Abstract
Background
The Occupational Safety and Health Research Institute (OSHRI) of the Korea had not recognized gastrointestinal cancer as work-related disease during their evaluation. However, in 2018 OSHRI recognized gastric and rectal cancers as work-related disease in asbestos-exposed workers. We present 2 such cases along supportive evidence of causation.
Case presentation
Patient A: A 57-year-old man had worked for about 40 years since 1978 as an oxygen cutter at workplaces that dismantle ships, buildings, boilers, and thermal power plants. In November 2016, endoscopy and biopsy confirmed the diagnosis of advanced gastric cancer, for which he underwent subtotal gastrectomy and chemotherapy; however, he later died of the cancer. Patient B: A 71-year-old man had worked in shipbuilding and repair workplaces for approximately 49 years, being employed in pipe laying, asbestos insulation installation, grinding, and other ship repair work. In 2003, he was diagnosed of rectal cancer by abdominal computed tomography. He accordingly underwent surgical removal of the cancer. Based on the occupational history of the 2 patients and our review of the relevant literature addressing the occupational environment, we concluded that both patients had continuous exposure to high levels of asbestos while performing their jobs for 40 and 49 years, respectively.
Conclusion
Both patients had a history of smoking and drinking (non-occupational personal risk factors). However, the possibility of an increased risk of gastric and rectal cancers from asbestos exposure cannot be excluded. Therefore, we considered that occupational exposure to asbestos had contributed to the cancer diagnosis in these cases. Workers exposed to asbestos should be made aware of the possibility of gastric or rectal cancer, and should undergo monitoring and medical examinations. Appropriate compensation for gastric and rectal cancers that occur in workers exposed to asbestos are anticipated in future.
Keywords: Gastrointestinal cancer, Gastric cancer, Rectal cancer, Asbestos, Work-relatedness
BACKGROUND
The Occupational Safety and Health Research Institute (OSHRI) receives consultation requests from the Korea Workers' Compensation & Welfare Service, the Ministry of Employment and Labor, and other entities regarding the work-related evaluation of workers' disease. OSHRI consultation is required for cases where a certain disease has not been recognized as an occupational disease, or where the determination of work-relatedness is difficult. This particularly applies when there are no precedents recognizing the condition as occupational disease, or when a new case raises a social controversy. When such requests are received, the OSHRI provides scientific consultations on the work-relatedness of a worker's disease. This is done by determining the correlation between physical and chemical hazards, to which a worker has been exposed while performing his or her job and their disease. The investigation is based on the information obtained through interviews with the worker, visits to the workplace, and review of relevant literature. From the work-related evaluation received by the OSHRI from 1992 to 2000, a total of 108 cases were cancer-related, 7 of which were gastrointestinal (GI) cancer; none of the 7 that were submitted were recognized as having occurred in relation to work [1,2]. From 2001–2009, 368 work-related evaluations were performed related to cancer; 25 concerned GI cancer. In 2010 to 2018, 437 such evaluations were performed, 51 of which addressed GI cancer. However, only 2 cases conducted in 2018 were recognized as being work-related. The 2 cases of GI cancer mentioned included gastric and rectal cancer, that occurred in workers exposed to asbestos. These were the first of the GI cancer cases to be recognized by the OSHRI as work-related. These were also the first cases wherein OSHRI would recognize causality between occupational exposure to asbestos and the occurrence of gastric and rectal cancer.
There were 951,600 new cases of gastric cancer and 723,100 deaths worldwide in 2012, establishing it as the fourth and fifth most common type of cancer in men and women, respectively [3]. In the Korea, gastric cancer was the most frequently reported type of cancer in 2016, affecting 20,509 males and 9,995 females [4]. Known predisposing factors to gastric cancer include gastric polyps [5,6], gastric ulcers [7], history of surgical removal of the GI tract, and chronic atrophic gastritis [6]. Non-occupational factors associated with gastric cancer include Helicobacter pylori infection [8], smoking, drinking, obesity, and dietary habits (such as intake of nitrate, smoked food, and/or salted food) [3,6]. Occupational-related factors include industrial exposures to rubber [9], ionizing radiation [10], asbestos [11,12], or lead [13], which are known to be associated with gastric cancer. Colorectal cancer is the third and second most common cancer found in men and women globally [3]. As of 2016, it was the third most common cancer reported by Korean men and women, with men outnumbering women [4]. The causes of colorectal cancer can be divided into non-occupational and occupational factors. Non-occupationally, smoking, drinking, dietary habits [14], inflammatory bowel disease [15], and genetic factors [16] are associated with colorectal cancer. Exposures to ionizing radiation [17,18] and asbestos [19,20] are listed as occupational factors.
In this report, we present the aforementioned 2 cases, one with gastric cancer and the other with rectal cancer, that were each recognized as having occurred in relation to the respective occupational exposures to asbestos. The basis for the recognition has also been presented.
CASE PRESENTATION
Patient A
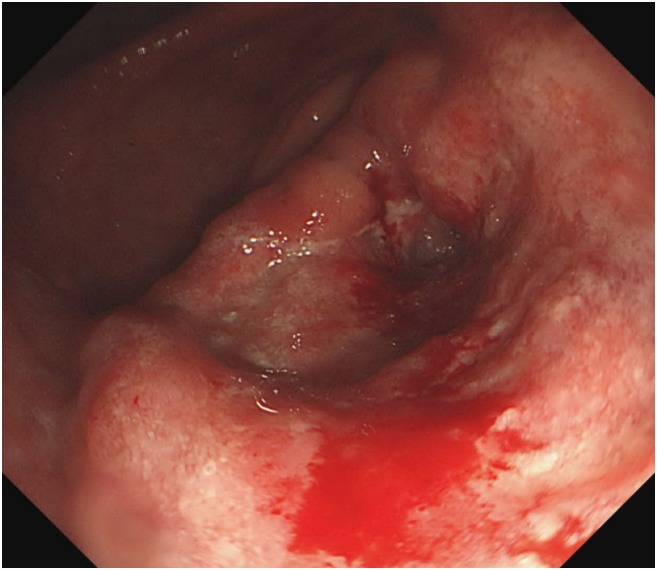
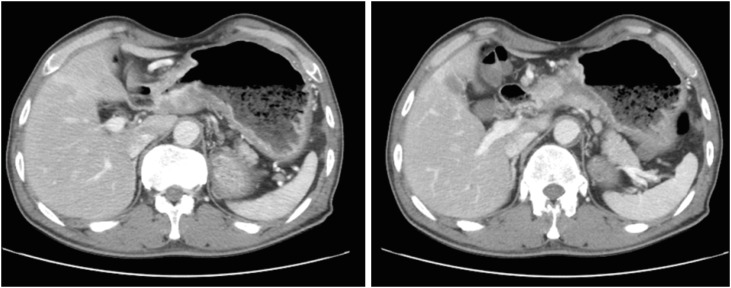
A 57-year-old man was informed regarding a possible diagnosis of gastric cancer at a local clinic on November 15, 2016, based on the results of endoscopic examination previously performed following his complaints of weight loss, abdominal pain, and indigestion. On the same day, the patient visited the department of gastroenterology at a university hospital for endoscopy including biopsy. The tests revealed advanced gastric cancer in the distal antrum (Fig. 1). Abdominal computed tomography (CT) scans subsequently confirmed the presence of cancer and metastasis. The results showed invasion of the entire wall of the antrum with regional lymph node metastasis (Fig. 2). He was diagnosed with stage IIIB gastric cancer (T4N2M0). On December 6, 2016, the patient underwent subtotal gastrectomy with Billroth I anastomosis and cholecystectomy, and was started on chemotherapy. Despite the treatment, he died of gastric cancer on January 9, 2018. Apart from having received treatment for chronic hepatitis B, the patient had never been diagnosed with any disease of the GI tract prior to the gastric cancer diagnosis. During the endoscopy performed on November 15, 2016, he was not tested for H. pylori infection; in addition, he never underwent any tests for H. pylori infection. We were thus unable to determine the presence of H. pylori infection. He had a 1–10 pack-years history of smoking, did not drink, and had no particular relevant family medical history. At the time of his job history survey, the patient was working as a day laborer; his official job history could not therefore be established. We accordingly reviewed his employment insurance records, the National Tax Service (NTS) reports, and his own statements. Based on the combined information, we summarized that the patient had worked as an oxygen cutter at shipbreaking workplaces from 1978 to the 1990s (for approximately 17 years). Furthermore, during the 1990s and 2010s (until 2016), he worked for 7 years at building demolition workplaces as a member of the boiler dismantling crew, and also spent another 14 years in dismantling factories and various thermal power generation plants. Altogether, the patient had presumably worked for about 40 years since 1978 as an oxygen cutter at workplaces that dismantle ships, buildings, boilers, and thermal power plants.
Fig. 1. Patient A: endoscopic findings. Endoscopy showing advanced gastric cancer with distal antrum and near gastric outlet obstruction.
Fig. 2. Patient A: abdominal computed tomography images. Abnormal wall thickening and enhancement in the gastric antrum with regional lymph node enlargement.
Patient B
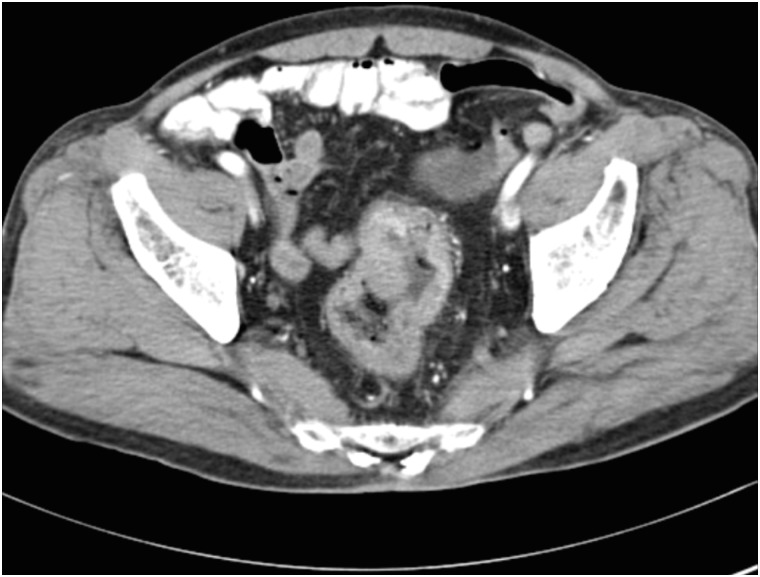
A 71-year-old male patient was diagnosed with rectal cancer on July 31, 2003. The results of his abdominal CT scans performed at a university hospital earlier in the same month demonstrated rectal wall thickening, wall enhancement, and masses protruding into the rectal lumen (Fig. 3). On August 18, 2003, he underwent surgery for the removal of the rectal cancer. His final diagnosis was that of rectal tubular adenocarcinoma, Borrmann type II. The patient had diabetes and hypertension, and was diagnosed with pneumoconiosis in 2004, based on the results of a chest CT scan, which was performed to investigate his breathing difficulty, coughing, and sputum production. Owing to varying statements in his medical records, his smoking history could only be estimated to have ranged between 2.5 and 25 pack-years. He admitted to drinking alcohol 2–3 times per week and had stopped drinking since 2000. There was no family history of cancer. Patient B was also a day laborer; hence we reviewed his employment insurance records and NTS tax reports as well as his own statements, to summarize his job history. From 1954 to 1975 (about 21 years), the patient worked at shipbuilding and repair workplaces. He then worked at ship repair yards for approximately 28 years (1975–2003). While at work, he laid pipes in ships, installed asbestos insulation on pipes and outer walls of frozen storage vessels, performed surface cleaning and preparation for painting (i.e., grinding or abrasive machining and sandblasting), and executed ship interior repairs.
Fig. 3. Patient B: abdominal computed tomography images. The rectal wall exhibits abnormal thickening and enhancement with protruding masses in the rectal lumen.
Exposure assessment
Patient A stated that personal protective equipment (PPE) including masks had not been provided adequately until the 1990s, and masks were provided intermittently from the 2000s. Park et al. [21] reported the continued use of asbestos in the Korean shipbuilding industry throughout the decades preceding 2005; this indicated the possibility of worker exposure to asbestos during the 1970–1990s, when Patient A was engaged in shipbreaking operations. The inadequate supply of PPE during those times had presumably made protection against asbestos infeasible. However, some of the workplaces where patient A had worked were closed, and the work environment had changed in the remaining workplaces where the patient was hired. Furthermore, asbestos exposures in shipbreaking and power plant demolition operations in the Korea asbestos job exposure matrix has not been evaluated [22]. Therefore, we mainly estimated exposure levels of asbestos based on a review of foreign literature. Wu et al. [23] reported their findings on the concentration of asbestos, to which workers were exposed during shipbreaking operations where 4,155 workers had been engaged for a minimum of one year from 1975. Oxygen cutters were found to have been exposed to, 0.196 f/cc of asbestos on an arithmetic average. Burdorf et al. [24] reviewed the literature published between 1970 and 1996, that addressed the types of work that exposed workers to asbestos. They studied the extent to which workers on different jobs were exposed to asbestos. Ship dismantling workers were reportedly exposed to 0.5-–0 f/cc of asbestos. Patient A had worked at building, boiler, and thermal power plant dismantling workplaces from the 1990s to the 2010s. If any of his building demolition workplaces included roofing and interior materials that use asbestos, it is highly likely that he was exposed to asbestos during building demolitions. According to Choi et al. [25], the geometric mean concentration of airborne asbestos released during the hospital building deconstruction in the Korea in 2000 was measured to be 0.053 f/cc (range: 0.002–0.419 f/cc). In 2006, Kim et al. [26] measured air asbestos concentrations in Korean demolition workplaces including shops, apartments, schools, and houses. The researchers detected asbestos concentrations in excess of the occupational exposure limit in 12 of 82 cases where airborne asbestos concentrations were measured. In particular, a study of boiler and power plant dismantling jobs by Burdorf et al. [24] including a job-specific asbestos exposure matrix mentioned that boiler demolition operations had resulted in asbestos exposures at concentrations of 0.5–2 f/cc (from 1986 to 1995); the levels for power plant demolition were 4.8–28 f/cc.
Patient B had also worked in the shipbuilding and repair industries between the 1950s and the 1970s; this preceded the beginning of the restrictions imposed on the use of asbestos in vessels [21]. In addition, he worked at ship repair workplaces between the 1970s and the early part of the 2000s. Therefore, he was presumed to have been exposed to asbestos while working at those places. Addressing asbestos exposure at ship repairing workplaces, Paek et al. [27] measured the levels of asbestos exposure in Korean ship repair operations in 1994. Their investigation confirmed the use of asbestos in the piping of ships at the time. The concentrations of asbestos in ambient air during the insulation and pipe repair operations were measured to be 6.3–7.8 f/cc. Yoon et al. [28] reported a case of lung cancer in Korea that occurred in a worker at a ship repair workplace. The level of workplace asbestos exposure at the time of measurement was reported to be 1.423 f/cc in 2003. In foreign literature, Burdorf et al. [24] estimated that workers at shipbuilding workplaces during the 1950s to 1970s had possibly been exposed to at least 5 f/cc of asbestos. Williams et al. [29] investigated the levels of asbestos exposure at workplaces that used related products between 1940 and 2006. According to their findings, the majority of operations performed at asbestos-involving ship repair workplaces provided asbestos exposures of 10 f/cc or above.
Combining the results of the studies on the levels of asbestos exposure that had either presumably or factually taken place at the ship dismantling workplaces, it was assumed that patient A was likely to have been exposed to a high level of asbestos while being engaged in shipbreaking operations. Although we found no data regarding measured asbestos exposures at Korean shipbreaking workplaces, we found that high levels of asbestos exposure were measured at ship repairing workplaces, where some of the same operations took place as in the shipbreaking industry. Therefore, ship dismantling workplaces in the Republic of Korea probably offered a high level of exposure to asbestos. Studies investigating the levels of asbestos exposure during building, boiler, and power plant demolitions mention the occurrence of high levels of asbestos exposure during the performance of the aforementioned jobs. However, since work environments at domestic and foreign workplaces differ, it is difficult to simply apply the levels of exposure mentioned in foreign sources to domestic workplaces. Moreover, the Korean sources that we investigated did not offer sufficient data on the measured levels of asbestos exposure in the country. We were thus unable to quantify the total cumulative exposure to asbestos. Despite the limitations, considering the results from literature and patient A's job history, we speculated that he was exposed to high levels of asbestos during the 40 years of work.
According to the studies that reported on work environment evaluation, the shipbuilding and repair jobs performed by patient B also conferred high levels of asbestos exposure. Data on asbestos exposure levels at ship repair workplaces in Korea are relatively recent; therefore, there are limitations in applying the data to the entire period of patient B's involvement in the operations and the jobs he had held. However, considering the results of both, Korean and foreign studies, we concluded that patient B was also exposed to high levels of asbestos over the period of 49 years at the shipbuilding and repair workplaces. Considering the respective duration of both patients' employment at the workplaces and the types of jobs they had, we speculate that the levels of cumulative asbestos exposure were very likely to exceed 25 f/cc-years. Reports suggest that according to the Helsinki criteria [30], the relative risk (RR) of lung cancer had doubled, and the RR of gastric and rectal cancers increased to 1.2 and 1.1, respectively.
Ethics statement
The Occupational Safety and Health Research Institute institutional review board (IRB) approved this study protocol (IRB No. 2019-IRB-10). Written informed consent was obtained from the patient for publication of this case report and any accompanying data.
DISCUSSION AND CONCLUSION
The International Agency for Research on Cancer (IARC) reported that there is limited evidence on the association between gastric cancer and asbestos exposure [31]. The Institute of Medicine (IOM) investigated the association between asbestos exposure and gastric cancer occurrence by reviewing 42 cohort studies and 5 case-control studies. In the cohort studies, the RR of gastric cancer was shown to have significantly increased to 1.17 (95% confidence interval [CI]: 1.07–1.28) [32]. Notably, some of the results of the cohort studies presented to the IOM showed dose-response relationships. For instance, the study by Selikoff et al. [12] mentioned that exposure to asbestos for 20–35 years would lead to a standardized mortality ratio (SMR) of 4.00 (95% CI: 1.47–8.71) for gastric cancer, and exposures of 35 years or longer would result an SMR of 3.42 (95% CI: 1.82–5.85); both indicate a significant increase in the SMR. In the cohort study by Liddell et al. [11] that investigated the carcinogenic risks of asbestos in 10,918 mine and mill workers who were exposed to asbestos in Quebec, Canada, the SMR of gastric cancer was 1.24 (95% CI: 1.07–1.48) and the increase in the level of cumulative asbestos exposure showed a positive correlation with the SMR of gastric cancer. However, the 5 case-control studies did not show a significant increase in the risks of gastric cancer from exposure to asbestos, with a RR of 1.11 (95% CI: 0.76–1.64). Hence, the IOM stated that there was suggestive, but not sufficient evidence of the association between asbestos exposure and the occurrence of gastric cancer [32]. After the IOM and the IARC studies, study reports have indicated significant association between asbestos exposure and gastric cancer occurrence. Lin et al. [33] conducted cohort studies for 26 years targeting 1,539 chrysotile miners. They found a dose-response relation between asbestos exposure and gastric cancer occurrence, with a hazard ratio (HR) of gastric cancer occurrence per 10 mg·years/m3 of 1.011 (95% CI: 1.010–1.013). According to the meta-analyses conducted by Sun et al. [34] using 69 cohort studies, by Fortunato et al. [35] using 40 cohort studies, and by Peng et al. [36] using 32 cohort studies, the SMR of gastric cancer was found to be 1.20 (p<0.01), 1.15 (95% CI: 1.03–1.27), and 1.19 (95% CI: 1.06–1.34), respectively. Although no significant increases were found in the risk of gastric cancer in our review of case-control studies, we considered that the results of the cohort studies and meta-analyses consistently showed significant increases in the risk of gastric cancer. We therefore recognized that exposure to high concentrations of asbestos for extended periods of time were highly likely to lead to increased risks of gastric cancer. Therefore, in the case of patient A with a career span of 40 years and presumed exposures to highly concentrated asbestos during the period of his employment, we concluded that his occupational exposure to asbestos had possibly increased the risk of gastric cancer occurrence. Although patient A had a history of 1–10 pack-years of smoking (a non-occupational risk factor for gastric cancer), and his H. pylori infection status was not available for verification, we concluded that his occupational asbestos exposure had contributed to the occurrence of gastric cancer.
The IARC stated that there was limited evidence to support the association between asbestos exposure and colorectal cancer occurrence [31]. According to the IOM, 41 cohort studies and 13 case-control studies were reviewed, and the cohort studies found a significant increase in the risk of colorectal cancer with asbestos exposure; RR = 1.15 (95% CI: 1.02–1.31) [32]. Some of the cohort studies presented to the IOM reported that workers who had worked for longer periods of time (i.e., a minimum of 20 years), and/or had been exposed to high asbestos concentrations (above 40 f/cc-year) demonstrated a significant increase in the incidence of colorectal cancer compared with their peers with shorter periods of exposure and/or lower levels of cumulative asbestos exposure [12,37]. However, 13 case-control studies reported a RR of 1.16 (95% CI: 0.90–1.49), which did not indicate a significant increase in the risks of colorectal cancer. Combining the results of the studies, the IOM concluded that exposure to asbestos offers suggestive, but not sufficient evidence towards the occurrence of rectal cancer [32]. Among the more recent studies on the association between asbestos exposure and rectal cancer occurrence, Offermans et al. [38] conducted cohort studies with 58,279 male workers. They reported a significant increase in the risk of rectal cancer, i.e., HR: 2.15 (95% CI: 1.23–3.77), when the workers were exposed to high levels of asbestos equivalent to score 4 of the job-exposure matrix from the Netherlands [39]. Paris et al. [20] observed 14,515 male workers with occupational exposure to asbestos by following up them for 10.2 years. According to their findings, the HR of rectal cancer increased 4.57-fold (95% CI: 1.14–18.27) after 20–40 years from the time of first exposure to asbestos. We noted consistency in the results of the cohort studies and considered an association between the patient's long-term exposure to highly concentrated asbestos and his diagnosis of rectal cancer. Therefore, considering that patient B had a 49-year career span, with exposure to high levels of asbestos at the workplaces, we concluded that his occupational exposure to asbestos had increased the risk of his rectal cancer. Patient B also had history of smoking and drinking, i.e., non-occupational personal risk factors for rectal cancer. Nevertheless, as in the case of patient A, we recognized that occupational asbestos exposure had contributed to the diagnosis of rectal cancer in this case.
According to the work-relatedness evaluations regarding GI cancer conducted in the 2000s and the 2010s, no case has been recognized for an association between work and GI cancer except for the above 2 cases. Our review of the 60 reports currently available, which were not recognized for work-relatedness showed that in 49 of the 60 cases, workers were not exposed to the risk factors known to cause GI cancer while performing their jobs. In 8 cases, the workers were exposed to ionizing radiation and/or other known carcinogens such as inorganic lead (in the case of gastric cancer); however, the levels of carcinogen exposure were extremely low in our work environment evaluations. Furthermore, 40 cases (i.e., more than 50% of the mentioned 57 cases) had non-occupational personal risk factors such as smoking, drinking, personal dietary habits, and infections.
In the remaining 3 cases, workers were exposed to occupational risk factors associated with GI cancer; however, the association was not recognized owing to the presence of non-occupational personal risk factors for GI cancer. The first of these cases was hepatocellular carcinoma in a worker who had worked for 22 years in the manufacture of polyvinyl chloride products. During the work, he was exposed to vinyl chloride, which may cause hepatocellular carcinoma [9,40]; however, this was not recognized as work-related cancer as he had chronic hepatitis B, a non-occupational personal risk factor for hepatocellular carcinoma. The second case was that of rectal cancer in a worker who may have been exposed to asbestos while handling asbestos gaskets in an automobile factory for approximately 6 years. However, the rectal cancer was not recognized as work-related owing to his 0.5 pack-year smoking history and a history of drinking one bottle of Soju (alcohol) per week; these are non-occupational personal risk factors. The third case was gastric cancer in a worker of an asbestos textile factory who was employed for 10 years. The worker had cumulative asbestos exposures of 40 f/cc-years or more during the period, and was diagnosed with asbestosis with pleural plaques and calcification on a chest CT scan. However, the gastric cancer was not recognized as work-related owing to a 23-pack-years smoking history (not smoking 20 years) and a diagnosis of H. pylori infection on the CLO test at the time of gastric cancer evaluation. In these 3 cases, workers were exposed to occupational risk factors; however, their cancers were not usually recognized as related to work based on the evidence that non-occupational personal risk factors have a greater impact on GI cancer incidence.
However, recent work-related evaluations indicated that patients A and B have had high levels of asbestos exposure for prolonged periods; epidemiological studies have also indicated that asbestos can significantly increase the risk of gastric and rectal cancer. Although patients A and B had non-occupational personal risk factors such as smoking and drinking, that are extremely common among Koreans [41,42], the possibility of an increased risk of gastric and rectal cancer owing to asbestos exposure cannot be excluded. Although no experimental studies have clearly explained the mechanisms for the development of gastric and rectal cancers from asbestos exposure [31], epidemiological studies have consistently demonstrated an increased risk of gastric and rectal cancer in workers with prolonged exposure to highly concentrated asbestos. Based on the evidence, we estimated that the levels of asbestos exposure that our patients were subjected to while performing their jobs, contributed to their respective diagnoses of gastric and rectal cancers. Recent reports suggest that work-relatedness should be assessed based on epidemiological studies when determining the causal relationship for occupational diseases. Myong et al. [43] described a case where although the worker exposed to asbestos was diagnosed with lung cancer and had a history of smoking, his illness was recognized as an occupational disease based on findings of epidemiological studies; they suggested the need to conduct work-related evaluation based on epidemiological studies.
Asbestos does not break down easily once it enters the human system; it is also not easily released from the body. It stays in the body for a long time, continuously exerting toxic effects. Hence, the latent period of asbestos-related diseases may extend to 40 years [44]; this implies that the effects of occupational asbestos exposure on the health of workers (including gastric and rectal cancers) are likely to continue. The findings suggest that workers on jobs that expose them to asbestos need to monitor their health aggressively, and undergo medical examinations. In addition, when patients with gastric or rectal cancer have had environmental and occupational asbestos exposures, the attending occupational and environmental physicians should actively consider the possibility that the patients' disease may have occurred in relation to their work. In the Korea, workers whose health has been affected by occupational asbestos exposure are eligible for compensation in accordance with the Enforcement Decree of the Industrial Accident Compensation Insurance Act. However, at the present moment, the aforementioned compensation for cancer cases is only provided for lung cancer, laryngeal cancer, malignant mesothelioma, and ovarian cancer [45]. The 2 cases presented herein are anticipated to serve as the index cases for expanding the scope of compensation to include gastric and rectal cancer cases associated with asbestos exposure.
Acknowledgements
The present study utilized worker's data, which were formally obtained from the Occupational Safety and Health Research Institute (OSHRI), Korean Occupational Safety and Health Agency (KOSHA), and did not include identifiable personal information.
Abbreviations
- CI
confidence interval
- CT
computed tomography
- GI
gastrointestinal
- H. pylori
Helicobacter pylori
- HR
hazard ratio
- IARC
International Agency for Research on Cancer
- IOM
Institute of Medicine
- NTS
National Tax Service
- OSHRI
Occupational Safety and Health Research Institute
- PPE
personal protective equipment
- RR
relative risk
- SMR
standardized mortality ratio
Footnotes
Funding: This study was funded by the Occupational Safety and Health Research Institute (OSHRI), and Korean Occupational Safety and Health Agency (KOSHA).
Competing interests: The authors declare that they have no competing interests.
Availability of data and materials: All data generated or analyzed during this study are included in this published article.
- Conceptualization: Choi BJ, Lee S2.
- Formal analysis: Choi BJ, Lee S1, Lee S2.
- Investigation: Choi BJ, Lee IJ, Lee S2.
- Visualization: Choi BJ, Park SW.
- Writing - original draft: Choi BJ, Lee S2.
- Writing - review & editing: Choi BJ, Lee S2.
Lee S1, Saerom Lee; Lee S2, Sanggil Lee.
References
- 1.Kang SK, Kim KS, Kim Y, Choi JK, Ahn YS, Jin YW, et al. Analysis of claimed cases as an occupational disease at Korea Occupational Safety and Health Agency from 1992 to 1999. Korean J Occup Environ Med. 2000;12(2):292–301. [Google Scholar]
- 2.Kang SK, Ahn YS, Chung HK. Occupational cancer in Korea in the 1990s. Korean J Occup Environ Med. 2001;13(4):351–359. [Google Scholar]
- 3.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 4.National Cancer Center. 2019. [Accessed 27 Dec 2019]. http://ncc.re.kr/
- 5.Antonioli DA. Precursors of gastric carcinoma: a critical review with a brief description of early (curable) gastric cancer. Hum Pathol. 1994;25(10):994–1005. doi: 10.1016/0046-8177(94)90058-2. [DOI] [PubMed] [Google Scholar]
- 6.Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12(3):354–362. doi: 10.3748/wjg.v12.i3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hansson LE, Nyrén O, Hsing AW, Bergström R, Josefsson S, Chow WH, et al. The risk of stomach cancer in patients with gastric or duodenal ulcer disease. N Engl J Med. 1996;335(4):242–249. doi: 10.1056/NEJM199607253350404. [DOI] [PubMed] [Google Scholar]
- 8.Sepulveda AR. Helicobacter, inflammation, and gastric cancer. Curr Pathobiol Rep. 2013;1(1):9–18. doi: 10.1007/s40139-013-0009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.International Agency for Research on Cancer. Chemical agents and related occupations: IARC monographs on the evaluation of carcinogenic risks to humans, volume 100F. Lyon: International Agency for Research on Cancer; 2012. [PMC free article] [PubMed] [Google Scholar]
- 10.Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev. 2014;23(5):700–713. doi: 10.1158/1055-9965.EPI-13-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liddell FD, McDonald AD, McDonald JC. The 1891–1920 birth cohort of Quebec chrysotile miners and millers: development from 1904 and mortality to 1992. Ann Occup Hyg. 1997;41(1):13–36. doi: 10.1016/S0003-4878(96)00044-0. [DOI] [PubMed] [Google Scholar]
- 12.Selikoff IJ, Hammond EC, Seidman H. Mortality experience of insulation workers in the United States and Canada, 1943--1976. Ann N Y Acad Sci. 1979;330:91–116. doi: 10.1111/j.1749-6632.1979.tb18711.x. [DOI] [PubMed] [Google Scholar]
- 13.Steenland K, Boffetta P. Lead and cancer in humans: where are we now? Am J Ind Med. 2000;38(3):295–299. doi: 10.1002/1097-0274(200009)38:3<295::aid-ajim8>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 14.Watson AJ, Collins PD. Colon cancer: a civilization disorder. Dig Dis. 2011;29(2):222–228. doi: 10.1159/000323926. [DOI] [PubMed] [Google Scholar]
- 15.Bernstein CN, Blanchard JF, Kliewer E, Wajda A. Cancer risk in patients with inflammatory bowel disease: a population-based study. Cancer. 2001;91(4):854–862. doi: 10.1002/1097-0142(20010215)91:4<854::aid-cncr1073>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 16.Tiwari AK, Roy HK, Lynch HT. Lynch syndrome in the 21st century: clinical perspectives. QJM. 2016;109(3):151–158. doi: 10.1093/qjmed/hcv137. [DOI] [PubMed] [Google Scholar]
- 17.Preston DL, Ron E, Tokuoka S, Funamoto S, Nishi N, Soda M, et al. Solid cancer incidence in atomic bomb survivors: 1958–1998. Radiat Res. 2007;168(1):1–64. doi: 10.1667/RR0763.1. [DOI] [PubMed] [Google Scholar]
- 18.Sugiyama H, Misumi M, Brenner A, Grant EJ, Sakata R, Sadakane A, et al. Radiation risk of incident colorectal cancer by anatomical site among atomic bomb survivors: 1958–2009. Int J Cancer. 2020;146(3):635–645. doi: 10.1002/ijc.32275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berry G, Newhouse ML, Wagner JC. Mortality from all cancers of asbestos factory workers in east London 1933–80. Occup Environ Med. 2000;57(11):782–785. doi: 10.1136/oem.57.11.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paris C, Thaon I, Hérin F, Clin B, Lacourt A, Luc A, et al. Occupational asbestos exposure and incidence of colon and rectal cancers in French men: the Asbestos-Related Diseases Cohort (ARDCo-Nut) Environ Health Perspect. 2017;125(3):409–415. doi: 10.1289/EHP153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park SH, Chung EK, Kwon JW, Kim KB, Chung KJ, Yi GY, et al. A study on the status of asbestos use on ships. J Korean Soc Occup Environ Hyg. 2011;21(3):123–127. [Google Scholar]
- 22.Choi S, Kang D, Park D, Lee H, Choi B. Developing asbestos job exposure matrix using occupation and industry specific exposure data (1984–2008) in Republic of Korea. Saf Health Work. 2017;8(1):105–115. doi: 10.1016/j.shaw.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu WT, Lin YJ, Shiue HS, Li CY, Tsai PJ, Yang CY, et al. Cancer incidence of Taiwanese shipbreaking workers who have been potentially exposed to asbestos. Environ Res. 2014;132:370–378. doi: 10.1016/j.envres.2014.04.026. [DOI] [PubMed] [Google Scholar]
- 24.Burdorf A, Swuste P. An expert system for the evaluation of historical asbestos exposure as diagnostic criterion in asbestos-related diseases. Ann Occup Hyg. 1999;43(1):57–66. [PubMed] [Google Scholar]
- 25.Choi CG, Kim CN, Lim NG, Roh YM, Roh JH. Exposure level of releasing asbestos during building destruction work. J Korean Soc Occup Environ Hyg. 2002;12(3):195–201. [Google Scholar]
- 26.Kim JY, Lee SK, Lee JH, Lim MH, Kang SW, Phee YG. A study on the factors affecting asbestos exposure level from asbestos abatement in building demolition sites. Korean Ind Hyg Assoc J. 2009;19(1):8–15. [Google Scholar]
- 27.Paek DM, Paik NW, Choi JD, Son MA, Im JG, Lee WJ, et al. Prevalence of asbestosis in Korean asbestos industry. Korean J Occup Environ Med. 1995;7(1):46–57. [Google Scholar]
- 28.Yoon DY, Kang JW, Lee HJ, Kim JI, Son JE, Jung KY, et al. A case of lung cancer caused by long-term asbestos exposure. Korean J Occup Environ Med. 2004;16(4):499–507. [Google Scholar]
- 29.Williams PR, Phelka AD, Paustenbach DJ. A review of historical exposures to asbestos among skilled craftsmen (1940–2006) J Toxicol Environ Health B Crit Rev. 2007;10(5):319–377. doi: 10.1080/10937400601034191. [DOI] [PubMed] [Google Scholar]
- 30.Wolff H, Vehmas T, Oksa P, Rantanen J, Vainio H. Asbestos, asbestosis, and cancer, the Helsinki criteria for diagnosis and attribution 2014: recommendations. Scand J Work Environ Health. 2015;41(1):5–15. doi: 10.5271/sjweh.3462. [DOI] [PubMed] [Google Scholar]
- 31.International Agency for Research on Cancer. Arsenic, metals, fibres, and dusts: IARC monographs on the evaluation of carcinogenic risks to humans, volume 100C. Lyon: International Agency for Research on Cancer; 2011. [Google Scholar]
- 32.Institute of Medicine (US) Committee on Asbestos: Selected Health Effects. Asbestos: selected cancers. Washington, D.C.: National Academies Press (US); 2006. [PubMed] [Google Scholar]
- 33.Lin S, Wang X, Yano E, Yu I, Lan Y, Courtice MN, et al. Exposure to chrysotile mining dust and digestive cancer mortality in a Chinese miner/miller cohort. Occup Environ Med. 2014;71(5):323–328. doi: 10.1136/oemed-2013-101360. [DOI] [PubMed] [Google Scholar]
- 34.Sun TD, Chen JE, Zhang XJ, Li XY. Cohort studies on cancer mortality of digestive system among workers exposed to asbestos: a meta-analysis. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2008;26(10):605–608. [PubMed] [Google Scholar]
- 35.Fortunato L, Rushton L. Stomach cancer and occupational exposure to asbestos: a meta-analysis of occupational cohort studies. Br J Cancer. 2015;112(11):1805–1815. doi: 10.1038/bjc.2014.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peng WJ, Jia XJ, Wei BG, Yang LS, Yu Y, Zhang L. Stomach cancer mortality among workers exposed to asbestos: a meta-analysis. J Cancer Res Clin Oncol. 2015;141(7):1141–1149. doi: 10.1007/s00432-014-1791-3. [DOI] [PubMed] [Google Scholar]
- 37.Albin M, Jakobsson K, Attewell R, Johansson L, Welinder H. Mortality and cancer morbidity in cohorts of asbestos cement workers and referents. Br J Ind Med. 1990;47(9):602–610. doi: 10.1136/oem.47.9.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Offermans NS, Vermeulen R, Burdorf A, Goldbohm RA, Keszei AP, Peters S, et al. Occupational asbestos exposure and risk of esophageal, gastric and colorectal cancer in the prospective Netherlands Cohort Study. Int J Cancer. 2014;135(8):1970–1977. doi: 10.1002/ijc.28817. [DOI] [PubMed] [Google Scholar]
- 39.Offermans NS, Vermeulen R, Burdorf A, Peters S, Goldbohm RA, Koeman T, et al. Comparison of expert and job-exposure matrix-based retrospective exposure assessment of occupational carcinogens in The Netherlands Cohort Study. Occup Environ Med. 2012;69(10):745–751. doi: 10.1136/oemed-2011-100556. [DOI] [PubMed] [Google Scholar]
- 40.International Agency for Research on Cancer. 1,3-butadiene, ethylene oxide and vinyl halides (vinyl fluoride, vinyl chloride and vinyl bromide): IARC monographs on the evaluation of carcinogenic risks to humans, volume 97. Lyon: International Agency for Research on Cancer; 2008. [PMC free article] [PubMed] [Google Scholar]
- 41.Choi S, Kim Y, Park S, Lee J, Oh K. Trends in cigarette smoking among adolescents and adults in South Korea. Epidemiol Health. 2014;36:e2014023. doi: 10.4178/epih/e2014023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ryu SY, Crespi CM, Maxwell AE. Drinking patterns among Korean adults: results of the 2009 Korean community health survey. J Prev Med Public Health. 2013;46(4):183–191. doi: 10.3961/jpmph.2013.46.4.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Myong JP, Kim H, Lee K, Chang SH. Trends in the of epidemiological perspectives on the causality of occupational diseases. J Korean Med Assoc. 2018;61(8):466–473. [Google Scholar]
- 44.Burdorf A, Dahhan M, Swuste P. Occupational characteristics of cases with asbestos-related diseases in The Netherlands. Ann Occup Hyg. 2003;47(6):485–492. doi: 10.1093/annhyg/meg062. [DOI] [PubMed] [Google Scholar]
- 45.Enforcement Decree of the Industrial Accident Compensation Insurance Act. Presidential Decree No. 29950. 2019. [Google Scholar]