Abstract
Background
Primary pulmonary myxoid sarcoma (PPMS) is an extremely rare lung sarcoma that is characterized in most cases by recurrent balanced chromosomal translocation t(2;22)(q33;q12) leading to the oncogenic fusion gene EWSR1-CREB1.
Case presentation
We report a case of PPMS with molecular confirmation using fluorescence in situ hybridization (FISH) and DNA sequencing in a 45-year-old female patient. Computer tomography (CT) scanning revealed a peripheral circumscribed solid mass of 2.1 × 2 cm in the right lung superior lobe. Histologically, the tumor cells ranged from stellate, polygonal to chondrocyte-like or physaliferous-like, forming reticular network of delicate lace-like cellular strands and cords in abundant myxoid stroma. The tumor cell immunophenotype was positive for vimentin, EMA and negative for CK-pan, TTF-1, CAM5.2, S-100, calponin, SMA, desmin, ALK, CD31 and CD34. Molecular analysis demonstrated EWSR1-CREB1 gene fusion in this tumor. During 38 months of follow-up, the patient was alive with no clinical or radiological evidence of recurrence or metastasis.
Conclusion
PPMS is a rare low-grade sarcoma with distinct histological and genetic features. We add another case to the literature of this rare tumor and report for the first time occurrence of chondrocyte-like and physaliferous-like tumor cells in this tumor, thus enriching its morphologic and cytologic spectrum.
Keywords: Pulmonary, Myxoid, Sarcoma, Chondrocyte-like, Physaliferous-like, EWSR1, CREB1
Background
Primary pulmonary sarcomas are extremely rare with prevalence of about 0.2% [1]. They comprise a heterogeneous group of sarcomas morphologically similar to the soft tissue counterparts. Among these rare sarcomas, primary pulmonary myxoid sarcoma (PPMS) are even more infrequent. PPMS is a recently described lung sarcoma more prevalent in young females with a characteristic genetic EWSR1-CREB1 fusion in most cases [2, 3].. To the best of our knowledge, 25 cases have been reported in the English literature [2–11], among which only 17 cases were confirmed with presence of the EWSR1-CREB1 gene fusion. We report another case of PPMS harboring the EWSR1-CREB1 gene fusion confirmed by molecular method, with review of the literature.
Case presentation
A 45-year-old woman was referred to our hospital for physical checkup. She was asymptomatic with no pulmonary obstructive symptoms or pneumonia. The patient was a non-user of alcohol and tobacco products. General physical and laboratory examination was unremarkable. Chest computed tomography (CT) revealed a 2.1 × 2 cm peripheral solid mass in the right lung superior lobe featuring moderate heterogeneous enhancement (Fig. 1). There was no abnormality on bronchoscopic and cytological examinations. Routine blood tests and tumor marker levels were normal. Although CT findings suggested the possibility of hemangioma, the patient insisted on surgical excision. During surgery, a mass was detected in the right lung superior lobe which adhered to the superior vena cava with no invasion to the adjacent lung parenchyma or bronchus. Adjuvant chemotherapy was not given post-operatively. At follow-up 38 months after surgery, there was no evidence of recurrence or metastasis.
Fig. 1.
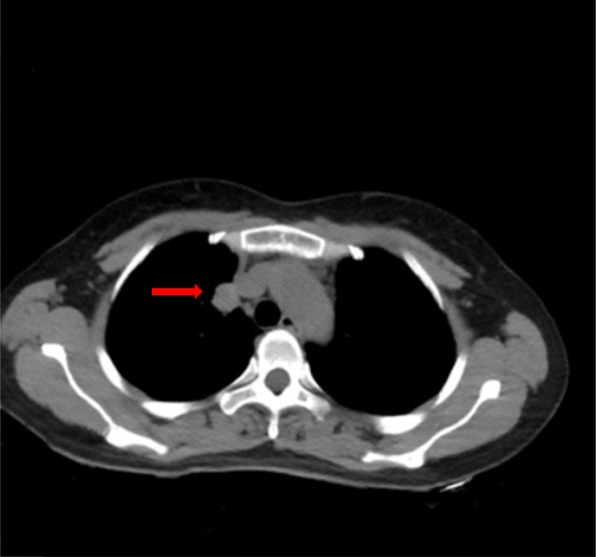
Chest computed tomography showed a 2.1 × 1.7 cm well-defined round mass exhibiting mild, heterogeneous internal enhancement at the periphery of the right superior lobe
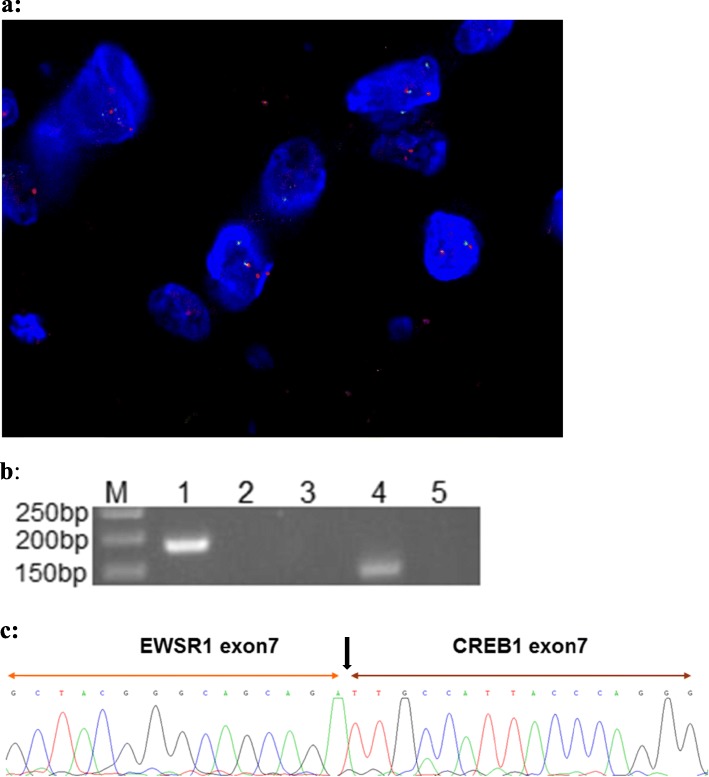
Macroscopically, the tumor was a solitary, well-circumscribed mass with a fleshy homogeneous white and gelatinous cut surface, measuring 2 × 1.7 × 1 cm without invasion to the bronchus. The tumor was histologically multinodular and composed of oval, or polygonal cells with vesicular nuclei, reminiscent of chondrocyte-like or physaliferous-like cells, in a background of myxoid stroma (Fig. 2a-d). There were rare mitotic figures and rich lymphoplasmacytic cell infiltration was also evident (Fig. 2e). There was no evidence an endobronchial component. Immunophenotypically, the tumor was positive for vimentin (Fig. 2f), epithelial membrane antigen (EMA) (Fig. 2g) and negative for CK-pan, CAM5.2, CD31, CD34, smooth muscle actin (SMA), desmin (Fig. 2h), anaplastic lymphoma kinase (ALK) (Fig. 2i), calponin, TTF-1 and S-100 protein (Fig. 2j) (Table 1). Molecular analysis was performed on formalin fixed paraffin embedded (FFPE) material by fluorescence in situ hybridization (FISH) using LSI EWSR1 dual-color break-apart probe (Break Apart Rearrangement probe, Ambipin,China), and specific gene fusion transcripts by reverse transcription-polymerase chain reaction (RT-PCR). Primers for the RT-PCR were located in exon 7 of EWSR1 (5’TCCTACAGCCAAGCTCCAAGTC3’) and in exon 7 of CREB1 (5’GTACCCCATCGGTACCATTGT3’). FISH showed a clear separation of red and green signals within a single tumor cell, demonstrating the presence of a EWSR1 gene rearrangement (Fig. 3a). The RT-PCR gene fusion products were confirmed by DNA sequencing with Sanger method (ABI3730, Japan) to show in-frame fusion of the 5′ region of EWSR1 (exon 7) to the 3′ region of CREB1 (exon 7) (Fig. 3b & c). The histological, immunophenotypic and molecular findings confirmed the diagnosis of primary pulmonary myxoid sarcoma with EWSR1-CREB1 gene fusion.
Fig. 2.
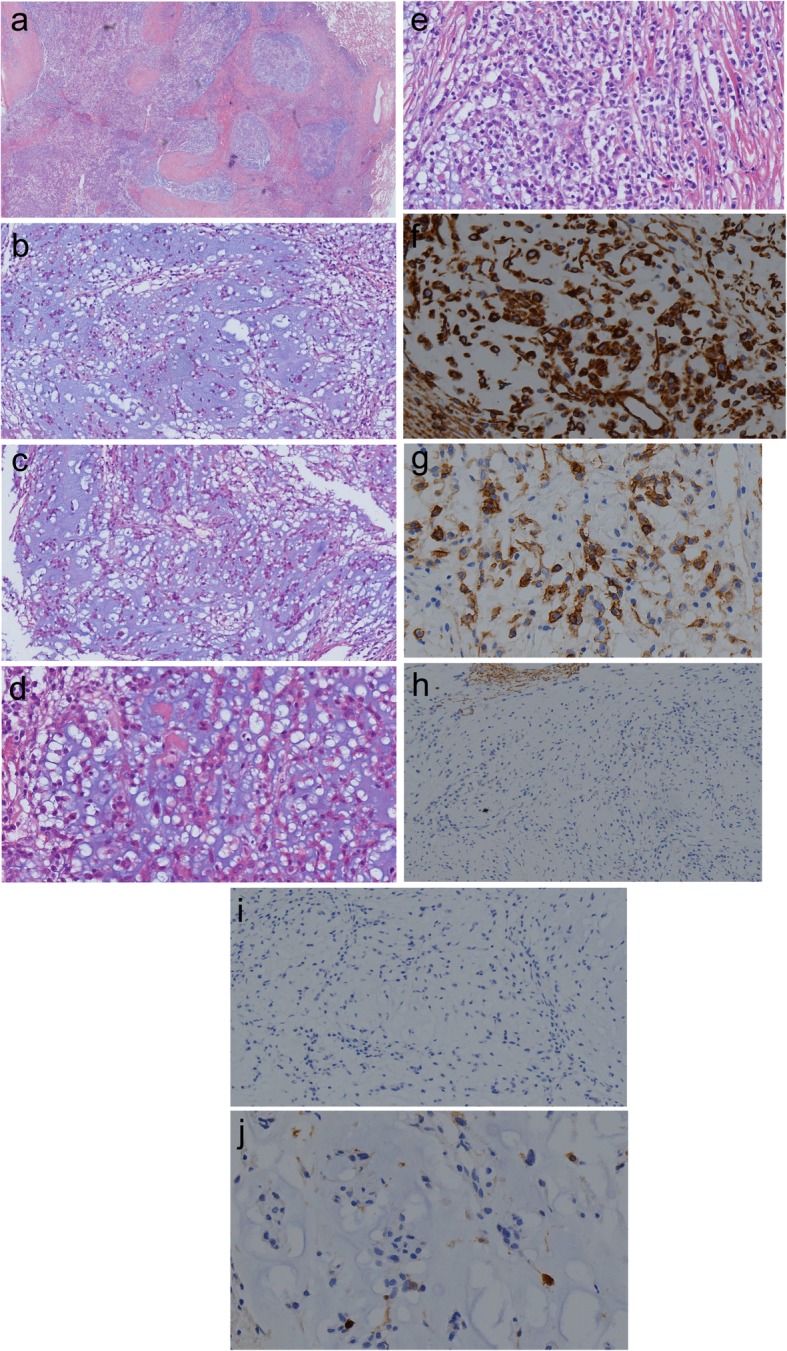
a. The tumor had abundant myxoid stroma and a multinodular architecture at low-magnification (a: magnification × 20 and b: magnification ×100). b & c & d The tumor showed variable cellularity with polygonal, stellate to chondrocyte-like or physaliferous-like tumor cells organized in prominent reticular network of delicate lace-like cellular strands and cords within prominent myxoid stroma (b & c magnification × 100, d: magnification × 400). e. Abundant lymphoid cells and plasma cells at the periphery or within the tumor (magnification × 400) f. Tumor cell immunohistochemical positive expression of vimentin (magnification × 400). g. Tumor cell immunohistochemical positive expression of EMA (magnification × 400). h. Tumor cell immunohistochemical negative expression of Desmin (magnification × 200). i. Tumor cell immunohistochemical negative expression of ALK (magnification × 400). j. Tumor cell immunohistochemical negative expression of S-100 (magnification × 400)
Table 1.
List of antibodies
| Antigen | Clone | Dilution | Manufacturer |
|---|---|---|---|
| Vimentin | MX034 | 1:200 | Fuzhou MXB Biotechnology Co,Ltd. China |
| EMA | GP1.4 | 1:100 | Fuzhou MXB Biotechnology Co,Ltd. China |
| CK-pan | polyclonal | 1:200 | Fuzhou MXB Biotechnology Co,Ltd. China |
| CAM5.2 | CAM5.2 | 1:100 | Fuzhou MXB Biotechnology Co,Ltd. China |
| CD31 | JC/70A | 1:100 | Fuzhou MXB Biotechnology Co,Ltd. China |
| CD34 | QBEnd/10 | 1:100 | Fuzhou MXB Biotechnology Co,Ltd. China |
| SMA | 1A4 | 1:200 | Fuzhou MXB Biotechnology Co,Ltd. China |
| Calponin | MX023 | 1:300 | Fuzhou MXB Biotechnology Co,Ltd. China |
| TTF-1 | 8G7G3/1 | 1:200 | Fuzhou MXB Biotechnology Co,Ltd. China |
| S-100 | polyclonal | 1:200 | Fuzhou MXB Biotechnology Co,Ltd. China |
| Desmin | D33 | 1:200 | Guangzhou Onco Care Biotechnology Co,Ltd. China |
| ALK | MX064 | 1:200 | Guangzhou Onco Care Biotechnology Co,Ltd. China |
Fig. 3.
a: Dual color interphase fluorescence in situ hybridization utilizing the EWSR1 break-apart probe. Split red and green signals within a single tumor cell demonstrated the presence of EWSR1 rearrangement. b: Gel electrophoresis of the RT-PCR products using EWSR1 and CREB1 primers; confirming presence of ESWR1-CREB1 fusion in the patient’s sample (Lane 4). M:50 bp markers; Lane 1: Internal control, PGK; Lane 2: Negative control with EWSR1 exon 7 + CREB1 exon 7 fusion primer; Lane 3: Negative control with EWSR1 exon 7 + CREB1 exon 8 fusion primer; Lane 4: Patient’s sample with EWSR1 exon 7 + CREB1 exon 7 fusion primer; Lane 5: Patient’s sample with EWSR1 exon 7 + CREB1 exon 8 fusion primer. c: Sanger sequencing result of the patient’s RT-PCR product demonstrated in Lane 4 of b. The sequence was the same as the EWSR1 - CREB1 fusion gene as reported in the literature
Discussion
Nicholson et al. first described a rare primary pulmonary myxoid sarcoma in 1999 as a novel low-grade malignant myxoid endobronchial neoplasm [4]. Further studies showed that this tumor has a characteristic genetic fingerprint characterized by the oncogenic fusion gene EWSR1-CREB1 [2], which has been first included in the latest WHO fascicle as a characteristic of this tumor [12]. To date, 25 cases of this sarcoma have been described in the English literature [2–11],with only 17 confirmed by presence of EWSR1-CREB1 fusion [2, 3, 5–10]. Our case is the eighteenth case of PPMS with confirmed EWSR1-CREB1 gene fusion. All 26 cases (including the 5 cases with no EWSR1-CREB1 gene fusion, 1 case with ESWR1 rearrangement not involving CREB1 and 2 cases without molecular genetic workup) are reviewed and summarized in Table 2. The patients had a broad age range from 21 to 80 years (mean 43 years), with female predominance (female: male, 1.5:1). The clinical manifestations are relatively nonspecific and include cough, hemoptysis and weight loss. There was no definite site of predilection of this tumor in the lung. Although initially reported mostly as endobronchial growths related to the bronchial tree, there were some tumors not associated with the bronchus [7, 8, 10], as in our case. One such case was a EWSR1-CREB1 gene rearranged low grade myxoid sarcoma of the pulmonary artery [10]. Grossly, the excised lesions are well circumscribed, ranged in size from 1.5 to 14 cm (average 4 cm) with pale, glistening cut surface. Microscopically, the characteristic features of most cases are lobulated architecture with cords of ovoid, spindle or stellate cells embedded in a prominent myxoid matrix. Most cases have a patchy background of inflammatory cells consisting mainly of lymphocytes and plasma cells. In distinct contrast, the tumor cells in our case are chondrocyte-like or physaliferous-like. Predominance of chondrocyte-like and physaliferous-like tumor cells in PPMS has not been reported in the literature previously. The inflammatory infiltrate in our case is also more intense. These unique findings of our case enrich the morphologic and cytologic spectrum of PPMS. Regarding genetics of PPMS, genetic study was not performed in two of the 26 cases. Among the remaining 24 cases, 18 (including our case) showed EWSR1-CREB1 gene fusion, one showed EWSR1 rearrangement but not fusion with CREB1 (case 16) [6], and 5 were negative for ESWR1-CREB1 gene fusion. This makes a 75% positive rate for EWSR1-CREB1 fusion, 79% for EWSR1 rearrangement, and a negative rate of 21% for ESWR1-CREB1 fusion or ESWR1 rearrangement in the 24 cases where genetic study was performed in the reviewed series of PPMS. These findings are broadly similar to the previously reported EWSR1-CREB1 gene fusion rate of 70% [2] and 63% [3], and EWSR1 and/or CREB1 gene rearrangement rate of 89% [3] in two smaller series of PPMS. Thway K et al [2] detected EWSR1-CREB1 fusion gene in 7 PPMS, with the fusion loci located in exon 7 of EWSR1 and exon 7 of CREB1 in 6 of the 7 cases. One other case showed fusion loci in exon 7 of EWSR1 and exon 8 of CREB1. There was no histological difference in the tumors bearing the different gene fusions. Furthermore, one case in this series with no EWSR1-CREB1 fusion died from brain metastasis, while another case bearing the EWSR1-CREB1 fusion of the same series had kidney metastasis but was alive and well after 3 years [2]. In another series [7], one case with EWSR1-CREB1 fusion developed metastasis to the contralateral lung, but remained disease free 72 months after removal of the lung metastasis. It appears that PPMS with EWSR1-CREB1 fusion fares better than those without EWSR1-CREB1 fusion. However, whether presence of EWSR1-CREB1 fusion connotates better prognosis in PPMS requires further study.
Table 2.
Clinicopathological and genetic features of current case and previously reported cases of primary pulmonary myxoid sarcoma
| Case No. (Ref. No.) | Age | sex | EBC (+/−) | Size (cm) | Pathological features | IHC | Molecular genetics | Treatment | Follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|
| 1(3) | 27 | F | EBC+ | 4 | Ill-defined nodules of myxoid stroma, interweaving cords of small uniform, round or slightly elongated cells with eosinophilic cytoplasm, occasional mitoses. | Vimentin+, CK-, S100-, desmin-, SMA-, CD34-. | ND | Surgery | NEOD after 36 |
| 2(3) | 43 | F | EBC+ | 13 | Ill-defined nodules of myxoid stroma, interweaving cords of small uniform, round or slightly elongated cells with eosinophilic cytoplasm, occasional mitoses. | Vimentin+, CK-, S100-, desmin-, SMA-, CD34-. | ND | Surgery | NEOD after 6 |
| 3(2) | 27 | F | EBC+ | 4 | Well circumscribed, lobulated, reticular network with delicate lacelike strands and cords of cells within prominent myxoid stroma, tumor cells showed no or minimal atypia. | CK-, S100-, desmin-. | EWSR1-CREB1 fusion | Surgery | NEOD after 180 |
| 4(2) | 33 | F | EBC+ | 3.5 | Lobulated, reticular network with delicate lacelike strands and cords of cells within prominent myxoid stroma, tumor cells showed mild to moderate atypia. | CK-, S100-, desmin-. | EWSR1-CREB1 fusion | Surgery | NEOD after 144 |
| 5(2) | 45 | F | EBC+ | 1.5 | Circumscribed, lobulated, cellular sheets or patternless, tumor cells showed mild to moderate atypia. | S100 focal+, CK-, desmin-, p63-. | Negative | Surgery | NEOD after 12 |
| 6(2) | 36 | F | NR | NR | Circumscribed with fibrous pseudocapsule. Reticular network with delicate lacelike strands and cords of cells within prominent myxoid stroma, tumor cells showed minimal atypia. | CK-, EMA-, TTF1-, S100-, desmin-. | Negative | Surgery | DOD with brain metastases a few months after diagnosis |
| 7(2) | 32 | F | EBC+ | NR | Lobulated. Reticular network with delicate lacelike strands and cords of cells within prominent myxoid stroma, tumor cells showed moderate atypia. | CK-, EMA-,S100-, desmin-. | EWSR1-CREB1 fusion | Surgery | NR |
| 8(2) | 28 | M | EBC+ | 2.8 | Infiltrative and lobulated, cellular sheets or patternless, tumor cells showed mild to moderate atypia, | EMA weak+, CK-, TTF1-, S100-, HMB45-, melan A-, desmin-. | EWSR1-CREB1 fusion | Surgery | Left renal metastasis, alive and well after 3 years. |
| 9(2) | 67 | M | EBC+ | 2.8 | Well circumscribed and lobulated, reticular network with delicate lacelike strands and cords of cells within a prominent myxoid stroma., tumor cells showed minimal atypia. | EMA weak+, CK-, TTF-1-, S100-, desmin-. | EWSR1-CREB1 fusion | Surgery | NR |
| 10(2) | 68 | F | EBC+ | 2.0 | Well circumscribed and lobulated, reticular network with delicate lacelike strands and cords of cells within a prominent myxoid stroma, tumor cells showed moderate to marked atypia. | EMA weak+, CK-, p63-, TTF-1-, S100-, desmin-. | Negative | Surgery | NR |
| 11(2) | 63 | F | EBC+ | NR | Lobulated, cellular sheets or patternless, tunor cells showed mild to minimal atypia. | EMA weak +, CK-, TTF-1-, S100-, HMB45-, melan A-, desmin-. | EWSR1-CREB1 fusion | Surgery | NEOD after 48 |
| 12(2) | 51 | M | NR | 2.0 | Well circumscribed and lobulated, reticular network with delicate lacelike strands and cords of cells within a prominent myxoid stroma, tumor cells showed mild to moderate atypia. | NR | EWSR1-CREB1 fusion | Surgery | NR |
| 13(5) | 31 | M | EBC+ | 2.7 | Well circumscribed, reticular cords of oval, short spindle or polygonal cells with mild atypia, rare mitotic figures, an abundant myxoid stroma, scattered lymphoplasmacytic infiltrates. |
Vimentin+, EMA focal+, CK-, TTF-1-, napsin A-, S-100-, CD34-, desmin-, SMA-, CD10-, p63-, calponin- caldesmon-, c-kit-, HMB-45-, synaptophysin-, GFAP- |
EWSR1-CREB1 fusion | Surgery | NEOD after 68 |
| 14(6) | 66 | F | EBC+ | 4 | Polygonal to spindled cells, reticular network with delicate lacelike strands and cords of cells within a prominent myxoid stroma, tumor cells showed mild atypia. | EMA focal+, CK-, p63-, S100-, desmin-. | EWSR1-CREB1 fusion | Surgery | NR |
| 15(6) | 28 | M | NR | 8.5 | Lobulated, biphasic, ~ 40% composed of myxoid pools, exuberant fibroinflammatory reaction with confluent plasma cells, tumor cells showed moderate atypia. | Desmin+, EMA focal+, CK-, p63-. S100-. | Negative | Surgery | NR |
| 16(6) | 28 | M | EBC+ | 6 | Infiltrative, focal necrosis and inflammation, tumor cells showed severe atypia. | EMA focal+, CK-, p63-, S100-. | EWSR1 rearrangement,. but not CREB1 | Surgery | NR |
| 17(7) | 26 | M | EBC+ | 9 | Multinodular, reticular network with delicate lacelike strands and cords of cells within prominent myxoid stroma, tumor cells showed mild to moderate atypia. | Vimentin+, EMA focal+, CD99 focal weak+, SMA-, desmin-, caldesmon-H-, calponin-, S100-, CK-, CD31-, CD34-, p63-, CD56-, synaptophysin-. | EWSR1-CREB1 fusion | Surgery | NEOD after 19 |
| 18(7) | 49 | F | EBC- | 4 | Multinodular, reticular network with delicate lacelike strands and cords of cells within prominent myxoid stroma, tumor cells showed mild to moderate atypia. | Vimentin+, EMA focal+, CD99 focal weak+, SMA focal+, desmin-, caldesmon-H, calponin-, S100-, CK-, CD31-, CD34-, p63-, CD56-, synaptophysin-. | EWSR1-CREB1 fusion | Surgery | NEOD after 117 |
| 19(7) | 54 | F | EBC+ | 4.5 | Multinodular, reticular network with delicate lacelike strands and cords of cells within prominent myxoid stroma, tumor cells showed moderate atypia. |
Vimentin+, EMA focal+, CD99 focal+, SMA-, desmin-, caldesmon-H-, calponin-, S100-, CK-, CD31-, CD34-, p63-, CD56-, synaptophysin-. |
EWSR1-CREB1 fusion | Surgery | NEOD after 152 |
| 20(7) | 65 | M | EBC+ | 13 | Multinodular, reticular network with delicate lacelike strands and cords of cells within prominent myxoid stroma, tumor cells showed mild atypia. |
Vimentin+, EMA focal+, CD99 focal+, SMA-, desmin-, caldesmon-H-, calponin-, S100-, CK-, CD31-, CD34-, p63-, CD56-, synaptophysin-. |
EWSR1-CREB1 fusion | Surgery | Metastasis to contralateral lung, NEOD 72 months after removal of metastasis. |
| 21(8) | 29 | F | EBC- | 3 | Well circumscribed, short spindle, ovoid or stellate cells in reticular network, myxoid stroma, lymphoplasmacytic infiltration, tumor cells showed mild atypia. | Vimentin+, EMA+. SMA-, SMMHC-, calretinin-, TTF-1-, CK-, p63-, S-100-, CD34-, CD56-. | EWSR1-CREB1 fusion | Surgery | NEOD after 17 |
| 22(4) | 80 | F | EBC+ | NR | Multinodular, spindle cells arranged in reticular pattern within prominent myxoid stroma, tumor cells showed moderately atypia | Vimentin+, EMA focal+. CK-, S100-, HMB45-, CD31-, CD34-, SMA-, caldesmon-H-, desmin-, GFAP-. | EWSR1-CREB1 fusion | Surgery | NEOD after 36. |
| 23(9) | 32 | F | NR | 3.5 | Well-delineated lobulated, anastomosing cords and small nests of epithelioid cells admixed with stellate cells in chondromyxoid matrix. | Vimentin+, CD68 weak+, CD163 weak+, synaptophysin weak+. CK-, EMA-, calponin-, GFAP-, SMA-, desmin-, caldesmon-H-, S-100-, HMB-45-, CD34-, CD31-, chromogranin-. | EWSR1-CREB1 fusion | Surgery | NEOD after 96. |
| 24(10) | 21 | F | PA/EBC- | NR | Polypoid tumor, trabecular networks, rare solid areas, tumor cells showed oval nuclei and eosinophilic cytoplasm, | CK weak+, SMA+, INI1-, EMA-, S100-, desmin-, ERG-, MDM2-, CDK4-. | EWSR1-CREB1 fusion | Surgery | NEOD after 38 |
| 25(11) | 48 | M | NR | 14 | Bland looking medium-sized oval to round epithelioid cells arranged in prominent reticular and microcystic lace-like chordoid pattern in highly myxoid stroma. | Vimentin+, CD10 focal+、EMA focal+, CK-, TTF-1-, ERG-, CD31-, p63-, desmin-, SMA-, S100-, CD34-, CD30- , MUC4-, TLE1-, STAT6-. | Negative | Surgery | NEOD after 23 |
| 26 (our case) | 45 | F | EBC- | 2.1 | Well circumscribed, multinodular, reticular network of delicate lace-like cellular strands and cords in abundant myxoid stroma, chondrocyte or physaliferous-like tumor cells with mild atypia. | Vimentin+, EMA+, CK-, TTF-1-, CAM5.2-, S-100-, calponin-, SMA-, desmin-, ALK-, CD31-, CD34-. | EWSR1-CREB1 fusion | Surgery | NEOD after 38 |
F female, M male, EBC+/− endobronchial component involved (+) or not involved (−). IHC immunohistochemical stains, ND not done, NR not reported, PA pulmonary artery, DOD died of disease, NEOD no evidence of disease, CK cytokeratin(s), EMA epithelial membrane antigen, SMA smooth muscle actin, SMMHC smooth muscle myosin heavy chain, TTF-1 thyroid transcription factor-1
PPMS should be differentiated from extraskeletal myxoid chondrosarcoma (EMC), which can also arise in the lung [13, 14]. Histologically, EMC is composed of cords of cells with scarce cytoplasm immersed in abundant myxoid matrix similar to PPMS. EMC, however, at least focally expresses S-100 which is negative in PPMS, as in our case. Genetically, EMC may harbor EWSR1-NR4A3、TAF15-NR4A3 or TFG-NR4A3 gene fusion [15, 16], and may thus confound with PPMS. However, PPMS exhibits the characteristic EWSR1-CREB1 fusion gene, allowing distinction from EMC. Differentiation between PPMS and angiomatoid fibrous histiocytoma (AFH), which may occur in the lung [17], is more problematic. Although both tumors are located predominantly endobronchially and harbor the EWSR1-CREB1 fusion gene, they differ in morphology. PPMS is composed of cords and clusters of spindle, stellate, ovoid, and in our case chondrocyte-like and physaliferous-like tumor cells arranged in a reticular pattern within prominent alcian blue positive myxoid stroma. In contrast, AFH comprises sheets and islands of spindle to epithelioid cells with bland ovoid vesicular nuclei and abundant eosinophilic cytoplasm within loose stroma. PPMS has more abundant myxoid stroma and lacks the prominent peripheral cuff of lymphocytes usually present in AFH. Moreover, desmin is expressed in 50% of AFH, which is not present in PPMS. Furthermore, ALK expression is common in AFH, which is negative in PPMS. Finally, AFH may show other fusion genes, such as EWSR1-ATF1 and FUS-ATF1, both of which are absent in PPMS. PPMS should be also be distinguished from myoepithelial tumors, which can also arise in the lung [18] with endobronchial growth pattern and EWSR1 rearrangements. Myoepithelial tumors are immunohistochemically positive for CK, p63, SMA, calponin and S-100 protein, which are negative in PPMS. Pulmonary microcystic fibromyxoma (PMF) are composed of bland spindle to stellate cells with uniform nuclei widely spaced within fibromyxoid stroma which is alcian blue positive and hyaluronidase sensitive [19]. However, PMF are not endobronchially located and are much less cellular with bland stellate cells disposed in a microcystic pattern. Furthermore, PMF does not harbor the EWSR1-CREB1 gene fusion, distinct from PPMS. Another differential diagnosis is inflammatory myofibroblastic tumor (IMT) which may possess myxoid stroma and inflammatory infiltrates. The stellate tumor cells in IMT are immunophenotypically positive for SMA, desmin and ALK, which are negative in PPMS. Since the physaliferous-like tumor cells in PPMS exhibit cytoplasmic bubbles, they can potentially be confused with lipoblasts. PPMS thus needs to be differentiated from low grade primary or metastatic myxoid liposarcoma [20]. Myxoid liposarcoma, however, often harbors rearrangement involving the DDIT3 gene, which is not present in PPMS. The various differential diagnoses and their features are summarized in Table 3.
Table 3.
Salient features of primary pulmonary myxoid sarcoma and differential diagnoses
| Tumors | Pathological features | IHC | Molecular genetics | Differentiating features from PPMS |
|---|---|---|---|---|
| Primary pulmonary myxoid sarcoma (PPMS) | Well-circumscribed, lobulated, reticular network of delicate lace-like cellular strands and cords in abundant myxoid stroma, tumor cells are stellate, polygonal with also chondrocyte-like or physaliferous-like tumor cells first reported in our case, prominent lymphoplasmacytic infiltrates within and at periphery of tumor. | Vimentin+, EMA+, CK--, TTF-1-, S-100-, calponin-, SMA-, desmin-, ALK-, CD31-, CD34-. | EWSR1-CREB1 fusion | NA |
| Extraskeletal myxoid chondrosarcoma (EMC) | Well-circumscribed, multinodular, tumor lobules separated by fibrous septae, umor cells epithelioid to spindled arranged in cords, strands, or clusters embedded in abundant myxoid stroma. | Vimentin+, S-100+, rarely EMA+, keratins+. |
,EWSR1-NR4A3, TFG-NR4A3, HSPA8-NR4A3, TCF12-NR4A3, FUS-NR4A3 or TAF15-NR4A3 gene fusion |
S-100+, different molecular genetics. Rare as primary in lungs, may present as lung metastasis. |
| Angiomatoid fibrous histiocytoma (AFH) | Sheets and islands of spindle to epithelioid cells with bland ovoid vesicular nuclei and abundant eosinophilic cytoplasm within loose myxoid stroma. | ALK+, desmin+. | EWSR1-ATF1, FUS-ATF1 gene fusion | No lobular or reticular architecture, no chondrocyte-like or physaliferous-like cells, ALK+ and desmin+, with different molecular genetics in AFH. |
| Myoepithelial tumors (MT) | Well-circumscribed, solid sheets, nested or cord-like growth pattern, hyalinized or myxoid stroma, moderate to severe nuclear pleomorphism. | Cytokeratins+, EMA+, S100+, calponin+, SMA+, p63+, GFAP+. |
EWSR1-FUS, EWSR1-PBX1, EWSR1-ZNF444, EWSR1-POU5F1 gene fusions |
No reticular pattern, no chondrocyte-like or physaliferous-like cells, different 1HC and molecular genetics in MT. |
| Pulmonary microcystic fibromyxoma (PMF) | Well-circumscribed, bland spindled to stellate cells widely spaced within prominent fibromyxoid stroma with prominent cystic change. | Vimentin+, CD34-, CD31-, HMB45-, SMA-, desmin-, S-100-, ALK-, CKpan-, EMA-, calretinin-, TTF1- | none | Prominent cystic pattern, much less cellular, no chondrocyte-like or physaliferous-like cells, no diagnostic molecular genetic change and not endobronchially located in PMF. |
| Inflammatory myofibroblastic tumor (IMT) | Areas of myxoid stroma with prominent vessels or hyalinized collagenous stroma, and contain a prominent infltrate of plasma cells and lymphocytes. | SMA+, desmin+, ALK+, rarely keratins+ |
RANBP2-ALK, RRBP1-ALK, ETV6-NTRK3 gene fusions |
No reticular pattern, prominent inflammatory component, no chondrocyte-like or physaliferous-like cells, SMA+, ALK+ with different molecular genetics in IMT. |
| Low grade myxoid liposarcoma (LGML) | Large well-circumscribed, monotonous small ovoid cells with fine chromatin, inconspicuous nucleoli, and scant cytoplasm., many characteristic lipoblasts., prominent plexiform vasculature, myxoid background with areas of mucin pooling, imparting a “pulmonary edema-like” pattern. | S-100+, rarely MDM2+ and CDK4+ | DDIT3-FUS and DDIT3-EWSR1 gene fusions | Characteristic prominent plexiform vasculature, “pulmonary edema-like” pattern, S-100+, different molecular genetics in LGML. Lung is rare site for primary LGML. |
NA not applicable, CK cytokeratin, EMA epithelial membrane antigen, SMA smooth muscle actin
Although most patients treated by adequate surgery had no local recurrences, three patients developed metastasis within a follow up period from 4 months to 15 years. The metastatic sites included the contralateral lung [7], the left kidney and brain [2]. The patient with brain metastasis died a few months after surgery. The patient with metastasis to the left kidney is still alive and well [2]. The patient with metastasis to the contralateral lung occurred 7 months after surgery [7]. Though PPMS is considered a low-grade sarcoma, it appears that there are no reliable histological or clinical features for predicting its prognosis and outcome. Some cases histologically showing nuclear atypia, necrosis and high mitotic figures, with increased Ki-67 proliferative index did not fare worse prognosis [2]. On the other hand, endobronchial location, capsule-like fibrosis, absence of solid architecture and lack of necrosis might be predictive of more favorable outcome, though presence of endobronchial component and capsule-like features had also been observed in metastatic cases [2]. Molecular fingerprint may play a prognostic role, as patients with EWSR1 rearrangement [11] or EWSR1-CREB1 gene fusion [2, 7] may have more favorable prognosis, while those with no EWSR1-CREB1 fusion or wild type EWSR1 may portend poor clinical outcome [2, 11].
Conclusion
PPMS is a rare low-grade sarcoma that occurs mostly in middle age women with most harboring the characteristic EWSR1-CREB1 fusion gene. Our case adds to the literature of this rare tumor and enriches its morphologic and cytologic spectrum with occurrence of chondrocyte-like and physaliferous-like tumor cells. Early diagnosis of PPMS requires high index of suspicion in pulmonary myxoid tumors and is based on clinical features, histological and molecular studies. The clinical behavior of this tumor is still uncertain due to its rarity and limited follow up of reported cases. There are no reliable histological or clinical features to predict prognosis and outcome of this tumor.
Acknowledgements
We thank Xianbin-tang and Yinghua-Hao, Department of Pathology, Taihe Hospital, Hubei University of Medicine, for their technical support (FISH and RT-PCR).
Abbreviations
- AFH
Angiomatoid fibrous histiocytoma
- ALK
Anaplastic lymphoma kinase
- CAM5.2
Cytokeratin
- CK-pan
Cytokeratin (Pan)
- CT
Computer tomography
- EMA
Epithelial membrane antigen
- EMC
Extraskeletal myxoid chondrosarcoma
- FFPE
Formalin fixed and paraffin embedded
- FISH
Fluorescence in situ hybridization
- IMT
Inflammatory myofibroblastic tumor
- PMF
Pulmonary microcystic fibromyxoma
- PPMS
Primary pulmonary myxoid sarcoma
- RT-PCR
Reverse transcription-polymerase chain reaction
- SMA
Smooth muscle actin
- TTF-1
Thyroid transcription factor 1
Authors’ contributions
ZWC and RMC performed histological evaluation, made the pathological diagnosis and drafted the manuscript. YHY and HQS performed the immunohistochemistry. CSN advised on manuscript preparation and made revision to the final manuscript. All authors read and approved the final manuscript.
Funding
Nil.
Availability of data and materials
The dataset supporting the conclusion of this article is included within the article.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient for the publication of this case report and any accompanying images. A copy of the consent form is available for review by the Editor of Diagnostic Pathology.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zhenwei Chen, Email: chenzhenwei110@126.com.
Yihui Yang, Email: 790039153@qq.com.
Rongming Chen, Email: chenrm_2011@126.com.
Chi Sing Ng, Email: ngcspeter@gmail.com.
Hongqi Shi, Email: zyq10311@126.com.
References
- 1.Attanoos RL, Appleton MA, Gibbs AR. Primary sarcomas of the lung: a clinicopathological and immunohistochemical study of 14 cases. Histopathology. 1996;29:29–36. doi: 10.1046/j.1365-2559.1996.d01-481.x. [DOI] [PubMed] [Google Scholar]
- 2.Thway K, Nicholson AG, Lawson K, Gonzalez D, Rice A, Balzer B, et al. Primary pulmonary myxoid sarcoma with EWSR1-CREB1 fusion: a new tumor entity. Am J Surg Pathol. 2011;35:1722–1732. doi: 10.1097/PAS.0b013e318227e4d2. [DOI] [PubMed] [Google Scholar]
- 3.Prieto-Granada CN, Ganim RB, Zhang L, Antonescu C, Mueller J. Primary pulmonary myxoid sarcoma: A newly described entity-report of a case and review of the literature. Int J Surg Pathol. 2017;25:518–525. doi: 10.1177/1066896917706413. [DOI] [PubMed] [Google Scholar]
- 4.Nicholson AG, Baandrup U, Florio R, Sheppard MN, Fisher C. Malignant myxoid endobronchial tumour: a report of two cases with a unique histological pattern. Histopathology. 1999;35:313–318. doi: 10.1046/j.1365-2559.1999.00724.x. [DOI] [PubMed] [Google Scholar]
- 5.Matsukuma S, Hisaoka M, Obara K, Kono T, Takeo H, Sato K, et al. Primary pulmonary myxoid sarcoma with EWSR1-CREB1 fusion, resembling extraskeletal myxoid chondrosarcoma: case report with a review of literature. Pathol Int. 2012;62:817–822. doi: 10.1111/pin.12014. [DOI] [PubMed] [Google Scholar]
- 6.Smith SC, Palanisamy N, Betz BL, Tomlins SA, Mehra R, Schmidt LA, et al. At the intersection of primary pulmonary myxoid sarcoma and pulmonary angiomatoid fibrous histiocytoma: observations from three new cases. Histopathology. 2014;65:144–146. doi: 10.1111/his.12354. [DOI] [PubMed] [Google Scholar]
- 7.Jeon YK, Moon KC, Park SH, Chung DH. Primary pulmonary myxoid sarcomas with EWSR1-CREB1 translocation might originate from primitive peribronchial mesenchymal cells undergoing (myo) fibroblastic differentiation. Virchows Arch. 2014;465:453–461. doi: 10.1007/s00428-014-1645-z. [DOI] [PubMed] [Google Scholar]
- 8.Kim S, Song SY, Yun JS, Choi YD, Na KJ. Primary pulmonary myxoid sarcoma located in interlobar fissure without parenchymal invasion. Thorac Cancer. 2017;8:535–538. doi: 10.1111/1759-7714.12469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yanagida R, Balzer BL, Mckenna RJ, Fuller CB. Primary pulmonary myxoid sarcoma, a potential mimic of metastatic extraskeletal myxoid chondrosarcoma. Pathology. 2017;49:792–794. doi: 10.1016/j.pathol.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 10.Opitz I, Lauk O, Schneiter D, Ulrich S, Maisano F, Weder W, et al. Intraluminal EWSR1-CREB1 gene rearranged, low-grade myxoid sarcoma of the pulmonary artery resembling extraskeletal myxoid chondrosarcoma. Histopathology. 2019;74:526–530. doi: 10.1111/his.13773. [DOI] [PubMed] [Google Scholar]
- 11.Agaimy A, Duell T, Morresi-Hauf AT. EWSR1-fusion-negative, SMARCB1-deficient primary pulmonary myxoid sarcoma. Pol J Pathol. 2017;68:261–267. doi: 10.5114/pjp.2017.71535. [DOI] [PubMed] [Google Scholar]
- 12.Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. Lyon: IAPR press; 2016. pp. P129–P131. [DOI] [PubMed] [Google Scholar]
- 13.Balanzá R, Arrangoiz R, Cordera F, Muñoz M, Luque-de-León E, Moreno E, et al. Pulmonary extraskeletal myxoid chondrosarcoma: A case report and literature review. Int J Surg Case Rep. 2016;27:96–101. doi: 10.1016/j.ijscr.2016.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou Q, Lu G, Liu A, Kohno T. Extraskeletal myxoid chondrosarcoma in the lung: asymptomatic lung mass with severe anemia. Diagn Pathol. 2012;7:112. doi: 10.1186/1746-1596-7-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thway K, Fisher C. Tumors with EWSR1-CREB1 and EWSR1-ATF1 fusions: the current status. Am J Surg Pathol. 2012;36:e1–e11. doi: 10.1097/PAS.0b013e31825485c5. [DOI] [PubMed] [Google Scholar]
- 16.Hisaoka M, Hashimoto H. Extraskeletal myxoid chondrosarcoma: updated clinicopathological and molecular genetic characteristics. Pathol Int. 2005;55:453–463. doi: 10.1111/j.1440-1827.2005.01853.x. [DOI] [PubMed] [Google Scholar]
- 17.Thway K, Nicholson AG, Wallace WA, AI-Nafussi A, Pilling J, Fisher C. Endobronchial pulmonary angiomatoid fibrous histiocytoma: two cases with EWSR1-CREB1 and EWSR1-ATF1 fusions. Am J Surg Pathol. 2012;36:883–888. doi: 10.1097/PAS.0b013e31824b1ee0. [DOI] [PubMed] [Google Scholar]
- 18.Leduc C, Zhang L, Öz B, Luo J, Fukuoka J, Antonescu CR, et al. Thoracic myoepithelial tumors: a pathologic and molecular study of 8 cases with review of the literature. Am J Surg Pathol. 2016;40:212–223. doi: 10.1097/PAS.0000000000000560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shilo K, Miettinen M, Travis WD, Timens W, Nogueira R, Franks TJ. Pulmonary microcystic fibromyxoma: report of 3 cases. Am J Surg Pathol. 2006;30:1432–1435. doi: 10.1097/01.pas.0000213279.53338.32. [DOI] [PubMed] [Google Scholar]
- 20.Chen M, Yang J, Zhu L, Zhou C, Zhao H. Primary intrathoracic liposarcoma: a clinicopathologic study and prognostic analysis of 23 cases. J Cardiothorac Surg. 2014;9:119. doi: 10.1186/1749-8090-9-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset supporting the conclusion of this article is included within the article.