Abstract
Retinal diseases, such as age-related macular degeneration (AMD), are the leading cause of blindness in the elderly population. Since no known cures are currently present, it is crucial to diagnose the condition in its early stages so that disease progression is monitored. Recent advances show that the mechanical elasticity of the posterior eye changes with the onset of AMD. In this work, we present a quantitative method of mapping the mechanical elasticity of the posterior eye using confocal shear wave acoustic radiation force optical coherence elastography (SW-ARF-OCE). This technique has been developed and validated with both an ex-vivo porcine tissue model and a customized in-vivo rabbit model, which both showed the quantified elasticity variations between different layers. This study verifies the feasibility of using this technology for the quantification and diagnosis of retinal diseases from the in-vivo posterior eye.
Keywords: shear wave elastography, ARF, OCT, retina, in-vivo study
I. Introduction
Age-related macular degeneration (AMD) is a progressive disease of the retina and is the leading cause of severe vision loss in the western population over 50 years of age [1]. AMD most often induces drusen formation in the dry form, and neovascularization in wet AMD. Drusen is present in more than half of the population over 70 years of age and often acts as an early sign of AMD [2]. Neovascularization occurs when excess blood vessels leak into the layers of the retina, signifying a later stage of disease. Although age is the primary factor that contributes to AMD, there are a number of environmental and genetic factors that are also associated and contribute to the onset and progression of AMD, including smoking, obesity, sunlight exposure, etc. [3].
Current methods of diagnosis include fundus photography, which provides surface structural information of the retina, and fluorescein angiography, which is used to visualize blood vessels and leakages if neovascularization is suspected [4]. For more accurate visualization of the entire depth of the retinal layers, optical coherence tomography (OCT) is used for non-invasive optical imaging [5]. OCT angiography is also used to produce the en face images of retinal blood vessels and diagnose abnormal regions. Once AMD is diagnosed, there is currently no known cure so disease management is necessary in various forms, including antiangiogenic drugs, radiation and laser treatments, and photodynamic therapy [6]. Since it is important to slow down the progression of the disease through treatment, early diagnosis is essential. Although it is possible to see anatomical changes that occur with drusen formation and neovascularization. It is very difficult to diagnose in the early stages, before structural changes are evident.
Recent studies show that the mechanical properties of the posterior eye also change with the onset of disease, such as in the case of angiogenesis or retinal degeneration [7–8]. In particular, the mechanical stress on the retina gets altered during the early stages when drusen deposits begin to form and also later when blood vessels infiltrate. Since it is often difficult to visualize drusen when the deposits are on the micron level, it would be helpful to use an alternate means of diagnosis [9]. In addition, since the layers of the posterior eye are made of a tight network of cells and tissues, the elasticity of different layers is expected to differ [8, 10].
Several methods for in-vivo measurements of mechanical properties of the eye have been reported [8, 10–11]. Elastography methods based on magnetic resonance imaging, ultrasound, and optical coherence tomography, have been widely used in determining the elasticity of tissues [12–19]. Optical coherence elastography (OCE) has the advantage in ocular imaging due to its high resolution (<10 um) and the transparency of ocular tissues [20–23]. However, few investigations of the mechanical properties of the posterior eye have been reported. Ex-vivo retinal elasticity has been studied previously, where elasticity of only two different retinal layers were visualized and quantified based on shear wave OCE using a phased array transducer as excitation [24]. However, their ultrasound excitation and OCT detection are on the opposite side, and this configuration is not feasible for in-vivo animal studies where the posterior side is not accessible. Therefore, we propose this confocal shear wave acoustic radiation force optical coherence elastography (SW-ARF-OCE) system, where an ultrasound ring transducer and optical scan head are co-aligned to facilitate in-vivo study of the retinal elasticity. Although in-vivo corneal elastography studies have been performed, due to the difficulty in penetrating to the posterior globe through the anterior eye and vitreous combined with the need for high sensitivity and resolution, in-vivo measurements of the retinal stiffness have yet to be studied.
In this study, we report on the development of a shear wave ARF-OCE system that enables in-vivo imaging of the mechanical properties of the retina. To the best of our knowledge, we demonstrate the first in-vivo elasticity mapping of the retina. A rabbit model was measured, and the elasticities of the different retinal layers were identified in increasing stiffness from the ganglion side to the photoreceptor portions.
II. Materials & Methods
A. System setup
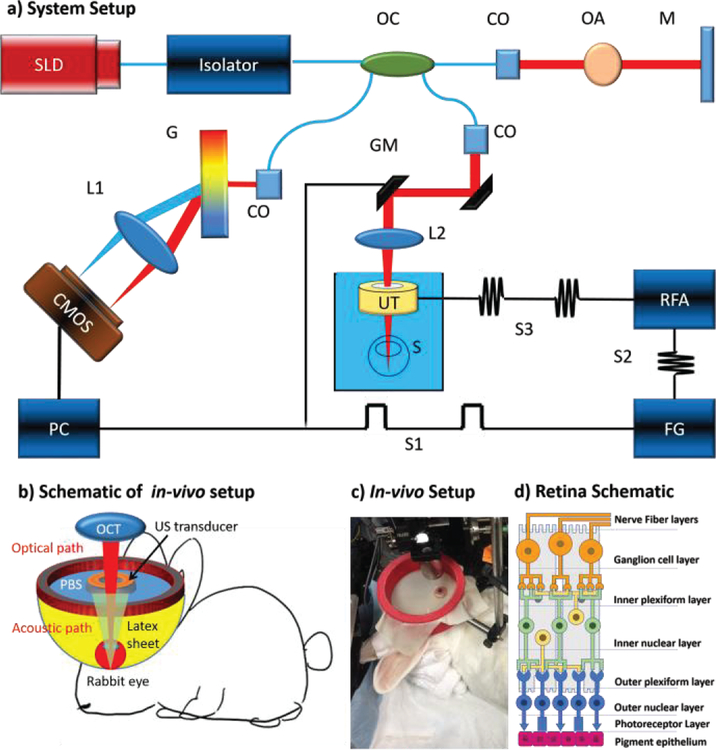
A customized 50 kHz spectral domain optical coherence tomography (SD-OCT) system with a central wavelength of 890 nm and bandwidth of 144 nm is used for the detection of tissue structure and response to stimulation. The imaging depth range is 2.9 mm while the penetration depth in tissue is approximately 1.5 mm. The light emitted from the superluminescent diode is filtered through the optical isolator and split with an optical coupler. For the safety purpose of in-vivo retinal imaging, 20% of the light is transmitted to the sample, which is well within the ANSI safety limits, and 80% is redirected to a reference mirror. Glass imaging windows are placed in the stationary reference arm for dispersion compensation. In the sample arm, galvo mirrors are used for B-M mode scanning, and a scan lens is used with a focal length of 54 mm to penetrate through the ring transducer and into the posterior eye globe. The scattering signal from the sample arm is coupled together with the reflected reference arm signal and sent to the detector arm. The interference signal is separated by wavelength with a diffraction grating and focused onto a line scan CMOS camera. The signal is processed and transformed into depth-resolved intensity and phase information.
A 4.5 MHz ring ultrasound transducer was used for pulsed tissue excitation. The excitation duration was limited to 1–2 ms while the optical detection speed was 50 kHz. The optical setup and the ex-vivo sample setup are shown in figure 1a, where a phosphate buffered saline (PBS) is used as the medium for ultrasound propagation as well as preservation of ocular tissue. For in-vivo experiments, the rabbit eye is proptosed within a rubber drape that can serve as a container to immerse the eye in PBS fluid as shown in figure 1c. The ultrasound transducer was removed in figure 1c in order to visualize the ocular proptosis of the rabbit. The drape system imitates the steridrapes that are used in clinical ultrasound. PBS is then added to the draped construct once again to serve as the medium for propagation as well as for lubrication of the rabbit eye. A detail description of this drape system can be illustrated in figure 1b.
Figure 1.
System set up. a) SW-ARF-OCE system schematic with ex-vivo sample. SLD: superluminescent diode, OC: optical coupler, CO: collimator, OA: optical attenuator, M: mirror, GM: galvanometer mirrors, L1/L2: lens, UT: ultrasound transducer, S: sample, RFA: radiofrequency amplifier, FG: function generator, G: grating, S1: baseband signal, S2: modulated signal, S3: amplified modulated signal. b) Schematic of in-vivo experimental setup. c) Photograph of in-vivo rabbit experimental setup. d) Schematic of retina layered anatomy.
B. System synchronization
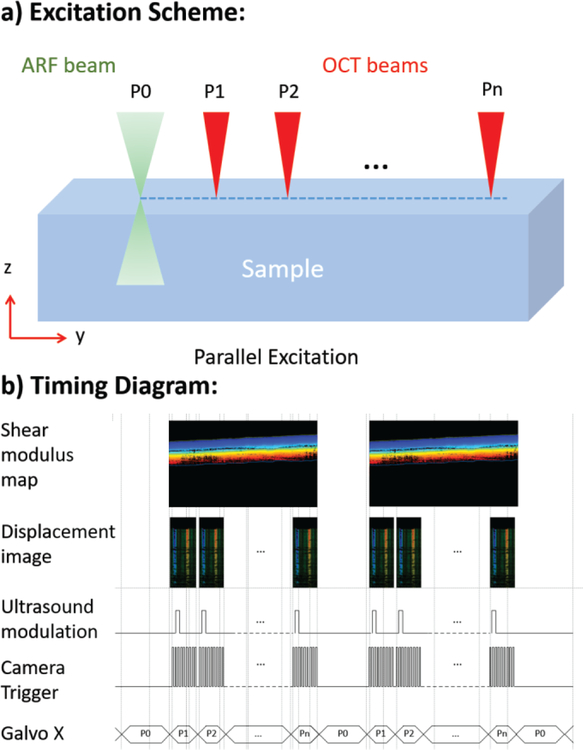
For the shear wave excitation, a baseband signal (S1) is given by the PC to the function generator, which converts it into a sinusoidal modulated pulse signal with 1–2 msec duration. This pulse is amplified by approximately 42dB and fed to the ultrasound transducer. A ultrasound generated pressure is applied onto the sample, initiating the propagation of the shear wave from the focal region to the peripheral areas. The detection scanning scheme is shown in figure 2a, where the ultrasound excitation beam is given at location P0, and B-M mode detection occurs along the lateral direction from P1 to Pn. At each location, an excitation pulse of 1–2 ms is given and a series of 400 A-lines, which corresponds to 8.8 ms, is obtained in M mode before the galvanometer moves to the next location. The number of A-lines is chosen such that the entire duration and progression of the shear wave can be captured.
Figure 2.
a) Scanning scheme of SW-ARF-OCE system. b) Timing diagram.
In order to achieve efficient and effective imaging, the entire excitation and detection process must be synchronized. The timing diagram is shown in figure 2b. To obtain a B-scan showing the full lateral scanning area, an ultrasound modulation pulse is given for every 400 A-lines while the camera DAQ trigger is given for each A-line to capture the intensity and phase information at each location for 8.8 ms total, in increments of 22 us. This synchronization generates 400 B-Scan images with lateral dimension of 600 um in a total of approximately 3.5 s. After detection is completed, the galvanometer moves to the next location in increments of 1.5 um, which is well within the lateral resolution of the optical system. The M-mode displacement image is obtained at every B-scan location, and the phase-resolved displacement is post-processed to obtain the shear modulus map. SW B-scan images are generated by reslicing the M-scan images, and therefore all the B-Scan images are generated at the same time after the imaging is completed.
C. Imaging processing
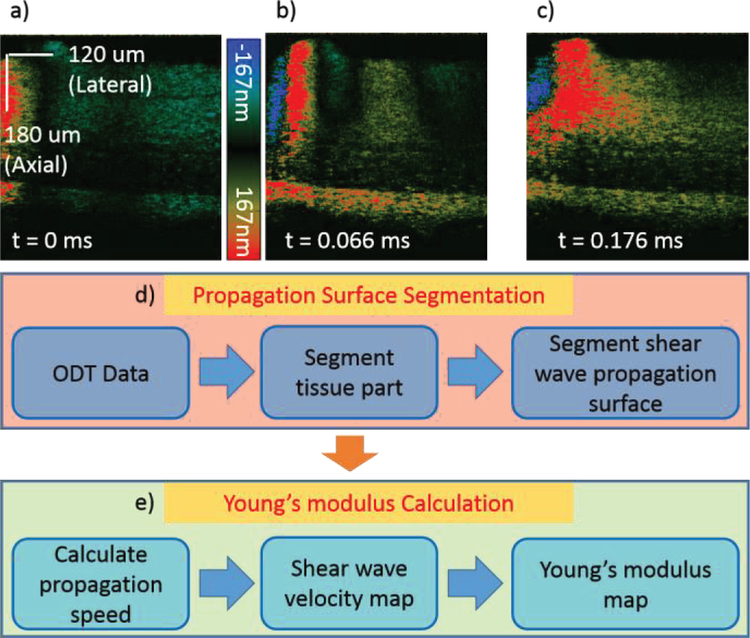
Within the 400 A-lines in M-mode at each location, the entire shear wave propagation through that point can be captured. In figure 3a–c, a sample raw data for the porcine retina is shown where it is apparent that different locations on the retina correspond to different propagation speeds. Note that only 330 out of 400 A-lines were shown in the figure below for visualization purpose. The transducer focal area is on the left side of the images, and propagation is to the right. For example, the bottom most layer of the retina propagates the fastest and corresponds to a stiffer tissue component. It is also important to note that the ultrasound wave intensity decreases at regions of stiffer tissue, and the intensity of the displacement is not considered in the velocity calculations.
Figure 3.
a–c) Cross sectional raw data showing wave propagation of retinal layers at different time points for an ex-vivo pig retina. Shear wave induced axial displacement is shown by different colors corresponding to the color bar. d–e) Flow diagram demonstrating post-processing of raw data. DOCT: Doppler optical coherence tomography.
One of the challenges in analyzing retinal OCE data is segmentation. It is necessary to segment the boundaries across layers so that data from each individual layer can be isolated for layer specific elasticity analysis. Another challenge of shear wave elasticity analysis is to trace the location of the wave surface over time for the purpose of wave speed calculation. With layered Doppler OCT data and the segmentation algorithm, the wave surface as a function of time in each layer can be segmented to calculate the shear wave velocity at every location.
In the SW-ARF-OCE setup, the raw data is obtained, and post-processing is performed to segment the layers and calculate the shear wave propagation speed. As shown in figure 3d–e, the OCT intensity and Doppler phase data are first obtained, and the OCT image is segmented based on a customized dynamic programming segmentation algorithm to isolate different retinal layers [25]. Then the segmented layers are applied to the displacement map, where the shear wave propagation surfaces for each layer is segmented as well. Next, the propagation speed is calculated for each location based on the slope of the segmented wave propagation for each layer. The velocity is based on the change in location over the time period, and the shear wave velocity map can be obtained. The relationship between the velocity Cs and the shear modulus μ, can be described with the equation , where ρ is the tissue density of approximately 1 kg/m3. The elastic modulus is approximately 3 times the shear modulus as demonstrated by previous literature [26] and can be calculated and mapped out as an elastogram.
D. Ex-vivo porcine eye preparation
The porcine eyeball was obtained within 24 hours of death. Since the eyeball was no longer fully transparent due to degradation, the anterior portion of the eye, including the cornea and the lens was removed along the iris. The vitreous was still attached to the retina and was kept in place during imaging to avoid retinal detachment. A 0.8% agar phantom was moulded around the posterior globe and used to keep the eye in place during imaging as well as to help preserve the shape of the posterior globe and prevent detachment. The sample was kept in phosphate-buffered saline during imaging to preserve freshness and as a medium for ultrasound propagation.
E. In-vivo rabbit experiment preparation
All rabbit experiments were performed according to the University of California, Irvine (UCI) Institutional Animal Care and Use Committee (IACUC) protocol. The rabbit was used for an AMD study, where it was exposed to a high fat diet, blue light, and nicotine for 8 weeks. Since the disease was localized, most of the central retina was still relatively healthy. The healthy region was used for this study. The rabbit was given 35 mg/kg of ketamine and 5 mg/kg of xylazine subcutaneously for initial anesthesia. Two drops of proparacaine HCl and atropine solution were applied topically for further anesthesia and dilation of the eye for imaging, respectively. The unconscious rabbit was propped onto the imaging stage and the eye was proptosed. Additional anesthesia was given via subcutaneous injection of ketamine (17.5 mg/kg) if the heart rate or oxygen levels indicate distress. After imaging was completed, the rabbit was euthanized with an intravenous injection of euthasol. When death was confirmed, the rabbit eye was enucleated and fixed in 10% buffered formalin for histological analysis.
III. Results
A. Ex-vivo porcine study
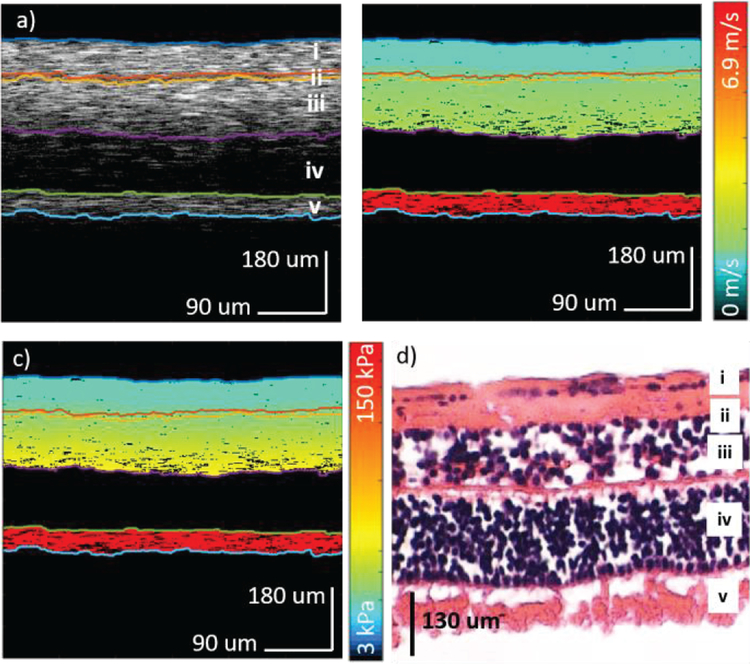
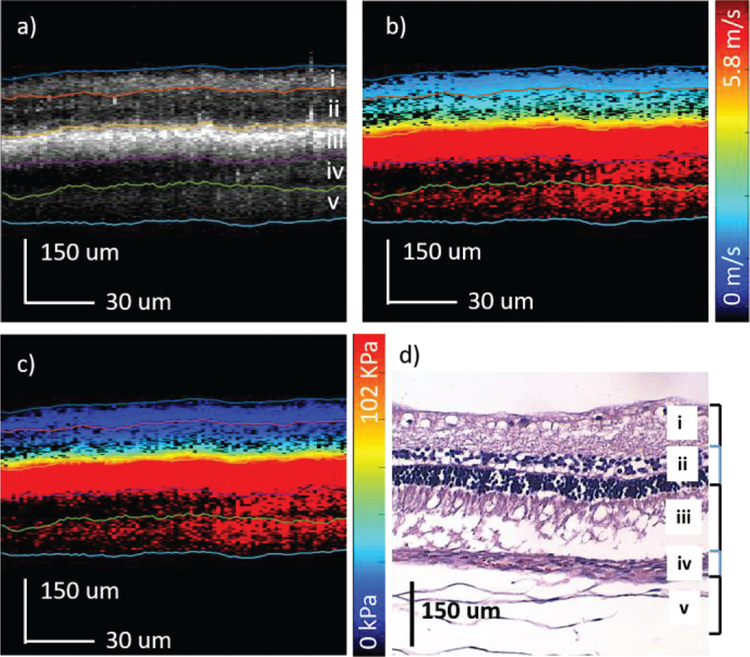
In order to test the confocal excitation and detection setup, imaging was first performed on an ex-vivo porcine retina using the same OCT system. The central retina region approximately 2 mm from the optic nerve on the temporal side was identified and imaged. The shear wave travels for over 700 um in the ex-vivo porcine retinal tissue and the system is capable of visualizing wave propagation in the whole depth of view. The results are shown in figure 4. The OCT intensity map is shown in figure 4a where segmentation was performed, and 5 different layers were isolated. Next, the corresponding shear wave velocity map and elastogram were calculated according to the above algorithm and shown in figure 4b and 4c, respectively. Finally, an H&E histology slide of a porcine retina is shown in figure 4d, where the 5 layers could be matched. Layer iv was omitted during the analysis due to low OCT scattering signal in the outer nuclear layer. Retinal detachment was observed and most likely due to detachment during the removal of the anterior portion and also due to the histology process.
Figure 4.
Ex-vivo porcine retina results. a) OCT of porcine central retina. b) Velocity map of the shear wave propagation. c) Elastogram of the corresponding region. d) H&E staining of the porcine retinal layers. i: optic nerve fibers (ONF) & ganglion cell layer (GCL); ii: inner plexiform layer (IPL); iii: inner nuclear layer (INL) & outer plexiform layer (OPL); iv: outer nuclear layer (ONL); v: photoreceptors (PR).
The elasticity results of the porcine retina are summarized in table 1, where the mean and standard deviations for each layer are listed and plotted. The elasticity increased from approximately 6 kPa on the ganglion side to over 140 kPa on the photoreceptor side. Since the photoreceptor side is close to the sclera of the eye globe, it is expected to be stiffer. The close-knit structure of the retinal layers most definitely interferes with the mechanical elasticity for each and is demonstrated by the gradual gradient increase in the elasticity over the layers from the ganglion side to the photoreceptors.
Table 1.
Summary of ex-vivo elasticity maps of each porcine retinal layer. ONL has been omitted due to low OCT signal in the outer nuclear layer. SD (standard deviation)
| Layer | Mean | Std | 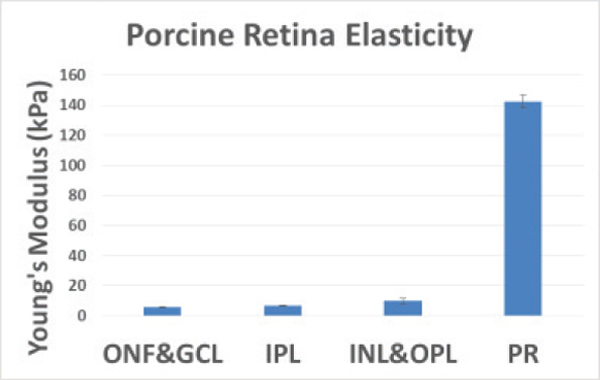 |
| ONF&GCL | 5.8 | 0.3 | |
| IPL | 6.7 | 0.26 | |
| INL&OPL | 10 | 1.97 | |
| ONL | N/A | N/A | |
| PR | 143 | 4.18 |
B. In-vivo rabbit study
Although the elasticity contrast between different retinal layers is apparent in the ex-vivo porcine model, the presence of intraocular pressure and blood vessel perfusion is lost. Therefore, it is not an accurate representation of the retinal structure in its natural environment. In order to address these issues, an in-vivo rabbit model was designed and imaged. For this shear wave elastography study, a healthy region of the central retina was chosen. The rabbit was put under anesthesia according to protocol and proptosed in the imaging setup as shown in figure 1c.
Imaging was performed on the central retina approximately 1.5 mm from the optic disc on the temporal side. The same B-M mode scanning scheme was used to capture the shear wave propagation. The OCT intensity image is shown in figure 5a, where segmentation was performed to isolate 5 different layers in the posterior globe. The shear velocity map is demonstrated in figure 5b, and a speed of up to 5.8 m/s can be visualized. The velocity was converted to the Young’s modulus in figure 5c. The elasticity of the first three layers from the ganglion side to the photoreceptor side are: 12.6±1.5, 35.7±18.9, 101.1±5.1 kPa. The bottom two layers of the eye could not be differentiated due to the fast propagation speed where the Young’s modulus is over 100 kPa. The shear wave is attenuated within 200 um in the lateral direction and the current system setup is not fast enough to capture the wave propagation within such a distance for the bottom three layers. This issue can be resolved by increasing the imaging speed or extending the traveling distance of the shear wave with a higher ultrasound excitation power. According to the histology, the bottom two layers are close to the sclera and are expected to be stiffer than the retina and have a higher propagation velocity.
Figure 5.
In-vivo rabbit elastography results. a) OCT of rabbit central retina. b) Shear wave velocity map. c) Elastogram of corresponding region. d) H&E histology showing some retinal detachment. i. nerve fiber, ganglion cell, & inner plexiform; ii. inner nuclear, outer plexiform, & outer nuclear; iii. RPE; iv. choroid; v. sclera
After the rabbit was euthanized, the posterior globe was fixed and processed for histological analysis. H&E staining is shown in figure 5d, where the layers of the retina can be corresponded to the OCT figure similar to literature [27]. The retinal detachment is likely a fixation artifact while the swelling in the sclera or layer v is caused by the repeated proptosis procedure. Three layers of the live rabbit retina have been distinguished with different elasticity values. The feasibility of using the SW-ARF-OCE method to probe the mechanical properties of the retina has been confirmed.
IV. Discussion & Conclusion
We have presented a SW-ARF-OCE method of measuring and quantifying the in-vivo elasticity map of the retina for the first time, to the best of our knowledge. This technology offers high resolution imaging and highly sensitive velocity maps that are used to quantitatively assess retinal layers. Furthermore, the confocal setup allows for easy access to the posterior eye during the in-vivo study. In addition, the system is non-invasive since OCT and ultrasound are readily used in ophthalmic clinics today. Ultrasound gel and steridrape based waterbaths are commonly used in clinics to couple ultrasound into the eye and are adaptable in the translation of our technology. This technology will allow researchers and physicians to study the mechanisms behind changes in the mechanical elasticity of the retina during disease onset and progression, which is crucial in both basic pathological research as well as clinical diagnosis.
Although the quantification of retinal mechanical properties has been demonstrated in-vivo, a few challenges remain to be addressed before the technology can be translated. First, a higher imaging speed is necessary to visualize stiffer posterior layers, such as the sclera. A faster line scan camera can address this issue. A faster line scan camera will help speed up the scan time and allow for spatial imaging of a single ARF pulse. The temporal resolution of shear wave propagation will be increased so that faster shear wave speeds can be tracked within stiffer tissue such as the sclera. Second, the propagation distance of the shear wave is limited to a few hundred microns due to the fast attenuation of the signal. A larger field of view can be achieved with a high power excitation pulse, more precise confocal alignment of the ultrasound excitation on retinal tissue, or more ideally, the implementation of an array transducer for a larger region of excitation and detection. It is also important to study the changes in the mechanical properties using a diseased model and examine its correlation to clinical impairments.
The mechanical index (MI) of the current system exceeds the federal ophthalmic ultrasound limit (0.23) by approximately 10 fold. However, since we use phase-Doppler to measure the displacement in hundreds-nanometer range and the displacement sensitivity of this imaging system is less than 1 nm [28], it will be possible to scale down the excitation ARF by at least 1 order of magnitude and still obtain sufficient signal. In addition, it will be possible to measure the shear wave propagation across a spatial region using a single pulse with the incorporation of a faster camera. This will also decrease the patient exposure to ARF and help the translation of this technique to clinics. In addition, our current OCT image is not optimal due to the bulk motion and noise that occurs during in-vivo imaging. There is a significant amount of bulk motion from the rabbit breathing and reflex motions. Furthermore, since imaging took place in a PBS bath, some signal loss was observed. Imaging in fluids also changes the natural focal characteristics of the rabbit eye since the refractive index change from air to cornea is different than that from PBS to cornea, so it is more difficult to obtain a perfect focus on the retina. The in-vivo optical setup may be improved by considering the use of ophthalmic gel to substitute for the PBS bath, fine tuning the optical focus, and also bulk motion removal.
In conclusion, we have demonstrated a novel method to quantify the mechanical elasticity of the retinal layers in-vivo using SW-ARF-OCE based on layer segmentation and shear wave analysis. We have first tested the feasibility on an ex-vivo porcine model, where 5 different retinal layers have been isolated and the mechanical elasticity was distinguished. In order to validate the feasibility of translating this technology, imaging was performed on an in-vivo New Zealand white rabbit retina model, where 3 layers of the retina could be analyzed and quantified for different mechanical properties. This study is a crucial stepping stone to the translation of the SW-ARF-OCE technology for clinical diagnosis and disease studies.
Acknowledgment
The authors would like to thank Dr. Don Minckler for his help in histological analysis. Dr. Z. Chen has a financial interest in OCT Medical Imaging Inc., which, however, did not support this work. Please address all correspondence to Dr. Q. Zhou and Dr. Z. Chen.
This work was supported by grant from the National Institutes of Health (R01HL-125084, R01HL-127271, R01EY-026091, R01EY-021529, P41EB-015890, 1 F31 EY027666), and the Air Force Office of Scientific Research (FA9550-14-1-0034).
Biography

Youmin He received the B.S and M.S degrees from the School of Electronics and Information Engineering, Beijing Jiaotong University, Beijing, China, in 2011 and 2014, respectively. In 2017, he received the M.S. degree in biomedical engineering from the University of California, Irvine, CA, USA and is currently a Ph.D. candidate working under the guidance of Dr. Z. Chen. His research interests include fast image segmentation, parallel computing for real time OCT imaging, high resolution OCT system for airway imaging, and ARF-OCE technology development and application on ophthalmology and cardiology.

Yueqiao Qu received the B.S. degree in biomedical engineering from the Johns Hopkins University in 2011 and the M.S. degree in biomedical engineering from the University of California, Irvine in 2015. She is currently a Ph.D. candidate working under the guidance of Dr. Z. Chen. In 2014, She was awarded the Cardiovascular Applied Research and Entrepreneurship Fellowship, offered by the Edwards Lifesciences Center for Advanced Cardiovascular Technology and the National Institutes of Health T32 grant. Her research interests include ARF-OCE technology development and applications in ophthalmology and cardiology, integrated intravascular ultrasound (IVUS) and OCT imaging, and miniature optical fiber catheter development for photoacoustic imaging, IVUS-OCT, and ARF-OCE.

Jiang Zhu received the Ph.D. degree in biology from Tsinghua University in 2009. He is now a postdoctoral researcher in Beckman Laser Institute. His interests include optical coherence elastography and Doppler OCT.

Yi Zhang received his Ph. D. degree from the Department of Mechanical and Electrical Engineering of Xiamen University, China in 2008. He is currently a Postdoctoral Research Associate at the Roski Eye Institute and the Department of Ophthalmology at the University of Southern California (USC), Los Angeles, CA. Before joining USC in 2012, he worked in the Division of Biomedical Engineering at University of Glasgow in Scotland, United Kingdom. Dr. Zhou is a member of the IEEE Ultrasonics, Ferroelectrics, and Frequency Control (UFFC) Society. His current research interests include the development of ophthalmic medical devices, MEMS technology, ultrasound transducers and arrays for biomedical applications. He has published more than 30 journal papers in this area.

Arya Saidi received his B.S. degree in Biomedical Engineering from the University of California, Irvine in 2010. He is currently a Doctor of Optometry candidate at the Southern California College of Optometry, and continuing his research in ophthalmic imaging at the Beckman Laser Institute and Medical Clinic under the guidance of Dr. Z. Chen. Arya was awarded the Chancellor’s Award of Excellence in Undergraduate Research at UC Irvine. His research interests include ARF-OCE applications in optometry, the development of animal models for ophthalmic imaging, and OCT imaging.

Teng Ma received his B.S.E degree from University of Michigan, Ann Arbor, USA majoring in Biomedical Engineering in 2011. He received his M.S. degree and Ph.D degree in Biomedical Engineering from University of Southern California, Los Angeles, USA in 2013 and 2015. He joined NIH Resource Center for Medical Ultrasonic Transducer Technology as a Research Assistant and Ph.D. candidate under supervision of Dr. K. Kirk Shung and Dr. Qifa Zhou. In 2013, two of his papers were selected as “Best Student Paper Finalist” and featured in the 2013 Joint UFFC, EFTF and PFM Symposium. His research interests include medical ultrasound technology and multi-modality intravascular imaging by combining ultrasonic and optical techniques, such as intravascular ultrasound (IVUS), intravascular optical coherence tomography (IV-OCT), intravascular photoacoustic imaging (IVPA), and acoustic radiation force optical coherence elastography (ARF-OCE). He is also actively working in translational research and medical device commercialization with entrepreneurial spirit to translate innovative technology from research to clinical benefits.

Qifa Zhou received his Ph. D. degree from the Department of Electronic Materials and Engineering of Xi’an Jiaotong University, China in 1993. He is currently a Research Professor at the NIH Resource on Medical Ultrasonic Transducer Technology and the Department of Biomedical Engineering and Industry & System Engineering at the University of Southern California (USC), Los Angeles, CA. Before joining USC in 2002, he worked in the Department of Physics at Zhongshan University in China, the Department of Applied Physics, Hong Kong Polytechnic University, and the Materials Research Laboratory, Pennsylvania State University. Dr. Zhou is a fellow of International Society for Optics and Photonics (SPIE) and American Institute for Medical and Biological Engineering (AIMBE). He is also a senior member of the IEEE Ultrasonics, Ferroelectrics, and Frequency Control (UFFC) Society and a member of the UFFC Society’s Ferroelectric Committee. He is a member of the Technical Program Committee of the IEEE International Ultrasonics Symposium. He is an Associate Editor of the IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control. His current research interests include the development of ferroelectric thin films, MEMS technology, nano-composites, and modeling and fabrication of high-frequency ultrasound transducers and arrays for medical imaging applications, such as photoacoustic imaging and intravascular imaging. He has published more than 130 journal papers in this area.

Zhongping Chen received the B.S. degree in applied physics from Shanghai Jiao Tong University, Shanghai, China, in 1982, the M.S. degree in electrical engineering from Cornell University, NY, USA, in 1987, and the Ph.D. degree in applied physics from Cornell University in 1993. He is currently a Professor of biomedical engineering and the Director of F-OCT Laboratory at the University of California, Irvine, CA, USA. He is a cofounder and the Board Chairman of OCT Medical Imaging, Inc. His research interests encompass the areas of biomedical photonics, microfabrication, biomaterials, and biosensors. His research group has pioneered the development of functional optical coherence tomography, which simultaneously provides high-resolution 3-D images of tissue structure, blood flow, and birefringence. He has published more than 250 peer-reviewed papers and review articles and holds a number of patents in the fields of biomaterials, biosensors, and biomedical imaging. Dr. Chen is a Fellow of the American Institute of Medical and Biological Engineering (AIMBE), a Fellow of SPIE, and a Fellow of the Optical Society of America.
Contributor Information
Youmin He, Department of Biomedical Engineering and the Beckman Laser Institute, University of California, Irvine, CA 92697 USA.
Yueqiao Qu, Department of Biomedical Engineering and the Beckman Laser Institute, University of California, Irvine, CA 92697 USA.
Jiang Zhu, Department of Biomedical Engineering and the Beckman Laser Institute, University of California, Irvine, CA 92697 USA.
Yi Zhang, Roski Eye Institute, Department of Ophthalmology and Biomedical Engineering, University of Southern California, Los Angeles, CA 90089 USA.
Arya Saidi, Marshall B. Ketchum University. Southern California College of Optometry, 2575 Yorba Linda Blvd, Fullerton, CA 92831 USA.
Teng Ma, Roski Eye Institute, Department of Ophthalmology and Biomedical Engineering, University of Southern California, Los Angeles, CA 90089 USA.
Qifa Zhou, Roski Eye Institute, Department of Ophthalmology and Biomedical Engineering, University of Southern California, Los Angeles, CA 90089 USA.
Zhongping Chen, Department of Biomedical Engineering and the Beckman Laser Institute, University of California, Irvine, CA 92697 USA.
References
- [1].Jager Rama D., Mieler William F., and Miller Joan W.. “Age-related macular degeneration.” New England Journal of Medicine 358, no. 24 (2008): 2606–2617. [DOI] [PubMed] [Google Scholar]
- [2].Bressler Neil M., Bressler Susan B., and Fine Stuart L.. “Age-related macular degeneration.” Survey of ophthalmology 32, no. 6 (1988): 375–413. [DOI] [PubMed] [Google Scholar]
- [3].Lim Laurence S., Mitchell Paul, Seddon Johanna M., Holz Frank G., and Wong Tien Y.. “Age-related macular degeneration.” The Lancet 379, no. 9827 (2012): 1728–1738. [DOI] [PubMed] [Google Scholar]
- [4].Chakravarthy Usha, Evans Jennifer, and Rosenfeld Philip J.. “Age related macular degeneration.” Bmj 340, no. 7745 (2010): 526–530. [DOI] [PubMed] [Google Scholar]
- [5].Hee Michael R., Baumal Caroline R., Puliafito Carmen A., Duker Jay S., Reichel Elias, Wilkins Jason R., Coker Jeffery G., Schuman Joel S., Swanson Eric A., and Fujimoto James G.. “Optical coherence tomography of age-related macular degeneration and choroidal neovascularization.” Ophthalmology 103, no. 8 (1996): 1260–1270. [DOI] [PubMed] [Google Scholar]
- [6].la Cour Morten, Jens Folke Kiilgaard, and Mogens Holst Nissen. “Age-related macular degeneration.” Drugs & aging 19, no. 2 (2002): 101–133. [DOI] [PubMed] [Google Scholar]
- [7].Krishnan L, Hoying JB, Nguyen H, Song H, Weiss JA. Interaction of angiogenic microvessels with the extracellular matrix. Am J Physiol Heart Circ Physiol 2007;293:H3650–H3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chen Kinon, Rowley Adrian P., and Weiland James D.. “Elastic properties of porcine ocular posterior soft tissues.” Journal of Biomedical Materials Research Part A 93, no. 2 (2010): 634–645. [DOI] [PubMed] [Google Scholar]
- [9].Sarks SH, Arnold JJ, Killingsworth MC, and Sarks JP. “Early drusen formation in the normal and aging eye and their relation to age related maculopathy: a clinicopathological study.” British Journal of Ophthalmology 83, no. 3 (1999): 358–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Friberg Thomas R., and Lace John W.. “A comparison of the elastic properties of human choroid and sclera.” Experimental eye research 47, no. 3 (1988): 429–436. [DOI] [PubMed] [Google Scholar]
- [11].Jones IL, Warner M, and Stevens JD. “Mathematical modelling of the elastic properties of retina: a determination of Young’s modulus.” Eye 6, no. 6 (1992): 556–559. [DOI] [PubMed] [Google Scholar]
- [12].Litwiller Daniel V., Lee Sung J., Kolipaka Arunark, Mariappan Yogesh K., Glaser Kevin J., Pulido Jose S., and Ehman Richard L.. “MR elastography of the ex vivo bovine globe.” Journal of magnetic resonance imaging 32, no. 1 (2010): 44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gennisson J-L, Thomas Deffieux, Mathias Fink, and Michaël Tanter. “Ultrasound elastography: principles and techniques.” Diagnostic and interventional imaging 94, no. 5 (2013): 487–495. [DOI] [PubMed] [Google Scholar]
- [14].Detorakis Efstathios T., Drakonaki Eleni E., Tsilimbaris Miltiadis K., Pallikaris Ioannis G., and Giarmenitis Spiridon. “Real-time ultrasound elastographic imaging of ocular and periocular tissues: a feasibility study.” Ophthalmic Surgery, Lasers and Imaging Retina 41, no. 1 (2010): 135–141. [DOI] [PubMed] [Google Scholar]
- [15].Wang Shang, and Larin Kirill V.. “Shear wave imaging optical coherence tomography (SWI-OCT) for ocular tissue biomechanics.” Optics letters 39, no. 1 (2014): 41–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Qu Y, Ma T, He Y, Yu M, Zhu J, Miao Y, Dai C, Patel P, Shung KK, Zhou Q and Chen Z, 2017. Miniature probe for mapping mechanical properties of vascular lesions using acoustic radiation force optical coherence elastography. Scientific Reports, 7, p.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zhu J, Miao Y, Qi L, Qu Y, He Y, Yang Q and Chen Z, 2017. Longitudinal shear wave imaging for elasticity mapping using optical coherence elastography. Applied Physics Letters, 110(20), p.201101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zhu J, Qi L, Miao Y, Ma T, Dai C, Qu Y, He Y, Gao Y, Zhou Q and Chen Z, 2016. 3D mapping of elastic modulus using shear wave optical micro-elastography. Scientific reports, 6, p.35499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zhu J, Qu Y, Ma T, Li R, Du Y, Huang S, Shung KK, Zhou Q and Chen Z, 2015. Imaging and characterizing shear wave and shear modulus under orthogonal acoustic radiation force excitation using OCT Doppler variance method. Optics letters, 40(9), pp.2099–2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ford Matthew R., Dupps William J., Rollins Andrew M., Sinha Roy Abhijit, and Hu Zhilin. “Method for optical coherence elastography of the cornea.” Journal of biomedical optics 16, no. 1 (2011): 016005–016005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Han Zhaolong, Aglyamov Salavat R., Li Jiasong, Singh Manmohan, Wang Shang, Vantipalli Srilatha, Wu Chen, Liu Chih-hao, Twa Michael D., and Larin Kirill V.. “Quantitative assessment of corneal viscoelasticity using optical coherence elastography and a modified Rayleigh–Lamb equation.” Journal of biomedical optics 20, no. 2 (2015): 020501–020501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wang Shang, and Larin Kirill V.. “Noncontact depth-resolved micro-scale optical coherence elastography of the cornea.” Biomedical optics express 5, no. 11 (2014): 3807–3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Qu Yueqiao, Ma Teng, He Youmin, Zhu Jiang, Dai Cuixia, Yu Mingyue, Huang Shenghai et al. “Acoustic radiation force optical coherence elastography of corneal tissue.” IEEE Journal of Selected Topics in Quantum Electronics 22, no. 3 (2016): 288–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Song S, Le NM, Huang Z, Shen T, & Wang RK (2015). Quantitative shear-wave optical coherence elastography with a programmable phased array ultrasound as the wave source. Optics letters, 40(21), 5007–5010. [DOI] [PubMed] [Google Scholar]
- [25].Huang S, Piao Z, Zhu J, Lu F, & Chen Z (2015). In vivo microvascular network imaging of the human retina combined with an automatic three-dimensional segmentation method. Journal of biomedical optics, 20(7), 076003–076003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Razani Marjan, Mariampillai Adrian, Sun Cuiru, Timothy WH Luk Victor XD Yang, and Kolios Michael C.. “Feasibility of optical coherence elastography measurements of shear wave propagation in homogeneous tissue equivalent phantoms.” Biomedical optics express 3, no. 5 (2012): 972–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Cohen Ethan, Agrawal Anant, Connors Megan, Hansen Barry, Charkhkar Hamid, and Pfefer Joshua. “Optical coherence tomography imaging of retinal damage in real time under a stimulus electrode.” Journal of neural engineering 8, no. 5 (2011): 056017. [DOI] [PubMed] [Google Scholar]
- [28].Zhang J, Rao B, Yu L and Chen Z High-dynamic-range quantitative phase imaging with spectral domain phase microscopy. Optics Letters, (2009). [DOI] [PMC free article] [PubMed]