Abstract
Nitric oxide (NO) is a ubiquitous signaling molecule in the human body with well-known roles in many different processes and organ systems. In cancer, the two-concentrations hypothesis of NO has dictated that low levels of NO are cancer promoting, while high levels of NO are protective against cancer. Although prostate cancer is a hormonally driven malignancy, research has been shifting away from androgen-responsive epithelial cells and evolving to focus on NO therapies, the tumor microenvironment (TME), and inflammation. NO is reported to be able to inhibit activity of the androgen receptor. This may prevent prostate growth, but low levels of NO could conversely select for castration-resistant prostate cells, creating an aggressive cancer phenotype. At high levels, nitrosative stress created from NO overproduction can be protective against prostate neoplasia. In this review, we discuss development and possibilities of NO-based therapies for prostate cancer.
Keywords: CRPC, nitric oxide, NO and cancer
Nitric oxide (NO) is a ubiquitous signaling molecule in the human body with well-delineated physiologic functions in multiple organ systems. It is a free radical with a short half-life, less than 5 s in vivo (Hasan et al., 2019). NO is synthesized as a by-product from the process of L-arginine becoming L-citrulline, which requires oxygen and NADPH. The enzyme responsible for this is nitric oxide synthase (NOS), of which there are three main isotypes: neuronal NOS (nNOS or NOS1), inducible NOS (iNOS or NOS2), and endothelial NOS (eNOS or NOS3). The three enzymes exist separately in the different cell types for which they are named and produce NO in different concentrations and for different durations. For example, eNOS and nNOS are considered constitutive enzymes and produce concentrations in the nanomolar range for seconds or minutes; iNOS is known to produce greater concentrations (micromolar range) for longer durations. The concentration and duration of NO action dictates its biological action and consequences, especially in the context of tumor biology (Fukumura et al., 2006; Mocellin et al., 2007; Vannini et al., 2015).
NO signaling occurs mainly through two pathways, the cyclic GMP (cGMP)–dependent and cGMP-independent pathways. In the cGMP-dependent pathway, signaling occurs through a complex soluble cyclase, producing cGMP, which interacts with a specific kinase. The overall result of this is downstream cellular phosphorylation and the effects include, but are not limited to, smooth muscle relaxation and decreased platelet aggregation (Vannini et al., 2015). In the cGMP-independent pathway, NO as a reactive nitrogen species (RNS) creates effects mainly by S-nitrosylation of proteins. Some of these effects include DNA damage, p53 mutations, and other genetic effects such as cell viability and cancer survival (Mocellin et al., 2007; Weiming et al., 2002). Nitrosative and oxidative stresses and their role in cancer have been thoroughly investigated for many years and as a result, much is known regarding this topic.
NO has also been identified as a key modulator in immune functions such as the growth and death of T cells, B cells, and mast cells (Tripathi, 2007). Because NO has been reported to have immune-modulating effects, this has led to a vast amount of research and discovery that has borne successful therapeutic advances such as NO-donating nonsteroidal anti-inflammatory drugs, which have shown promising results for the growth inhibition of cancer cells (Vannini et al., 2015). Recently, studies on the tumor-suppressing actions of NO have been demonstrated in prostate cancer, which is the most prevalent and second most lethal cancer in American men (Cookson et al., 2015). Death from prostate cancer generally results from the development of an antiandrogen-resistant or castration-resistant phenotype (castration-resistant prostate cancer [CRPC]), which often coincides with metastatic spread (mCRPC). Arora and colleagues demonstrated that increased NO levels suppress the CRPC tumor burden in murine models of CRPC by targeting the tumor microenvironment (TME; Arora et al., 2018). This review focuses on understanding the developments in NO-based therapy to identify the various impacts that it can have on multiple cancers.
NO and Cancer
The Biphasic Role of NO
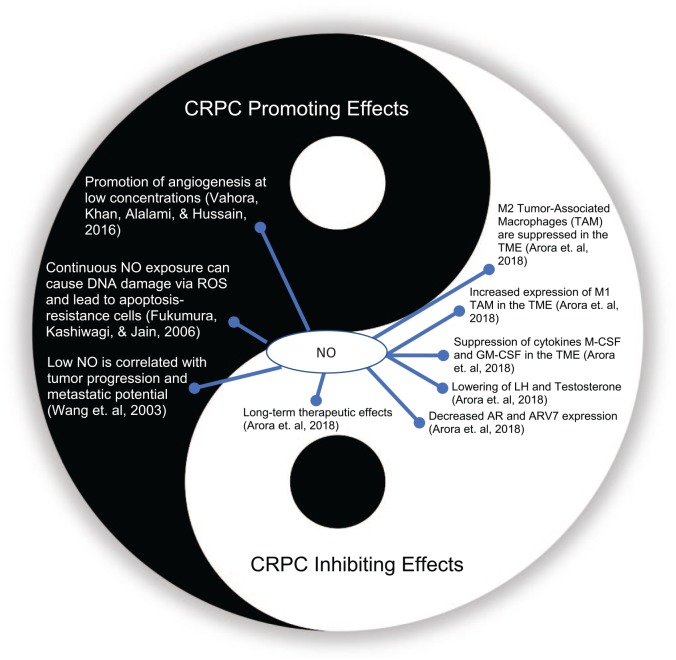
The role of NO in cancer has been simplified as the tale of two concentrations, with a large body of research supporting this biphasic simplification. At low concentrations, NO is known to promote cell growth and proliferation through its role as a signaling molecule (Frederiksen et al., 2007). At high concentrations, the generation of reactive species such as peroxynitrite damages the cell membranes and inhibits cancerous cell growth (Burke et al., 2013). In the inflammatory state, supraphysiologic concentrations of reactive oxygen species (ROS) and RNS via iNOS upregulation are carcinogenic through a variety of mechanisms including cellular lipid modification, angiogenesis, and antiapoptosis (Cronauer et al., 2007). Depending on a variety of factors, including redox status, cell cycle, concentration, and distribution, NO can act as a pro or anticancer agent. Some of the studied mechanisms of NO in cancer biology include the promotion or inhibition of angiogenesis, cell proliferation, apoptosis, radiosensitivity, and epigenetic modification (Fukumura et al., 2006; Weiming et al., 2002; Figure 1).
Figure 1.
The biphasic role of nitric oxide (NO) on castrate-resistant prostate cancer (CRPC).
AR = androgen receptor; GM-CSF = granulocyte-macrophage colony-stimulating factor; LH = luteinizing hormone; M-CSF = macrophage colony-stimulating factor; ROS = reactive oxygen species; TAM = tumor-associated macrophage; TME = tumor microenvironment.
Numerous studies support the role of NO in either promoting or inhibiting a wide variety of cancer cell types (Choudhari et al., 2013). For example, studies with keratinocytes in vitro identify an increased proliferation rate with a decreased cellular differentiation at low doses of NO. When cultured at higher concentrations of NO, cell growth was reduced and differentiation of the cells increased. This supports the general schema of the two-concentrations hypothesis of NO (Krischel et al., 1998). In addition, a study exploring the molecular determinants of lethal prostate cancer showed that lethal prostate cancer is associated with a high iNOS concentration in the tumor epithelium (Erlandsson et al., 2018). A study in rats reported that inhibiting NOS caused an increase in preneoplastic lesions in colon cancer, thereby suggesting an antitumor role for NO (Schleiffer et al., 2000). NO also plays a key role in the development and regulation of blood and lymphatic vessels (Lahdenranta et al., 2009). For example, NOS expression is known to be directly correlated with lymphatic metastasis. In vitro and in vivo studies identified that inhibition of eNOS (through both genetic and pharmacologic methods) in vascular endothelial growth factor (VEGF)–producing cancer cells led to a decrease in the proliferation of lymphatic vessels, decreased lymphatic dissemination of metastatic cells, and decreased gross evidence of metastatic disease. This work provided the first link between eNOS, VEGF, and lymphatic spread and metastasis in human cancers (Lahdenranta et al., 2009). In contrast, a murine model of oral squamous cell carcinoma transfected with iNOS to upregulate NO production showed reductions in cell and tumor growth as well as metastasis to cervical lymph nodes (Lahdenranta et al., 2009). The high concentrations were produced due to tightly packed transfectant cells, which are thought to be required in the pathway to promote tumor cell apoptosis (Harada et al., 2004). This work demonstrated that increased levels of NO can lead to tumor suppression.
The Role of NO in Inflammation
Inflammation is well known to be a precursor to carcinogenesis in many organs, including the prostate, and NO plays a central role in this. In experimental studies, iNOS has been increased in a number of inflammatory-related cancers, including Barrett’s esophagus and esophageal carcinoma. The study of NO in inflammatory-promoted carcinogenesis has given further insight into the role of NO in cancer and how therapies may incorporate this molecule, and its downstream effects, in the future (Kundu & Surh, 2012). By using NO as an inflammatory mediator, the role that inflammation plays in cancer can be mediated by NO-based therapies.
The Role of NO in Prostate Cancer
The Impact of NO on the TME in Prostate Cancer
Research into the TME and the role of NO has been especially fruitful in prostate cancer (Table 1). Defining the signaling profile of the cells in the stromal compartment that surrounds the prostate has been the starting point for a lot of ongoing research. A study of testosterone and 17β-estradiol administration in rats identified that the hormonal milieu increases expression of NO and oxide-producing enzymes, which implicates the epithelial cell and the surrounding stroma itself, separate from the inflammatory process, in the redox imbalance mechanism of prostatic intraepithelial neoplasia (PIN) and prostate cancer pathogenesis (Tam et al., 2007). The creation of a further proinflammatory state by the production of free radicals following hormonal stimulation and NOS upregulation creates a “feed-forward” mechanism of redox imbalance, inflammation, and cellular damage that can hasten neoplastic changes (Tam et al., 2007). In the setting of CRPC, androgen receptors (ARs) may become sensitized to lower levels of androgens present in the microenvironment, even though systemic androgen levels are undetectable. NO production has been identified to play an important part in these AR-independent cancers (Cronauer et al., 2007). Because these receptors are able to detect extremely low levels of androgens, the production of intraprostatic androgens through the microenvironment is a major target of current approved therapies for advanced disease.
Table 1.
Role of Nitric Oxide (NO) in Various Cancer Types.
| Cancer type | Role of NO | Outcome | Reference |
|---|---|---|---|
| Prostate | Positive | In vivo tumor inhibition: This study validates the significance of NO on inhibition of castration-resistant prostate cancer (CRPC) tumors through tumor microenvironment (TME) | Arora et al. (2018) |
| Shows the ability of NO to attenuate hypoxia-induced progression of prostate cancer | Siemens et al. (2009) | ||
| Small molecules able to inhibit WNT and androgen receptor (AR) signaling via NO release represent a promising platform for the development of new compounds for the treatment of CRPC | Laschak et al. (2012) | ||
| Inhibits epithelial–mesenchymal transition. Treatment of human prostate metastatic cell lines with the NO donor, DETANONOate, inhibits epithelial–mesenchymal transition and reverses both the mesenchymal phenotype and the cell-invasive properties | Baritaki et al. (2010) | ||
| Inhibits cellular proliferation. GIT-27NO, an NO donor, inhibited in vivo prostate cancer cell growth of PC3 and LnCap cells | Donia et al. (2009) | ||
| Lung | Positive | Decrease in epithelial–mesenchymal transition. NO serves a critical role in preserving an epithelial phenotype and in attenuating epithelial–mesenchymal transition in alveolar epithelial cells | Vyas-Read et al. (2007) |
| Negative | Promotes angiogenesis. In vivo, NO has a role in maintaining tumor blood supply, and we provide early clinical evidence that inhibition of NO synthesis has tumor antivascular activity | Ng et al. (2007) | |
| Gastric | Positive | Inhibits cellular proliferation. Cell growth suppression via NO may be mediated through Akt signaling | Sang et al. (2011) |
| Ovarian | Negative | Promotes cellular proliferation. While NO was reduced, there was inhibited cell proliferation in HOC-7 cells | Keith Bechtel and Bonavida (2001) |
| Breast | Negative | Promotes cellular proliferation. Via inactivation of RAS, there is an NO-induced increase in proliferation | Pervin et al. (2007) |
| Hepatic | Positive | Promotes apoptosis. In high doses, NO was able to promote apoptosis via p38MAP-kinase | Wang et al. (2011) |
| Negative | Inhibits apoptosis. In low doses, NO inhibited apoptosis via iNOS/akt/surviving axis | Wang et al. (2011) |
The Role of NO in Inflammatory Processes in Prostate Cancer
Prostate cancer is promoted by inflammation, of which iNOS is an important player (Fukumura et al., 2006). ROS damage and downregulated antioxidants are risk factors for PIN and prostate cancer, and oxidative damage is found in greater quantities in cancerous versus normal prostatic epithelium. Antioxidants such as copper-zinc oxide dismutase (SOD1) and manganese superoxide dismutase (SOD2) are reported to have lower expressions in the epithelium of PIN and prostate cancer (Bostwick et al., 2000; Oberley et al., 2000). NO is shown to inhibit AR activity in a dose-dependent manner. This is mediated through nitrosative stress, likely the S-nitrosylation of the zinc fingers, preventing AR from binding to DNA (Cronauer et al., 2007). Some argue that this action may only inhibit the proliferation of AR-dependent cancers and create a selection pressure promoting the growth of AR-independent cancers (CRPC). This line of reasoning could provide mechanistic support for previous assertions linking increasing iNOS expression with inferior survival in prostate cancer (Cronauer et al., 2007). At the same time, however, the knowledge that NO can prevent the intracellular DNA binding of the AR may have implications not just for androgen-sensitive tumors but also for CRPC.
Genome-Wide Studies of NO in Prostate Cancer
Genome-wide association studies have identified polymorphisms in the NOS genes that are linked with prostate cancer and outcomes (Vahora et al., 2016). In a case-controlled study of 170 men with prostate cancer, two polymorphisms of the eNOS gene were reported to be linked with an increased risk of prostate cancer (Safarinejad et al., 2013). A meta-analysis identifying over 3,000 patients with prostate cancer and a similar number of controls identified a significant association between the 849G>T polymorphism on the eNOS gene and the development of prostate cancer; eNOS is expressed in endothelial cells and was reported to be a regulator for angiogenesis in both normal and neoplastic conditions. The authors detailed that the link between eNOS mutation and cancer development may be the decreased NO in the microenvironment, allowing unchecked tumor growth (Wu et al., 2014). Yet another study identified a variant of the NOS3 (eNOS) gene, which was linked not only to an increased risk of prostate cancer but also with a less differentiated tumor type (higher Gleason score) and a higher TNM cancer stage classification, implying later stage cancer with increased tumor size, lymph node spread, and metastasis (Nikolić et al., 2015). While results such as these are promising, other studies have identified a lack of correlation and almost all studies are limited by a case-controlled retrospective design (Wu et al., 2014). Through these studies, it is evident that prostate cancer has a clear connection with eNOS genes that undergo mutations in the form of polymorphisms.
Prostate Cancer and NO Therapeutics
The theoretical underpinning of NO therapies is progressing well, as the in vivo and in vitro studies mentioned previously have shown positive effects of NO therapy on cancer. The practical development of NO-releasing drugs is the obvious but challenging next step. There are several different methods of using NO as a drug, but the method of delivery must be tailored so that the proper concentration is delivered for the correct duration to create the desired effect (e.g., an erroneously low concentration may promote, rather than inhibit, tumor growth; Vannini et al., 2015). Methods are also available to limit the amount of NO available to cells. These methods include viral transfection of genes, cell-based methods, NO prodrugs, free radical scavengers, and pharmacologic inhibition of NOS and/or NO itself. The NO dose necessary for cytotoxicity is generally 10–100 times greater than the dose for tumor promotion (Mocellin et al., 2007; Weiming et al., 2002; Xie et al., 2018). While the first challenge of NO-based therapy is dose modulation, the other major challenge is how to direct the NO to the tumor cells and avoid systemic toxicity (Xie et al., 2018).
Glyceryl trinitrate (GTN), sodium nitroprusside (SNP), and other NO donors have all been reported to have beneficial effects on prostate cancer, via apoptotic pathways and altered redox states, in reducing cancer invasiveness (Xie et al., 2018). Some new methods include prodrug development with conjugation to antigens, which will utilize the enzymatic activity of the tumor (i.e., glutathione S-transferase [GST]), as well as conjugation to tumor-specific antigens. An NO donor tagged to an antigen aptamer can optimize prostate cancer–specific delivery while avoiding systemic toxicity (Tan et al., 2017; Xie et al., 2018). JS-K, one such NO prodrug, is activated by GST, which is overexpressed by prostate cancer cells. JS-K is known to reduce prostate CA growth and promote the apoptotic pathway through the ubiquitin-proteasome pathway. Its role as an effective prodrug, which only produces NO in the presence of tumor enzymes, has made it a hot target for prostate cancer treatment, specifically in CRPC (Tan et al., 2017). Low-dose NO is classically thought of as promoting tumorigenic growth. However, in a controlled study of men with recurrent prostate cancer receiving low-dose concentrations of GTN, prostate-specific antigen (PSA) doubling time was significantly lower in men receiving the low-dose NO treatment than in age-matched controls receiving no treatment (Siemens et al., 2009). Another proposed mechanism of tumor suppression is sensitization to chemotherapy, as GTN given with doxorubicin reported more effective cellular killing than doxorubicin given alone (Frederiksen et al., 2007; Xie et al., 2018).
In CRPC, NO has been identified to play a role in the downregulation of AR, mediating the mechanism of resistance to this mainstay of treatment (Arora et al., 2018). The suggestion that NO downregulates transcription of the AR gene, causing an evolutionary selection pressure for aggressive CRPC, has been reproduced elsewhere (Cronauer et al., 2003). Inducing antiandrogen resistance in mice via treatment with bicalutamide created a mouse model of CRPC. This model clarified the role of NO in the suppression of AR activity, showing increased eNOS in CRPC specimens. When eNOS was inhibited, sensitivity to antiandrogens was reinstated (Yu et al., 2013). Components of the NO signaling pathway, soluble guanylyl cyclase alpha1 (sGCa1) in particular, have been reported to promote prostate cancer growth even independent of NO signaling (Cai et al., 2012). Administration of an NO prodrug, JS-K, decreased intracellular expression of the AR through the Wnt pathway, supporting the finding that high-dose NO delivery can impair intracellular nuclear receptor function (Laschak et al., 2012).
Nitrosative stress has been shown to cause secondary hypogonadism by reducing gonadotropin-releasing hormone (GnRH) receptors in the brain. Therefore, it is possible that NO may also reduce the tumor burden in an androgen-dependent cancer. Mice treated with NO prodrugs showed a decreased tumor burden mediated through the TME in a cell nonautonomous fashion, where NO is not acting on the malignant cells but on the environment they are in (Masterson et al., 2018). Another therapeutic option stems from the impact of NO on activity and dedifferentiation of tumor-associated macrophages (TAMs), which play a role in the progression of CRPC through the TME and have been reported to be suppressed by NO. Additionally, the cytokines responsible for the differentiation of TAMs are also suppressed by NO. When xenografted mice were treated with NO, the tumor burden was suppressed for a number of weeks even though the half-life of NO is known to be seconds, indicating a potential long-term therapeutic effect (Arora et al., 2018). Another approach has been flavonoid administration from citrus peels, which have reduced tumor size, metastasis, and angiogenic growth in mouse models of human prostate cancer (Lai et al., 2013).
Conclusion
In conclusion, while low-dose NO is thought to be cancer promoting and high-dose NO cancer inhibiting, this is a generalization as substantial research contradicts this assumption. In prostate cancer there is evolving research, which demonstrates that NO is upregulated from hormonal stimulation and inflammation, both of which are precursors to prostatic neoplasia. NO has also been reported to downregulate the AR, which may implicate it in the proliferation of AR-independent cancers. Further studies should explore this area of prostate cancer to analyze this cancer type. NO can impact DNA binding of the AR intracellularly, which has been a therapeutic target in CRPC. Research on the genetic polymorphisms in the NOS genes as well as case-controlled studies to link them to cancer are well underway. Finally, there are in vitro and in vivo cellular and animal studies that show promise for NO production as well as inhibition as therapeutic targets. These studies are a starting point for the next step, early-phase human trials, which will ultimately decide the role of NO production or inhibition in the fight against prostate cancer. The new bottom line is that NO action and its consequences on cancer are cell and context specific and cannot be explained or predicted with simple dogma. Understanding the tumor type and its dependence on NO for growth may aid in suggesting whether NO can be useful to cancer therapy (Burke et al., 2013).
Footnotes
Author Contributions: KS, YS, HA, and RR wrote the manuscript; HA and YS created the tables and figures.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Yash Soni  https://orcid.org/0000-0001-9944-9972
https://orcid.org/0000-0001-9944-9972
References
- Arora H., Panara K., Kuchakulla M., Kulandavelu S., Burnstein K. L., Schally A. V., Hare J. M., Ramasamy R. (2018). Alterations of tumor microenvironment by nitric oxide impedes castration-resistant prostate cancer growth. Proceedings of the National Academy of Sciences of the United States of America, 115(44), 11298–11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baritaki S., Huerta-Yepez S., Sahakyan A., Karagiannides I., Bakirtzi K., Jazirehi A., Bonavida B. (2010). Mechanisms of nitric oxide-mediated inhibition of EMT in cancer: Inhibition of the metastasis-inducer Snail and induction of the metastasis-suppressor RKIP. Cell Cycle, 9(24), 4931–4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostwick D. G., Alexander E. E., Singh R., Shan A., Qian J., Santella R. M., Oberley L. W., Yan T., Zhong W., Jiang X., Oberley T. D. (2000). Antioxidant enzyme expression and reactive oxygen species damage in prostatic intraepithelial neoplasia and cancer. Cancer, 89(1), 123–134. https://www.ncbi.nlm.nih.gov/pubmed/10897009 [PubMed] [Google Scholar]
- Burke A. J., Sullivan F. J., Giles F. J., Glynn S. A. (2013). The yin and yang of nitric oxide in cancer progression. Carcinogenesis, 34(3), 503–512. [DOI] [PubMed] [Google Scholar]
- Cai C., Hsieh C.-L., Gao S., Kannan A., Bhansali M., Govardhan K., Dutta R., Shemshedini L. (2012). Soluble guanylyl cyclase α1 and p53 cytoplasmic sequestration and down-regulation in prostate cancer. Molecular Endocrinology, 26(2), 292–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhari S. K., Chaudhary M., Bagde S., Gadbail A. R., Joshi V. (2013). Nitric oxide and cancer: A review. World Journal of Surgical Oncology, 11(1), 118. doi: 10.1186/1477-7819-11-118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cookson M. S., Lowrance W. T., Murad M. H., Kibel A. S., & American Urological, A. (2015). Castration-resistant prostate cancer: AUA guideline amendment. Journal of Urology, 193(2), 491–499. [DOI] [PubMed] [Google Scholar]
- Cronauer M. V., Ince Y., Engers R., Rinnab L., Weidemann W., Suschek C. V., Burchardt M., Kleinert H., Wiedenmann J., Sies H., Ackermann R., Kröncke K. D. (2007). Nitric oxide-mediated inhibition of androgen receptor activity: Possible implications for prostate cancer progression. Oncogene, 26(13), 1875–1884. [DOI] [PubMed] [Google Scholar]
- Cronauer M. V., Schulz W. A., Burchardt T., Anastasiadis A. G., de la Taille A., Ackermann R., Burchardt M. (2003). The androgen receptor in hormone-refractory prostate cancer: Relevance of different mechanisms of androgen receptor signaling (Review). International Journal of Oncology, 23(4), 1095–1102. [DOI] [PubMed] [Google Scholar]
- Donia M., Mijatovic S., Maksimovic-Ivanic D., Miljkovic D., Mangano K., Tumino S., Biondi A., Basile F., Al-Abed Y., Stosic-Grujicic S., Nicoletti F. (2009). The novel NO-donating compound GIT-27NO inhibits in vivo growth of human prostate cancer cells and prevents murine immunoinflammatory hepatitis. European Journal of Pharmacology, 615(1–3), 228–233. [DOI] [PubMed] [Google Scholar]
- Erlandsson A., Carlsson J., Andersson S. O., Vyas C., Wikstrom P., Andren O., Davidsson S., Rider J. R. (2018). High inducible nitric oxide synthase in prostate tumor epithelium is associated with lethal prostate cancer. Scandinavian Journal of Urology, 52(2), 129–133. [DOI] [PubMed] [Google Scholar]
- Frederiksen L. J., Sullivan R., Maxwell L. R., Macdonald-Goodfellow S. K., Adams M. A., Bennett B. M., Siemens D. R., Graham C. H. (2007). Chemosensitization of cancer in vitro and in vivo by nitric oxide signaling. Clinical Cancer Research, 13(7), 2199–2206. [DOI] [PubMed] [Google Scholar]
- Fukumura D., Kashiwagi S., Jain R. K. (2006). The role of nitric oxide in tumour progression. Nature Reviews Cancer, 6(7), 521. [DOI] [PubMed] [Google Scholar]
- Harada K., Kawaguchi S.-I., Tomitaro O., Yoshida H., Sato M. (2004). Overexpression of iNOS gene suppresses the tumorigenicity and metastasis of oral cancer cells. In Vivo, 18(4), 449–455. [PubMed] [Google Scholar]
- Hasan N., Cao J., Lee J., Naeem M., Hlaing S. P., Kim J., Jung Y., Lee B. L., Yoo J. W. (2019). PEI/NONOates-doped PLGA nanoparticles for eradicating methicillin-resistant Staphylococcus aureus biofilm in diabetic wounds via binding to the biofilm matrix. Materials Science and Engineering C: Materials for Biological Applications, 103, 109741. doi: 10.1016/j.msec.2019.109741 [DOI] [PubMed] [Google Scholar]
- Keith Bechtel M., Bonavida B. (2001). Inhibitory effects of 17beta-estradiol and progesterone on ovarian carcinoma cell proliferation: A potential role for inducible nitric oxide synthase. Gynecologic Oncology, 82(1), 127–138. [DOI] [PubMed] [Google Scholar]
- Krischel V., Bruch-Gerharz D., Suschek C., Kröncke K.-D., Ruzicka T., Kolb-Bachofen V. (1998). Biphasic effect of exogenous nitric oxide on proliferation and differentiation in skin derived keratinocytes but not fibroblasts. Journal of Investigative Dermatology, 111(2), 286–291. [DOI] [PubMed] [Google Scholar]
- Kundu J. K., Surh Y.-J. (2012). Emerging avenues linking inflammation and cancer. Free Radical Biology and Medicine, 52(9), 2013–2037. [DOI] [PubMed] [Google Scholar]
- Lahdenranta J., Hagendoorn J., Padera T. P., Hoshida T., Nelson G., Kashiwagi S., Jain K., Fukumura D. (2009). Endothelial nitric oxide synthase mediates lymphangiogenesis and lymphatic metastasis. Cancer Research, 69(7), 2801–2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C.-S., Li S., Miyauchi Y., Suzawa M., Ho C.-T., Pan M.-H. (2013). Potent anti-cancer effects of citrus peel flavonoids in human prostate xenograft tumors. Food & Function, 4(6), 944–949. [DOI] [PubMed] [Google Scholar]
- Laschak M., Spindler K.-D., Schrader A. J., Hessenauer A., Streicher W., Schrader M., Cronauer M. V. (2012). JS-K, a glutathione/glutathione S-transferase-activated nitric oxide releasing prodrug inhibits androgen receptor and WNT-signaling in prostate cancer cells. BMC Cancer, 12(1), 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masterson T. A., Arora H., Kulandavelu S., Carroll R. S., Kaiser U. B., Gultekin S. H., Hare J. M., Ramasamy R. (2018). S-nitrosoglutathione reductase (GSNOR) deficiency results in secondary hypogonadism. The Journal of Sexual Medicine, 15(5), 654–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocellin S., Bronte V., Nitti D. (2007). Nitric oxide, a double edged sword in cancer biology: Searching for therapeutic opportunities. Medicinal Research Reviews, 27(3), 317–352. [DOI] [PubMed] [Google Scholar]
- Ng Q. S., Goh V., Milner J., Stratford M. R., Folkes L. K., Tozer G. M., Saunders M. I., Hoskin P. J. (2007). Effect of nitric-oxide synthesis on tumour blood volume and vascular activity: A phase I study. Lancet Oncology, 8(2), 111–118. [DOI] [PubMed] [Google Scholar]
- Nikolić Z. Z., Pavićević D. L., Romac S. P., Brajušković G. N. (2015). Genetic variants within endothelial nitric oxide synthase gene and prostate cancer: A meta-analysis. Clinical and Translational Science, 8(1), 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberley T. D., Zhong W., Szweda L. I., Oberley L. W. (2000). Localization of antioxidant enzymes and oxidative damage products in normal and malignant prostate epithelium. Prostate, 44(2), 144–155. https://www.ncbi.nlm.nih.gov/pubmed/10881024 [DOI] [PubMed] [Google Scholar]
- Pervin S., Singh R., Hernandez E., Wu G., Chaudhuri G. (2007). Nitric oxide in physiologic concentrations targets the translational machinery to increase the proliferation of human breast cancer cells: Involvement of mammalian target of rapamycin/eIF4E pathway. Cancer Research, 67(1), 289–299. [DOI] [PubMed] [Google Scholar]
- Safarinejad M. R., Safarinejad S., Shafiei N., Safarinejad S. (2013). Effects of the T-786C, G894T, and Intron 4 VNTR (4a/b) polymorphisms of the endothelial nitric oxide synthase gene on the risk of prostate cancer. Urologic Oncology: Seminars and Original Investigations, 31(7), 1132–1140. [DOI] [PubMed] [Google Scholar]
- Sang J., Chen Y., Tao Y. (2011). Nitric oxide inhibits gastric cancer cell growth through the modulation of the Akt pathway. Molecular Medicine Reports, 4(6), 1163–1167. [DOI] [PubMed] [Google Scholar]
- Schleiffer R., Duranton B., Gossé F., Bergmann C., Raul F. (2000). Nitric oxide synthase inhibition promotes carcinogen-induced preneoplastic changes in the colon of rats. Nitric Oxide, 4(6), 583–589. [DOI] [PubMed] [Google Scholar]
- Siemens D. R., Heaton J. P., Adams M. A., Kawakami J., Graham C. H. (2009). Phase II study of nitric oxide donor for men with increasing prostate-specific antigen level after surgery or radiotherapy for prostate cancer. Urology, 74(4), 878–883. [DOI] [PubMed] [Google Scholar]
- Tam N. N., Leav I., Ho S.-M. (2007). Sex hormones induce direct epithelial and inflammation-mediated oxidative/nitrosative stress that favors prostatic carcinogenesis in the noble rat. The American Journal of Pathology, 171(4), 1334–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan G., Qiu M., Chen L., Zhang S., Ke L., Liu J. (2017). JS-K, a nitric oxide pro-drug, regulates growth and apoptosis through the ubiquitin-proteasome pathway in prostate cancer cells. BMC Cancer, 17(1), 376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi P. (2007). Nitric oxide and immune response. Indian Journal of Biochemistry and Biophysics, 44(5), 310–319. https://www.ncbi.nlm.nih.gov/pubmed/18341206 [PubMed] [Google Scholar]
- Vahora H., Khan M. A., Alalami U., Hussain A. (2016). The potential role of nitric oxide in halting cancer progression through chemoprevention. Journal of Cancer Prevention, 21(1), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannini F., Kashfi K., Nath N. (2015). The dual role of iNOS in cancer. Redox Biology, 6, 334–343. doi: 10.1016/j.redox.2015.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas-Read S., Shaul P. W., Yuhanna I. S., Willis B. C. (2007). Nitric oxide attenuates epithelial-mesenchymal transition in alveolar epithelial cells. American Journal of Physiology Lung Cellular and Molecular Physiology, 293(1), L212–L221. [DOI] [PubMed] [Google Scholar]
- Wang K., Brems J. J., Gamelli R. L., Holterman A. X. (2011). iNOS/NO signaling regulates apoptosis induced by glycochenodeoxycholate in hepatocytes. Cell Signal, 23(10), 1677–1685. [DOI] [PubMed] [Google Scholar]
- Weiming X., Liu L. Z., Loizidou M., Ahmed M., Charles I. G. (2002). The role of nitric oxide in cancer. Cell Research, 12(5), 311. [DOI] [PubMed] [Google Scholar]
- Wu J. H., Yang K., Ma H. S., Xu Y. (2014). Association of endothelia nitric oxide synthase gene rs1799983 polymorphism with susceptibility to prostate cancer: a meta-analysis. Tumor Biology, 35(7), 7057–7062. [DOI] [PubMed] [Google Scholar]
- Xie J.-D., Chen L.-Q., Lai N.-L., Chen J.-Y. (2018). Nitric oxide donors for prostate cancer therapy. Medical Exploration, 2(01), 05–12. [Google Scholar]
- Yu S., Jia L., Zhang Y., Wu D., Xu Z., Ng C.-F., To K. K., Huang Y., Chan F. L. (2013). Increased expression of activated endothelial nitric oxide synthase contributes to antiandrogen resistance in prostate cancer cells by suppressing androgen receptor transactivation. Cancer Letters, 328(1), 83–94. [DOI] [PubMed] [Google Scholar]