Abstract
Background:
The importance of local failure (LF) after treatment of high-grade prostate cancer (PCa) with definitive radiotherapy (RT) remains unknown.
Objective:
To evaluate the clinical implications of LF after definitive RT.
Design, setting, and participants:
Individual patient data meta-analysis of 992 patients (593 Gleason grade group [GG] 4 and 399 GG 5) enrolled in six randomized clinical trials.
Outcome measurements and statistical analysis:
Multivariable Cox proportional hazard models were developed to evaluate the relationship between overall survival (OS), PCa-specific survival (PCSS), and distant metastasis (DM)-free survival (DMFS) and LF as a time-dependent covariate. Markov proportional hazard models were developed to evaluate the impact of specific transitions between disease states on these endpoints.
Results and limitations:
Median follow-up was 6.4 yr overall and 7.2 yr for surviving patients. LF was significantly associated with OS (hazard ratio [HR] 1.70 [95% confidence interval {CI} 1.37–2.10]), PCSS (3.10 [95% CI 2.33–4.12]), and DMFS (HR 1.92 [95% CI 1.54–2.39]), p < 0.001 for all). Patients who had not transitioned to the LF state had a significantly lower hazard of transitioning to a PCa-specific death state than those who transitioned to the LF state (HR 0.13 [95% CI 0.04–0.41], p < 0.001). Additionally, patients who transitioned to the LF state had a greater hazard of DM or death (HR 2.46 [95% CI 1.22–4.93], p = 0.01) than those who did not.
Conclusions:
LF is an independent prognosticator of OS, PCSS, and DMFS in high-grade localized PCa and a subset of DM events that are anteceded by LF events. LF events warrant consideration for intervention, potentially suggesting a rationale for upfront treatment intensification. However, whether these findings apply to all men or just those without significant comorbidity remains to be determined.
Patient summary:
Men who experience a local recurrence of high-grade prostate cancer after receiving upfront radiation therapy are at significantly increased risks of developing metastases and dying of prostate cancer.
Keywords: Local failure, Radiotherapy, High grade
1. Introduction
The addition of prostate-directed radiotherapy (RT) to lifelong androgen deprivation therapy (ADT) in patients with locally advanced prostate cancer (PCa) has been shown to improve overall survival (OS) in two randomized trials [1,2]. However, the prognostic implications of local failure (LF) events following definitive RT for high-grade PCa (ie, Gleason grade group [GG] 4–5 disease) remain unclear. This is relevant not only for the consideration of managing LFs, but also for local treatment intensification, such as RT dose escalation. A central hypothesis underlying the concept of RT dose escalation for PCa is that locally recurrent disease eventually “seeds” distant metastases (DMs), leading to a “second wave” of DMs [3,4]. Multiple retrospective studies have identified a significant association between local control and DM-free survival (DMFS) and/or PCa-specific survival (PCSS), lending credence to this theory [4–9]. However, the early competing risk of DMs in high-grade disease calls into question the importance of local control, and of the multiple randomized trials of dose escalation [10], only two have suggested a benefit in freedom from DMs [11,12] and none has shown an improvement in PCSS or OS. Alternatively, three large randomized trials demonstrated OS, PCSS, and DMFS benefits when ADT was combined with RT for high-risk PCa, and multiple subsequent trials have established that long-term ADT (LTADT) should be the standard of care when treating high-risk PCa with RT [10]. Although ADT has radiosensitizing effects that improve local control, it is generally believed that a major role in the setting of high-risk disease is a systemic cytostatic effect that would affect both the primary and the occult micrometastatic disease at presentation. Notably, the retrospective evidence supporting the “second wave” theory was derived from patients treated with minimal amounts of ADT, generally with low-grade disease [4–9].
Recent retrospective data suggest a PCSS and DMFS benefit to extremely dose-escalated RT compared with standard dose-escalated RT in patients with GG 5 disease [13]. To explore the importance of LF events in high-grade PCa, we obtained individual patient-level data from six randomized trials of definitive RT with varying durations of ADT that included LF as a prespecified endpoint.
2. Patients and methods
The identification of eligible trials for this meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement [14] and followed the schema of a previous meta-analysis of these trials (Supplementary Fig. 1 and Supplementary Table 1) [15]. The duration of LTADT was 28–36 mo, while that of short-term ADT (STADT) was 4–6 mo. Trial-specific definitions of LF are provided in Table 1. All times to event were measured from study entry (ie, date of randomization) such that the follow-up period began from randomization. Patients were considered censored for a given endpoint if they did not experience this endpoint during their follow-up period. The effects of ADT duration on LF were examined using a network meta-analysis approach (Supplementary material) [15,16]. Cumulative incidence rates of LF and DM, with death as the competing event, were estimated in a competing risk framework. Subsequently, multivariable Cox proportional hazard models were developed to evaluate the relationship between OS, PCSS, and DMFS and LF as a time-dependent covariate, while adjusting for GG, ADT treatment group, T stage, age, and potential interaction between GG and ADT treatment group [15]. We also performed Fine and Gray competing risk regression for PCSS and DMFS with death as the competing event; in these analyses, LF was a time-independent covariate. Within each ADT treatment group, hazard rates to DM over 2-yr intervals were determined using the life-table method for patients with and without LF as a time-independent covariate [4]. Among patients who experienced DM, the differences of median times to DM between patients with and without LF, with LF treated as a time-independent covariate, were assessed by Wilcoxon rank sum test.
Table 1 –
Trial-specific definitions of local failure
| Trial | Definition of local failure |
|---|---|
| RTOG 8531 | Reappearance of palpable tumor after initial clearance, progression of palpable tumor at any time, persistence of palpable tumor beyond 2 yr after study entry, and the biopsy-proven presence of carcinoma of the prostate ≥2 yr after study entry |
| RTOG 8610 | PSA >4 at ≥1 yr after randomization, additional hormonal therapy in the absence of metastatic disease, an increase of >50% in tumor size (cross-sectional area), recurrence of a palpable tumor after initial clearance, or a biopsy specimen revealing adenocarcinoma of the prostate ≥2 yr after study entry |
| RTOG 9202 | Tumor growth of 25% or local persistence of palpable tumor beyond 18 mo |
| EORTC 22863 | Recurrence of a palpable tumor after initial regression |
| EORTC 22961 | Palpable enlargement of a previously regressed prostate gland by ≥25%, assessed on the basis of the product of its two largest diameters, or urethral obstruction |
| EORTC 22991 | Palpable enlargement of a previously regressed prostate gland by ≥25%, assessed on the basis of the product of its two largest diameters, or urethral obstruction |
PSA = prostate-specific antigen.
To simultaneously analyze the multiple possible endpoints describing the course of PCa, two multistate models were developed (Fig. 1 and the Supplementary material) [17]. The first was a four-state model consisting of a relapse-free survival state, an LF state, a DM state, and a death state; patients who did not experience a PCa-specific mortality (PCSM) event were coded as “censored” for PCSS. With a semiparametric approach, Markov proportional hazard models for the four-state model were developed to evaluate the effects of the aforementioned covariates on PCSS and OS, and additionally evaluate the proportional hazard between patients transitioning from the relapse-free survival state versus the LF state to the “death” state. The second model was a three-state model consisting of a relapse-free survival state, an LF state, and the state “DM or death” (Fig. 1); this was designed to investigate the effect of LF on the specific endpoint DMFS. A Markov proportional hazard model was developed to explore the relationship between DMFS and LF as a time-dependent covariate [18,19]. In both frameworks, patients can experience different types of transitions, although no patient can experience all transitions. The potential heterogeneity between trials was accounted for by including a random effect in Cox and Markov models. The proportional hazard assumption was examined via the diagnostic plot method. Finally, the chi-square test of independence (or Fisher’s exact test when indicated) was used to assess the association between entering the DM state from the relapse-free survival and LF states, defined by the time intervals “0–5 yr” or “beyond 5 yr.” All analyses were completed using R v.3.3.2 [20] with the survival [21], surv2sampleComp [22], KMsurv [23], coxme [24], and mstate [25] packages at a two-tailed level of significance of 0.05.
Fig. 1 –
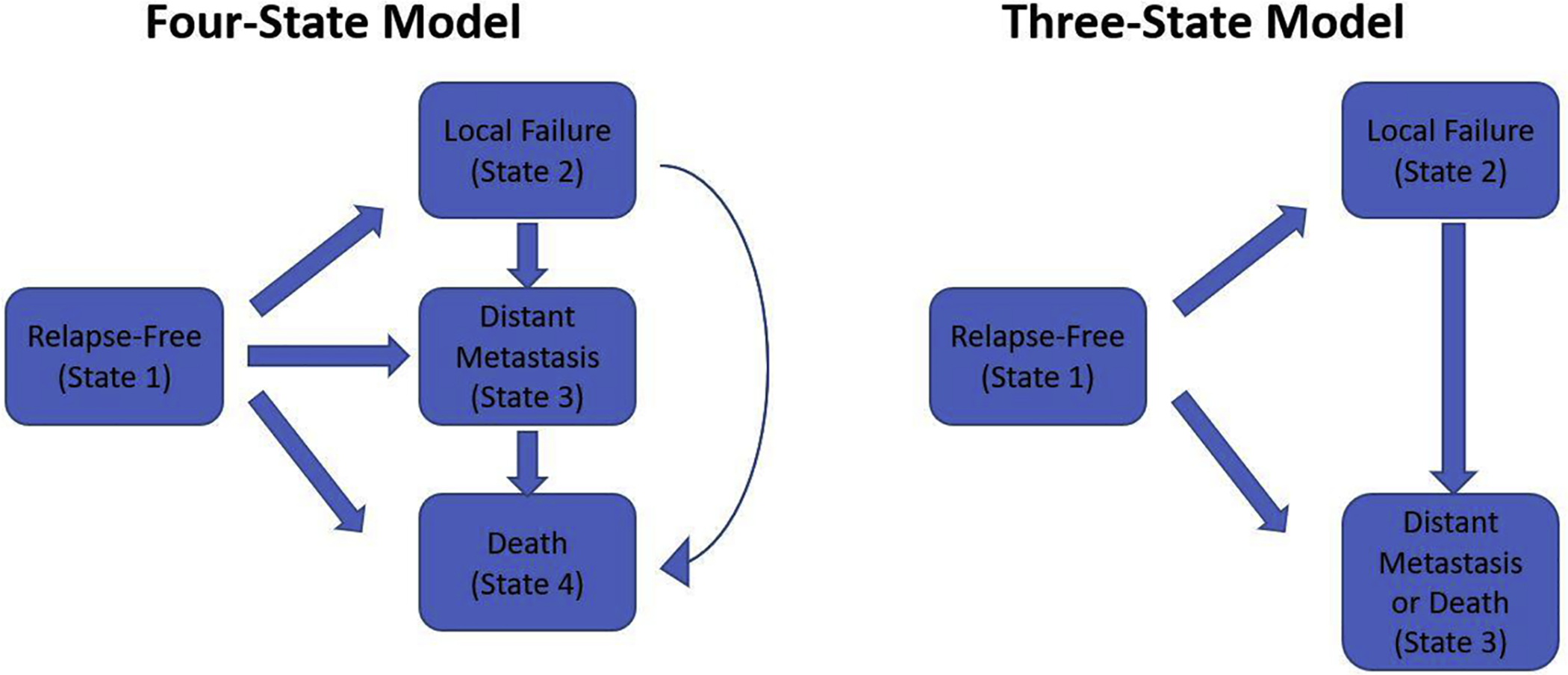
Schematic depiction of the (A) four-state and (B) three-state models developed to evaluate various disease states experienced and traversed by patients with high-grade localized prostate cancer. The four-state model was designed to evaluate the outcomes PCSS and OS; patients who did not experience a PCa-specific mortality event were coded as “censored” for PCSS. OS = overall survival; PCa = prostate cancer; PCSS = PCa-specific survival.
3. Results
A total of 992 patients (593 with GG 4 and 399 with GG 5 cancers) were identified (Table 2 and Supplementary Table 2). The median follow-up was 6.4 yr overall and 7.2 yr for living patients. The median follow-up was 6.3 yr among patients without an LF event, 7.1 yr among those without DM or death, and 7 yr among those without a PCSM event. Crude rates of LF, DM, PCSM, and all-cause mortality were 24% (241/992), 37% (371/992), 30% (296/992), and 67% (661/992), respectively (Supplementary Table 3). Cumulative incidence estimates of LF and DM are provided in Supplementary Table 4. The results of individual trial Cox proportional hazard models did not identify a significant interaction between ADT treatment group and GG, but a network meta-analysis evaluating the effect of ADT durations on LF identified that the effect differed between GG 4 and GG 5 patients and between different durations of ADT (Supplementary Tables 5 and 6). As such, further analyses included GG, ADT treatment group, and an interaction term between them as covariates.
Table 2 –
Summary of trials included in meta-analysis with breakdown by Gleason grade group
| Trial | Inclusion a | Number of patients randomized | Number of patients extracted | Arms | GG 4 | GG 5 | Total |
|---|---|---|---|---|---|---|---|
| RTOG 8531 (1987–1992) | cT1-T2N+ or cT3–4 <25 cm2 b | 977 | 216 | RT alone | 61 | 47 | 108 |
| Lifelong ADT | 59 | 49 | 108 | ||||
| RTOG 8610 (1987–1991) | cT2-T4 ≥25 cm2 | 456 | 128 | RT alone | 33 | 36 | 69 |
| STADT | 29 | 30 | 59 | ||||
| RTOG 9202 (1992–1995) | cT2–4N0-X, PSA <150c | 1554 | 337 | STADT | 93 | 78 | 171 |
| LTADT | 92 | 74 | 166 | ||||
| EORTC 22863 (1987–1995) | cT1–2N0 WHO grade 3 cT3–4N0 | 415 | 43d | RT alone | 17 | 7 | 24 |
| LTADT | 13 | 6 | 19 | ||||
| EORTC 22961 (1997–2001) | cT1c-2bN+, cT3– 4N0 PSA <40 × ULN |
970 | 186 | STADT | 56 | 34 | 90 |
| LTADT | 75 | 21 | 96 | ||||
| EORTC 22991 (2001–2008) | cT1b-c with PSA ≥10 or GS ≥7, cT2a with PSA ≤50 | 819 | 82 | RT alone | 29 | 8 | 37 |
| STADT | 36 | 9 | 45 | ||||
| Group | GG 4 | GG 5 | Total | ||||
| RT alone | 140 | 98 | 238 | ||||
| STADT | 214 | 151 | 365 | ||||
| LTADT | 180 | 101 | 281 | ||||
| Lifelong ADT | 59 | 49 | 108 | ||||
| Total | 593 | 399 | 992 | ||||
ADT = androgen deprivation therapy; GG = Gleason grade group; GS = Gleason score; LTADT = long-term ADT; PSA = prostate-specific antigen; RT = radiation therapy; STADT = short-term ADT; ULN = upper limit of normal; WHO = World Health Organization.
Patients with cN+ or pN+ disease were included in several protocols but not included in our analyses.
RTOG 8531 also included patients with high-risk features after radical prostatectomy, who were not included in our analysis.
PSA values are in units of ng/ml unless otherwise indicated.
The 43 patients from EORTC 22863 are drawn from a subgroup of 132 patients who underwent Gleason grading.
In a competing risk regression model adjusted for GG, ADT treatment group, T stage, and age, LF as a time-independent covariate was associated with doubling of the rate of PCSM among men who were either alive or had died of a cause other than PCa (subdistribution hazard ratio 2.04 [95% confidence interval {CI} 1.54–2.71], p < 0.001; Table 3). LF was significantly associated with an increased hazard of death in a time-dependent Cox model adjusted for GG, ADT treatment group, T stage, and age (hazard ratio [HR] for OS of 1.70 [95% CI 1.37–2.10], p < 0.001; Supplementary Table 7). It was also significantly associated with PCSS and DMFS (HRs 3.10 [95% CI 2.33–4.12] and 1.92 [95% CI 1.54–2.39]; p < 0.001 for both). When attempting to adjust the competing risk model for initial prostate-specific antigen (PSA) in a subset of 492 patients with available PSA data, the model could no longer be fit. However, we also developed a Cox model for this subset and found similar results with respect to the prognostic power of LF (Supplementary Table 8). The hazard rates of DM development in 2-yr intervals within each ADT treatment group suggest an increasing hazard over time in patients with LF (as a time-independent covariate) and a decreasing hazard in those without LF (Fig. 2 and Supplementary Table 9). The only exception was the RT-alone group, in which the hazard of DM was the highest in the first 2 yr in both groups. Among patients who experienced DM events, the median time to event was significantly longer in those who had LF than in those without LF among patients receiving RT + STADT (4.58 vs 2.38 yr, p < 0.01 by Wilcoxon rank sum test; Supplementary Table 10). No significant differences in median time to DM could be identified in any of the other groups.
Table 3 –
Multivariable competing risk analyses with local failure as a time-independent variable
| PCSS | DMFS | |||
|---|---|---|---|---|
| SHR (95% CI) | p value | SHR (95% CI) | p value | |
| LF (time independent) | 2.04 (1.54–2.71) | <0.001 | 1.29 (0.9–1.87) | 0.17 |
| GG 5 vs GG 4 | 1.46 (1.07–2.00) | 0.016 | 1.54 (1.19–2.00) | <0.01 |
| RT + STADT vs RT alone | 0.65 (0.51–0.83) | <0.001 | 0.61 (0.43–0.87) | <0.01 |
| RT + LTADT vs RT alone | 0.43 (0.30–0.62) | <0.001 | 0.35 (0.23–0.53) | <0.001 |
| RT + lifelong ADT vs RT alone | 0.59 (0.55–0.64) | <0.001 | 0.46 (0.42–0.51) | <0.001 |
| T3/4 vs T1/2 | 0.99 (0.83–1.19) | 0.9 | 0.98 (0.78–1.23) | 0.8 |
| Age (per 10 yr)a | 0.85 (0.73–0.99) | 0.043 | 0.75 (0.61–0.93) | <0.01 |
ADT = androgen deprivation therapy; CI = confidence interval; DMFS = distant metastasis–free survival; GG = Gleason grade group; LF = local failure; LTADT = long-term ADT; PCSS = prostate cancer–specific survival; RT = radiation therapy; SHR = subdistribution hazard ratio; STADT = short-term ADT.
Subdistribution hazard ratios are shown above, with 95% confidence intervals in parentheses.
Age is rescaled for feasible interpretation.
Fig. 2 –
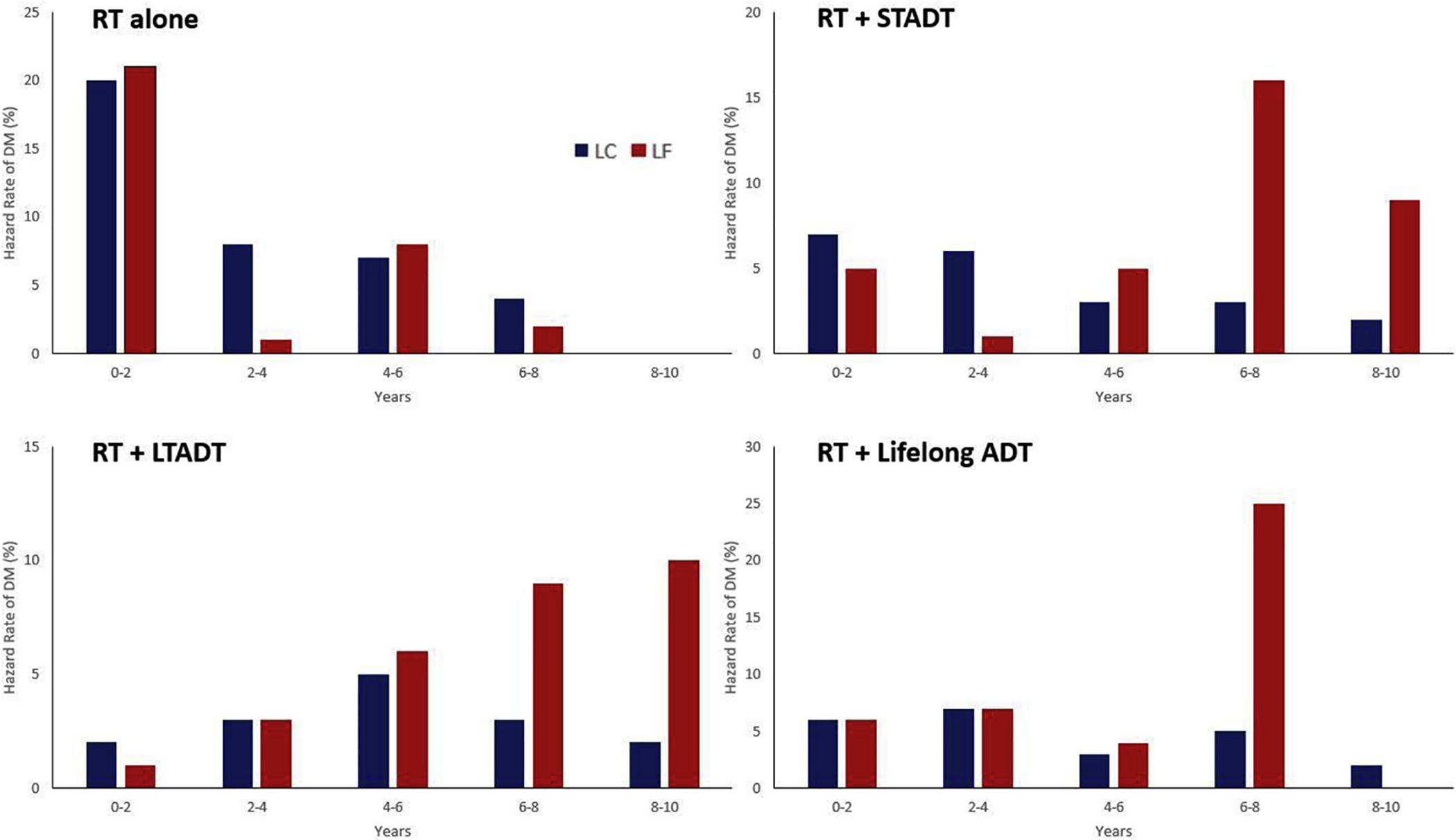
Hazard rate of distant metastasis development in 2-yr intervals in patients with local control (LC, blue) and local failure (LF, red). Here, these are treated as time-independent variables, meaning that all patients with LF at any point (before or after distant metastasis development) are included in the LF group, whereas patients in the LC group never had an LF event. ADT = androgen deprivation therapy; DM = distant metastasis; LTADT = long-term ADT; RT = radiotherapy; STADT = short-term ADT.
To further explore the implications of an LF event on subsequent disease progression and survival, we evaluated Markov models derived from two multistate models (Fig. 1). Patients who had not transitioned to the LF state had a significantly lower hazard of transitioning to a PCSM state than those who transitioned to the LF state (HR 0.14 [95% CI 0.04–0.42], p < 0.001; Table 4 and Supplementary Fig. 2). The hazard of PCSM and death among patients who transitioned to the LF state was significantly reduced by LTADT (HRs of 0.34 [95% CI 0.21–0.54] and 0.34 [95% CI 0.20–0.59], p < 0.001]) and lifelong ADT (HRs of 0.30 [95% CI 0.16–0.56] and 0.17 [95% CI 0.06–0.49], p < 0.001]), compared with treatment with RT alone. A Markov model for the three-state model found that patients who had transitioned to the LF state had a greater hazard of DM or death than those who did not (HR 1.86 [95% CI 1.03–3.36], p = 0.04; Supplementary Fig. 3 and Supplementary Table 11).
Table 4·–
Markov proportional hazard models for overall survival and prostate cancer–specific survival in four-state model
| OS | PCSS | |||
|---|---|---|---|---|
| HR (95% CI) | p– value | HR (95% CI) | p– value | |
| Relative hazards between specific transitions | ||||
| RFS →– death vs LF →– death | 0.64 (0.25–1.66) | 0.4 | 0.14 (0.04–0.42) | <0.001 |
| DM → death vs LF → death | 2.91 (1.18–7.22) | 0.021 | 4.27 (2.04–8.95) | <0.001 |
| Effect on the RFS → LF transition | ||||
| GG 5 vs GG 4 | 1.38 (0.96–1.99) | 0.079 | 1.17 (0.87–1.58) | 0.3 |
| RT + STADT vs RT alone | 0.68 (0.42–1.09) | 0.11 | 0.68 (0.46–1.01) | 0.054 |
| RT + LTADT vs RT alone | 0.34 (0.20–0.59) | <0.001 | 0.34 (0.21–0.54) | <0.001 |
| RT + lifelong ADT vs RT alone | 0.17 (0.06–0.49) | <0.001 | 0.30 (0.16–0.56) | <0.001 |
| Effect on the RFS → DM transition | ||||
| GG 5 vs GG 4 | 1.78 (1.33–2.39) | <0.001 | 1.51 (1.20–1.91) | <0.001 |
| RT + STADT vs RT alone | 0.45 (0.31–0.66) | <0.001 | 0.45 (0.32–0.63) | <0.001 |
| RT + LTADT vs RT alone | 0.25 (0.16–0.38) | <0.001 | 0.26 (0.18–0.39) | <0.001 |
| RT + lifelong ADT vs RT alone | 0.24 (0.13–0.45) | <0.001 | 0.42 (0.28–0.63) | <0.001 |
| Effect on the RFS → death transition | ||||
| GG 5 vs GG 4 | 1.07 (0.80–1.42) | 0.6 | 2.10 (0.92–4.80) | 0.079 |
| RT + STADT vs RT alone | 0.77 (0.47–1.27) | 0.3 | 0.50 (0.18–1.41) | 0.19 |
| RT + LTADT vs RT alone | 0.74 (0.45–1.23) | 0.2 | 0.25 (0.07–0.88) | 0.031 |
| RT + lifelong ADT vs RT alone | 0.97 (0.52–1.78) | 0.9 | 0.61(0.18–2.11) | 0.4 |
| Effect on the LF → DM transition | ||||
| GG 5 vs GG 4 | 1.67 (1.05–2.65) | 0.03 | 1.57 (1.04–2.38) | 0.031 |
| RT + STADT vs RT alone | 1.10 (0.65–1.88) | 0.7 | 1.08 (0.68–1.72) | 0.7 |
| RT + LTADT vs RT alone | 0.80 (0.40–1.61) | 0.5 | 0.85 (0.44–1.67) | 0.6 |
| RT + lifelong ADT vs RT alone | 0.93 (0.21–4.06) | 0.9 | 0.74 (0.27–2.03) | 0.6 |
| Effect on the LF → death transition | ||||
| GG 5 vs GG 4 | 0.84 (0.44–1.58) | 0.6 | 0.92 (0.42–2.02) | 0.8 |
| RT + STADT vs RT alone | 0.79 (0.34–1.81) | 0.6 | 0.67 (0.26–1.68) | 0.4 |
| RT + LTADT vs RT alone | 0.89 (0.36–2.22) | 0.8 | 1.04 (0.38–2.84) | 0.9 |
| RT + lifelong ADT vs RT alone | 1.61 (0.34–7.58) | 0.6 | 0.39 (0.05–3.03) | 0.4 |
| Effect on the DM → death transition | ||||
| GG 5 vs GG 4 | 1.20 (0.89–1.61) | 0.2 | 1.25 (0.97–1.62) | 0.087 |
| RT + STADT vs RT alone | 1.06 (0.73–1.56) | 0.8 | 1.41 (0.99–2.02) | 0.057 |
| RT + LTADT vs RT alone | 1.09 (0.70–1.68) | 0.7 | 1.47 (0.95–2.28) | 0.085 |
| RT + lifelong ADT vs RT alone | 1.14 (0.62–2.08) | 0.7 | 1.39 (0.87–2.23) | 0.17 |
| Homogeneous effect across transitions | ||||
| T3/T4 vs Tl/T2 | 1.05 (0.90–1.22) | 0.6 | NA | NA |
| Age (per 10 yr)a | 1.11 (1.00–1.23) | 0.057 | 0.87 (0.78–0.97) | 0.01 |
ADT = androgen deprivation therapy; CI = confidence interval; DM = distant metastasis; GG = Gleason grade group; HR = hazard ratio; LF = local failure; LTADT = long-term ADT; OS = overall survival; PCSS = prostate cancer–specific survival; RFS = relapse-free survival; RT = radiation therapy; STADT = short-term ADT.
Hazard ratios are shown above, with 95% confidence intervals in parentheses.
Age is rescaled for feasible interpretation.
To specifically evaluate whether an increasing proportion of patients would transition from the LF state to the DM state over time, the proportions of patients entering the DM state from the relapse-free survival state versus the DM state were calculated and compared over time periods “0–5 yr” and “beyond 5 yr”. The proportion of men transitioning to the DM state from the relapse-free survival state ranged from 81% to 96% (89/110–96/100) in the first 5 yr (Supplementary Fig. 4). In the RT-alone and RT + STADT groups, however, 50% (6/12) and 51% (20/39) of metastases after 5 yr transitioned from the LF state, respectively. Within these two treatment arms, the time since completion of RT, defined by the time intervals 0–5 yr or beyond 5 yr, was significantly associated with entering the DM state from the relapse-free survival or LF state (p = 0.025 and p < 0.001 via chi-square test). This association was not identified for the patients treated with RT + LTADT or RT + lifelong ADT.
4. Discussion
In this individual patient-level meta-analysis of six prospective trials of patients with high-grade PCa, LF was identified as a significant predictor of OS, PCSS, and DMFS. The hazard rate of developing DMs increased over time in patients with LF in all treatment groups except for RT-alone group, where the rates in years 0–2 were 20–21% regardless of LF status. Using multistate models, the transition to an LF state before a DM event was identified as an independent predictor of impaired PCSS, and LF as both a time-dependent and a time-independent event was a significant predictor of increased risk of the composite outcome DM or death. Finally, in patients treated with RT alone or RT + STADT, around half of the patients entering the DM state beyond 5 yr were transitioning from the LF state, whereas in the first 5 yr, >80% were transitioning from the relapse-free survival state.
Taken together, these data provide high-level evidence that an LF event after definitive-intent RT portends a poor prognosis. The mechanism for this, however, remains unclear. This could simply be ascribed to biological “predeterminism” of cancer, wherein an LF event merely indicates the same aggressive biology that would have independently led to a DM event [26]. It is widely acknowledged that DMs, and not LFs, lead to PCSM, and DMFS is a validated surrogate endpoint for PCSS [27]. However, all patients included in the analysis had both an elevated tendency to develop early DMs and an elevated chance of having occult micrometastatic disease at presentation, and despite this, LF prior to DM remained a strong predictor of outcome even after adjustment for ADT use and pretreatment clinical factors.
The data confirm that the most predominant pathway to metastatic failure is directly from a disease-free state, presumably caused by the emergence of occult metastases. Over the entirety of follow-up, this accounts for 73–90% of DMs across ADT treatment groups. However, the results also suggest that some DM events arise subsequent to LF events—these account for a minority of transitions overall but constitute an increasing proportion over time (~5–20% in the first 5 yr of follow-up and ~20–50% in the second 5 yr of follow-up). While this does not prove that LF events have caused the subsequent DM events, this evidence presents, to our knowledge, the first time that this hypothesized “second wave” has been identified in a large, prospectively followed cohort of patients. Notably, prior studies were conducted retrospectively in patient populations enriched with low-grade tumors and generally included few patients receiving ADT [4–9] (Supplementary Table 12).
One explanation of this pattern would be the traditional interpretation of the second wave hypothesis: locally recurrent disease can eventually seed DMs. However, it should be recognized that even if this mechanistic explanation were true, the primary mode of metastatic failure would remain a direct transition to the DM state. An alternate explanation of these data is that an intraprostatic LF recapitulates properties of the primary lesion that promote metastatic development [28]. Robust data suggest a survival benefit to treating the prostatic primary lesion in patients with a low burden of metastatic disease [29], and perhaps appropriately managing—or preventing—intraprostatic failure is important even in the context of occult micrometastatic disease.
4.1. Limitations
There are several limitations to this work. We intentionally restricted inclusion to patients with GG 4–5 disease to evaluate the importance of LF in a setting where the competing risk of DM, particularly from micrometastatic disease at presentation, was high. As such, we were able to include only a subset of patients enrolled in any of the included trials, thus not allowing a comprehensive analysis of surrogacy due to limited sample size. The results should also be taken within the context that we have limited analysis to patients with GG 4–5 disease, which was not an initial stratification variable in any trial; thus, this violates the randomization of the original trials. Additionally, despite pooling across multiple trials, some subgroups are still small in size, potentially limiting generalizability. Heterogeneity between trials is a significant limitation for any meta-analysis. Unfortunately, the variation in cohort size between trials, the relatively small sample size within any given trial, and the use of multiple analytical models precluded the identification of stable estimates within trials considered in isolation. This limits our ability to truly assess heterogeneity. We have attempted to limit the impact of this heterogeneity by employing random effects in our modeling [30], but this is an imperfect solution. Important data that were lacking, and therefore could not be adjusted for, include comorbidity status, race, and burden of Gleason pattern 4 or 5 disease. Further, the determination of LF was not entirely consistent across the six studies analyzed (and details of how each LF was identified are not available), and the most common detection method was via a palpable lesion on digital rectal examination, with biopsy confirmation neither required nor available for review. However, LF was a prespecified endpoint in all studies, and while digital rectal examination is not sensitive for recurrent disease, it has a specificity of the order of 86–90% [5,31]. Our definition of local recurrence provides a lower bound on the true rate, and alternative definitions would likely increase the proportion of patients developing LF before DM; the manner in which this would have impacted our analyses is unclear. Advanced imaging at enrollment would likely have identified a significant proportion of patients with extraprostatic disease [32]. This could conceivably have overlapped with the proportion of patients with early DMs. If these patients were excluded, the study population would have been enriched for patients in whom the true impact of local control, outside of the competing risk for DMs, could be explored. It is also likely that the relative proportion of patients developing DMs subsequent to LFs (vs those directly from a relapse-free survival state) would have increased if advanced imaging were used. Conversely, advanced imaging at subsequent time points would likely have identified metastases earlier than LF events in patients otherwise categorized as having developed DMs after LFs. This would have had an opposite effect. Finally, if advanced imaging scans identified LF at earlier time points, the observed rates of LF would have increased. Additionally, the management standard for LF was not prespecified, and therefore heterogeneous management practices after LF cannot be accounted for in the analysis. However, most patients with LF were likely started on salvage ADT, which would have delayed or prevented development of metastases and prolonged survival. There was no central pathology review for this study. However, while there has been a gradual shift in GG over time, this grade shift has mostly manifested in an increase in GG 2–3 lesions, while the numbers of GG 4–5 cancers, and particularly GG 5 cancers, have remained stable [33]. While we were able to obtain individual patient data from six randomized trials, multiple other trials were excluded due to a lack of availability of data. It is possible that the effects observed in the six included trials may not be generalizable to these other randomized trials, most (but not all) of which are more modern studies. In general, OS metrics have improved over time, likely due to advancements in both general medical are and PCa-specific therapies (eg, salvage therapies after initial treatment failure and treatments for metastatic disease). Given this gradual improvement in OS over time, it is a limitation that we have focused our analysis on an older series of trials. It is conceivable that with death removed as a competing event, there could be more of an impact of LF, ultimately leading to a DM event; however, better salvage therapies may also limit the significance of an LF event to begin with.
5. Conclusions
In conclusion, this patient-level meta-analysis provides high-level evidence that LF is an important prognostic endpoint in high-grade PCa and that the development of LF temporally precedes the development of DMs in a subset of patients. To our knowledge, this is the first time the latter observation, the so-called “second wave,” has been identified in prospectively treated patients with high-grade PCa. However, it is unknown whether these local recurrences seed DMs, encourage the development of DMs via other means, or simply correlate with an underlying aggressiveness that predicts for both DM and LF. Nonetheless, if locally recurrent lesions are indeed mechanistically related to subsequent DM events, these data suggest a benefit to local treatment intensification. For example, these results provide a mechanistic explanation for the robust DMFS and PCSS benefits seen for treatment with external beam radiotherapy (EBRT) with a brachytherapy boost versus EBRT alone in a large retrospective study focusing on GG 5 disease [13].
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosures: Amar U. Kishan certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
References
- [1].Fossa SD, Wiklund F, Klepp O, et al. Ten- and 15-yr prostate cancer-specific mortality in patients with nonmetastatic locally advanced or aggressive intermediate prostate cancer, randomized to lifelong endocrine treatment alone or combined with radiotherapy: final results of the Scandinavian Prostate Cancer Group-7. Eur Urol 2016;70:684–91. [DOI] [PubMed] [Google Scholar]
- [2].Mason MD, Parulekar WR, Sydes MR, et al. Final report of the intergroup randomized study of combined androgen-deprivation therapy plus radiotherapy versus androgen-deprivation therapy alone in locally advanced prostate cancer. J Clin Oncol 2015;33:2143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Grossman HB, Batata M, Hilaris B, Whitmore WF Jr. 125I implantation for carcinoma of prostate. Further follow-up of first 100 cases. Urology 1982;20:591–8. [DOI] [PubMed] [Google Scholar]
- [4].Coen JJ, Zietman AL, Thakral H, Shipley WU. Radical radiation for localized prostate cancer: local persistence of disease results in a late wave of metastases. J Clin Oncol 2002;20:3199–205. [DOI] [PubMed] [Google Scholar]
- [5].Freiha FS, Bagshaw MA. Carcinoma of the prostate: results of post-irradiation biopsy. Prostate 1984;5:19–25. [DOI] [PubMed] [Google Scholar]
- [6].Kuban DA, el-Mahdi AM, Schellhammer PF. Effect of local tumor control on distant metastasis and survival in prostatic adenocarcinoma. Urology 1987;30:420–6. [DOI] [PubMed] [Google Scholar]
- [7].Zagars GK, von Eschenbach AC, Ayala AG, Schultheiss TE, Sherman NE. The influence of local control on metastatic dissemination of prostate cancer treated by external beam megavoltage radiation therapy. Cancer 1991;68:2370–7. [DOI] [PubMed] [Google Scholar]
- [8].Fuks Z, Leibel SA, Wallner KE, et al. The effect of local control on metastatic dissemination in carcinoma of the prostate: long-term results in patients treated with 125I implantation. Int J Radiat Oncol Biol Phys 1991;21:537–47. [DOI] [PubMed] [Google Scholar]
- [9].Zelefsky MJ, Reuter VE, Fuks Z, Scardino P, Shippy A. Influence of local tumor control on distant metastases and cancer related mortality after external beam radiotherapy for prostate cancer. J Urol 2008;179:1368–73; discussion 1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Nguyen PL. Optimization of the radiation management of high-risk prostate cancer. Semin Radiat Oncol 2017;27:43–9. [DOI] [PubMed] [Google Scholar]
- [11].Kuban DA, Tucker SL, Dong L, et al. Long-term results of the M. D. Anderson randomized dose-escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys 2008;70:67–74. [DOI] [PubMed] [Google Scholar]
- [12].Michalski JM, Moughan J, Purdy J, et al. Effect of standard vs dose-escalated radiation therapy for patients with intermediate-risk prostate cancer: the NRG Oncology RTOG 0126 randomized clinical trial. JAMA Oncol 2018;4:e180039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kishan AU, Cook RR, Ciezki JP, et al. Radical prostatectomy, external beam radiotherapy, or external beam radiotherapy with brachytherapy boost and disease progression and mortality in patients with Gleason score 9–10 prostate cancer. JAMA 2018;319:896–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264–269, w264. [DOI] [PubMed] [Google Scholar]
- [15].Kishan AU, Wang X, Seiferheld W, et al. Association of Gleason grade with androgen deprivation therapy duration and survival outcomes: a systematic review and patient-level meta-analysis. JAMA Oncol 2019;5:91–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Rucker G Network meta-analysis, electrical networks and graph theory. Res Synth Methods 2012;3:312–24. [DOI] [PubMed] [Google Scholar]
- [17].Andersen PK, Keiding N. Multi-state models for event history analysis. Stat Methods Med Res 2002;11:91–115. [DOI] [PubMed] [Google Scholar]
- [18].Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi-state models. Stat Med 2007;26:2389–430. [DOI] [PubMed] [Google Scholar]
- [19].de Wreede LC, Fiocco M, Putter H. mstate: An R package for the analysis of competing risks and multi-state models. J Stat Softw 2011;38:30. [Google Scholar]
- [20].Team RC. R: a language and environment for statistical computing. 2016. https://www.R-project.org/
- [21].Therneau T. A package for survival analysis in S. version 2.38. 2015 https://CRAN.R-project.org/package=survival.
- [22].Tian LU H; Horiguchi M surv2sampleComp: inference for model-free between-group parameters for censored survival data. R package version 1.0–5 2017. 2019. https://CRAN.R-project.org/package=surv2sampleComp
- [23].Yan J KMsurv: data sets from Klein and Moeschberger, survival analysis. Version 0.1–5 1997. https://CRAN.R-project.org/package=KMsurv
- [24].Terry MT. coxme: Mixed effects Cox models. R package version 2.2–5 2015. https://CRAN.R-project.org/package=coxme
- [25].Putter H, de Wreede L, Fiocco M. mstate: Data preparation, estimation and prediction in multistate models. Version 0.2.11 2018. https://CRAN.R-project.org/package=mstate
- [26].Macdonald I Biological predeterminism in human cancer. Surg Gynecol Obstet 1951;92:443–52. [PubMed] [Google Scholar]
- [27].Xie W, Regan MM, Buyse M, et al. Metastasis-free survival is a strong surrogate of overall survival in localized prostate cancer. J Clin Oncol 2017;35:3097–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Yuan Y, Kishan AU, Nickols NG. Treatment of the primary tumor in metastatic prostate cancer World J Urol. In press. 10.1007/s00345-018-2552-8 [DOI] [PubMed] [Google Scholar]
- [29].Parker CC, James ND, Brawley CD, et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet 2018;392:2353–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Michiels S, Baujat B, Mahe C, Sargent DJ, Pignon JP. Random effects survival models gave a better understanding of heterogeneity in individual patient data meta-analyses. J Clin Epidemiol 2005;58:238–45. [DOI] [PubMed] [Google Scholar]
- [31].Pucar D, Shukla-Dave A, Hricak H, et al. Prostate cancer: correlation of MR imaging and MR spectroscopy with pathologic findings after radiation therapy-initial experience. Radiology 2005;236:545–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Calais J, Kishan AU, Cao M, et al. Potential impact of (68)Ga-PSMA-11 PET/CT on the planning of definitive radiation therapy for prostate cancer. J Nucl Med 2018;59:1714–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Danneman D, Drevin L, Robinson D, Stattin P, Egevad L. Gleason inflation 1998–2011: a registry study of 97,168 men. BJU Int 2015;115:248–55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


