Abstract
The use of electronic cigarettes (e-cigarettes) is increasing despite insufficient information concerning their long-term effects, including the effects of maternal e-cigarette use on pre- and postnatal development. Our previous study demonstrated that developmental exposure to 1,2-propanediol (a principal component of e-cigarette liquid) affected early development of zebrafish, causing reduced growth, deformities, and hyperactive swimming behavior in larvae. The current study extends assessment of the developmental toxicity of 1,2-propanediol by examining additional long-term behavioral effects. We demonstrate that embryonic/larval exposure of zebrafish to 1,2-propanediol (0.625% or 1.25%) not only affected behavioral parameters in the larvae, but also caused persisting behavioral effects in adults after early developmental exposure. Additional parameters, including neural and vascular development in larvae, stress response in adults, and concentration of neurotransmitters dopamine and serotonin in adult brain were examined, in order to explain the behavioral differences. These additional assessments did not find 1,2-propanediol exposure to significantly affect Tg(Neurog1:GFP) or the transcript abundance of neural genes (Neurog1, Ascl1a, Elavl3, and Lef1). Vascular development was not found to be affected by 1,2-propanediol exposure, as inferred from experiments with Tg(Flk1:eGFP) zebrafish; however, transcript abundance of vascular genes (Flk1, Vegf, Tie-2, and Angpt1) was decreased. No statistically significant changes were noted for plasma cortisol or brain neurotransmitters in adult fish. Lastly, analysis of gene transcripts involved with 1,2-propanediol metabolism (Adh5, Aldh2.1, and Ldha) showed an increase in Adh5 transcript. This is the first study to demonstrate that developmental exposure to 1,2-propanediol has long-term neurobehavioral consequences in adult zebrafish, showing that e-cigarettes contain substances potentially harmful to neurodevelopment.
Keywords: Electronic cigarettes; 1,2-Propanediol (propylene glycol); Zebrafish; Behavior; Neural and vascular development; Xenobiotic metabolism
1. Introduction
In 2015, electronic cigarette (e-cigarette) sales were estimated at $3.5 billion in the US (Hiles, 2015). The use of e-cigarettes among US adults, who smoke tobacco cigarettes, could be regarded as a tool to reduce or even quit smoking (Hajek et al., 2014). In contrast, e-cigarette use among US youth and young adults, which increased 900% from 2011 to 2015, is now a major public health concern (US Department of Health and Human Services, 2016), and it was suggested that the use of e-cigarettes may encourage adolescents to use tobacco cigarettes (Dutra and Glantz, 2014).
Moreover, it has been suggested that the perception of e-cigarettes as a safe alternative to tobacco cigarettes may lead to e-cigarette smoking during pregnancy (Baeza-Loya et al., 2014). In fact, recent data suggest that the prevalence of e-cigarette use during pregnancy is similar to that of tobacco cigarettes, and that pregnant women view e-cigarettes as a safer alternative (Wagner et al., 2017). This is an important concern because most e-cigarettes also contain nicotine, which can adversely impact the developing brain of fetuses (via maternal smoking) or adolescents (US Department of Health and Human Services, 2016). In addition to nicotine, e-cigarette liquid contains several other chemicals, depending on the brand, but >90% of e-cigarette liquid is composed of the humectants 1,2-propanediol (also known as propylene glycol) and glycerin (Schober et al., 2014). Other chemicals, including various alcohols, polycyclic aromatic hydrocarbons, flavors, are also present in e-cigarettes (Goniewicz et al., 2014; Schober et al., 2014).
The FDA has classified the use of 1,2-propanediol as safe (Agency for Toxic Substances and Disease Registry, 2007); however, this decision has been made prior to the increased use of e-cigarettes. It has been estimated that humans are primarily exposed to 1,2-propanediol through ingestion of various foods, an average of 34 mg/kg/day. 1,2-Propanediol is also a well-known excipient, used in medicinal products that are administered topically, orally, or intravenously (European Medicines Agency, 2014). The increased use of e-cigarettes is likely to increase human exposure to 1,2-propanediol.
Studies in animal models and humans show that 1,2-propanediol is rapidly absorbed (within ~1 h) after oral or intravenous administration. The half-life of 1,2-propanediol in humans is estimated at ~4 h, depending on the dose. Upon absorption, 1,2-propanediol is uniformly distributed in total body water without a significant distribution to specific tissues (European Medicines Agency, 2014). 1,2-Propanediol is primarily metabolized in the liver by alcohol dehydrogenase (ADH; EC 1.1.1.1) into lactaldehyde, which is then metabolized by aldehyde dehydrogenase (ALDH; EC 1.2.1.3) into lactic acid. It is further metabolized by lactate dehydrogenase (LDH; EC 1.1.1.27) into pyruvate (Fowles et al., 2013) that is eventually metabolized into CO2 and H2O within the citric acid cycle. About 45% of absorbed 1,2-propanediol is excreted in urine unchanged or as the glucuronide conjugate (Agency for Toxic Substances and Disease Registry, 2007).
Although the use of 1,2-propanediol is presumed safe, several case studies have documented that exposure to 1,2-propanediol as an excipient in medicinal products may lead to hyperosmolarity, metabolic acidosis, hemolysis, neurotoxicity, and reduced renal clearing. In fact, 1,2-propanediol toxicity appears to manifest at exposures of 1 g/kg a day, with serious clinical symptoms occurring at 3 g/kg/day (European Medicines Agency, 2014). Neonates and infants are particularly susceptible to the effects of 1,2-propanediol and several cases have reported toxicity of 1,2-propanediol when administered in infants (European Medicines Agency, 2014). Importantly, the epidemiological data on the potential developmental toxicity of 1,2-propanediol is lacking, especially in the context of e-cigarette use during pregnancy, and its potential for altering the toxicity (e.g. synergism) of known chemicals, such as nicotine. Interestingly, a recent study demonstrated that prenatal and early postnatal exposure of mice to e-cigarettes that contain 1,2-propanediol, but not nicotine, leads to lower weights in offspring, while exposure to e-cigarettes that contain both nicotine and 1,2-propanediol also alters offspring behavior (Smith et al., 2015). Certainly, additional studies on the toxicity of various e-cigarette components are necessary to better understand the potential dangers associated with e-cigarette use, especially during pregnancy.
Consequently, in this study we investigate the developmental toxicity of 1,2-propanediol and its potential implications for e-cigarette use. Our previous study demonstrated that developmental exposure to 1,2-propanediol in zebrafish embryos reduced growth, increased incidence of deformities, and resulted in hyperactive swimming behavior in larvae, suggesting that 1,2-propanediol could contribute to the overall toxicity of e-cigarettes (Massarsky et al., 2017). Based upon this initial investigation, the current study expands on the short- and long-term consequences of 1,2-propanediol developmental toxicity. Thus, we examined the effects of 1,2-propanediol on behavior in larvae and adult fish and whether or not these changes are associated with potential alterations in neural and vascular development, since the synchronized development of these two systems during embryogenesis is essential for central nervous system (CNS) growth and maturation (Bautch and James, 2009). We also examined the stress response in adult fish and concentration of neurotransmitters dopamine and serotonin in adult brain, since alteration of these physiological parameters could underlie behavioral changes. Lastly, we examined whether 1,2-propanediol induces the gene expression of metabolic enzymes, similarly to mammalian models. Ultimately, the study aims to encourage additional research that would examine the contribution of 1,2-propanediol to the developmental toxicity of e-cigarettes.
2. Materials and methods
2.1. Chemicals
All reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise specified. 1,2-Propanediol was purchased from Sigma-Aldrich (57–55-6, 99%). The stock solution of 1,2-propanediol was diluted to working concentrations in 30% Danieau’s (in mM: 58 NaCl, 0.7 KCl, 0.4 MgSO4, 0.6 Ca(NO3)2, 5 HEPES).
2.2. Zebrafish maintenance
Adult wild type EkkWill zebrafish (EkkWill Waterlife Resources, Ruskin, FL, USA) were maintained in holding tanks on a 14:10 h light–dark cycle (lights on at 9 AM) at 28°C in re-circulating AHAB system (Aquatic Habitats, Apopka, FL, USA) in 60 mg/L salt water (Instant Ocean, Foster & Smith, Rhinelander, WI, USA). Fish were fed brine shrimp in the morning and Zeigler’s Adult Zebrafish Complete Diet (Aquatic Habitats) in the afternoon. Breeding tanks were set at 5 PM, and embryos were collected the following morning within 1 h of spawning between 9 and 10 AM and kept in Danieau’s at 28°C until separated into experimental groups. Transgenic zebrafish Mitfa mutant Tg(Neurog1:GFP) (a generous gift from Dr. Linney, Duke University) and Tg(Flk1:eGFP) (a generous gift from Dr. Tobin, Duke University) were maintained in a separate re-circulating AHAB system under similar conditions. All procedures were approved by the Duke University Institutional Animal Care and Use Committee (A279–08-10).
2.3. Experimental setup
At 6 hours post fertilization (hpf), zebrafish embryos were screened under a dissecting microscope (SMZ1500, Nikon, Tokyo, Japan) and distributed into 6 cm glass petri dishes (15 embryos/dish), containing 14 mL Danieau’s to which 1 mL 1,2-propanediol working solution was added. Wild type zebrafish were used to assess behavior and gene expression. Each treatment group had five replicate dishes per treatment: two Petri dishes were used for larval behavior, one Petri dish was used for gene expression, and the remainder two Petri dishes were used to raise larvae to adulthood for adult behavior assessments. Tg(Neurog1:GFP) and Tg(Flk1:eGFP) zebrafish were exposed in a similar manner with two replicate dishes per treatment. The final nominal concentrations of 1,2-propanediol were 0.625% and 1.25%; these concentrations were shown not to cause malformations in our previous study (Massarsky et al., 2017). Throughout the exposure the embryos were kept in a 28°C incubator (Model 3326, Forma Scientific, Inc., Marietta, OH, USA) on a 14:10 h light–dark cycle. The study was repeated at least 3 times, using independent zebrafish cohorts.
2.4. Behavioral assessments
2.4.1. Larval behavior
At the end of the exposure, the 72 hpf larvae were transferred into 50 mL beakers, containing 25 mL of fresh Danieau’s, and transported to Dr. Levin’s laboratory, where they were kept under a 14:10 h light-dark cycle at 28°C. At 144 hpf, larvae were randomly transferred to a 96-well plate (1 larva/well, 30 wells/treatment) and allowed to acclimate in dark for 1 h before being transferred to a DanioVision™ observation chamber (Noldus Inc., Wageningen, The Netherlands). Swimming distance was monitored for 50 min in alternating 10 min dark (“0% illumination”, <1 lux) and light (“100% illumination”, 5,000 lux) phases, starting with a 10 min habituation phase in dark. Larval motion was recorded at a sample rate of 30 times/s via an infrared camera. Video data were analyzed by computer tracking software EthoVision XT® (Noldus Inc., Wageningen, The Netherlands), in order to calculate total distance moved for each individual larva over the course of the session.
2.4.2. Adult behavior
At the end of the exposure, the 72 hpf larvae were transferred to clean system water and raised to adulthood in the recirculating AHAB system. The viability and sex ratios of adult zebrafish (~5 months) were assessed prior to behavioral testing. The fish were then transported to the testing facility and acclimated for 3 weeks prior to testing. The fish were tested in a series of four behavioral tests, including novel tank dive test, startle tap test, shoaling test, and predator avoidance test. These tests were performed exactly as outlined in Glazer et al. (2017). Each test was conducted on a separate day. All testing was conducted between 10 AM and 5 PM, and testing times were counterbalanced across all experimental groups. Each testing day began after the routine morning brine shrimp feeding. Fish tanks designated for testing were transferred to the behavior testing room, and fish were acclimated for 30 min. Fresh system water was used in all testing apparatuses. A HD camcorder (VIXIA HFR700; Canon Inc., Melville, NY, USA) was used for video recording in all tests, and the videos were analyzed with the EthoVision XT® software.
2.4.2.1. Novel tank dive test
Adult zebrafish were tested for novel environment response and recovery. The experimental setup consisted of two adjacent 1.5 L plastic tanks filled with 10 cm of system water. Each tank was a trapezoid: 22.9 cm along the bottom, 27.9 cm at the top, 15.2 cm high, and 15.9 cm along the diagonal side. It was 6.4 cm wide at the top, and tapered to 5.1 cm at the bottom. The tanks were video recorded from the side. At the beginning of each trial two fish were individually placed in the testing tanks and recorded for 6 min. Measurements extracted were total distance traveled and the mean distance to bottom of tank for each minute. A dive recovery value was calculated for each treatment group by subtracting the distance to bottom in the first minute of testing from the mean distance in the last five minutes of testing.
2.4.2.2. Startle tap test
Sensorimotor startle response and habituation were tested using a custom-built apparatus. The testing apparatus consisted of flat white 23 × 39 cm surface with white 23 × 27 cm frontal and rear blocking barriers attached. Eight acrylic cylindrical arenas, 5.7 cm in diameter, were attached onto the flat surface in two rows. Each arena was clear with horizontal bottoms and slightly angled sides to enable visibility to the camera fixed overhead. Opaque screens were used to separate the arenas, isolating fish from each other. Each arena contained 40 mL system water that was replaced after each trial. Below each arena was a central 24-volt DC push solenoid that provided a sudden tap when activated by the EthoVision XT® software. The fish were individually placed in the testing arenas (8 fish/trial). The testing sequence consisted of a 30 s acclimation period followed by 10 consecutive taps at 1 min intervals. Total distance traveled 5 s before (pre) and after (post) each tap was measured.
2.4.2.3. Shoaling test
Individual social interaction was tested using a custom-built adult behavior testing tank called Multiple Use Partitioned Experimental Tank (MUPET). The tank was a 519.85 cm long and 327.15 cm wide (outer measurements). The sides and bottom were made of 12.7 mm thick transparent acrylic sheets. The bottom sheet was sandblasted to reduce glare, and divided into a 5 × 3 grid by a network of slots that were 6.35 mm wide and 10 mm deep. The grid slots continued up the walls of the tank, and along the walls on the inner bottom perimeter. Three 16 × 31.4 mm black partitions were inserted to create two adjacent lanes across the tank width. The MUPET was situated on two metal bars and an A2 60 × 40 cm light box (Huion Technology, Shenzhen, China) was placed underneath the tank bottom, providing even light throughout the tank. Two 50 cm LCD monitors flanked the narrow ends of the two lanes. A digital video camcorder was placed above the tank.
Adult fish were individually isolated in 1.5 L tanks surrounded by opaque dividers for 30 min before being netted into the MUPET lanes described above and recorded for 7 min. During the first 2 min of the test, each monitor screen displayed a background of static ovals approximately the size of an adult zebrafish and displaying the pattern and colors of a zebrafish. At the end of the first two min one of the monitors began to display a video recording of a zebrafish shoal for the remaining 5 min. Measurements extracted were total distance traveled and the mean distance to the side of the tank on which the video was displayed (distance to zone). A pre-post video difference value was calculated for each treatment group by subtracting the mean distance to zone during the two minutes after the video started playing from the mean distance to zone during the two minutes before the video began.
2.4.2.4. Predator avoidance test
Threat recognition and evasion behavior were tested using a testing apparatus and setup as described in the previous section. Individual fish were placed in the MUPET lanes and recorded for 9 min, consisting of one min acclimation followed by 8 min of alternating minute-long stimulus/no stimulus events. The stimulus was a power point presentation showing either a blue slow-growing dot (4 s) or a red fast-growing dot (1 s), appearing repeatedly on one of the screens. The blue dot appeared in the first two stimulus events and the red dot appeared in the last two stimulus events. Measurements extracted were total distance traveled and the mean distance to the side of the tank on which the stimulus was displayed (distance to zone). A flee response value was calculated for each treatment group as the difference in mean distance to zone pre and post stimulus.
2.5. Potential factors contributing to behavioral changes
2.5.1. Larval neural development
Neural development was assessed in Tg(Neurog1:GFP) Mitfa mutant zebrafish (do not generate pigment). Images of five randomly selected fish from each treatment were captured at 72 hpf, using Zeiss Lumar.V12 stereoscope (Oberkochen, Germany). To minimize any potential biases, the images were evaluated independently by two researchers. We also quantified the relative fluorescence in the head region using Image J software (expressed as fold change), in order to further minimize any potential biases.
In addition, transcript abundance of neural markers Neurog1, Asc11a, Elavl3, and Lef1 was assessed in wild type 72 hpf larvae (see section 2.7 for details), in order to assess whether exposure to 1,2-propanediol results in changes on a molecular level. Neurog1 and Ascl1a are proneural transcription factors that are essential for proliferation of neural progenitor cells, while Elavl3 (formerly known as Huc) and Lef1 are transcription factors that are essential for neuronal maturation (Schmidt et al., 2013).
2.5.2. Larval vascular development
Vascular development was assessed in Tg(Flk1:eGFP) zebrafish that were treated with 0.2 mM phenylthiourea (PTU) at 26 hpf, in order to prevent pigment generation. Images of five randomly selected fish from each treatment were captured at 72 hpf, using Zeiss Lumar.V12 stereoscope. To minimize any potential biases, the images were evaluated independently by two researchers. We also quantified the relative fluorescence in the head and trunk regions using Image J software (expressed as fold change), in order to further minimize any potential biases.
In addition, transcript abundance of vascular markers Flk1, Vegf, Tie-2, and Angpt1 was assessed in wild type 72 hpf larvae (see section 2.7 for details), in order to assess whether exposure to 1,2-propanediol results in changes on a molecular level. Flk1 (a membrane receptor) and its ligand Vegf facilitate a signaling cascade of a suite of genes that promote angiogenesis (Leung et al., 2006), while Tie-2 (a cell membrane receptor kinase) and its ligand Angpt1 facilitate the signaling cascade of a suite of genes that are essential for endothelial cell survival, proliferation, and migration (Huang et al., 2010).
2.5.3. Adult stress response
Stress response was assessed in adult zebrafish (~12 months; older fish were used to ensure sufficient blood volumes for sample collection) that were exposed to 1,2-propanediol up to 72 hpf and were previously tested for behavior at ~5 months. Zebrafish were stressed using the standard netting stress and euthanized in ice-cold water according to Massarsky et al. (2014). The fish were weighed and blood was collected into a 0.2 mL PCR tube and kept on ice. The collected blood was centrifuged at 7000 rpm for 2 min and the resulting plasma was collected into a fresh 0.2 mL PCR tube, flash-frozen in liquid nitrogen, and kept at −80°C until analyzed. Whole brain tissues from individual fish were also collected (see section 2.5.4 for details). All samples were collected between 1 and 3 PM. Samples from two fish were pooled. The experiment was repeated on 3 independent zebrafish cohorts (n=3). Plasma cortisol concentration was assessed using a Cortisol ELISA kit (07M-21603; MP Biomedicals, Solon, OH, USA) according to manufacturer’s instructions.
2.5.4. Neurotransmitters in adult brain
Whole brain tissues were collected into 0.5 mL Eppendorf tubes (1 brain/tube), flash-frozen in liquid nitrogen, and kept at −80°C until analyzed. Individual brain samples were processed according to Chatterjee and Gerlai (2009) with slight modifications. Briefly, 90 μL artificial cerebrospinal fluid (in mM: 119 NaCl, 26.2 NaHCO3, 2.5 KCl, 1 NaH2PO4, 1.3 MgCl2, 10 glucose) were added into each tube and sonicated 5 s on ice. The homogenates were centrifuged at 10,000 g for 10 min at 4°C and the supernatants were collected into fresh 0.5 mL Eppendorf tubes. The supernatants were used to assess dopamine and serotonin using commercial kits (dopamine: KA3838, serotonin: KA2518; Abnova, Taipei, Taiwan) according to manufacturer’s instructions. The concentration of neurotransmitters was normalized to protein concentration in the supernatant using BCA assay.
2.6. Markers of xenobiotic metabolism
Transcript abundance of Adh5, Aldh2.1, and Ldha was assessed in wild type 72 hpf larvae (see section 2.7 for details), in order to assess whether exposure to 1,2-propanediol induces the metabolic detoxification pathway. As aforementioned, these enzymes sequentially metabolize 1,2-propanediol into pyruvate.
2.7. mRNA transcript abundance
At the end of the exposure, 72 hpf larvae were euthanized on ice, collected into 1.5 mL Eppendorf tubes, and flash-frozen in liquid nitrogen. The samples were kept at −80°C until analyzed. Total RNA from frozen larvae was extracted using a Direct-zol RNA MiniPrep kit (Zymo Research, Irvine, CA, USA). mRNA concentration and purity were determined using NanoDrop ND-100 (NanoDrop Technologies, Wilmington, DE, USA). cDNA synthesis was performed using Omniscript® RT kit (Qiagen, Hilden, Germany). Quantitative RT-PCR was performed using SYBR Green Lightcycler Master Mix (Life Technologies, Carlsbad, CA, USA) on an ABI 7300 quantitative real-time PCR machine. The thermal cycle included 5 min at 95°C, followed by 40 cycles of 15 s at 95°C, 1 min at 60°C, and a dissociation curve. Samples were run in duplicate.
The primers for RT-PCR were acquired from Integrated DNA Technologies (IDT), Inc. (Coralville, IA, USA). Unless otherwise specified, the primers were designed using the PrimerQuest Tool (IDT) (Table 1). Data were analyzed on the ABI PRISM 7300 Sequence Detection System, Version 1.1 (Applied Biosystems, Inc., Foster City, CA, USA). The Ct values of target genes were compared to those of reference gene Eif1b (eukaryotic translation initiation factor 1b) and the average fold change was calculated according to methods published by Livak and Schmittgen (2001).
Table 1.
List of primers that were used for quantitative real-time PCR analysis.
| Gene | GenBank ID | Primer sequence (5’-3’) |
|---|---|---|
| Eif1b1 | NM_199588 | Fwd: GCCTTCAAGAAGAAATTTGCC Rev: CCGTGGACTTTGAGCTG |
| Ascl1a | NM_131219 | Fwd: ACCCTCTGAGTCCAGAAGAA |
| Rev: CCCAAGCGAGTGCTGATATT | ||
| Elavl3 | NM_131449.1 | Fwd: ATTGGTCAGAGACAAGATCACAG |
| Rev: ACCGTTGAGCGTGTTGATAG | ||
| Lef1 | AF136454 | Fwd: CCATTCCCAGAACGTCGAATA |
| Rev: GAAGTGCTCGTCACTGTATGT | ||
| Neurog1 | NM_131041 | Fwd: AAGCGTTCTCATGTCGGTATAG |
| Rev: TACCTAACGTTGTGCTCTTGG | ||
| Angpt1 | AF379602 | Fwd: GAACTCGCTGTCCACCAATAA Rev: CTGCATCTTCTCCTCCAGAAAC |
| Flk1 | AF487829 | Fwd: CACAGTCCTCCTCCAGACTATAA Rev: CAGAATGGACCGATCAGACTTC |
| Tie-2 | AF053632 | Fwd: GCCGTCAAGAGGATGAAAGA Rev: CCCAGCAGGTGTATGATGTT |
| Vegf | AF016244 | Fwd: CGGATGTGTTACTTTGACCCACGA Rev: GCAGCCTTTACAGCAGACAGATGGAGGA |
| Adh52 | NM_131849 | Fwd: CTTTCCATCGAGGAGGTGGAG |
| Rev: GAGAAGGTGCTGGTGCCCATGAAGTGG | ||
| Aldh2.13 | NM_200490 | Fwd: CTGATGTGGATAAAGCGGTG |
| Rev: CTAAATAGGCAGCATCTCTC | ||
| Ldha4 | NM_131246 | Fwd: TCCTTCTCAAGGATCTGACCGA |
| Rev: TGTGCGTCTTGAGAAACAGGC |
Dasmahapatra et al. (2001); Adh5 was previously known as Adh3.
2.8. Statistical analysis
All data are presented as means and standard error of the mean (SEM). Statistical analyses were conducted using SigmaPlot (SPW 12; Systat Software, Inc., San Jose, CA, USA). For larval behavior, a two-way ANOVA was used to assess significant effects of exposure and time (i.e. dark/light conditions). For adult viability and sex ratios, a one-way ANOVA was used to assess significant effects of developmental exposure. For novel tank dive test, a three-way ANOVA was used to assess significant effects of exposure, sex, and test duration on total distance traveled and distance to bottom, and a two-way ANOVA was used to assess significant effects of exposure and sex on dive recovery. For startle tap test, a three-way ANOVA was used to assess significant effects of exposure, sex, and tap number on distance moved post or pre tap. For shoaling test, a three-way ANOVA was used to assess significant effects of exposure, sex, and test duration on total distance traveled and distance to zone, and a two-way ANOVA was used to assess significant effects of exposure and sex on pre-post distance. For predator avoidance test, a three-way ANOVA was used to assess significant effects of exposure, sex, and test duration on total distance traveled and distance to zone, and a three-way ANOVA was used to assess significant effects of exposure, sex, and blue/red stimulus on flee response. For gene expression and relative fluorescence, a one-way ANOVA was used to assess significant effect of exposure. For plasma cortisol, a three-way ANOVA was used to assess significant effects of exposure, sex, and presence/absence of stress. For brain neurotransmitters, a two-way ANOVA was used to assess significant effects of exposure and sex. In all cases, a post hoc Tukey method was used whenever significant differences were detected by the ANOVA test. In all cases a P ≤ 0.050 was considered significant. The normality of the data was verified by calculating the correlation coefficient between the data and the corresponding z-scores, which were ≥0.95, as well as plotting the corresponding graphs, which had r2 values of ≥0.95.
3. Results
3.1. Behavioral assessments
3.1.1. Larval behavior
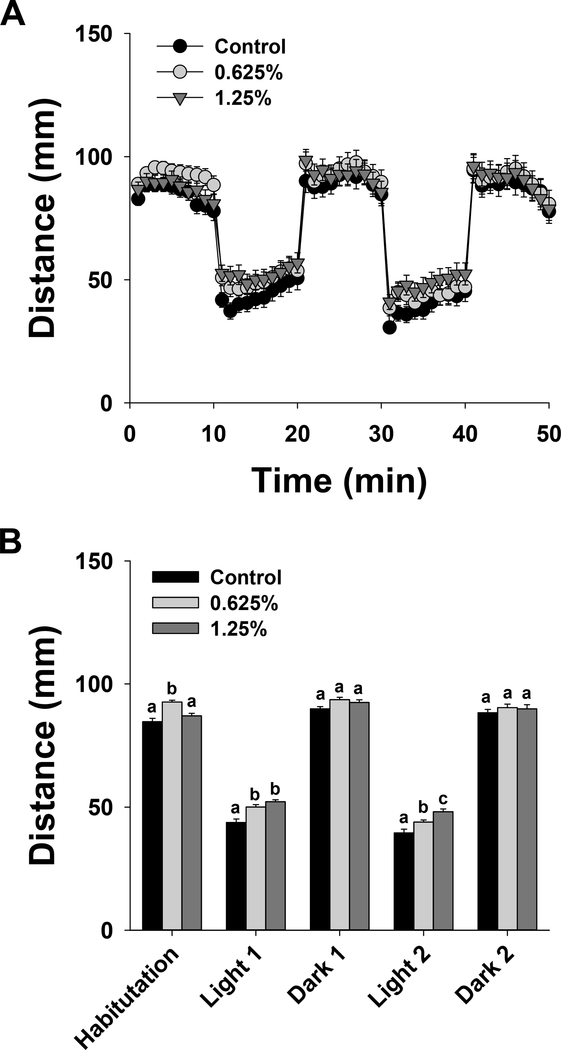
The results of two-way ANOVA are provided in Table S1. Exposure to 1,2-propanediol altered swimming behavior (Fig. 1). During the initial habituation phase in the dark (1–10 min), swimming hyperactivity was noted in 0.625% group, but not 1.25% group (Fig. 1B). No significant differences were noted in the subsequent dark phases (21–30 and 41–50 min). Swimming hyperactivity was noted during both light phases (11–20 min and 31–40 min) in 0.625% and 1.25% groups. Moreover, during the second light phase 1.25% group was significantly different from 0.625% group. Both the control and exposed larvae were able to respond to changing light conditions by increasing activity in dark and decreasing activity in light.
Figure 1.
Larval locomotor activity in 144 hpf zebrafish exposed to 0% (control), 0.625% or 1.25% 1,2-propanediol from 6 hpf until 72 hpf. A. Total distance moved per minute over the course of the test. B. Average distance moved during each 10-min illumination phase. Letters indicate significant differences determined using two-way ANOVA with a post hoc Tukey test; P ≤ 0.05 was considered significant.
3.1.2. Adult behavior
Survival of adult zebrafish was not significantly different across groups despite a lower survival at 1.25% 1,2-propanediol (Table 2). Sex ratios were also not significantly different across groups, such that ~61% were males and ~39% were females. About 20 fish of each sex were used for behavioral testing.
Table 2.
Survival and sex ratio of adult zebrafish (~5 months) that were exposed to 0.625% or 1.25% 1,2-propanediol from 6 to 72 hpf and raised to adulthood. No statistically significant differences were detected [P = 0.072 (survival); P = 0.999 (sex ratios)].
| Survival (%) | Males (%) | Females (%) | |
|---|---|---|---|
| Control | 50.4±9.3 | 61.4±8.7 | 38.6±8.7 |
| 0.625% | 44.4±7.1 | 61.3±2.8 | 38.7±2.8 |
| 1.25% | 23.0±3.2 | 62.3±7.9 | 37.7±7.9 |
3.1.2.1. Novel tank dive test
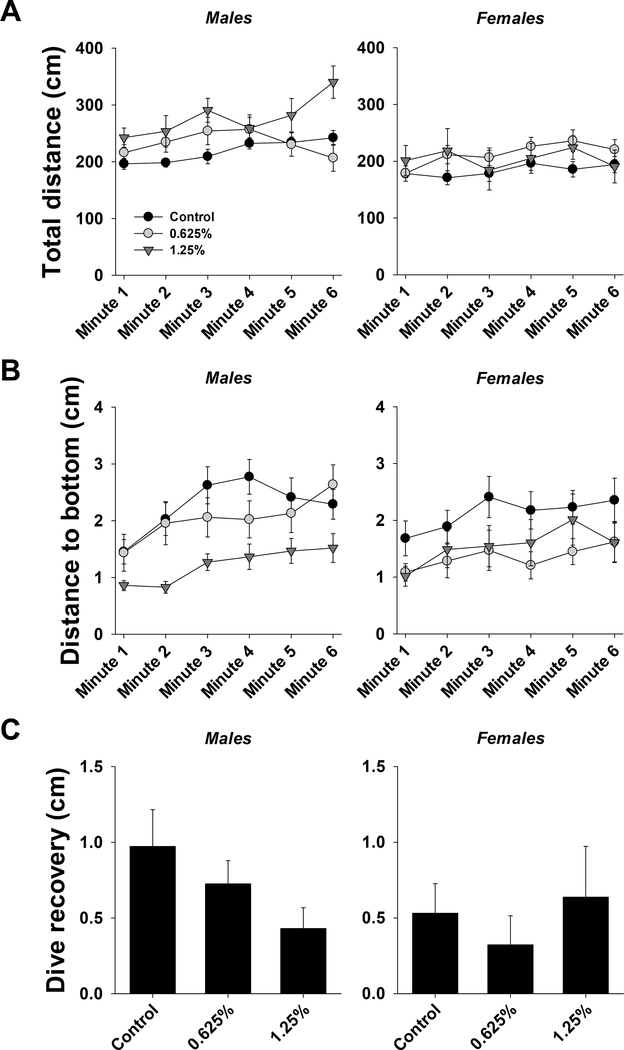
The results of three-way ANOVA for total distance and distance to bottom, as well as two-way ANOVA for dive recovery are provided in Table S2. Total distance traveled during the test across all treatments and both sexes was significantly affected by time, such that the traveled distance increased over time (Fig. 2A). There was also a significant effect of exposure. In males, total distance traveled was significantly higher in 1.25% group compared to control and 0.625% groups. In females, total distance traveled was significantly higher in 0.625% group compared to the control group. There was also a significant effect of sex. Overall, total distance traveled was higher in males compared to females. In addition, sex-specific differences were noted within control and 1.25% groups, such that total distance traveled was higher in males than females.
Figure 2.
Novel tank dive test in ~5 months old adult zebrafish exposed to 0%, 0.625% or 1.25% 1,2-propanediol from 6 hpf until 72 hpf. A. Total distance moved per minute over the course of the test for males and females. B. Distance to bottom of the tank per minute over the course of the test for males and females. C. Dive recovery for males and females. See section 3.1.2.1 for details on statistical differences.
Distance to bottom was significantly affected by time, such that the distance to bottom at minutes 3–6 was significantly higher than at minute 1 (Fig. 2B). There was also a significant effect of exposure. In males, distance to bottom was significantly lower in 1.25% group compared to control and 0.625% groups. In females, distance to bottom was significantly lower in 0.625% and 1.25% groups compared to the control group. There was also a significant effect of sex noted within the 0.625% group, such that the distance to bottom was higher in males compared to females.
Dive recovery was not significantly different across groups in males or females. There was also no significant effect of sex on dive recovery.
3.1.2.2. Startle tap test
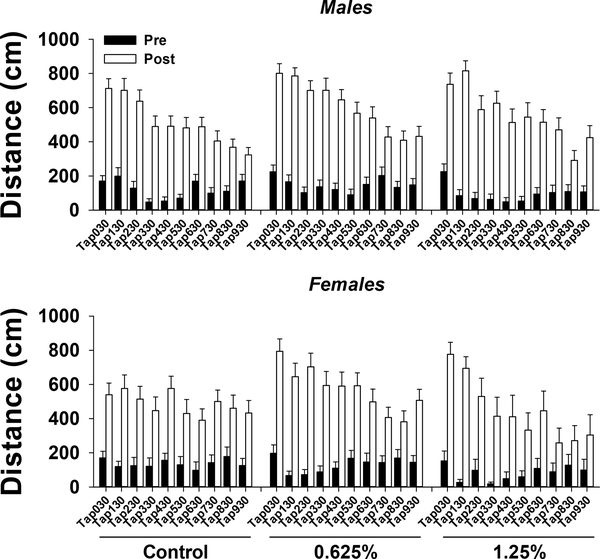
The results of three-way ANOVA for post tap and pre tap are provided in Table S3. Distance traveled post tap was significantly affected by time, such that overall there was a decrease in traveled distance over time (Fig. 3). There was also a significant effect of exposure on distance traveled post tap, such that distance traveled was higher in 0.625% group compared to control group. There was also a significant effect of sex, such that distance traveled was higher in males compared to females.
Figure 3.
Startle tap test in ~5 months old adult zebrafish exposed to 0%, 0.625% or 1.25% 1,2-propanediol from 6 hpf until 72 hpf. Total distance moved in the 5 s pre (black bars) and post (white bars) each tap over the course of the test is presented for males (top panel) and females (bottom panel). See section 3.1.2.2 for details on statistical differences.
Distance traveled pre tap was significantly affected by time, such that overall there was a decrease in traveled distance at taps 2–6 compared to tap 1, while taps 7–10 were not significantly different from tap 1. There was also a significant effect of treatment on distance traveled pre tap, such that distance traveled was lower in 1.25% group compared to 0.625% group. There was no significant effect of sex on distance traveled pre tap.
3.1.2.3. Shoaling test
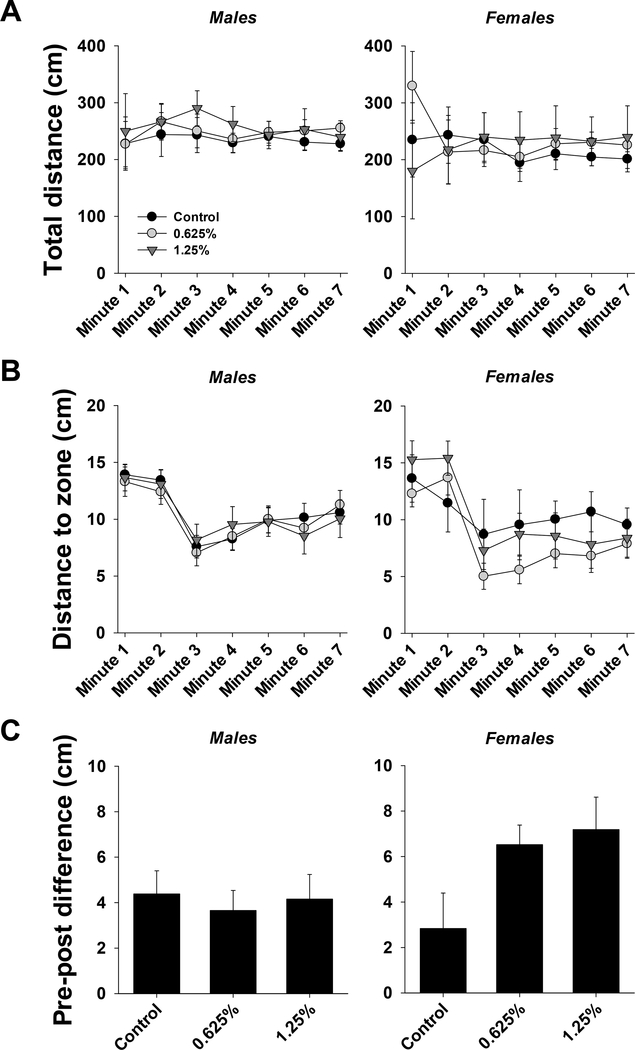
The results of three-way ANOVA for total distance and distance to zone, as well as two-way ANOVA for pre-post difference are provided in Table S4. Total distance traveled during the test was not significantly affected by time, exposure, or sex (Fig. 4A). Total distance traveled was similar across all groups in both males and females.
Figure 4.
Shoaling test in ~5 months old adult zebrafish exposed to 0%, 0.625% or 1.25% 1,2-propanediol from 6 hpf until 72 hpf. A. Total distance moved per minute over the course of the test for males and females. B. Distance to zone per minute over the course of the test for males and females. C. Pre-post difference for males and females. See section 3.1.2.3 for details on statistical differences.
Distance to zone was significantly affected by time, such that the distance to zone at minutes 3–7 was significantly lower than at minute 1 or 2 (Fig. 4B). There was also a significant effect of exposure, such 0.625% group was different from control. There was no significant effect of sex.
Pre-post difference was not significantly different across groups in males or females. There was also no significant effect of sex on pre-post difference.
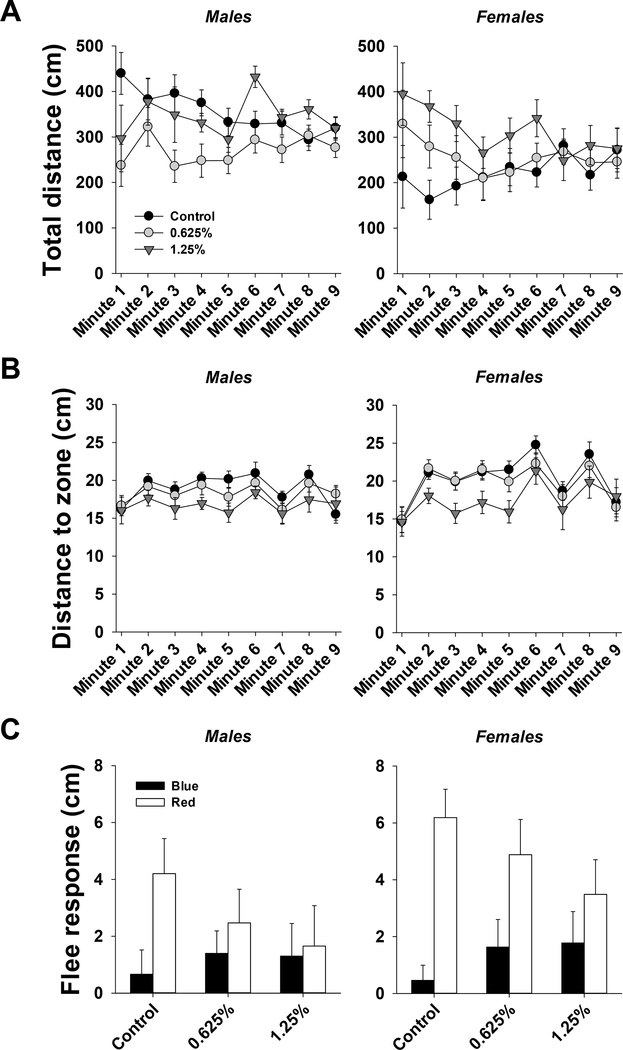
3.1.2.4. Predator avoidance test
The results of three-way ANOVA for total distance and distance to bottom, and flee response are provided in Table S5. Total distance traveled during the test was not significantly affected by time (Fig. 5A). There was, however, a significant effect of exposure. In males, total distance traveled was significantly lower in 0.625% group compared to control and 1.25% groups. In females, total distance traveled was significantly higher in 1.25% group compared to the control and 0.625% groups. There was also a significant effect of sex within the control group, such that distance traveled was higher in males compared to females.
Figure 5.
Predator avoidance test in ~5 months old adult zebrafish exposed to 0%, 0.625% or 1.25% 1,2-propanediol from 4 hpf until 72 hpf. A. Total distance moved per minute over the course of the test for males and females. B. Distance to zone per minute over the course of the test for males and females. C. Flee response from blue (black bars) and red (white bars) stimuli for males and females. See section 3.1.2.4 for details on statistical differences.
Distance to zone was significantly affected by time, such that the distance to zone was higher in presence of stimulus (minutes 2, 4, 6, and 8) compared to absence of stimulus (minutes 1, 3, 5, 7, and 9) (Fig. 5B). There was also a significant effect of exposure. In both males and females, distance to zone was significantly lower in 1.25% group compared to control group.
Flee response was not significantly different across groups in males or females in the presence of blue or red stimulus, despite a trend for a reduced flee response to red stimulus in both males and females with increased concentration of 1,2-propanediol (Fig. 5C). There was a signifnicat difference between response to blue and red stimulus, such that the response was higher with red stimulus.
3.2. Potential factors contributing to behavioral changes
3.2.1. Neural development
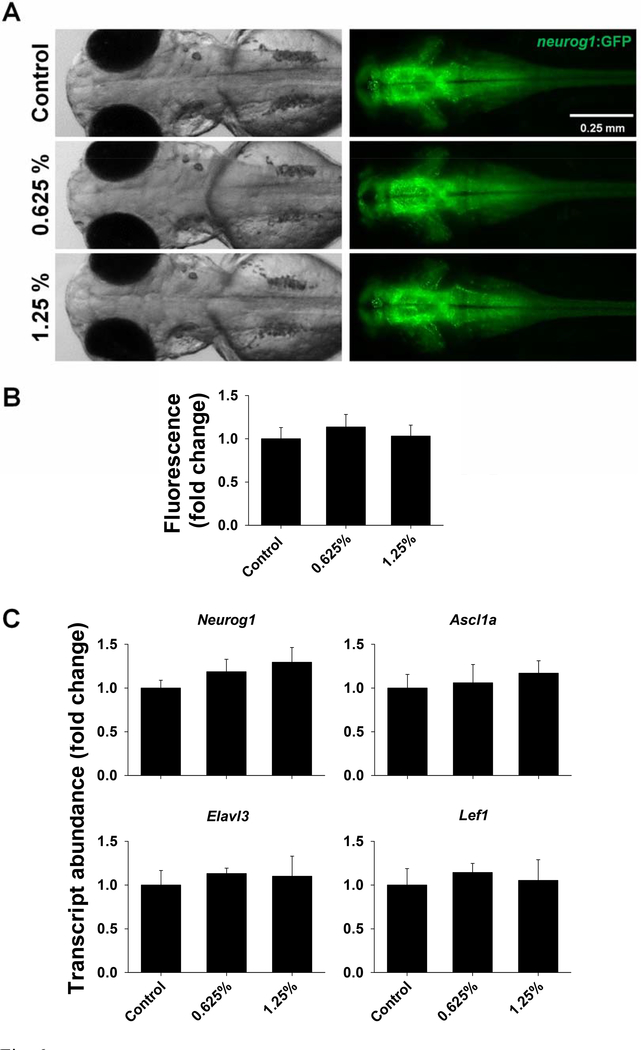
The results of one-way ANOVA for markers of neural development are provided in Tables S6 and S7. Neural development at 72 hpf did not appear to be affected by 1,2-propanediol, since the expression of Neurog1:GFP (Fig. 6A) and the relative fluorescence in the brain region (Fig. 6B) were not significantly different across treatment groups. Exposure to 1,2-propanediol did not significantly affect the transcript abundance of Neurog1, Asc11a, Elavl3, and Lef1 (Fig. 6C).
Figure 6.
Neural development in zebrafish exposed to 0%, 0.625% or 1.25% 1,2-propanediol from 6 hpf until 72 hpf. Tg(Neurog1:GFP) zebrafish were used to visualize the expression of Neurog1; brightfield (left) and fluorescence (right) images of zebrafish were captured at 72 hpf (A), and fluorescent signal in the head region was quantified using Image J (B). Transcript abundance of Neuorg1, Ascl1a, Elavl3, and Lef1 was assessed in wild type zebrafish at 72 hpf (C). No significant differences were detected.
3.2.2. Vascular development
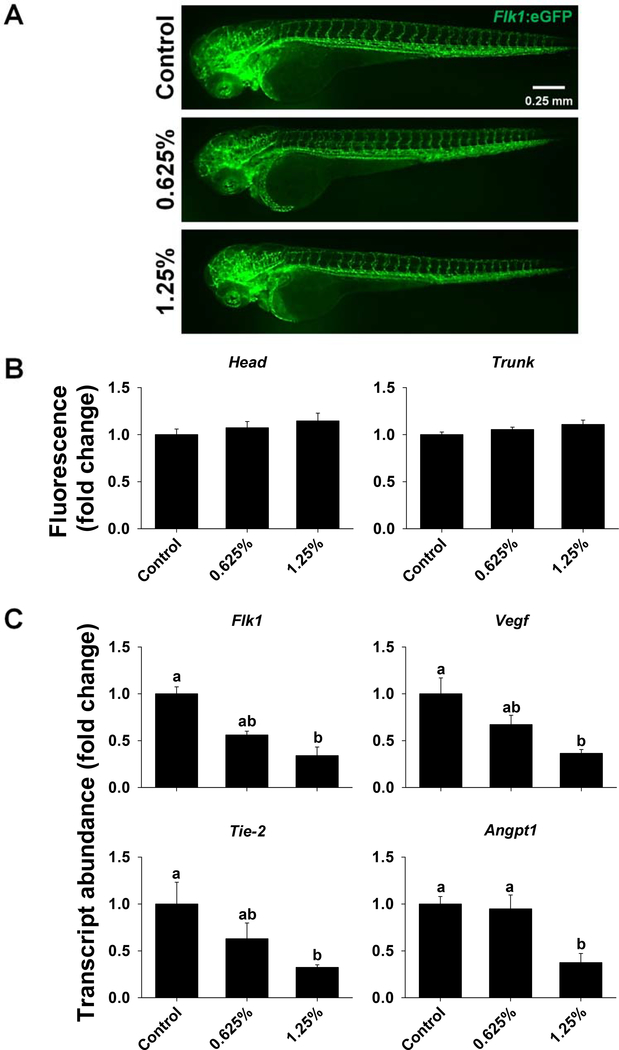
The results of one-way ANOVA for markers of vascular development are provided in Tables S6 and S7. Vascular development at 72 hpf did not appear to be affected by 1,2-propanediol, since the overall vascular patterning, as evident by Flk1:eGFP expression in endothelial cells (Fig. 7A), and the relative fluorescence in the head and trunk regions (Fig. 7B) were not significantly different across treatment groups. Exposure to 1,2-propanediol significantly decreased Flk1, Vegf, Tie-2, and Angpt1 transcripts in a dose-dependent manner (Fig. 7C).
Figure 7.
Vascular development in zebrafish exposed to 0%, 0.625% or 1.25% 1,2-propanediol from 6 hpf until 72 hpf. Tg(Flk1:eGFP) zebrafish were used to visualize the expression of Flk1; fluorescence images of zebrafish were captured at 72 hpf (A), and fluorescent signal in the head and trunk regions was quantified using Image J (B). Transcript abundance of Flk1, Vegf, Tie-2, and Angpt1 was assessed in wild type zebrafish at 72 hpf (C). Letters indicate significant differences that were assessed using one-way ANOVA with a post hoc Tukey test; P ≤ 0.05 was considered significant.
3.2.3. Adult stress response
The results of three-way ANOVA for plasma cortisol concentrations are provided in Table S8. Overall, cortisol concentrations were significantly higher during stress condition, but no significant interactions with sex or 1,2-propanediol concentration were noted (Fig. S1).
3.2.4. Neurotransmitters in adult brain
The results of two-way ANOVA for concentrations of dopamine and serotonin in the brain are provided in Table S9. Neurotransmitter concentrations were not significantly different across groups in males or females (Fig. S2).
3.3. Markers of xenobiotic metabolism
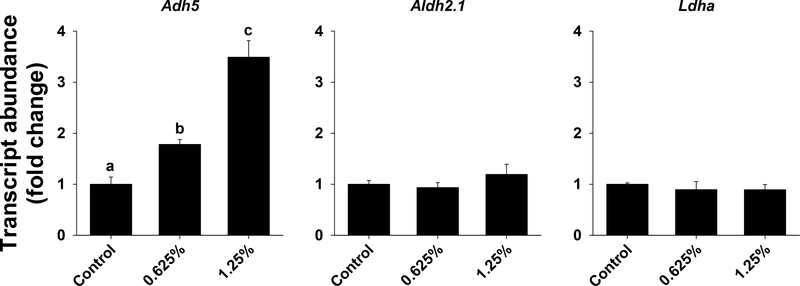
The results of one-way ANOVA for markers of xenobiotic metabolism are provided in Table S7. Exposure to 1,2-propanediol significantly increased Adh5 transcript in a dose-dependent manner (Fig. 8). The transcript abundance of Aldh2.1 and Ldha was not significantly affected by exposure to 1,2-propanediol.
Figure 8.
Markers of 1,2-propanediol metabolism in zebrafish exposed to 0%, 0.625% or 1.25% 1,2-propanediol from 6 hpf until 72 hpf. Transcript abundance of Adh5, Aldh2.1, and Ldha was assessed in wild type zebrafish at 72 hpf. Letters indicate significant differences that were assessed using one-way ANOVA with a post hoc Tukey test; P ≤ 0.05 was considered significant.
Discussion
E-cigarette liquid contains potentially toxic compounds and thus cannot be considered harmless. The increased use of e-cigarettes among adolescents and the perception of e-cigarettes as a safe alternative to tobacco cigarettes during pregnancy is a major public health concern, since their potential adverse neurobehavioral effects have not been studied adequately. We have examined the developmental toxicity of 1,2-propanediol (a major component of e-cigarette liquid) in a zebrafish model. Our previous study has demonstrated that exposure to 1,2-propanediol from 6 hpf until 72 hpf resulted in higher incidence of deformities, reduced growth, and swimming hyperactivity. The current study expands on the developmental toxicity of 1,2-propanediol by examining larval and adult behavior, neural and vascular development, stress response, neurotransmitters concentration, and markers of 1,2-propanediol metabolism.
It should be noted that it is unclear to what extent the results of the current study are pertinent to the developmental e-cigarette exposure in humans. As noted earlier, there is a lack of epidemiological data on the developmental toxicity of e-cigarettes. We hope that the results of the current study will stimulate additional research on developmental toxicity of e-cigarettes, including the contribution of 1,2-propanediol (and other humectants) to the overall toxicity of e-cigarettes.
We demonstrate that exposure of zebrafish embryos to 1,2-propanediol resulted in behavioral changes. Larvae that were exposed to 0.625% displayed swimming hyperactivity during the initial habituation phase, as well as the two light phases. Larvae exposed to 1.25% 1,2-propanediol displayed swimming hyperactivity during both light phases. These results are similar to our previous study, showing that exposure to 1.25% 1,2-propanediol led to swimming hyperactivity during both light phases and the last dark phase (Massarsky et al., 2017). Together, the two studies conclusively demonstrate 1,2-propanediol-induced hyperactive swimming during the light phases. It is unclear how exposure to 1,2-propanediol impacts swimming activity; however, it has been previously shown that exposure of zebrafish to ethanol (0.1–1%) increases swimming activity during dark phases, while 2.0% ethanol increases activity in both light and dark phases, and that these changes are associated with changes in neurotransmitters involved with norepinephrine, dopamine, and serotonin pathways (Guo et al., 2015). Whether 1,2-propanediol and ethanol act through similar mechanisms to induce hyperactivity in larval zebrafish is unknown, but 1,2-propanediol has been reported to have ‘alcohol-like’ effects in humans (Fowles et al., 2013), suggesting that ethanol and 1,2-propanediol may have similar effects on the central nervous system (CNS).
Furthermore, the adult behavioral tests demonstrated that developmental exposure to 1,2-propanediol leads to long lasting behavioral effects, which could be sex-specific. The behavioral effects of 1,2-propanediol were especially noticeable in the novel tank dive test and predator avoidance test. The novel tank dive test suggested that developmental exposure to 1,2-propanediol increases anxiety-like behavior, since the distance to bottom was lower in exposed fish (1.25% - males; 0.625% and 1.25% - females). The results also suggested that females were more susceptible to the effects of 1,2-propanediol. This is further collaborated by the total distance traveled, which was lower in females than males. The predator avoidance test suggested that exposed fish may have a reduced perception of danger, since distance to zone was lower in exposed fish (1.25% - males and females), especially in females. Interestingly, males were less responsive to the aversive stimuli, as inferred from the lower distance to zone in comparison to females. In addition, total distance traveled was higher in control males compared to females.
The behavioral effects of 1,2-propanediol were less noticeable in the startle tap test and shoaling test. The startle tap test suggested that developmental exposure to 1,2-propanediol does not appear to interfere with the habituation response to tap stimulus, since distance traveled post tap decreased over time in both males and females. However, there were sex-specific differences in response to tap stimulus, such that distance traveled post tap was higher in males compared to females, whereas, distance traveled pre tap in males, but not females, followed a U-shaped pattern. The shoaling test suggested that developmental exposure to 1,2-propanediol does not appear to interfere with the shoaling behavior, since distance to zone decreased to similar extent in most fish once the shoaling video was displayed. There were no sex-specific differences in shoaling behavior or total distance traveled.
The advantage of testing a variety of behavioral functions is that differential effects of exposure on behavioral function provide information about the specific neurobehavioral systems affected by exposure. Moreover, the behavioral functions may be affected by sex, and previous studies do suggest that males and females may respond differently to chemical exposure (Weber et al., 2015). Although effects of 1,2-propanediol exposure on adult zebrafish behavior have not been reported, previous studies have demonstrated that exposure of adult zebrafish to ethanol does alter behavior, including reduced anxiety, decreased shoaling, and decreased response to predator, and that these changes could be associated with impairment or death of neuronal cells, and/or impairment of the ocular system [the reader is referred to a detailed review by Cole et al. (2012) for more details]. Interestingly, the reported behavioral effects of ethanol on anxiety and shoaling contrast the effects of 1,2-propanediol on these two parameters. This suggests that although 1,2-propanediol may have ‘alcohol-like’ effects, the two compounds may affect the CNS differently.
Consequently, we assessed markers of neural development, in order to assess whether the behavioral effects are associated with impaired development of the nervous system. Our results suggest that early developmental exposure to 1,2-propanediol does not impair neural development per se. We demonstrate that expression of Neurog1 in Tg(Neurog1:GFP) zebrafish larvae was similar across treatment groups, and no significant differences were noted in the fluorescent signal. Moreover, transcript abundance of Neurog1, Asc11a, Elavl3, and Lef1 (involved with neuronal proliferation and maturation) was not significantly different across treatment groups. However, given that long lasting behavioral differences were observed in both larvae and adults, it is likely that exposure to 1,2-propanediol leads to functional changes within CNS. Alternatively, developmental exposure to 1,2-propanediol may alter other physiological processes, leading to behavioral differences. For example, it has been previously shown that a developmental exposure to ethanol altered behavior in larval and adult zebrafish, displaying less anxiety, which coincided with an attenuated stress response in exposed fish (Baiamonte et al., 2016). Although plasma cortisol measurements herein did not reveal treatment-dependent differences, the variation between tested individuals was quite high, making it difficult to conclude whether developmental exposure to 1,2-propanediol could attenuate the stress response. It has also been shown that a 1 h exposure of adult zebrafish to ethanol results in increased levels of neurotransmitters dopamine and serotonin (Chatterjee and Gerlai, 2009). Interestingly, the concentrations of dopamine and serotonin reported herein were not significantly different across treatment groups. Therefore, future studies should examine additional aspects of the nervous system, as well as other physiological processes, in order to determine the underlying causes of the behavioral changes induced by 1,2-propanediol exposure.
Also, we examined vascular development, since optimal vascular development is essential for proper development of CNS. Assessment of vascular development in Tg(Flk1:eGFP) zebrafish at 72 hpf did not reveal major changes in vessel formation, and fluorescence in the brain and trunk regions was not significantly different. Interestingly, transcript abundance of Flk1, Vegf, Tie-2, and Angpt1 was decreased in a dose-dependent manner. These contrasting results suggest that the reduction in transcript abundance of Flk1, Vegf, Tie-2, and Angpt1 is not sufficient to induce marked phenotypic changes in vessel formation. However, it should be noted that the transcript abundance was assessed at 72 hpf, which means that it is possible that the downregulation was gradual over the course of the exposure, allowing sufficient transcription for proper vessel development prior to 72 hpf. The reduction in transcript abundance of Flk1, Vegf, Tie-2, and Angpt1 could also imply that any potential vascular injury may not be adequately repaired.
The effects of 1,2-propanediol on vascular development in zebrafish have not been examined previously, which limits the discussion on this topic; however, several studies examined the effects of ethanol on angiogenesis. It has been reported that developmental exposure to ethanol (1% or 2%) disrupts vessel formation in Tg(Fli1:eGFP) zebrafish (similar to Flk1:eGFP), but angiogenic markers were not assessed (Li et al., 2016). Prenatal exposure to ethanol has also been shown to disrupt angiogenesis in brain of neonatal mice, evidenced by reduced vascular density and decreased expression of VEGF receptors (Jégou et al., 2012). It has also been suggested that the ethanol-induced reduction in VEGF expression could be due to ethanol metabolites (Radek et al., 2008). Therefore, a more rigorous study on the effects of 1,2-propanediol on vascular development is warranted, including exposure to the potential metabolites, which could contribute to the observed effects.
Moreover, we assessed markers of 1,2-propanediol metabolism. Exposure to 1,2-propanediol significantly increased Adh5 transcript. The increase in Adh5 was dose-dependent, and suggests that Adh5 is a sensitive marker of 1,2-propanediol exposure. Moreover, increase in Adh5 transcript suggests that 1,2-propanediol is taken up by the embryos/larvae. In contrast, the transcript abundance of Aldh2.1 and Ldha was not significantly affected by 1,2-propanediol exposure. While 1,2-propanediol metabolism has not been assessed previously in zebrafish, exposure to ethanol resulted in increased activity of ADH, such that a dose-dependent response was observed for the low and middle doses (0.25% and 0.5%), but at a higher dose (1%), ADH activity was similar to control (Tran et al., 2015). In addition, the activity of ALDH was significantly decreased in response to ethanol exposure (Tran et al., 2015). We noted that the inhibitory effect of ethanol on ALDH activity is commonly reported in mammalian studies and it could occur due to the production of reactive oxygen species (ROS) upon exposure to ethanol. This suggests that similar inhibition of ALDH could occur with 1,2-propanediol in the current study; thus, it is possible that 1,2-propanediol is not adequately metabolized and that the observed toxicity could be due to the production of lactaldehyde, which is produced after the action of ADH on 1,2-propanediol. Future studies should examine the activity of ADH, ALDH, and LDH, and verify whether production of ROS occurs.
Lastly, it should be noted that zebrafish embryos/larvae in the current study were exposed from 6 hpf until 72 hpf, in order to capture the very early developmental stages. However, zebrafish development continues well after 72 hpf. Therefore, it is possible that a longer exposure could have produced more substantial effects than reported herein. Nonetheless, the fact that behavioral effects were observed in larval fish and persisted to adulthood highlights the sensitivity of the zebrafish model to developmental toxicity of 1,2-propanediol.
In summary, the current study demonstrates that developmental exposure to 1,2-propanediol affects larval behavior and has long-term effects, since behavioral differences were noted in adults. Interestingly, behavioral effects in larvae were not associated with neural or vascular deficits, although transcript abundance of angiogenic genes decreased. Behavioral effects in adults were not associated with changes in plasma cortisol levels or the concentrations of dopamine or serotonin in the brain. Lastly, exposure to 1,2-propanediol induced xenobiotic metabolism. This is the first study to demonstrate that a developmental exposure to 1,2-propanediol has later life sex-specific consequences in adult zebrafish, thereby adding to the growing evidence that e-cigarettes contain potentially harmful chemicals, such as 1,2-propanediol, and cannot be presumed harmless.
Supplementary Material
Acknowledgments
The authors thank all members of the Di Giulio lab, Dr. Y. Gao (Duke LMCF), as well as Dr. A. Hawkey and K.A. Odamah (Levin lab) for support and technical assistance. This research was supported by RJR-Leon Golberg Postdoctoral Fellowship to A. Massarsky.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agency for Toxic Substances and Disease Registry, 2007. Ethylene glycol and propylene glycol toxicity. Case studies in environmental medicine (CSEM). Course WB 1103. https://www.atsdr.cdc.gov/csem/egpg/docs/egpg.pdf Retrieved March 17th 2017.
- Arnold MC, Forte JE, Osterberg JS, Di Giulio RT, 2016. Antioxidant rescue of selenomethionine-induced teratogenesis in zebrafish embryos. Arch. Environ. Toxicol 70, 311–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeza-Loya S, Viswanath H, Carter A, Molfese DL, Velasquez KM, Baldwin PR, Thompson-Lake DGY, Sharp C, Fowler JC, De La Garza R II, Salas R, 2014. Perceptions about e-cigarette safety may lead to e-smoking during pregnancy. Bull. Menninger Clin 78, 243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baiamonte M, Parker MO, Vinson GP, Brennan CH, 2016. Sustained effects of developmental exposure to ethanol on zebrafish anxiety-like behaviour. PLoS ONE 11, e0148425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautsch VL, James JM, 2009. Neurovascular development: the beginning of a beautiful friendship. Cell Adh. Migr 3, 199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee D, Gerlai R, 2009. High precision liquid chromatography analysis of dopaminergic and serotoninergic responses to acute alcohol exposure in zebrafish. Behav. Brain Res 200, 208–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole GJ, Zhang C, Ojiaku P, Bell V, Devkota S, Mukhopadhyay. S., 2012. Effects of ethanol exposure on nervous system development in zebrafish. Int. Rev. Cell Mol. Biol. 299, 255–315. [DOI] [PubMed] [Google Scholar]
- Dasmahapatra AK, Doucet HL, Bhattacharyya C, Carvan MJ III, 2001. Developmental expression of alcohol dehydrogenase (ADH3) in zebrafish (Danio rerio). Biochem. Biophys. Res. Commun. 286, 1082–1086. [DOI] [PubMed] [Google Scholar]
- Dutra LM, Glantz SA, 2014. Electronic cigarettes and conventional cigarette use among US adolescents. JAMA Pediatr. 168, 610–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Medicines Agency, 2014. Background review for the excipient propylene glycol. http://www.ema.europa.eu/docs/en_GB/document_library/Report/2014/12/WC500177937.pdf. Retrieved December 23rd, 2016.
- Fowles JR, Banton MI, Pottenger H, 2013. A toxicological review of the propylene glycols. Crit. Rev. Toxicol 43, 363–390. [DOI] [PubMed] [Google Scholar]
- Glazer L, Wells CN, Drastal M, Odamah KA, Galat RE, Behl M, Levin ED, 2017. Developmental exposure to low concentrations of two brominated flame retardants, BDE-47 and BDE-99, causes life-long behavioral alterations in zebrafish. Neurotoxicology. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goniewicz ML, Knysak J, Gawron M, Kosmider L, Sobczak A, Kurek J, Prokopowicz MJ-C, Rosik-Dulewska C, Havel C, Jacob P 3rd, Benowitz N, 2014. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob. Control 23, 133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo N, Lin J, Peng X, Chen H, Zhang Y, Liu X, Li Q, 2015. Influences of acute ethanol exposure on locomotor activities of zebrafish larvae under different illumination. Alcohol 49, 727–737 [DOI] [PubMed] [Google Scholar]
- Hajek P, Etter J-F, Benowitz N, Eissenberg T, McRobbie H, 2014. Electronic cigarettes: review of use, content, safety, effects on smokers and potential for harm and benefit. Addiction 109, 1801–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiles S, 2015. What’s in all that e-cig vapor? The Washington Post. https://www.washingtonpost.com/national/health-science/whats-in-all-that-vaping-smoke/2015/08/31/57fe8e58-2700-11e5-b72c-2b7d516e1e0e_story.html. Retrieved March 17th, 2017
- Huang H, Bhat A, Woodnutt G, Lappe R 2010. Targeting the ANGPT-TIE2 pathway in malignancy. Nat. Rev. Cancer 10, 575–585. [DOI] [PubMed] [Google Scholar]
- Jégou S, El Ghazi F, de Lendeu PK, Marret S, Laudenbach V, Uguen A, Marcorelles P, Roy V, Laquerrière, Gonzalez BS, 2012. Prenatal alcohol exposure affects vasculature development in the neonatal brain. Ann. Neurol 72, 952–960. [DOI] [PubMed] [Google Scholar]
- Lassen N, Estey T, Tanguay RL, Pappa A, Reimers MJ, Vasiliou V, 2005. Molecular cloning, baculovirus expression, and tissue distribution of the zebrafish aldehyde dehydrogenase 2. Drug Metabol. Dispos 33, 649–656. [DOI] [PubMed] [Google Scholar]
- Leung T, Chen H, Stauffer AM, Giger KE, Sinha S, Horstick EJ, Humbert JE, Hansen CA, Robishaw JD, 2006. Zebrafish G protein g2 is required for VEGF signaling during angiogenesis. Blood 108, 160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Gao Y, Chen M, Peng J, Yan H, Zhao X, Feng X, Chen D, 2016. Alcohol exposure leads to unrecoverable cardiovascular defects along with edema and motor function changes in developing zebrafish larvae. Biol. Open 5, 1128–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD, 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Massarsky A, Abdel A, Glazer L, Levin ED, Di Giulio RT, 2017. Exposure to 1,2-propanediol impacts early development of zebrafish (Danio rerio) and induces hyperactivity. Zebrafish 14, 216–222. [DOI] [PubMed] [Google Scholar]
- Massarsky A, Strek L, Craig PM, Eisa-Beygi S, Trudeau VL, Moon TW, 2014. Acute embryonic exposure to nanosilver or silver ion does not disrupt the stress response in zebrafish (Danio rerio) larvae and adults. Sci. Total Environ. 478, 133–140. [DOI] [PubMed] [Google Scholar]
- Ngan AK, Wang YS, 2009. Tissue-specific transcriptional regulation of monocarboxylate transporters (MCTs) during short-term hypoxia in zebrafish (Danio rerio). Comp. Biochem. Physiol. B 154, 396–405. [DOI] [PubMed] [Google Scholar]
- Radek KA, Kovacs EJ, Gallo RL, DiPietro LA, 2008. Acute ethanol exposure disrupts VEGF receptor signaling in endothelial cells. Am. J. Physiol. Heart Circ. Physiol 295, H174–H184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R, Strähle U, Scholpp S, 2013. Neurogenesis in zebrafish – from embryo to adult. Neural Dev. 8:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schober W, Szendrei K, Matzen W, Osiander-Fuchs H, Heitmann D, Schettgen T, Jörres RA, Fromme H, 2014. Use of electronic cigarettes (e-cigarettes) impairs indoor air quality and increases FeNO levels of e-cigarette consumers. Int. J. Hyg. Envir. Heal, 217, 628–637. [DOI] [PubMed] [Google Scholar]
- Smith D, Aherrera A, Lopez A, Neptune E, Winickoff JP, Klein JD, Chen G, Lazarus P, Collaco JM, McGrath-Morrow SA, 2015. Adult behavior in male mice exposed to e-cigarette nicotine vapors during late prenatal and early postnatal life. PLoS ONE 10, e0137953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran S, Nowicki M, Chatterjee D, Gerlai R, 2015. Acute and chronic ethanol exposure differentially alters alcohol dehydrogenase and aldehyde dehydrogenase activity in the zebrafish liver. Prog. Neuropsychopharmacol. Biol. Psychiatry 56, 221–226. [DOI] [PubMed] [Google Scholar]
- US Department of Health and Human Services, 2016. E-cigarette use among youth and young adults: a report of the surgeon general–executive summary. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. [Google Scholar]
- Wagner NJ, Camerota M, Propper C, 2017. Prevalence and perceptions of electronic cigarette use during pregnancy. Matern. Child Health J, 21, 1655–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber DN, Hoffmann RG, Hoke ES, Tanguay RL, 2015. Bisphenol A exposure during early development induces sex-specific changes in adult zebrafish social interactions. J. Toxicol. Environ. Health 78A, 50–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.