Abstract
The Endocrine Disruptor Screening Program (EDSP) is transitioning from traditional testing methods to integrating ToxCast/Tox21 in vitro high-throughput screening assays for identifying chemicals with endocrine bioactivity. The ToxCast high-throughput H295R steroidogenesis assay may potentially replace the lowthroughput assays currently used in the EDSP Tier 1 battery to detect chemicals that alter the synthesis of androgens and estrogens. Herein, we describe an approach for identifying in vitro candidate reference chemicals that affect the production of androgens and estrogens in models of steroidogenesis. Candidate reference chemicals were identified from a review of H295R and gonad-derived in vitro assays used in methods validation and published in the scientific literature. A total of 29 chemicals affecting androgens and estrogen levels satisfied all criteria for positive reference chemicals, while an additional set of 21 and 15 chemicals partially fulfilled criteria for positive reference chemicals for androgens and estrogens, respectively. The identified chemicals included pesticides, pharmaceuticals, industrial and naturally-occurring chemicals with the capability to increase or decrease the levels of the sex hormones in vitro. Additionally, 14 and 15 compounds were identified as potential negative reference chemicals for effects on androgens and estrogens, respectively. These candidate reference chemicals will be informative for performance-based validation of in vitro steroidogenesis models.
Keywords: steroidogenesis, androgens, estrogens, H295R, gonadal models, sex hormones
1. Introduction
The U.S Environmental Protection Agency’s (EPA) Endocrine Disruptor Screening Program (EDSP) was established to identify potential endocrine bioactivity of pesticides and chemicals found in sources of drinking water (U.S.EPA, 1998). The EDSP is focused on identifying chemicals perturbing the estrogen, androgen, and thyroid hormone pathways, following a two-tiered approach consisting of a battery of 11 in vitro and in vivo Tier 1 screening assays (U.S.EPA, 2009), and four Tier 2 in vivo tests to characterize adverse outcomes (U.S.EPA, 2016). While there are approximately 10,000 chemicals covered by the EDSP chemical universe (U.S.EPA, 2012), to date, only 52 of these chemicals have undergone Tier 1 screening (U.S.EPA, 2015b). In order to more quickly and cost-effectively evaluate potential endocrine bioactivity of chemicals, the EDSP has been transitioning from traditional Tier 1 screening methods to high-throughput screening (HTS) in vitro assays and computational models to prioritize chemical screening and to provide alternative data for Tier 1 endpoints (U.S.EPA, 2015a).
One of the best characterized mechanism of endocrine disruption involves the direct interaction of chemicals with hormone nuclear receptors, mimicking or antagonizing the activity of endogenous hormones (Lee et al., 2013). The EPA’s ToxCast program (https://www.epa.gov/chemical-research/toxicity-forecasting) includes 18 in vitro HTS assays measuring perturbations in the estrogen receptor (ER) pathway (Judson et al., 2015), and 11 assays evaluating chemical interactions across the androgen receptor (AR) pathway (Kleinstreuer et al., 2016). The 18 HTS ER assays have been integrated in an ER model whose scores strongly correlate with reported potencies of estrogenic reference chemicals. These ER model scores are accepted by EPA as an alternative for the Tier 1 ER binding, ER transactivation, and rodent uterotrophic assays (Browne et al., 2015; Judson et al., 2015; U.S.EPA, 2015a). A similar modelling approach for the AR pathway has been validated against reference (anti)androgenic chemicals (Kleinstreuer et al., 2016), demonstrating robust performance that may indicate feasibility of using the AR pathway model as an alternative to the EDSP Tier 1 AR binding assay.
In addition to receptor-mediated mechanisms, chemicals may also affect processes involved in the synthesis, release, metabolism, transport, and elimination of endogenous hormones, which can lead to alterations in the levels of circulating sex hormones and potentially cause adverse health outcomes (Crisp et al., 1998). The gonads are the primary site of sex hormone synthesis, and the rat sliced testes steroidogenesis assay to detect chemicals that affect the synthesis of testosterone was initially considered for inclusion in the EDSP Tier 1 screening assay battery. However, implementation of the rat sliced testes assay was terminated due to variability of the assay and lack of ability to discern between cytotoxicity of Leydig and other testicular cells (U.S.EPA, 2008). The in vitro human H295R cell-based assay was validated as an international test guideline (US EPA OPPTS 890.1550; OECD TG 456; Hecker et al., 2011; Hecker et al., 2007), and is currently used in the EDSP Tier 1 battery in conjunction with a recombinant human aromatase inhibition assay (US EPA OPPTS 890.1200) for the identification of chemicals with the potential to alter the levels of androgens and estrogens (U.S.EPA, 2009). The H295R assay synthesizes most major steroid hormones produced in the human adult adrenal gland and male and female gonads, allowing testing for effects on the production of corticosteroids, progestagens, androgens, and estrogens (OECD, 2011). Additionally, chemical-induced cytotoxicity can be easily evaluated in the H295R cells in contrast to primary tissue cultures, and the model is suitable for the detection of chemicals that inhibit and induce steroid synthesis as opposed to enzymatic cell-free assays which are limited to assessing inhibition of steroidogenic enzymes (OECD, 2011).
A high-throughput H295R (HT-H295R) assay has been developed to facilitate the screening of large number of chemicals for effects on steroid synthesis (Karmaus et al., 2016). This HT-H295R assay could provide alternative data for the current Tier 1 steroidogenesis assays used for screening chemicals for potential effects on steroidogenesis. However, to establish confidence in the HT-H295R assay, its performance must be demonstrated against a robust set of reference chemicals. Herein, we describe an approach for identifying candidate reference chemicals for evaluating in vitro steroidogenesis assays. Candidate reference chemicals were identified from a review of primary scientific literature assessing effects of chemicals on the synthesis of androgens and estrogens in H295R and gonad-derived in vitro and ex vivo assays. The candidate reference chemicals were selected based on fulfillment of defined criteria for positive and negative chemicals and include chemicals that have the capability to alter the levels of androgens or estrogens in vitro or that are confirmed negatives for effects on these steroid hormones. These reference chemicals can potentially be used for validation and performance-evaluation of the HT-H295R assay and other in vitro steroidogenesis models.
2. Methods
2.1. Literature review
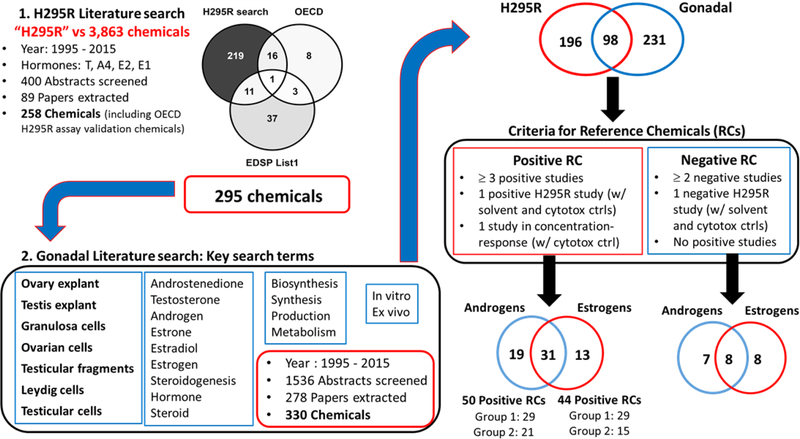
A two-step systematic literature review was performed for the identification of candidate reference chemicals for steroidogenesis (Fig. 1). Only studies compiled from the literature were used for identification of candidate reference chemicals. First, a literature search was designed to identify studies in the broad literature that used H295R cells to evaluate steroidogenesis. The H295R cells were selected as a starting point because the EDSP Tier 1 H295R assay uses the same cell model, and the literature review focused on identifying chemicals that perturb the synthesis of androgens and estrogens. PubMed and Web of Science searches targeting articles published from years 1995 to 2015 were conducted using the keyword “H295R” against a library of 3,863 unique substances (Supplemental File 1), which included chemicals run through ToxCast assays, used in the Organization for Economic Cooperation and Development (OECD) validation of the H295R assay (Hecker et al., 2011; Hecker et al., 2007), and the 52 chemicals for which there are EDSP Tier 1 data (U.S.EPA, 2015b). Searches were performed using the chemical name, Medical Subject Heading (MeSH) term, and Chemical Abstracts Service Registry Number (CASRN) as identifiers. Abstracts from retrieved articles were screened using the DistillerSR software (Evidence Partners, Ottawa, Canada), and abstracts reporting effects of a chemical on the levels of estrogens (17β-estradiol [E2] and/or estrone [E1]) or androgens (androstenedione [A4] and/or testosterone [T]) in H295R cells were identified for full text review and data extraction.
Figure 1.
Representation of the literature search scheme used to identify candidate reference chemicals for in vitro steroidogenesis assays. A two-step systematic literature was performed to identify chemicals affecting the synthesis of androgens and estrogens. The first search was designed to identify chemicals altering the synthesis of androgens (Testosterone [T] and/or androstenedione [A4]) and/or estrogens (17β-estradiol [E2] and/or estrone [E1]) in H295R cells. Chemicals with H295R data were then used in a second literature search targeting testicular and ovarian models. RC = Reference chemical
Following the first literature review, a second review was undertaken to further support the ability of potential reference chemicals to alter steroidogenesis in gonadal in vitro and ex vivo test systems. The search terms included “granulosa cell”, “Leydig cell”, “ovarian cell”, “testicular cell”, “ovary explant”, “testis explant” and “testicular fragments”, as well as terms related to hormone synthesis (Fig. 1). The articles identified in the second search were linked to the chemicals with H295R data based on chemical synonyms, CASRN, and MeSH terms. Abstracts from the gonadal literature search were similarly screened and abstracts reporting effects of chemicals on the levels of androgens (A4 and T) or estrogens (E1 and E2) in gonadal models proceeded to full text review and data extraction. Some abstracts reported effects on androgens primarily produced by immature or progenitor mammalian Leydig cells (3α-androstanediol and androsterone) (Ge and Hardy, 1998; Xiao et al., 2010; Zhao et al., 2012) or androgens produced in fish gonads (11-ketotestosterone and 11β-hydroxyandrostenedione) (Amiri et al., 1999; Beitel et al., 2014; Garcia-Lopez et al., 2010; Loomis and Thomas, 2000; Martins et al., 2009), and these abstracts were also included for full text review and data extraction. Only studies where chemical treatment was performed in vitro or ex vivo were extracted. The types of information extracted from each chemical-study combination for H295R and gonadal studies are provided in Supplemental Table 1. The LEL (lowest effective level; lowest concentration with a statistically significant effect on hormone levels) values and/or AC50 (half-maximal activity concentration) values were recorded for compounds with positive results. The highest concentration tested (HCT) was recorded for chemicals with negative results. For studies that reported cytotoxicity measurements, the highest concentration that affected ≤ 80% cell viability was recorded.
2.2. Criteria for candidate reference chemicals for steroidogenesis
Results from the in vitro H295R and gonadal literature searches, chemicals used in the OECD H295R validation studies, and EDSP Tier 1 data were used to identify candidate reference chemicals for steroidogenesis. In order to compare results from the literature review with data from guideline H295R assays, studies identified from literature searches were only evaluated if androgen or estrogen levels were measured in the culture medium. The criteria used for identification of candidate reference chemicals were reviewed by the Interagency Coordinating Committee on the Validation of Alternative Methods (ICCVAM) Reference Chemical Work Group Subcommittee.
For identification of candidate reference chemicals, studies were considered to be independent when: (i) the same in vitro model in different publications (but potentially the same authors); (ii) different in vitro models of the same species in a single publication (e.g. pig granulosa cells, pig ovarian follicles); (iii) different species in a single publication (e.g. testes slices from rat and mouse); (iv) different developmental stages evaluated in the same species in a single publication (e.g. granulosa cells from prepubertal and adult rats); (v) basal and stimulated experimental conditions using the same model (e.g. stimulation with gonadotropins or forskolin); and (vi) different steroidogenic precursors using the same model (e.g. hydroxycholesterol and progesterone). A chemical and its salt forms (e.g., valproic acid/sodium valproate; perfluorooctane sulfonate/potassium perfluoroctane sulfonate) were considered as the same substance for the purpose of identifying candidate reference chemicals.
In order to qualify as a positive candidate reference chemical, the following criteria had to be met for each test chemical:
The chemical had to be tested in at least three independent studies that demonstrated a significant effect of the chemical on the levels (increase or decrease) of androgens or estrogens;
One of the studies that demonstrated a significant effect of the chemical on androgen or estrogen levels had to be conducted in H295R cells;
The study conducted in H295R cells had to have both solvent control and cell viability of ≥ 80%;
One of the H295R or gonadal studies had to demonstrate a concentration-response effect (≥ 2 adjacent concentrations significantly affected in the same direction; consistent with the criterion for a positive effect in the OECD H295R TG 456 (OECD, 2011));
The study demonstrating a concentration-response effect needed to include a parallel cell viability control.
Chemicals reported to have an effect on estrogen or androgen levels in at least one H295R study that met all five criteria were categorized as Group 1 reference chemicals, while those that fulfilled four of five criteria were considered Group 2 candidate reference chemicals (Table 1). Studies without reported statistical analyses were also included for identifying candidate reference chemicals; in this case, chemicals were considered to have a positive effect if a minimum 2-fold change (increase or decrease) in hormone levels was reported. Chemical effects reported in measures derived from curve fitting (e.g., AC50) were considered positive and to have demonstrated a concentration-response effect.
Table 1.
Selection criteria for positive candidate reference chemicals for steroidogenesis.
| Chemical | Number positive studies | H295R study | Study in concentration-response | Reference Chemical Group | |||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | ≥ 3 | H295R Positive | Solvent control | Cell viability control | ≥ 2 adjacent concentrations affected | Cell viability control | ||
| Chemical 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Chemical 2 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 2 |
| Chemical 3 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 2 |
| Chemical 4 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 2 |
| Chemical 5 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 2 |
| Chemical 6 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | NA | 2 |
| Chemical 7 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 2 |
| Criteria 1 | Criteria 2 | Criteria 3 | Criteria 4 | Criteria 5 | |||||
Characterization of group 1 reference chemicals is dependent on the fulfillment of five criteria: at least three independent positive studies, one of which conducted in H295R cells with both solvent and cell viability (cytotoxicity) controls, and at least one positive H295R or gonadal study with a parallel cell viability control indicating a concentration-response effect (at least two adjacent concentrations affected in the same direction). Group 2 reference chemicals satisfy four of the five criteria, as exemplified above. 1 = Yes; 0 = No; NA = not applicable
In order to be classified as a candidate negative reference chemical for steroidogenic disruption of androgens or estrogens, the chemical had to meet the following criteria:
The chemical had to be tested in at least two independent studies (one of which must have been conducted in H295R cells with solvent and cytotoxicity controls) demonstrating no significant effect of the chemical on the levels of androgens or estrogens;
No H295R or gonadal studies reporting significant effects of the chemical on the synthesis of androgens or estrogens.
2.3. Potency characterization for positive candidate reference chemicals
For characterizing chemical potency, the LEL or AC50 values reported from all positive studies that measured T and E2 were converted into log10 micromolar (μM) units and values were used to derive a median representative effect concentration (potency value) for each candidate reference chemical. Results from all positive H295R and gonadal studies that tested the chemical in at least two concentrations were considered, even in cases where the no-effect-level (NEL) was not reported in the study.
2.4. Curation of Candidate Reference Chemicals
The candidate reference chemical list was run against EPA’s Chemistry Dashboard (https://comptox.epa.gov/dashboard) to ensure that reported chemical name and CASRN correctly aligned. In cases where chemical information was missing from the dashboard, the chemical curation was performed using a hierarchy of chemical search databases (Supplemental Table 2). The CASRNs and reported names of candidate reference chemicals that matched a valid synonym from a regulatory source from the EPA’s Substance Registry System (SRS database) were considered “clean” matches. In all other cases, reported chemical names had to be linked to the same valid synonym, INCHI/canonical SMILEs, and same CASRN. Discrepancies were resolved using the hierarchy of chemical search databases. Concordance of results across all available sources was evaluated in resolving a substance identity.
3. Results
3.1. Chemicals identified from H295R literature review
The first literature search for studies conducted in H295R cells returned 400 papers, and 89 of these papers reported the effect of a chemical on the levels of androgens (androstenedione [A4] or testosterone [T]) and/or estrogens (estrone [E1] or 17β-estradiol [E2]), and thus, advanced to full text evaluation and data extraction. Publications reporting on the OECD H295R validation studies were also captured in the H295R literature search (Hecker et al., 2011; Hecker et al., 2007). A total of 540 studies were extracted from the 89 articles, representing 258 unique substances (H295R DB, Supplemental File 3). The effects of at least two chemical concentrations were evaluated in 420 H295R studies (78%), while the remaining studies only tested a single chemical concentration. The majority of the positive H295R studies reported a LEL, and an AC50 was calculated for 49 studies.
The effects of 250 substances on androgens were evaluated, with effects on T and A4 levels reported for 237 and 69 chemicals, respectively (Supplemental Table 3). A total of 81 chemicals had multiple H295R studies that measured T levels, while 156 chemicals were tested in only one H295R study. Among the 237 chemicals with data on T, a total of 96 chemicals were associated with positive results only (increases or decreases in T levels), 109 chemicals displayed negative results only (no effect on T levels), and 32 showed mixed results (both positive and negative results). There was a predominance of studies that indicated inhibitory effects on T among chemicals with at least one positive result (104/128 chemicals) (Fig. 2A, Supplemental Table 3). Similar to the results observed for T, the majority of the chemicals with positive data on A4 displayed inhibitory effects on A4 levels (Supplemental Fig. 1 and Supplemental Table 3).
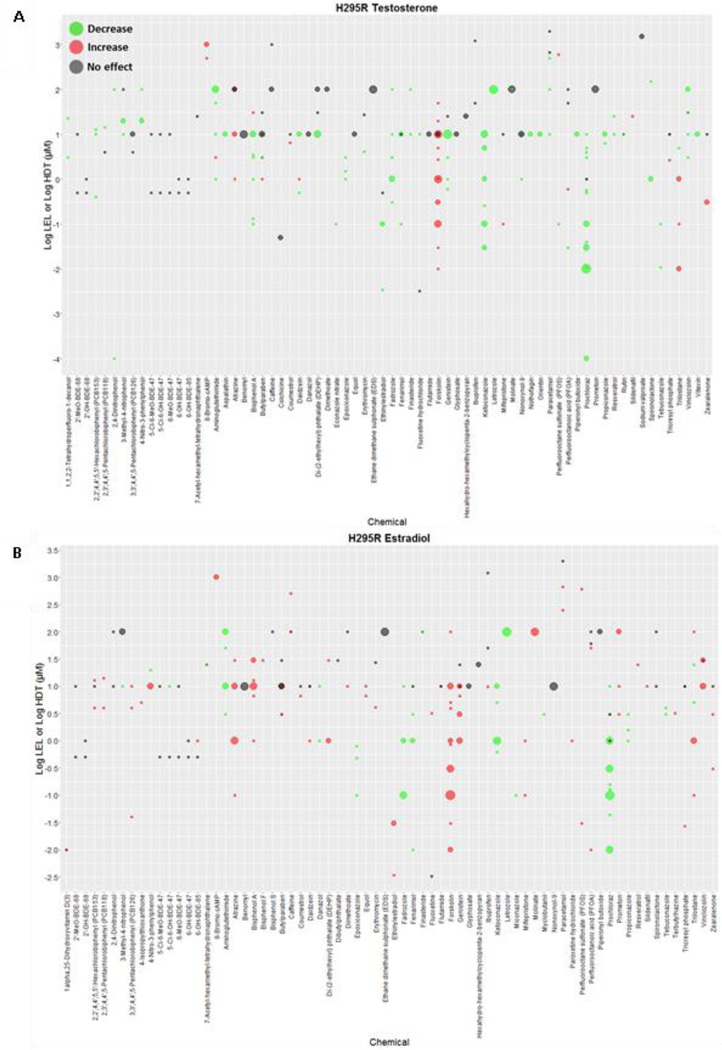
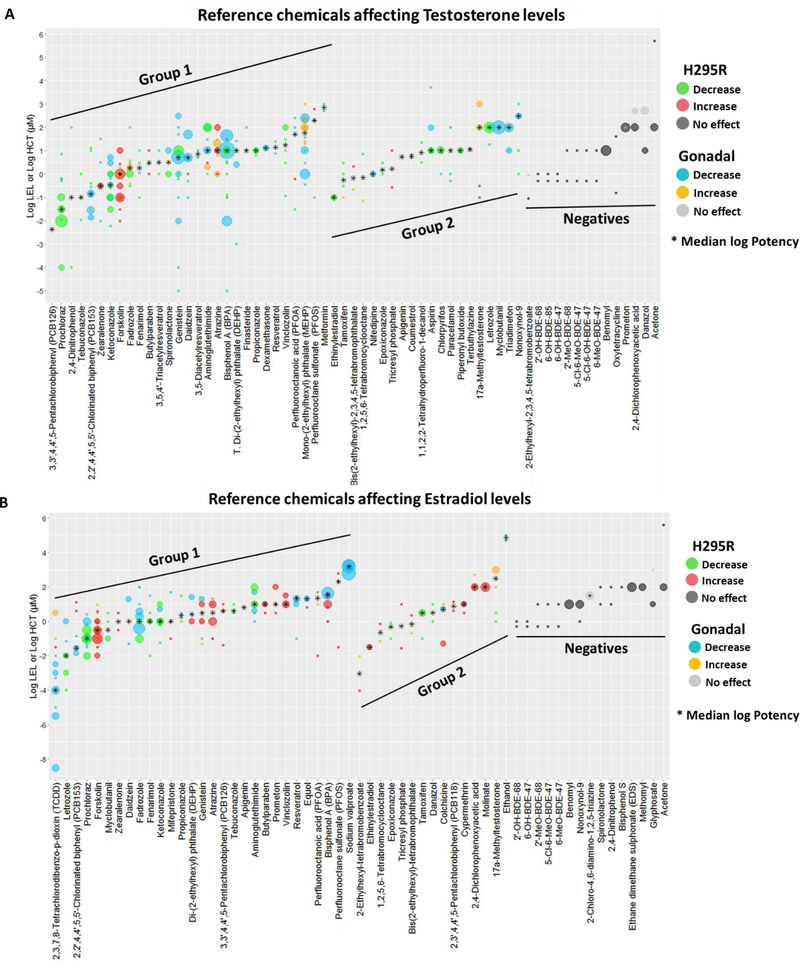
Figure 2. Results of the effect of 70 chemicals identified in H295R studies on T and E2 levels.
Chemicals are listed along the x-axis and the log10 transformed concentrations along the y-axis. Green and red dots represent LELs (or AC50s) of studies reporting decreases and increases, respectively, in T (Panel A) or E2 (Panel B). Gray dots indicate HCT for negative results. The size of the dots is proportional to the number of studies (range 1 – 7). Plot includes OECD H295R validation results and H295R literature studies of chemicals tested in one or more concentrations. Similar plots for chemical effects on A4 and E1 are provided in supplemental figures 1 and 2, respectively. (LEL = Lowest effect level; HCT = highest concentration tested)
Studies measuring changes in estrogen levels were identified for 215 chemicals, which represented 208 and 41 chemicals with data on E2 and E1, respectively (Supplemental Table 3). Among the 208 chemicals with data on E2, 73 chemicals had multiple H295R studies measuring E2 levels, while 135 chemicals were tested in only one H295R study. A total of 92 chemicals significantly altered E2 levels, while 85 chemicals displayed negative outcomes only; mixed results for effects on E2 were identified for 31 chemicals. Contrary to the results on T, there was a predominance of studies indicating increases in E2 levels for chemicals with at least one positive study (83/122 chemicals) (Fig. 2B, Supplemental Table 3). H295R studies measuring effects on E1 are summarized in Supplemental Table 3 and Supplemental Fig. 2.
3.2. Chemicals identified from the gonadal steroidogenesis literature review
To add further confidence in the steroidogenic effect of chemicals for which H295R assay results were identified (Supplemental Files 2 and 3), a total of 295 chemicals identified from the H295R literature search with EDSP List 1 substances added were used in a literature search that evaluated chemical effects on steroidogenesis in ovarian and testicular-derived models (Fig. 1). A total of 1,536 abstracts were screened, 278 of which advanced to full-text review and data extraction (Gonadal DB, Supplemental File 4). During the data extraction phase, all chemicals evaluated in the 278 papers for effects on the synthesis of androgens and/or estrogens were extracted, even if no studies had been previously identified in H295R cells, resulting in extraction of data from 2109 studies on 330 chemicals.
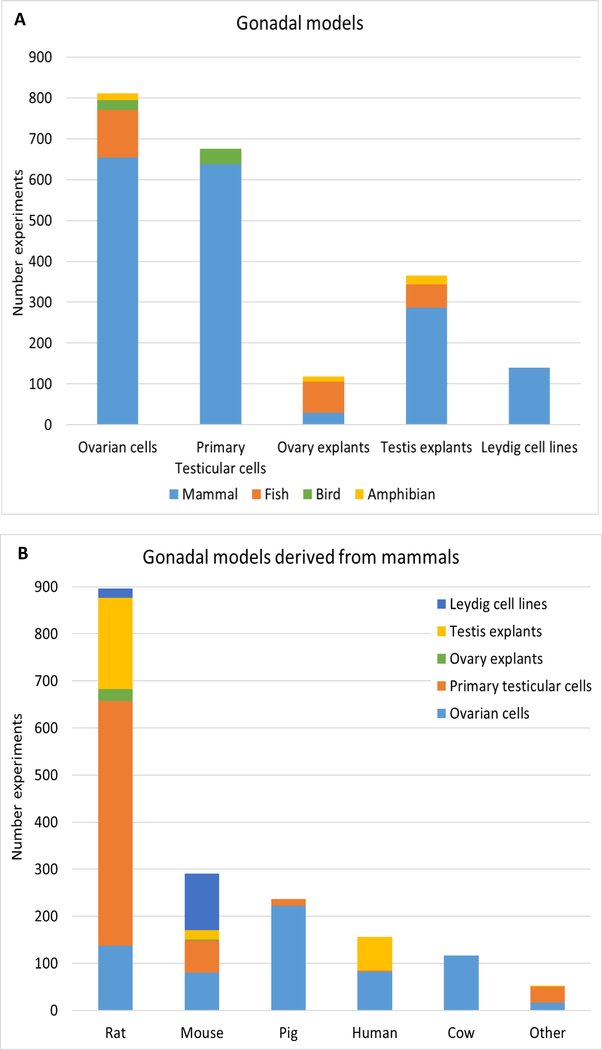
Ovarian-derived cells constituted 38% of the gonadal models reported in the studies (811/2109 studies), including granulosa cells, theca cells, granulosa-theca co-cultures, ovarian follicles, and oocyte fragments (Fig. 3A). Primary testicular cells were used in 32% of the studies (675/2109), mostly represented by purified Leydig cells. Leydig cell lines isolated from tumors (MA-10, MLTC-1, BLTK-1, and R2C) or immature testes (TM3) were also represented in 7% (140/2109) of the studies. Testis and ovary explants, including whole tissue cultures, fragments, and slices accounted for 17% (365/2109) and 6% (118/2109) of studies, respectively. The majority of the gonadal models represented in the studies were derived from mammals (83%; 1748/2109), followed by fish (12%; 250/2109), bird (3%, 62/2109), and amphibian (2%, 49/2109) (Fig. 3A; Supplemental Table 4). Among mammalian studies, 68% (1186/1748) comprised rat and mouse models, while 9% (156/1748) of the gonadal models originated from human sources (Fig. 3B).
Figure 3. Gonadal models represented in the literature search.
The number of studies indicating the distribution of gonadal models per animal class is represented in A. Ovarian cells include granulosa cells, theca cells, co-cultures and ovarian follicles. Primary testicular cells are represented mostly by purified Leydig cells. Leydig cell lines constitute tumor-derived cells (BLTK-1, R2C, MA-10, MLTC-1) and Leydig cells isolated from normal immature testis (TM-3). Ovary and testis explants constitute whole tissue cultures, fragments and slices. The distribution of gonadal models per mammalian animal sources are indicated in B. “Other” refers to studies using bank vole, hamster, buffalo, monkey, dog, sheep, and rabbit gonadal models.
Several gonadal publications described multiple experimental protocols including basal or stimulated conditions with gonadotropins (LH: luteinizing hormone, FSH: follicle-stimulating hormone, CG: chorionic gonadotropin), chemical inducers (e.g. forskolin, analogues of 3’, 5’-cyclic adenosine monophosphate; cAMP), and steroidogenic precursors (e.g. 22R-hydroxycholesterol, pregnenolone, progesterone), as well as different concentrations of stimulants and incubation time with chemicals. Most studies investigating chemical effects on androgen synthesis (i.e. either increasing or decreasing levels of androgens in cell media) reported effects on T (1448 studies), representing 264 chemicals (Fig. 4A), followed by A4 and other androgens (androsterone, 3α-androstanediol, 11β-OH-androstenedione, and 11-Ketotestosterone) (Supplemental Table 5). Effects on E2 were reported in 894 studies for 187 chemicals (Fig. 4B; Supplemental Table 4), and E1 measurements were identified for 7 chemicals.
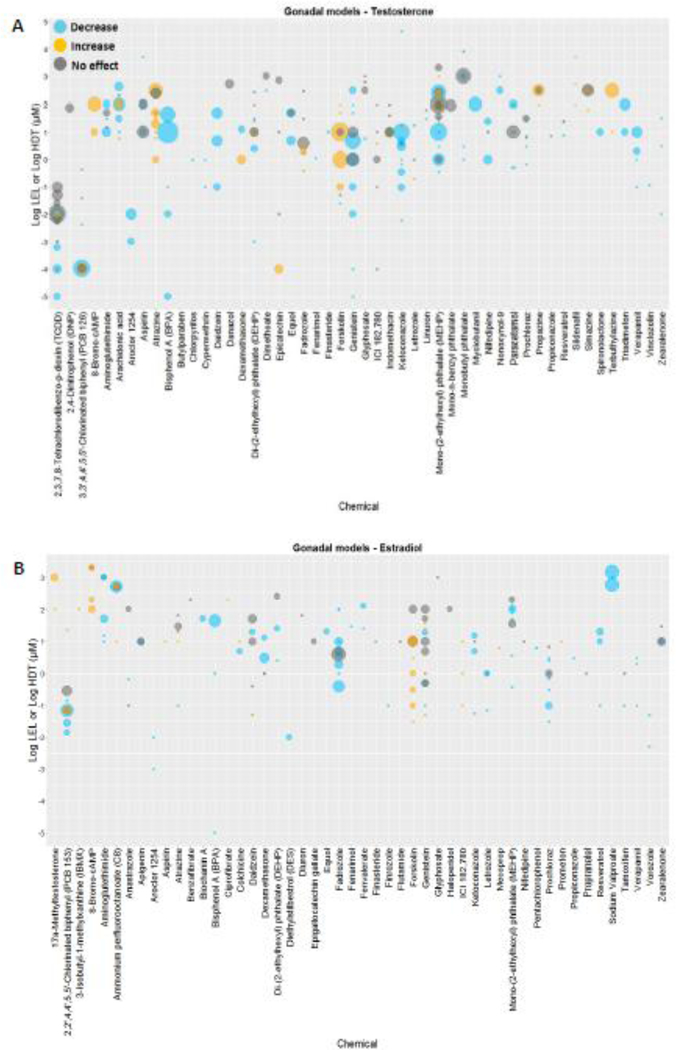
Figure 4. Results of the effect of 50 chemicals identified in gonadal studies on T and E2 levels.
Chemicals are listed along the x-axis and the log10 transformed concentrations along the y-axis. Blue and orange dots represent LELs (or AC50s) of studies reporting decreases and increases, respectively, in T (Panel A) or E2 (Panel B). Gray dots indicate HCT for negative results. The size of the dots is proportional to the number of studies (T range: 1 – 18; E2 range: 1 – 13). The plot represents literature studies testing the effect of a chemical in one or more concentrations in ovarian or testicular-derived models. (LEL = Lowest effect level; HCT = highest concentration tested).
Results of steroidogenic effects in both H295R and gonadal assays were identified for 98 substances, representing a total of 89 and 57 chemicals with steroidogenic data for effects on androgens and estrogens, respectively (Supplemental Table 5).
3.3. Identification of Candidate Reference Chemicals
Candidate Reference chemicals affecting androgen synthesis
Using the criteria defined in the Section 2.2, a total of 29 chemicals satisfied all five criteria (i.e. group 1) positive reference chemicals affecting androgen levels (Table 1; Supplemental File 5). The group 1 reference chemicals included forskolin, an inducer of steroidogenesis, prochloraz and other known inhibitors of steroidogenic enzymes, such as aminoglutethimide (Cash et al., 1967), ketoconazole (Loose et al., 1983), propiconazole (Laville et al., 2006), and fenarimol (Laville et al., 2006).
Group 2 reference chemicals that met four of five criteria included 21 chemicals, 13 of which were positive in two instead of three independent studies, and satisfied all the other criteria (Table 1; Supplemental File 5). Eight group 2 chemicals had at least three positive studies, but failed to fulfill one of the other conditions (Supplemental File 5). For example, the H295R assays conducted with triadimefon (Goetz et al., 2009) and aspirin (Albert et al., 2013) did not report solvent or cytotoxicity controls, respectively. It was unclear whether significant effects were observed in two adjacent concentrations for myclobutanil and trilostane. Myclobutanil inhibited the synthesis of androgens at the highest concentration tested (100 μM) in H295R and gonadal studies (Goetz et al., 2009; U.S.EPA, 2015b). While a clear trend in inhibition of T synthesis was observed for myclobutanil at concentrations ≥ 10 μM in H295R cells (Goetz et al., 2009), the inhibitory effects were not indicated as statistically significant. Studies of trilostane identified in H295R and gonadal literature searches tested effects at one concentration only, and reported inhibitory effects of the chemical on androgen levels (Garcia-Lopez et al., 2010; Nakamura et al., 2011; Rijk et al., 2012). Trilostane increased T levels at more than one adjacent concentration in all studies from the OECD H295R assay validation; however, a concentration-response effect could not be clearly defined in the validation studies due to cross-reactivity with the hormone detection system (Hecker et al., 2011). A concentration-response effect was observed for nifedipine, nonoxynol-9 and letrozole in studies without cytotoxicity measurement (Freyberger et al., 2010; Hwang et al., 2009; Kudoh et al., 1995). Overall, 22 out of the 50 positive chemicals (groups 1 and 2) were tested in guideline studies (OECD H295R assay validation or EDSP Tier 1 studies) (Supplemental Table 6), and 21 chemicals had positive outcomes in guideline studies. The exception was di-(2-ethylhexyl) phthalate (DEHP), which tested negative in the H295R assay validation studies, but had several positive outcomes in gonadal and H295R studies identified in the broad literature search.
Fourteen chemicals were classified as candidate negative reference chemicals, including derivatives of polybrominated diphenyl ethers (BDEs), the flame retardant 2-ethylhexyl-2,3,4,5-tetrabromobenzoate, the pharmaceuticals oxytetracycline and danazol, the pesticides 2,4-dichlorophenoxyacetic acid, prometon and benomyl, and the solvent acetone. The latter five negative chemicals were tested in H295R guideline studies (Supplemental Table 6). The highest concentration tested (HCT) for all negative chemicals were ≥ 1 μM, with the exception of 2-ethylhexyl-2,3,4,5-tetrabromobenzoate, which was tested at the maximum concentration of 0.09 μM and 0.9 μM in H295R and primary testicular cells, respectively (Mankidy et al., 2014; Saunders et al., 2013). All H295R and gonadal studies that supported the characterization of positive and negative candidate reference chemicals affecting the synthesis of androgens are listed in Supplemental File 6.
Potency of candidate reference chemicals affecting androgen synthesis
For characterization of potency of positive reference chemicals, the log10 LELs (or log10 AC50s in the absence of LELs) of studies measuring T levels in at least two concentrations were used to obtain a median potency value of all positive studies (Supplemental File 7). Prochloraz and forskolin, often used as in vitro positive controls for inhibition and induction of steroidogenesis, respectively, had median LELs of 0.03 and 1 μM (Table 2; Fig. 5A). Additionally, PCB126 and PCB153, tebuconazole, ketoconazole, zearalenone, and 2,4-dinitrophenol (group 1 chemicals) and ethinylestradiol, tamoxifen, bis(2-ethylhexyl)-tetrabromophthalate, tetrabromocyclooctane, and nifedipine (group 2) presented median potency values ≤ 1 μM. The median potency values ranged between 1 and 50 μM for 27 chemicals (17 from group 1 and 10 from group 2 chemicals; Table 2; Fig. 5A). Median potency values were greatest (≥ 50 μM) for perfluorooctanoic acid (PFOA), perfluorooctane sulfonate (PFOS), mono-(2ethylhexyl) phthalate (MEHP) and metformin (group 1), and methyltestosterone, letrozole, myclobutanil, triadimefon and nonoxynol-9 (group 2; Table 2; Fig. 5A).
Table 2.
List of Candidate Reference Chemicals affecting androgen levels.
| Chemical | CASRN | Potency values * | Direction of Effect (% studies) † | ||
|---|---|---|---|---|---|
| Lowest (μM) | Median (μM) | Highest (μM) | |||
| GROUP 1 | |||||
| 3,3',4,4',5-Pentachlorobiphenyl (PCB126) | 57465-28-8 | - | 4.2E-03 | - | 74 |
| Prochloraz | 67747-09-5 | 1.0E-04 | 3.5E-02 | 7.4E+00 | 100 |
| 2,4-Dinitrophenol | 51-28-5 | 1.0E-04 | 1.0E-01 | 1.0E+02 | 67 |
| Tebuconazole | 107534-96-3 | 1.1E-02 | 1.0E-01 | 1.0E+00 | 100 |
| 2,2',4,4',5,5'-Chlorinated biphenyl (PCB153) | 35065-27-1 | 1.4E-02 | 1.4E-01 | 1.3E+01 | 93 |
| Zearalenone | 17924-92-4 | 1.0E-02 | 3.0E-01 | 3.1E+01 | 60 |
| Ketoconazole | 65277-42-1 | 6.0E-03 | 3.4E-01 | 5.0E+00 | 98 |
| Forskolin | 66575-29-9 | 1.0E-02 | 1.0E+00 | 1.0E+01 | 99 |
| Fadrozole | 102676-31-3 | 3.0E-01 | 1.8E+00 | 1.0E+02 | 71 |
| Fenarimol | 60168-88-9 | 1.0E-01 | 1.8E+00 | 1.0E+01 | 100 |
| Butylparaben | 94-26-8 | 1.0E+00 | 3.0E+00 | 1.0E+01 | 50 |
| 3,5,4'-Triacetylresveratrol | 42206-94-0 | 1.0E+00 | 3.2E+00 | 1.0E+01 | 100 |
| Spironolactone | 52-01-7 | 1.0E+00 | 3.2E+00 | 1.0E+01 | 100 |
| Genistein | 446-72-0 | 1.0E-05 | 5.0E+00 | 3.0E+02 | 100 |
| Daidzein | 486-66-8 | 1.0E-01 | 5.0E+00 | 5.0E+01 | 93 |
| 3,5-Diacetylresveratrol | 411233-14-2 | 5.0E+00 | 7.1E+00 | 1.0E+01 | 100 |
| Aminoglutethimide | 125-84-8 | 1.0E+00 | 1.0E+01 | 1.0E+02 | 78 |
| Atrazine | 1912-24-9 | 1.0E+00 | 1.0E+01 | 1.0E+02 | 93 |
| Bisphenol A (BPA) | 80-05-7 | 1.0E-05 | 1.0E+01 | 4.4E+01 | 96 |
| Di-(2-ethylhexyl) phthalate (DEHP) | 117-81-7 | 1.0E-03 | 1.0E+01 | 2.6E+01 | 80 |
| Finasteride | 98319-26-7 | 1.0E+01 | 1.0E+01 | 1.0E+02 | 100 |
| Propiconazole | 60207-90-1 | 6.3E+00 | 1.0E+01 | 1.0E+01 | 100 |
| Dexamethasone | 50-02-2 | 1.3E+01 | 1.3E+01 | 1.3E+01 | 73 |
| Resveratrol | 501-36-0 | 7.5E+00 | 1.4E+01 | 2.5E+01 | 100 |
| Vinclozolin | 50471-44-8 | 1.2E-01 | 1.7E+01 | 1.0E+02 | 88 |
| Perfluorooctanoic acid (PFOA) | 335-67-1 | 3.0E-02 | 5.0E+01 | 2.5E+02 | 91 |
| Mono-(2-ethylhexyl) phthalate (MEHP) | 4376-20-9 | 3.6E-01 | 5.7E+01 | 1.0E+03 | 71 |
| Perfluorooctane sulfonate (PFOS) | 1763-23-1 | 1.0E-01 | 2.0E+02 | 6.0E+02 | 75 |
| Metformin | 657-24-9 | 5.0E+02 | 7.1E+02 | 1.0E+03 | 100 |
| GROUP 2 | |||||
| Ethinylestradiol | 57-63-6 | 1.0E-01 | 1.0E-01 | 1.0E-01 | 100 |
| Tamoxifen | 10540-29-1 | 1.0E-01 | 5.5E-01 | 3.0E+00 | 75 |
| Bis(2-ethylhexyl)-2,3,4,5-tetrabromophthalate | 26040-51-7 | 2.1E-01 | 6.7E-01 | 2.1E+00 | 100 |
| 1,2,5,6-Tetrabromocyclooctane | 3194-57-8 | 7.0E-02 | 7.0E-01 | 7.0E+00 | 50 |
| Nifedipine | 21829-25-4 | 1.0E+00 | 1.0E+00 | 1.0E+00 | 100 |
| Epoxiconazole | 133855-98-8 | 1.0E+00 | 1.5E+00 | 3.0E+00 | 100 |
| Tricresyl phosphate | 1330-78-5 | 2.7E-01 | 1.6E+00 | 1.0E+01 | 100 |
| Apigenin | 520-36-5 | - | 5.5E+00 | - | 100 |
| Coumestrol | 479-13-0 | 5.0E+00 | 5.8E+00 | 6.7E+00 | 50 |
| 1,1,2,2-Tetrahydroperfluoro-1-decanol | 678-39-7 | 3.1E+00 | 8.3E+00 | 2.2E+01 | 100 |
| Aspirin | 50-78-2 | 1.0E+01 | 1.0E+01 | 5.0E+03 | 100 |
| Chlorpyrifos | 2921-88-2 | 1.0E+00 | 1.0E+01 | 1.0E+01 | 100 |
| Paracetamol | 103-90-2 | 5.0E-01 | 1.0E+01 | 1.0E+02 | 100 |
| Piperonyl butoxide | 51-03-6 | 1.0E+01 | 1.0E+01 | 1.0E+01 | 100 |
| Terbuthylazine | 5915-41-3 | 1.0E+01 | 1.1E+01 | 1.3E+01 | 100 |
| 17α-Methyltestosterone | 58-18-4 | 1.0E-01 | 1.0E+02 | 1.0E+03 | 100 |
| Letrozole | 112809-51-5 | 1.8E+01 | 1.0E+02 | 1.0E+02 | 88 |
| Myclobutanil | 88671-89-0 | 1.0E+02 | 1.0E+02 | 1.0E+02 | 93 |
| Triadimefon | 43121-43-3 | 1.0E+01 | 1.0E+02 | 1.0E+02 | 92 |
| Nonoxynol-9 | 26027-38-3 | 1.0E+00 | 3.0E+02 | 1.0E+03 | 100 |
| Trilostane | 13647-35-3 | NA | NA | NA | 50 |
| NEGATIVES | HCT (μM) | ||||
| 2-Ethylhexyl-2,3,4,5-tetrabromobenzoate | 183658-27-7 | 9.1E-01 | |||
| 6-OH-BDE-47 | 1017894-49-3 | 1.0E+00 | |||
| 6-OH-BDE-85 | 1219628-90-6 | 1.0E+00 | |||
| 2'-OH-BDE-68 | 79755-43-4 | 1.0E+00 | |||
| 2'-MeO-BDE-68 | 96920-28-4 | 1.0E+01 | |||
| 5-Cl-6-MeO-BDE-47 | 497106-81-7 | 1.0E+01 | |||
| 5-Cl-6-OH-BDE-47 | 497069-18-8 | 1.0E+01 | |||
| 6-MeO-BDE-47 | 102739-99-1 | 1.0E+01 | |||
| Benomyl | 17804-35-2 | 1.0E+01 | |||
| Oxytetracycline | 6153-64-6 | 4.0E+01 | |||
| Prometon | 1610-18-0 < | 1.0E+02 | |||
| 2,4-Dichlorophenoxyacetic acid | 94-75-7 | 5.0E+02 | |||
| Danazol | 17230-88-5 | 5.0E+02 | |||
| Acetone | 67-64-1 | 5.1E+05 | |||
Potency values: Positive H295R and gonadal studies testing the effect of a chemical in at least two concentrations on T levels were used for median potency calculation; AC50s were considered when LELs were not reported. For negative chemicals, the HCT is reported. Only one study reporting effects on T included for PCB126 and apigenin.
All H295R and gonadal studies and protocols demonstrating an effect on androgen levels were included for characterization of direction of effect. A cut-off of 75% was applied to determine a predominant effect direction. The colors green and red indicate chemicals predominantly causing decreases and increases in androgen levels, respectively, and the numbers reflect the percentage of studies affected. Chemicals without a predominant effect are not colored, and the number represents the percentage of studies showing a decrease in androgen levels.
LEL: Lowest Effect Level; HCT: Highest concentration tested
Figure 5. Potency values for candidate reference chemicals affecting T and E2 levels.
The log LELs (or log AC50s) are indicated for positive Group 1 and Group 2 candidate reference chemicals, and the HCT is indicated for candidate negative reference chemicals. A. Plot of candidate reference chemicals affecting T levels. A total of 359 positive and 42 negative studies are plotted. The log LEL and log AC50 are represented for 336 and 23 studies, respectively. Details on studies used for potency characterization is in Supplemental file 7. B. Candidate reference chemicals affecting E2 levels. A total of 322 positive and 45 negative studies are plotted. The log LEL and log AC50 are represented for 302 and 20 studies, respectively. Details on studies used for potency characterization is in Supplemental file 10. Only studies testing the effect of a chemical in at least two concentrations are plotted and included for calculation of median log potency values. Green and blue dots indicate, respectively, H295R and gonadal studies showing a decrease (inhibition) on the levels of hormones; red and yellow dots represent H295R and gonadal studies indicating an increase (induction) on hormone levels. Black asterisk indicates the median log potency value. Dark and light gray dots indicate HCTs for negative chemicals. The size of the dots is proportional to the number of studies. (LEL = Lowest effect level; HCT = highest concentration tested).
Results of H295R and gonadal assays were also used to characterize whether test chemicals induced or inhibited androgen synthesis (Table 2). In some cases, a chemical was reported to both increase and decrease androgens in different studies, and 25 out of the 50 candidate positive reference chemicals had studies indicating both decreases and increases in androgen levels. For this reason, the direction of the effect was determined by consistent results in at least 75% of the positive studies. The majority of the chemicals (33/50 chemicals; 66%) reduced androgen levels in vitro, including azole pesticides known to inhibit the activity of cytochrome P450 enzymes involved in the synthesis of sterols and steroid hormones (e.g. prochloraz, tebuconazole, propiconazole; Table 1) (Laville et al., 2006; Trosken et al., 2006; Trosken et al., 2004). Several phytochemicals (e.g. genistein, daidzein, resveratrol and derivatives) and pharmaceutical compounds (e.g. aminoglutethimide, finasteride, spironolactone, aspirin, paracetamol) also had a predominant inhibitory effect on androgen synthesis. A clear trend in induction of androgen levels was observed for forskolin, atrazine, PFOS, and four group 2 chemicals (bis(2-ethylhexyl)-tetrabromophthalate, tricresyl phosphate, terbuthylazine, and 17α-methyltestosterone) (Table 2). Mixed effects (less than 75% studies with consistent results) were observed for 2,4-dinitrophenol, PCB126, fadrozole, zearalenone, butylparaben, MEHP, tetrabromocyclooctane, dexamethasone and coumestrol.
Reference chemicals affecting estrogen synthesis
A total of 29 compounds satisfied the criteria for group 1 candidate reference chemicals affecting estrogen synthesis, and 15 chemicals were assigned to the group 2 category (Table 3; Supplemental Files 8 and 9). Among group 2 chemicals, 12 were positive in two instead of three studies, and satisfied all other criteria. PCB118 and molinate displayed positive results in at least three studies, but lacked concentration-response data (Gregoraszczuk et al., 2008; Hecker et al., 2011; Kraugerud et al., 2010; Tremoen et al., 2014). Moreover, no viability control was indicated in studies demonstrating a concentration-response effect on E2 levels for colchicine (Gregoraszczuk et al., 1996) (Supplemental Files 8 and 9). Trilostane tested positive in all OECD H295R interlaboratory validation studies, but it was excluded from the reference chemical list as its effect on E2 was entirely associated with cross-reactivity with the hormone immunoassay (Hecker et al., 2011). Twenty-one out of the 44 positive chemicals (groups 1 and 2) were tested in H295R assay validation or Tier 1 studies (Supplemental Table 6), and the steroidogenic effect of the 21 chemicals was supported in the guideline studies.
Table 3.
List of Candidate Reference Chemicals affecting estrogen levels.
| Chemical | CASRN | Potency values * | Direction of Effect (% studies) † | ||
|---|---|---|---|---|---|
| Lowest LEL (μM) | Median LEL (μM) | Highest LEL (μM) | |||
| GROUP 1 | |||||
| 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) | 1746-01-6 | 3.1E-09 | 1.0E-04 | 3.1E+00 | 90 |
| Letrozole | 112809-51-5 | 1.0E-04 | 1.0E-02 | 1.0E+00 | 100 |
| 2,2',4,4',5,5'-Chlorinated biphenyl (PCB153) | 35065-27-1 | 1.4E-02 | 2.8E-02 | 1.3E+01 | 63 |
| Prochloraz | 67747-09-5 | 1.0E-02 | 1.0E-01 | 2.5E+00 | 100 |
| Forskolin | 66575-29-9 | 1.0E-02 | 3.0E-01 | 9.3E+00 | 98 |
| Myclobutanil | 88671-89-0 | 1.0E-01 | 3.2E-01 | 3.0E+00 | 100 |
| Zearalenone | 17924-92-4 | 3.0E-01 | 9.5E-01 | 3.0E+00 | 50 |
| Daidzein | 486-66-8 | 5.0E-02 | 9.9E-01 | 2.0E+01 | 50 |
| Mifepristone | 84371-65-3 | 1.0E-01 | 1.0E+00 | 5.0E+01 | 60 |
| Fadrozole | 102676-31-3 | 1.0E-01 | 1.0E+00 | 1.0E+02 | 100 |
| Fenarimol | 60168-88-9 | 1.0E-02 | 1.0E+00 | 3.0E+01 | 100 |
| Ketoconazole | 65277-42-1 | 5.6E-02 | 1.0E+00 | 1.0E+01 | 100 |
| Genistein | 446-72-0 | 5.0E-02 | 3.0E+00 | 2.5E+01 | 32 |
| Propiconazole | 60207-90-1 | 1.0E+00 | 2.2E+00 | 3.0E+01 | 100 |
| Di-(2-ethylhexyl) phthalate (DEHP) | 117-81-7 | 1.0E+00 | 2.6E+00 | 2.6E+01 | 60 |
| Atrazine | 1912-24-9 | 1.0E-01 | 3.2E+00 | 2.0E+01 | 86 |
| 3,3',4,4',5-Pentachlorobiphenyl (PCB126) | 57465-28-8 | 4.0E-02 | 4.0E+00 | 1.0E+01 | 100 |
| Tebuconazole | 107534-96-3 | 3.0E+00 | 4.0E+00 | 1.0E+01 | 100 |
| Apigenin | 520-36-5 | 6.4E+00 | 6.5E+00 | 6.4E+00 | 100 |
| Aminoglutethimide | 125-84-8 | 1.0E+00 | 1.0E+01 | 1.0E+02 | 100 |
| Butylparaben | 94-26-8 | 3.0E+00 | 1.0E+01 | 1.0E+01 | 100 |
| Prometon | 1610-18-0 | 3.0E+00 | 1.0E+01 | 1.0E+02 | 100 |
| Vinclozolin | 50471-44-8 | 1.0E+01 | 1.0E+01 | 3.0E+01 | 100 |
| Resveratrol | 501-36-0 | 1.0E-01 | 2.0E+01 | 2.5E+01 | 86 |
| Equol | 94105-90-5 | 6.6E+00 | 2.1E+01 | 2.1E+01 | 67 |
| Perfluorooctanoic acid (PFOA) | 335-67-1 | 1.0E-02 | 2.2E+01 | 5.0E+01 | 63 |
| Bisphenol A (BPA) | 80-05-7 | 1.0E+00 | 3.6E+01 | 4.4E+01 | 52 |
| Perfluorooctane sulfonate (PFOS) | 1763-23-1 | 3.0E-02 | 2.0E+02 | 6.0E+02 | 100 |
| Sodium valproate | 1069-66-5 | 6.0E+02 | 1.5E+03 | 1.7E+03 | 100 |
| GROUP 2 | |||||
| 2-Ethylhexyl-tetrabromobenzoate | 183658-27-7 | 9.1E-05 | 9.1E-04 | 9.1E-03 | 100 |
| Ethinylestradiol | 57-63-6 | 3.0E-02 | 3.0E-02 | 3.0E-02 | 100 |
| 1,2,5,6-Tetrabromocyclooctane | 3194-57-8 | 7.0E-02 | 2.2E-01 | 7.0E-01 | 100 |
| Epoxiconazole | 133855-98-8 | 1.0E-01 | 4.8E-01 | 8.0E-01 | 100 |
| Tricresyl phosphate | 1330-78-5 | 2.7E-02 | 5.2E-01 | 1.0E+01 | 100 |
| Bis(2-ethylhexyl)-tetrabromophthalate | 26040-51-7 | 2.1E-01 | 6.7E-01 | 2.1E+00 | 100 |
| Tamoxifen | 10540-29-1 | 1.0E-01 | 3.0E+00 | 3.0E+00 | 100 |
| Danazol | 17230-88-5 | 1.0E+00 | 3.2E+00 | 1.0E+01 | 100 |
| Colchicine | 64-86-8 | 5.0E-02 | 5.0E+00 | 1.0E+01 | 40 |
| 2,3',4,4',5-Pentachlorobiphenyl (PCB118) | 31508-00-6 | 4.0E+00 | 7.5E+00 | 1.4E+01 | 100 |
| Cypermethrin | 52315-07-8 | 3.1E+00 | 1.0E+01 | 1.0E+01 | 100 |
| 2,4-Dichlorophenoxyacetic acid | 94-75-7 | 1.0E+02 | 1.0E+02 | 5.0E+02 | 100 |
| Molinate | 2212-67-1 | 1.0E+02 | 1.0E+02 | 1.0E+02 | 100 |
| 17α-Methyltestosterone | 58-18-4 | 1.0E+00 | 3.2E+02 | 1.0E+03 | 33 |
| Ethanol | 64-17-5 | 5.0E+04 | 7.1E+04 | 1.0E+05 | 100 |
| NEGATIVES | HCT (μM) | ||||
| 2'-OH-BDE-68 | 79755-43-4 | 1.0E+00 | |||
| 6-OH-BDE-47 | 1017894-49-3 | 1.0E+00 | |||
| 2'-MeO-BDE-68 | 96920-28-4 | 1.0E+01 | |||
| 5-Cl-6-MeO-BDE-47 | 497106-81-7 | 1.0E+01 | |||
| 6-MeO-BDE-47 | 102739-99-1 | 1.0E+01 | |||
| Benomyl | 17804-35-2 | 1.0E+01 | |||
| Nonoxynol-9 | 26027-38-3 | 1.0E+01 | |||
| 2-Chloro-4,6-diamino-1,2,5-triazine | 3397-62-4 | 3.0E+01 | |||
| 2,4-Dinitrophenol | 51-28-5 | 1.0E+02 | |||
| Bisphenol S | 80-09-1 | 1.0E+02 | |||
| Ethane dimethane sulphonate (EDS) | 4672-49-5 | 1.0E+02 | |||
| Methomyl | 16752-77-5 | 1.0E+02 | |||
| Spironolactone | 52-01-7 | 1.0E+02 | |||
| Glyphosate | 1071-83-6 | 1.0E+03 | |||
| Acetone | 67-64-1 | 5.1E+05 | |||
Median LELs: Positive H295R and gonadal studies testing the effect of a chemical in at least two concentrations on E2 levels were used for median potency calculation; AC50s were considered when LELs were not reported. For negative chemicals, the HCT is reported. Only one study reporting effects on E2 included for apigenin.
All H295R and gonadal studies and protocols demonstrating an effect on estrogen levels were included for characterization of direction of effect. A cut-off of 75% was applied to determine a predominant effect direction. The colors green and red indicate chemicals predominantly causing decreases and increases in estrogen levels, respectively, and the numbers reflect the percentage of studies affected. Chemicals without a predominant effect are not colored, and the number represents the percentage of studies showing a decrease in estrogen levels.
LEL: Lowest Effect Level; HCT: Highest concentration tested
Fifteen chemicals met the criteria for negative reference chemicals, and eight of these inactive chemicals were tested in guideline studies, including methomyl, glyphosate, benomyl, 2,4-dinitrophenol, acetone, spironolactone, ethane-1,2-dimethylsulphonate and nonoxynol-9 (Supplemental Table 6; Supplemental Files 8 and 9). The highest concentration tested for all negative chemicals were ≥ 1 μM.
Potency of candidate reference chemicals affecting estrogen synthesis
Among group 1 positive candidate reference chemicals, 2,3,7,8-tetrachlorodibenzodioxin (TCDD) displayed the lowest median potency value (0.0001 μM), followed by letrozole, PCB153, prochloraz, forskolin, myclobutanil, zearalenone, daidzein, mifepristone, fadrozole, fenarimol, and ketoconazole (median potency values ≤ 1 μM). The median potency values for 44% group 1 and 2 chemicals (20/44) ranged between 1 and 50 μM, and six chemicals, including PFOS, sodium valproate, 2,4-dichlorophenoxyacetic acid and molinate displayed a median potency value ≥ 50 μM (Fig. 5B; Table 3; Supplemental File 10).
A total of 15 candidate positive reference chemicals had studies indicating both decreases and increases in estrogen levels, and the direction of the effect was determined by consistent results in at least 75% of the positive studies. Results of studies identified in literature searches indicated 17 chemicals decreased and 16 chemicals increased estrogen synthesis (Table 3). Similar to androgen results, pharmaceuticals and pesticides known to act as inhibitors of steroidogenic enzymes, including aromatase, caused a decrease in estrogen levels. Forskolin, atrazine, prometon, and vinclozolin, among others, consistently induced estrogens in vitro. Inconsistent effects (less than 75% total studies with a predominant effect in a specific direction) were observed for 11 chemicals, including daidzein, genistein, DEHP and bisphenol A (BPA) (Table 3).
3.4. Overlap of candidate reference chemicals affecting androgens and estrogen synthesis
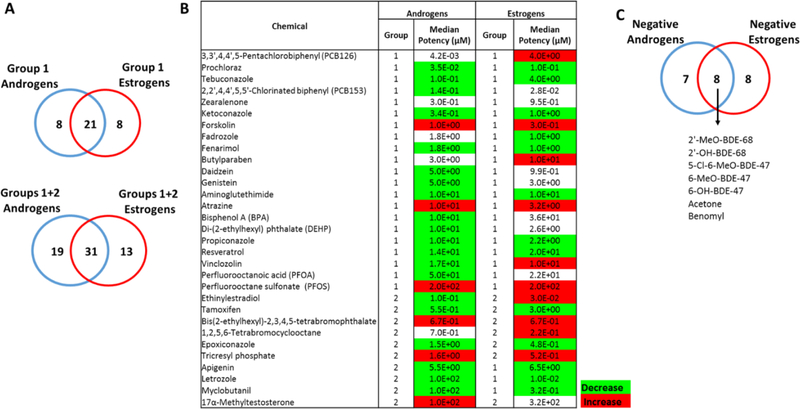
Using the defined list of candidate reference chemicals, we identified sets of compounds affecting both androgen and estrogen levels (Fig. 6A–B). A total of 21 chemicals met all criteria for group 1 reference chemicals that altered synthesis of both androgens and estrogens, including forskolin, atrazine, several azole and azole-like chemicals (prochloraz, tebuconazole, ketoconazole, fenarimol, fadrozole, and propiconazole). Additionally, the phytochemicals genistein, daidzein, resveratrol, zearalenone, PCBs, BPA, DEHP, and perfluorinated compounds were commonly categorized as group 1 reference chemicals for androgens and estrogens. Inclusion of group 2 candidate reference chemicals resulted in a total of 31 chemicals affecting both androgens and estrogens, which included other azoles (letrozole, myclobutanil, epoxiconazole), apigenin, and tamoxifen. Seven chemicals had no effect on either androgen or estrogen synthesis (Fig. 6C).
Figure 6. Candidate reference chemicals affecting both the levels of androgens and estrogens.
A. Venn diagram indicating number of group 1 and group 2 reference chemicals commonly affecting the levels of androgens and estrogens. B. Median potency values of candidate reference chemicals for T and E2. The colors designate the predominant direction of effect in the positive studies. Green and red indicate, respectively, that chemicals caused a decrease (inhibition) and increase (induction) on the levels of the sex hormones in at least 75% studies. Absence of color indicates no predominant effect. C. Venn overlap of negative chemicals for both androgens and estrogens.
The median LELs for 42% of chemicals (13/31) ranged from 1 and 50 μM, and PFOS and methyltestosterone were the weakest chemicals, with median LELs ≥ 50 μM for both T and E2. Five group 1 and three group 2 reference chemicals displayed median potency values ≤ 1 μM for both T and E2: prochloraz, forskolin, ketoconazole, PCB153, zearalenone, ethinylestradiol, tetrabromophthalate, and tetrabromocyclooctane. Letrozole and myclobutanil were clearly stronger inhibitors for E2 compared to T, displaying over 100-fold preferential inhibitory activity on E2 synthesis based on the median potency values. On the contrary, the median potency values obtained from the studies suggest that tebuconazole has a stronger inhibitory effect on T, with a 40-fold higher potency in affecting T synthesis compared to E2. A total of 12 chemicals, including azoles, aminogluthetimide, resveratrol, apigenin, and tamoxifen consistently inhibited both androgen and estrogen synthesis, while forskolin, atrazine, bis (2-ethylhexyl)-2,3,4,5-tetrabromophthalate, tricresyl phosphate, and PFOS increased the levels of both sex hormones. Vinclozolin displayed opposite steroidogenic effects on androgens and estrogens, predominantly causing decreases on androgen levels, while increasing estrogens (Fig. 6B).
4. Discussion
The US EPA’s EDSP is transitioning from using traditional testing methods to high throughput screening in vitro assays and predictive models to identify chemicals with potential endocrine bioactivity. Herein, we identified a set of chemicals that affect the synthesis of androgens (A4 and T) and estrogens (E1 and E2) in cell-based assays that may be useful for the validation of in vitro steroidogenesis models. This reference chemical set was identified from H295R studies reported in the scientific literature, data from the OECD interlaboratory validation of the guideline H295R assay, and studies submitted to the EPA in response to EDSP Tier 1 test orders (List 1 chemicals; SI File 2). A literature search for in vitro steroidogenesis assays conducted in gonadal models was also performed to increase the confidence and corroborate steroidogenic activities of the list of the candidate reference chemicals.
Demonstration of activity in H295R assays was required as an initial criterion for candidate reference chemicals, as the H295R assay is used to screen chemicals in the EDSP Tier 1 battery, and ToxCast includes data from the HT-H295R alternative to the Tier 1 assay (Karmaus et al., 2016). Moreover, solvent and cytotoxicity controls were included in 74% (400/540) of H295R studies identified in the literature search, indicating that altered steroid levels were likely a result of altered steroidogenesis, rather than confounding effects. Testicular and ovarian-derived cell models identified in the second literature search are relevant tools for identifying chemicals that alter sex steroid hormone synthesis, but were only used to support the H295R data due to lack of harmonized test guideline, difficulties measuring cytotoxicity in whole tissue cultures (e.g., slices and explants), lack of clear indication of viability controls, or difficulties in discernment between an effect on steroidogenesis or on differentiation/maturation of cells in primary cultures.
Twenty-nine compounds satisfied five criteria defined herein for identification of candidate reference chemicals (group 1 chemicals) (Tables 2 and 3). This set of reference chemicals could be expanded for the chemicals that met criteria for group 2 candidate reference chemicals by conducting additional studies, in particular for those compounds tested without discernable cell viability controls or without a characterized concentration-response effect.
It should be noted that cytotoxicity or non-specific interactions within the steroidogenic model could contribute to altered steroid levels. For example, the surfactant nonoxynol-9 altered T levels in OECD H295R validation studies only at the highest non-cytotoxic concentration (1–10 μM) (Hecker et al., 2011); in testis fragments, where no viability measurements were performed, nonoxynol-9 decreased T levels at 300 and 1000 μM. The effect of nonoxynol-9 may possibly be attributed to disruption of H295R and Leydig cell membranes due to the detergent properties of the compound (Freyberger et al., 2010). Additionally, nonoxynol-9 has presented uncertainties related to the nature of the substance tested in the H295R and gonadal studies. No CASRN was provided for nonoxynol-9 in the studies using testis fragments (Freyberger et al., 2010), and the CASRN indicated for nonoxynol-9 in the OECD H295R validation studies corresponds to a mixture of repeating ethoxy (oxy-1,2ethanediyl) groups of undefined composition (Hecker et al., 2011). Therefore, the CASRN provided for nonoxynol-9 cannot be definitively linked to a specific substance.
Many of the candidate reference chemicals are well characterized and have additional in vitro and in vivo data supporting steroidogenic effects. The list of candidate reference chemicals includes pesticides or pharmaceuticals (e.g. prochloraz, tebuconazole, ketoconazole, fadrozole, propiconazole, epoxiconazole, letrozole, myclobutanil, and aminogluthethimide) which are known inhibitors of enzymes related to sterol and steroid hormone synthesis (Mason et al., 1987; Roelofs et al., 2013; Trosken et al., 2004; Vanden Bossche et al., 1994; Warrilow et al., 2013). Candidate reference chemicals also included known estrogen receptor (ER) ligands, such as BPA, genistein, daidzein, ethynilestradiol, zearalenone, tamoxifen, and resveratrol, which suggests that perturbation of ER signaling could be involved in the mechanism of action of these chemicals. Several steroid hormone receptors including the ERs are expressed in H295R cells (Montanaro et al., 2005), and evidence of involvement of the ERs in steroidogenic processes have been reported in gonad-derived models (Adashi and Hsueh, 1982; Akingbemi et al., 2003; Taniguchi et al., 2007). Moreover, published studies also suggest mode of action for flavonoid compounds (genistein, daidzein, apigenin) via direct inhibition of steroidogenic enzymes (Le Bail et al., 1998). Forskolin induces steroidogenesis by activating adenylyl cyclase, resulting in increased cAMP levels and activation of the PKA (protein kinase A) signaling pathway, and atrazine may also affect steroid levels by a cAMP-mediated mechanism (Kucka et al., 2012).
Characterization of the candidate reference chemicals based on median potency levels and direction of effect was performed considering the H295R results and the gonad-derived data. Differences in the potency or direction of observed effects may be attributed to different sensitivities of models, experimental conditions (e.g., basal or stimulated protocols, exposure duration), biokinetics of the chemical based on the in vitro model and format, compensatory mechanisms or functional signaling pathways dependent on cell/tissue type or species, or developmental stage of the animal from which the gonad test systems were derived. While assessing differences in responses across models is outside the scope of this study, the database can be used to identify a subset of data that may be used to characterize a model, developmental stage, or species-specific effects. For example, BPA predominantly inhibited T production in H295R, ovarian follicles, granulosa cells, and Leydig cells (Supplemental File 6). However, BPA induced E2 levels in all identified H295R studies, whereas it inhibited E2 levels in the gonadal models (Supplemental File 9), possibly indicating different mechanisms of aromatase regulation/estrogen metabolism in gonadal models vs. H295R cells. The selective aromatase inhibitor, fadrozole, inhibited E2 synthesis in all positive H295R and gonadal studies identified in the literature. While fadrozole induced T levels in the majority of the positive Leydig cell studies, it displayed inhibitory effects on T synthesis in H295R cells, indicating that fadrozole may also inhibit enzyme(s) upstream of aromatase, activate different compensatory molecular mechanisms and/or affect metabolism/clearance of estrogen precursors depending on cell type/species.
Moreover, the comparison of H295R and gonadal data can also be useful for characterizing specificity and sensitivity of test systems and can potentially identify the need to include additional gonadal models in the ToxCast/Tox21 steroidogenesis screening panel. For example, H295R cells may not capture effects on sex steroid hormone synthesis mediated by gonadotropin hormone receptors, as these cells lack fully functional luteinizing hormone (LH) and follicle-stimulating hormone (FSH) receptors (Hecker et al., 2011; Rao Ch et al., 2004). Therefore, compounds that alter steroidogenesis by affecting gonadotropin receptors may be inactive in H295R cells.
Limitations and future directions
These literature reviews were designed to specifically identify studies reporting the effect of a chemical on the synthesis of androgens or estrogens, such that studies selected for full test screening and data extraction had to mention the effect of the chemical substance on androgens or estrogens in the abstract. This might have been limiting for identifying inactive chemicals, as negative outcomes may not be reported in abstracts. As such, the current list of inactive reference chemicals is enriched with derivatives of brominated diphenyl ethers (BDEs) and inclusion of other compounds would be essential to increase the structural diversity of negative chemicals. Additionally, although the H295R literature search included over 3800 substances (Supplemental File 1), the search targeted chemicals with ToxCast HTS data and substances used to validate the EDSP Tier 1 assays. Thus, pharmaceuticals not listed in ToxCast whose mode of action is associated with alteration of sterol/steroid hormone synthesis, such as some cancer therapy drugs, fungicides, and modulators of specific signaling pathways relevant for steroidogenesis (kinase activators and inhibitors), may have failed to be captured in the H295R search. Inclusion of pharmaceutical chemicals that are likely to be selective and strongly active would be valuable additions to the reference chemical list as the majority of the positive chemicals have relatively weak activity (median potency values higher than 1 μM).
An additional challenge surrounds the curation of chemicals in literature studies. As previously indicated for nonoxynol-9, many of the publications contained incomplete or inconsistent information on chemical names, CASRNs, or chemical structures. Given the prospect of tracking data from hundreds of labs to verify the particulars of a chemical tested, the authors used a hierarchy of chemical search tools and their professional judgement to identify the most likely test substance when the chemical information was not ideal. Ultimately, this issue highlights the need for journals to require that the authors of toxicological studies provide more robust chemical information in their papers.
Previous efforts for identification of reference chemicals for validation of in vitro assays have considered percentage of positive studies as a criterion for determining candidate positive reference chemicals. At least 70% positive studies were required as a threshold for identification of candidate reference chemicals for androgen receptor activity (Kleinstreuer et al., 2016). Percentage of positive studies was not considered for identification of candidate reference chemicals for in vitro steroidogenesis assays due to the great variability in responses depending on experimental protocols, exposure windows, basal or stimulated conditions, and sensitivity of the models. As an example, E2 secretion was inhibited in human granulosa cells after treatment for 8, 12, and 24 h with 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), while exposures for 4, 36, and 48 h did not significantly affect E2 levels (Baldridge et al., 2015). Another publication reported that TCDD modulated E2 synthesis under all the tested protocols in human granulosa cells (n=4 studies) (Moran et al., 2000), while no effect on E2 secretion was reported for TCDD under any of the experimental conditions (n=34 studies) in a paper evaluating rat granulosa cell steroidogenesis (Son et al., 1999). Although 14 out of the 20 publications that evaluated the effect of TCDD on E2 synthesis had at least one protocol yielding a positive result, TCDD affected E2 levels in less than 50% of all extracted studies (Supplemental File 9). Therefore, percentage of positive studies was not included as criterion for candidate reference chemical determination, as results may be skewed depending on number of studies/protocols used in the publications.
While mechanistic information was not required for the selection of the candidate reference chemicals, identification of molecular targets associated with the disruptive activity of these compounds may be important for potential refinement of this reference chemical list and may aid in developing computational models of steroidogenesis via identification of additional pathways that contribute to the effects measured on androgen and estrogen production (Saito et al., 2016). Therefore, studies assessing alterations in the activity of aromatase and other steroidogenic enzymes, effects on cAMP and other second messengers, changes in the expression of cholesterol and steroid hormone biosynthesis genes, reactive oxygen production, activation of nuclear and membrane receptors associated with steroidogenic processes, and other mechanistic data could be captured in future literature search strategies. Finally, criteria for reference chemicals could be redefined based on mechanistic data, extent of perturbation of the steroidogenic pathway, efficacy (integration of fold change magnitude), number and percentage of positive studies, consistency in the direction of effects across models, and other pertinent factors.
In summary, we identified candidate reference chemicals for in vitro steroidogenesis assays based on their capability to alter androgen and estrogen synthesis in guideline and non-guideline H295R assays and in gonadal cell models. The list includes chemicals with different potency levels that are capable of increasing or decreasing the levels of the sex hormones in vitro. These candidate reference chemicals will be useful for the validation and/or development of in vitro assays and predictive models for steroidogenesis.
Supplementary Material
Highlights.
A literature review of in vitro steroidogenesis studies was performed.
Chemical effects on the levels of androgens/estrogens were extracted into a database.
Candidate reference chemicals for in vitro steroidogenesis assays were identified.
The reference chemicals may be used to validate in vitro steroidogenesis models.
Acknowledgements
The research described in this article has been funded wholly or in part by the United States Environmental Protection Agency (EPA) administered by the Oak Ridge Institute for Science and Education (ORISE) through an interagency agreement between the U.S. Department of Energy and EPA. It has been subjected to review by the Office of Science Coordination and Policy and approved for publication. Approval does not signify that the contents reflect the views of the EPA or OECD, nor does mention of trade names or commercial products constitute endorsement or recommendation for use. The authors would like to acknowledge the Interagency Coordinating Committee on the Validation of Alternative Methods (ICCVAM) Reference Chemical Work Group for supporting the criteria for identification of candidate reference chemicals for steroidogenesis. We would like to thank Drs. Katie Paul-Friedman (National Center for Computational Toxicology [NCCT], U.S. EPA) and Agnes Karmaus (Integrated Laboratory Systems [ILS], Research Triangle Park, NC) for their valuable comments and contributions to the manuscript. We also wish to thank Dr. Anthony Williams (NCCT, U.S. EPA) for help in the curation of the candidate reference chemicals.
Abbreviations:
- EDSP
Endocrine Disruptor Screening Program
- OPPTS
Office of Prevention, Pesticides and Toxic Substances
- U.S EPA
United States Environmental Protection Agency
- OECD
Organization for Economic Cooperation and Development
- A4
Androstenedione
- T
Testosterone
- E2
17β-estradiol
- E1
Estrone
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adashi EY, Hsueh AJ, 1982. Estrogens augment the stimulation of ovarian aromatase activity by follicle-stimulating hormone in cultured rat granulosa cells. J Biol Chem 257, 6077–6083. [PubMed] [Google Scholar]
- Akingbemi BT, Ge R, Rosenfeld CS, Newton LG, Hardy DO, Catterall JF, Lubahn DB, Korach KS, Hardy MP, 2003. Estrogen receptor-alpha gene deficiency enhances androgen biosynthesis in the mouse Leydig cell. Endocrinology 144, 84–93. [DOI] [PubMed] [Google Scholar]
- Albert O, Desdoits-Lethimonier C, Lesne L, Legrand A, Guille F, Bensalah K, Dejucq-Rainsford N, Jegou B, 2013. Paracetamol, aspirin and indomethacin display endocrine disrupting properties in the adult human testis in vitro. Hum Reprod 28, 1890–1898. [DOI] [PubMed] [Google Scholar]
- Amiri BM, Maebayashi M, Adachi S, Moberg GP, Doroshov SI, Yamauchi K, 1999. In vitro steroidogenesis by testicular fragments and ovarian follicles in a hybrid sturgeon, Bester. Fish Physiology and Biochemistry 21, 1–14. [Google Scholar]
- Baldridge MG, Marks GT, Rawlins RG, Hutz RJ, 2015. Very low-dose (femtomolar) 2,3,7,8tetrachlorodibenzo-p-dioxin (TCDD) disrupts steroidogenic enzyme mRNAs and steroid secretion by human luteinizing granulosa cells. Reproductive Toxicology 52, 57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beitel SC, Doering JA, Patterson SE, Hecker M, 2014. Assessment of the sensitivity of three North American fish species to disruptors of steroidogenesis using in vitro tissue explants. Aquat Toxicol 152, 273–283. [DOI] [PubMed] [Google Scholar]
- Browne P, Judson RS, Casey WM, Kleinstreuer NC, Thomas RS, 2015. Screening Chemicals for Estrogen Receptor Bioactivity Using a Computational Model. Environ Sci Technol 49, 8804–8814. [DOI] [PubMed] [Google Scholar]
- Cash R, Brough AJ, Cohen MN, Satoh PS, 1967. Aminoglutethimide (Elipten-Ciba) as an inhibitor of adrenal steroidogenesis: mechanism of action and therapeutic trial. J Clin Endocrinol Metab 27, 1239–1248. [DOI] [PubMed] [Google Scholar]
- Crisp TM, Clegg ED, Cooper RL, Wood WP, Anderson DG, Baetcke KP, Hoffmann JL, Morrow MS, Rodier DJ, Schaeffer JE, Touart LW, Zeeman MG, Patel YM, 1998. Environmental endocrine disruption: an effects assessment and analysis. Environ Health Perspect 106 Suppl 1, 11–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freyberger A, Weimer M, Lofink W, Ahr HJ, 2010. Short-term dynamic culture of rat testicular fragments as a model to assess effects on steroidogenesis--potential use and limitations. Reproductive Toxicology 30, 36–43. [DOI] [PubMed] [Google Scholar]
- Garcia-Lopez A, de Jonge H, Nobrega RH, de Waal PP, van Dijk W, Hemrika W, Taranger GL, Bogerd J, Schulz RW, 2010. Studies in zebrafish reveal unusual cellular expression patterns of gonadotropin receptor messenger ribonucleic acids in the testis and unexpected functional differentiation of the gonadotropins. Endocrinology 151, 2349–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge RS, Hardy MP, 1998. Variation in the end products of androgen biosynthesis and metabolism during postnatal differentiation of rat Leydig cells. Endocrinology 139, 3787–3795. [DOI] [PubMed] [Google Scholar]
- Goetz AK, Rockett JC, Ren H, Thillainadarajah I, Dix DJ, 2009. Inhibition of rat and human steroidogenesis by triazole antifungals. Syst Biol Reprod Med 55, 214–226. [DOI] [PubMed] [Google Scholar]
- Gregoraszczuk E, Stoklosowa S, Duda M, Slomczynska M, 1996. Does ovarian granulosa and theca cell interaction in co-culture affect the cytoplasmic microtubule organization? Cytobios 88, 133–140. [PubMed] [Google Scholar]
- Gregoraszczuk EL, Ptak A, Karniewska M, Ropstad E, 2008. Action of defined mixtures of PCBs, p,p’-DDT and its metabolite p,p’-DDE, on co-culture of porcine theca and granulosa cells: steroid secretion, cell proliferation and apoptosis. Reproductive Toxicology 26, 170–174. [DOI] [PubMed] [Google Scholar]
- Hecker M, Hollert H, Cooper R, Vinggaard AM, Akahori Y, Murphy M, Nellemann C, Higley E, Newsted J, Laskey J, Buckalew A, Grund S, Maletz S, Giesy J, Timm G, 2011. The OECD validation program of the H295R steroidogenesis assay: Phase 3. Final inter-laboratory validation study. Environ Sci Pollut Res Int 18, 503–515. [DOI] [PubMed] [Google Scholar]
- Hecke M., Holler H., Coope R., Vinggaar AM., Akahor Y., Murph M., Nelleman C., Higle E., Newste J., W R., La P., Laske J., Buckale A., Grun S., Naka M., Tim G., Gies J., 2007. The OECD validation program of the H295R steroidogenesis assay for the identification of in vitro inhibitors and inducers of testosterone and estradiol production. Phase 2: Inter-laboratory pre-validation studies. Environmental Science and Pollution Research 14, 23–30. [DOI] [PubMed] [Google Scholar]
- Hwang GS, Chen ST, Chen TJ, Wang SW, 2009. Effects of hypoxia on testosterone release in rat Leydig cells. Am J Physiol Endocrinol Metab 297, E1039–1045. [DOI] [PubMed] [Google Scholar]
- Judson RS, Magpantay FM, Chickarmane V, Haskell C, Tania N, Taylor J, Xia M, Huang R, Rotroff DM, Filer DL, Houck KA, Martin MT, Sipes N, Richard AM, Mansouri K, Setzer RW, Knudsen TB, Crofton KM, Thomas RS, 2015. Integrated Model of Chemical Perturbations of a Biological Pathway Using 18 In Vitro High-Throughput Screening Assays for the Estrogen Receptor. Toxicol Sci 148, 137–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmaus AL, Toole CM, Filer DL, Lewis KC, Martin MT, 2016. High-Throughput Screening of Chemical Effects on Steroidogenesis Using H295R Human Adrenocortical Carcinoma Cells. Toxicological Sciences 150, 323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinstreuer NC, Ceger P, Watt ED, Martin M, Houck K, Browne P, Thomas RS, Casey WM, Dix DJ, Allen D, Sakamuru S, Xia M, Huang R, Judson R, 2016. Development and Validation of a Computational Model for Androgen Receptor Activity. Chem Res Toxicol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraugerud M, Zimmer KE, Dahl E, Berg V, Olsaker I, Farstad W, Ropstad E, Verhaegen S, 2010. Three structurally different polychlorinated biphenyl congeners (Pcb 118, 153, and 126) affect hormone production and gene expression in the human H295R in vitro model. J Toxicol Environ Health A 73, 1122–1132. [DOI] [PubMed] [Google Scholar]
- Kucka M, Pogrmic-Majkic K, Fa S, Stojilkovic SS, Kovacevic R, 2012. Atrazine acts as an endocrine disrupter by inhibiting cAMP-specific phosphodiesterase-4. Toxicol Appl Pharmacol 265, 19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudoh M, Susaki Y, Ideyama Y, Nanya T, Okada M, Shikama H, Fujikura T, 1995. The potent and selective inhibition of estrogen production by non-steroidal aromatase inhibitor, YM511. J Steroid Biochem Mol Biol 54, 265–271. [DOI] [PubMed] [Google Scholar]
- Laville N, Balaguer P, Brion F, Hinfray N, Casellas C, Porcher JM, Ait-Aissa S, 2006. Modulation of aromatase activity and mRNA by various selected pesticides in the human choriocarcinoma JEG-3 cell line. Toxicology 228, 98–108. [DOI] [PubMed] [Google Scholar]
- Le Bail JC, Laroche T, Marre-Fournier F, Habrioux G, 1998. Aromatase and 17beta-hydroxysteroid dehydrogenase inhibition by flavonoids. Cancer Lett 133, 101–106. [DOI] [PubMed] [Google Scholar]
- Lee HR, Jeung EB, Cho MH, Kim TH, Leung PC, Choi KC, 2013. Molecular mechanism(s) of endocrinedisrupting chemicals and their potent oestrogenicity in diverse cells and tissues that express oestrogen receptors. J Cell Mol Med 17, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis AK, Thomas P, 2000. Effects of estrogens and xenoestrogens on androgen production by Atlantic croaker testes in vitro: evidence for a nongenomic action mediated by an estrogen membrane receptor. Biol Reprod 62, 995–1004. [DOI] [PubMed] [Google Scholar]
- Loose DS, Kan PB, Hirst MA, Marcus RA, Feldman D, 1983. Ketoconazole blocks adrenal steroidogenesis by inhibiting cytochrome P450-dependent enzymes. J Clin Invest 71, 1495–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankidy R, Ranjan B, Honaramooz A, Giesy JP, 2014. Effects of novel brominated flame retardants on steroidogenesis in primary porcine testicular cells. Toxicol Lett 224, 141–146. [PubMed] [Google Scholar]
- Martins RS, Fuentes J, Almeida O, Power DM, Canario AV, 2009. Ca(2+)-Calmodulin regulation of testicular androgen production in Mozambique tilapia (Oreochromis mossambicus). Gen Comp Endocrinol 162, 153–159. [DOI] [PubMed] [Google Scholar]
- Mason JI, Carr BR, Murry BA, 1987. Imidazole antimycotics: selective inhibitors of steroid aromatization and progesterone hydroxylation. Steroids 50, 179–189. [DOI] [PubMed] [Google Scholar]
- Montanaro D, Maggiolini M, Recchia AG, Sirianni R, Aquila S, Barzon L, Fallo F, Ando S, Pezzi V, 2005. Antiestrogens upregulate estrogen receptor beta expression and inhibit adrenocortical H295R cell proliferation. J Mol Endocrinol 35, 245–256. [DOI] [PubMed] [Google Scholar]
- Moran FM, Conley AJ, Corbin CJ, Enan E, VandeVoort C, Overstreet JW, Lasley BL, 2000. 2,3,7,8tetrachlorodibenzo-p-dioxin decreases estradiol production without altering the enzyme activity of cytochrome P450 aromatase of human luteinized granulosa cells in vitro. Biol Reprod 62, 1102–1108. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Xing Y, Hui XG, Kurotaki Y, Ono K, Cohen T, Sasano H, Rainey WE, 2011. Human adrenal cells that express both 3beta-hydroxysteroid dehydrogenase type 2 (HSD3B2) and cytochrome b5 (CYB5A) contribute to adrenal androstenedione production. J Steroid Biochem Mol Biol 123, 122–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OECD, 2011. Test No. 456: H295R Steroidogenesis Assay. OECD Publishing. [Google Scholar]
- Rao Ch V, Zhou XL, Lei ZM, 2004. Functional luteinizing hormone/chorionic gonadotropin receptors in human adrenal cortical H295R cells. Biol Reprod 71, 579–587. [DOI] [PubMed] [Google Scholar]
- Rijk JC, Peijnenburg AA, Blokland MH, Lommen A, Hoogenboom RL, Bovee TF, 2012. Screening for modulatory effects on steroidogenesis using the human H295R adrenocortical cell line: a metabolomics approach. Chem Res Toxicol 25, 1720–1731. [DOI] [PubMed] [Google Scholar]
- Roelofs MJE, Piersma AH, van den Berg M, van Duursen MBM, 2013. The relevance of chemical interactions with CYP17 enzyme activity: Assessment using a novel in vitro assay. Toxicol Appl Pharmacol 268, 309–317. [DOI] [PubMed] [Google Scholar]
- Saito R, Terasaki N, Yamazaki M, Masutomi N, Tsutsui N, Okamoto M, 2016. Estimation of the Mechanism of Adrenal Action of Endocrine-Disrupting Compounds Using a Computational Model of Adrenal Steroidogenesis in NCI-H295R Cells. J Toxicol 2016, 4041827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders DM, Higley EB, Hecker M, Mankidy R, Giesy JP, 2013. In vitro endocrine disruption and TCDD-like effects of three novel brominated flame retardants: TBPH, TBB, & TBCO. Toxicol Lett 223, 252–259. [DOI] [PubMed] [Google Scholar]
- So DS., Ushinoham K., Ga X., Taylo CC., Rob KF., Rozma KK., Terranov PF., 1999. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) blocks ovulation by a direct action on the ovary without alteration of ovarian steroidogenesis: lack of a direct effect on ovarian granulosa and thecal-interstitial cell steroidogenesis in vitro. Reproductive Toxicology 13, 521–530. [DOI] [PubMed] [Google Scholar]
- Taniguchi F, Couse JF, Rodriguez KF, Emmen JM, Poirier D, Korach KS, 2007. Estrogen receptor-alpha mediates an intraovarian negative feedback loop on thecal cell steroidogenesis via modulation of Cyp17a1 (cytochrome P450, steroid 17alpha-hydroxylase/17,20 lyase) expression. FASEB J 21, 586–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremoen NH, Fowler PA, Ropstad E, Verhaegen S, Krogenaes A, 2014. Exposure to the three structurally different PCB congeners (PCB 118, 153, and 126) results in decreased protein expression and altered steroidogenesis in the human adrenocortical carcinoma cell line H295R. J Toxicol Environ Health A 77, 516–534. [DOI] [PubMed] [Google Scholar]
- Trosken ER, Adamska M, Arand M, Zarn JA, Patten C, Volkel W, Lutz WK, 2006. Comparison of lanosterol-14 alpha-demethylase (CYP51) of human and Candida albicans for inhibition by different antifungal azoles. Toxicology 228, 24–32. [DOI] [PubMed] [Google Scholar]
- Trosken ER, Scholz K, Lutz RW, Volkel W, Zarn JA, Lutz WK, 2004. Comparative assessment of the inhibition of recombinant human CYP19 (aromatase) by azoles used in agriculture and as drugs for humans. Endocr Res 30, 387–394. [DOI] [PubMed] [Google Scholar]
- U.S.EPA, 1998. EDSP: Statement of Police Notice, Federal Register Notice, December 1998. https://www.epa.gov/sites/production/files/2015-08/documents/122898frnotice.pdf.
- U.S.EPA, 2008. Technical Review Document for Endocrine Disruptor Screening Program (EDSP):Proposed Tier 1 Screening Battery.
- U.S.EPA, 2009. Endocrine Disruptor Screening Program (EDSP); Announcing the Availability of the Tier 1 [Google Scholar]
- Screening Battery and Related Test Guidelines. https://www.regulations.gov/document?D=EPA-HQ-OPPT-20080521–0001.
- U.S.EPA, 2012. Endocrine Disruptor Screening Program Universe of Chemicals and General Validation Principles. https://www.epa.gov/sites/production/files/2015-07/documents/edsp_chemical_universe_and_general_validations_white_paper_11_12.pdf.
- U.S.EPA, 2015a. EDSP: Use of High Throughput Assays and Computational Tools, Federal Register Notice, June 2015. http://www.gpo.gov/fdsys/pkg/FR-2015-06-19/pdf/2015-15182.pdf.
- U.S.EPA, 2015b. Endocrine Disruptor Screening Program Tier 1 Assessments. https://www.epa.gov/ingredientsused-pesticide-products/endocrine-disruptor-screening-program-tier-1-assessments.
- U.S.EPA, 2016. Endocrine Disruptor Screening Program (EDSP) Overview. https://www.epa.gov/endocrinedisruption/endocrine-disruptor-screening-program-edsp-overview.
- Vanden Bossche HV, Moereels H, Koymans LM, 1994. Aromatase inhibitors--mechanisms for non-steroidal inhibitors. Breast Cancer Res Treat 30, 43–55. [DOI] [PubMed] [Google Scholar]
- Warrilow AG, Parker JE, Kelly DE, Kelly SL, 2013. Azole affinity of sterol 14alpha-demethylase (CYP51) enzymes from Candida albicans and Homo sapiens. Antimicrob Agents Chemother 57, 1352–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao YC, Huang YD, Hardy DO, Li XK, Ge RS, 2010. Glucocorticoid Suppresses Steroidogenesis in Rat Progenitor Leydig Cells. Journal of Andrology 31, 365–371. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Ao H, Chen L, Sottas CM, Ge RS, Li L, Zhang Y, 2012. Mono-(2-ethylhexyl) phthalate affects the steroidogenesis in rat Leydig cells through provoking ROS perturbation. Toxicology in Vitro 26, 950–955. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.