modulate aging. Many membrane ion channels and receptors are expressed in distinct tissues to sense various environmental stimuli. For example, TRP channels, CNG channels, and many GPCRs play essential roles in temperature sensation, smell and taste, light sensation, and chemesthesis. Consistent with their roles in sensing environmental physical and chemical cues, these ion channels and receptors have been shown to play important roles in the environmental modulation of longevity (e.g., TRPA-1, TAX-2 and TAX-4, PPK23, PPK28, and Or83b) (Gendron et al., 2014; Libert et al., 2007; Waterson et al., 2014; Xiao et al., 2015). Since membrane ion channels and receptors in both olfactory and gustatory systems are known to modulate lifespan in C. elegans and Drosophila, it would be interesting to examine whether sensory ion channels and receptors have similar roles in mammalian aging.
In addition to the environmental cues, membrane ion channels and receptors also detect and relay many internal signals such as neurotransmitters, hormones, cytokines, and metabolites. In this regard, these membrane proteins may integrate systemic signals and coordinate the aging processes among distinct tissues and organs. Insulin and insulin-like growth factor receptors are well established to act in a cell-non-autonomous fashion and play important roles in systemic aging across taxa (Alic et al., 2014; Apfeld and Kenyon, 1998). In addition, several neurotransmitters and neuropeptides are also involved in the cell-non-autonomous regulation of lifespan. In C. elegans, the neurotransmitter octopamin mediates the neuronal CRTC-1 modulation of intestinal mitochondrial metabolism and longevity (Burkewitz et al., 2015). Additionally, serotonin and neuropeptide FLP-2 mediate the cell-non-autonomous mitochondrial unfolded protein response and lifespan modulation from neurons to the intestine (Berendzen et al., 2016; Shao, Niu, & Liu, 2016). Lastly, serotonin and its intestinal membrane receptor SER-7 are required for the cell-non-autonomous modulation of lifespan by neuronal hypoxia-inducible factor-1 (HIF-1) (Leiser et al., 2015). Nevertheless, it should be noted that the aging-related membrane receptors for most neurotransmitters and neuropeptides discussed above are not known yet. Genetic and molecular characterization of these receptors could provide important insights into the mechanisms of systemic aging.
Many aging-related signaling pathways converge on several key transcription factors (FOXO/DAF-16, Nrf2/SKN-1, HSF1/HSF-1, and FOXA/PHA-4), nuclear hormone receptors (DAF-12 and NHR-49), and histone modifiers (sirtuins and histone demethylases) to regulate animal lifespan (Finch & Ruvkun, 2001; Kenyon, 2010; Riera et al., 2016). Interestingly, multiple G protein subunits, GABAB receptor, CNG channels, and TRP channels all modulate lifespan in a somewhat FOXO/DAF-16-dependent fashion in C. elegans (Artan et al., 2016; Chun et al., 2015; Lans & Jansen, 2007; Xiao et al., 2013). These results support an important role of FOXO transcription factors in animal aging. Remarkably, some membrane ion channels and receptors may use distinct nuclear factors to regulate longevity under different cellular contexts. For example, TAX-2/TAX-4 CNG channels sense environmental temperature changes in AFD neuron and modulate lifespan through a DAF-12 steroid signaling pathway (Lee & Kenyon, 2009). In contrast, TAX-4 suppresses lifespan in a subset of sensory neurons through FOXO/DAF-16 signaling. Except a few cases, the signaling cascades from membrane ion channels and receptors to nuclear modulators of aging are largely unknown. The identification of the aging-related signaling pathways downstream these membrane sensors may reveal novel regulators of animal lifespan.
In summary, membrane ion channels and receptors function in the forefront to detect various environmental inputs and intrinsic growth signals. As a result, they may initiate distinct downstream signaling pathways involved in lifespan modulation. Studying the functions and mechanisms of membrane ion channels and receptors in lifespan regulation may provide important information about the environmental modulation of longevity.
ACKNOWLEDGMENTS
We thank the members of the Xiao lab for comments. Work in the lab is supported by the UF Cancer-Aging Collaborative Grant, UF Pepper Center Pilot Grant, and the UF Start-Up Funds (to R.X.).
Funding information
UF Cancer-Aging Collaborative Grant; UF Pepper Center Pilot Grant; UF Start-Up Funds
REFERENCES
- Alcedo J, & Kenyon C (2004). Regulation of C. elegans longevity by specific gustatory and olfactory neurons. Neuron, 41(1), 45–55. [DOI] [PubMed] [Google Scholar]
- Alfonso-De Matte MY, Moses-Soto H, & Kruk PA (2002). Calcium-mediated telomerase activity in ovarian epithelial cells. Arch Biochem Biophys, 399(2), 239–244. [DOI] [PubMed] [Google Scholar]
- Alic N, Tullet JM, Niccoli T, Broughton S, Hoddinott MP, Slack C,… Partridge L (2014). Cell-nonautonomous effects of dFOXO/DAF-16 in aging. Cell Rep, 6(4), 608–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews ZB, & Horvath TL (2009). Uncoupling protein-2 regulates lifespan in mice. Am J Physiol Endocrinol Metab, 296(4), E621–E627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annaert WG, Levesque L, Craessaerts K, Dierinck I, Snellings G, Westaway D, … De Strooper B (1999). Presenilin 1 controls gamma-secretase processing of amyloid precursor protein in pre-golgi compartments of hippocampal neurons. J Cell Biol, 147(2), 277–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apfeld J, & Kenyon C (1998). Cell nonautonomy of C. elegans daf-2 function in the regulation of diapause and life span. Cell, 95(2), 199–210. [DOI] [PubMed] [Google Scholar]
- Apfeld J, & Kenyon C (1999). Regulation of lifespan by sensory perception in Caenorhabditis elegans. Nature, 402(6763), 804–809. [DOI] [PubMed] [Google Scholar]
- Artan M, Jeong DE, Lee D, Kim YI, Son HG, Husain Z,… Lee SJ (2016). Food-derived sensory cues modulate longevity via distinct neuroendocrine insulin-like peptides. Genes Dev, 30(9), 1047–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagal S, Brown AD, Cox PJ, Omoto K, Owen RM, Pryde DC, … Swain NA (2013). Ion channels as therapeutic targets: A drug discovery perspective. J Med Chem, 56(3), 593–624. [DOI] [PubMed] [Google Scholar]
- Barbieri M, Boccardi V, Esposito A, Papa M, Vestini F, Rizzo MR, & Paolisso G (2012). A/ASP/VAL allele combination of IGF1R, IRS2, and UCP2 genes is associated with better metabolic profile, preserved energy expenditure parameters, and low mortality rate in longevity. Age, 34(1), 235–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargmann CI (1998). Neurobiology of the Caenorhabditis elegans genome. Science, 282(5396), 2028–2033. [DOI] [PubMed] [Google Scholar]
- Bargmann CI (2006). Chemosensation in C. elegans. WormBook, 1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzilai N, Guarente L, Kirkwood TB, Partridge L, Rando TA, & Slagboom PE (2012). The place of genetics in ageing research. Nat Rev Genet, 13(8), 589–594. [DOI] [PubMed] [Google Scholar]
- Berendzen KM, Durieux J, Shao LW, Tian Y, Kim HE, Wolff S, … Dillin A (2016). Neuroendocrine co-ordination of mitochondrial stress signaling and proteostasis. Cell, 166(6), 1553–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biteau B, & Jasper H (2011). EGF signaling regulates the proliferation of intestinal stem cells in Drosophila. Development, 138(6), 1045–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown-Borg HM, Borg KE, Meliska CJ, & Bartke A (1996). Dwarf mice and the ageing process. Nature, 384(6604), 33. [DOI] [PubMed] [Google Scholar]
- Burkewitz K, Morantte I, Weir HJ, Yeo R, Zhang Y, Huynh FK, … Mair WB (2015). Neuronal CRTC-1 governs systemic mitochondrial metabolism and lifespan via a catecholamine signal. Cell, 160(5), 842–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero-Gonzalez FJ (2015). Calcium channel blockers in the management of hypertension in the elderly. Cardiovasc Hematol Agents Med Chem, 12(3), 160–165. [DOI] [PubMed] [Google Scholar]
- Cai SQ, & Sesti F (2009). Oxidation of a potassium channel causes progressive sensory function loss during aging. Nat Neurosci, 12(5), 611–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron P, Hiroi M, Ngai J, & Scott K (2010). The molecular basis for water taste in Drosophila. Nature, 465(7294), 91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrano AC, Liu Z, Dillin A, & Hunter T (2009). A conserved ubiquitination pathway determines longevity in response to diet restriction. Nature, 460(7253), 396–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, & Julius D (1997). The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature, 389(6653), 816–824. [DOI] [PubMed] [Google Scholar]
- Cavaliere S, Malik BR, & Hodge JJ (2013). KCNQ channels regulate age-related memory impairment. PLoS ONE, 8(4), e62445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatzigeorgiou M, Yoo S, Watson JD, Lee WH, Spencer WC, Kindt KS, … Schafer WR (2010). Specific roles for DEG/ENaC and TRP channels in touch and thermosensation in C. elegans nociceptors. Nat Neurosci, 13(7), 861–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Bronson RT, Klaman LD, Hampton TG, Wang JF, Green PJ, … Neel BG (2000). Mice mutant for Egfr and Shp2 have defective cardiac semilunar valvulogenesis. Nat Genet, 24(3), 296–299. [DOI] [PubMed] [Google Scholar]
- Christensen K, Johnson TE, & Vaupel JW (2006). The quest for genetic determinants of human longevity: Challenges and insights. Nat Rev Genet, 7(6), 436–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun L, Gong J, Yuan F, Zhang B, Liu H, Zheng T, … Liu J (2015). Metabotropic GABA signalling modulates longevity in C. elegans. Nat Commun, 6, 8828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirelli C, Bushey D, Hill S, Huber R, Kreber R, Ganetzky B, & Tononi G (2005). Reduced sleep in Drosophila shaker mutants. Nature, 434(7037), 1087–1092. [DOI] [PubMed] [Google Scholar]
- Clancy DJ, Gems D, Harshman LG, Oldham S, Stocker H, Hafen E, … Partridge L (2001). Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science, 292(5514), 104–106. [DOI] [PubMed] [Google Scholar]
- Clapham DE (2003). TRP channels as cellular sensors. Nature, 426(6966), 517–524. [DOI] [PubMed] [Google Scholar]
- Colbert HA, Smith TL, & Bargmann CI (1997). OSM-9, a novel protein with structural similarity to channels, is required for olfaction, mechanosensation, and olfactory adaptation in Caenorhabditis elegans. J Neurosci, 17(21), 8259–8269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consortium, A. (2000). Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet, 26(3), 345–348. [DOI] [PubMed] [Google Scholar]
- Conti B, Sanchez-Alavez M, Winsky-Sommerer R, Morale MC, Lucero J, Brownell S, … Bartfai T (2006). Transgenic mice with a reduced core body temperature have an increased life span. Science, 314(5800), 825–828. [DOI] [PubMed] [Google Scholar]
- Coschigano KT, Clemmons D, Bellush LL, & Kopchick JJ (2000). Assessment of growth parameters and life span of GHR/BP gene-disrupted mice. Endocrinology, 141(7), 2608–2613. [DOI] [PubMed] [Google Scholar]
- Cosens DJ, & Manning A (1969). Abnormal electroretinogram from a Drosophila mutant. Nature, 224(5216), 285–287. [DOI] [PubMed] [Google Scholar]
- De Strooper B, Saftig P, Craessaerts K, Vanderstichele H, Guhde G, Annaert W, … Van Leuven F (1998). Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature, 391(6665), 387–390. [DOI] [PubMed] [Google Scholar]
- Enwere E, Shingo T, Gregg C, Fujikawa H, Ohta S, & Weiss S (2004). Aging results in reduced epidermal growth factor receptor signaling, diminished olfactory neurogenesis, and deficits in fine olfactory discrimination. J Neurosci, 24(38), 8354–8365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erben RG, & Andrukhova O (2016). FGF23-klotho signaling axis in the kidney. Bone. pii: S8756-3282(16)30256-3. [DOI] [PubMed] [Google Scholar]
- Fagerberg L, Jonasson K, von Heijne G, Uhlen M, & Berglund L (2010). Prediction of the human membrane proteome. Proteomics, 10(6), 1141–1149. [DOI] [PubMed] [Google Scholar]
- Finch CE, & Ruvkun G (2001). The genetics of aging. Annu Rev Genomics Hum Genet, 2, 435–462. [DOI] [PubMed] [Google Scholar]
- Flachsbart F, Caliebeb A, Kleindorp R, Blanche H, von Eller-Eberstein H, Nikolaus S, … Nebel A (2009). Association of FOXO3A variation with human longevity confirmed in German centenarians. Proc Natl Acad Sci USA, 106(8), 2700–2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman DB, & Johnson TE (1988). A mutation in the age-1 gene in Caenorhabditis elegans lengthens life and reduces hermaphrodite fertility. Genetics, 118(1), 75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendron CM, Kuo TH, Harvanek ZM, Chung BY, Yew JY, Dierick HA, & Pletcher SD (2014). Drosophila life span and physiology are modulated by sexual perception and reward. Science, 343(6170), 544–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazi A, Henis-Korenblit S, & Kenyon C (2007). Regulation of Caenorhabditis elegans lifespan by a proteasomal E3 ligase complex. Proc Natl Acad Sci USA, 104(14), 5947–5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracheva EO, Ingolia NT, Kelly YM, Cordero-Morales JF, Hollopeter G, Chesler AT, … Julius D (2010). Molecular basis of infrared detection by snakes. Nature, 464(7291), 1006–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahm JH, Kim S, & Paik YK (2009). Endogenous cGMP regulates adult longevity via the insulin signaling pathway in Caenorhabditis elegans. Aging Cell, 8(4), 473–483. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Yamaguchi T, Sakakibara Y, Taguchi K, Maeda M, Kuzuya M, & Hattori Y (2014). ENOS-dependent antisenscence effect of a calcium channel blocker in human endothelial cells. PLoS ONE, 9(2), e88391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose M, Schilf P, Lange F, Mayer J, Reichart G, Maity P, … Ibrahim SM (2016). Uncoupling protein 2 protects mice from aging. Mitochondrion, 30, 42–50. [DOI] [PubMed] [Google Scholar]
- Holzenberger M, Dupont J, Ducos B, Leneuve P, Geloen A, Even PC, … Le Bouc Y (2003). IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature, 421(6919), 182–187. [DOI] [PubMed] [Google Scholar]
- Hou PF, Liu ZH, Li N, Cheng WJ, & Guo SW (2015). Knockdown of STIM1 improves neuronal survival after traumatic neuronal injury through regulating mGluR1-dependent Ca(2+) signaling in mouse cortical neurons. Cell Mol Neurobiol, 35(2), 283–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutter D, Yo Y, Chen W, Liu P, Holbrook NJ, Roth GS, & Liu Y (2000). Age-related decline in Ras/ERK mitogen-activated protein kinase cascade is linked to a reduced association between Shc and EGF receptor. J Gerontol Series A Biol Sci Med Sci, 55(3), B125–B134. [DOI] [PubMed] [Google Scholar]
- Iwasa H, Yu S, Xue J, & Driscoll M (2010). Novel EGF pathway regulators modulate C. elegans healthspan and lifespan via EGF receptor, PLC-gamma, and IP3R activation. Aging Cell, 9(4), 490–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon C, Chang J, Gensch E, Rudner A, &Tabtiang R (1993). A C-elegans mutant that lives twice as long as wild-type. Nature, 366(6454), 461–464. [DOI] [PubMed] [Google Scholar]
- Kenyon CJ (2010). The genetics of ageing. Nature, 464(7288), 504–512. [DOI] [PubMed] [Google Scholar]
- Kimura KD, Tissenbaum HA, Liu Y, & Ruvkun G (1997). Daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science, 277(5328), 942–946. [DOI] [PubMed] [Google Scholar]
- Kroemer G, Galluzzi L, & Brenner C (2007). Mitochondrial membrane permeabilization in cell death. Physioll Rev, 87(1), 99–163. [DOI] [PubMed] [Google Scholar]
- Krueger JN, Moore SJ, Parent R, McKinney BC, Lee A, & Murphy GG (2016). A novel mouse model of the aged brain: Over-expression of the L-type voltage-gated calcium channel Ca V1.3. Behav Brain Res. pii: S0166-4328(16)30418-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, … Nabeshima YI (1997). Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature, 390(6655), 45–51. [DOI] [PubMed] [Google Scholar]
- Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt KP, … Kuro-o M (2006). Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem, 281(10), 6120–6123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lans H, & Jansen G (2007). Multiple sensory G proteins in the olfactory, gustatory and nociceptive neurons modulate longevity in Caenorhabditis elegans. Dev Biol, 303(2), 474–482. [DOI] [PubMed] [Google Scholar]
- Lee BH, & Ashrafi K (2008). A TRPV channel modulates C. elegans neurosecretion, larval starvation survival, and adult lifespan. PLoS Genet, 4(10), e1000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, & Kenyon C (2009). Regulation of the longevity response to temperature by thermosensory neurons in Caenorhabditis elegans. Curr Biol, 19(9), 715–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiser SF, Miller H, Rossner R, Fletcher M, Leonard A, Primitivo M, … Kaeberlein M (2015). Cell nonautonomous activation of flavin-containing monooxygenase promotes longevity and health span. Science, 350(6266), 1375–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonov A, & Titorenko VI (2013). A network of interorganellar communications underlies cellular aging. IUBMB Life, 65(8), 665–674. [DOI] [PubMed] [Google Scholar]
- Li W, Gao B, Lee SM, Bennett K, & Fang D (2007). RLE-1, an E3 ubiquitin ligase, regulates C. elegans aging by catalyzing DAF-16 polyubiquitination. Dev Cell, 12(2), 235–246. [DOI] [PubMed] [Google Scholar]
- Li Y, Wang WJ, Cao HQ, Lu JH, Wu C, Hu FY, … Tian XL (2009). Genetic association of FOXO1A and FOXO3A with longevity trait in Han Chinese populations. Hum Mol Genet, 18(24), 4897–4904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libert S, Zwiener J, Chu X, Vanvoorhies W, Roman G, & Pletcher SD (2007). Regulation of Drosophila life span by olfaction and food-derived odors. Science, 315(5815), 1133–1137. [DOI] [PubMed] [Google Scholar]
- Lin YJ, Seroude L, & Benzer S (1998). Extended life-span and stress resistance in the Drosophila mutant methuselah. Science, 282(5390), 943–946. [DOI] [PubMed] [Google Scholar]
- Linford NJ, Kuo TH, Chan TP, & Pletcher SD (2011). Sensory perception and aging in model systems: From the outside in. Ann Rev Cell Dev Biol, 27, 759–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Rogers J, Murphy CT, & Rongo C (2011). EGF signalling activates the ubiquitin proteasome system to modulate C. elegans lifespan. EMBO J, 30(15), 2990–3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb J, & Northrop JH (1916). Is there a temperature coefficient for the duration of life? Proc Natl Acad Sci USA, 2, 456–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeski AE, & Dice JF (2004). Mechanisms of chaperone-mediated autophagy. Int J Biochem Cell Biol, 36(12), 2435–2444. [DOI] [PubMed] [Google Scholar]
- Marchese A, George SR, Kolakowski LF Jr., Lynch KR, & O'Dowd BF (1999). Novel GPCRs and their endogenous ligands: Expanding the boundaries of physiology and pharmacology. Trends Pharmacol Sci, 20(9), 370–375. [DOI] [PubMed] [Google Scholar]
- Mathie A (2010). Ion channels as novel therapeutic targets in the treatment of pain. J Pharm Pharmacol, 62(9), 1089–1095. [DOI] [PubMed] [Google Scholar]
- McGarrigle D, & Huang XY (2007). Methuselah antagonist extends life span. Nat Chem Biol, 3(7), 371–372. [DOI] [PubMed] [Google Scholar]
- Montell C, & Rubin GM (1989). Molecular characterization of the Drosophila trp locus: A putative integral membrane protein required for phototransduction. Neuron, 2(4), 1313–1323. [DOI] [PubMed] [Google Scholar]
- Morris BJ, Wilicoxa DC, Donlon TA, & Willcox BJ (2015). FOX O3: A major gene for human longevity—A mini-review. Gerontology, 61(6), 515–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino I, Fu J, Tanji K, Yamada T, Shimojo S, Koori T, … Hirano M (2000). Primary LAMP-2 deficiency causes X-linked vacuolar cardiomyopathy and myopathy (Danon disease). Nature, 406(6798), 906–910. [DOI] [PubMed] [Google Scholar]
- Nunez-Santana FL, Oh MM, Antion MD, Lee A, Hell JW, & Disterhoft JF (2014). Surface L-type Ca2+ channel expression levels are increased in aged hippocampus. Aging Cell, 13(1), 111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocorr K, Reeves NL, Wessells RJ, Fink M, Chen HS, Akasaka T, … Bodmer R (2007). KCNQ potassium channel mutations cause cardiac arrhythmias in Drosophila that mimic the effects of aging. Proc Natl Acad Sci USA, 104(10), 3943–3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi M, Nakatani T, Lanske B, & Razzaque MS (2009). In vivo genetic evidence for suppressing vascular and soft-tissue calcification through the reduction of serum phosphate levels, even in the presence of high serum calcium and 1,25-dihydroxyvitamin d levels. Circ Cardiovasc Genet, 2(6), 583–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JS, Kim YS, & Yoo MA (2009). The role of p38b MAPK in age-related modulation of intestinal stem cell proliferation and differentiation in Drosophila. Aging, 1(7), 637–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SK, Link CD, & Johnson TE (2010). Life-span extension by dietary restriction is mediated by NLP-7 signaling and coelomocyte endocytosis in C. elegans. FASEB J, 24(2), 383–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patapoutian A, Peier AM, Story GM, & Viswanath V (2003). ThermoTRP channels and beyond: Mechanisms of temperature sensation. Nat Rev Neurosci, 4(7), 529–539. [DOI] [PubMed] [Google Scholar]
- Pierce SB, Costa M, Wisotzkey R, Devadhar S, Homburger SA, Buchman AR, … Ruvkun G (2001). Regulation of DAF-2 receptor signaling by human insulin and ins-1, a member of the unusually large and diverse C. elegans insulin gene family. Genes Dev, 15(6), 672–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzaque MS (2009). The FGF23-Klotho axis: Endocrine regulation of phosphate homeostasis. Nat Rev Endocrinol, 5(11), 611–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard VR, Leonov A, Beach A, Burstein MT, Koupaki O, Gomez-Perez A, … Titorenko VI (2013). Macromitophagy is a longevity assurance process that in chronologically aging yeast limited in calorie supply sustains functional mitochondria and maintains cellular lipid homeostasis. Aging, 5(4), 234–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riera CE, Huising MO, Follett P, Leblanc M, Halloran J, Van Andel R, … Dillin A (2014). TRPV1 pain receptors regulate longevity and metabolism by neuropeptide signaling. Cell, 157(5), 1023–1036. [DOI] [PubMed] [Google Scholar]
- Riera CE, Merkwirth C, De Magalhaes Filho CD, & Dillin A (2016). Signaling networks determining life span. Annu Rev Biochem, 85,35–64. [DOI] [PubMed] [Google Scholar]
- Rodier F, Campisi J, & Bhaumik D (2007). Two faces of p53: Aging and tumor suppression. Nucleic Acids Res, 35(22), 7475–7484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose G, Crocco P, De Rango F, Montesanto A, & Passarino G (2011). Further support to the uncoupling-to-survive theory: The genetic variation of human UCP genes is associated with longevity. PLoS ONE, 6(12), e29650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousset S, Alves-Guerra MC, Mozo J, Miroux B, Cassard-Doulcier AM, Bouillaud F, & Ricquier D (2004). The biology of mitochondrial uncoupling proteins. Diabetes, 53(Suppl 1), S130–S135. [DOI] [PubMed] [Google Scholar]
- Roux AE, Leroux A, Alaamery MA, Hoffman CS, Chartrand P, Ferbeyre G, & Rokeach LA (2009). Pro-aging effects of glucose signaling through a G protein-coupled glucose receptor in fission yeast. PLoS Genet, 5(3), e1000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selman C, Lingard S, Choudhury AI, Batterham RL, Claret M, Clements M, … Withers DJ (2008). Evidence for lifespan extension and delayed age-related biomarkers in insulin receptor substrate 1 null mice. FASEB J, 22(3), 807–818. [DOI] [PubMed] [Google Scholar]
- Selman C, Partridge L, & Withers DJ (2011). Replication of extended lifespan phenotype in mice with deletion of insulin receptor substrate 1. PLoS ONE, 6(1), e16144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao LW, Niu R, & Liu Y (2016). Neuropeptide signals cell non-autonomous mitochondrial unfolded protein response. Cell Res, 26(11), 1182–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamura Y, Hamada K, Inoue K, Ogata K, Ishihara M, Kagawa T, … Terada Y (2012). Serum levels of soluble secreted alpha-klotho are decreased in the early stages of chronic kidney disease, making it a probable novel biomarker for early diagnosis. Clin Exp Nephrol, 16(5), 722–729. [DOI] [PubMed] [Google Scholar]
- Skaper SD (2011). Ion channels on microglia: Therapeutic targets for neuroprotection. Cns Neurol Disord-Dr, 10(1), 44–56. [DOI] [PubMed] [Google Scholar]
- Smith IF, Green KN, & LaFerla FM (2005). Calcium dysregulation in Alzheimer's disease: Recent advances gained from genetically modified animals. Cell Calcium, 38(3–4), 427–437. [DOI] [PubMed] [Google Scholar]
- Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, … Patapoutian A (2003). ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell, 112(6), 819–829. [DOI] [PubMed] [Google Scholar]
- Stubbs JR, Liu S, Tang W, Zhou J, Wang Y, Yao X, & Quarles LD (2007). Role of hyperphosphatemia and 1,25-dihydroxyvitamin D in vascular calcification and mortality in fibroblastic growth factor 23 null mice. J Am Soc Nephrol, 18(7), 2116–2124. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Guhde G, Suter A, Eskelinen EL, Hartmann D, Lullmann-Rauch R, … Saftig P (2000). Accumulation of autophagic vacuoles and cardiomyopathy in LAMP-2-deficient mice. Nature, 406(6798), 902–906. [DOI] [PubMed] [Google Scholar]
- Tandon A, & Fraser P (2002). The presenilins. Genome Biol, 3(11), reviews3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatar M, Kopelman A, Epstein D, Tu MP, Yin CM, & Garofalo RS (2001). A mutant Drosophila insulin receptor homolog that extends lifespan and impairs neuroendocrine function. Science, 292(5514), 107–110. [DOI] [PubMed] [Google Scholar]
- Tempel BL, Papazian DM, Schwarz TL, Jan YN, & Jan LY (1987). Sequence of a probable potassium channel component encoded at shaker locus of Drosophila. Science, 237(4816), 770–775. [DOI] [PubMed] [Google Scholar]
- Tobin DM, Madsen DM, Kahn-Kirby A, Peckol EL, Moulder G, Barstead R, … Bargmann CI (2002). Combinatorial expression of TRPV channel proteins defines their sensory functions and subcellular localization in C. elegans neurons. Neuron, 35(2), 307–318. [DOI] [PubMed] [Google Scholar]
- Tonelli M, Wiebe N, Culleton B, House A, Rabbat C, Fok M, … Garg AX (2006). Chronic kidney disease and mortality risk: A systematic review. J Am Soc Nephrol, 17(7), 2034–2047. [DOI] [PubMed] [Google Scholar]
- Troemel ER, Sagasti A, & Bargmann CI (1999). Lateral signaling mediated by axon contact and calcium entry regulates asymmetric odorant receptor expression in C. elegans. Cell, 99(4), 387–398. [DOI] [PubMed] [Google Scholar]
- Tu H, Nelson O, Bezprozvanny A, Wang Z, Lee SF, Hao YH, … Bezprozvanny I (2006). Presenilins form ER Ca2+ leak channels, a function disrupted by familial Alzheimer's disease-linked mutations. Cell, 126(5), 981–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, … Yamashita T (2006). Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature, 444(7120), 770–774. [DOI] [PubMed] [Google Scholar]
- vB Hjelmborg J, Iachine I, Skytthe A, Vaupel JW, McGue M, Koskenvuo M, … Christensen K (2006). Genetic influence on human lifespan and longevity. Hum Genet, 119(3), 312–321. [DOI] [PubMed] [Google Scholar]
- Venkatachalam K, & Montell C (2007). TRP channels. Annu Rev Biochem, 76, 387–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanath V, Story GM, Peier AM, Petrus MJ, Hwang SW, Patapoutian A, & Jegla T (2003). Ion channels-Opposite thermosensor in fruitfly and mouse. Nature, 423(6942), 822–823. [DOI] [PubMed] [Google Scholar]
- Wang M, Gamo NJ, Yang Y, Jin LE, Wang XJ, Laubach M, … Arnsten AF (2011). Neuronal basis of age-related working memory decline. Nature, 476(7359), 210–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterson MJ, Chung BY, Harvanek ZM, Ostojic I, Alcedo J, & Pletcher SD (2014). Water sensor ppk28 modulates Drosophila lifespan and physiology through AKH signaling. Proc Natl Acad Sci USA, 111(22), 8137–8142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilicox BJ, Donlon TA, He Q, Chen R, Grove JS, Yano K, … Curb JD (2008). FOXO3A genotype is strongly associated with human longevity. Proc Natl Acad Sci USA, 105(37), 13987–13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe MS, Xia W, Ostaszewski BL, Diehl TS, Kimberly WT, & Selkoe DJ (1999). Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and gamma-secretase activity. Nature, 398(6727), 513–517. [DOI] [PubMed] [Google Scholar]
- Xiao R, Liu J, & Xu XZ (2015). Thermosensation and longevity. J Comp Physiol A Neuroethol Sens Neural Behav Physiol, 201(9), 857–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao R, & Xu XZS (2009). Function and regulation of TRP family channels in C. elegans. Pflugers Arch, 458(5), 851–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao R, Zhang B, Dong YM, Gong JK, Xu T, Liu JF, & Xu XZS (2013). A genetic program promotes C. elegans longevity at cold temperatures via a thermosensitive TRP channel. Cell, 152(4), 806–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Bhat S, Terrillion CE, Smith RJ, Tonelli LH, & Gould TD (2015). Sex-dependent modulation of age-related cognitive decline by the L-type calcium channel gene Cacna1c (Cav 1.2). Eur J Neurosci, 42(8), 2499–2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelle KM, Lu B, Pyfrom SC, & Ben-Shahar Y (2013). The genetic architecture of degenerin/epithelial sodium channels in Drosophila. G3 (Bethesda), 3(3), 441–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Xiao R, Ronan EA, He YQ, Hsu AL, Liu JF, & Xu XZS (2015). Environmental temperature differentially modulates C. elegans longevity through a thermosensitive TRP channel. Cell Rep, 11(9), 1414–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]