Abstract
We have recently completed the largest GWAS on lung cancer including 29,266 cases and 56,450 controls of European descent. The goal of our study has been to integrate the complete GWAS results with a large-scale expression quantitative trait loci (eQTL) mapping study in human lung tissues (n = 1,038) to identify candidate causal genes for lung cancer. We performed transcriptome- wide association study (TWAS) for lung cancer overall, by histology (adenocarcinoma, squamous cell carcinoma and small cell lung cancer) and smoking subgroups (never- and ever-smokers). We performed replication analysis using lung data from the Genotype- Tissue Expression (GTEx) project. DNA damage assays were performed in human lung fibroblasts for selected TWAS genes. As expected, the main TWAS signal for all histological subtypes and ever-smokers was on chromosome 15q25. The gene most strongly associated with lung cancer at this locus using the TWAS approach was IREB2 (pTWAS = 1.09E-99), where lower predicted expression increased lung cancer risk. A new lung adenocarcinoma susceptibility locus was revealed on 9p13.3 and associated with higher predicted expression of AQP3 (pTWAS = 3.72E-6). Among the 45 previously described lung cancer GWAS loci, we mapped candidate target gene for 17 of them. The association AQP3-adenocarcinoma on 9p13.3 was replicated using GTEx (pTWAS = 6.55E-5). Consistent with the effect of risk alleles on gene expression levels, IREB2 knockdown and AQP3 overproduction promote endogenous DNA damage. These findings indicate genes whose expression in lung tissue directly influences lung cancer risk.
Keywords: GWAS, lung cancer, lung eQTL, transcriptome-wide association study
Introduction
Genome‐wide association studies (GWAS) to date have reported 45 lung cancer susceptibility loci in European and Asian populations.1 Identifying the causal genes underpinning these loci remains a major challenge. Expression quantitative trait loci (eQTL) in disease‐relevant tissues are known to complement GWAS results by providing the specific genes whose expression levels are associated with disease‐associated SNPs.2 This strategy has been applied in lung cancer by directly testing disease‐associated SNPs for association with expression levels of nearby genes in lung tissues.3
Recent development in bioinformatics now allows transcriptome‐wide association study (TWAS), which is a more advanced approach to integrate GWAS and eQTL results and identify candidate causal genes underlying diseases.4, 5 TWAS requires a set of individuals for whom both gene expression and genetic variants have been measured, that is, an eQTL dataset. The part of gene expression that can be explained by cis‐acting SNPs can then be modeled in the eQTL dataset and used to impute the genetic component of expression in a second (usually larger) set of individuals with only SNP GWAS data. The approach can be conceptualized as having imputed expression data for all cases and controls used in a GWAS without directly measuring expression levels in these samples. The association between imputed gene expression and the disease (or biological trait) of interest is then evaluated by performing a TWAS.
In our study, we combined the largest GWAS on lung cancer6 and the largest lung eQTL study7 to perform a TWAS on lung cancer, histological subtypes and smoking subgroups. The objective is to identify candidate target genes for lung cancer residing within and outside GWAS‐nominated loci.
Materials and Methods
Lung eQTL dataset
The lung eQTL dataset consists of whole‐genome genotyping (Illumina Human1M‐Duo BeadChip) and gene expression (Affymetrix) in nontumor lung tissues from patients who underwent lung surgery at three academic sites, Laval University, University of British Columbia and University of Groningen, henceforth referred to as Laval, UBC and Groningen, respectively. All lung specimens from Laval were obtained from patients undergoing lung cancer surgery and were harvested from a site distant from the tumor. At UBC, the majority of samples were from patients undergoing resection of small peripheral lung lesions. Additional samples were from autopsy and at the time of lung transplantation. At Groningen, the lung specimens were obtained at surgery from patients with various lung diseases, including patients undergoing therapeutic resection for lung tumors, harvested from a site distant from the tumor, and lung transplantation. Lung tissue processing and storage, DNA and RNA extraction, genotyping, microarray‐based gene expression and lung cis‐eQTL analyses have been described previously.7, 8 Following standard microarray and genotyping quality controls, data on 1,038 patients were available. At Laval and UBC, written informed consent was obtained from all subjects and the study was approved by their respective ethics committee. At Groningen, lung specimens were provided by the local tissue bank of the Department of Pathology and the study protocol was consistent with the Research Code of the University Medical Center Groningen and Dutch national ethical and professional guidelines (“Code of conduct; Dutch federation of biomedical scientific societies”; http://www.federa.org).
GWAS dataset
The GWAS data were derived from the Transdisciplinary Research in Cancer of Lung team of the International Lung Cancer Consortium (TRICL‐ILCCO) OncoArray project comprising 29,266 lung cancer cases and 56,450 controls of European ancestry based on OncoArray and other Illumina genome‐wide arrays.6 The GWAS was performed using logistic regression to evaluate the association of genetic variants with overall lung cancer and the predominant histological subtypes including adenocarcinoma (n = 11,273), squamous cell carcinoma (n = 7,426) and small cell lung cancer (SCLC; n = 2,664). Genetic variants were also tested for association in never‐ (n = 2,355) and ever‐smokers (n = 23,223). For our study, summary statistics were available for more than 10 million genotyped and imputed SNPs for overall lung cancer, histological subtypes and smoking subgroups (range 10,333,102–11,268,805). All participating studies in the TRICL‐ILCCO OncoArray project were approved by their local ethics committee and all participants signed an informed consent.
Transcriptome‐wide association study
The TWAS was performed for lung cancer overall, histological subtypes and smoking subgroups using two approaches, that is, S‐PrediXcan5 and FUSION.4 The lung eQTL dataset was used as the training set to derive the expression weights. Gene expression normalized for age, sex and smoking status from Laval, UBC and Groningen were combined with ComBat.9
For analysis with S‐PrediXcan, gene expression traits were first trained with elastic net linear models (alpha = 0.5, n_k_folds = 10, window = 500 Kb) using the lung eQTL set. Models with false‐discovery rate (FDR) < 0.05 as implemented in S‐PrediXcan were obtained for 19,889 probe sets. Predicted expression levels from the lung in the TRICL‐ILCCO OncoArray project were then tested for association with lung cancer.5
For analysis with FUSION, expression prediction models for each gene were evaluated in cis, using markers within 500 Kb on both sides of the expression probe sets. Probe sets that passed QC in the lung eQTL dataset (n = 41,738) were evaluated and significant cis‐heritability (p < 0.01) were observed for 12,587 annotated probe sets. The best performing prediction models implemented in FUSION were LASSO regression and elastic net regression (enet) for 8,254 and 4,333 probe sets, respectively. Once the expression weights were obtained, expression imputation was performed using the summary statistics from the TRICL‐ILCCO OncoArray project.
For both approaches, genome‐wide significant TWAS was considered at pTWAS < 0.05 based on Bonferroni correction (S‐PrediXcan pTWAS = 0.05/19,889 = 2.51E−6; FUSION pTWAS = 0.05/12,587 = 3.97E−6). A more liberal significant threshold was also used (pTWAS < 0.0001) to explore the top TWAS signals not reaching genome‐wide significance for some histological or smoking subgroups. Finally, the top TWAS genes in previously established lung cancer risk loci that showed some evidence of association (pTWAS < 0.05) with both S‐PrediXcan and FUSION were considered. For both TWAS approaches, we reported well‐annotated probe sets. LocusCompare10 was used to visualize GWAS and eQTL colocalization events.
Published GWAS loci for lung cancer
Lung cancer GWAS loci were derived from our recent review.1 The boundaries of each locus were defined by adding 1 Mb downstream and upstream of lung cancer‐associated SNPs derived from published GWAS on lung cancer. The genomic locations of TWAS genes were then overlapped with these lung cancer loci to delineate those residing within or outside GWAS loci.
TWAS replication
Lung eQTL data from 383 individuals available in the Genotype‐Tissue Expression (GTEx) project (GTEx, version 7)11 were used for TWAS replication. The TWAS was performed using S‐PrediXcan and FUSION as described above.
In vitro assays
Cell line, plasmids and reagents
MRC‐5V2 (male, SV40‐immortalized human lung fibroblasts, Research Resource Identifier (RRID): CVCL_2627, source: Stephen P. Jackson Lab) cell line was maintained in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Catalog #: 41965) supplemented with 10% fetal bovine serum (Gibco, Catalog #: 10438034), 2 mM L‐glutamine, 100 μg/ml penicillin, and 100 μg/ml streptomycin as previously described.12 The human cell line has been authenticated using STR profiling within the last 3 years and all experiments were performed with mycoplasma‐free cells. Gateway compatible AQP3 entry clone was obtained from ccsbBroad gene libraries (ccsbBroadEn_00089). We subcloned AQP3 into a mammalian expression vector containing a GFP epitope tag (pcDNA6.2/N‐EmGFP‐DEST, Invitrogen), which allows us to separate the transfected and nontransfected cell populations. Overproduction plasmids transfections were performed using GenJet (SignaGen, Catalog #: SL100488). SMARTpool IREB2 and NEXN siRNAs as well as nontargeting (NT) siRNA were purchased from Dharmacon. siRNA transfections were carried out with lipofectamine RNAiMax (Invitrogen #13778150) following the manufacturer’s instructions. Knockdown efficiency was evaluated by real‐time quantitative reverse transcription PCR (qRT‐PCR). RNeasy mini kit (Qiagen #74106) was used to extract from MRC‐5V2 cells that were transfected with siRNA for 72 hr. About 300 ng of total RNA from each sample was used to synthesize cDNA by the Superscript III first‐strand synthesis system (Invitrogen, #18080051). qPCR reactions were performed using iTaq Universal SYBR Green Supermix (BioRad #172‐5121). qPCR experiments were performed on the QuantStudio 3 Real‐Time PCR System (Applied Biosystems). For each gene, three replicates were analyzed and the average threshold cycle (Ct) was calculated. The relative expression levels were calculated with the 2−ΔΔCt method.13 Primers used included IREB2 forward: TCTTGGTATTACAAAGCACCTCAG; IREB2 reverse: TCACATTGTCAACAGGGAAAAAG; GADPH forward: CAATGACCCCTTCATTGACC; GADPH reverse: GATCTCGCTCCTGGAAGATG; NEXN forward: ACTGTGAAGGGTAGATTTGCTG; NEXN reverse: TTCTGCGTTTTCGTTCCTCCT. Knockdown efficiency was 88% for IREB2 and 95% for NEXN.
DNA damage assays by flow cytometry
Flow‐cytometric DNA damage assays and quantification signals were performed as previously described.12 Briefly, cells were fixed, permeabilized and stained with γH2AX antibody (Sigma, Catalog #05‐636), then samples were measured by a BD LSRFortessa flow cytometer and analyzed using FlowJo software. For overproduction experiments, cells with mock transfection were used to set the threshold gating to determine the percentage of GFP− and γH2AX− cells, with 0.5% of control cells gated as the damage threshold as previously validated. The DNA‐damage ratio caused by protein overproduction is defined by (Q2/Q3)/(Q1/Q4), where Q2 is the number of transfected damage‐positive cells; Q3 is the number of transfected damage‐negative cells; Q1 is the number of untransfected damage positive cells, and Q4 is the number of untransfected damage‐negative cells.
Results
Genes with cis‐genetic component of expression in the lung
A total of 1,038 individuals for whom both gene expression and genetic variants were measured (i.e., the lung eQTL dataset) were used to impute the cis genetic component of expression into the larger set of 29,266 cases and 56,450 controls from the TRICL‐ILCCO OncoArray project using their SNP genotype data (i.e., GWAS data). Integration of the lung eQTL and lung cancer GWAS was performed by two TWAS approaches, namely, S‐PrediXcan and FUSION. To be assessed by TWAS, a significant portion of gene expression had to be explained by SNPs. For S‐PrediXcan, expression prediction models were obtained for 19,889 probe sets. On average, SNPs explained 4.95% of the probe sets expression variance, including 62.2% of probe sets that showed a prediction performance (R2) of at least 0.01 (Supporting Information Fig. S1a). For FUSION, significant cis‐heritability was observed for 12,587 annotated probe sets. On average, SNPs explained 7.39% of the probe sets expression variance, including 80.4% of probe sets for which their expression variance is explained by more than 1% (Supporting Information Fig. S1b). Significant cis‐heritability was observed for 12,099 probe sets in both S‐PrediXcan and FUSION (Supporting Information Fig. S1c) and the expression variance explained by SNPs for these probe sets was tightly correlated between the two methods (Supporting Information Fig. 1d).
Overall lung cancer
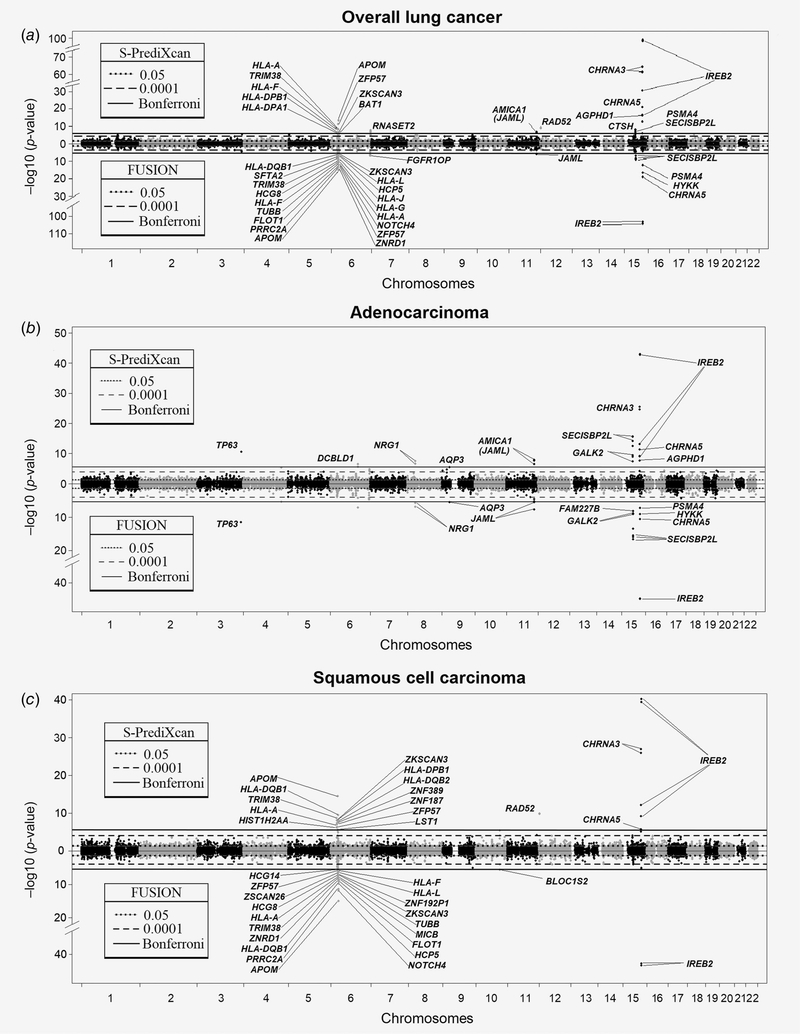
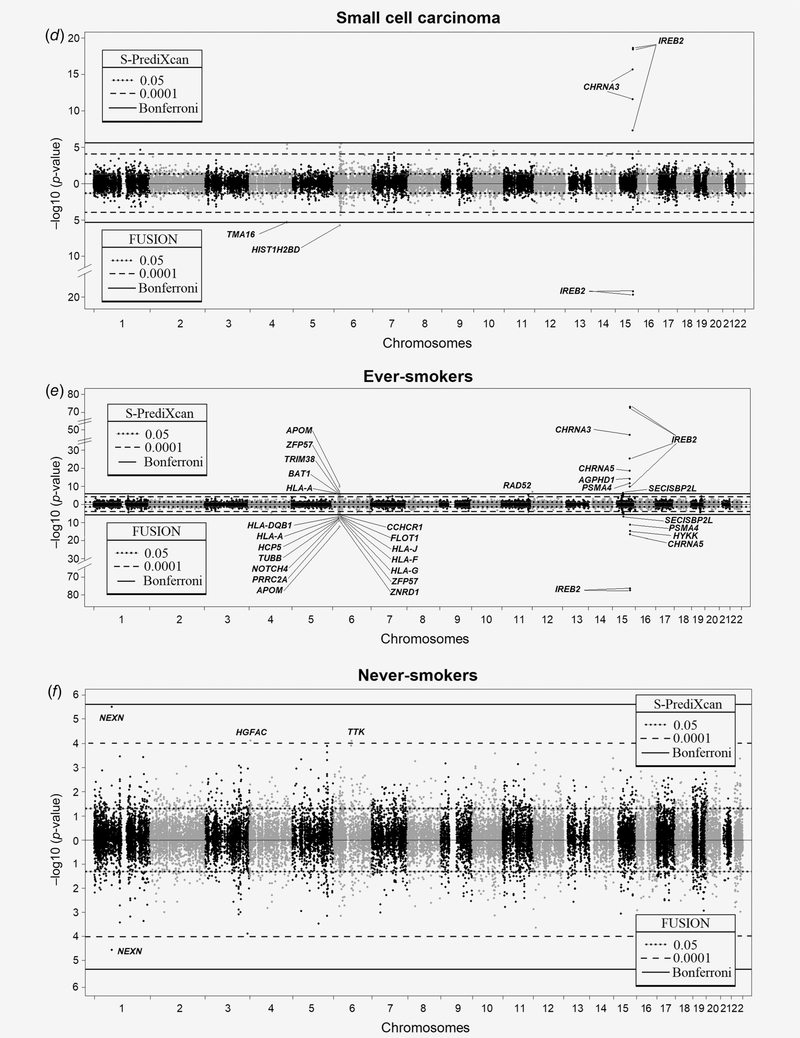
The TWAS results for overall lung cancer are illustrated in Figure 1a. TWAS genes that are statistically significant after Bonferroni correction are indicated in Table 1. The top TWAS signal is on chromosome 15q25, which is well‐established as the strongest lung cancer susceptibility locus derived from GWAS. Interestingly, IREB2 is the lead TWAS target gene on 15q25 by S‐PrediXcan (pTWAS = 1.09E−99). Other statistically significant TWAS genes include CHRNA3 (pTWAS = 4.66E−65), CHRNA5 (pTWAS = 6.01E−22), HYKK (pTWAS = 6.57E−17) and PSMA4 (pTWAS = 1.42E−13). In FUSION, IREB2 also has a level of significance stronger (pTWAS = 4.97E−104) than other significant TWAS genes at this locus including CHRNA5 (pTWAS = 5.26E−20), HYKK (pTWAS = 2.04E−17) and PSMA4 (pTWAS = 4.15E−13). Lower predicted expression of IREB2 is associated with increased lung cancer risk. Figure 2 shows the colocalization of the GWAS and lung eQTL signals on 15q25 as well as the effect of the top GWAS SNP on the expression of IREB2. LocusCompare plots show the colocalization events for IREB2 as well as other significant TWAS genes on 15q25 (Supporting Information Fig. S2). The lung cancer risk allele is associated with lowered expression of IREB2 in lung tissues.
Figure 1.
TWAS results for lung cancer overall, histological subtypes and smoking subgroups. Manhattan plots for S-PrediXcan (top) and FUSION (bottom) are illustrated in a mirror view to show similarities and differences between the two TWAS approaches. Each point represents a probe set with physical position plotted on the x-axis. The p values for gene expression-lung cancer associations are on the y- axis in -log10 scale. Annotations for the significant probe sets are indicated. (a) Overall lung cancer; (b) adenocarcinoma; (c) squamous cell carcinoma; (d) small cell lung cancer; (e) ever-smokers; and (f) never-smokers.
Table 1.
TWAS genes identified by lung cancer and smoking subgroups
| Bonferroni-corrected TWAS genes1 |
GTEx |
||||
|---|---|---|---|---|---|
| Subgroups | Loci | S-PrediXcan (direction) | FUSION (direction) | S-PrediXcan (direction, PTWAS) | FUSION (direction, PTWAS) |
| Overall lung cancer | 15q25 | IREB2 (−) > CHRNA3 (−) > CHRNA5 (−) > HYKK (−) > PSMA4 (+) | IREB2 (−) > CHRNA5 (−) > HYKK (−) > PSMA4 (+) |
IREB2 (no model) CHRNA3 (−, 1.33E −12) CHRNA5 (−, 1.70E −14) HYKK (no model) PSMA4 (no model) |
IREB2 (no model) CHRNA3 (−, 3.54E −23) CHRNA5 (−, 1.71 E −14) HYKK (no model) PSMA4 (no model) |
| 12p13.33 | RAD52 (+) | RAD52 (+, 2.10E-9) | RAD52 (+, 1.43E −09) | ||
| 15q21.1 | CTSH (+) > SECISBP2L (−) | SECISBP2L (−) | CTSH (+, 0.00015) SECISBP2L (−, 5.79E −8) | CTSH (+, 0.0001) SECISBP2L (−, 4.42E-08) | |
| 6p-MHC |
APOM (−) > ZFP57 (+) > HLA-A (−) > TRIM38 (−) > HLA-F (+) > ZKSCAN3 (−) > HLA-DPB1 (−) > BAT1 (−) > HLA-DPA1 (+) |
APOM (−) > ZNRD1 (−) > PRRC2A (−) > ZFP57 (+) > FLOU (+) > NOTCH4 (−) > TUBB (+) > HLA-A (−) > HLA-F (+) > HLA-G (−) > HCG8 (−) > HLA-J (−) > TRIM38 (−) > HCP5 (−) > SFTA2 (+) > HLA- L (−) > HLA-DQB1 (−) > ZKSCAN3 (−) > PSORS1C3 (−) > CCHCR1 (+) |
CCHCR1 (+, 5.08E-16)2 | FLOT1 (+, 6.05 E −16)2 | |
| 6q27 | RNASET2 (+) | FGFRIOP (−) | RNASET2 (+, 1.16E-6) FGFRIOP (no model) |
RNASET2 (+, 1.33E −07) FGFRIOP (−, 6.23E −04) |
|
| 11q23.3 | AMICA1/JAML (−) | JAML (−) | JAML (no model) | JAML (no model) | |
| Adenocarcinoma | 15q25 | IREB2 (−) > CHRNA3 (−) > CHRNA5 (−) > HYKK (−) | IREB2 (−) > CHRNA5 (−) > HYKK (−) > PSMA4 (+) |
IREB2 (no model) CHRNA3 (−, 1.96E −07) CHRNA5 (−, 1.23E-8) HYKK (no model) PSMA4 (no model) |
IREB2 (no model) CHRNA3 (−, 1.87E −12) CHRNA5 (−, 7.16E −09) HYKK (no model) PSMA4 (no model) |
| 15q21.1 | SECISBP2L (−) > GALK2 (+) | SECISBP2L (−) > GALK2 (+) > FAM227B (−) |
SECISBP2L (−, 2.57E −14) GALK2 (no model) FAM227B (+, 1.13E-7) |
SECISBP2L (−, 1.54E-14) GALK2 (no model) FAM227B (+, 8.53E −05) | |
| 3q28 | TP63 (−) | TP63 (−) | TP63 (−, 3.64E-6) | TP63 (no model) | |
| 11q23.3 | JAML (−) | JAML (−) | JAML (−, 0.158) | JAML (no model) | |
| 8p12 | NRG1 (−) | NRG1 (−) | NRG1 (−, 2.67E-6) | NRG1 (no model) | |
| 9p13.3 | AQP3 (+, PTWAS = 3.72E-6) | AQP3 (+) | AQP3 (+, 6.55E-5) | AQP3 (+, 1.72E-05) | |
| 6q22.1 | DCBLD1 (−) | DCBLD1 (−, 8.69E-7) | DCBLD1 (−, 4.63 E −07) | ||
| Squamous cell | 15q25 | IREB2 (−) > CHRNA3 (−) > CHRNA5 (−) | IREB2 (−) | IREB2 (no model) CHRNA3 (−, 0.00095) CHRNA5 (−, 0.00063) |
IREB2 (no model) CHRNA3 (−, 4.40E −07) CHRNA5 (−, 6.78E −04) |
| 6p-MHC |
APOM (−) > HLA-DQB1 (−) > TRIM38 (−) > ZKSCAN3 (−) > HLA-DPB1 (−) > HLA-DQB2 (−) > ZNF389 (−) > ZNF187 (−) HFA-A (−) > ZFP57 (+) > HIST1H2AA (−) > LST1 (+) |
APOM (−) > NOTCH4 (−) > PRRC2A (−) > HCP5 (−) > HLA-DQB1 (−) > FLOT1 (+) > ZNRD1 (−) > MICB (+) > TRIM38 (−) > TUBB (+) > HFA-A (−) > ZKSCAN3 (−) > HCG8 (−) > ZNF192P1 (−) > ZSCAN26 (−) > HLA-L (−) > ZFP57 (+) > HLA-F (+) > HCG14 (+) |
ATF6B (+, 3.70E-15)2 | C4A (−, 7.52E-15)2 | |
| 10q24.31 | BLOC1S2 (−) | BLOC1S2 (−, 4.99E-7) | BLOC1S2 (−, 8.36E −07) | ||
| 12p13.33 | RAD52 (+) | RAD52 (+, 1.22E-10) | RAD52 (+, 5.53E −12) | ||
| SCLC | 15q25 | IREB2 (−) > CHRNA3 (−) | IREB2 (−) | IREB2 (no model) CHRNA3 (−, 0.023) | IREB2 (no model) CHRNA3 (−, 3.51E −05) |
| 6p-MHC | HIST1H2BD (+) | HIST1H2BD (no model) | HIST1H2BD (no model) | ||
| 4q32.2 | TMA16 (+, PTWAS = 4.20E—6) | TMA16 (no model) | TMA16 (−, 9.38E −04) | ||
| Ever-smokers | 15q25 | IREB2 (−) > CHRNA3 (−) > CHRNA5 (−) > HYKK (−) > PSMA4 (+) | IREB2 (−) > CHRNA5 (−) > HYKK (−) > PSMA4 (+) |
IREB2 (no model) CHRNA3 (−, 9.19E −12) CHRNA5 (−, 1.36E −13) HYKK (no model) PSMA4 (no model) |
IREB2 (no model) CHRNA3 (−, 3.47E −20) CHRNA5 (−, 4.55E −13) HYKK (no model) PSMA4 (no model) |
| 6p-MHC | APOM (−) > ZFP57 (+) > TRIM38 (−) > BAT1 (−) > HFA-A (−) | APOM (−) > ZNRD1 (−) > PRRC2A (−) > ZFP57 (+) > NOTCH4 (−) > HLA-G (−) > TUBB (+) > HLA-F (+) > HCP5 (−) > HLA-J (−) > HLA-A (−) > FLOT1 (+) > HFA-DQB1 (−) > CCHCR1 (+) | CCHCR1 (+, 5.51 E-15)2 | FLOT1 (+, 1.38E −14)2 | |
| 15q21.1 | SECISBP2L (−) | SECISBP2L (−) | SECISBP2L (−, 8.73 E −7) | SECISBP2L (−, 9.70E-7) | |
| 12p13.33 | RAD52 (+) | RAD52 (+, 1.06E-9) | RAD52 (+, 2.23E −09) | ||
| Never-smokers | 1p31.1 | NEXN (−, PTWAS = 3.11E-6) | NEXN (−, PTWAS = 2.64E-5) | NEXN (−, 0.0059) | NEXN (−, 0.0028) |
Bold indicates new loci or new susceptibility genes. Novel loci are defined as not overlapping (±500 Kb) with a previously reported GWAS lung cancer locus.1 (+) and (−) indicate predicted gene expression positively or negatively associated with lung cancer risk.
Specific PTWAS values are provided for genes that did not pass the Bonferroni significance threshold.
Only the top TWAS gene is indicated for the MHC locus.
Figure 2.
IREB2 is the top candidate target gene on 15q25. The upper left panel shows the genetic associations with overall lung cancer in TRICL-ILCCO OncoArray. The bottom left panel shows the lung eQTL statistics for IREB2. The location of genes is illustrated at the bottom. The right panel shows boxplots of gene expression levels in the lung according to genotype groups for Laval, Groningen and UBC samples. The y- axis shows the mRNA expression levels. The x-axis shows the three genotype groups for the SNP most strongly associated with lung cancer (upper right) and the SNP most strongly associated with mRNA expression of IREB2 (lower right) with the number of individuals in parenthesis. The risk allele in TRICL-ILCCO OncoArray is shown in red. [Color figure can be viewed at wileyonlinelibrary.com]
Significant TWAS genes are also identified at two loci on chromosome 6. The most significant, and containing the largest number of TWAS genes, is the MHC locus, including 23 significant genes (Table 1). The top TWAS gene is APOM in both S‐PrediXcan and FUSION. In the MHC locus, lower predicted expression of 16 genes and higher predicted expression of seven genes are associated with increased lung cancer risk. The direction of effect is consistent for the six genes in common between S‐PrediXcan and FUSION (Supporting Information Fig. S3). The second locus on chromosome 6 (6q27) identifies RNASET2 and FGFR1OP as the TWAS gene in S‐PrediXcan (pTWAS = 2.33E−8) and FUSION (pTWAS = 7.68E−8), respectively.
Significant genes are observed at three additional loci. First, RAD52 on 12p13.33 (pTWAS = 6.58E−10) by S‐PrediXcan with higher predicted expression associated with higher lung cancer risk. Second, SECISBP2L on 15q21.1 by S‐PrediXcan (pTWAS = 5.44E−9) and FUSION (pTWAS = 8.01E−10), which we have recently identified as the candidate target gene.6 Third, JAML on 11q23.3 by S‐PrediXcan (pTWAS = 2.64E−7) and FUSION (pTWAS = 1.39E−6) with lower predicted expression associated with higher lung cancer risk.
Overall, TWAS genes are identified in six lung cancer susceptibility loci previously established by GWAS (Table 1). A potentially novel susceptibility gene is identified for 6q27‐FGFR1OP. For the other five loci, the TWAS results refined putative causal genes suspected by GWAS and demonstrated their direction of effects with lung cancer risk. LocusCompare plots for these TWAS hits are provided in Supporting Information Figure 4.
Histological subtypes
TWAS results by histological subtypes are shown in Figures 1b–1d and Table 1. IREB2 is the top TWAS gene for the three predominant subtypes, namely adenocarcinoma, squamous cell carcinoma and SCLC. Consistent with overall lung cancer, lower predicted expression is associated with increased risk of all histological subtypes.
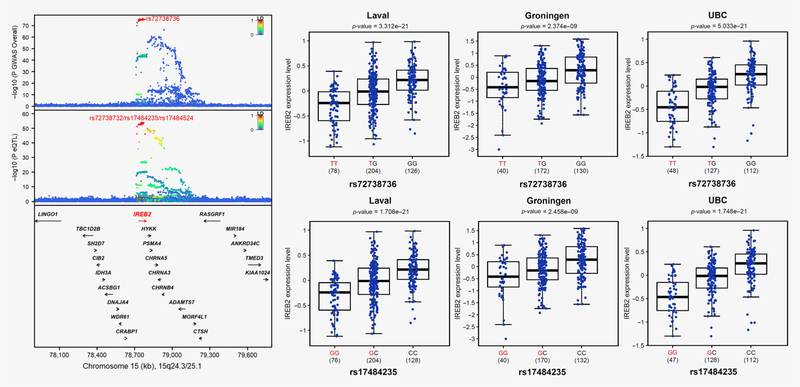
For adenocarcinoma, consistent results between S‐PrediXcan and FUSION are observed for NRG1 on 8p12 (S‐PrediXcan pTWAS = 3.29E−8, FUSION pTWAS = 1.21E−7) and AQP3 on 9p13.3 (S‐PrediXcan pTWAS = 3.72E−6, FUSION pTWAS = 3.49E−6). The latter is a new lung cancer susceptibility locus. Figure 3 and Supporting Information Figure S5 show the colocalization of the GWAS and lung eQTL signals on 9p13.3 as well as the effect of the top GWAS SNP on the expression of AQP3. The lung cancer risk allele is associated with higher expression of AQP3 in lung tissues. Additional TWAS genes for adenocarcinoma identified by S‐PrediXcan and FUSION include SECISBP2L on 15q21.1 (S‐PrediXcan pTWAS = 1.92E−16 and FUSION pTWAS = 2.50E−17), TP63 on 3q28 (S‐PrediXcan pTWAS = 2.50E−11 and FUSION pTWAS = 3.35E−12) and JAML on 11q23.3 (S‐PrediXcan pTWAS = 1.21E−8 and FUSION pTWAS = 2.09E−8). S‐PrediXcan identifies DCBLD1 on 6q22.1 (pTWAS = 3.59E−7). Lower predicted expression of all these genes (DCBLD1, TP63, SECISBP2L and JAML) is associated with increased risk of adenocarcinoma. All these loci were associated with lung cancer before. Interestingly, no significant TWAS gene in the MHC region was observed for adenocarcinoma.
Figure 3.
A novel susceptibility locus for adenocarcinoma on 9p13.3 with AQP3 as the underlying gene. The upper left panel shows the genetic associations with adenocarcinoma in TRICL-ILCCO OncoArray. The bottom left panel shows the lung eQTL statistics for AQP3. The location of genes is illustrated at the bottom. The right panel shows boxplots of gene expression levels in the lung according to genotype groups for Laval, Groningen, and UBC samples. The y-axis shows the mRNA expression levels. The x-axis shows the three genotype groups for the SNP most strongly associated with lung cancer (upper right) and the SNP most strongly associated with mRNA expression of AQP3 (lower right) with the number of individuals in parenthesis. The risk allele in TRICL-ILCCO OncoArray is shown in red.
For squamous cell carcinoma, the MHC region includes many TWAS genes (Fig. 1c and Table 1). Similar to results observed for overall lung cancer, the top TWAS gene using S‐PrediXcan and FUSION is APOM. There is one additional TWAS gene for squamous cell carcinoma by S‐PrediXcan on 12p13.33. The target gene is RAD52 (pTWAS = 1.24E−10) and the direction of effect indicates that higher expression is associated with an increased risk of squamous cell carcinoma. In FUSION, one more TWAS gene is identified for squamous cell carcinoma, namely, BLOC1S2 (pTWAS = 2.16E−6) on 10q24.31 with lower predicted expression associated with squamous cell carcinoma. BLOC1S2 is a new candidate causal gene for squamous cell carcinoma.
For SCLC, the only significant TWAS gene other than IREB2 and CHRNA3 on 15q25 was HIST1H2BD on 6p22.2 (MHC locus) by FUSION (pTWAS = 1.54E−6) with predicted expression positively associated with SCLC. A second TWAS gene that just missed genome‐wide significance is TMA16 (pTWAS = 4.2E−6) on 4q32.2, which is a locus not yet reported for lung cancer. Higher predicted expression of TMA16 is associated with higher risk of SCLC. S‐PrediXcan did not provide a significant gene expression model for TMA16.
Smoking subgroups
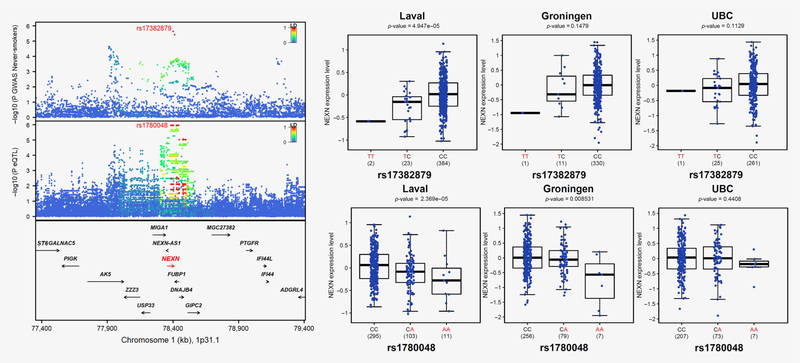
TWAS results for ever‐ and never‐smokers are in Figures 1e and 1f and Table 1. The TWAS in ever‐smokers parallel results observed for overall lung cancer, albeit at lower significance levels. This includes IREB2, CHRNA3 and CHRNA5 on 15q25, SECISBP2L on 15q21.1, RAD52 on 12p13.33 by S‐PrediXcan, and many genes in the MHC locus. The direction of effects is also consistent with overall lung cancer. For never‐smokers, no TWAS gene reach genome‐wide significance. One gene is identified using a more liberal significant threshold (pTWAS < 0.0001) using both TWAS approaches, namely, NEXN on 1p31.1 with predicted expression negatively associated with lung cancer in never‐smokers. Figure 4 shows the GWAS results for never‐smokers on 1p31.1 and lung eQTL signals for NEXN. Colocalization events can further be visualized in Supporting Information Figure S6. The lung cancer risk allele is associated with lower expression of NEXN in lung tissues. NEXN has never been reported as a lung cancer susceptibility gene.
Figure 4.
NEXN is the candidate target gene underpinning the lung cancer susceptibility locus for never-smokers on 1p31.1. The upper left panel shows the genetic associations with lung cancer in never-smokers in TRICL-ILCCO OncoArray. The bottom left panel shows the lung eQTL statistics for NEXN. The location of genes is illustrated at the bottom. The right panel shows boxplots of gene expression levels in the lung according to genotype groups for Laval, Groningen, and UBC samples. The y-axis shows the mRNA expression levels. The x-axis shows the three genotype groups for the SNP most strongly associated with lung cancer (upper right) and the SNP most strongly associated with mRNA expression of NEXN (lower right) with the number of individuals in parenthesis. The risk allele in TRICL-ILCCO OncoArray is shown in red. [Color figure can be viewed at wileyonlinelibrary.com]
Lung cancer risk loci from GWAS
We also explored the top TWAS genes in known lung cancer risk loci derived from previous GWAS. The boundaries of each locus were defined (see Methods) and the top TWAS genes by S‐PrediXcan and FUSION for overall lung cancer are indicated in Table 2. The top TWAS gene (pTWAS < 0.05) is consistent for both S‐PrediXcan and FUSION at six additional loci (not in Table 1): ORMDL1 on 2q32.2, SLC22A5 on 5q31, TRIM38 on 6p22.2, MTAP on 9p21.3, N4BP2L2 on 13q13.1 and MTMR3 on 22q12.2. Colocalization of GWAS and lung eQTL signals support ORMDL1, SLC22A5 and TRIM38 as candidate causal genes at these loci (Supporting Information Fig. S7). In contrast, the strongest lung eQTL variants for MTAP, N4BP2L2 and MTMR3 have weak GWAS p values (Supporting Information Fig. S7), suggesting the possibility of false‐positive TWAS genes and the need to use alternative approaches to find the causal genes at these loci. Overall, we map candidate causal genes for 17 out of the 45 known lung cancer GWAS loci. Supporting Information Figure S8 summarizes candidate target genes for lung cancer identified in our study residing within and outside GWAS‐nominated loci.
Table 2.
Top TWAS genes in GWAS-nominated loci for overall lung cancer
| Top TWAS genes (direction, PTWAS) |
Replication in GTEx (direction, PTWAS) |
||||
|---|---|---|---|---|---|
| GWAS loci | Suspected causal genes by GWAS alone1 | S-PrediXcan | FUSION | S-PrediXcan | FUSION |
| 1p36.32 | AJAP1, NPHP4 | NPHP4 (+, 0.146) | NPHP4 (+, 0.102) | NPHP4 (+, 0.379) | NPHP4 (+, 0.408) |
| 1p31.1 | FUBP, DNAJB4 | GIPC2 (+, 0.209) | PIGK (−, 0.037) | GIPC2 (+, 0.187) PIGKi−, 0.176) | GIPC2 (+, 0.194) PIGK (−, 0.2) |
| lq22 | MUC1, ADAM15, THBS3 | THBS3 (+, 1.15E-05) | DCST2 (+, 0.000172) | THBS3 (+, 1.69E-09) | THBS3 (+, 2.76E-05) |
| 2p16.3 | NRXN1 | ||||
| 2q32 | NUP35 | DUSP19 (−, 0.097) | NUP35 (+, 0.2349) | DUSP19 (−, 0.702) NUP35 (−, 0.458) | DUSP19 (ho model) NUP35 (−, 0.341) |
| 2q32.2 | HIBCH, INPP1, PMS1, STAT1 | ORMDL1 (−, 0.029) | ORMDL1 (−, 0.028) | ORMDL1 (−, 0.082) | ORMDL1 (−, 0.058) |
| 3p26 | No genes. Deletions associated with cancer | SUMF1 (+, 0.083) | SUMF1 (+, 0.0918) | SUMF1 (+, 0.095) | SUMF1 (+, 0.122) |
| 3q28 | TP63 | TP63 (−, 5.67E-06) | TP63 (−, 1.99E-5) | TP63 (−, 0.005) | TP63 (no model) |
| 3q29 | C3orf21 | CPN2 (+, 0.122) | No model | No model | |
| 4p15.2 | KCNIP4 | KCNIP4 (−, 0.137) | PACRGL (+, 0.057) | KCNIP4 (−, 0.293) PACRGL (+, 0.009) | KCNIP4 (no model) PACRGL (+, 0.0193) |
| 5p15 | TERT, CLPTM1L | SLC6A19 (+, 7.99E-06) | SLC6A3 (+, 0.0019) | SLC6A19 (ho model) SLC6A3 (+, 0.00068) | SLC6A19 (ho model) SLC6A3 (+, 0.00045) |
| 5q14.2 | XRCC4 | XRCC4 (+, 0.756) | XRCC4 (+, 0.59) | No model | No model |
| 5q31 | PAHA2, CSF2, IL3, SLC22A5, ACSL6 | SLC22A5 (−, 0.0047) | SLC22A5 (−, 0.005) | SLC22A5 (−, 0.025) | SLC22A5 (−, 0.018) |
| 5q32 | STK32A, PPP2R2B, DPYSL3 | STK32A (+, 0.069) | SPINK1 (−, 0.238) | STK32A (+, 0.569) SPINK1 (−, 0.524) | STK32A (+, 0.424) SPINK1 (−, 0.498) |
| 6p22.2 | HIST1H1E | TRIM38 (−, 5.07E-09) | TRIM38 (−, 5.32E-08) | No model | No model |
| 6p21 | BAGÓ, APOM, TNXB, MSH5, BTNL2, PRRC2A, FKBPL, HSPA1B, FOXP4, FOXP4-AS1, GTF2H4, LRFN2, HLA-A, HLA-DQB1 | APOM (−, 3.16E-14) | APOM (−, 9.29E-16) | APOM (−, 0.0011) | APOM (no model) |
| 6q22 | DCBLD1, ROS1 | DCBLD1 (−, 0.0019) | DCBLD1 (−, 0.0019) | DCBLD1 (−, 0.0109) | DCBLD1 (−, 0.00352) |
| 6q27 | RNASET2 | RNASET2 (+, 2.33E-08) | FGFR10P (−, 7.68E-08) | RNASET2 (+, 1.16E-06) FGFRIOP (no model) |
RNASET2 (+, 1.33E −07) FGFRIOP (−, 6.23E −04) |
| 7p15.3 | SP4, DNAH11 | FAM126A (−, 0.207) | No model | No model | |
| 8p21.1 | EPHX2, CHRNA2 | CLU (+, 1.78E-05) | CLU (−, 0.00672) | CLU (no model) | No model |
| 8p12 | NRG1 | NRG1 (−, 3.37E-05) | NRG1 (−, 9.69E-05) | NRG1 (−, 0.0003) | No model |
| 9p21.3 | CDKN2A, CDKN2B, CDKN2B-AS1, MTAP | MTAP (—, 0.0013) | MTAP (−, 0.0271) | No model | MTAP (−, 0.0178) |
| 10p14 | GATA3 | GATA3 (−,0.633) | No model | ||
| 10q23.33 | FFAR4 | HECTD2 (+, 0.026) | PPP1R3C (+, 0.364) | No model | No model |
| 10q24.3 | OBFC1 | LZTS2 (−, 0.056) | TMEM180 (+, 0.24) | LZTS2 (No model) TMEM180 (+, 0.193) | LZTS2 (No model) TMEM180 (+, 0.183) |
| 10q25.2 | VTI1A | VT11A (−, 0469) | VT11A (−, 0.34) | No model | No model |
| Ilq23.3 | MPZL3, JAML (also known asAMICA1) | JAML (−, 2.64E-07) | JAML (−, 1.39E-06) | JAML (−, 0.135) | No model |
| 12p13.33 | RAD52 | RAD52 (+, 6.58E-10) | RAD52 (+, 0.000363) | RAD52 (+, 2.10E-09) | RAD52 (+, 1.43E-09) |
| 12q13.13 | ACVR1B, NR4A1 | ACVR1B (+, 0.0199) | SLC11A2 (+, 0.0297) | ACVR1B (+, 0.039) SLC11A2 (no model) | ACVR1B (+, 0.186) SLC11A2 (no model) |
| 12q23.1 | NR1H4, SLC17A8 | ARL1 (+, 0.041) | GOLGA2P5 (+, 0.036) | ARL1 (+, 0.592) GOLGA2P5 (no model) | ARL1 (+, 0.155) GOLGA2P5 (no model) |
| 12q24 | SH2B3 | TMEM116 (+, 0.006) | ATXN2 (2, 0.019) | TMEM116 (−, 0.234) ATXN2 (No model) | TMEM116 (−,0.348) ATXN2 (No model) |
| 13q12.12 | Ml PEP, TNFRSF19 | MIPEP (−, 0.169) | MIPEP (−, 0.089) | MIPEP (−, 0.205) | MIPEP (−, 0.205) |
| 13q13.1 | BRCA2 | N4BP2L2 (−, 0.049) | N4BP2L2 (+, 0.027) | No model | No model |
| 13q31.3 | GPC5 | GPC5 (−,0.387) | MIR17HG (−, 0.947) | GPC5 (−, 0.173) MIR17HG (No model) | GPC5 (−.0.211) MIR17HG (No model) |
| 15q21.1 | SEMA6D, SECISBP2L | SECISBP2L (−, 5.44E −09) | SECISBP2L (−, 8.01 E −10) | SECISBP2L (−, 5.79E-8) | SECISBP2L (−, 4.42E −8) |
| 15q25 | CHRNA5, CHRNA3, CHRNB4, IREB2, PSMA4, HYKK | IREB2 (−, 1.09E-99) | IREB2 (−, 4.97E-104) | No model | No model |
| 17q24.3 | BPTF | BPTF (−, 0.019) | BPTF (−, 0.016) | No model | No model |
| 18p11.22 | FAM38B (also known as FAM38B2), APCDD1, NAPG | FAM38B2 (−, 0.167) | APCDD1 (−, 0.318) | No model | No model |
| 18q12.1 | GAREM | GALNT1 (−, 0.187) | GAREM (−, 0.00045) | No model | No model |
| 19q13.2 | TGFB1, CYP2A6 | ZNF565 (−, 0.005) | C19orf54 (−, 0.005) | ZNF565 (−, 0.02) C19orf54 (−, 3.44E −05) | ZNF565 (−, 0.006) C19orf54 (−, 2.89E −05) |
| 20q11.21 | B PI FBI | CPNE1 (−, 0.051) | PIGU (−, 0.027) | CPNE1 (−, 0.453) PIGU (No model) | CPNE1 (−, 0.41) PIGU (No model) |
| 20q13.2 | CYP24A1 | CBLN4 (−, 0.119) | CBLN4 (−, 0.09) | No model | No model |
| 20q13.33 | RTEL1 | RTEL1 (+, 0.007) | No model | No model | |
| 22q12.1 | CHEK2 | PIK3IP1 (+, 0.009) | XBP1 (+, 0.085) | PIK3IP1 (No model) XBP1 (+, 0.107) | PIK3IP1 No model) XBP1 (+, 0.047) |
| 22q12.2 | LIE, HORMAD2, MTMR3 | MTMR3 (+, 0.026) | MTMR3 (+, 0.025) | MTMR3 (+, 0.027) | MTMR3 (+, 0.031) |
Replication in GTEx
The lung eQTL data from 383 individuals available in GTEx was used to validate the results. We first evaluated the new adenocarcinoma locus on 9p13.3‐AQP3. The association AQP3‐adenocarcinoma is strongly validated in GTEx (S‐PrediXcan pTWAS = 6.55E−5 and FUSION pTWAS = 1.72E−5) with a consistent direction of effect, that is, the risk allele increases the expression levels of AQP3 in lung tissues. Second, we assessed NEXN as the new target gene underlying the 1p31.1 locus in never‐smokers. The association and direction of effect were replicated (S‐PrediXcan pTWAS = 0.006 and FUSION pTWAS = 0.003) with predicted expression negatively associated with lung cancer in never‐smokers.
We also compared candidate target genes identified in GWAS‐nominated loci. Note that replication of S‐PrediXcan and FUSION results in GTEx lung data is only feasible for genes with significant prediction models. The sample size available for building lung models in GTEx is smaller (n = 383) compared to our lung eQTL dataset (n = 1,038). Therefore, replication is not feasible for a fraction of genes in GTEx lung, that is, some genes will have no significant prediction model. This is the case for IREB2 on 15q25 that did not yield a prediction model in GTEx lung. The top TWAS gene on 15q25 for overall lung cancer in GTEx lung is CHRNA5 (pTWAS = 1.70E−14). Replication of all Bonferroni‐corrected TWAS genes by histology and smoking subgroups is indicated in Table 1. Excluding the 15q25 and 6p‐MHC loci, replication of TWAS genes was observed for 3 out of 4 for overall lung cancer, 6 out of 6 for adenocarcinoma, 2 out of 2 for squamous cell carcinoma, 1 out of 1 for SCLC, 2 out of 2 for ever‐smokers and 1 out of 1 for never‐smokers. Among the six additional loci showing the same top TWAS gene for both S‐PrediXcan and FUSION, 4 could be evaluated in GTEx and 3 were replicated: 5q31‐SLC22A5, 9p21.3‐MTAP and 22q12.2‐MTMR3 (Table 2). Overall, for the 17 TWAS genes located in the 45 GWAS‐nominated loci, 14 could be evaluated in GTEx and 12 were replicated.
Endogenous DNA damage assays
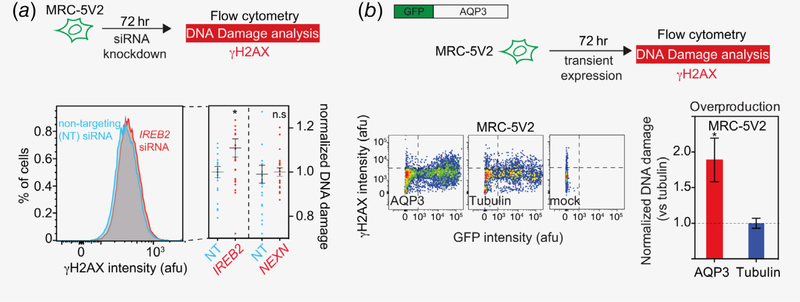
We hypothesized that some of the TWAS‐nominated genes might promote cancer by increasing endogenous DNA damage, and subsequently lead to genome instability. Three TWAS genes were selected for in vitro assays: IREB2 on 15q25, AQP3 on 9p13.3 and NEXN on 1p31.1. The choice between knockdown and overproduction assays was guided by the direction of effect observed in the TWAS. For IREB2 and NEXN, knockdown assays were performed to corroborate lower predicted expression associated with increased lung cancer risk, whereas overproduction assays were performed for AQP3 to mimic higher predicted expression associated with increased risk of lung cancer. We discovered that knockdown of IREB2 increased endogenous DNA damage in human lung fibroblasts (Fig. 5). In contrast, knockdown of NEXN had no effect on DNA damage. For AQP3, overproduction promotes endogenous DNA damage in lung fibroblasts (Fig. 5).
Figure 5.
IREB2 knockdown and AQP3 overproduction promote endogenous DNA damage. (a) Knocking down IREB2, but not NEXN by siRNA causes increased γH2AX level in MRC-5V2 cell line. Upper: scheme for a siRNA DNA damage assay. Lower left: representative flow cytometric histogram showing increased γH2AX in cells with IREB2 knockdown compared to cells that were transfected with nontargeting siRNA. Lower right, a summary of at least three independent experiments for both IREB2 and NEXN, n > 20. Mean fluorescence intensity of each knockdown experiment was normalized to its corresponding nontargeting siRNA control. Error bar: SEM. (b) AQP3 overproduction increases endogenous γH2AX levels in MRC-5V2. Upper: full-length sequence-verified AQP3 fused with N-terminal GFP fusion (and GFP-Tubulin as a control) were transiently overproduced in MRC-5V2 cell line and the DNA damage levels for both nongreen and green cells were quantified by flow cytometry (details see Methods). Lower left: representative flow cytometric histograms of GFP-AQP3, GFP-Tubulin, and mock transfection. Lower right: summary, mean ± SEM, n ≥ 8.
Discussion
Our study is the largest lung tissue based TWAS on lung cancer; gene expression prediction models built with a lung eQTL dataset of 1,038 individuals and association analyses of predicted gene expression with lung cancer risk using summary statistics derived from a GWAS on 29,266 cases and 56,450 controls. We revealed a new lung adenocarcinoma locus on 9p13.3 associated with the expression levels of AQP3 in lung tissues. We also identified candidate causal genes at GWAS‐nominated lung cancer loci including IREB2 on 15q25 for all histological subtypes. Cellular DNA damage assays further supported the potential causality of lower predicted expression of IREB2 and higher predicted expression of AQP3 in increasing the risk of lung cancer. Overall, we mapped putative causal genes for 17 out of the 45 known lung cancer risk loci derived from GWAS.
During the last 10 years, GWAS have identified 45 susceptibility loci for lung cancer.1 The genes underlying these genetic associations are largely unknown. As with other complex diseases, the GWAS risk variants for lung cancer are mostly located in noncoding regions and are thus believed to mediate their effects by influencing gene expression of nearby genes. In our study, we used a TWAS approach that captures the aggregate effects of multiple SNPs on gene expression and then tested the association of genetically predicted gene expression and disease risk. As a gene‐based strategy, TWAS has the ability to identify the most likely target genes residing within GWAS‐nominated loci, and also to reveal novel risk loci by the resulting power of combining GWAS and eQTL results. In our study, TWAS was performed using two competing approaches, that is, S‐PrediXcan and FUSION. Both belong to the same family of methods to discover gene‐trait associations using models trained in eQTL datasets and summary‐level GWAS data. The difference lies in the prediction models, that is, S‐PrediXcan uses elastic net (enet), while FUSION evaluates different prediction schemes (herein: enet, LASSO, top1) and selects the best performing model. Using default parameters, we obtained more expression prediction models in S‐PrediXcan compared to FUSION (19,889 vs. 12,587 probe sets with significant cis‐heritability). However, the prediction performance of the 12,099 probe sets in common between S‐PrediXcan and FUSION were tightly correlated, even when different prediction models (enet vs LASSO) were used (Supporting Information Fig. S1D).
The majority of TWAS genes identified in our study lie around known GWAS loci. The only SNP‐level subgenome‐wide significant locus that yields genome‐wide significant results by TWAS is 9p13.3‐AQP3 for adenocarcinoma. This novel susceptibility locus for adenocarcinoma (9p13.3‐AQP3) was observed in S‐PrediXcan and FUSION, and was also replicated in GTEx lung (Table 1). The direction of effect indicates that higher AQP3 expression is associated with an increased risk of lung adenocarcinoma. AQP3 (aquaporin 3) encodes a water channel protein that is expressed in the normal respiratory track and upregulated in NSCLC, especially adenocarcinoma.14, 15 Knockdown of AQP3 has been shown to suppress proliferation and invasion of lung cancer cells16, 17 as well as to inhibit tumor growth in human NSCLC xenografts.18 The direction of effect observed in our study is thus concordant with these functional studies. In the current study, we further demonstrated that AQP3 overproduction promotes endogenous DNA damage in human lung fibroblasts. All together these observations support AQP3 as the causal gene for lung adenocarcinoma on 9p13.3. The genetic association between AQP3 and lung adenocarcinoma will require further validation.
Novel susceptibility genes were identified in previously established GWAS loci. In never‐smokers, we have identified NEXN (nexilin F‐actin binding protein) as the putative causal gene on 1p31.1. Nexilin is an actin‐binding protein known to play a role in cell adhesion and migration. Mutations in this gene have been associated with cardiomyopathy.19, 20 The 1p31.1 locus was first demonstrated to be associated with lung cancer as part of a genome‐wide investigation of SNPs within all long noncoding RNA (lncRNA) genes.21 SNP rs114020893 located in lncRNA NEXN‐AS1 was associated with lung cancer and with a similar association between adenocarcinoma and squamous cell carcinoma subgroups. In silico analysis then predicted that rs114020893 could change the folding structure of NEXN‐AS1. However, it was unclear if the lung cancer‐associated SNP was acting through NEXN‐AS1 or by regulating the expression of its corresponding gene, NEXN. Our current study supports the later. In this lncRNA study,21 analyses by smoking subgroups were not performed. In McKay et al.,6 the 1p31.1 locus was GWAS significant for overall lung cancer as well as for adenocarcinoma and ever‐smoker subgroups but did not reach significance in never‐smokers. By using a TWAS approach, we demonstrated that this locus might also be relevant for the development of lung cancer in never‐smokers and, at least in this subgroup, the susceptibility locus may mediate its effect by down‐regulating the expression of NEXN in lung tissues. Interestingly, a recent study demonstrated that the expression levels of NEXN‐AS1 and NEXN are decreased in human atherosclerotic plaques and NEXN deficiency promotes atherosclerosis in an experimental mouse model.22NEXN seems to confer protection against atherosclerosis by suppressing inflammatory cytokines (IL‐6 and TNFα), adhesion molecules (ICAM1, VCAM1 and MCP1) and extracellular matrix‐degrading enzymes (MMP1 and MMP9). The control exerted by NEXN on these molecular processes may also come into play in lung cancer. Here we showed that NEXN knockdown lung fibroblasts do not show altered endogenous DNA damage, implying the need for investigating alternative mechanisms of action in future functional studies.
15q25 is the locus most strongly associated with lung cancer,1 but also a leading susceptibility locus for smoking behavior23 and other traits related to lung disease such as chronic obstructive pulmonary disease (COPD).24 COPD and lung cancer‐associated variants in 15q25 are known expression and methylation QTL (eQTL and meQTL) for multiple genes and tissues.3, 25 It has not been possible so far to definitely identify all of the causal gene(s) at this locus, but most evidence points toward CHRNA5 (cholinergic receptor nicotinic alpha 5 subunit) or IREB2 (iron‐responsive element binding protein 2). In our study, we focused specifically on gene expression in lung tissues with the hope to identify genes directly involved in lung cancer development. More than one Bonferroni‐corrected TWAS gene were identified at 15q25. The top one was IREB2, and then in order of significance, CHRNA3, CHRNA5, HYKK and PSMA4. IREB2 was also the top significant TWAS gene at this locus for COPD,8 and the results are in line with previous analysis specifically focused on 15q25.26IREB2 encodes a RNA‐binding protein that regulates iron levels in cells. Alteration of iron metabolism has been observed in NSCLC27 and iron has been shown to influence apoptosis of lung cancer cells (A549).28 Silencing of IREB2 in these cells has been shown to modulate the expression of iron metabolism‐related genes (transferrin receptor and ferritin)29 and injection of wild‐type IREB2 in mice was shown to stimulate growth of tumor xenografts.30 Previous studies have thus demonstrated a potential biological link between IREB2 and lung cancer. In the current study, knockdown of IREB2 was shown to increase endogenous DNA damage in human lung fibroblasts, supporting a potential cancer‐promoting role in the lung by elevated DNA damage and genomic instability. However, the 15q25 locus harbors additional candidate genes including three nicotinic receptors, namely, CHRNA3, CHRNA5 and CHRNB4. Variation in these genes have been strongly associated with smoking behavior and other aspects of addiction, thus indirectly affecting lung cancer risk through modulation of smoking behavior.31 It should be emphasized that our study is relevant for lung expression only and that causal genes of addiction to smoking on 15q25 may be complemented by future brain eQTL studies. Similarly, other forms of genetic variation may be modulating function at this locus, for example, one most associated SNP at this locus encodes a missense change in CHRNA5 (rs16969968). Our results nevertheless suggest the possibility that one or more genes acting in the lung, brain or other tissues may mediate the risk of lung cancer on 15q25. The IREB2 locus shows linkage disequilibrium with the CHRNA3/CHRNA5/CHRNB4 locus complicating our ability to distinguish between these genetic effects. Clearly, more research will be needed to pinpoint the causal gene(s) or pathway(s) underpinning this lung cancer susceptibility locus.
On 6p22‐p21 (MHC locus), multiple candidate causal genes were identified for overall lung cancer, squamous cell carcinoma and ever‐smokers. However, no TWAS gene was found for adenocarcinoma, which is consistent with previous GWAS showing stronger association with squamous cell carcinoma at the MHC locus.6, 32 The interpretation of TWAS results in the MHC locus is complicated by the extended LD structure in this region. TWAS cannot distinguish causal relationship and pleiotropy. For example, if the same SNPs affect the expression level of more than one gene, TWAS cannot delineate the causal one. Here, we identified multiple candidate genes on 6p22‐p21 that varied by histological subtypes and that showed some similarity, but also differences between S‐PrediXcan and FUSION. Although the top TWAS gene with both TWAS approaches was APOM, our study does not provide firm conclusion about the most likely causal gene(s) in the MHC locus and suggests the need of using alternative methods to reach this goal in this region.
It should be emphasized that TWAS genes do not imply causality. TWAS genes are more appropriately interpreted as prioritized or ranked candidate causal genes at loci.33 In addition, TWAS cannot distinguish causal relationship and pleiotropy. For example, if the same SNPs affect the expression level of more than one gene, TWAS cannot delineate the causal one. In our study, we intentionally highlighted the top TWAS finding at each locus. It is not uncommon to observe multiple TWAS genes per locus, which is caused by co‐regulation and shared eQTL.34 Further functional experiments will be needed to demonstrate causality of one or more genes at each locus. One of the main limitation of TWAS is to study only genes with a significant cis‐heritability, that is, genes for which a part of expression can be explained by SNPs. This leaves out a large proportion of genes including known and potential cancer genes, particularly variants that influence gene product function through other ways. On the other hand, by focusing on the genetic component of expression, we avoid confounding effects of other factors (measured or not) on gene expression. This however does not preclude confounders of the SNP‐expression correlation derived from the lung eQTL mapping study. We have used bulk gene expression data from the lung in both the discovery and validation (GTEx) sets. The lung is a heterogeneous tissue containing many cell types (organ‐specific and migratory) with relative proportions that can vary based on the underlying lung disease, harvesting location, histological subtypes and environmental factors.35 These factors may have limited our ability to derive lung eQTL signals and subsequently study by TWAS the association between the cis‐genetic component of expression and lung cancer. In addition, with our approach, we were unable to identify cell type‐specific eQTL signals including from rare (or less frequent) cell types that may give rise to cancer that are not well represented in bulk expression data.
In conclusion, this work outlines a new lung adenocarcinoma locus on 9p13.3 with AQP3 as the most likely underlying causal gene. Within known lung cancer GWAS loci, we map IREB2 on 15q25 for all histological subtypes and ever‐smokers, NEXN on 1p31.1 in never‐smokers and provide putative causal genes for 15 additional loci. The cancer‐promoting role of IREB2 and AQP3 were further supported by endogenous DNA damage assays in human lung fibroblasts. TWAS genes are key to understand disease etiology, facilitate biological interpretation of GWAS results, and prioritize follow‐up functional studies.
Supplementary Material
Acknowledgements
The authors would like to thank the teams at the IUCPQ site of the Respiratory Health Network (RHN) Tissue Bank of the FRQS and the Biomedical Telematics Laboratory for their valuable assistance. Yohan Bossé holds a Canada Research Chair in Genomics of Heart and Lung Diseases. Our study was supported by grants from the Chaire de Pneumologie de la Fondation JD Bégin de l’Université Laval, the Fondation de l’Institut universitaire de cardiologie et de pneumologie de Québec and the Canadian Institutes of Health Research (MOP‐123369) to Y.B. and Institut National du Cancer (France) to J.M. (TABAC 17‐022). CARET is funded by the National Cancer Institute, National Institutes of Health through grants U01‐CA063673, UM1‐CA167462 and U01‐CA167462. The genetic data was supported by NIH U19 CA148127 and U19 CA203654. The in vitro assays were supported by National Institutes of Health (NIH) Director’s Pioneer Award DP1‐CA174424 and R35‐GM122598 (to S.M.R.); the WM Keck Foundation (to S.M.R.); the BCM Cytometry and Cell Sorting Core with funding from the NIH P30‐AI036211, P30‐CA125123 and S10‐RR024574.
References
- 1.Bossé Y, Amos CI. A decade of GWAS results in lung cancer. Cancer Epidemiol Biomarkers Prev 2018; 27: 363–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bossé Y Genome‐wide expression quantitative trait loci analysis in asthma. Curr Opin Allergy Clin Immunol 2013; 13: 487–94. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen JD, Lamontagne M, Couture C, et al. Susceptibility loci for lung cancer are associated with mRNA levels of nearby genes in the lung. Carcinogenesis 2014; 35: 2653–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gusev A, Ko A, Shi H, et al. Integrative approaches for large‐scale transcriptome‐wide association studies. Nat Genet 2016; 48: 245–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbeira AN, Dickinson SP, Bonazzola R, et al. Exploring the phenotypic consequences of tissue specific gene expression variation inferred from GWAS summary statistics. Nat Commun 2018; 9: 1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McKay JD, Hung RJ, Han Y, et al. Large‐scale association analysis identifies new lung cancer susceptibility loci and heterogeneity in genetic susceptibility across histological subtypes. Nat Genet 2017; 49: 1126–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hao K, Bossé Y, Nickle DC, et al. Lung eQTLs to help reveal the molecular underpinnings of asthma. PLoS Genet 2012; 8:e1003029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lamontagne M, Berube JC, Obeidat M, et al. Leveraging lung tissue transcriptome to uncover candidate causal genes in COPD genetic associations. Hum Mol Genet 2018; 27: 1819–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson WE, Li C, Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics 2007; 8: 118–27. [DOI] [PubMed] [Google Scholar]
- 10.Liu B, Gloudemans MJ, Rao AS, et al. Abundant associations with gene expression complicate GWAS follow‐up. Nat Genet 2019; 51: 768–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Consortium GTEx. Genetic effects on gene expression across human tissues. Nature 2017; 550: 204–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xia J, Chiu LY, Nehring RB, et al. Bacteria‐to‐human protein networks reveal origins of endogenous DNA damage. Cell 2019; 176: 127–43.e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real‐time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 2001; 25: 402–8. [DOI] [PubMed] [Google Scholar]
- 14.Liu YL, Matsuzaki T, Nakazawa T, et al. Expression of aquaporin 3 (AQP3) in normal and neoplastic lung tissues. Hum Pathol 2007; 38: 171–8. [DOI] [PubMed] [Google Scholar]
- 15.Hanada S, Maeshima A, Matsuno Y, et al. Expression profile of early lung adenocarcinoma: identification of MRP3 as a molecular marker for early progression. J Pathol 2008; 216: 75–82. [DOI] [PubMed] [Google Scholar]
- 16.Xiong G, Chen X, Zhang Q, et al. RNA interference influenced the proliferation and invasion of XWLC‐05 lung cancer cells through inhibiting aquaporin 3. Biochem Biophys Res Commun 2017; 485: 627–34. [DOI] [PubMed] [Google Scholar]
- 17.Hou SY, Li YP, Wang JH, et al. Aquaporin‐3 inhibition reduces the growth of NSCLC cells induced by hypoxia. Cell Physiol Biochem 2016; 38: 129–40. [DOI] [PubMed] [Google Scholar]
- 18.Xia H, Ma YF, Yu CH, et al. Aquaporin 3 knockdown suppresses tumour growth and angiogenesis in experimental non‐small cell lung cancer. Exp Physiol 2014; 99: 974–84. [DOI] [PubMed] [Google Scholar]
- 19.Hassel D, Dahme T, Erdmann J, et al. Nexilin mutations destabilize cardiac Z‐disks and lead to dilated cardiomyopathy. Nat Med 2009; 15: 1281–8. [DOI] [PubMed] [Google Scholar]
- 20.Wang H, Li Z, Wang J, et al. Mutations in NEXN, a Z‐disc gene, are associated with hypertrophic cardiomyopathy. Am J Hum Genet 2010; 87: 687–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yuan H, Liu H, Liu Z, et al. A novel genetic variant in long non‐coding RNA gene NEXN‐AS1 is associated with risk of lung cancer. Sci Rep 2016; 6: 34234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu YW, Guo FX, Xu YJ, et al. Long noncoding RNA NEXN‐AS1 mitigates atherosclerosis by regulating the Actin‐binding protein NEXN. J Clin Invest 2019; 129: 1115–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tobacco Genetics Consortium. Genome‐wide meta‐analyses identify multiple loci associated with smoking behavior. Nat Genet 2010; 42: 441–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakornsakolpat P, Prokopenko D, Lamontagne M, et al. Genetic landscape of chronic obstructive pulmonary disease identifies heterogeneous cell‐type and phenotype associations. Nat Genet 2019; 51: 494–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nedeljkovic I, Carnero‐Montoro E, Lahousse L, et al. Understanding the role of the chromosome 15q25.1 in COPD through epigenetics and transcriptomics. Eur J Hum Genet 2018; 26: 709–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fehringer G, Liu G, Pintilie M, et al. Association of the 15q25 and 5p15 lung cancer susceptibility regions with gene expression in lung tumor tissue. Cancer Epidemiol Biomarkers Prev 2012; 21: 1097–104. [DOI] [PubMed] [Google Scholar]
- 27.Kukulj S, Jaganjac M, Boranic M, et al. Altered iron metabolism, inflammation, transferrin receptors, and ferritin expression in non‐small‐cell lung cancer. Med Oncol 2010; 27: 268–77. [DOI] [PubMed] [Google Scholar]
- 28.Choi SJ, Oh JM, Choy JH. Toxicological effects of inorganic nanoparticles on human lung cancer A549 cells. J Inorg Biochem 2009; 103: 463–71. [DOI] [PubMed] [Google Scholar]
- 29.Cheng Z, Dai LL, Song YN, et al. Regulatory effect of iron regulatory protein‐2 on iron metabolism in lung cancer. Genet Mol Res 2014; 13: 5514–22. [DOI] [PubMed] [Google Scholar]
- 30.Maffettone C, Chen G, Drozdov I, et al. Tumorigenic properties of iron regulatory protein 2 (IRP2) mediated by its specific 73‐amino acids insert. PLoS One 2010; 5:e10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen LS, Baker TB, Piper ME, et al. Interplay of genetic risk factors (CHRNA5‐CHRNA3‐CHRNB4) and cessation treatments in smoking cessation success. Am J Psychiatry 2012; 169: 735–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Timofeeva MN, Hung RJ, Rafnar T, et al. Influence of common genetic variation on lung cancer risk: meta‐analysis of 14 900 cases and 29 485 controls. Hum Mol Genet 2012; 21: 4980–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wainberg M, Sinnott‐Armstrong N, Mancuso N, et al. Opportunities and challenges for transcriptome‐wide association studies. Nat Genet 2019; 51: 592–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gusev A, Mancuso N, Won H, et al. Transcriptome‐wide association study of schizophrenia and chromatin activity yields mechanistic disease insights. Nat Genet 2018; 50: 538–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCall MN, Illei PB, Halushka MK. Complex sources of variation in tissue expression data: analysis of the GTEx lung transcriptome. Am J Hum Genet 2016; 99: 624–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.