Abstract
Purpose of Review
This review will focus on the long-term outcomes in offspring exposed to in utero hyperglycemia and gestational diabetes (GDM), including obesity, adiposity, glucose metabolism, hypertension, hyperlipidemia, nonalcoholic fatty liver disease, and puberty.
Recent Findings
There is evidence, mostly from observational studies, that offspring of GDM mothers have increased risk of obesity, increased adiposity, disorders of glucose metabolism (insulin resistance and type 2 diabetes), and hypertension. In contrast, evidence from the two intervention studies of treatment of mild GDM and childhood measures of BMI, adiposity, and glucose tolerance do not demonstrate that GDM treatment significantly reduces adverse childhood metabolic outcomes. Thus, more evidence is needed to understand the impact of maternal GDM on offspring’s adiposity, glucose metabolism, lipid metabolism, risk of fatty liver disease, and pubertal onset.
Summary
Offspring of GDM mothers may have increased risk for metabolic and cardiovascular complications. Targeting this group for intervention studies to prevent obesity and disorders of glucose metabolism is one potential strategy to prevent adverse metabolic health outcomes.
Keywords: Exposure to gestational diabetes mellitus, Pregnancy, Childhood metabolic disease, Long-term offspring outcomes, Developmental programming of diabetes, Gestational diabetes treatment effect on offspring
Introduction
The prevalence of diabetes has increased worldwide, especially in developing countries, over the last few decades [1]. More concerning is that the average age of onset of diabetes has decreased resulting in an increasing number of reproductive age women with diabetes [1]. From a public health standpoint, hyperglycemia during pregnancy has identifiable adverse offspring outcomes in the short and long term. Offspring outcomes following exposure to diabetes in pregnancy are directly related to glycemic levels during pregnancy [2–5]. Potential adverse offspring outcomes vary by age group, and avoidance of one outcome does not indicate reduction in risk of other adverse outcomes [4••, 6, 7]. This review will focus specifically on the long-term offspring outcomes following in utero exposure to gestational diabetes mellitus (GDM) and the milder form of hyperglycemia during pregnancy. Exposure to type 1 diabetes will not be addressed in this review and it is important to point out that it has been associated with a unique set of complications.
The effects of maternal hyperglycemia start in utero with a relative fetal hypoxia that may result in the risk of birth asphyxia and stillbirth [8]. Fetal hypoxia is also thought to induce increased erythropoietin production, the precursor to known complications in neonates born to mothers with GDM, i.e., polycythemia and hyperbilirubinemia [9]. Delivery complications in offspring born to women with GDM include high risk of cesarean section, shoulder dystocia, and birth injury and are often due to a large size fetus. Large size at birth is a consequence of fetal hyperinsulinemia and subsequent increased fetal production of insulin-like growth factor-1. Studies have demonstrated that offspring of mothers with GDM have higher amounts of fat mass as opposed to fat-free muscle mass and it is the increased fat mass that results in increased birthweight [10, 11]. Neonates are also predisposed to respiratory distress syndrome (RDS) secondary to fetal hyperinsulinemia that alters lung surfactant synthesis [12]. RDS is a life-threatening condition in the neonatal period that often times requires admission to the neonatal intensive care unit. Lastly, and the most common adverse outcome in neonates born to women with GDM, is hypoglycemia, secondary to fetal hyperinsulinemia [10]. Most often, the hypoglycemia is self-limited and resolves as the hyperinsulinemia resolves.
While recent improvements in maternal glycemic control have resulted in a reduction of perinatal complications, the frequency of adverse pregnancy outcomes remains higher in mothers with GDM than the general population [11, 13]. Although our focus for this review is to report on the long-term offspring outcomes, it is important to note that many of the studies that investigate associations between maternal hyperglycemia and long-term offspring outcomes were composed of mothers with different types of diabetes in pregnancy.
Childhood Complications
Obesity
Some observational studies have shown that offspring of mothers with glycemic disorders in pregnancy have increased obesity in childhood compared to offspring not exposed to maternal hyperglycemia. Specifically, the Pima Indian and Northwestern University studies, which included women with pre-gestational, type 1, type 2, and GDM, were long-term follow-up studies with repeat measures of weight and glucose metabolism throughout childhood. [5, 14] While the Pima Indian population had a high prevalence of obesity and type 2 diabetes mellitus (T2DM), the Northwestern cohort was primarily composed of White women, and obesity and T2DM rates were similar to the general population. These two studies reported higher weight among children exposed to diabetes in pregnancy compared to children who were not exposed. Interestingly, not all offspring exposed to maternal hyperglycemia were large at birth [15]. The timing of obesity development was different in these two cohorts. The offspring of Pima mothers with diabetes in pregnancy weighed more compared to offspring of non-diabetic mothers at every age throughout childhood [15]. However, the Northwestern cohort reported that offspring of mothers with diabetes were noted to have emerging obesity in the peri-pubertal years [16]. The sibling pair studies in the Pima Indian population of offspring born prior to maternal diabetes compared to offspring born after maternal diagnosis of diabetes support the hypothesis that hyperglycemia in utero results in an elevated risk of obesity in the offspring, separate or perhaps additive to the genetic risk and home environment [17]. This finding was replicated in a study of Swedish male siblings born to mothers with diabetes in pregnancy, and similar to the Pima Indians and Northwestern cohorts, the Swedish cohort included mothers with type 1, type 2, and gestational diabetes [18]..
The Australian Carbohydrate Intolerance Study (ACHOIS), a multicenter randomized control trial, studied treatment of mild GDM and perinatal and offspring outcomes. This trial demonstrated that infants born to mothers with treated GDM were less likely to be LGA/macrosomic at birth [11]. A follow-up study of the 4–5-year-old children found that there was no difference in the BMI of the children in the intervention group compared to the routine care control group [19]. Similarly, in a separate randomized study of treatment for mild GDM conducted in the USA, children born to mothers with untreated mild GDM did not have higher BMI Z-scores compared to children born to mothers without GDM [20]. A possible explanation for these negative studies, compared to other positive observational studies, is that the childhood metabolic impact of maternal GDM exposure may not appear until later in childhood.
In summary, many studies, in separate cohorts, have demonstrated associations between maternal GDM and higher childhood BMI [4••, 21–24]; yet, treatment of mild GDM in the few randomized studies did not lead to differences in childhood BMI.
Adiposity
More recent studies have explored body composition measures beyond weight or BMI in offspring of mothers with GDM. In the Hyperglycemia and Adverse Pregnancy Outcomes Follow Up Study (HAPO FUS) offspring exposed to mild, untreated maternal hyperglycemia at a mean age of 11.4 years had increased adiposity, as measured by air displacement plethysmography, skinfold thickness with calipers, and waist circumference [4••]. Figure 1 demonstrates the odds ratio of adiposity outcomes in the 11.4-year-old children exposed to GDM. HAPO FUS corroborates the findings of other studies which have used different methodologies of anthropometry [25], BIA [26], DEXA [27], and MRI [28] to demonstrate increased adiposity among offspring of mothers with GDM. In all these studies, maternal BMI attenuated the results.
Fig. 1.
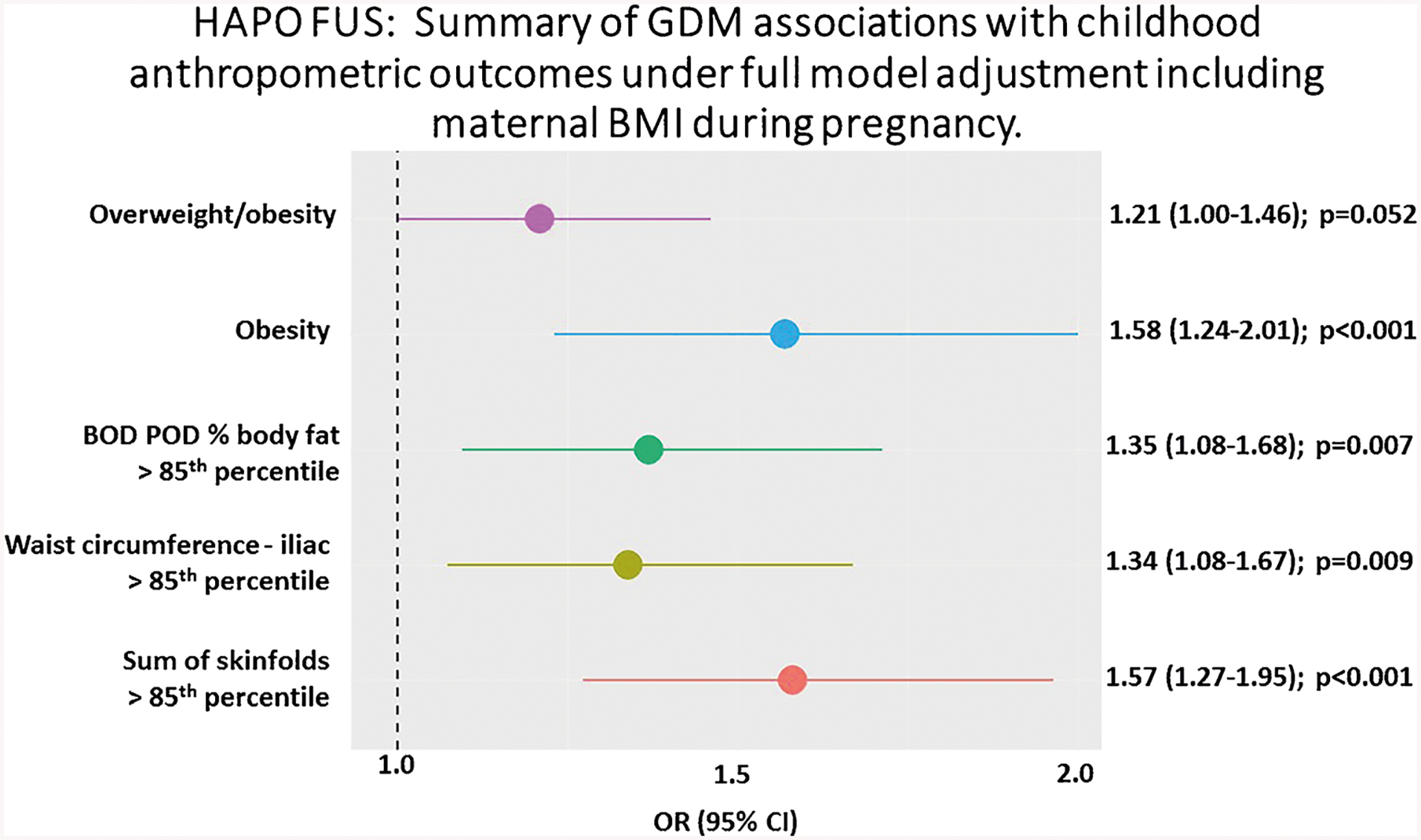
This Forest plot shows that the five dichotomous measures of child adiposity at mean age of 11.4 years are significantly associated with GDM and the associations are strongest for obesity alone and sum of skinfolds
There are studies that have reported no association between GDM and adiposity. For example, in Project Viva, increased adiposity was initially demonstrated among children at 3 years of age born to mothers with hyperglycemia, but by adolescence, that association was no longer demonstrated [29, 30]. It is possible that cohort size, timing of puberty in relation to the study, and differences in glycemic control of mothers enrolled in the study played a role in the contrasting association between maternal glycemia and childhood adiposity. Interestingly, in one of the randomized studies of treatment for mild GDM described above [20], maternal glycemia was associated with increased childhood sum of skinfolds, a measure of adiposity, but not BMI Z-score in the offspring of women with untreated mild GDM compared to offspring of mothers without GDM. Higher adiposity, as opposed to higher weight, has important implications for cardiometabolic diseases and early death [31]. Separating the independent effects of maternal hyperglycemia and maternal obesity will help guide targeting of preventative strategies to decrease risk of obesity in the offspring.
Glucose Metabolism
Potential long-term complications among offspring exposed to GDM include insulin resistance, impaired glucose tolerance, and type 2 diabetes mellitus (T2DM), collectively termed disorders of glucose metabolism. For example, HAPO FUS demonstrated that there is an increased risk of impaired glucose tolerance in the offspring of mothers with mild, untreated hyperglycemia [32, 33] as displayed in Fig. 2 [34•]. It is important to clarify that this association is observed independent of maternal BMI. In the study of women with mild GDM randomized to treatment or usual care discussed earlier, female offspring of treated mothers had lower fasting blood glucose [35]. As reviewed by Kawasaki et al. [36], measurement of glucose tolerance following a glucose load, rather than a fasting glucose level, is better at detecting abnormalities in glucose metabolism in children exposed to GDM. In addition, disposition index, a measure of pancreatic beta cell function and a predictor of progression to T2DM among youth with disorders of glucose metabolism, was significantly lower among offspring of GDM mothers [33, 37].
Fig. 2.
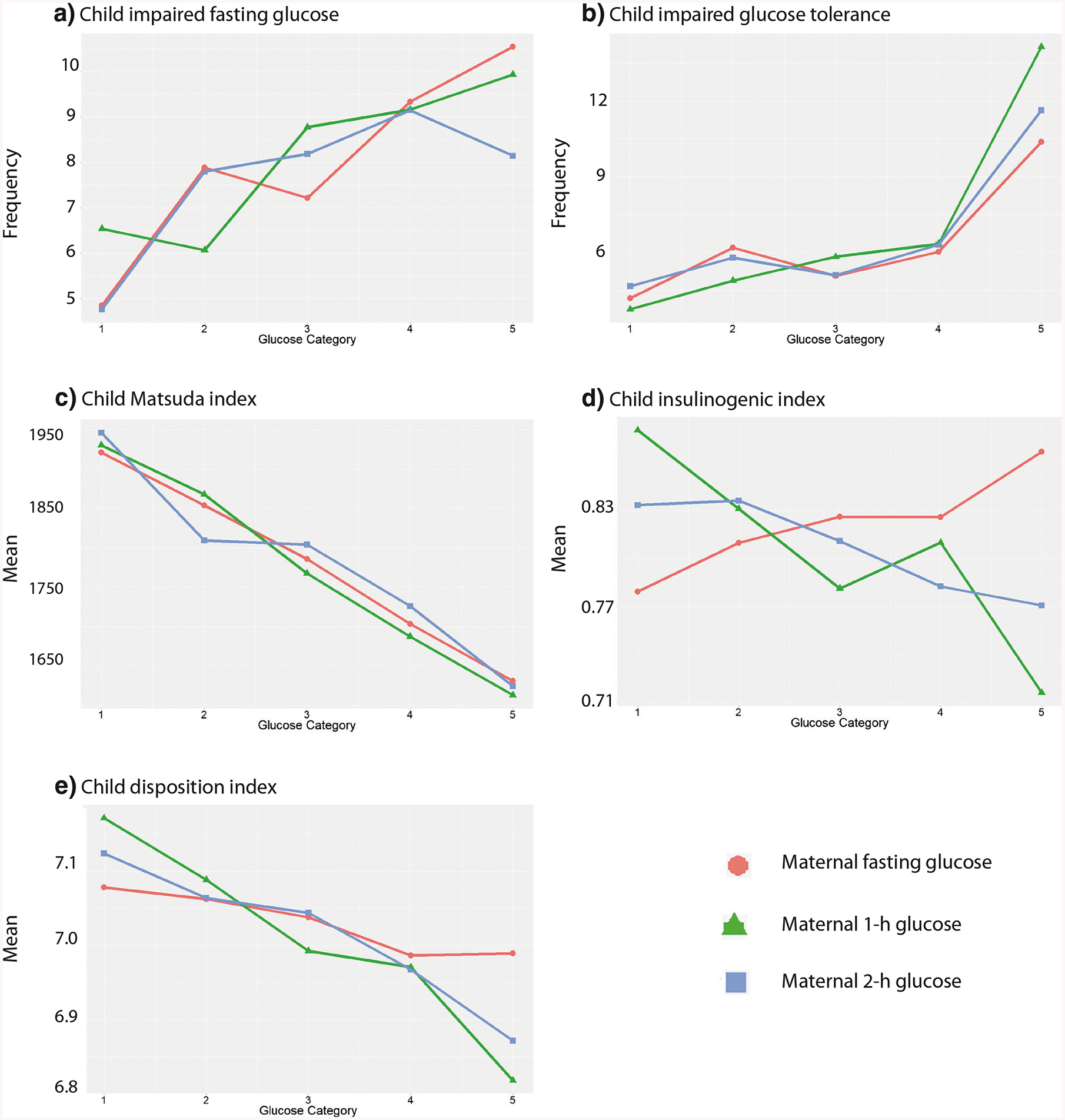
Child glucose outcomes across categories of maternal glucose levels. The frequency of childhood IFG (a) and IGT (b) and means of the Matsuda index (c), insulinogenic index (d), and disposition index (e) across categories of fasting, 1-h, and 2-h PG is shown. Glucose categories are defined as follows: fasting PG level—category 1, < 4.2 mmol/L; category 2, 4.2–4.4 mmol/L; category 3, 4.5–4.7 mmol/L; category 4, 4.8–5.0 mmol/L; and category 5, 5.1 mmol/L or more; 1-h PG level—category 1, 5.8 mmol/L or less; category 2, 5.9–7.3 mmol/L; category 3, 7.4–8.6 mmol/L; category 4, 8.7–9.9 mmol/L; and category 5, ≥ 10.0 mmol/L; and 2-h PG level—category 1, ≤ 5.0 mmol/L; category 2, 5.1–6.0 mmol/L; category 3, 6.1–6.9 mmol/L; category 4, 7.0–8.4 mmol/L; and category 5, ≥8.5 mmol/L (from: Scholtens et al. Diabetes Care. 2019;42 [3]:381–92. American Diabetes Association [Diabetes Care, American Diabetes Association, 2019]. Copyright and all rights reserved. Material from this publication has been used with permission of American Diabetes Association) [34•]
Many epidemiological studies have found higher rates of T2DM in youth who were exposed to maternal GDM as assessed by maternal recall or a national database [37–39]. HAPO FUS provides further evidence that in utero exposure to hyperglycemia, below the diagnostic criteria for GDM, increases future risk of disorders of glucose metabolism potentially as a result of adverse intrauterine fetal programming of the pancreas [34•]. However, it is important to note that smaller studies have not found that exposure to GDM is associated with insulin resistance or other glycemic outcomes in offspring [30, 40]. It is unclear if this difference is an issue of power, a difference in study population (well-controlled GDM vs poorly controlled GDM), or a difference in the timing of offspring follow-up study (pre-pubertal vs pubertal).
Hypertension
Studies have also evaluated hypertension as a potential adverse outcome in offspring of GDM mothers. Hypertension is associated with changes in the adolescent cardiac structure [41–43]. In adults, a 2 mmHg rise in SBP is associated with increased mortality [44]. Intrauterine hyperglycemia, throughout pregnancy or only in the latter two-thirds of the pregnancy, in animal studies has demonstrated decreased nephron numbers, increased angiotensin converting enzyme activity in cardiovascular system, and disrupted fetal vascular development all resulting in increasing the risk for hypertension in the offspring [45–48]. Hypertension is an important contributor to cardiovascular mortality and morbidity and discerning if a relationship between maternal GDM increases the risk of offspring hypertension is a public health concern.
As with disorders of glucose metabolism, sorting out which contributors are responsible for the differences in blood pressure is difficult. There are a number of studies examining the association of GDM on offspring blood pressure with conflicting findings. The initial Pima Indian studies reported elevated systolic blood pressure in the offspring exposed to maternal diabetes (T2DM or GDM), independent of adiposity, compared to offspring born to mothers with a normal glucose tolerance during pregnancy who subsequently developed T2DM after pregnancy but before 40 years of age [49]. A small prospective Chinese cohort studied at a median age of 8 years showed that children born to mothers with GDM had significantly higher systolic and diastolic blood pressures. However, when these children were later restudied at a mean age of 15 years old, there was no significant difference in blood pressure between the control group and those born to mothers with GDM [40, 50]. Interestingly, a larger Chinese cohort from the Hong Kong HAPO FUS site was studied at a median age of 7 years and offspring of GDM mothers were noted to have higher blood pressures at that follow-up compared to those born to mothers without GDM [32]. However, the KiGGS cross-sectional study in Germany of children 3–17 years old reported that there was no difference in blood pressure in offspring of GDM mothers compared to those of non-GDM mothers [51]. A meta-analysis including 15 different studies published before 2012 concluded that children born to mothers with GDM had higher systolic blood pressures but similar diastolic blood pressures compared to non-GDM offspring [52]. A more recent prospective population-based cohort in Portugal, which followed children at ages 4, 7, and 10, reported increased blood pressure in offspring of mothers with mixed types of diabetes at 10 years, with boys being at higher risk than girls [53••].
Lipids
There are few studies on the effects of maternal glycemia on lipid metabolism in the offspring. A rat study found no significant changes in lipid metabolism on offspring of GDM rats from birth to 12 months [54]. In humans, the KiGGS study also reported that there was no differences in cholesterol levels in offspring of GDM mothers at mean age of 10.3 years compared to those of non-GDM mothers [51]. A small Chinese cohort reported lower high-density lipoprotein cholesterol at a median age of 8 years, but at a mean age of 15 years old, there were no significant differences in the lipid profiles of those born to mothers with GDM compared to controls [40, 50]. With the limited data available, it appears that in childhood, we cannot conclude on associations between maternal GDM and offspring lipid metabolism.
Nonalcoholic Fatty Liver Disease
Considering the effect of GDM on disorders of glucose metabolism and hypertension, one could hypothesize that in utero hyperglycemia would also have a negative impact on liver metabolism. In rat models induced to have severe hyperglycemia throughout pregnancy, researchers found that the offspring of the normal weight diabetic rats had more hepatic steatosis than control rats on the same diet [55]. However, with the prevalence of obesity at reproductive age increasing, there is a concern that this model is not typical of what is seen in humans. In a different study, obese GDM rats were studied and the offspring again had higher hepatic steatosis and an altered lipid metabolism within the liver [56]. Nevertheless, this makes separating the effects of obesity from those of GDM on hepatic steatosis difficult. A small human study of 25 neonates found that pre-pregnancy BMI was more strongly associated with neonatal hepatic fat storage than maternal GDM. This suggests that maternal obesity may be a stronger predictor of NAFLD [57]. A more recent study from the EPOCH cohort supports this finding as well as demonstrating that maternal obesity is associated with higher hepatic fat fraction in children and adolescents independent of maternal GDM [58]. Further studies are needed to better understand the relationship between maternal GDM exposure and risk of NAFLD.
Puberty
Associations between GDM exposure and earlier pubertal onset in offspring are currently being explored. An earlier study that did not distinguish between boys and girls reported that there were no differences in age of pubertal onset between offspring of GDM mothers and those of non-GDM mothers [59]. Two recent studies have found that girls born to mothers with GDM have an earlier onset of puberty but not boys [60, 61]. However, it is important to mention that increased BMI and fat mass in childhood have also been associated with earlier onset of puberty [62, 63]. It is unclear if the female offspring of GDM mothers are at increased risk for earlier puberty because of the in utero environment or if earlier puberty is occurring in this group because of the increased fat mass/BMI. In a more recent study, which also demonstrated earlier onset of puberty in girls born to GDM mothers, this association remained even after adjusting for the girls’ BMI [64]. This association is important to note because a more rapid decline in insulin response and disposition index across pubertal Tanner Stages is seen in offspring of GDM mothers [59].
GDM Treatment and Effects on Offspring Outcomes
Considering associations between GDM exposures and short-and long-term offspring outcomes, some studies have attempted to understand whether GDM is a modifiable risk factor. An observational study on the associations of maternal glycemia on offspring obesity found that worsening hyperglycemia in pregnancy was associated with an increased risk of obesity at ages 5–7 [22]. In this study, there was a small group of mothers with GDM that were treated and the association with childhood obesity disappeared, suggesting that GDM treatment may decrease the risk for childhood obesity. Studies have also looked at the long-term offspring outcomes of different pharmacotherapy modalities of GDM. The MiG TOFU randomized control trial reported that offspring of GDM mothers randomized to treatment with metformin or insulin had similar adiposity and metabolic measures at age 7–9 years old [65]. Other randomized control studies comparing metformin to insulin treatment have reported no significant growth or development differences between the two treatment modalities in offspring at age 2 [66, 67]. At this time, it is early to make a definitive conclusion; however, the available data suggests that there is no significant difference between offspring born to metformin- or insulin-treated GDM mothers.
Conclusion
Rates of GDM have been increasing and with the rising prevalence of obesity among women of reproductive age, it is likely that they will continue to increase. More troubling is the evidence from observational long-term follow-up studies that substantiates the presence of a cycle of diabetes between mother and child. Children exposed to GDM and hyperglycemia in pregnancy represent a population at risk for development of metabolic diseases. To date, the few intervention studies comparing children of euglycemic to untreated GDM mothers have failed to demonstrate metabolic differences in young children under the age of 10 years. Yet, the large, epidemiological observational HAPO FUS provides evidence that untreated maternal hyperglycemia is associated with increased adiposity and disorders of glucose metabolism in pubertal offspring. Longer follow-up evaluation of offspring from mothers involved in prior GDM intervention studies is necessary to definitively address the impact of maternal GDM on offspring metabolic health. In conclusion, optimizing diagnoses and treatment of GDM may be an important strategy to prevent adverse metabolic health outcomes in offspring.
Footnotes
Conflict of Interest Monica E. Bianco and Jami Josefson declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Diabetes and Pregnancy
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Nanditha A, Ma RC, Ramachandran A, Snehalatha C, Chan JC, Chia KS, et al. Diabetes in Asia and the Pacific: implications for the global epidemic. Diabetes Care. 2016;39(3):472–85. [DOI] [PubMed] [Google Scholar]
- 2.Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358(19):1991–2002. [DOI] [PubMed] [Google Scholar]
- 3.Falavigna M, Schmidt MI, Trujillo J, Alves LF, Wendland ER, Torloni MR, et al. Effectiveness of gestational diabetes treatment: a systematic review with quality of evidence assessment. Diabetes Res Clin Pract. 2012;98(3):396–405. [DOI] [PubMed] [Google Scholar]
- 4.••.Lowe WL Jr, Scholtens DM, Lowe LP, Kuang A, Nodzenski M, Talbot O, et al. Association of Gestational Diabetes With Maternal Disorders of Glucose Metabolism and Childhood Adiposity. Jama. 2018;320(10):1005–16. [DOI] [PMC free article] [PubMed] [Google Scholar]; Findings in this study suggest that untreated, mild GDM is assoicated with increased adiposity in childhood.
- 5.Pettitt DJ, Knowler WC. Long-term effects of the intrauterine environment, birth weight, and breast-feeding in Pima Indians. Diabetes Care. 1998;21(Suppl 2):B138–41. [PubMed] [Google Scholar]
- 6.Schaefer-Graf UM, Buchanan TA, Xiang A, Songster G, Montoro M, Kjos SL. Patterns of congenital anomalies and relationship to initial maternal fasting glucose levels in pregnancies complicated by type 2 and gestational diabetes. Am J Obstet Gynecol. 2000;182(2):313–20. [DOI] [PubMed] [Google Scholar]
- 7.Wendland EM, Torloni MR, Falavigna M, Trujillo J, Dode MA, Campos MA, et al. Gestational diabetes and pregnancy outcomes–a systematic review of the World Health Organization (WHO) and the International Association of Diabetes in Pregnancy Study Groups (IADPSG) diagnostic criteria. BMC pregnancy and childbirth. 2012;12:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dudley DJ. Diabetic-associated stillbirth: incidence, pathophysiology, and prevention. Obstet Gynecol Clin N Am. 2007;34(2):293–307 ix. [DOI] [PubMed] [Google Scholar]
- 9.Salvesen DR, Brudenell JM, Snijders RJ, Ireland RM, Nicolaides KH. Fetal plasma erythropoietin in pregnancies complicated by maternal diabetes mellitus. Am J Obstet Gynecol. 1993;168(1 Pt1):88–94. [DOI] [PubMed] [Google Scholar]
- 10.Metzger BE, Persson B, Lowe LP, Dyer AR, Cruickshank JK, Deerochanawong C, et al. Hyperglycemia and adverse pregnancy outcome study: neonatal glycemia. Pediatrics. 2010;126(6):e1545–52. [DOI] [PubMed] [Google Scholar]
- 11.Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005;352(24):2477–86. [DOI] [PubMed] [Google Scholar]
- 12.Moore TR. A comparison of amniotic fluid fetal pulmonary phospholipids in normal and diabetic pregnancy. Am J Obstet Gynecol. 2002;186(4):641–50. [DOI] [PubMed] [Google Scholar]
- 13.Wexler DJ, Powe CE, Barbour LA, Buchanan T, Coustan DR, Corcoy R, et al. Research gaps in gestational diabetes mellitus: executive summary of a National Institute of Diabetes and Digestive and Kidney Diseases workshop. Obstet Gynecol. 2018;132(2):496–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silverman BL, Rizzo TA, Cho NH, Metzger BE. Long-term effects of the intrauterine environment. The Northwestern University Diabetes in Pregnancy Center. Diabetes Care. 1998;(21 Suppl 2): B142–9. [PubMed] [Google Scholar]
- 15.Pettitt DJ, Knowler WC, Bennett PH, Aleck KA, Baird HR. Obesity in offspring of diabetic Pima Indian women despite normal birth weight. Diabetes Care. 1987;10(1):76–80. [DOI] [PubMed] [Google Scholar]
- 16.Silverman BL, Rizzo T, Green OC, Cho NH, Winter RJ, Ogata ES, et al. Long-term prospective evaluation of offspring of diabetic mothers. Diabetes. 1991;40(Suppl 2):121–5. [DOI] [PubMed] [Google Scholar]
- 17.Dabelea D, Hanson RL, Lindsay RS, Pettitt DJ, Imperatore G, Gabir MM, et al. Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships. Diabetes. 2000;49(12):2208–11. [DOI] [PubMed] [Google Scholar]
- 18.Lawlor DA, Lichtenstein P, Langstrom N. Association of maternal diabetes mellitus in pregnancy with offspring adiposity into early adulthood: sibling study in a prospective cohort of 280,866 men from 248,293 families. Circulation. 2011;123(3):258–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gillman MW, Oakey H, Baghurst PA, Volkmer RE, Robinson JS, Crowther CA. Effect of treatment of gestational diabetes mellitus on obesity in the next generation. Diabetes Care. 2010;33(5):964–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Landon MB, Mele L, Varner MW, Casey BM, Reddy UM, Wapner RJ, et al. The relationship of maternal glycemia to childhood obesity and metabolic dysfunction(double dagger). J Matern Fetal Neonatal Med. 2018:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nehring I, Chmitorz A, Reulen H, von Kries R, Ensenauer R. Gestational diabetes predicts the risk of childhood overweight and abdominal circumference independent of maternal obesity. Diabet Med. 2013;30(12):1449–56. [DOI] [PubMed] [Google Scholar]
- 22.Hillier TA, Pedula KL, Schmidt MM, Mullen JA, Charles MA, Pettitt DJ. Childhood obesity and metabolic imprinting: the ongoing effects of maternal hyperglycemia. Diabetes Care. 2007;30(9): 2287–92. [DOI] [PubMed] [Google Scholar]
- 23.Wang J, Wang L, Liu H, Zhang S, Leng J, Li W, et al. Maternal Gestational Diabetes and Different Indicators of Childhood Obesity – A Large Study. Endocr Connect. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hockett CW, Harrall KK, Moore BF, Starling AP, Bellatorre A, Sauder KA, et al. Persistent effects of in utero overnutrition on offspring adiposity: the exploring perinatal outcomes among children (EPOCH) study. Diabetologia. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vohr BR, McGarvey ST, Tucker R. Effects of maternal gestational diabetes on offspring adiposity at 4–7 years of age. Diabetes Care. 1999;22(8):1284–91. [DOI] [PubMed] [Google Scholar]
- 26.Zhao P, Liu E, Qiao Y, Katzmarzyk PT, Chaput JP, Fogelholm M, et al. Maternal gestational diabetes and childhood obesity at age 9–11: results of a multinational study. Diabetologia. 2016;59(11): 2339–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kearney M, Perron J, Marc I, Weisnagel SJ, Tchernof A, Robitaille J. Association of prenatal exposure to gestational diabetes with offspring body composition and regional body fat distribution. Clin Obes. 2018;8(2):81–7. [DOI] [PubMed] [Google Scholar]
- 28.Crume TL, Ogden L, West NA, Vehik KS, Scherzinger A, Daniels S, et al. Association of exposure to diabetes in utero with adiposity and fat distribution in a multiethnic population of youth: the exploring perinatal outcomes among children (EPOCH) study. Diabetologia. 2011;54(1):87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wright CS, Rifas-Shiman SL, Rich-Edwards JW, Taveras EM, Gillman MW, Oken E. Intrauterine exposure to gestational diabetes, child adiposity, and blood pressure. Am J Hypertens. 2009;22(2): 215–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gingras V, Rifas-Shiman SL, Derks IPM, Aris IM, Oken E, Hivert MF. Associations of gestational glucose tolerance with offspring body composition and estimated insulin resistance in early adolescence. Diabetes Care. 2018;41(12):e164–e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Freedman DS, Mei Z, Srinivasan SR, Berenson GS, Dietz WH. Cardiovascular risk factors and excess adiposity among overweight children and adolescents: the Bogalusa Heart Study. J Pediatr. 2007;150(1):12–7.e2. [DOI] [PubMed] [Google Scholar]
- 32.Tam WH, Ma RCW, Ozaki R, Li AM, Chan MHM, Yuen LY, et al. In utero exposure to maternal hyperglycemia increases childhood Cardiometabolic risk in offspring. Diabetes Care. 2017;40(5):679–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lowe WL Jr, Scholtens DM, Kuang A, Linder B, Lawrence JM, Lebenthal Y, et al. Hyperglycemia and adverse pregnancy outcome follow-up study (HAPO FUS): maternal gestational diabetes mellitus and childhood glucose metabolism. Diabetes Care. 2019;42(3):372–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.•.Scholtens DM, Kuang A, Lowe LP, Hamilton J, Lawrence JM, Lebenthal Y, et al. Hyperglycemia and adverse pregnancy outcome follow-up study (HAPO FUS): maternal Glycemia and childhood glucose metabolism. Diabetes Care. 2019;42(3):381–92. [DOI] [PMC free article] [PubMed] [Google Scholar]; Findings from this study suggest that hyperglycemia in utero is associated with increased childhood glucose and insulin resistance.
- 35.Landon MB, Rice MM, Varner MW, Casey BM, Reddy UM, Wapner RJ, et al. Mild gestational diabetes mellitus and long-term child health. Diabetes Care. 2015;38(3):445–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kawasaki M, Arata N, Miyazaki C, Mori R, Kikuchi T, Ogawa Y, et al. Obesity and abnormal glucose tolerance in offspring of diabetic mothers: a systematic review and meta-analysis. PLoS One. 2018;13(1):e0190676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holder T, Giannini C, Santoro N, Pierpont B, Shaw M, Duran E, et al. A low disposition index in adolescent offspring of mothers with gestational diabetes: a risk marker for the development of impaired glucose tolerance in youth. Diabetologia. 2014;57(11): 2413–20. [DOI] [PubMed] [Google Scholar]
- 38.Dabelea D, Mayer-Davis EJ, Lamichhane AP, D’Agostino RB Jr, Liese AD, Vehik KS, et al. Association of intrauterine exposure to maternal diabetes and obesity with type 2 diabetes in youth: the SEARCH case-control study. Diabetes Care. 2008;31(7):1422–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blotsky AL, Rahme E, Dahhou M, Nakhla M, Dasgupta K. Gestational diabetes associated with incident diabetes in childhood and youth: a retrospective cohort study. CMAJ. 2019;191(15): E410–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tam WH, Ma RC, Yang X, Li AM, Ko GT, Kong AP, et al. Glucose intolerance and cardiometabolic risk in adolescents exposed to maternal gestational diabetes: a 15-year follow-up study. Diabetes Care. 2010;33(6):1382–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hao G, Wang X, Treiber FA, Harshfield G, Kapuku G, Su S. Blood Pressure Trajectories From Childhood to Young Adulthood Associated With Cardiovascular Risk: Results From the 23-Year Longitudinal Georgia Stress and Heart Study. Hypertension. 2017;69(3):435–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Theodore RF, Broadbent J, Nagin D, Ambler A, Hogan S, Ramrakha S, et al. Childhood to Early-Midlife Systolic Blood Pressure Trajectories: Early-Life Predictors, Effect Modifiers, and Adult Cardiovascular Outcomes. Hypertension. 2015;66(6):1108–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Urbina EM, Khoury PR, McCoy C, Daniels SR, Kimball TR, Dolan LM. Cardiac and vascular consequences of prehypertension in youth. J Clin Hypertens. 2011;13(5):332–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.National Clinical Guideline C. National Institute for Health and Clinical Excellence: Guidance Hypertension: The Clinical Management of Primary Hypertension in Adults: Update of Clinical Guidelines 18 and 34. London: Royal College of Physicians (UK) National Clinical Guideline Centre; 2011. [Google Scholar]
- 45.Amri K, Freund N, Vilar J, Merlet-Benichou C, Lelievre-Pegorier M. Adverse effects of hyperglycemia on kidney development in rats: in vivo and in vitro studies. Diabetes. 1999;48(11):2240–5. [DOI] [PubMed] [Google Scholar]
- 46.Ritz E, Amann K, Koleganova N, Benz K. Prenatal programming-effects on blood pressure and renal function. Nat Rev Nephrol. 2011;7(3):137–44. [DOI] [PubMed] [Google Scholar]
- 47.Wichi RB, Souza SB, Casarini DE, Morris M, Barreto-Chaves ML, Irigoyen MC. Increased blood pressure in the offspring of diabetic mothers. Am J Phys Regul Integr Comp Phys. 2005;288(5):R1129–33. [DOI] [PubMed] [Google Scholar]
- 48.Pinter E, Haigh J, Nagy A, Madri JA. Hyperglycemia-induced vasculopathy in the murine conceptus is mediated via reductions of VEGF-A expression and VEGF receptor activation. Am J Pathol. 2001;158(4):1199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bunt JC, Tataranni PA, Salbe AD. Intrauterine exposure to diabetes is a determinant of hemoglobin a(1)c and systolic blood pressure in pima Indian children. J Clin Endocrinol Metab. 2005;90(6):3225–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tam WH, Ma RC, Yang X, Ko GT, Tong PC, Cockram CS, et al. Glucose intolerance and cardiometabolic risk in children exposed to maternal gestational diabetes mellitus in utero. Pediatrics. 2008;122(6):1229–34. [DOI] [PubMed] [Google Scholar]
- 51.Beyerlein A, Nehring I, Rosario AS, von Kries R. Gestational diabetes and cardiovascular risk factors in the offspring: results from a cross-sectional study. Diabet Med. 2012;29(3):378–84. [DOI] [PubMed] [Google Scholar]
- 52.Aceti A, Santhakumaran S, Logan KM, Philipps LH, Prior E, Gale C, et al. The diabetic pregnancy and offspring blood pressure in childhood: a systematic review and meta-analysis. Diabetologia. 2012;55(11):3114–27. [DOI] [PubMed] [Google Scholar]
- 53.••.Miranda JO, Cerqueira RJ, Barros H, Areias JC. Maternal Diabetes Mellitus as a Risk Factor for High Blood Pressure in Late Childhood. Hypertens. 2019;73(1):e1–7. [DOI] [PubMed] [Google Scholar]; Finding of this study suggest that maternal diabetes is associated with higher blood pressure in childhood.
- 54.Blondeau B, Joly B, Perret C, Prince S, Bruneval P, Lelievre-Pegorier M, et al. Exposure in utero to maternal diabetes leads to glucose intolerance and high blood pressure with no major effects on lipid metabolism. Diabetes Metab. 2011;37(3):245–51. [DOI] [PubMed] [Google Scholar]
- 55.Song Y, Li J, Zhao Y, Zhang Q, Liu Z, Li J, et al. Severe maternal hyperglycemia exacerbates the development of insulin resistance and fatty liver in the offspring on high fat diet. Exp Diabetes Res. 2012;2012:254976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pereira TJ, Fonseca MA, Campbell KE, Moyce BL, Cole LK, Hatch GM, et al. Maternal obesity characterized by gestational diabetes increases the susceptibility of rat offspring to hepatic steatosis via a disrupted liver metabolome. J Physiol. 2015;593(14):3181–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brumbaugh DE, Tearse P, Cree-Green M, Fenton LZ, Brown M, Scherzinger A, et al. Intrahepatic fat is increased in the neonatal offspring of obese women with gestational diabetes. J Pediatr. 2013;162(5):930–6.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bellatorre A, Scherzinger A, Stamm E, Martinez M, Ringham B, Dabelea D. Fetal Overnutrition and Adolescent Hepatic Fat Fraction: the Exploring Perinatal Outcomes in Children Study. J Pediatr. 2018;192:165–70.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Davis JN, Gunderson EP, Gyllenhammer LE, Goran MI. Impact of gestational diabetes mellitus on pubertal changes in adiposity and metabolic profiles in Latino offspring. J Pediatr. 2013;162(4):741–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grunnet LG, Hansen S, Hjort L, Madsen CM, Kampmann FB, Thuesen ACB, et al. Adiposity, Dysmetabolic traits, and earlier onset of female puberty in adolescent offspring of women with gestational diabetes mellitus: a clinical study within the Danish National Birth Cohort. Diabetes Care. 2017;40(12):1746–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lauridsen LLB, Arendt LH, Ernst A, Brix N, Parner ET, Olsen J, et al. Maternal diabetes mellitus and timing of pubertal development in daughters and sons: a nationwide cohort study. Fertil Steril. 2018;110(1):35–44. [DOI] [PubMed] [Google Scholar]
- 62.Aksglaede L, Juul A, Olsen LW, Sorensen TI. Age at puberty and the emerging obesity epidemic. PLoS One. 2009;4(12):e8450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Crocker MK, Stern EA, Sedaka NM, Shomaker LB, Brady SM, Ali AH, et al. Sexual dimorphisms in the associations of BMI and body fat with indices of pubertal development in girls and boys. J Clin Endocrinol Metab. 2014;99(8):E1519–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kampmann FB, Thuesen ACB, Hjort L, Olsen SF, Pires SM, Tetens I, et al. Exposure to gestational diabetes is a stronger predictor of Dysmetabolic traits in children than size at birth. J Clin Endocrinol Metab. 2019;104(5):1766–76. [DOI] [PubMed] [Google Scholar]
- 65.Rowan JA, Rush EC, Plank LD, Lu J, Obolonkin V, Coat S, et al. Metformin in gestational diabetes: the offspring follow-up (MiG TOFU): body composition and metabolic outcomes at 7–9 years of age. BMJ Open Diabetes Res Care. 2018;6(1): e000456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ijas H, Vaarasmaki M, Saarela T, Keravuo R, Raudaskoski T. A follow-up of a randomised study of metformin and insulin in gestational diabetes mellitus: growth and development of the children at the age of 18 months. BJOG. 2015;122(7):994–1000. [DOI] [PubMed] [Google Scholar]
- 67.Tertti K, Eskola E, Ronnemaa T, Haataja L. Neurodevelopment of two-year-old children exposed to metformin and insulin in gestational diabetes mellitus. J Dev Behav Pediatr. 2015;36(9): 752–7. [DOI] [PubMed] [Google Scholar]


